Abstract
Vesicular transporter proteins are an essential component of the presynaptic machinery that regulates neurotransmitter storage and release. They also provide a key point of control for homeostatic signaling pathways that maintain balanced excitation and inhibition following changes in activity levels, including the onset of sensory experience. To advance understanding of their roles in the developing auditory forebrain, we tracked the expression of the vesicular transporters of glutamate (VGluT1, VGluT2) and GABA (VGAT) in primary auditory cortex (A1) and medial geniculate body (MGB) of developing mice (P7, P11, P14, P21, adult) before and after ear canal opening (~P11–P13). RNA sequencing, in situ hybridization, and immunohistochemistry were combined to track changes in transporter expression and document regional patterns of transcript and protein localization. Overall, vesicular transporter expression changed the most between P7 and P21. The expression patterns and maturational trajectories of each marker varied by brain region, cortical layer, and MGB subdivision. VGluT1 expression was highest in A1, moderate in MGB, and increased with age in both regions. VGluT2 mRNA levels were low in A1 at all ages, but high in MGB, where adult levels were reached by P14. VGluT2 immunoreactivity was prominent in both regions. VGluT1+ and VGluT2+ transcripts were co-expressed in MGB and A1 somata, but co-localization of immunoreactive puncta was not detected. In A1, VGAT mRNA levels were relatively stable from P7 to adult, while immunoreactivity increased steadily. VGAT+ transcripts were rare in MGB neurons, whereas VGAT immunoreactivity was robust at all ages. Morphological changes in immunoreactive puncta were found in two regions after ear canal opening. In the ventral MGB, a decrease in VGluT2 puncta density was accompanied by an increase in puncta size. In A1, peri-somatic VGAT and VGluT1 terminals became prominent around the neuronal somata. Overall, the observed changes in gene and protein expression, regional architecture, and morphology relate to—and to some extent may enable— the emergence of mature sound-evoked activity patterns. In that regard, the findings of this study expand our understanding of the presynaptic mechanisms that regulate critical period formation associated with experience-dependent refinement of sound processing in auditory forebrain circuits.
Keywords: Glutamate, GABA, Fox-3, Protein, RNA, Development, Sequencing, Cortex, Thalamus, Geniculate, A1, Juvenile, Critical period, Homeostatic plasticity, BDNF, MeCP2
Introduction
In the central nervous system, vesicular transporters are responsible for packaging neurotransmitters into synaptic vesicles and play important roles in the release machinery (Blakely and Edwards 2012; Martin and Krantz 2014; Takamori et al. 2006). Several classes of vesicular transporters have been characterized, each associated with a different neurotransmitter: acetylcholine (VAChT); monoamines (VMAT2); GABA and glycine (VGAT); and glutamate (VGluT1, VGluT2, VGluT3). Activity-dependent regulation of their expression can alter the number of transporters localized to each vesicle, impacting vesicle filling, quantal size, and the amount and probability of neurotransmitter release (Blakely and Edwards 2012; Edwards 2007; Erickson et al. 2006; Fei et al. 2008; Hnasko and Edwards 2012; Omote et al. 2011; Santos et al. 2009; Takamori et al. 2006; Wilson et al. 2005; Wojcik et al. 2004). Accordingly, these transporters contribute to pre- and postsynaptic homeostatic mechanisms that regulate the balance of excitatory and inhibitory neurotrans-mission (Coleman et al. 2010; De Gois et al. 2005; Lazarevic et al. 2013; Rich and Wenner 2007; Turrigiano 2008; Turrigiano and Nelson 2004; Wilson et al. 2005).
Vesicular transporter expression in the forebrain undergoes substantial changes across development. Prenatally, VGluT1 and VGluT2 mRNA is detected in preplate and marginal zones of mice by E10 (Ina et al. 2007; Schuurmans et al. 2004). Postnatally, changes in VGluT and VGAT expression levels are correlated with the onset of sensory experience (e.g., eye opening), and can be modified by altering sensory experience (Boulland and Chaudhry 2012; Boulland et al. 2004; Chattopadhyaya et al. 2004; De Gois et al. 2005; Liguz-Lecznar and Skangiel-Kramska 2007; Minelli et al. 2003a, b; Nakamura et al. 2005; Takayama and Inoue 2010). Accordingly, these transporters are thought to play an important role in the plastic changes that shape critical period formation (Griffen and Maffei 2014; Hensch 2005; Kotak et al. 2008; Kuhlman et al. 2013; Lefort et al. 2013; Levelt and Hubener 2012; Maffei and Turrigiano 2008; Nahmani and Turrigiano 2014; Wang and Maffei 2014).
