Abstract
The aryl hydrocarbon receptor (Ahr) is an important regulator of the development and function of both innate and adaptive immune cells through roles associated with Ahr's ability to respond to cellular and dietary ligands. Recent findings have revealed tissue and context-specific functions for Ahr in both homeostasis and in during an immune response. I review these findings here, and integrate them into the current understanding of the mechanisms that regulate Ahr transcription and function. I propose a conceptual framework in which Ahr function is determined by three factors: the amount of Ahr in any given cell, the abundance and potency of Ahr ligands within certain tissues, and the tissue microenvironment wherein Ahr+ cells reside. This complexity emphasizes the necessity cell-type specific genetic approaches towards the study of Ahr function.
Introduction
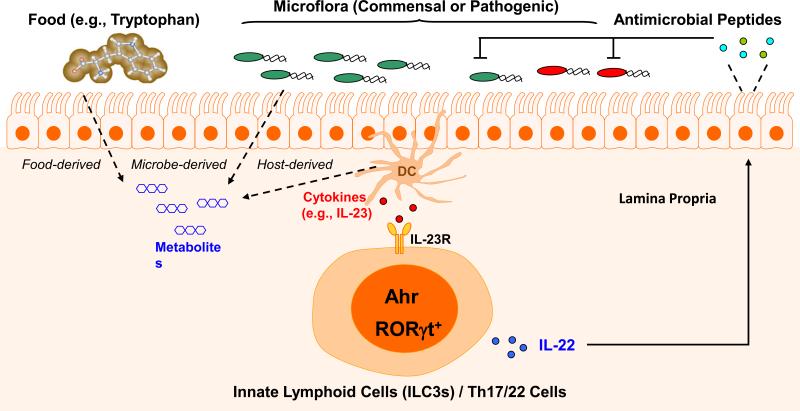
The aryl hydrocarbon receptor (Ahr) is a ligand-dependent transcription factor, best known for mediating the biotransformation and carcinogenic/teratogenic effects of certain environmental toxins, such as for example, 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD), a prototypical xenobiotic ligand for Ahr). The Ahr gene was cloned in the early 1990's [1-4], and much of our understanding of Ahr function initially came from studies in toxicology and pharmacology, which focused on its role in response to xenobiotics [5]. The perspective on Ahr changed when it was revealed to be an important regulator of the development and function of both innate and adaptive immune cells, and that this role was mediated by the ability of Ahr to respond to endogenous ligands generated from the host cell, diet, and from microbiota [6-8]. Ahr is currently considered to function as an environmental sensor, connecting “outside” environmental signals to “inside” cellular processes, with important consequences in immune cell function (Figure 1).
Figure 1.
Control of mucosal immunity by Ahr at the interface between the host and external environment. The gut is enriched with metabolites derived from either food or microflora, and some of these metabolites can function as Ahr ligands, binding to Ahr to induce its nuclear translocation and transcriptional activiation. The gut also has a cytokine mileau resulting from the cytokine production by immune cells such as dendritic cells, likely in response to gut microbiota. These environmental cues instruct the differentiation programs of immune cells (such as innate lymphoid cells and T cells), promoting the secretion of IL-22, which activates gut epithelial cells to produce antimicrobial peptides that control bacterial infections.
Recent findings have provided new insights into the role of Ahr in different settings, revealing complex regulatory pathways that guide tissue and context-specific functions for Ahr in both homeostasis and in during an immune response [9-11]. I review these findings here, and integrate them into the current understanding of the mechanisms that regulate Ahr transcription and function, and the physiological and pathological roles of Ahr upon activation by endogenous ligands. I propose a conceptual framework in which Ahr function is determined by three factors: the amount of Ahr in any given cell, the abundance and potency of Ahr ligands within certain tissues, and the tissue microenvironment wherein Ahr+ cells reside.
Ahr structure and function
Ahr is a ligand-dependent nuclear receptor and belongs to the basic helix-loop-helix (bHLH)/Per-Arnt-Sim (PAS) family of proteins (Figure 2) [12]. In the absence of a ligand, Ahr is kept in the cytosol and complexes with the chaperone proteins, HSP90, AIP (also known as XAP2 or ARA9), and p23. Ligand binding results in a conformational change that in turn leads to its nuclear translocation. Release of Ahr from its chaperones requires the dimerization of Ahr with another bHLH/PAS-domain transcription factor - the Ahr nuclear translocator (ARNT) (also known as Hif1β), which has constitutive nuclear localization. The Ahr-ARNT complex binds to the cognate DNA binding motifs referred to as Ahr responsive elements (AhRE), dioxin response elements (DRE), or xenobiotic response elements (XRE) to initiate transcription of the target genes to regulate gene transcription (Figure 2b). It is unclear whether dimerization with ARNT is absolutely required for Ahr transcriptional activity, or whether Ahr can regulate transcription by interacting with factors other than ARNT. ARNT can dimerize with Hif1α [13], and thus ARNT likely has gene targets (and associated functions) that are independent from Ahr. Direct comparison of the role of Ahr and ARNT in different cell types using conditional loss-of-function approaches may shed light on these questions.
Figure 2.
The structure and transcriptional activation of Ahr. (A) Functional domains of Ahr. bHLH (basic helix – loop – helix); PAS (Per – Arnt – Sim) domain containing PAS A and B repeats; Q-rich (glutamine rich) region; the location of functional domains are indicated by double arrows. (B) Transcriptional activation of Ahr. Ligands diffuse into the cell and are bound by the cytosolic Ahr complex. The ligand-bound receptor complex translocates into the nucleus and heterodimerizes with ARNT. Ahr-ARNT complex binds to AhRE, leading to transcriptional activation of target genes.
The PAS domains of Ahr consist of two regions, PAS-A and PAS-B, which function as interfaces for dimerization with ARNT and for ligand binding, respectively [14]. Although both the bHLH and the PAS-A domains have been shown to be involved in dimerization with ARNT, a recent report suggests that only the PAS-A domain of Ahr is essential [15]. Of note, designation of a chemical as an Ahr ligand is typically based on Ahr-dependent transcription and gel shift assays, which do not necessarily demonstrate binding to the PAS-B region [16]. Ahr ligands with different structural characteristics have been reported [17], and it is unclear whether the PAS-B domain of Ahr is capable of accommodating different compounds due to structural flexibility, or whether these ligands bind to Ahr at locations other than its ligand-binding pocket. A detailed structural analysis of Ahr (or PAS-B) bound to physiological ligands will help to differentiate these possibilities.
