Abstract
In well-recovered stroke patients with preserved hand movement, motor dysfunction relates to interhemispheric and intracortical inhibition in affected hand muscles. In less fully recovered patients unable to move their hand, the neural substrates of recovered arm movements, crucial for performance of daily living tasks, are not well understood. Here, we evaluated interhemispheric and intracortical inhibition in paretic arm muscles of patients with no recovery of hand movement (n=16, upper extremity Fugl-Meyer Assessment = 27.0 ± 8.6). We recorded silent periods (contralateral and ipsilateral) induced by transcranial magnetic stimulation (TMS) during voluntary isometric contraction of the paretic biceps and triceps brachii muscles (correlates of intracortical and interhemispheric inhibition respectively), and investigated links between the silent periods and motor recovery, an issue that has not been previously explored. We report that interhemispheric inhibition, stronger in the paretic triceps than biceps brachii muscles, significantly correlated with the magnitude of residual impairment (lower Fugl-Meyer scores). In contrast, intracortical inhibition in the paretic biceps brachii, but not in the triceps, correlated positively with motor recovery (Fugl-Meyer scores) and negatively with spasticity (lower Modified Ashworth scores). Our results suggest that interhemispheric inhibition and intracortical inhibition of paretic upper arm muscles relate to motor recovery in different ways. While interhemispheric inhibition may contribute to poorer recovery, muscle-specific intracortical inhibition may relate to successful motor recovery and lesser spasticity.
Keywords: Stroke, Transcranial Magnetic Stimulation, Upper Extremity Paresis, Cerebrovascular Accident, Rehabilitation, Arm, Interhemispheric Inhibition
Introduction
Over the past nearly two decades, there has been a great deal of investigation into mechanisms of impairment and recovery of hand movement after human stroke. This work has demonstrated that limitations in recovery of functional hand movements post-stroke are often linked to abnormalities in intracortical and interhemispheric inhibition. These findings have provided insight into the mechanisms of behavioral rehabilitation approaches, such as constraint-induced movement therapy (Liepert et al., 2000, Liepert, 2006, Boake et al., 2007, Sawaki et al., 2008, Bolognini et al., 2011), and have informed the development of cortical stimulation paradigms to improve hand recovery (Ward and Cohen, 2004, Nowak et al., 2009, Dimyan and Cohen, 2011) though see also (Di Pino et al., 2014).
Previous studies have used transcranial magnetic stimulation (TMS) to investigate intracortical inhibition of primary motor cortex (M1) hand representations in well-recovered stroke patients with at least partial recovery of hand function. Paired-pulse measurements of short-interval intracortical inhibition (SICI) (Kujirai et al., 1993), associated with GABAA–mediated intracortical inhibition (Werhahn et al., 1999), have shown abnormally decreased levels of intracortical inhibition targeting the paretic hand (Liepert et al., 2000, Manganotti et al., 2002, Cicinelli et al., 2003, Liepert, 2006, Huynh et al., 2013, Takechi et al., 2014). In contrast, intracortical inhibition reflected by the contralateral silent period (cSP), associated with GABAB receptor-mediated inhibition (Werhahn et al., 1999), is reported to be abnormally increased in the paretic hand (Haug and Kukowski, 1994, Braune and Fritz, 1995, Ahonen et al., 1998, Liepert et al., 2000, Kim et al., 2008, Takechi et al., 2014) and to decrease with recovery (Haug and Kukowski, 1994). Thus, it appears that SICI, reflecting GABAA–mediated intracortical inhibition, is abnormally decreased while cSP, reflecting GABAB receptor-mediated inhibition, is abnormally increased in the paretic hand post-stroke.
In addition to intracortical inhibition, interhemispheric inhibition between M1 hand representations in stroke patients with hand recovery has also been widely studied, and like intracortical inhibition, it has been studied using both paired-pulse and silent period TMS techniques. Paired-pulse measurements have shown that interhemispheric inhibition targeting the affected hemisphere (i.e. paretic hand) is stronger than that targeting the unaffected hemisphere (Nair et al., 2007, Butefisch et al., 2008, Kirton et al., 2010) and abnormally persistent during paretic finger movement preparation (Murase et al., 2004, Duque et al., 2005), particularly in those with poorer hand recovery. Ipsilateral silent period measurements have provided further support for the notion that interhemispheric inhibition targeting the paretic hand is stronger than that targeting the non-paretic hand (Takeuchi et al., 2012, Takechi et al., 2014) and than that measured in controls (Netz et al., 1997).
Mechanisms of upper arm motor recovery in stroke patients unable to use their hands, however, are not well understood. To examine interhemispheric and intracortical inhibition in paretic elbow flexors and extensors, we evaluated silent periods during voluntary isometric contractions of paretic arm biceps (flexor) or triceps (extensor) brachii, and measured the correlation between these measures and clinical and behavioral tests of motor ability, reaching performance, and spasticity. Recognizing that specific electrophysiological measurements, such as silent periods, reflect only a portion of the larger processes of intracortical and interhemispheric inhibition, we emphasize that when we refer to intracortical and interhemispheric inhibition we are referring only to that reflected by the contralateral and ipsilateral silent periods, respectively.
Given that many patients have particular difficulty de-activating elbow flexors, we postulated that inhibition targeting an elbow flexor muscle (biceps brachii) would be less than that targeting an elbow extensor (triceps brachii), and that biceps inhibition would correlate negatively with motor impairment. We report that interhemispheric inhibition and intracortical inhibition of these paretic upper arm muscles relate to paretic arm motor recovery differently in this population.
Materials and Methods
Participants
Sixteen individuals with chronic hemispheric stroke participated in the study (8 female; age: 59.0 ± 9.0 years; time post-stroke: 5.0 ± 3.8 years; Table 1) after providing written informed consent according to a protocol approved by the local ethical review boards. Testing was conducted at 1 of 2 sites, the National Institutes of Health Clinical Center (Bethesda, MD) or MedStar National Rehabilitation Hospital (Washington DC), using identical equipment and methods. Inclusion criteria included being at least 6 months post-stroke and having the ability to reach forward at least 5 cm without compensatory trunk movement. Potential participants were excluded if they were less than 18 years of age, pregnant, had cerebellar or brainstem lesions, or any contraindications to TMS (e.g., metal objects inside eyes or skull, history of seizures). We also excluded patients with voluntary wrist and finger movement on the paretic side because we wished to specifically target patients with less complete recovery (i.e. more residual motor dysfunction) than those who have been typically studied in the past. Thus, all participants had relatively severe arm impairment and lacked voluntary finger and wrist movement. On average, they scored 27.0 ± 8.6 on the Upper Extremity Fugl-Meyer Assessment. All participants had subcortical lesions, some of which extended into the cortex, but all of which spared the primary motor cortex (M1). In general, the lesions resulted from large ischemic or hemorrhagic infarcts of the middle cerebral artery.