Less is known about the maturation of vesicular transporter expression in the developing central auditory system. However, many other developmental milestones for changes in protein expression and neurophysiological responses around the onset of hearing have been established, which provide context for the present studies. The delayed onset of hearing in altricial animals has provided researchers with a convenient window to study activity-dependent changes in basic neuronal response properties (Barkat et al. 2011; Brown and Harrison 2010; Carrasco et al. 2013; Chang et al. 2005; de Villers-Sidani et al. 2007; Insanally et al. 2010; Kral et al. 2005; Mrsic-Flogel et al. 2003, 2006; Polley et al. 2013; Razak and Fuzessery 2007; Rosen et al. 2010; Sarro et al. 2011; Trujillo et al. 2013) and associated mechanisms (Dorrn et al. 2010; Metherate and Cruikshank 1999; Oswald and Reyes 2008, 2011; Venkataraman and Bartlett 2013, 2014). Critical periods for sound processing are also contained within this window (Barkat et al. 2011; Fitch et al. 2013; Froemke and Jones 2011; Keating and King 2013; Kral et al. 2013; Polley et al. 2013; Sanes and Bao 2009; Sanes and Kotak 2011; Sanes and Woolley 2011; Takesian et al. 2009; Whitton and Polley 2011). As observed in other sensory systems, the vesicular transporters are likely an important component of the synaptic machinery associated with maturation in the auditory forebrain, but their maturational trajectories are yet to be co-registered with established markers of central auditory system development.
Given their potential importance in development and plasticity, and the absence of data on their expression in young animals, the main purpose of the current study was to document the expression of VGluT1, VGluT2, and VGAT in the primary auditory cortex (A1) and medial geniculate body (MGB) of mice at various postnatal ages surrounding the onset of hearing: P7, P11, P14, P21, and adult. We tracked mRNA expression using next-generation RNA sequencing (RNAseq) and in situ hybridization (ISH), which primarily reflects transcript expression in neuronal somata. These assays were complemented by immunohistochemistry (IHC) to index protein expression, which localizes to the axon terminals of neurons that express each gene. The multimodal profiling approach (i.e., RNAseq, ISH, and IHC) enabled the identification of maturational changes in the expression of each transporter and also permitted localization of each gene and protein within single neurons across all layers of A1 and within ventral (MGv) and dorsal (MGd) divisions of the MGB.
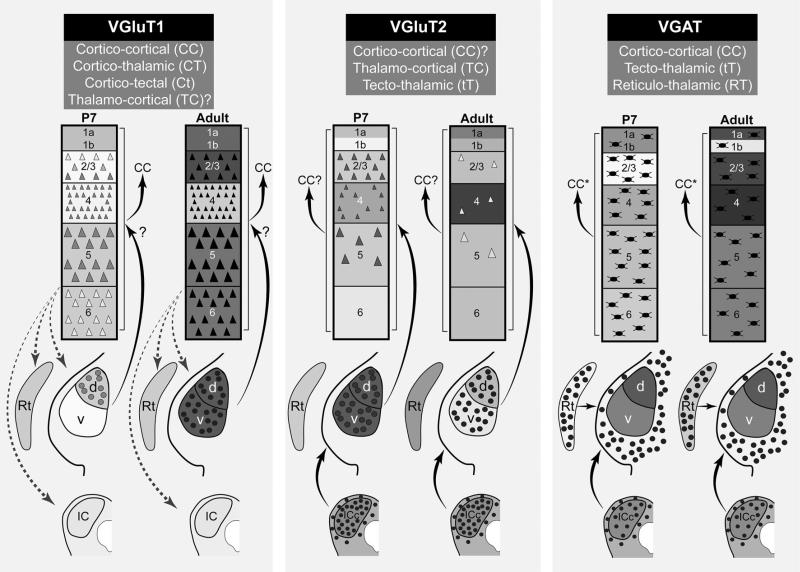
Based on known or predicted connectivity, the data in this paper reflect the maturation of several systems of projections: corticocortical (CC) (VGluT1 and VGAT); corticothalamic (CT) (VGluT1); thalamocortical (TC) (VGluT2); tectothalamic (tT) (VGluT2 and VGAT); and thalamorecticular (TRN) (VGAT). In addition, the data support the utility of RNAseq as an efficient means to screen for and identify genes of interest in any brain region, prior to anatomical characterization using ISH or IHC. Altogether, these data add to our understanding of changes in the presynaptic signaling machinery that accompany alterations in sensory experience during postnatal development.