Interestingly, an Ahr deletion mutant lacking the PAS-B domain (ΔPAS-B) has been shown to constitutively dimerize with ARNT, bind to DNA, and activate transcription in a ligand-independent manner (i.e., constitutively active (CA)-Ahr) [18]. Three groups generated transgenic mice using this construct to examine the impact of CA-Ahr in vivo. Transgenic mice expressing CA-Ahr under the control of the SV40 promoter developed gastric tumors, suggesting a role of Ahr in oncogenesis and cell proliferation [19]. Mice bearing CA-Ahr under the control of keratin 14 promoter (Keratin-CA-Ahr) developed inflammatory skin lesions, and exhibited high amounts of serum IgE and IgG1 and a dominant Th2 response. To examine Ahr function in T cells, CA-Ahr was also expressed under the regulation of human CD2 promoter. The hCD2-CA-Ahr transgenic mice exhibited lower numbers of total thymocytes as compared to wild-type mice, although the percentage of CD8+CD4− thymocytes was higher in these mice; interestingly, the peripheral T cell compartments seemed less affected [20]. Of note, the expression of CAAhr was controlled not by the Ahr endogenous regulatory elements, instead by the artificial hCD2 minigene in these mice, which might result in expression of CA-Ahr in a non-physiological context, and confound the phenotypes of the hCD2-CA-Ahr transgenic mice.
Studying Ahr function has also been achieved through administration of ligands to activate Ahr in vitro or in vivo. However, the complex effects of Ahr activation have been reported, presumably caused by various ligands used in vitro, or in vivo through different administration routes [11, 21, 22]. Therefore, the development of a mouse model wherein expression of CA-Ahr is controlled by endogenous regulatory elements within the Ahr locus will be important to clarify the functions of Ahr in different cell types.
Transcriptional regulation of Ahr expression
Ahr is expressed in barrier tissues (e.g., the gut, the skin, and the lung) by both immune cells such as lymphocytes and tissue structural cells such as epithelial and stromal cells and in the liver by hepatocytes, consistent with its role as a sensor for environmental stimuli. Ahr expression is regulated by environmental cues, such as cytokines (e.g., IL-6, IL-21, TGF-β, and others) [22-24]. The available evidence suggests that Ahr expression is high in T helper (Th)17 cells, low in Foxp3+ T regulatory cells (Treg cells), and almost undetectable in Th1 or Th2 cells (reviewed in [11]). Of note, these studies assessed the Ahr mRNA expression in bulk cell population, and the absence of data on Ahr expression on a per cell basis (e.g., using the Ahr reporter mice or single cell sequencing approaches) confounds the interpretation of these results. Furthermore, a given cell type may have variable levels of Ahr expression in different tissues, and lack of Ahr may conceivably have a disparate impact on the differentiation of specific cells in these tissues, such as Treg cells in the gut (as discussed below) and lung γδ T cells [25], arguing for the importance of determining Ahr expression in any given cells within a specific tissue milieu. Furthermore, as noted earlier, cytokines can induce Ahr in certain cell types, such as T cells, and thus cell culture conditions may have a significant impact on the levels of Ahr expression.
The mechanisms that control Ahr transcription are poorly understood, especially when considering cell type-specific regulation. A recent report suggested that the Ahr transcription might be directly promoted by RORγt, based on ChIP-Seq analyses of RORγt binding in Th17 cells, a type of T helper cells that express RORγt and secrete signature cytokine IL-17, and histone acetyltransferase p300 and histone H3K4– dimethylation profiles [26]. By extrapolating these Th17 cell data to group 3 innate lymphoid cells (ILC3s), a subset of lymphocytes that are analogous to T helper cells in their transcriptional regulation and function but do not express antigen receptors, an intriguing hypothesis was proposed that RORγt can directly regulate Ahr transcription in ILC3s via enhancer interactions. This hypothesis is consistent with the observation of high amounts of Ahr in RORγt+ cells (e.g., Th17/22 cells and ILC3s). However, it is worthwhile to note that Ahr is expressed in other cell types (e.g., innate immune cells including dendritic cells (DCs) or macrophages and other innate lymphoid cells) (Li, Bostick and Zhou, unpublished) [27-30] in which RORγt expression is presumably negligible or low. In addition, RORγt deficiency prevents Th17 cell differentiation, but does not affect Ahr expression (Zhou, unpublished), suggesting a dispensable role for RORγt in regulating Ahr at least in certain cell types.
Of note, although Th17 cells and ILC3s share marked similarity in cytokine production and transcription regulation, ILC3s are not simply a counter part of Th17 cell population that lacks T cell receptor. There are clear differences between these two cell types in terms of transcriptional regulation. IL-6 can induce Th17 cells in vitro and in vivo via a Stat3-dependent pathway, and regulates Ahr expression in CD4+ T cells [22-24, 31, 32] (Zhou, unpublished). Genome-wide ChIP studies in Th17 cells detected binding of Stat3 to the Ahr promoter, suggesting that Stat3 directly controls Ahr transcription [33]. However, genetic ablation of Stat3 in RORγt+ cells has no effect on the Ahr expression in ILC3s, or on ILC3 development [34]. In addition, whether IL-6 can influence ILC3 development remains unclear. Therefore, the role of RORγt in regulating the Ahr transcription needs careful examination in primary ILC3s, including determining the Ahr locus occupancy by RORγt using ChIP assay and transcriptional regulation of Ahr by RORγt using functional assays.
Recent studies have reported direct binding of Ikaros, a zinc finger transcription factor, to the Ahr locus by ChIP assay, and concomitant reduced levels of Ahr transcription in Ikaros-null T cells or Ikaros mutant T cells lacking DNA-binding zinc finger 4 [35, 36], suggesting a positive role for Ikaros in Ahr transcription. However, the amount of Il22 transcripts is increased in T cells that express mutant Ikaros lacking zinc finger 4, whereas Cyp1a1 expression is not; this is surprising because both genes are canonical Ahr targets [36]. Thus, Ikaros may regulate Ahr activity in a gene-specific manner.
TGF-β, retinoic acid (RA) and short-chain fatty acids are abundantly present in the gut and can influence Treg cell differentiation by promoting Foxp3 expression [37]. Furthermore, TGF-β and RA have been shown to promote Ahr expression in T cells and mouse embryo fibroblast cells [22, 23, 38]. Recently, calcitriol, an active form of vitamin D, was shown to inhibit Ahr transcription in human CD4+ T cells, which in turn inhibits BATF-induced IL-9 production [39]. Whether these cytokine and metabolite signals regulate Treg cell differentiation through the Ahr pathway remains to be determined.
Regulation of Ahr activity
Ahr activity is regulated in various ways. First, Ahr protein levels are controlled via ubiquitin-mediated proteosomal degradation: Ligand binding induces Ahr ubiquitination and subsequent degradation by the proteosome [5]. AIP, a component of the Ahr chaperone complex, stabilizes Ahr by inhibiting its ubiquitination [40-42]. Second, an auto-regulatory feedback loop is in place, in which Ahr induces the expression of negative regulators that in turn prevent excessive Ahr activation. Ahr repressor (Ahrr), encoded by a target gene of Ahr, is a member of the bHLH protein family and structurally similar to Ahr but lacks a ligand-binding domain (PAS-B domain) and an activation domain [43]. Ahrr is thought to disrupt the interaction between Ahr and ARNT complex to inhibit Ahr-ARNT binding to DNA, although the precise mechanisms involved are not fully understood [44]. TiPARP (TCDD-inducible poly-ADP-ribose polymerase (also known as ARTD14) is a mono-ADP-ribosyltransferase and a ligand-induced negative regulator of Ahr transactivation, which may play a role in regulating the Ahr protein level [45-47]. Third, Ahr activity can be regulated indirectly, through availability of its ligands. Induction of cytochrome P450 enzymes (e.g., Cyp1a1), which degrade Ahr ligands prevent prolonged Ahr activation [11].