Table 1.
Patient Characteristics
| Subject | Gender | Age | Time post stroke (years) | Fugl-Meyer Assessment | Hemisphere Involved | Unaffected Hemisphere RMT (% MSO) | Affected Hemisphere RMT (% MSO) | Stroke type (Artery) | Sub-type | Lesion location |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62.4 | 3.4 | 33 | R | 42 | > 100 | Ischemic (ICA) | Subcortical | Lacunar infarct from basal ganglia to centrum semiovale |
| 2 | M | 58.0 | 3.6 | 22 | L | 53 | > 100 | Hemorrhagic (MCA) | Subcortical | Tail of putamen, insula, thalamus, PLIC, corona radiata, centrum semiovale |
| 3 | M | 58.2 | 9.0 | 29 | L | 43 | > 100 | Ischemic (MCA) | Subcortical/ Cortical | Anterior temporal lobe, insula, frontal operculum, centrum semiovale, |
| 4 | F | 43.8 | 3.0 | 27 | R | 62 | > 100 | Ischemic (MCA) | Subcortical/ Cortical | Basal ganglia, frontal operculum, superior temporal lobe |
| 5 | M | 51.8 | 4.3 | 21 | L | 59 | > 100 | Hemorrhagic (MCA) | Subcortical | Basal ganglia, internal capsule, extending to level of left lateral ventricle |
| 6 | M | 59.9 | 1.7 | 33 | L | 52 | > 100 | Ischemic (MCA) | Subcortical | Tail of putamen, PLIC, corona radiata, centrum semiovale, |
| 7 | M | 59.7 | 12.1 | 20 | L | 60 | > 100 | Ischemic (MCA) | Subcortical/ Cortical | Temporal lobe, basal ganglia, centrum semiovale |
| 8 | M | 56.0 | 9.4 | 22 | L | 54 | > 100 | Ischemic (MCA) | Subcortical/ Cortical | Temporal lobe, insula, basal ganglia, corona radiata, centrum semiovale |
| 9 | F | 50.1 | 11.8 | 23 | R | 34 | > 100 | Ischemic (MCA) | Subcortical/ Cortical | Temporal lobe, basal ganglia, subinsular regions, corona radiata |
| 10 | F | 50.5 | 5.5 | 37 | R | 40 | > 100 | Ischemic (MCA) | Subcortical/ Cortical | Frontal operculum, insula, anterior temporal lobe |
| 11 | F | 52.9 | 8.2 | NA | R | 68 | > 100 | Ischemic (MCA) | Subcortical/ Cortical | Temporal lobe, insula, basal ganglia, corona radiata, centrum semiovale |
| 12 | M | 61.5 | 2.8 | 42 | L | 48 | > 100 | Ischemic (MCA) | Subcortical | Basal ganglia, PLIC |
| 13 | F | 83.0 | 1.3 | 40 | L | 47 | > 100 | Ischemic (MCA) | Subcortical/Cortical | Basal ganglia, internal capsule, posterior parietal lobe |
| 14 | F | 62.1 | 1.2 | 23 | R | 91 | > 100 | Ischemic (MCA) | Subcortical | Basal ganglia, PLIC |
| 15 | M | 64.1 | 1.9 | 23 | R | 47 | > 100 | Ischemic (MCA) | Subcortical | Basal ganglia, PLIC, corona radiata |
| 16 | F | 69.5 | 1.0 | 10 | R | 74 | > 100 | Ischemic (MCA) | Subcortical | Basal ganglia, PLIC, insula, corona radiata |
| Group | F = 8 | 59.0 ± 9.0 | 5.0 ± 3.8 | 27.0 ± 8.6 | R = 8 | 54.6 ± 14.3 | > 100 | Ischemic = 14 MCA = 15 |
Subcortical = 8 Subcortical/Cortical = 8 |
|
Abbreviations: RMT = resting motor threshold, MSO = maximum stimulator output, ICA = internal carotid artery, MCA = middle cerebral artery, PLIC = posterior limb of the internal capsule, NA = not available
Procedures
Participants were seated in an adjustable high-backed chair with arm rests. Participants' shoulders were positioned at 00 flexion and elbows at 900 flexion, with forearms supported by cushioned arm rests. EMG signals were recorded using active differential surface EMG electrodes (B&L Engineering, Santa Ana, CA) placed on the biceps brachii (short head) and triceps brachii (long head) of both arms. Standard skin preparation and muscle identification procedures were used to ensure the reliability of EMG signals. The electrodes had a pre-amplified gain of 330 and input impedance greater than 100 MΩ, providing good electromagnetic artifact suppression when used with TMS. EMG signals were digitized at 10 kHz, 16-bit precision (Cambridge Electronic Design Ltd, Cambridge, England) and subsequently high-pass filtered at 10 Hz (2nd order Butterworth) in MATLAB (MathWorks, Natick, MA) to reduce direct current offsets and movement artifacts.
A figure-of-eight double 70 mm TMS coil attached to a Magstim 200 stimulator (Magstim Company Ltd, Wales, UK) was used to deliver the stimuli. When stimulating the unaffected hemisphere M1, coil position (oriented to induce posterior-to-anterior current flow in the underlying cortex) was determined by identifying the location on the scalp where MEPs in biceps and triceps of the non-paretic arm could be optimally elicited by single-pulse TMS with the muscles at rest, the so-called “hotspot”. Resting motor threshold (RMT) for each muscle was determined by identifying the lowest stimulation intensity to elicit MEPs with peak-to-peak amplitude larger than 50 μV in 5 out of 10 trials. Since RMTs for non-paretic arm biceps and triceps were similar (54.6 ± 14.3% of maximum stimulator output for biceps, 53.90± 13.2% for triceps), the average of RMTs for both muscles was used to determine subsequent stimulation intensities for each subject.