Materials and methods
Tissue acquisition
All procedures were approved by the Animal Care and Use Committee at Massachusetts Eye and Ear Infirmary and followed the guidelines established by the National Institutes of Health for the care and use of laboratory animals. The morning that a new litter of pups was first observed was designated as P0. Brains collected from adult (8–10 weeks) and juvenile (P7, P11, P14, and P21) male and female C57BL/6J mice (Jackson Labs 000664) were used in this study. Animals were given a lethal dose of ketamine and xylazine (200/50 mg/kg, respectively) intraperitoneally and then perfused transcardially with 20–30 ml of perfusion saline (0.1 % NaNO2 and 0.9 % NaCl in H2O), followed by 20–30 ml of 4 % paraformaldehyde dissolved in 0.1 M phosphate-buffered (PB) saline using a medium flow peristaltic pump (Fisher Scientific 13-876-2; 0.4–85.0 ml/min). The brains were sunk in 30 % sucrose dissolved in 0.1 M PB and then stored at –20 °C. Brains were cut frozen in the coronal plane on a sliding microtome at 40 or 50 μm. Sections were stored at 4 °C in 0.1 M PB saline containing 1 % sodium azide. Animals designated for RNAseq (P11 omitted) (N = 6 per age, total = 24) and multi-fluorescence in situ hybridization (N = 6) were euthanized in the same manner, but not perfused. Brains from these animals were removed immediately, flash frozen on dry ice, and stored at –80 °C prior to sectioning.
Next-generation sequencing of total RNA
Sample acquisition
For harvesting of RNAseq samples, fresh-frozen brains from 6 animals in each age group (3 male, 3 female) were sectioned at 40–100 μm in the coronal plane (rostral to caudal) on a sliding microtome and viewed through a surgical microscope. The gross anatomical features illustrated in Fig. 1 and described in “Architectonic features of A1 and MGB” were used as a guide to identify areas targeted for sampling (A1, primary auditory cortex; MGB, medial geniculate body). As target regions became visible, they were extracted using a sterile tissue punch or curette of a size appropriate to the brain region. A1 samples were obtained using a 0.5 mm-diameter punch, with the ventral edge beginning approximately 1 mm dorsal to the rhinal fissure. MGB samples were harvested with a curette after using a micro-dissecting scalpel to circumscribe its perimeter. Auditory cortex (AC) samples were centered on A1, but potentially also included some tissue in the adjacent auditory field dorsal to A1. For consistency with the ISH results, all RNAseq samples from the AC were designated as A1, but with the foregoing disclaimer. For the MGB, the microdissection procedure was designed to exclude the lateral geniculate nucleus (LGN) and adjoining nuclei dorsal, medial, and ventral to the MGB (Figs. 1, 2c). The extreme rostral and caudal poles of the MGB were largely excluded from these samples. Punches from homologous areas of both hemispheres were combined in sterile tube containing 400 μl of Trizol, homogenized for 45 s using a mechanized sterile pestle, flash frozen on dry ice, then stored at –80 °C. Samples from the IC were stored, but not further processed for RNAseq.