Ahr binding partners present another level of regulation. RORγt can facilitate the binding of Ahr to its target gene Il22, potentiating transcription [48]. Although activation of liver X receptors (LXRs) by metabolites of cholesterol leads to decreased Ahr transcription in T cells, LXR-induced Srebp-1 has been shown to physically interact with Ahr and to prevent Ahr-mediated Il17 transcription by interfering with DNA binding [49]. ID proteins inhibit E protein-mediated transcription by interfering with DNA binding [50]. ID2-deficient ILC3s have reduced amounts of Ahr [51]. E2A protein has been shown to interact with Ahr in EL4 cell lines, and to suppresses Ahr binding to the Il22 locus, presumably by interfering with RORγt-Ahr complex. These data are consistent with the reduced levels of IL-22 produced by ID2-deficient ILC3s in which E2A protein transcriptional activity is enhanced [51].
Variety of Ahr ligands
The impact of xenobiotic ligands (e.g., TCDD) on Ahr function in the immune system has been reviewed elsewhere [52, 53]. Here, I focus on endogenous and physiological Ahr ligands, made by cells, the microbiota or from dietary sources, that have been shown to impact lymphocyte development and function.
The high affinity Ahr ligand 6-formylindolo[3,2-b]carbazole (FICZ) is an ultraviolet photoproduct of L-tryptophan[54]. Recent data suggest that FICZ can also be generated by other metabolic pathways such as enzymatic deamination of tryptamine and oxidation of tryptophan by intracellular oxidants [9]. FICZ promotes Th17 differntiation in vitro and in vivo, and treatment with FICZ results in increased numbers of gut ILC3s, via an Ahr-dependent pathway [22-24, 48]. Another high-affinity Ahr ligand, indolo-[3,2-b]-carbazole (ICZ), is generated with 3,3’-diindolylmethane (DIM) through indole-3-carbinal (I3C) under acidic conditions in the stomach [55]. I3C is enzymatically generated from glucobrassicin, an L-tryptophan derived glucosinolate that is enriched in cruciferous vegetables, suggesting a mechanism for immune regulation by dietary components. Indeed, mice fed on a diet that lacks Ahr ligand have reduced Ahr activity and concomitant reduction of immune cell compartments including γδT cells and ILC3s in the gut. Restoration of these compartments could be achieved by I3C dietary supplementation [7, 56].
Kynurenine, an Ahr agonist, is a tryptophan metabolite generated by the enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). IDO expression is induced by Ahr [57, 58], suggesting positive feedback in this pathway. It has been proposed that activation of Ahr with different ligands can lead to different cell fates depending on the surrounding milieu [23]. Consistent with this notion, FICZ and kynurenine have different impact on T cell differentiation in vitro. FICZ, but not kynurenine, promotes Th17 cell differentiation [22-24]; kynurenine promotes TGF-β-induced Treg (iTreg) cell differentiation, while FICZ is inhibitory [27]. Different effects for FICZ and TCDD in Th17 and Treg lineage specification in vitro and in vivo have also been reported [23]. While concerns regarding toxicity and long-term/secondary effects of TCDD should be taken into account [11], it will nevertheless be important to examine the impact of different Ahr ligands in vivo, in a cell type specific manner.
Microbe-derived ligands can also activate Ahr. Malassezia, a commensal yeast in human skin, can metabolize tryptophan into several Ahr activating compounds including FICZ and ICZ [59]. Lactobacillus converts tryptophan into indole-3-aldehyde (IAld), which can activate Ahr and promote IL-22 production by gut ILC3s [8]. Bacterial pigmented virulence factors that are structurally similar to TCDD, such as phenazines produced by Pseudomonas aeruginosa and naphthoquinone phthiocol from Mycobacterium tuberculosis, have been recently proposed by bind Ahr. Degradation of these virulent pigments is dependent on Ahr, as is the inflammatory response by host cells to eradicate these bacterial infections [60]. Ahr thus serves dual roles, neutralizing the virulent factors and functioning as a pattern recognition receptor (PRR) that detects these danger-associated molecular patterns (DAMPs) (phenazines/naphthoquinones) and activates host immunity.
Complex roles for Ahr in T cell differentiation
In sharp contrast to earlier data showing that Ahr promotes Th17 cell differentiation and IL-17/22 production by γδT cells in vitro [11, 22], recent findings revealed that Ahr-deficient mice have increased Th17 and IL-17/22-producing γδT cell responses in a skin-inflammation model [61]. The role of Ahr in Treg cell differentiation is also unclear. While several groups [22, 23, 62] reported the expression of Ahr in Treg cells, others reported that splenic Tregs express low levels of Ahr, as compared to Th17 cells [24]. Our data suggest that Treg cells in the gut express high amounts of Ahr, while Tregs in other anatomic locations do not (Ye and Zhou, unpublished data), consistent with the importance of Ahr in gut-tissue associated Treg cells [30]. The determinants of this tissue-specific expression are unknown, and require further investigation.
Impact of Ahr in Th17 and Th22 cells
Differentiation of naïve CD4+ T cells into Th17 cells requires the transcription factor RORγt [63], and these cells are characterized by a signature cytokine profile which includes IL-22, GM-CSF, IL-17 (also known as IL-17A) and IL-17F [64]. IL-17 and IL-17F are encoded by genes located on the same chromosome and may share similar regulatory mechanisms [65]. As noted above, in vitro studies suggest that Ahr promotes Th17 cell differentiation, and several mechanisms by which Ahr promotes the expression of IL-17 have been proposed. Ahr may bind to the Il17 gene locus directly and induce its transcription [49]. Under Th17-polarizing conditions, Ahr inhibits STAT1 phosphorylation, thus blocking alternative Th1 cell differentiation and reinforcing Th17 cell differentiation [22]. IL-2 is known to interfere with Th17 cell differentiation [66-70]. Ahr was shown to interact with Stat3 to induce the expression of Aiolos, a member of Ikaros family of proteins that directly silences Il2 expression, thus promoting Th17 cell differentiation [71]. Ahr also limits the activation of IL-2-induced Stat5 signaling in Th17 cells, thereby interfering with the inhibitory signals provided by IL-2 on Th17 cell differentiation [72]. Together with IL-6, TGF-β1 promotes Th17 cells that co-express IL-10 and are thought to be regulatory/non-pathogenic [73], whereas TGF-β3 induces pathogenic Th17 cells that do not express IL-10 and cause tissue inflammation [74]. Interestingly, the expression of Ahr varies in Th17 cells that are differentiated in these conditions with a reduced expression in the pathogenic Th17 cells differentiate by TGF-β3 and IL-6 [74].