Unable to elicit MEPs in the paretic arm at rest even using high stimulation intensities (Table 1), we instead asked participants to produce isometric contractions of the target muscle by either pulling up against a fixed strap placed just proximal to the wrist (elbow flexion) or pushing down into the chair's arm rest (elbow extension) to activate biceps or triceps, respectively. Real-time visual feedback of the EMG activity, a low target level (around 30 μV), and frequent rest periods were used to ensure that background activation remained constant during coil localization and subsequent testing. Background activation of the target muscle was constantly monitored by the investigator throughout the experiment. Any trial in which there was little or no EMG activation in the target muscle was immediately discarded and, after a rest period, an additional trial was collected to replace it. Participants were relatively accurate in maintaining the target activation level (mean pre-stimulus EMG was 25.9 ± 3.7 μV for biceps and 23.6 ± 3.0 μV for triceps).
We used the highest stimulation intensity tolerated by the participant to identify the location that, at least intermittently, produced a facilitatory EMG response or, as was more frequently the case, a period of suppression of the ongoing EMG activation. In addition, a stereotactic neuro-navigation system (Brainsight, Rogue Research Inc., Montreal Quebec, Canada) was used to confirm that the hotspot locations for both hemispheres were located on the precentral gyrus, the affected hemisphere hotspot was approximately in the mirror location of that for the unaffected hemisphere, and to ensure location accuracy of each TMS delivery throughout the experiment. Even with background muscle activation and high stimulation intensities, facilitatory responses (i.e. MEPs) were rarely elicited and thus it was not possible to determine active motor threshold.
Unaffected Hemisphere Stimulation
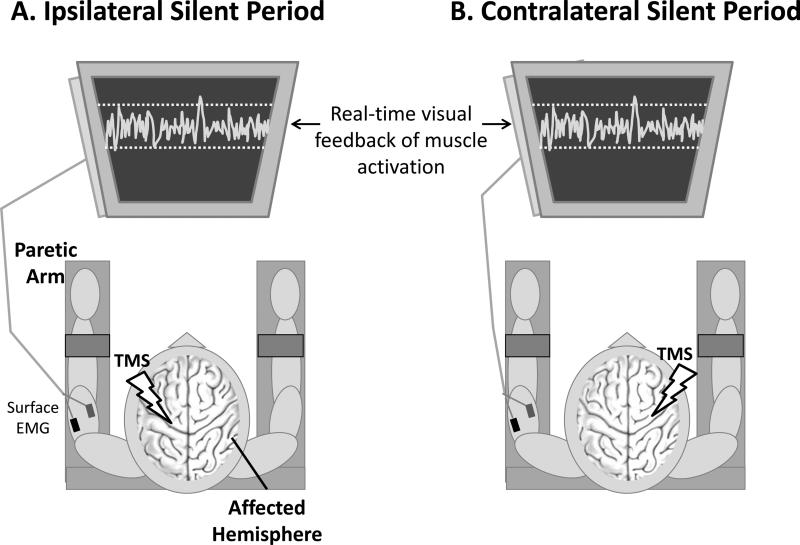
During sustained muscle activation, stimulation of the hemisphere ipsilateral to the active muscle produces a transcallosal volley that elicits a period of inhibition of the ongoing EMG activity known as an “ipsilateral silent period” (iSP; (Ferbert et al., 1992, Meyer et al., 1995, Chen et al., 2003). To measure the iSP, EMG was recorded from the paretic arm during biceps or triceps isometric muscle contraction while TMS was delivered to the unaffected (ipsilateral) hemisphere M1 hotspot at 110%, 130%, and 150% of the non-paretic RMT (20 trials at each intensity for each muscle; Fig. 1A).
Figure 1.
To elicit an ipsilateral silent period (A), patients activated their paretic arm by isometrically contracting the target muscle (biceps or triceps) while TMS was applied to the unaffected hemisphere primary motor cortex. The resulting period of decreased EMG activity reflects interhemispheric inhibition from the unaffected to the affected hemisphere. To elicit a contralateral silent period (B), the paretic arm was activated while TMS was applied to the affected hemisphere primary motor cortex, eliciting a period of decreased EMG activity that reflects intracortical inhibition.
EMG = electromyography; TMS = transcranial magnetic stimulation
Affected Hemisphere Stimulation
During voluntary muscle activation, stimulation of the contralateral M1 produces a period of reduced EMG activity known as the contralateral silent period (cSP; (Davey et al., 1994), which reflects GABAB-mediated intracortical inhibition (Werhahn et al., 1999). To measure the cSP, EMG was recorded from the affected arm during biceps or triceps isometric muscle contraction while TMS was delivered to the affected (contralateral) hemisphere M1 hotspot at 80%, 90%, and 100% of maximum stimulator output (10 trials at each intensity for each muscle; Fig. 1B).
Measurements of Motor Recovery
To characterize motor recovery we collected metrics of overall arm impairment, spasticity at the elbow, and performance of a proximal arm reaching task. The upper extremity portion of the Fugl-Meyer Assessment (Fugl-Meyer et al., 1975) and the Modified Ashworth Scale (Bohannon and Smith, 1987), administered by a licensed physical therapist, served as measures of motor impairment/ability (Crow et al., 2014) and spasticity, respectively. To specifically measure proximal arm motor performance, patients performed a forward reaching task which required shoulder flexion with elbow extension, but no finger or wrist movement. Participants were seated at a table in a high-backed chair with crossed non-elastic straps across the torso to prevent compensatory movements of the trunk. In response to a randomly-timed visual “Go” cue, participants reached forward as quickly as possible to contact a large circular button on the table (the reaching “target”). The target was placed at 80% of each participant's maximum forward reaching distance (21.4 ± 7.7 cm from the front edge of the table). After 10 familiarization trials, participants performed 20 test trials. Reaching response time was defined as the time elapsed from the appearance of the “Go” cue to button contact and was quantified as the median of the 20 trials for each individual. Note that this measure does not differentiate between reaction time and movement time and therefore represents the time required for not only movement execution but movement preparation as well.