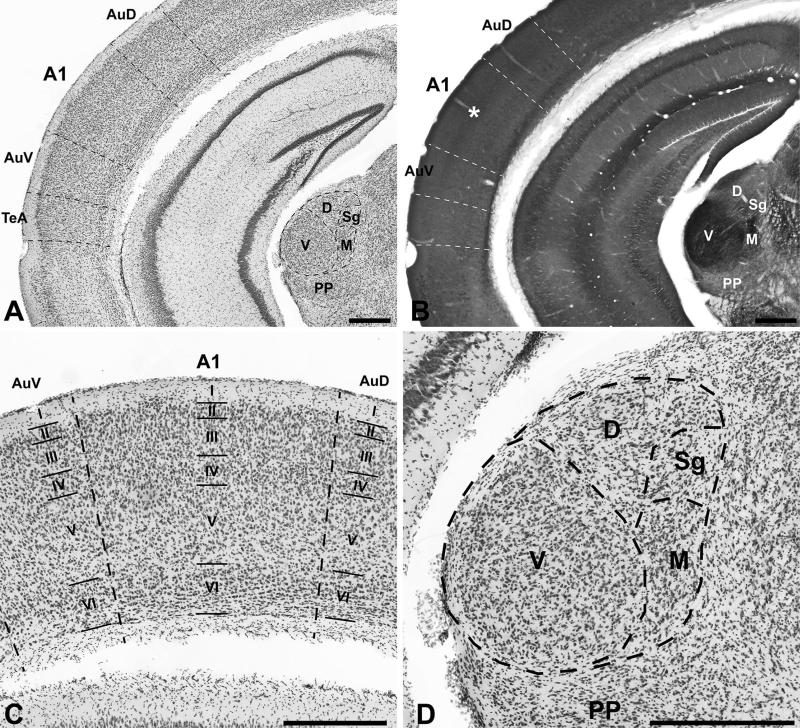
Fig. 1.
Architectonic delineation of A1 and MGB. Coronal sections at the level of A1 and MGB. a Nissl stain at low magnification showing A1 and adjoining areas. b Cytochrome oxidase stain at the same level as a. Asterisk denotes darker staining in L4. c Nissl stain at higher magnification showing laminar details of A1 and adjoining areas. d Nissl stain showing cytoarchitectonic details of MGB. A1 primary auditory cortex area 1, AuD dorsal auditory cortex, AuV ventral auditory cortex, TeA temporal cortex area A, V ventral division of MGB, D dorsal of MGB, M magnocellular/medial division of MGB, PP peripeduncular, Sg suprageniculate nucleus. Roman numerals denote cortical layers. Scale bars a–c 500 μm, d 250 μm
RNA extraction and sequencing
For each Trizol lysate, 100 μl of reagent grade chloroform (Fisher Scientific, S25248) was added. The samples were centrifuged for 3 min on a desktop centrifuge to fractionate the aqueous and organic layers. After centrifugation, the resulting aqueous layer was carefully removed and transferred to 2.0 ml Sarstedt tubes (Sarstedt, 72.694) which were run on the QIAsymphony using the QIAsymphony RNA Kit (Qiagen, 931636) and protocol RNA_CT_400_V7 which incorporates DNAse treatment. Prior to each run, the desk was UV-irradiated using a programmed cycle. The resulting RNA was eluted to 100 μl of RNase-free water and stored at –80 °C in 2.0 ml Sarstedt tubes until use. Samples were initially quantitated using a Qubit RNA assay. Additional analyses of purity and the quantitation of total RNA were performed using a NanoDrop spectrophotometer (Thermo Scientific) and Agilent RNA 6000 Pico chip (Agilent) according to themanufacturer's protocol using the reagents, chips, and ladder provided in the kit. Quality control data for the 48 samples sequenced are contained in Supplementary Table S1.
RNAseq was performed by the Vanderbilt Technologies for Advanced Genomics core (VANTAGE). Total RNA was isolated with the Aurum Total RNA Mini Kit. All samples were quantified on the QuBit RNA assay. RNA quality was verified using an Agilent Bioanalyzer. RNAseq data were obtained by first using the Ribo-Zero Magnetic Gold Kit (Human/Mouse/Rat) (Epicente) to perform ribosomal reduction on 1 μg total RNA following the manufacturer's protocol. After ribosomal RNA (rRNA) depletion, samples were then purified using the Agencourt RNAClean XP Kit (Beckman Coulter) according to the Epicentre protocol specifications. After purification, samples were eluted in 11 μl RNase-free water. Next, 1 μl ribosomal depleted samples were run on the Agilent RNA 6000 Pico Chip to confirm rRNA removal. After confirmation of rRNA removal, 8.5 μl of rRNA-depleted sample was input into the Illumina TruSeq Stranded RNA Sample Preparation kit (Illumina) for library preparation. Libraries were multiplexed six per lane and sequenced on the HiSeq 2500 to obtain at least 30 million paired end (2 × 50 bp) reads per sample.
