Abstract
Background
Positive psychological constructs, especially optimism, have been linked with superior cardiovascular health. However, there has been minimal study of positive constructs in patients with acute coronary syndrome (ACS), despite the prevalence and importance of this condition. Furthermore, few studies have examined multiple positive psychological constructs and multiple cardiac-related outcomes within the same cohort to determine specifically which positive construct may affect a particular cardiac outcome.
Materials and methods
The Gratitude Research in Acute Coronary Events (GRACE) study examines the association between optimism/gratitude 2 weeks post-ACS and subsequent clinical outcomes. The primary outcome measure is physical activity at 6 months, measured via accelerometer, and key secondary outcome measures include levels of prognostic biomarkers and rates of nonelective cardiac rehospitalization at 6 months. These relationships will be analyzed using multivariate linear regression, controlling for sociodemographic, medical, and negative psychological factors; associations between baseline positive constructs and subsequent rehospitalizations will be assessed via Cox regression.
Results
Overall, 164 participants enrolled and completed the baseline 2-week assessment; the cohort had a mean age of 61.5 +/− 10.5 years and was 84% men; this was the first ACS for 58% of participants.
Conclusion
The GRACE study will determine whether optimism and gratitude are prospectively and independently associated with physical activity and other critical outcomes in the 6 months following an ACS. If these constructs are associated with superior outcomes, this may highlight the importance of these constructs as independent prognostic factors post-ACS.
Keywords: optimism, gratitude, physical activity, acute coronary syndrome, coronary artery disease
1. Introduction
Each year, 1.1 million Americans are hospitalized for an acute coronary syndrome (ACS; myocardial infarction [MI] or unstable angina [UA]).1 Among post-ACS patients, approximately 20% will be re-hospitalized for ischemic heart disease or suffer mortality within the next year.2 It is therefore critical to identify factors that may protect against adverse events and improve overall prognosis during the high risk post-ACS period.
Psychological factors may play an important role in post-ACS prognosis. Depression following ACS has been associated with recurrent cardiac events, rehospitalizations, and death, independent of sociodemographic factors or medical illness severity,3 and has been declared an official risk factor for poor prognosis following ACS by the American Heart Association.4 Likewise, elevated anxiety symptoms and formal anxiety disorders have been associated with adverse events in patients with cardiovascular disease, including those with an ACS.5,6 In contrast, positive psychological factors may have a beneficial impact on cardiac prognosis. Several syntheses of the literature have found that positive psychological well-being is associated with superior cardiac health7,8 and reduced mortality in patients with medical illness;9 such connections are typically independent of traditional risk factors and above and beyond the adverse effects of depression. Optimism (a general expectation that the future will be favorable) in particular may be associated with superior medical outcomes in those with and without known heart disease, with several large longitudinal studies and a large meta-analysis finding links between optimism and superior cardiac prognosis.8,10,11 These positive constructs may be especially related to physical activity and other cardiac health behaviors, as several prior studies have found links between positive psychological well-being and increased activity, healthier diet, and reduced rates of smoking.12–14
However, critical gaps in the literature exist. First, there has been minimal study of the prognostic impact of positive psychological constructs following an ACS, despite the high rates of adverse events in this population. Such studies could inform post-ACS assessment and interventions. Second, gratitude (a general disposition to appreciate and be thankful for people, events, and experiences in one’s life) is a commonly experienced psychological state following an ACS; approximately one-half of post-ACS patients experience increased gratitude.15 However, there has been minimal study of the association of gratitude with cardiac prognosis. Third, relatively few studies exploring positive psychological constructs and health outcomes have examined more than one positive state to assess whether one construct may be more or less prognostically important. Finally, very few investigations in this field have simultaneously examined the prospective effects of positive psychological well-being on biological, behavioral, and clinical outcomes to parse the potential mechanistic effects of positive constructs.
Accordingly, in the Gratitude Research in Acute Coronary Events (GRACE) study, we will examine the prospective effect of optimism and gratitude, measured 2 weeks post-ACS, on subsequent health behaviors, prognostic biomarkers, and clinical outcomes over the subsequent 6 months.
2. Study methods
2.1 Overview
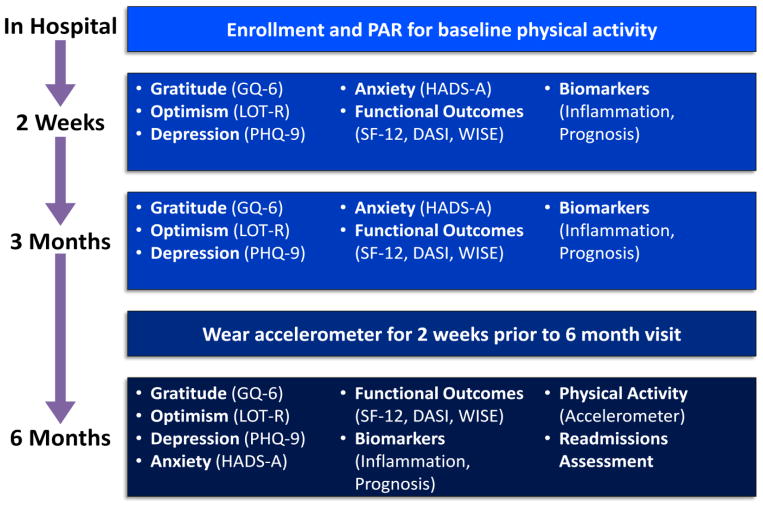
This is a prospective observational study of the impact of baseline gratitude and optimism over a 6 month follow up period on health-related outcomes of patients hospitalized for an ACS. Patients were enrolled in the hospital, will have in-person study visits 2 weeks post-ACS and 6 months later, and will complete interim self-report assessments by phone at 3 months (Figure 1).
Figure 1.
Timeline of study assessments.
DASI=Duke Activity Status Index; GQ-6=Gratitude Questionnaire Six Item Form; HADS-A=Hospital Anxiety and Depression Scale—Anxiety Subscale; LOT-R=Life Orientation Test-Revised; MOS-SAS=Medical Outcomes Study Specific Adherence Scale; PAR=Physical activity recall; PHQ-9=Patient Health Questionnaire-9; SF-12=Short Form 12; WISE=Women’s Ischemia Symptom Evaluation Scale.
The primary outcome measure for the study is physical activity, measured by accelerometer, at 6 months. The enrollment goal for the project was a minimum of 150 patients; enrollment is now complete. Approval from the Partners Healthcare Institutional Review Board (IRB) for the full protocol was obtained prior to commencement of study procedures.
2.2 Eligibility criteria
To be potentially eligible, patients were required to be admitted to one of three cardiac units at MGH, an urban academic medical center, for an ACS.
2.2.1 Cardiac inclusion criteria
To receive a diagnosis of ACS, patients had to meet criteria for MI or UA. For MI, potential subjects met established consensus criteria,16 specifically: (1) elevation of cardiac biomarkers (cardiac troponin) in addition to: (2) symptoms of ischemia (e.g., acute chest pain), (3) ischemic changes on electrocardiogram (e.g., ST-segment elevation or ST-depression and T-wave inversions), or (4) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. For UA, subjects met formal standardized criteria used in prior cardiac studies:17, 18 (1) crescendo angina, (2) new onset (within 1 month) angina with minimal exertion, or (3) angina with minimal exertion or at rest. When unclear, diagnoses were adjudicated by the study cardiologist (J.J.).