Data Analysis
Physiological data were analyzed off-line using custom software programmed in MATLAB. Pre-stimulus mean EMG was calculated from the averaged rectified waveform and was defined as the mean value during a 100-ms time window (from 150 ms to 50 ms prior to TMS onset). For iSP, the onset of the silent period was defined as the point at which the averaged rectified EMG dropped and stayed below the pre-stimulus level for at least 5 ms. For cSP, the onset was defined as the time of stimulus delivery since, though they were rare in this population, any MEPs preceding the silent period could obscure cSP onset. Trials in which onsets were obscured by prolonged TMS artifact were discarded. Silent period offsets were defined as the point after the onset at which the averaged rectified EMG returned to and stayed at or above the pre-stimulus level for at least 5 ms. Silent period (iSP and cSP) measurements included percent inhibition (Ferbert et al., 1992), calculated from the rectified and ensemble averaged waveform and expressed as a percentage of pre-stimulus EMG according to the following equation:
Calculated in this manner, a higher value indicates stronger inhibition. We also measured silent period duration (Cantello et al., 1992, Kukowski and Haug, 1992, Inghilleri et al., 1993) and rate of occurrence, a unitless value between 0 and 1 defined as the number of trials in which a silent period was successfully elicited divided by the total number of trials (0 = silent period was not elicited in any of the trials; 1 = silent period elicited in all trials). To measure the rate of occurrence, each trial was analyzed individually to determine the presence or absence of a silent period. A trial was defined as positive for a silent period when it contained a post-stimulus period lasting at least 25 ms during which the mean rectified EMG values remained below the mean rectified pre-stimulus EMG level. Each trial was also visually inspected to validate the result of the algorithm.
Dependent variables were analyzed using a 2 (Muscles) X 3 (Stimulation Intensities) ANOVA with repeated measures. In the case of significant Interaction effects, paired t-tests with a Bonferroni correction were used for post-hoc comparisons. Pearson Product Moment Correlation Coefficients were calculated between the physiological measures and Fugl-Meyer score and reaching response time, since the latter measures produce interval and ratio data, respectively. Since the physiological measures were characterized using multiple dependent measures, to minimize the number of comparisons, we chose the measure most commonly used to quantify each type of silent period (i.e. iSP percent inhibition and cSP duration) and used the average value across stimulation intensities for calculating the correlation coefficients. To test for differences between how physiological measures recorded in biceps vs. triceps correlated with motor recovery, we calculated the Steiger's Z score to statistically compare correlation coefficients (Steiger, 1980). Steiger's Z is a method for statistically comparing “overlapping” correlation coefficients (i.e. correlations that share a common variable) from a single sample. Each correlation is converted into a z score using Fisher's r-to-z transformation and the z-scores are used in the significance testing formula. We compared the correlations between biceps silent periods (cSP or iSP) and motor recovery (Fugl-Meyer Score, reaching response time, and elbow spasticity) to those between triceps silent periods and motor recovery to determine whether measurements of physiology in biceps and triceps have different relationships with motor recovery. Spearman's Rank Correlation Coefficient (ρ) was used to test for statistical dependence between the physiological measures and spasticity, since it is measured by the ordinal Modified Ashworth Scale. All statistical analyses were performed using IBM® SPSS® Statistics 21 with significance level (α) set at 0.05.
Results
We observed clear distinctions between inhibition targeting paretic arm flexor vs. extensor muscles (biceps and triceps, respectively), as well as muscle-specific correlations between inhibition and paretic arm motor recovery.
Ipsilateral Silent Period (iSP)
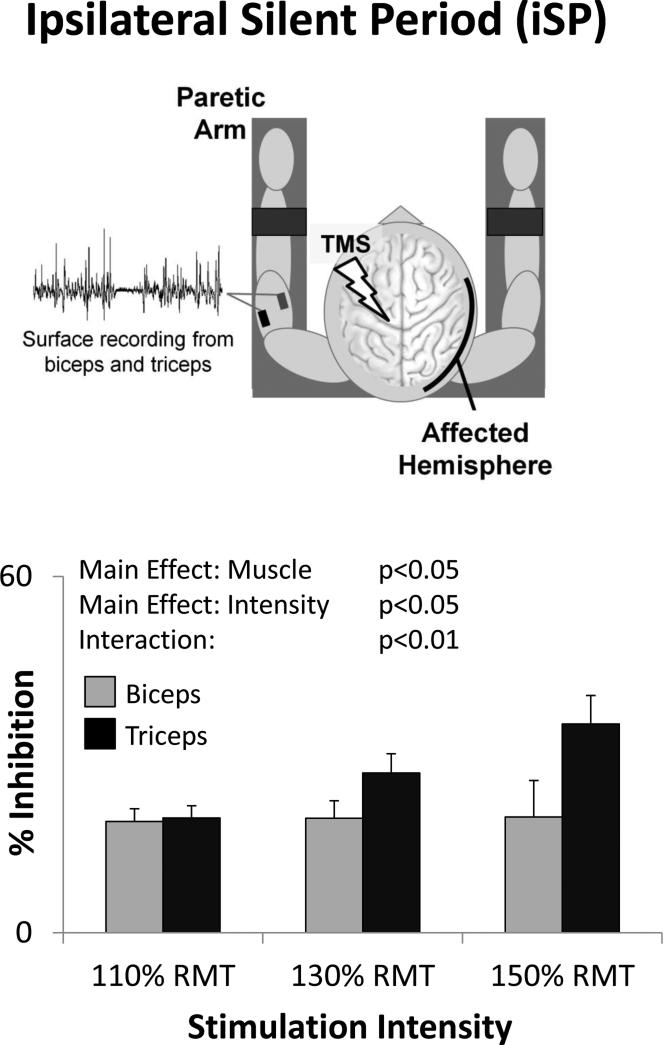
iSPs were readily elicited in both muscles with unaffected hemisphere stimulation (ipsilateral to pre-activated paretic arm muscles). They were present in 71± 5% and 75 ± 5% of trials for biceps and triceps respectively. However, these muscles showed marked differences in iSP % inhibition (Fig 2). There was a significant main effect of Muscle (F(1,10)=5.88, p=0.036) indicating greater inhibition in triceps than in biceps. There was also a significant Main Effect of Intensity (F(2,20)=4.9 p=0.018) indicating that inhibition increased with increasing stimulation intensity. Additionally, there was a significant stimulus Intensity x Muscle Interaction Effect (F(2,20)=6.61, p=0.006). Post-hoc testing revealed no significant difference between muscles at the lowest stimulation intensity (110% RMT: t(14)=0.782, p=0.447), but greater inhibition in triceps than biceps with increasing stimulation intensities (130% RMT: t(14)=2.318, p=0.036; 150% RMT: t(11)=2.949, p=0.013) (Fig. 2). iSP duration and rate of occurrence each showed a significant Main Effect of Intensity (F(2,20)=10.17, p=0.001 and F(2,20)=4.11, p=0.032, respectively) indicating that inhibition increased with increasing stimulus intensity, but no significant Main Effect of Muscle (F(1,10)=0.54, p=0.479 for iSP duration; F(1,10)=1.37, p=0.269 for iSP rate of occurrence) and no significant Interactions (F(2,20)=1.00, p=0.387 for iSP duration; F(2,20)=1.07, p=0.363 for iSP rate of occurrence).