RNAseq data processing
The RNAseq data went through multiple stages of thorough quality control as recommended by Guo et al. (2013). Raw data and alignment quality control were performed using QC3 (Guo et al. 2014a), and gene quantification quality control was conducted using MultiRankSeq (Guo et al. 2014b). Differ-entialexpressionanalysesbetween all postnatal ages and brain regions were performed using MultiRankSeq [53], which combines three independent methods for RNAseq analysis: DESeq [57]; edgeR [58]; baySeq [59]. Raw data were aligned with TopHat2 (Kim et al. 2013) against mouse transcript genome mm 10, and read counts per gene were obtained using HTSeq (Anders et al. 2014). Normalized read counts (used in all plots) were obtained by normalizing each gene's read count against the sample's total read count and then multiplying by a constant (1 × 106). Hierarchical clustering analysis and heatmaps were produced using the Heatmap3 (Zhao et al. 2014) package from R. Normalized read counts for VGluT1 (SLC17A7), VGluT2 (SLC17A6), and VGAT (SLC32A1) were averaged over all samples for each age (P7, P14, P21, adult) and brain region (AC, MGB). Analysis of variance (ANOVA) with Tukey post hoc testing was used to screen for significant differences in expression between ages for each brain area and gene (see Tables 2, 3). Raw sequencing files have been uploaded to the National Center for Biotechnology Information (NCBI) database (accession #SRP053237). Analyses of the complete RNAseq dataset is included in Hackett et al. (2015).
In situ hybridization (ISH)
Single colorimetric ISH assays for VGluT1, VGluT2, VGAT, and the housekeeping gene, GAPDH (glyceraldehyde-3- phosphate dehydrogenase), were performed in adjacent sections from each brain. This minimized differences between individual animals and permitted within-subject normalization of ISH levels using GAPDH as a reference. Multiplex fluorescence ISH (FISH) was performed simultaneously in a separate series of brain sections, permitting visualization of all genes in each tissue section.
Preparation of probes for single colorimetric ISH
Plasmids with inserts of specific sequences to each gene were prepared using the conventional TA-cloning technique. Sequences of primer sets are summarized in Table 1. The sequences were amplified by RT-PCR from mouse whole brain cDNA (Zyagen, San Diego, CA, USA) and inserted into pCR®II-TOPO plasmid vectors (Invitrogen, Carlsbad, CA, USA). Those plasmids were amplified by transfecting into competent cells (E. coli) (Invitrogen) and purified into a 1.0 μg/μl solution. Digoxigenin (DIG)-labeled antisense and sense ribo-probes were prepared from these plasmids using a DIG-dUTP labeling kit (Roche Diagnostics, Indianapolis, IN, USA). RNA Probes were then purified with ProbeQuant G-50 Micro Columns (GE Healthcare Life Schience, Pittsburg, PA, USA) and stored as a 100 μg/ml solution in TE [tris-ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA) buffer, pH 8.0].
Table 1.
Details of probes designed for in situ hybridization
| Gene | Forward primer | Reverse primer | Accession no. | Position | Product length |
|---|---|---|---|---|---|
| Colorimetric probes | |||||
| VGLUT1 (SLC17A7) | cttctacctgctcctcatctct | acacttctcctcgctcatct | NM_182993 | 972–1545 | 574 |
| VGLUT2 (SLC17A6) | catggtcaacaacagcactatc | ctctccaatgctctcctctatg | XM_006540602 | 298–871 | 574 |
| VGAT (SLC32A1) | taagaacctcaaggccgtgtccaa | cacataaatggccatgagcagcgt | NM_009508 | 1255–1832 | 578 |
| GAPDH | tgctgagtatgtcgtggagtct | ggtccagggtttcttactcctt | NM_001289726 | 359–1107 | 749 |
| Gene | Channel (color) | Catalog number | Accession no. | Position |
|---|---|---|---|---|
| RNAscope multiplex fluorescence probes | ||||
| GAPDH | 1 (FITC) | 314091 | NM_008084.2 | 21–935 |
| VGLUT1 (SLC17A7) | 2 (Cy3) | 416631-C2 | NM_182993.2 | 464–1415 |
| VGLUT2 (SLC17A6) | 3 (Alexa 647) | 319171-C3 | NM_080853.3 | 1986–2998 |
| VGAT (SLC32A1) | 4 (Alexa 750) | 319191-C4 | NM_009508.2 | 894–2037 |
| 3-Plex positive control probe | ||||
| UBC | 1 | 320881 | NM_019639.4 | 34–860 |
| POLR2A | 2 | NM_009089.2 | 2802–3678 | |
| PPIB | 3 | NM_011149.2 | 98–856 | |
| 3-Plex negative control probe | ||||