2.2.2. Exclusion criteria
Patients were excluded if they had: (1) a periprocedural ACS (ACS occurring in the setting of another medical procedure), (2) a condition likely to alter biomarkers of interest, (3) a medical condition likely to be terminal within the timeframe of the study, (4) an unrelated condition limiting physical activity, (5) an inability to communicate in English, or (6) a cognitive disturbance that precluded participation or informed consent, as identified using a six-item cognitive screen designed to assess suitability for research participation.19
Patients with a periprocedural ACS (e.g., type 4 or type 5 MI16) were excluded due to concerns that such events may occur in the absence of structural heart disease and likely represent a different pathophysiology, course, and prognosis than those with ‘endogenous’ ACS. It is true that such patients, who may have cardiovascular systems that are especially prone to (physical or emotional) stress-related cardiac events, may have even greater associations between well-being and cardiac outcomes. However, to maintain the most homogeneous cohort for this project, they were excluded from this initial trial.
Conditions likely to alter biomarkers of interest included renal failure requiring hemodialysis, and some inflammatory illnesses (systemic lupus erythematosus, inflammatory bowel disease, and Wegener’s granulomatosis) that could substantially alter markers of inflammation. Those unable to complete physical activity due to an unrelated medical condition (e.g., arthritis) were excluded because such a condition would impair measurement of the primary study outcome (physical activity in steps). Determination of exclusion criteria were made in conjunction with the primary medical team (e.g., regarding comorbid or terminal conditions) with consultation and adjudication from the study team cardiologist (JJ) as needed.
2.3 Recruitment, enrollment, and informed consent
Identification of potentially eligible patients was performed via several steps. Patients admitted for an ACS to one of the three cardiac units were identified via a daily review of the patient census. If a potential participant was identified, a study staff member notified one of the patient’s clinicians (physician, nurse, or nurse practitioner), who then asked the patient if they were willing to hear about an optional study. If the patient was amenable, a physician investigator explained the rationale, procedures, risks, and benefits of the study. The study staff member then assessed for inclusion and exclusion criteria (e.g., presence of cognitive deficits on six-item screen) via interview. For patients meeting all study criteria, written informed consent was obtained by a study investigator and a baseline assessment of physical activity was obtained.
3. Study aims and outcome assessments
3.1 Specific aims
The aims of the study are to prospectively assess the association between optimism/gratitude 2 weeks after ACS and (1) subsequent adherence to physical activity (primary outcome measure) and other health behaviors known to improve post-ACS prognosis, (2) levels of inflammatory and prognostic biomarkers, and (3) rates of cardiac readmission (along with other medical/functional outcomes), over the next 6 months.
3.2 Outcome assessment procedures
Figure 1 displays the study assessments to be used at each time point. During in-hospital enrollment, participants completed a detailed measure of physical activity for the preceding 7 days via the Physical Activity Recall (PAR) 7-day recall scale.20 At the 2 week baseline in-person study visit, participants completed self-report measures of psychological status, health behavior adherence, as well as functional and medical status, and blood was collected for baseline biomarker assessment. Self-report measures will be repeated at 3 months. Immediately preceding the 6 month follow-up visit, participants will wear an accelerometer to measure physical activity. Finally, at the 6 month visit, we will repeat self-report measures and biomarkers, collect accelerometers, and gather information on readmissions from participants.
3.3 Data collection for baseline characteristics
Data for baseline characteristics was collected at the enrollment interview and using the electronic medical record at discharge to characterize our population and to control for specific variables in multivariate analyses. This data included sociodemographic variables (age, gender, ethnicity/race, and living status) and medical variables (hyperlipidemia, diabetes mellitus, hypertension, smoking status, prior ACS, admission diagnosis [MI or UA], body mass index, and length of hospitalization). We also gathered information about medications at discharge (aspirin, beta-blockers, angiotensin converting enzyme-inhibitors, angiotensin receptor blockers, statins, oral antiplatelet agents, antidepressants, and benzodiazepines).
3.4 Study outcome measures
3.4.1 Positive psychological variables
Optimism will be measured using the Life Orientation Test-Revised (LOT-R).21 The LOT-R has been validated and extensively used in a large number of studies examining connections between optimism and health outcomes.11,22,23 Example items include the positively worded “I expect more good things to happen to me than bad” and the negatively-worded “I hardly ever expect things to go my way.” Participants respond to each item with the following scale: 4=agree a lot, 3=agree a little, 2=neither agree nor disagree, 1=disagree a little, and 0=disagree a lot. Per convention,21 a total optimism score will be computed by summing the responses of all six items. In addition to the total optimism scale, the LOT-R contains two embedded 3-item optimism and pessimism subscales, with higher scores on each subscale representing higher optimism and lower pessimism, respectively.
Gratitude will be assessed using the Gratitude Questionnaire-6 (GQ-6).24 The GQ-6 is a brief, validated six-item measure of dispositional gratitude used in many prior studies.25–28 Example items include “I have so much in life to be thankful for” and the reverse scored “When I look at the world, I don’t see much to be grateful for.” We selected this scale because it has been widely used, it has excellent psychometric properties, and it is not strongly correlated with negative affective states (r=−0.2 to −0.3 correlation with anxiety and depression).24 Its brevity is also a major advantage.
3.4.2 Physical activity and related behaviors (Aim #1 and primary study outcome)
Physical activity in steps per day will be collected immediately prior to the 6 month assessment using the Fitlinxx Pebble uniaxial accelerometer (Fitlinxx, Shelton, CT). The Pebble is a small (size of a half dollar coin), silent device that hooks to a shoe or belt, measures activity in 20 minute epochs, and stores activity data for at least 2 weeks.
Devices will be sent to participants 14 days prior to their 6 month follow-up visit. Participants will wear the devices and complete parallel daily physical activity logs to allow correlation of accelerometer data and self-reported activity. The devices will be collected at the 6 month follow-up visit, and step data (identified with study ID number only) will be offloaded from the Pebbles to the Fitlinxx server via USB connection. The Pebble records activity data in 20 minute epochs, and study staff trained in the software/interface will review and consolidate the data for each participant. Similar to other published protocols for measuring physical activity,29 a minimum of 6 adequate wear days will be required with a total of 8 confirmed hours constituting an adequate wear day. If participants fail to achieve adequate step data collection, they will be asked to re-wear the devices following the visit and to return them by mail. We specifically chose Fitlinxx accelerometers because they are wearable on the belt or shoe with little participant burden, have been validated against other pedometers and accelerometers,30 and they have been successfully used in activity coaching31,32 as well as a study of physical activity in patients with pulmonary disease.33 Though these devices are classified as accelerometers, they have been most often validated and used to count steps rather than examine activity intensity or caloric expenditure, and therefore we decided upon step count as the primary activity variable.30–32
To control for baseline physical activity preceding the ACS, the PAR was completed at enrollment (recalling activity in the 7 days prior to admission). This scale has good test-retest reliability34 and correlates with activity measured by diary and by accelerometer in medically ill persons.35 We chose to administer this measure in the hospital given the level of detail required for this assessment; waiting until the 2 week visit to obtain this information may have resulted in less detailed or less accurate recollection of baseline activity prior to hospitalization. The PAR will be repeated at 6 months to allow cross-validation with step counter data.