Figure 2.
With stimulation of the unaffected hemisphere, the ipsilateral silent period (iSP) elicited in the paretic arm triceps muscle was greater than that in biceps (Main Effect of Muscle), increased with increasing stimulation intensity (Main Effect of Intensity), and increased more in triceps than biceps (Interaction Effect).
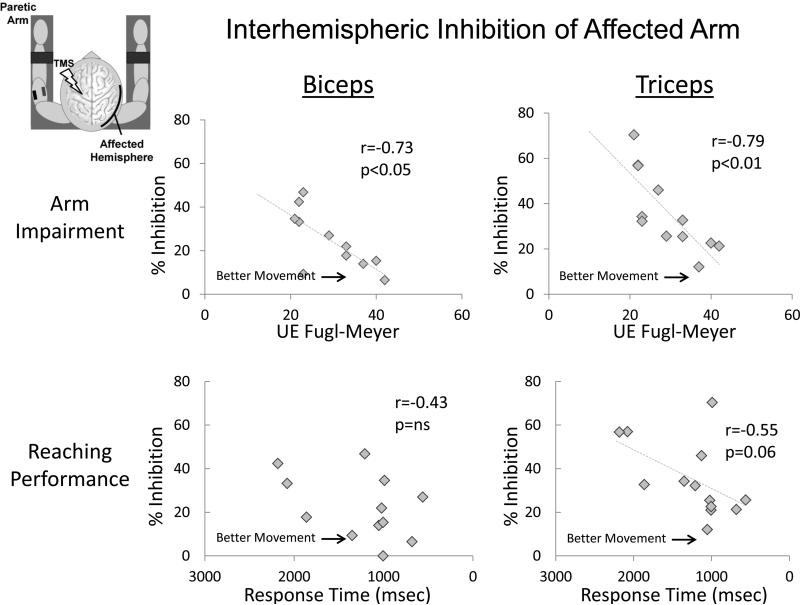
iSPs correlated negatively with Fugl Meyer scores (i.e. larger iSPs correlated with more severe impairment; biceps: r=−0.73, p=0.011, triceps: r=−0.79, p=0.004; Fig. 3, top row) and, in the triceps, tended to correlate positively with reaching response time (i.e. larger iSPs correlated with slower reaching response times; Fig. 3, bottom row, r=0.43; p=0.165 and r=0.55; p=0.063, for biceps and triceps, respectively). No correlation was observed between iSPs and spasticity (ρ=0.27, p=0.178 and ρ=−0.10, p=0.371 for biceps and triceps, respectively).
Figure 3.
Associations between interhemispheric inhibition, measured by the ipsilateral silent period (iSP), and overall motor impairment of the arm (measured by the Upper Extremity Fugl-Meyer Assessment) and proximal arm motor performance (measured by reaching response time). Note that response time values are displayed longest to shortest (left to right), since longer response time indicates slower task completion. For both biceps and triceps, higher iSPs correlated with more severe motor impairment of the arm (top row). For triceps, higher iSP values also tended to be associated with longer (i.e. slower) reaching response times (bottom right).
Contralateral Silent Period (cSP)
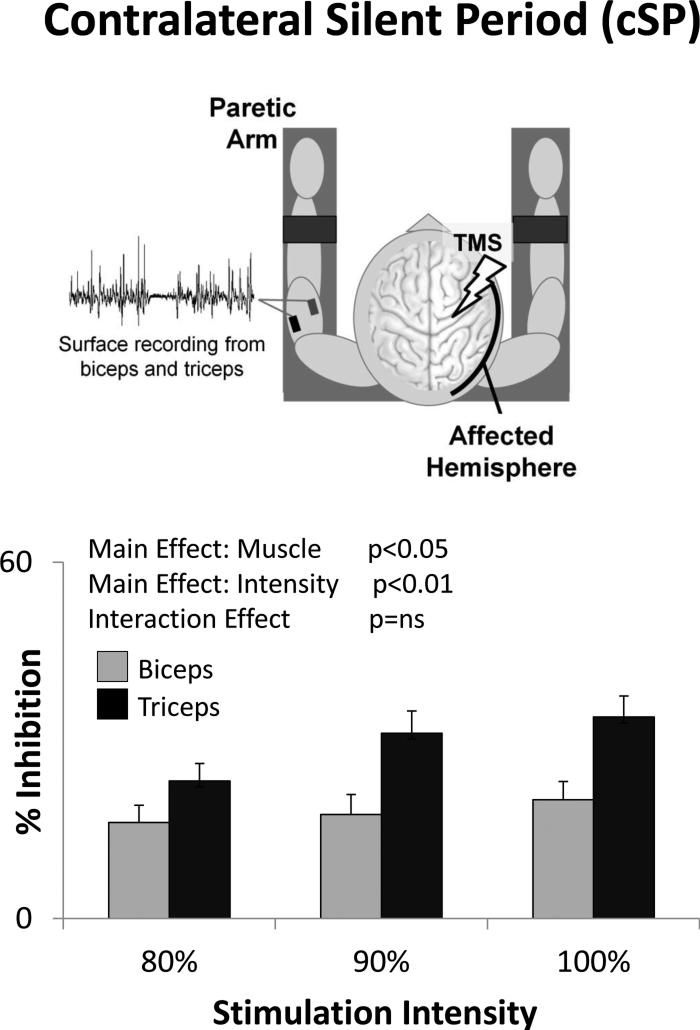
Like iSPs, cSPs were readily elicited in both biceps and triceps (83 ± 3% and 91 ± 3% of trials respectively). There was a significant Main Effect of Muscle (F(1,11)=7.34, p=0.020) and Intensity (F(2,22)=7.99, p=0.002) on cSP % inhibition, indicating significantly stronger cSP in triceps than biceps and increased cSP with increasing stimulation intensity (Fig. 4). There was no significant Interaction (F(2,22)=2.14, p=0.142). The rate of occurrence of cSP was significantly greater in triceps than biceps (Main Effect of Muscle F(1,12)=6.31, p=0.027) and increased as a function of stimulation intensity (Main Effect of Intensity F(2,24)=6.24, p=0.007), but the Interaction was not significant (F(2,24)=0.00, p=1.00). cSP duration showed a non-significant trend toward a Main Effect of Intensity (F(2,24)=2.90, p=0.075), but no significant effect of Muscle (F(1,12)=0.64, p=0.440) and no Interaction effect (F(2,24)=0.83, p=0.447).