| DAPB | 1–3 | 320871 | EF191515 | 414–862 |
Colorimetric in situ hybridization (ISH)
Free-floating sections were soaked in 4 % PFA/0.1 M PB (pH 7.4) overnight at 4 °C, permeabilized with 0.3 % Triton-X 100 for 20 min at room temperature, and treated with 1.0 μg/ml proteinase K for 30 min at 37 °C. After acetylation with acetylation buffer (0.13 % triethanolamine, 0.18 % HCl, 0.25 % acetic anhydride) for 10 min at room temperature, the sections were incubated in hybridization buffer [5× standard saline citrate (SSC 150 mM NaCl, 15 mM Na citrate, pH 7.0), 50 % formamide, 2 % blocking reagent (Roche Diagnostics), 0.1 % N-lauroylsarcosine (NLS), 0.1 % sodium dodecyl sulfate (SDS), 20 mM maleic acid buffer; pH 7.5] for 60 min at 60 °C and then transferred into the hybridization buffer containing 1.0 μg/ml DIG-labeled riboprobe at 60 °C overnight. Hybridized sections were washed by successive immersion in wash buffer (2× SSC, 50 % formamide, 0.1 % NLS; 60 °C, 20 min, twice), RNase A buffer (10 mM Tris–HCl, 10 mM EDTA, 500 mM NaCl; pH 8.0) containing 20 μg/ml RNase A (37 °C, 30 min), 2× SSC/0.1 % NLS (37 °C, 20 min), and 0.2× SSC/0.1 % NLS (37 °C, 15 min). Hybridization signals were visualized by alkaline phosphatase (AP) immunohistochemical staining using a DIG detection kit (Roche Diagnostics). Sections were mounted onto glass slides, dehydrated through a graded series of increasing ethanol concentrations followed by xylenes, and then coverslipped with Permount. Sense probes detected no signals stronger than background (see Supplementay Fig. S1).
Fluorescence in situ hybridization (FISH)
Tissue blocks (fresh, not fixed) were embedded in OCT compound (Tissue-Tek, Torrance, CA, USA), flash frozen on dry ice, sectioned at 10 μm on a cryostat, and then mounted directly onto Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA, USA). Quadruple fluorescence ISH (FISH) for GAPDH, VGluT1, VGluT2, and VGAT was conducted on sections containing A1 and MGB in two adult brains. Custom target and standard control probes were provided by Advanced Cell Diagnostics (ACD, Hayward CA, USA), as described previously (Wang et al. 2012a). In-house comparisons revealed that their assay (RNAscope) was vastly superior to results obtained using DIG-based conjugates. This was attributed to unique signal amplification and background suppression methodology that consistently yielded exceptionally high specificity and low background. Briefly, after a 30-min protease permeabilization step, two independent probes (double Z probe) were hybridized to each target sequence (~20 probe pairs per target molecule). The lower region of each probe is complementary to the target sequence, and the upper region is a 14-base tail sequence. Together, the dual probe construct provides a 28-base binding site for the preamplifiers, which were built up during a three-stage amplification cycle. In the final step, labeled probes containing the fluorescent conjugates were bound to each of the 20 binding sites on each preamplifier. All incubation steps were performed at 40 °C in a hybridization oven (HybEZ, ACD, Hayward, CA, USA) using the RNAscope Multiplex Fluorescent Reagent Kit, according to the manufacturer's instructions for fresh-frozen brain tissue.
Four types of controls were utilized to evaluate the specificity of the target probes and fluorescence amplification in each channel (Table 1): (1) GAPDH placed in channel 1 as a positive control and reacted along with the target probes in channels 2–4; (2) a three-plex positive control probe containing three highly characterized housekeeping genes (UBC, ubiquitin C; POLR2A, DNA-directed RNA polymerase II subunit RPB1; PPIB, cyclophilin B) in channels 1–3, respectively; (3) a negative control probe (DAPB, dihydrodipicolinate reductase), which is a gene from a soil bacterium (Bacillus subtilis strain SMY) that has never yielded specific signal in any tissue samples; (4) fluorescence amplification steps in the absence of any positive control or target probes. These controls revealed that all probes were highly specific with no cross-reactivity between any gene or color channel (see Supplementary Fig. S1).