Rationale for choice of physical activity as primary study outcome measure
We selected physical activity as the primary outcome measure for several reasons. First, physical activity plays a major role in cardiovascular and overall health, and is a key modifiable prognostic factor in ACS patients.36 Second, increasing physical activity is key to preventing the development or recurrence of many other medical illnesses;37–39 hence findings related to physical activity could be relevant to many medically ill persons. Third, there has been consistent evidence for psychological factors influencing physical activity40,41 and prior studies have found that both dispositional optimism and interventions targeting gratitude have been associated with increased physical activity.13,28 Finally, physical activity can be objectively measured in a relatively straightforward manner. We will use accelerometers to assess activity because they are often considered to be the standard for measuring habitual physical activity.42,43
Adherence to cardiac health behaviors (Aim #1 secondary outcome)
In addition to physical activity, broader self-reported adherence to health behaviors will be measured using items from the Medical Outcomes Study Specific Adherence Scale (MOS SAS). The selected items from the MOS SAS assess frequency of adherence to diet, physical activity, and medication over the preceding month. Our group and others have used this scale (and these items) to assess adherence in prior studies examining psychological factors in cardiac patients.44,45 At the baseline visit (2 weeks post-ACS), we inquired about health behaviors in the month preceding admission using this scale.
3.4.3 Biomarker outcomes (Aim #2 outcome)
For our Aim #2 analysis, we will examine several specific biomarkers. We will focus primarily on circulating markers related to systemic inflammation, given that coronary heart disease is increasingly understood as a disorder of inflammation, involving an ongoing inflammatory response and the presence of inflammatory cells in arterial plaques.46–48 Higher levels of circulating inflammatory markers have also been associated with increased mortality in cardiac patients,49–51 including specific studies in post-ACS populations.52 Furthermore, psychological factors, including positive psychological constructs, have been associated with inflammation in non-ACS populations.53–56
High sensitivity C-reactive protein (hsCRP) will be measured based on specific studies in patients finding that hsCRP shortly after an ACS is independently associated with mortality.57–59 Depression has been associated with higher levels of hsCRP,60 and prior studies examining positive psychological constructs and hsCRP have been mixed.56
Interleukin-6 (IL-6) will be measured based on its relationship with adverse cardiac events,61,62 and based on prior studies in patients with and without heart disease finding positive affect or optimism to be associated with lower levels of IL-6.56,63
Tumor necrosis factor-alpha (TNF-α) will be measured similarly because it is an important prognostic factor in the development of heart disease61 and is associated with recurrent cardiac events following ACS.64 As with IL-6, positive affect, measured using several different instruments, has been associated with lower levels of TNF-α.56
Soluble intracellular adhesion molecule-1 (sICAM-1) will be examined as an additional cardiac prognostic biomarker related to inflammation. Levels of these adhesion molecules are increased on the surface of vascular endothelial cells in response to stress-induced activation of inflammatory cytokines, and these molecules mediate pathways responsible for vascular inflammation.65 Prior studies of optimism have used this marker to assess endothelial function, and higher optimism levels have been linked in prior studies to lower levels of sICAM-1 in patients without pre-existing cardiac disease.63,66
Finally, in addition to these inflammation-related markers, we will measure N-terminal pro-brain natriuretic peptide (NT-proBNP). This marker is associated with overall mortality risk after ACS.67,68 Mood symptoms have been linked to NT-proBNP levels in some, but not all, studies,69,70 but this marker has not been closely examined in prior studies of positive psychological constructs.
For all biomarker sample collection at 2 week and 6 month visits, blood will be collected by the study research coordinator. Samples are allowed to clot at room temperature for 30 minutes and then centrifuged and the serum decanted. Samples (marked only with study ID number) are stored at −80°C, and will be analyzed in batch by immunoassay kits, as per published methods (and per the team’s prior experience analyzing these biomarkers) via the MGH Research Core Laboratory (hsCRP, sICAM-1) and the Singulex Corporation (IL-6, TNF-α, NT-proBNP).
3.3.4 Rehospitalizations (Aim #3 outcome)
Nonelective cardiac readmissions
Data on readmissions will be collected from multiple sources. Subjects will be queried about all readmissions at the 6 month follow-up assessment, with data gathered about timing of admission, symptoms, cause, and treatment. In addition, study staff will contact patients’ primary medical/cardiology providers at the end of the 6 month study period to inquire about readmissions and their cause. This systematic inquiry of providers will assist in adjudication of the cause of admissions and can identify admissions not mentioned by the participant. Finally, participants’ electronic medical records across the Partners Healthcare system (the healthcare system that includes MGH and numerous other hospitals, sub-acute care settings, and community health centers throughout the Boston metropolitan area) will be systematically reviewed over the 6 month follow-up timeframe. For any admissions outside of the Partners system, additional records will be obtained with appropriate release of information to identify principal diagnosis and other specific details. All future admissions that had been planned at the time of discharge (e.g., readmission for sequential cardiac stent placement in those with complex lesions) and any elective admissions will be excluded from the analysis. Determination of cardiac (vs. noncardiac) cause for readmissions will be completed based on all available data including principal diagnosis; when unclear, this determination will be adjudicated by the study cardiologist (J.J.).
All-cause nonelective readmissions
As an exploratory outcome, we will also collect data on all admissions, regardless of cause; as previously, we will exclude planned and elective admissions.
3.4.5 Additional patient-reported outcomes (Aim #3 secondary measures)
Function
We will assess function using the Duke Activity Status Index (DASI). The DASI71 is a 12-item yes/no instrument that has been used to assess health status among cardiac patients in a wide variety of prior studies.72–74 Questions ask about different functional tasks, for example, “Can you do light work around the house like dusting or washing dishes?”
Cardiac symptoms
We will measure the presence and severity of cardiac disease with ten symptoms adapted from a scale used in the NHLBI Women’s Ischemia Symptom Evaluation (WISE) study75 (chest pain/pressure, palpitations, lightheadedness, sweating, jaw pain, arm/shoulder pain, weakness, nausea, and indigestion) that are felt to best characterize the range of symptoms experienced by patients with prior ACS. We have used this scale in prior studies of cardiac patients,76,77 and have found it to be easy to use and well-accepted.
Health-related quality of life (HRQoL) will be measured using the Medical Outcomes Study Short Form-12 (SF-12) scale.78 Completion of the scale generates both a mental component score (MCS) and physical component score (PCS). This scale, and its parent scale, the SF-36, have been used in prior studies of patients with cardiovascular disease,79,80 including our prior trials in cardiac patients.81
Measures of negative psychological status
We will use two measures of negative psychological states to control for ill-being in our analyses and as a secondary outcome measure. For depression, we will utilize the Patient Health Questionnaire-9 (PHQ-9) score, a nine-item scale inquiring about the frequency of the nine symptoms of major depression in the prior two weeks. It has been found to have good sensitivity and specificity for major depression diagnosis in patients with heart disease.82–84 For anxiety, we will utilize the Hospital Anxiety and Depression Scale anxiety subscale (HADS-A).85 The HADS-A is an anxiety scale designed for use with medically ill patients; it has a minimum of somatic items, and has been used in multiple studies of cardiac patients,86–88 including our prior trials in hospitalized cardiac patients.18,76
At all assessments, patients who endorsed Item 9 on the PHQ-9 (which inquires about thoughts of death or self-harm) with any frequency other than “not at all,” will undergo additional questions via a scripted safety protocol89 that inquires about thoughts of death versus active suicidal ideation, prior history of suicide attempt, and other elements of suicide risk assessment (e.g., intent, plan, availability of plan elements, and mitigating factors). The study staff member will then discuss the specific elements of this evaluation with a study psychiatrist, who will speak with the patient as warranted, and intervene if necessary (including arranging transportation to emergency department if required).