Figure 4.
With transcranial magnetic stimulation (TMS) of the stroke-affected hemisphere, the contralateral silent period (cSP) was greater in triceps than biceps (Main Effect of Muscle) and increased with increasing stimulation intensity (Main Effect of Intensity).
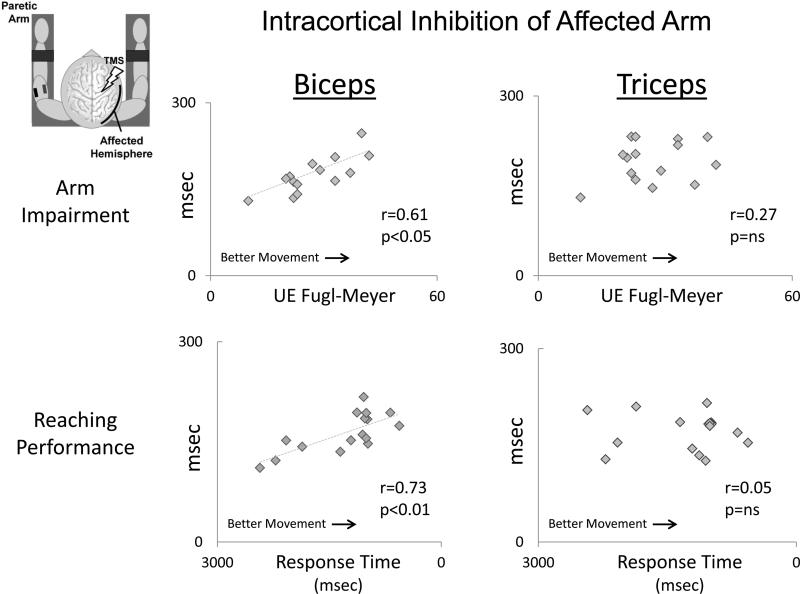
In contrast to the negative correlation observed between iSP and motor ability (Fig. 3), in the paretic biceps, cSPs correlated positively with Fugl-Meyer scores (i.e. larger cSPs were associated with higher levels of motor ability; r=0.61, p=0.017; Fig. 5, top left), and correlated negatively with reaching response times (i.e. faster reaching response times; r=0.73, p=0.001; Fig. 5, bottom left) and spasticity (i.e. lower levels of spasticity; ρ=−0.53, p=0.007). However, for triceps, no such relationship was observed between cSP and behavioral measures of motor recovery (r=0.07, p=0.799; r=0.05, p=0.855; and ρ=0.17, p=0.478, for Fugl-Meyer Score, reaching response time, and spasticity score, respectively). This difference between how cSPs in biceps vs. triceps correlated with motor recovery was statistically significant (Z=2.42, p=0.016 and Z=2.47, p=0.013 for response time and Fugl-Meyer score, respectively).
Figure 5.
Associations between intracortical inhibition, measured by the contralateral silent period (cSP), and overall motor impairment of the arm (measured by the Upper Extremity Fugl-Meyer Assessment) and proximal arm motor performance (measured by reaching response time). Note that biceps cSP (left) was positively associated with Fugl-Meyer score and reaching response time, while triceps cSP (right) showed no correlation with either measure.
Discussion
In summary, iSP was stronger in the paretic triceps than biceps brachii, and correlated negatively with motor recovery. Additionally, cSP correlated positively with motor recovery only in the paretic biceps but not triceps. Thus, in patients with severe stroke-related arm dysfunction, metrics of interhemispheric and intracortical inhibition differed across paretic upper arm muscles and in their relationship with motor recovery.
Ipsilateral Silent Period
The iSP produced when stimulating the unaffected hemisphere during isometric activation of the paretic arm reflects the strength of interhemispheric inhibition from the unaffected to the affected hemisphere and is thought to be transcallosally mediated (Meyer et al., 1995, Meyer et al., 1998, Fling et al., 2013) and to reflect the activity of networks similar to those measured using a more traditional paired-pulse TMS technique (Chen et al., 2003). In this patient population, interhemispheric inhibition targeting the paretic arm triceps muscle was stronger than that targeting the biceps, in spite of which the relationship between interhemispheric inhibition and motor recovery was similar for both muscles: stronger inhibition from unaffected to affected hemisphere M1 was associated with poorer motor recovery. This relationship is similar to that previously reported in hand muscles of more mildly impaired patients (Murase et al., 2004) and replicates our previous report (Harris-Love et al., 2011).
Cortical Silent Period
cSPs were elicited by stimulating affected hemisphere M1 during paretic arm muscle activation. The cSP primarily reflects intracortical inhibitory processes (Fuhr et al., 1991, Cantello et al., 1992, Inghilleri et al., 1993, Roick et al., 1993), (Triggs et al., 1993, Classen and Benecke, 1995, Chen et al., 1999, Tergau et al., 1999) to which GABAB receptors contribute (Ziemann et al., 1996, Siebner et al., 1998, Werhahn et al., 1999).