With the exception of Figs. 11 and 12, FISH-reacted sections were counterstained with DAPI (4′,6-diamidino-2-phenylindole) to improve identification of layers and subdivisions and provide a focal point for cytoplasmic probe labeling (Figs. 6, 7, 8, 9, 10). As these figures indicate, labeling yield for each target probe was high and readily visible at all magnifications, despite a neuronal density in the 10 μm sections that was roughly 20 % of the 50 μm colorimetric sections.
Antibody selection and immunohistochemistry (IHC)
Vesicular transporter antibody and secondary antibody selection was a lengthy process that began by evaluating the single chromagen staining quality and consistency of several commercially available antibodies against each target protein (for complete listing, see Supplementary Table S2). These assays were performed in parallel in adult mouse and macaque monkey tissue, in support of the present study of mice and prior studies of monkeys (Balaram et al. 2011; Hackett and de la Mothe 2009; Hackett et al. 2014). Antibody specificity was tested by incubating each antibody with a 10× concentration of the control protein provided by the manufacturer, when available. Negative controls, in which the primary antibody was omitted, were used in the testing of all antibodies. Optimal primary antibody concentrations were determined from these tests. Thereafter, antibodies that produced specific staining in single antibody assays were systematically combined in double- and triple-fluorescence assays, with positive and negative controls for both primary and secondary antibodies, to identify combinations that produced strong specific labeling with no cross-reactivity. The antibody combinations used in the present study reflect our judgment of the best combinations for this application (see Table 2). Note that the primary and secondary antibodies listed in Table 2 were included because they all produced reliably good results alone and in combination. To maximize continuity, however, the illustrated figures and analyses were obtained from assays using the primary and secondary antibody combinations indicated by asterisks. VGluT1gp (gp, guinea pig) was always combined with VGATrb (rb, rabbit), and VGluT1rb was combined with VGluT2gp. These distinctions were added to the text and figure legends where appropriate.
Table 2.
Primary and secondary antibodies used
| Antibody | Species | Supplier | Part number | Dilution | References |
|---|---|---|---|---|---|
| Primary antibodies | |||||
| VGAT* | rb | Synaptic Systems | 131002 | 1:1000 | Dudanova et al. (2007), Panzanelli et al. (2007) |
| VGluT1* | rb | Synaptic Systems | 135303 | 1:1000 | Herzog et al. (2006) |
| VGluT1* | Synaptic Systems | 135304 | 1:2500–5000 | Michalski et al. (2013), Siembab et al. (2010), Wouterlood et al. (2012) | |
| VGluT1 | rb | MABTech | VGT1-3 | 1:2000 | Raju et al. (2006), Villalba and Smith (2011), Wojcik et al. (2004) |
| VGluT2 | rb | Synaptic Systems | 135403 | 1:2000 | Gomez-Nieto and Rubio (2009), Herzog et al. (2006), Persson et al. (2006), Sergeeva and Jansen (2009), Toyoshima et al. (2009), Zhou et al. (2007) |
| VGluT2* | gp | Synaptic Systems | 135404 | 1:2000 | Michalski et al. (2013), Mikhaylova et al. (2014), Perederiy et al. (2013) |
| Fox3/NeuN* | ms | Covance | SIG39860 | 1:2000 | Wimmer et al. (2010) |
| Control proteins | |||||
| VGluT1 | CP | Millipore | AG208 | 10× | – |
| VGluT1 | CP | Synaptic Systems | 135-3P | 10× | – |
| VGluT2 | CP | Synaptic Systems | 135-4P | 10× | – |
| Antibody | Species | Supplier | Part number | Dilution | Primary antibody combination |
|---|---|---|---|---|---|
| Secondary antibodies | |||||
| Alexa 488 | G-ms | Lifetech | A21200 | 1:500 | |
| Alexa 488 | Ch-ms | Lifetech | A21200 | 1:500 | |
| Alexa 488* | Ch-rb | Lifetech | A21442 | 1:500 | VGAT 131002 |
| Alexa 594* | Ch-ms | Lifetech | A21201 | 1:500 | Fox3 SIG39860 |
| Alexa 594 | G-ms | Lifetech | A11032 | 1:500 | |
| Alexa 568 | G-ms | Lifetech | A11004 | 1:500 | |
| Alexa 647 | G-gp | Lifetech | A21450 | 1:500 | VGluT2 135403; VGluT1 135304 |
| Alexa 647 | G-rb | Lifetech | A21200 | 1:500 | VGluT1 135303 |