4. Data analysis
4.1 Specific Aim #1 (Primary Aim): To assess the association of baseline optimism/gratitude, measured 2 weeks post-ACS, with subsequent physical activity measured by accelerometer 6 months later
We will first examine univariate associations between baseline LOT-R (optimism) and GQ-6 (gratitude) scores with mean number of daily steps as measured by accelerometer at the 6 month follow-up using Pearson correlation. We will then utilize hierarchical creation of adjusted multivariate models. In the minimally-adjusted model (Model 1), we will include the positive psychological variable (optimism/gratitude), age, and gender. In Model 2 (social and medical factors), we will add a marker of social support (living alone), measures of ACS severity and history (peak troponin T during admission and prior ACS), and a measure of overall medical comorbidity (the Charlson comorbidity index).90 Finally, in the fully adjusted model (Model 3), we will add measures of depression and anxiety (PHQ-9 and HADS-A). For this outcome, we will also control for baseline physical activity using the PAR. Of note, all variables will be included in the models as described above, with no automated stepwise forward selection or backward elimination procedures used.91
As a secondary health behavior outcome, we will utilize summed MOS SAS scores for adherence to diet, exercise, and medications as a continuous outcome variable, utilizing the same iterative covariate adjustment, including controlling for baseline values, in the multivariate models. To account for missing data, we will complete exploratory analyses using random effects models with a random intercept for each patient, allowing us to include participants who did not provide 6 month data.
4.2 Specific Aim #2: To examine the association of baseline optimism/gratitude at 2 weeks with prognostic cardiac biomarkers at 6 months
We will first examine levels of each biomarker (hsCRP, IL-6, TNF-α, sICAM-1, NT-proBNP) at 6 months as the dependent variable. As with physical activity, we will perform univariate analyses using Pearson correlations, and multivariate analyses using linear regression analyses, with iterative adjustment over the three successive hierarchical models as in Aim #1. We will analyze these initially as absolute values without controlling for baseline biomarker values, then in main analyses will control for 2 week biomarker levels in the models. As a secondary analysis, we will also examine biomarkers as categorical variables (e.g., via median split).
4.3 Specific Aim #3: To examine the association of baseline optimism/gratitude with cardiac rehospitalizations and additional medical/functional outcomes
We will complete time-to-event analyses. In preliminary analyses, we will divide baseline LOT-R and GQ-6 scores at the median split and examine between-group differences in cardiac rehospitalizations using Kaplen-Meier curves and log-rank tests of significance. For our primary analyses of cardiac rehospitalizations, we will utilize multivariate Cox regression to examine connections between continuous LOT-R and GQ-6 scores. We will control for age and gender in the primary model; additional covariates will not be included in the model because of the risk of overfitting92 based on the number of expected rehospitalization events (approximately 20–25% of the sample). However, in an exploratory model we will additionally control for overall medical burden/comorbidity (Charlson Index90).
We will also perform a number of secondary analyses for Aim #3. First, we will examine all-cause rehospitalizations as an outcome variable, using identical methods to those used for cardiac rehospitalizations. For self-report measures of medical, psychological, and functional status (DASI, WISE, SF-12, PHQ-9, HADS-A), we will utilize Pearson correlations (univariate analyses) and linear regression (Models 1–3), in an identical manner as described for physical activity and biomarkers. To account for missing data, as in the analysis of self-reported adherence in Aim #1, we will complete exploratory analyses using random effects models with a random intercept for each patient.
All analyses will be performed using Stata statistical software (version 11.2, StataCorp, College Station, TX). Statistical significance will be set at p<.05, though a conservative correction for multiple comparisons for the Aim #2 biomarker analyses using a Bonferroni correction93 would have set the p value for significance at p<.01; this will be noted in reporting of results. All tests will be two-tailed.93
4.4 Power calculation for primary outcome measure
Prior studies of psychological factors and physical activity in medical patients have found that psychological states/symptoms have moderate to large effects on physical activity.41,94,95 For the originally planned enrollment of at least 150 participants, assuming follow-up data from 85% of subjects (consistent with our similar prior studies77,81) and a conservatively calculated moderate effect size (ρ=.3) of gratitude on activity (univariate), this study will be powered at 94% to detect a significant association between both positive psychological constructs and physical activity as measured in steps using a two-tailed alpha of .05 (calculated via G*Power 3.1.2). Given that we were able to enroll and obtain baseline data from 164 participants (see below), with a projected 85% of participants providing follow-up data, our power to detect between-group differences will be slightly greater (95%).
The study was not designed to be fully powered for Aim #2 outcomes, but using an estimated r=.2 correlation between positive constructs and inflammatory markers seen in a prior study by Brouwers and colleagues,96 the study will be powered at 67% to detect between-group differences using two-tailed tests and p<.05. For the Aim #3 functional and medical outcomes, using a prior study by Pelle and colleagues97 (r=.22–.25, depending on specific outcome variable), the study will be powered at 76–86% to detect between-group differences in these outcome measures.
5. Baseline data
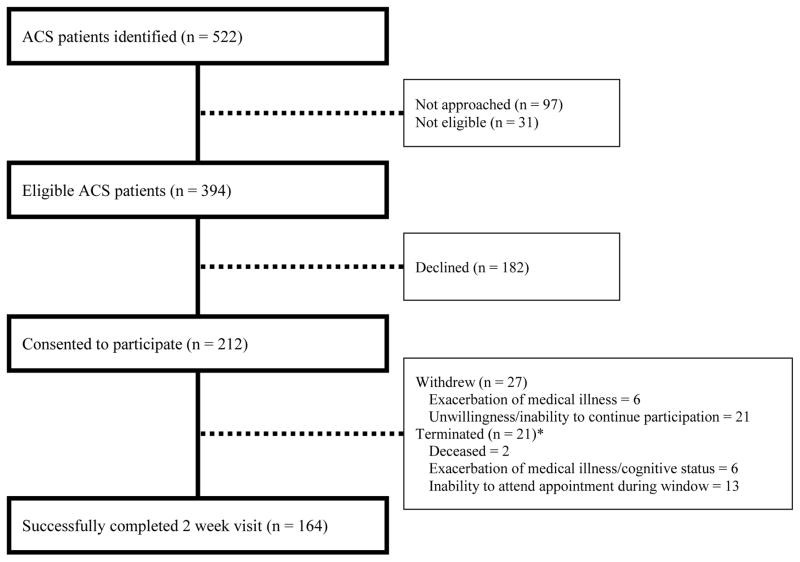
Figure 2 presents the CONSORT diagram for GRACE. Recruitment occurred from September 2012 through January 2014. A total of 522 potentially eligible patients were identified over that period. Among the 394 approached patients who met all eligibility criteria, 212 were enrolled and 164 successfully completed the 2-week visit.
Figure 2.
Study recruitment and enrollment.
* Terminated refers to participants who were withdrawn from the study by the study team, rather than those who actively requested to withdraw from the study.
When comparing baseline characteristics of patients who declined participation to those who enrolled, there were no significant differences in primary diagnosis (UA or MI) or race (White or non-White). However, patients who declined were significantly older (mean age: 66.1 [declined] vs. 62.0 [enrolled]; t=3.89; p<.001) and were more likely to be women (29.1% [declined] vs.19.4% women [enrolled]; χ2=6.01; p=.014). Given that, across all patients approached for participation, women were older (mean age: 67.4 [women] vs. 63.2 [men]; t=3.48; p<.001), we combined these variables in multivariate analysis (logistic regression) examining enrollment predictors and found that gender became non-significant and only older age was associated with declining participation (β=.029; p<.001).