IL-22 is a cytokine that has a wide spectrum of functions ranging from host immunity to metabolism [75]. In CD4+ T cells, IL-22 can be produced by Th17 cells that co-express other cytokines such as IL-17 and IL-17F. However, CD4+ T cells that express only IL-22 but not IL-17, namely Th22 cells, have also been identified in mice and in humans [76]. IL-6 or IL-21 alone induces IL-22 expression in T cells, and generates Th22 cells [77-79]. In contrast of the role of TGF-β in promoting IL-17 expression by CD4+ T cells [80-82], TGF-β has been shown to inhibit IL-22 expression in Th17 cells through induction of C-maf [77]. Recent data suggest that under the steady state, IL-22 is not only expressed by CD4+ T cells (Th17 and Th22) but also ILC3s, with the latter being the major producer [48].
Ahr is essential for IL-22 production by T cells and ILC3s [83]. Co-expression of Ahr and RORγt by retroviral transduction in a thymoma cell line, EL4, synergistically upregulates IL-22 expression [48]. The cooperativity between Ahr and RORγt has also been observed in primary T cells (Zhou unpublished). The physical association between Ahr and RORγt is detected in HEK293T cells by an overexpression study [48]. Recently, this interaction at the endogenous level has been confirmed in primary ILC3s using Proximity Ligation Assay (PLA), an assay that allows to detect protein-protein interaction on a per cell basis using immunofluorescence microscopy (Wang and Zhou, unpublished). The exact contribution of the interaction between Ahr and RORγt to the regulation of Il22 transcription remains to be determined. Nevertheless, the recruitment of Ahr to the Il22 locus can only be detected by ChIP assay when the cells co-express RORγt, while Ahr binding to the Cyp1a1 locus is independent of RORγt [48, 79]. These data point out an essential role for RORγt in facilitating Ahr binding at the chromatin level. Facilitated by RORγt, Ahr may achieve enhanced DNA binding activity, whereby directly binding to the AhREs at the Il22 locus to induce transcription. Alternatively, Ahr may indirectly bind to ROREs through interaction with RORγt (Figure 3).
Figure 3.
Cooperativity between Ahr and RORγt in the Il22 transcriptional regulation. RORγt and Ahr may act synergistically to enhance the Il22 transcription, and this synergy may result from multiple molecular interactions. The interaction between Ahr and RORγt is depicted at the Il22 promoter. Similar synergy could be present at the intron 1 of the Il22, where AhRE and RORE cluster.
Although RORγt is essential for IL-22 expression in wildtype CD4+ T cells [63, 84], it becomes completely dispensable for IL-22 production by mutant T cells that express a loss-of-function form of Ikaros that lacks the DNA-binding zinc finger 4 [36]. In these Ikaros-mutant T cells, however, Ahr is still absolutely required for IL-22 expression. One intriguing hypothesis is that some pioneering factors (e.g., RORγt) may create a permissive chromatin environment that allows subsequent binding of Ahr to the Il22 locus. Without RORγt, the accessibility of Ahr to the locus is limited, thus diminishing its potential in Il22 transcriptional promotion. However, in Ikaros-mutant cells, the chromatin environment may be changed so that RORγt is no longer required for Ahr binding to the locus. This model is consistent with the reported role of Ikaros in general as a chromatin repressor by interacting with chromatin modulators Mi-2 and HDAC [85]. However, detailed structural and functional studies of RORγt and Ahr at the Il22 locus are necessary to provide insight into the mechanisms of action of Ahr in promoting IL-22 expression in Th17/22 cells and ILC3s.
Ahr in Treg cell development and function
Although Ahr is expressed by Treg cells, especially in certain subtypes with an activated phenotype [23, 86, 87], the precise role of Ahr in Treg cell development/function remains controversial [88]. A substantial body of literature suggests that xenobiotic ligand TCDD administration in vivo suppresses immune responses [52]. This immunosuppressive effect of TCDD has been linked to the expansion or induction of Treg cells and promotion of Treg cell function in an Ahr-dependent manner in mice and in humans [23, 89, 90]. Consistently, TCDD has been shown to induce Foxp3 expression by activating Ahr, which binds to the Foxp3 locus and regulates its epigenetic status and transcription [23, 91]. However, it has also been suggested that the immunosuppressive nature of TCDD could be due to its cytotoxicity that might kill certain proinflammatory effector cells (e.g., Th17 cells) [16]. In contrast, kynurenine, a breakdown product in the IDO-dependent tryptophan degradation pathway, has recently been shown to function as an endogenous Ahr ligand and to enhance Treg cell differentiation through the activation of Ahr [27, 92, 93]. Another endogenous ligand of Ahr (i.e., 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE)) has also been shown to suppress autoimmunity by inducing Tregs [30]. Interestingly, it has been shown that activation of human intestinal lamina proprial cells, including Tregs, by Ahr ligand leads to downregulation of proinflammatory cytokines (e.g., IFN-γ)[94]. It remains to be determined whether the suppression of gut inflammation by Ahr ligand is mediated through Ahr-expressing Treg cells in humans. Despite these exciting results, the Treg cell-intrinsic role of Ahr remains to be determined. It is tempting to speculate that the expression of Ahr in Treg cells may have a favorable biological outcome for immune tolerance. For example, high Ahr expression in gut-associated Treg cells may render these cells readily activated by environmental cues (e.g., Ahr ligands that are abundantly present in the gut) to exert their suppressive function locally for intestinal immune homeostasis (Figure 4).
Figure 4.
Ahr+ Treg cells in immune homeostasis. Treg cells may respone to Ahr ligands present in the local tissue milieu, and pathways triggered downstream of this response may be relevant for the maintenance of immune homeostasis via the regulation of both adaptive (Th1/Th17) and innate (dendritic cell (DC) or macrophage (MΦ)) responses.
Previous studies to investigate the functions of Ahr in Treg cells consist of loss-of-function approach using Ahr complete null mice or gain-of-function approach by ligand administration. However, these approaches may confound the interpretation of the results. For example, the broad expression of Ahr in other cell types will most likely influence Treg cell development and/or function. For example, it has been shown that LPS induces expression of Ahr in macrophages and Ahr-deficient macrophages produce more IL-6 [28], a cytokine known to suppress Foxp3 expression [82]. In addition, our group has recently shown that Ahr deficiency in group 3 innate lymphoid cells (ILC3s) leads to aberrant outgrowth of gut commensal segmented filamentous bacteria (SFB) and elevated intestinal Th17 cells [95] that have a reciprocal relationship with Tregs during differentiation [82, 96]. It is worth mentioning that not only are the identities of endogenous ligands for Ahr elusive [17], administration of ligands to activate Ahr may result in the production of downstream metabolites that exert indirect effects on the immune system [16]. Thus, elucidating the cell-autonomous role of Ahr in Treg cells is crucial for future targeted manipulation of the Ahr pathway in the treatment of human disease (e.g., IBD).