We report stronger intracortical inhibition in paretic triceps than biceps. However, the relationship between intracortical inhibition and paretic arm motor recovery differed from that observed between interhemispheric inhibition and motor recovery. First, stronger intracortical inhibition in biceps was associated with better motor recovery, i.e. higher Fugl-Meyer scores, faster reaching response times, and lower Modified Ashworth scores (unlike interhemispheric inhibition, in which stronger inhibition was associated with poorer motor recovery). Second, the relationship between intracortical inhibition and motor recovery differed between biceps and triceps (unlike interhemispheric inhibition, in which the correlation with motor recovery was similar for biceps and triceps). Intracortical inhibition in the paretic biceps correlated with motor recovery, while intracortical inhibition in the paretic triceps did not (Fig. 5).
In general, cSPs elicited from hand muscles in well-recovered stroke patients are reported to be abnormally increased and to decrease with recovery (Haug and Kukowski, 1994, Braune and Fritz, 1995, Classen et al., 1997, Ahonen et al., 1998, Liepert et al., 2000). Our finding of a positive relationship between biceps cSP and motor ability (higher Fugl-Meyer scores), proximal arm motor performance (faster reaching response times) and spasticity (lower Modified Ashworth scores) appears to differ with these previously reported findings. However, in regards to the latter, even in patients with some recovery of hand movement there have been reports of a relationship between stronger intracortical inhibition in the affected hemisphere and lower Modified Ashworth scores (Uozumi et al., 1992, Catano et al., 1997, Catano et al., 1997{Cruz Martinez, 1998 #1445, Cruz Martinez et al., 1998). Interestingly, this association found with cSPs has been also reported when studying intracortical inhibition with paired-pulse TMS (a measure thought to reflect inhibition mediated by a different GABA subtype; (Liepert, 2006)). The reasons for this relationship are not known but studies from both human and non-human primates may provide some clues. It is possible that intracortical inhibition of corticoreticular excitation, either via the corticoreticular tract (Yeo et al., 2012) or via reticular collaterals of the corticospinal tract, which are most abundant in corticospinal neurons projecting to proximal arm muscles (Keizer and Kuypers, 1989), contribute to cSP. Alternatively, medial brainstem pathways such as those in the pontomedullary reticular formation (PMRF) project to proximal upper limb muscles (Davidson and Buford, 2006, Davidson et al., 2007, Sakai et al., 2009) and, following an extensive lesion of the corticospinal tract, projections from PMRF to upper limb flexor muscles have been shown to be preferentially strengthened (Zaaimi et al., 2012). The possibility that intracortical inhibition could interact with the preferential activation of flexors that may occur at the brainstem level post-stroke (and the relationship this may have with spasticity and other aspects of arm impairment) is an important topic for future investigation.
Limitations
There are difficulties inherent to the study of patients without hand function, thus the relative absence of information on this population in the literature. First, because MEPs could not be elicited at rest, it was not possible to determine resting motor thresholds in the affected hemisphere in distal or proximal arm muscles of these patients (Table 1). For this reason we were confined to comparing iSP and cSP across muscle groups and their relation to motor impairment. This limitation also made it difficult to compare our results in stroke patients with complete hand paralysis with less impaired individuals, in whom RMTs are more easily determined. Additionally, most of the information on intracortical and interhemispheric inhibition in the literature has been obtained by testing distal hand muscles, not feasible in our patients with hand paralysis.
The reaching response time results must be interpreted with caution in that this measurement does not differentiate between reaction time and movement time, and thus represents not only movement execution but movement preparation time as well. The response times recorded were quite long (most were between 1000 and 2000 msec), possibly due to the difficulty these patients had in completing the reaching task, but without the ability to differentiate reaction and movement time, it is impossible to know. It is critical for future studies to clarify this issue. Another important limitation to the present study is that we do not yet have information on how these measurements may differ between muscles in age-matched healthy volunteers. Thus, we cannot conclude that the between-muscle difference we observed was abnormal, per se, only that it was in some cases correlated with motor function. Finally, the average age of our sample (59 ± 9 years) is considerably younger than the average age of the overall stroke population (70-71 years (Hall et al., 2012). Thus, the generalizability of these results to the larger stroke population is an important topic for future investigation.
Summary and Conclusions
The results of this study indicate differences in post-stroke cortical physiology between upper arm muscles in stroke patients with severe arm dysfunction and their relationship to the level of motor recovery attained. While interhemispheric inhibition relates to motor dysfunction, muscle-specific intracortical inhibition may contribute to motor recovery and lesser spasticity.
Acknowledgements
We are grateful to all of the study participants for volunteering their time. We also thank Drs. Barbara Bregman, Stuart Baker, and Theresa Jones for helpful discussions on earlier versions of this manuscript.
Funding NIH K01HD060886 (MHL); NIH/NINDS Competitive Intramural Postdoctoral Fellowship (MHL); National Capital Area Rehabilitation Research Network (NCARRN) pilot award to MHL via NICHD/NINDS HD050845 (Barbara S. Bregman, PI)
Abbreviations
- cSP
contralateral silent period (reflects intracortical inhibition)
- iSP
ipsilateral silent period (reflects interhemispheric inhibition)
- M1
primary motor cortex
- RMT
resting motor threshold
- TMS
transcranial magnetic stimulation
Contributor Information
Michelle L. Harris-Love, Georgetown University Medical Center, MedStar National Rehabilitation Hospital.
Evan Chan, MedStar National Rehabilitation Hospital.
Alexander W. Dromerick, Georgetown University Medical Center, MedStar National Rehabilitation Hospital, District of Columbia VA Medical Center.
Leonardo G. Cohen, Human Cortical Physiology and Neurorehabilitation, Section, NINDS, NIH.