Baseline sociodemographic characteristics, medical variables, psychiatric status, and baseline study outcome variables are listed in Table 1. Overall, the mean age of subjects was 61.5 (standard deviation [SD] 10.5) years, 84% were men, and 84% were White. The majority of participants had more than one cardiac risk factor, with hyperlipidemia (81%) and hypertension (63%), the most common diagnosed risk factors. This was the first ACS for 58% of participants, and participants were hospitalized for a mean of 3.0 (SD 2.2) days.
Table 1.
Baseline socio-demographic and clinical characteristics.
| Characteristics (N=164) | N (%)* |
|---|---|
| Demographics and psychosocial characteristics | |
| Age (mean [SD]) | 61.5 (10.5) |
| Male sex | 137 (83.5) |
| White | 137 (83.5) |
| Married | 113 (68.9) |
| Living alone | 38 (23.2) |
| Medical history | |
| BMI (mean [SD]) | 28.9 (5.2) |
| Hypertension | 103 (62.8) |
| Diabetes mellitus | 34 (20.7) |
| Hyperlipidemia | 132 (80.5) |
| Current smoking | 21 (12.8) |
| Prior ACS | 69 (42.1) |
| Diagnosis: MI | 88 (53.7) |
| Length of stay (days) | 3 (2.2) |
| Labs (mean [SD]) | |
| Troponin T | 1.5 (3.5) |
| LVEF (n=159) | 0.58 (0.1) |
| Charlson score age adjusted | 3.3 (1.6) |
| Medications at discharge | |
| Aspirin | 159 (96.9) |
| Beta blocker | 144 (87.8) |
| ACEI/ARB | 90 (54.9) |
| Antiplatelet agents | 127 (77.4) |
| Statin | 153 (93.3) |
| Antidepressant | 27 (16.5) |
| Anxiolytic | 16 (9.8) |
| Scores at 2 week visit (mean [SD]) | |
| GQ-6 total (range: 6 – 42) | 36.5 (5.8) |
| MOS-SAS total (range: 4–24) | 16.9 (3.1) |
| SF-12 PCS (range: 0–100) | 40.8 (10.4) |
| SF-12 MCS (range: 0–100) | 50.8 (9.2) |
| PHQ-9 total (range: 0–27) | 4.4 (4.5) |
| HADS-A total (range: 0–21) | 4.3 (4) |
| LOT-R optimism (range: 0–12) | 9.0 (2.9) |
| LOT-R pessimism (range: 0–12) | 8.7 (3.3) |
| LOT-R total (range: 0–24) | 17.7 (5.6) |
| DASI (range:0–58.2) | 38.7 (15.8) |
All figures are N (%) unless otherwise specified.
ACEI=Angiotensin-Converting-Enzyme Inhibitor; ACS=Acute Coronary Syndrome; ARB=Angiotensin Receptor Blockers; BMI=Body Mass Index; DASI=Duke Activity Status Index; GQ-6=Gratitude Questionnaire Six Item Form; HADS-A=Hospital Anxiety and Depression Scale—Anxiety Subscale; LOT-R=Life Orientation Test-Revised; LVEF=Left Ventricular Ejection Fraction; MI=Myocardial Infarction; MOS-SAS=Medical Outcomes Study Specific Adherence Scale; PAR=Physical activity recall; PHQ-9=Patient Health Questionnaire-9; SF-12 MCS=Short Form 12 Mental Composite Score; SF-12 PCS=Short Form 12 Physical Composite Score.
With respect to baseline psychological variables (2 week visit), participants had a mean LOT-R score of 17.7 (SD 5.6), which was higher than general population norms for this age group (14.8 [SD 3.4]),98 and slightly higher than in a sample of non-depressed cardiac patients (16.9 [SD 3.7])99 and mean GQ-6 scores of 36.5 (SD 5.8), which is consistent with published norms in older adults (36.9 [SD 4.9]).24 Mean depression (PHQ-9: mean score 4.4 [SD 4.5]) and anxiety (HADS-A: mean score 4.3 [SD 4.0]) were slightly lower than mean values in other cardiac populations (PHQ-9=4.8; HADS-A=6.8).40,41 Using established cutoffs for clinically significant depression and anxiety, seventeen participants (10.4%) had PHQ-9 ≥10, and 32 (19.5%) had HADS-A≥8.
6. Comment
The GRACE study has been designed to answer several important questions about the prospective relationship between positive psychological constructs and subsequent outcomes in cardiac patients. It differs from prior work by studying ACS patients, a high-yield population at substantial risk for rehospitalizations and mortality. It also will examine simultaneously two positive psychological constructs, including the highly understudied construct of gratitude, to assess differential effects of these constructs on post-ACS prognosis. Finally, it will examine within the same study the prospective effects of these constructs on health behaviors, prognostic biomarkers, and clinical outcomes to begin to specify mechanisms by which these constructs may confer benefit. Interventions targeting optimism, including imagining a better possible future or a ‘best possible self,’ have led to improvements in well-being and optimism.100,101 Likewise, specific exercises in which participants express gratitude or list experiences for which they are grateful have led to increased positive emotions and, in some cases, to greater physical activity.28,102
The study has been successful in recruiting participants beyond the planned enrollment target. This should ensure adequate statistical power to test study hypotheses. The objective measurement of physical activity is an additional and important strength, given than most studies of psychological effects on activity and function in cardiac patients have utilized self-report.14,28,103 A final study strength is that analyses will control for sociodemographic factors, medical variables, depression, and anxiety to ensure that observed effects of positive psychological constructs are independent of these numerous potential confounders.
There may be several reasons for the relatively high rates at which patients declined participation. First, in recruiting participants from inpatient units, all patients were approached by clinical staff regarding their willingness to hear about an optional study. This is in contrast to many outpatient studies in which patients proactively identify studies in which they are interested in participating and therefore are more likely to participate upon learning of study details. Second, patients had just suffered a major cardiac event and may have not felt willing to consider participation as they came to terms with their illness. Finally, a meaningful proportion of ACS patients admitted to this urban academic medical center lived a substantial distance from the hospital, or had functional or logistical barriers to transportation, making a study with multiple in-person visits less viable.
Limitations of this study include recruitment from a single academic medical center and enrollment of a predominantly White and male cohort. Participants were also somewhat more optimistic than age-matched population norms, but similar to nondepressed patients in other cardiac studies.23 The moderate sample size and a relatively short follow-up period to assess readmissions also represent limitations; future studies could extend this follow-up period. Older ACS patients were more likely to decline participation, signaling a potential need in future studies to develop methods to enhance recruitment of older adults. We had a moderately high dropout rate between enrollment and the baseline visit, largely due to intervening medical issues that prevented return to the hospital at 2 weeks. Given that we will not have objective physical activity data at baseline and that rehospitalization data cannot have baseline values, we will only utilize techniques to account for missing data for the Aim #1 and Aim #3 self-report study outcomes. Finally, though physical activity will be objectively measured, we will measure steps rather than activity intensity, and other health behaviors, such as medication adherence, will be measured by self-report.
If this study finds that optimism and/or gratitude are associated with improved physical activity and other key outcomes following ACS, this would highlight psychological factors outside of depression and anxiety that may be of substantial prognostic importance. The next step would be to determine whether it is possible to successfully cultivate optimism, gratitude, and related states (e.g., positive affect) in patients with an ACS, and to learn whether boosting these states leads to greater health behavior participation and superior outcomes. There has been promising preliminary work suggesting that systematic positive psychological interventions are well-accepted and may reduce distress and improve outcomes in patients with chronic medical illnesses,104,105 including patients with heart disease.106–108 Whether such interventions can improve prognosis in the vulnerable population of post-ACS patients remains an open question and will be worth further study if it indeed appears that optimism or gratitude predict superior post-ACS outcomes in the GRACE study.