Ahr in Tr1 development and function
Type 1 regulatory T (Tr1) cells are characterized by the expression of IL-10 but not Foxp3 and are prominent in chronic infections and upon certain immune manipulations in vivo (e.g., peptide immunization or activation by anti-CD3) [97]. Because lineage-specific transcription factor has yet to be identified for Tr1 cells and IL-10 is a common cytokine that can be produced by different subsets of CD4+ T helper cells (e.g., Th17 cells), it is debatable whether Tr1 cells represent a bona fide independent T helper subset or they are derived from other lineages due to CD4+ T cell plasticity [11, 98]. It was reported that IL-27 promotes Tr1 cell differentiation in vitro and production of IL-10 [99-101]. In Tr1-skewing conditions, Ahr induced by IL-27 physically interacts with C-maf and cooperatively transactivates the human and mouse Il10 and Il21 promoters, which results in generation of Tr1 cells and amelioration of experimental autoimmune encephalomyelitis [90, 102]. Considering the cooperativity between Ahr and C-maf in promoting IL-10 expression, the mechanism remains unknown for the inverse regulation of IL-22 by Ahr and C-maf in Th17 cells [77]. It has recently been reported that Th17 cells can trans-differentiate into Tr1 cells in the presence of TGF-β1, and Ahr activation promotes this conversion [103]. Metabolic stress hypoxia through Hif1α activation suppresses Tr1 cell differentiation [104]. Ahr is shown to control the metabolism of Tr1 cells and reduces Hif1α cellular levels, participating in the late stages of Tr1 cell differentiation [104].
Ahr in innate-like lymphocytes
The role of Ahr in ILC3s has been discussed extensively in a previous review [83]. Here, I mainly focus on the Ahr-mediated crosstalk between T cells and ILC3s. RORγt-expressing ILCs (also known as ILC3s) strikingly resemble Th17 and Th22 cells in their cytokine profile (e.g., production of IL-22 and IL-17). Co-evolution of two systems may be a fail-safe mechanism to implement redundancy in host immunity to certain infections especially at the mucosal surfaces especially during different stages of the infection. It has been shown that intestinal Th17/22 responses are enhanced by Citrobacter rodentium, a murine pathogen that models human enterohemorrhagic E. coli and enteropathogenic E. coli infections. Most recently, our group and others have shown that ILC-produced IL-22 is essential for the clearance of C. rodentium in the intestines [48, 105]. Interestingly, even in the lymphocyte-replete hosts, mice lacking ILC3s died from the infection, highlighting an essential role for ILCs in gut immunity [105]. However, the molecular mechanism underlying the development and function of ILC3s regulated by Ahr is incompletely understood. There are at least three mechanisms of action of Ahr in ILC3s that have been described. Although, Ahr is dispensable for T cell survival, Ahr can increase ILC3 survival by IL-7/IL-7R pathway and anti-apoptotic gene expression and thus promote ILC3 maintenance [48]. Ahr can enhance ILC3 proliferation, leading to its expansion in the gut [56]. Ahr has also been shown to directly regulate the transcription of Notch 1 and Notch 2, which are important for ILC3 development [6]. Ahr is important for all ILC3 subsets including lymphoid tissue-inducer (LTi)-like ILC3s and NKp46+ ILC3s [6, 48, 56]. However, the precise function of each subset in gut immunity and inflammation has not been addressed until using conditional knockout mice to delete Ahr or RORγt in RORγt- and NKp46-expressing cells, thus lacking all ILC3s or NKp46+ ILC3s, respectively [106]. NKp46+ILC3s are sufficient to promote inflammatory monocyte accumulation in the anti-CD40-induced colitis model. In contrast, NKp46+ILC3s are not required to resist C.rodentium infection even in the absence of T cells, presumably because LTi-like ILC3s provide sufficient protection [105, 106].
Increasing evidence indicates that ILCs participate in a dialog with CD4+ T cells [107-110]. This dialog is mediated, in part, through the expression of MHC class II (MHCII) molecules on ILC3s [107]. Depending on the expression of co-stimulatory molecules, MHCII:TCR interaction can result in T cell activation, anergy, or deletion [107, 108, 111]. ILC3s in the intestine express MHCII molecules, but lack expression of CD80 and CD86 co-stimulatory molecules [107]. T cell receptor interaction with MHCII expressed on intestinal ILC3s is thought to promote tolerance due to the lack of co-stimulation. When commensal bacteria-specific T cells encounter antigen presented by MHCII molecules on CCR6+ ILC3s, T cell apoptosis is induced [112]. Ahr expression in ILC3s controls adaptive Th17 cell responses [95, 113]. Ahr deficiency in vivo leads to upregulated Th17 cell responses, correlating with the aberrant outgrown Th17-inducing commensal SFB in Ahr−/− mice [95]. Lack of innate expression of IL-22 by ILC3s in Ahr−/− mice at least in part accounts for the upregulation of SFB and Th17 cell responses [95, 114]. It remains to be determined if Ahr regulates MHCII expression in ILC3s.
The role of Ahr in innate T cells, such as γδT cells, has been reviewed elsewhere [115]. Although γδ T cells do express a unique T cell receptor (TCR), engagement of this TCR with MHC-antigen complexes is not a prerequisite for their activation. Unlike conventional αβ T cells, cytokine stimulation alone is sufficient for IL-17-secreting γδ T cell activation, rendering these cells rapid and potent innate mediators of inflammation [116]. Although expressed in all γδT cell subsets [7, 25, 117-119], including the systemic Vγ1 and Vγ2, epidermal Vγ3, reproductive tract/lung/oral mucosal Vγ4, and intestinal Vγ5, Ahr expression is higher in γδT cells in mucosal tissues (e.g., the skin, intestine, or lung) [11]. IL-22 production by some of systemic Vγ2 γδT cells is dependent on Ahr [119]. The survival of epidermal Vγ3 and intestinal Vγ5 γδ T cells requires the expression of Ahr, whereas Vγ4 γδ T cells that express high levels of Ahr in the lung are surprisingly present in the absence of Ahr [11, 25].
Mouse Ahr vs. human Ahr
Phylogenetic analysis shows that functional orthologues of the Ahr gene are present in mammals, amphibians, reptiles and birds [120]. Although rodent studies can yield invaluable insights into the function of Ahr, particularly in the human immune system, it needs to be kept in mind that there are several differences between the mouse and human Ahr-pathways that may complicate the interpretation of results. First, a decrease in stability of the human AHR/HSP90 interaction compared to mouse Ahr [121] has been reported. Secondly, although N-terminal half of the protein (~85% sequence homology) is well conserved, the mouse and human Ahr differ significantly at the C-terminal half of the receptor that interacts with co-activators for transactivation [122]. Studies using primary hepatocytes from humans, mice, and humanized mice have revealed that the human and mouse Ahr differentially regulate gene expression in hepatocytes [123-125]. It is conceivable that similar differences in gene regulation caused by Ahr might also be present in the mouse and human immune cells. Thirdly, the sensitivity and affinity to ligand activation is different between the mouse and human Ahr. For example, the mouse Ahr binds to TCDD with a 10-fold higher affinity than the human Ahr, due to the difference in a single amino acid residue in the ligand-binding PAS-B domain [126]. In contrast, the human Ahr binds endogenous indole derivatives such as indirubin and indoxyl sulfate with much higher affinity than the mouse Ahr [127, 128]. Finally, considering the importance of gut microbes in generating and metabolizing the ligands of Ahr, the differences between the mice and human gut microbiome will inevitably affect the function of Ahr in vivo. Furthermore, unlike most studies done with mice in a controlled environment (e.g., using defined diet and commensal conditions), the variations in dietary components and commensal communities among each individual will potentially impact the Ahr pathway in humans.