References Cited
- Ahonen JP, Jehkonen M, Dastidar P, Molnar G, Hakkinen V. Cortical silent period evoked by transcranial magnetic stimulation in ischemic stroke. Electroencephalogr Clin Neurophysiol. 1998;109(3):224–9. doi: 10.1016/s0924-980x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Boake C, Noser EA, Ro T, Baraniuk S, Gaber M, Johnson R, et al. Constraint induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21(1):14–24. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25(9):819–29. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- Braune HJ, Fritz C. Transcranial magnetic stimulation-evoked inhibition of voluntary muscle activity (silent period) is impaired in patients with ischemic hemispheric lesion. Stroke. 1995;26(4):550–3. doi: 10.1161/01.str.26.4.550. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22(1):4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42(10):1951–9. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- Catano A, Houa M, Noel P. Magnetic transcranial stimulation: clinical interest of the silent period in acute and chronic stages of stroke. Electroencephalogr Clin Neurophysiol. 1997;105(4):290–6. doi: 10.1016/s0924-980x(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Catano A, Houa M, Noel P. Magnetic transcranial stimulation: dissociation of excitatory and inhibitory mechanisms in acute strokes. Electroencephalogr Clin Neurophysiol. 1997;105(1):29–36. doi: 10.1016/s0924-980x(96)96515-7. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128(4):539–42. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89(3):1256–64. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired pulse transcranial magnetic stimulation study. Stroke. 2003;34(11):2653–8. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- Classen J, Benecke R. Inhibitory phenomena in individual motor units induced by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1995;97(5):264–74. doi: 10.1016/0013-4694(95)00099-k. [DOI] [PubMed] [Google Scholar]
- Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, et al. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997;120(Pt 4):605–19. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- Crow JL, Kwakkel G, Bussmann JB, Goos JA, Harmeling-van der Wel BC. Are the hierarchical properties of the Fugl Meyer assessment scale the same in acute stroke and chronic stroke? Phys Ther. 2014;94(7):977–86. doi: 10.2522/ptj.20130170. [DOI] [PubMed] [Google Scholar]
- Cruz Martinez A, Munoz J, Palacios F. The muscle inhibitory period by transcranial magnetic stimulation. Study in stroke patients. Electromyogr Clin Neurophysiol. 1998;38(3):189–92. [PubMed] [Google Scholar]
- Davey NJ, Romaiguere P, Maskill DW, Ellaway PH. Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. J Physiol. 1994;477(Pt 2):223–35. doi: 10.1113/jphysiol.1994.sp020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173(1):25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007;27(30):8053–8. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7(2):76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28(4):940–6. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–46. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Benson BL, Seidler RD. Transcallosal sensorimotor fiber tract structure-function relationships. Hum Brain Mapp. 2013;34(2):384–95. doi: 10.1002/hbm.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81(4):257–62. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in U.S. hospitals, 1989-2009. NCHS Data Brief. 2012(95):1–8. [PubMed] [Google Scholar]
- Harris-Love ML, Morton SM, Perez MA, Cohen LG. Mechanisms of short-term training induced reaching improvement in severely hemiparetic stroke patients: a TMS study. Neurorehabil Neural Repair. 2011;25(5):398–411. doi: 10.1177/1545968310395600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug BA, Kukowski B. Latency and duration of the muscle silent period following transcranial magnetic stimulation in multiple sclerosis, cerebral ischemia, and other upper motoneuron lesions. Neurology. 1994;44(5):936–40. doi: 10.1212/wnl.44.5.936. [DOI] [PubMed] [Google Scholar]
- Huynh W, Vucic S, Krishnan AV, Lin CS, Hornberger M, Kiernan MC. Longitudinal plasticity across the neural axis in acute stroke. Neurorehabil Neural Repair. 2013;27(3):219–29. doi: 10.1177/1545968312462071. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–34. [PMC free article] [PubMed] [Google Scholar]
- Keizer K, Kuypers HG. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp Brain Res. 1989;74(2):311–8. doi: 10.1007/BF00248864. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee HW, Cohen LG, Park KD, Choi KG. Motor cortical excitability in patients with poststroke epilepsy. Epilepsia. 2008;49(1):117–24. doi: 10.1111/j.1528-1167.2007.01231.x. [DOI] [PubMed] [Google Scholar]
- Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121(11):1922–9. doi: 10.1016/j.clinph.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukowski B, Haug B. Quantitative evaluation of the silent period, evoked by transcranial magnetic stimulation during sustained muscle contraction, in normal man and in patients with stroke. Electromyogr Clin Neurophysiol. 1992;32(7-8):373–8. [PubMed] [Google Scholar]
- Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006;19(1):41–7. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111(4):671–6. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113(6):936–43. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118(Pt 2):429–40. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Woiciechowsky C. Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol. 1998;43(3):360–9. doi: 10.1002/ana.410430314. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual-Leone A, Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage. 2007;34(1):253–63. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120(Pt 9):1579–86. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23(7):641–56. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- Roick H, von Giesen HJ, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Exp Brain Res. 1993;94(3):489–98. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Davidson AG, Buford JA. Reticulospinal neurons in the pontomedullary reticular formation of the monkey (Macaca fascicularis). Neuroscience. 2009;163(4):1158–70. doi: 10.1016/j.neuroscience.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, et al. Constraint induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22(5):505–13. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21(9):1209–12. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87(2):245–51. [Google Scholar]
- Takechi U, Matsunaga K, Nakanishi R, Yamanaga H, Murayama N, Mafune K, et al. Longitudinal changes of motor cortical excitability and transcallosal inhibition after subcortical stroke. Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.01.034. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Matsuo Y, Ikoma K. Low-frequency repetitive TMS plus anodal transcranial DCS prevents transient decline in bimanual movement induced by contralesional inhibitory rTMS after stroke. Neurorehabil Neural Repair. 2012;26(8):988–98. doi: 10.1177/1545968311433295. [DOI] [PubMed] [Google Scholar]
- Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, et al. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res. 1999;124(4):447–54. doi: 10.1007/s002210050640. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Cros D, Macdonell RA, Chiappa KH, Fang J, Day BJ. Cortical and spinal motor excitability during the transcranial magnetic stimulation silent period in humans. Brain Res. 1993;628(1-2):39–48. doi: 10.1016/0006-8993(93)90935-g. [DOI] [PubMed] [Google Scholar]
- Uozumi T, Ito Y, Tsuji S, Murai Y. Inhibitory period following motor potentials evoked by magnetic cortical stimulation. Electroencephalogr Clin Neurophysiol. 1992;85(4):273–9. doi: 10.1016/0168-5597(92)90116-s. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61(12):1844–8. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591–7. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SS, Chang MC, Kwon YH, Jung YJ, Jang SH. Corticoreticular pathway in the human brain: diffusion tensor tractography study. Neurosci Lett. 2012;508(1):9–12. doi: 10.1016/j.neulet.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135(Pt 7):2277–89. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40(3):367–78. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]