Acknowledgments
This work was supported by the Expanding the Science and Practice of Gratitude Project run by UC Berkeley’s Greater Good Science Center in partnership with UC Davis with funding from the John Templeton Foundation (grant ID: 15627, awarded to JH). Analysis and manuscript preparation time was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL113272 to JH.
Footnotes
The content is solely the responsibility of the authors and does not represent the official views of the funders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Yeo KK, Fam JM, Lim ST, et al. Comparison of clinical characteristics, 1-year readmission rates, cost and mortality amongst patients undergoing percutaneous coronary intervention for stable angina, acute coronary syndromes and ST-elevation myocardial infarction. EuroIntervention. 2012;8:295. [Google Scholar]
- 3.Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–69. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 5.Roest AM, Martens EJ, Denollet J, de Jonge P. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: a meta-analysis. Psychosom Med. 2010;72:563–9. doi: 10.1097/PSY.0b013e3181dbff97. [DOI] [PubMed] [Google Scholar]
- 6.Roest AM, Zuidersma M, de Jonge P. Myocardial infarction and generalised anxiety disorder: 10-year follow-up. Br J Psychiatry. 2012;200:324–9. doi: 10.1192/bjp.bp.111.103549. [DOI] [PubMed] [Google Scholar]
- 7.Boehm JK, Kubzansky LD. The heart’s content: the association between positive psychological well-being and cardiovascular health. Psychol Bull. 2012;138:655–91. doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: a meta-analytic review. Ann Behav Med. 2009;37:239–56. doi: 10.1007/s12160-009-9111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosom Med. 2008;70:741–56. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- 10.Kubzansky LD, Sparrow D, Vokonas P, Kawachi I. Is the glass half empty or half full? A prospective study of optimism and coronary heart disease in the normative aging study. Psychosom Med. 2001;63:910–6. doi: 10.1097/00006842-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Tindle H, Chang Y, Kuller L, et al. Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women’s Health Initiative. Circulation. 2009;120:656–62. doi: 10.1161/CIRCULATIONAHA.108.827642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steptoe A, Wright C, Kunz-Ebrecht SR, Iliffe S. Dispositional optimism and health behaviour in community-dwelling older people: associations with healthy ageing. Br J Health Psychol. 2006;11:71–84. doi: 10.1348/135910705X42850. [DOI] [PubMed] [Google Scholar]
- 13.Giltay EJ, Geleijnse JM, Zitman FG, Buijsse B, Kromhout D. Lifestyle and dietary correlates of dispositional optimism in men: The Zutphen Elderly Study. J Psychosom Res. 2007;63:483–90. doi: 10.1016/j.jpsychores.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Scheier MF, Matthews KA, Owens JF, et al. Dispositional optimism and recovery from coronary artery bypass surgery: the beneficial effects on physical and psychological well-being. J Pers Soc Psychol. 1989;57:1024–40. doi: 10.1037//0022-3514.57.6.1024. [DOI] [PubMed] [Google Scholar]
- 15.Laerum E, Johnsen N, Smith P, Larsen S. Myocardial infarction may induce positive changes in life-style and in the quality of life. Scand J Prim Health Care. 1988;6:67–71. doi: 10.3109/02813438809009293. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Weisz G, Moses JW, Teirstein PS, et al. Safety of sirolimus-eluting stenting and its effect on restenosis in patients with unstable angina pectoris (a SIRIUS substudy) Am J Cardiol. 2007;99:1044–50. doi: 10.1016/j.amjcard.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 18.Huffman JC, Beach SR, Suarez L, et al. Design and baseline data from the Management of Sadness and Anxiety in Cardiology (MOSAIC) randomized controlled trial. Contemp Clin Trials. 2013;36:488–501. doi: 10.1016/j.cct.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–81. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 21.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–78. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 22.Scheier MF, Matthews KA, Owens JF, et al. Optimism and rehospitalization after coronary artery bypass graft surgery. Arch Intern Med. 1999;159:829–35. doi: 10.1001/archinte.159.8.829. [DOI] [PubMed] [Google Scholar]
- 23.Tindle H, Belnap BH, Houck PR, et al. Optimism, response to treatment of depression, and rehospitalization after coronary artery bypass graft surgery. Psychosom Med. 2012;74:200–7. doi: 10.1097/PSY.0b013e318244903f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough ME, Emmons RA, Tsang JA. The grateful disposition: a conceptual and empirical topography. J Pers Soc Psychol. 2002;82:112–27. doi: 10.1037//0022-3514.82.1.112. [DOI] [PubMed] [Google Scholar]
- 25.Wood AM, Joseph S, PAL Coping style as a psychological resource of grateful people. J Soc Clin Psychol. 2007;26:1076–93. [Google Scholar]
- 26.Kashdan TB, Uswatte G, Julian T. Gratitude and hedonic and eudaimonic well-being in Vietnam war veterans. Behav Res Ther. 2006;44:177–99. doi: 10.1016/j.brat.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 27.McCullough ME, Tsang JA, Emmons RA. Gratitude in intermediate affective terrain: links of grateful moods to individual differences and daily emotional experience. J Pers Soc Psychol. 2004;86:295–309. doi: 10.1037/0022-3514.86.2.295. [DOI] [PubMed] [Google Scholar]
- 28.Emmons RA, McCullough ME. Counting blessings versus burdens: an experimental investigation of gratitude and subjective well-being in daily life. J Pers Soc Psychol. 2003;84:377–89. doi: 10.1037//0022-3514.84.2.377. [DOI] [PubMed] [Google Scholar]
- 29.Cain KL, Geremia CM. Accelerometer data collection and scoring manual for adult & senior studies. San Diego, CA: San Diego State University; 2012. Accessed at: http://sallis.ucsd.edu/measures.html. [Google Scholar]
- 30.Brown DK, Grimwade D, Martinez-Bussion D, Taylor MJ, Gladwell VF. The validity of the ActiPed for physical activity monitoring. Int J Sports Med. 2013;34:431–7. doi: 10.1055/s-0032-1323723. [DOI] [PubMed] [Google Scholar]
- 31.Coleman KR. Thesis. East Tennessee State University; 2010. The effects of an ActiPed pedometer intervention program on body composition and aerobic capacity of youth in a school system in East Tennessee. available at http://gradworks.umi.com/34/10/3410177.html. [Google Scholar]
- 32.Watson A, Bickmore T, Cange A, Kulshreshtha A, Kvedar J. An internet-based virtual coach to promote physical activity adherence in overweight adults: randomized controlled trial. J Med Internet Res. 2012;14:e1. doi: 10.2196/jmir.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moy ML, Matthess K, Stolzmann K, Reilly J, Garshick E. Free-living physical activity in COPD: assessment with accelerometer and activity checklist. J Rehabil Res Dev. 2009;46:277–86. doi: 10.1682/jrrd.2008.07.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dishman RK, Steinhardt M. Reliability and concurrent validity for a 7-d re-call of physical activity in college students. Med Sci Sports Exerc. 1988;20:14–25. doi: 10.1249/00005768-198802000-00003. [DOI] [PubMed] [Google Scholar]
- 35.McDermott MM, Liu K, O’Brien E, et al. Measuring physical activity in peripheral arterial disease: a comparison of two physical activity questionnaires with an accelerometer. Angiology. 2000;51:91–100. doi: 10.1177/000331970005100201. [DOI] [PubMed] [Google Scholar]
- 36.Pitsavos C, Kavouras SA, Panagiotakos DB, et al. Physical activity status and acute coronary syndromes survival The GREECS (Greek Study of Acute Coronary Syndromes) study. J Am Coll Cardiol. 2008;51:2034–9. doi: 10.1016/j.jacc.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 37.Loprinzi PD, Cardinal BJ, Winters-Stone K, Smit E, Loprinzi CL. Physical activity and the risk of breast cancer recurrence: a literature review. Oncol Nurs Forum. 2012;39:269–74. doi: 10.1188/12.ONF.269-274. [DOI] [PubMed] [Google Scholar]