Translational considerations by targeting the Ahr pathway
Modulating Ahr function offers exciting therapeutic potential in host immunity and inflammation. However, the emerging concept of Ahr function in a cell-type specific manner and the difference between the Ahr activation in vitro cell cultures and in vivo animals present challenges for targeting the Ahr pathway pharmacologically. Therefore, any attempt to activate or inhibit Ahr function in vivo using agonists (e.g., ligands) or antagonists (e.g., small molecular inhibitors) has to take into consideration the location and timing of administration of these Ahr modulators. Since Ahr is highly expressed in the barrier organs, local delivery of the Ahr modulators may be able to achieve more precise and potent effects than the systemic delivery. Indeed, it has been shown that local vs. systemic delivery of Ahr ligand FICZ has opposite effects on experimental autoimmune encephalitis [21, 23, 24]. In addition, Ahr is expressed by multiple cell types, and certain side effects could thus be circumvented by manipulation of Ahr function in a cell-type specific manner either in vitro or in vivo. Timing of administration of the Ahr modulators is also critical considering that Ahr expression is minimal in naïve T cells but could be induced in certain conditions, for example, by cytokines. Of note, unlike the effectiveness of administration of Ahr ligands or depletion of Ahr ligands by diet restriction in young mice from the birth, little or no impact on ILC3 compartment was observed when these pharmacological manipulations were done in adult mice [48, 56]. Abundant food- or microbiota-derived ligands for Ahr in adult mice might account for the lack of responses in these studies. The unresponsiveness to ligand manipulation might be apparent in cell types that have the high expression level of Ahr (e.g., ILC3s) and/or in an environment that endogenous Ahr ligands are abundant, where Ahr expression/function is saturated and thus resistant to further pharmacological manipulation.
Another consideration that needs our attention is that pharmacological agents may interfere with the Ahr pathway in a sustained/prolonged way or even a harmful way. Ahr activation leads to expression of cytochrome P450 enzymes, which could metabolize many Ahr ligands. The consequences of the enzyme-mediated degradation of Ahr ligands could be complex and detrimental, for example, it is known that the Ahr agonist benzo[a]pyrene can be metabolized into carcinogens by cytochrome P450 1A1 and 1B1 [129].
Human gut microbiota can metabolize many components of our diet and the drugs that we take. The microbes in the gut will determine the effectiveness and consequences of Ahr ligands, and are in turn shaped by their exposure to these compounds. Thus, manipulation of the Ahr pathway during host-microbe interactions will represent a novel microbiome-based therapeutic strategy for human diseases.
Concluding Remarks
Future research to understand the physiological and pathological role of Ahr in the immune system has to take into consideration the broad and inducible expression of Ahr in various tissues, the elusive nature of its endogenous ligands, and the similarities and differences among cell culture, animal, and human studies (Outstanding Questions). Ahr exerts its function in a context-dependent and very much cell type-specific manner. In vivo studies of Ahr function using ligands or other pharmacological means may be complicated by the diverse function of Ahr in different tissues and cells. Thus, understanding the role of Ahr in each cell type is crucial for the future design of therapeutics that targets the Ahr pathway pharmacologically. Considering the crosstalk between the Ahr pathway and other cytokine/metabolic pathways, mechanistic understanding of the action of Ahr will benefit from global analysis of Ahr-directed transcriptome, cistrome, interactome, and metabolome by next generation sequencing technologies in primary immune cells.
Outstanding Questions.
How do inflammation and infection impact the mechanisms that regulate Ahr in different cell types? Ahr expression can be induced in T cells in vitro upon cytokine treatment (e.g., IL-6, IL-21, TGF-β, and IL-27). It remains to be determined whether Ahr expression can be regulated in vivo in a similar fashion. Cis-acting genomic elements and transcription factors involved in the regulation of Ahr transcription remain undefined.
What mechanisms underlie cell-type specific regulation of Ahr transcription and function? For example, the role of Ikaros in regulating Ahr transcription and function in T cells and other lymphoid cells such as ILC3s requires further investigation.
How does RORγt regulate Ahr expression and activity in ILC3s? Of note, because of its indispensible role in ILC3 development, inhibitors of RORγt activity or genetic means to inhibit RORγt function and/or expression in mature ILC3s will be required.
Is there crosstalk between the Ahr pathway and other metabolic pathways (e.g., fatty acid metabolism, vitamin metabolism, and hypoxic stress), and what roles does this crosstalk play in the differentiation and function of T cells and ILC3s? What are the points of interaction, at a molecular level?
What are the fundamental differences between activation of Ahr by xenobiotic ligands as compared to endogenous ligands?
What are the similarities and differences in terms of the regulation of Ahr pathway in mice and in humans? What features of Ahr function translate from animal models to human biology, both in health and disease?
Trends Box.
Regulated expression of Ahr by immunological stimuli, such as cytokines, can determine its action in the corresponding cells, thus influencing host immunity.
Tissues that are exposed to the external environmental stimuli, such as the gut and the skin, are the host milieu that is enriched in both Ahr-expressing cells and in Ahr ligands.
Despite a myriad of genes that can be bound by Ahr in different cell types (e.g., hepatocytes) upon treatment with pollutants such as dioxin, unique gene targets of Ahr might be present, especially in physiological conditions, without its activation by xenobiotic compounds.
Ahr can also partner with other factors to exert its complex function in the immune system. The factors can have tissue and context-specific expression, thus presenting an additional regulatory level to Ahr function.
Identification and characterization of compounds that are either derived from food, generated endogenously by the host cells, or by indigenous microbes will facilitate drug discovery by presenting strategies towards targeting the Ahr pathway in different tissues and disease settings.
Acknowledgements
I thank the entire Zhou laboratory for help and suggestions, and John Bostick for critical reading the manuscript. The work was supported by the National Institutes of Health (AI089954 and DK105562 L.Z.), and by a Cancer Research Institute Investigator Award (LZ). Liang Zhou is a Pew Scholar in Biomedical Sciences, supported by the Pew Charitable Trusts, and an Investigator in the Pathogenesis of Infectious Disease, supported by Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no financial conflict of interest.