- 38.van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222,497 Australian adults. Arch Intern Med. 2012;172:494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 39.Zisser H, Gong P, Kelley CM, Seidman JS, Riddell MC. Exercise and diabetes. Int J Clin Pract Suppl. 2011:71–5. doi: 10.1111/j.1742-1241.2010.02581.x. [DOI] [PubMed] [Google Scholar]
- 40.Stavrakakis N, de Jonge P, Ormel J, Oldehinkel AJ. Bidirectional prospective associations between physical activity and depressive symptoms. The TRAILS Study. J Adolesc Health. 2012;50:503–8. doi: 10.1016/j.jadohealth.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 41.DuBois CM, Beach SR, Kashdan TB, Park ER, Celano CM, Huffman JC. Positive psychological attributes and cardiac outcomes: associations, mechanisms, and interventions. Psychosomatics. 2012 doi: 10.1016/j.psym.2012.04.004. in press. [DOI] [PubMed] [Google Scholar]
- 42.Westerterp KR. Assessment of physical activity level in relation to obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S522–5. doi: 10.1097/00005768-199911001-00006. [DOI] [PubMed] [Google Scholar]
- 43.Westerterp KR. Assessment of physical activity: a critical appraisal. Eur J Appl Physiol. 2009;105:823–8. doi: 10.1007/s00421-009-1000-2. [DOI] [PubMed] [Google Scholar]
- 44.Bauer LK, Caro MA, Beach SR, et al. Effects of depression and anxiety improvement on adherence to medication and health behaviors in recently hospitalized cardiac patients. Am J Cardiol. 2012;109:1266–71. doi: 10.1016/j.amjcard.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160:1818–23. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 46.Alie N, Eldib M, Fayad ZA, Mani V. Inflammation, atherosclerosis, and coronary artery disease: PET/CT for the evaluation of atherosclerosis and inflammation. Clin Med Insights Cardiol. 2014;8:13–21. doi: 10.4137/CMC.S17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linden F, Domschke G, Erbel C, Akhavanpoor M, Katus HA, Gleissner CA. Inflammatory therapeutic targets in coronary atherosclerosis-from molecular biology to clinical application. Front Physiol. 2014;5:455. doi: 10.3389/fphys.2014.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zernecke A, Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc Res. 2010;86:192–201. doi: 10.1093/cvr/cvp391. [DOI] [PubMed] [Google Scholar]
- 49.Kavsak PA, Ko DT, Newman AM, et al. Risk stratification for heart failure and death in an acute coronary syndrome population using inflammatory cytokines and N-terminal pro-brain natriuretic peptide. Clin Chem. 2007;53:2112–8. doi: 10.1373/clinchem.2007.090613. [DOI] [PubMed] [Google Scholar]
- 50.Hartford M, Wiklund O, Mattsson Hulten L, et al. C-reactive protein, interleukin-6, secretory phospholipase A2 group IIA and intercellular adhesion molecule-1 in the prediction of late outcome events after acute coronary syndromes. J Intern Med. 2007;262:526–36. doi: 10.1111/j.1365-2796.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 51.Abbate A, Biondi-Zoccai GG, Brugaletta S, Liuzzo G, Biasucci LM. C-reactive protein and other inflammatory biomarkers as predictors of outcome following acute coronary syndromes. Semin Vasc Med. 2003;3:375–84. doi: 10.1055/s-2004-815695. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 53.Ryff CD, Singer BH, Dienberg Love G. Positive health: connecting well-being with biology. Philos Trans R Soc Lond B Biol Sci. 2004;359:1383–94. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer T, Stanske B, Kochen MM, et al. Serum levels of interleukin-6 and interleukin-10 in relation to depression scores in patients with cardiovascular risk factors. Behav Med. 2011;37:105–12. doi: 10.1080/08964289.2011.609192. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Brouwers C, Mommersteeg P, Nyklicek I, et al. Positive affect dimensions and their association with inflammatory biomarkers in patients with chronic heart failure. Biol Psychol. 2013;92:220–6. doi: 10.1016/j.biopsycho.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro DR, Ramos AM, Vieira PL, et al. High-sensitivity C-reactive protein as a predictor of cardiovascular events after ST-elevation myocardial infarction. Arq Bras Cardiol. 2014;103:69–75. doi: 10.5935/abc.20140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James SK, Armstrong P, Barnathan E, et al. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: a GUSTO-IV substudy. J Am Coll Cardiol. 2003;41:916–24. doi: 10.1016/s0735-1097(02)02969-8. [DOI] [PubMed] [Google Scholar]
- 59.Zebrack JS, Anderson JL, Maycock CA, et al. Usefulness of high-sensitivity C-reactive protein in predicting long-term risk of death or acute myocardial infarction in patients with unstable or stable angina pectoris or acute myocardial infarction. Am J Cardiol. 2002;89:145–9. doi: 10.1016/s0002-9149(01)02190-7. [DOI] [PubMed] [Google Scholar]
- 60.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 61.Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35:578–89. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–8. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 63.Ikeda A, Schwartz J, Peters JL, et al. Optimism in relation to inflammation and endothelial dysfunction in older men: the VA Normative Aging Study. Psychosom Med. 2011;73:664–71. doi: 10.1097/PSY.0b013e3182312497. [DOI] [PubMed] [Google Scholar]
- 64.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 65.Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw. 2004;15:91–8. [PubMed] [Google Scholar]
- 66.Non AL, Rimm EB, Kawachi I, Rewak MA, Kubzansky LD. The effects of stress at work and at home on inflammation and endothelial dysfunction. PLoS ONE. 2014;9:e94474. doi: 10.1371/journal.pone.0094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Timoteo AT, Toste A, Ramos R, et al. Does admission NT-proBNP increase the prognostic accuracy of GRACE risk score in the prediction of short-term mortality after acute coronary syndromes? Acute Card Care. 2009;11:236–42. doi: 10.1080/17482940903177036. [DOI] [PubMed] [Google Scholar]
- 68.Jarai R, Iordanova N, Jarai F, et al. Prediction of clinical outcome in patients with non-ST-elevation acute coronary syndrome (NSTE-ACS) using the TIMI risk score extended by N-terminal pro-brain natriuretic peptide levels. Wien Klin Wochenschr. 2007;119:626–32. doi: 10.1007/s00508-007-0892-2. [DOI] [PubMed] [Google Scholar]
- 69.Brouwers C, Spindler H, Larsen ML, et al. Association between psychological measures and brain natriuretic peptide in heart failure patients. Scand Cardiovasc J. 2012;46:154–62. doi: 10.3109/14017431.2012.658579. [DOI] [PubMed] [Google Scholar]
- 70.Politi P, Minoretti P, Piaggi N, Brondino N, Emanuele E. Elevated plasma N-terminal ProBNP levels in unmedicated patients with major depressive disorder. Neurosci Lett. 2007;417:322–5. doi: 10.1016/j.neulet.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 71.Alonso J, Permanyer-Miralda G, Cascant P, Brotons C, Prieto L, Soler-Soler J. Measuring functional status of chronic coronary patients. Reliability, validity and responsiveness to clinical change of the reduced version of the Duke Activity Status Index (DASI) Eur Heart J. 1997;18:414–9. doi: 10.1093/oxfordjournals.eurheartj.a015260. [DOI] [PubMed] [Google Scholar]
- 72.Hsu J, Uratsu C, Truman A, et al. Life after a ventricular arrhythmia. Am Heart J. 2002;144:404–12. doi: 10.1067/mhj.2002.125497. [DOI] [PubMed] [Google Scholar]
- 73.Parissis JT, Nikolaou M, Birmpa D, et al. Clinical and prognostic value of Duke’s Activity Status Index along with plasma B-type natriuretic peptide levels in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2009;103:73–5. doi: 10.1016/j.amjcard.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 74.Erickson SR, Ellis JJ, Kucukarslan SN, Kline-Rogers E, Smith DE, Eagle KA. Satisfaction with current health status in patients with a history of acute coronary syndrome. Curr Med Res Opin. 2009 doi: 10.1185/03007990802714473. [DOI] [PubMed] [Google Scholar]
- 75.Krantz DS, Olson MB, Francis JL, et al. Anger, hostility, and cardiac symptoms in women with suspected coronary artery disease: the Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt) 2006;15:1214–23. doi: 10.1089/jwh.2006.15.1214. [DOI] [PubMed] [Google Scholar]
- 76.Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52:26–33. doi: 10.1016/j.psym.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 77.Huffman JC, Mastromauro CA, Beach SR, et al. Collaborative care for depression and anxiety disorders in patients with recent cardiac events: the Management of Sadness and Anxiety in Cardiology (MOSAIC) randomized clinical trial. JAMA Intern Med. 2014;174:927–35. doi: 10.1001/jamainternmed.2014.739. [DOI] [PubMed] [Google Scholar]
- 78.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Davidson KW, Bigger JT, Burg MM, et al. Centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression: CODIACS Vanguard randomized controlled trial. JAMA Intern Med. 2013:1–8. doi: 10.1001/jamainternmed.2013.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rollman BL, Belnap BH, LeMenager MS, et al. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA. 2009;302:2095–103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huffman JC, Mastromauro CA, Sowden G, Fricchione GL, Healy BC, Januzzi JL. Impact of a depression care management program for hospitalized cardiac patients. Circulation Cardiovascular Quality and Outcomes. 2011;4:198–205. doi: 10.1161/CIRCOUTCOMES.110.959379. [DOI] [PubMed] [Google Scholar]
- 82.Lichtman JH, Bigger JT, Blumenthal JA, et al. Depression and coronary heart disease. Recommendations for screening, referral, and treatment. Circulation. 2008;118:1768–75. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 83.Lin EH, Katon W, Von Korff M, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290:2428–9. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 84.Thombs BD, Ziegelstein RC, Whooley MA. Optimizing detection of major depression among patients with coronary artery disease using the patient health questionnaire: data from the heart and soul study. J Gen Intern Med. 2008;23:2014–7. doi: 10.1007/s11606-008-0802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 86.Doyle F, Conroy R, McGee H. Differential predictive value of depressive versus anxiety symptoms in the prediction of 8-year mortality after acute coronary syndrome. Psychosom Med. 2012;74:711–6. doi: 10.1097/PSY.0b013e318268978e. [DOI] [PubMed] [Google Scholar]
- 87.Frasure-Smith N, Lesperance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2008;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 88.Rothenbacher D, Hahmann H, Wusten B, Koenig W, Brenner H. Symptoms of anxiety and depression in patients with stable coronary heart disease: prognostic value and consideration of pathogenetic links. Eur J Cardiovasc Prev Rehabil. 2007;14:547–54. doi: 10.1097/HJR.0b013e3280142a02. [DOI] [PubMed] [Google Scholar]
- 89.Suarez L, Beach SR, Moore SV, et al. Use of the Patient Health Questionnaire-9 and a detailed suicide evaluation in determining imminent suicidality in distressed patients with cardiac disease. Psychosomatics. 2014 doi: 10.1016/j.psym.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 90.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 91.Pace NL. Independent predictors from stepwise logistic regression may be nothing more than publishable P values. Anesth Analg. 2008;107:1775–8. doi: 10.1213/ane.0b013e31818c1297. [DOI] [PubMed] [Google Scholar]
- 92.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 93.Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56:52. [Google Scholar]
- 94.Myers V, Gerber Y, Benyamini Y, Goldbourt U, Drory Y. Post-myocardial infarction depression: increased hospital admissions and reduced adoption of secondary prevention measures—a longitudinal study. J Psychosom Res. 2012;72:5–10. doi: 10.1016/j.jpsychores.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 95.Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med. 2006;21:1178–83. doi: 10.1111/j.1525-1497.2006.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pelle AJ, Pedersen SS, Szabo BM, Denollet J. Beyond Type D personality: reduced positive affect (anhedonia) predicts impaired health status in chronic heart failure. Qual Life Res. 2009;18:689–98. doi: 10.1007/s11136-009-9485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glaesmer H, Rief W, Martin A, et al. Psychometric properties and population-based norms of the Life Orientation Test Revised (LOT-R) Br J Health Psychol. 2012;17:432–45. doi: 10.1111/j.2044-8287.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- 98.Tindle HA, Chang YF, Kuller LH, et al. Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women’s Health Initiative. Circulation. 2009;120:656–62. doi: 10.1161/CIRCULATIONAHA.108.827642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stafford L, Berk M, Jackson HJ. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry. 2007;29:417–24. doi: 10.1016/j.genhosppsych.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Meevissen YM, Peters ML, Alberts HJ. Become more optimistic by imagining a best possible self: effects of a two week intervention. J Behav Ther Exp Psychiatry. 2011;42:371–8. doi: 10.1016/j.jbtep.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 101.Sheldon KM, Lyubomirsky S. How to increase and sustain positive emotion: the effects of expressing gratitude and visualizing best possible selves. J Positive Psychology. 2006;1:73–82. [Google Scholar]
- 102.Sheldon K, Lyubomirsky S. How to increase and sustain positive emotion: the effects of expressing gratitude and visualizing best possible selves. J Posit Psychol. 2006;1:73–82. [Google Scholar]
- 103.Versteeg H, Pedersen SS, Erdman RA, van Nierop JW, de Jaegere P, van Domburg RT. Negative and positive affect are independently associated with patient-reported health status following percutaneous coronary intervention. Qual Life Res. 2009;18:953–60. doi: 10.1007/s11136-009-9511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cohn MA, Pietrucha ME, Saslow LR, Hult JR, Moskowitz JT. An online positive affect skills intervention reduces depression in adults with type 2 diabetes. J Posit Psychol. 2014;9:523–34. doi: 10.1080/17439760.2014.920410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moskowitz JT, Hult JR, Duncan LG, et al. A positive affect intervention for people experiencing health-related stress: development and non-randomized pilot test. J Health Psychol. 2012;17:676–92. doi: 10.1177/1359105311425275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huffman J, Mastromauro C, Boehm J, et al. Development of a positive psychology intervention for patients with acute cardiovascular disease. Heart Int. 2011;6:e14. doi: 10.4081/hi.2011.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogedegbe GO, Boutin-Foster C, Wells MT, et al. A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African Americans. Arch Intern Med. 2012;172:322–6. doi: 10.1001/archinternmed.2011.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peterson JC, Charlson ME, Hoffman Z, et al. A randomized controlled trial of positive-affect induction to promote physical activity after percutaneous coronary intervention. Arch Intern Med. 2012;172:329–36. doi: 10.1001/archinternmed.2011.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]