References
- 1.Ema M, Sogawa K, Watanabe N, Chujoh Y, Matsushita N, Gotoh O, Funae Y, Fujii-Kuriyama Y. cDNA cloning and structure of mouse putative Ah receptor. Biochemical and biophysical research communications. 1992;184:246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- 2.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh S, Kamataki T. Human Ah receptor cDNA: analysis for highly conserved sequences. Nucleic acids research. 1993;21:3578. doi: 10.1093/nar/21.15.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Molecular pharmacology. 1993;44:911–917. [PubMed] [Google Scholar]
- 5.Hankinson O. The aryl hydrocarbon receptor complex. Annual review of pharmacology and toxicology. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nature immunology. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacological reviews. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 10.Julliard W, Fechner JH, Mezrich JD. The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Frontiers in immunology. 2014;5:458. doi: 10.3389/fimmu.2014.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annual review of immunology. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 12.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annual review of pharmacology and toxicology. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 13.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. The Journal of biological chemistry. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 14.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D, Potluri N, Kim Y, Rastinejad F. Structure and dimerization properties of the aryl hydrocarbon receptor PAS-A domain. Molecular and cellular biology. 2013;33:4346–4356. doi: 10.1128/MCB.00698-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends in immunology. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuire J, Okamoto K, Whitelaw ML, Tanaka H, Poellinger L. Definition of a dioxin receptor mutant that is a constitutive activator of transcription: delineation of overlapping repression and ligand binding functions within the PAS domain. The Journal of biological chemistry. 2001;276:41841–41849. doi: 10.1074/jbc.M105607200. [DOI] [PubMed] [Google Scholar]
- 19.Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, Hanberg A, Poellinger L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9990–9995. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nohara K, Pan X, Tsukumo S, Hida A, Ito T, Nagai H, Inouye K, Motohashi H, Yamamoto M, Fujii-Kuriyama Y, et al. Constitutively active aryl hydrocarbon receptor expressed specifically in T-lineage cells causes thymus involution and suppresses the immunization-induced increase in splenocytes. Journal of immunology. 2005;174:2770–2777. doi: 10.4049/jimmunol.174.5.2770. [DOI] [PubMed] [Google Scholar]
- 21.Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PloS one. 2013;8:e79819. doi: 10.1371/journal.pone.0079819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 24.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 25.Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. gammadelta T cells protect against lung fibrosis via IL-22. The Journal of experimental medicine. 2010;207:2239–2253. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebihara T, Song C, Ryu SH, Plougastel-Douglas B, Yang L, Levanon D, Groner Y, Bern MD, Stappenbeck TS, Colonna M, et al. Runx3 specifies lineage commitment of innate lymphoid cells. Nature immunology. 2015;16:1124–1133. doi: 10.1038/ni.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. Journal of immunology. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. The Journal of experimental medicine. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 32.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. The Journal of biological chemistry. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 33.Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo X, Qiu J, Tu T, Yang X, Deng L, Anders RA, Zhou L, Fu YX. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 2014;40:25–39. doi: 10.1016/j.immuni.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong LY, Hatfield JK, Brown MA. Ikaros sets the potential for Th17 lineage gene expression through effects on chromatin state in early T cell development. The Journal of biological chemistry. 2013;288:35170–35179. doi: 10.1074/jbc.M113.481440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heller JJ, Schjerven H, Li S, Lee A, Qiu J, Chen ZM, Smale ST, Zhou L. Restriction of IL-22-producing T cell responses and differential regulation of regulatory T cell compartments by zinc finger transcription factor Ikaros. Journal of immunology. 2014;193:3934–3946. doi: 10.4049/jimmunol.1401234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends in immunology. 2015;36:3–12. doi: 10.1016/j.it.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs H, Dennefeld C, Feret B, Viluksela M, Hakansson H, Mark M, Ghyselinck NB. Retinoic acid drives aryl hydrocarbon receptor expression and is instrumental to dioxin-induced toxicity during palate development. Environ Health Perspect. 2011;119:1590–1595. doi: 10.1289/ehp.1003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takami M, Fujimaki K, Nishimura MI, Iwashima M. Cutting Edge: AhR Is a Molecular Target of Calcitriol in Human T Cells. Journal of immunology. 2015;195:2520–2523. doi: 10.4049/jimmunol.1500344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazlauskas A, Poellinger L, Pongratz I. The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. The Journal of biological chemistry. 2000;275:41317–41324. doi: 10.1074/jbc.M007765200. [DOI] [PubMed] [Google Scholar]
- 41.Lees MJ, Peet DJ, Whitelaw ML. Defining the role for XAP2 in stabilization of the dioxin receptor. The Journal of biological chemistry. 2003;278:35878–35888. doi: 10.1074/jbc.M302430200. [DOI] [PubMed] [Google Scholar]
- 42.Morales JL, Perdew GH. Carboxyl terminus of hsc70-interacting protein (CHIP) can remodel mature aryl hydrocarbon receptor (AhR) complexes and mediate ubiquitination of both the AhR and the 90 kDa heat-shock protein (hsp90) in vitro. Biochemistry. 2007;46:610–621. doi: 10.1021/bi062165b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes & development. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans BR, Karchner SI, Allan LL, Pollenz RS, Tanguay RL, Jenny MJ, Sherr DH, Hahn ME. Repression of aryl hydrocarbon receptor (AHR) signaling by AHR repressor: role of DNA binding and competition for AHR nuclear translocator. Molecular pharmacology. 2008;73:387–398. doi: 10.1124/mol.107.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacPherson L, Tamblyn L, Rajendra S, Bralha F, McPherson JP, Matthews J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin poly(ADP-ribose) polymerase (TiPARP, ARTD14) is a mono-ADP-ribosyltransferase and repressor of aryl hydrocarbon receptor transactivation. Nucleic acids research. 2013;41:1604–1621. doi: 10.1093/nar/gks1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacPherson L, Ahmed S, Tamblyn L, Krutmann J, Forster I, Weighardt H, Matthews J. Aryl hydrocarbon receptor repressor and TiPARP (ARTD14) use similar, but also distinct mechanisms to repress aryl hydrocarbon receptor signaling. International journal of molecular sciences. 2014;15:7939–7957. doi: 10.3390/ijms15057939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Q, Baldwin KT, Renzelli AJ, McDaniel A, Dong L. TCDD-inducible poly(ADP-ribose) polymerase: a novel response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochemical and biophysical research communications. 2001;289:499–506. doi: 10.1006/bbrc.2001.5987. [DOI] [PubMed] [Google Scholar]
- 48.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, Sheng H, Xi B, Zhang JZ, Zang YQ. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. The Journal of clinical investigation. 2011;121:658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kee BL. E and ID proteins branch out. Nature reviews. Immunology. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 51.Guo X, Liang Y, Zhang Y, Lasorella A, Kee BL, Fu YX. Innate Lymphoid Cells Control Early Colonization Resistance against Intestinal Pathogens through ID2-Dependent Regulation of the Microbiota. Immunity. 2015;42:731–743. doi: 10.1016/j.immuni.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. International immunopharmacology. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 53.Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Annals of the New York Academy of Sciences. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafstrom AK. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. The Journal of biological chemistry. 1987;262:15422–15427. [PubMed] [Google Scholar]
- 55.Shertzer HG, Senft AP. The micronutrient indole-3-carbinol: implications for disease and chemoprevention. Drug metabolism and drug interactions. 2000;17:159–188. doi: 10.1515/dmdi.2000.17.1-4.159. [DOI] [PubMed] [Google Scholar]
- 56.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 57.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. Journal of immunology. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 58.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochemical and biophysical research communications. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magiatis P, Pappas P, Gaitanis G, Mexia N, Melliou E, Galanou M, Vlachos C, Stathopoulou K, Skaltsounis AL, Marselos M, et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. The Journal of investigative dermatology. 2013;133:2023–2030. doi: 10.1038/jid.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moura-Alves P, Fae K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, Barison N, Diehl A, Munder A, Constant P, et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512:387–392. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- 61.Di Meglio P, Duarte JH, Ahlfors H, Owens ND, Li Y, Villanova F, Tosi I, Hirota K, Nestle FO, Mrowietz U, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989–1001. doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5919–5924. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 64.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Zhang Y, Yang XO, Nurieva RI, Chang SH, Ojeda SS, Kang HS, Schluns KS, Gui J, Jetten AM, et al. Transcription of Il17 and Il17f is controlled by conserved noncoding sequence 2. Immunity. 2012;36:23–31. doi: 10.1016/j.immuni.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nature immunology. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature immunology. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, Hammerling G, Li MO, Cua DJ, McGeachy MJ. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 70.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, et al. Aiolos promotes T(H)17 differentiation by directly silencing Il2 expression. Nature immunology. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. The Journal of experimental medicine. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nature immunology. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 74.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. Induction and molecular signature of pathogenic TH17 cells. Nature immunology. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual review of immunology. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 76.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunological reviews. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 77.Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, Hackney J, Ding J, Singh H, Ouyang W. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nature immunology. 2011;12:1238–1245. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- 78.Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeste A, Mascanfroni ID, Nadeau M, Burns EJ, Tukpah AM, Santiago A, Wu C, Patel B, Kumar D, Quintana FJ. IL-21 induces IL-22 production in CD4+ T cells. Nature communications. 2014;5:3753. doi: 10.1038/ncomms4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 81.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 82.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 83.Qiu J, Zhou L. Aryl hydrocarbon receptor promotes RORgammat(+) group 3 ILCs and controls intestinal immunity and inflammation. Seminars in immunopathology. 2013;35:657–670. doi: 10.1007/s00281-013-0393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshida T, Georgopoulos K. Ikaros fingers on lymphocyte differentiation. International journal of hematology. 2014;100:220–229. doi: 10.1007/s12185-014-1644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and - independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 87.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. International immunology. 2013;25:335–343. doi: 10.1093/intimm/dxt011. [DOI] [PubMed] [Google Scholar]
- 89.Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn's disease. Toxicological sciences : an official journal of the Society of Toxicology. 2011;120:68–78. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nature immunology. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PloS one. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 93.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. 248, e231. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou L, Lopes JE, Chong MM, Ivanov, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nature reviews. Immunology. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 98.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature immunology. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 100.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nature immunology. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 101.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature immunology. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 102.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nature immunology. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gagliani N, Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nature medicine. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song C, Lee JS, Gilfillan S, Robinette ML, Newberry RD, Stappenbeck TS, Mack M, Cella M, Colonna M. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. The Journal of experimental medicine. 2015;212:1869–1882. doi: 10.1084/jem.20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, Liew FY. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. Journal of immunology. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 110.Korn LL, Thomas HL, Hubbeling HG, Spencer SP, Sinha R, Simkins HM, Salzman NH, Bushman FD, Laufer TM. Conventional CD4+ T cells regulate IL-22-producing intestinal innate lymphoid cells. Mucosal immunology. 2014;7:1045–1057. doi: 10.1038/mi.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.von Burg N, Chappaz S, Baerenwaldt A, Horvath E, Bose Dasgupta S, Ashok D, Pieters J, Tacchini-Cottier F, Rolink A, Acha-Orbea H, et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12835–12840. doi: 10.1073/pnas.1406908111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science. 2015;348:1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wagage S, Harms Pritchard G, Dawson L, Buza EL, Sonnenberg GF, Hunter CA. The Group 3 Innate Lymphoid Cell Defect in Aryl Hydrocarbon Receptor Deficient Mice Is Associated with T Cell Hyperactivation during Intestinal Infection. PloS one. 2015;10:e0128335. doi: 10.1371/journal.pone.0128335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shih VF, Cox J, Kljavin NM, Dengler HS, Reichelt M, Kumar P, Rangell L, Kolls JK, Diehl L, Ouyang W, et al. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22-mediated containment of commensal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13942–13947. doi: 10.1073/pnas.1323852111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stange J, Veldhoen M. The aryl hydrocarbon receptor in innate T cell immunity. Seminars in immunopathology. 2013;35:645–655. doi: 10.1007/s00281-013-0389-1. [DOI] [PubMed] [Google Scholar]
- 116.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Kadow S, Jux B, Zahner SP, Wingerath B, Chmill S, Clausen BE, Hengstler J, Esser C. Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. Journal of immunology. 2011;187:3104–3110. doi: 10.4049/jimmunol.1100912. [DOI] [PubMed] [Google Scholar]
- 118.Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, Kornfeld H, Xiong N, Cohen NR, Brenner MB, et al. Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nature immunology. 2012;13:511–518. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 120.Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Manchester DK, Gordon SK, Golas CL, Roberts EA, Okey AB. Ah receptor in human placenta: stabilization by molybdate and characterization of binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 3-methylcholanthrene, and benzo(a)pyrene. Cancer research. 1987;47:4861–4868. [PubMed] [Google Scholar]
- 122.Flaveny C, Reen RK, Kusnadi A, Perdew GH. The mouse and human Ah receptor differ in recognition of LXXLL motifs. Archives of biochemistry and biophysics. 2008;471:215–223. doi: 10.1016/j.abb.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Flaveny CA, Murray IA, Perdew GH. Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicological sciences : an official journal of the Society of Toxicology. 2010;114:217–225. doi: 10.1093/toxsci/kfp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Forgacs AL, Dere E, Angrish MM, Zacharewski TR. Comparative analysis of temporal and dose-dependent TCDD-elicited gene expression in human, mouse, and rat primary hepatocytes. Toxicological sciences : an official journal of the Society of Toxicology. 2013;133:54–66. doi: 10.1093/toxsci/kft028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Black MB, Budinsky RA, Dombkowski A, Cukovic D, LeCluyse EL, Ferguson SS, Thomas RS, Rowlands JC. Cross-species comparisons of transcriptomic alterations in human and rat primary hepatocytes exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicological sciences : an official journal of the Society of Toxicology. 2012;127:199–215. doi: 10.1093/toxsci/kfs069. [DOI] [PubMed] [Google Scholar]
- 126.Ramadoss P, Perdew GH. Use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Molecular pharmacology. 2004;66:129–136. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- 127.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010;49:393–400. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Flaveny CA, Murray IA, Chiaro CR, Perdew GH. Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Molecular pharmacology. 2009;75:1412–1420. doi: 10.1124/mol.109.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer science. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]