| Alexa 750* | G-ms | Lifetech | A21037 | 1:500 | Fox3 SIG39860 |
ms mouse, rb rabbit, gp guinea pig, G/g goat, Ch chicken, H horse, CP control protein
In addition to the vesicular transporters, an additional primary antibody was used in multifluorescence IHC to identify neuronal somata. NeuN (Fox-3) is one of many members of the RNA-binding protein family (Darnell 2013). Although these proteins are mainly involved in the regulation of mRNA, Fox-3/NeuN is widely used as a neuron-specific marker in adult and developing brain across species (Arellano et al. 2012; Fuentes-Santamaria et al. 2013; Hackett et al. 2014; Kim et al. 2009). Antibodies for NeuN produce strong somatic labeling of neurons in most brain areas. In the present study, multifluorescence IHC assays included NeuN in a separate color channel for cytoarchitectonic identification of cortical layers, subcortical nuclei, or particular brain areas.
Multifluorescence immunohistochemistry
Multifluorescence IHC was performed in series of coronal sections (section spacing 1:8). Sections were rinsed for 30 min in 0.01 M PBS-Tx (phosphate-buffered saline, 0.1 % triton), followed by three changes of 0.01 M PBS for 10 min (standard rinse procedure). Nonspecific labeling of myelin by fluorescent secondary antibodies was blocked by incubation in IT-Fx (Life Tech) for 60 min at RT, followed by a standard rinse. Sections were then incubated for 5 min at RT in a single purified glycoprotein blocking solution (Superblock, ScyTek Laboratories, Logan, UT, USA), followed by a single rinse in PBS. Superblock reagent reduces nonspecific antibody binding and was used in lieu of species-specific sera or bovine serum albumin (Buttini et al. 2002; Evans et al. 1996; Turtzo et al. 2014). Sections were then incubated for 48 h in the primary antibody cocktail at 4 °C, rinsed, and then incubated for 3–4 h in the secondary antibody cocktail at RT. All incubations and rinsing steps were performed on a laboratory shaker with constant agitation.
Sections from two animals in each age group were used for quantitative measurements and associated illustrations. In these brains, sections stained for a particular combination of antibodies (VGluT2gp + VGluT1rb + NeuNms or VGluT1gp + VGATrb + NeuNms) were reacted simultaneously for all five age groups in separate well plates under identical conditions (i.e., antibody concentrations, blocking and rinsing steps, solutions, incubation times, etc.). Aliquots of the antibody cocktails and all other solutions were distributed to wells from the same beakers in which they were prepared. These procedures ensured uniformity of conditions across age groups.
ISH image acquisition and analyses
Brightfield images of single colorimetric ISH tissue sections were obtained with a Nikon 80i microscope controlled by Neurolucida 10 software (MBF Bioscience). Fluorescence widefield images and image montages of FISH sections were obtained with a Nikon 90i epiflourescence microscope and Hamamatsu Orca 4.0 CCD camera, controlled by Nikon Elements AR software using 10×, 40×, and 100× objectives. Exposure parameters for brightfield and fluorescence images were maintained at the same levels across all samples to permit comparison of signal intensity measurements between age groups. Images were assembled into figures using Adobe Illustrator CS6 (Adobe Systems, Inc.). 100× images are z-plane stacks collapsed to two dimensions obtained using an Extended Depth of Focus plugin to the Nikon Elements software. This method was chosen over confocal microscopy because we did not have access to a microscope with an infrared laser.
Estimates of the magnitude of gene expression in colori-metric ISH sections were performed on inverted 10× images from A1, MGB, and IC in both hemispheres that were first converted to 8-bit grayscale using ImageJ software (NIH, nih.gov). For A1, raw grayscale intensity was measured using rectangular selection boxes drawn from the top of layer 2 (L2) to the bottom of L6 (avoiding the subplate layer when present). Raw grayscale values were corrected to minimize differences in tissue staining between samples by subtracting the grayscale intensity of the white matter beneath A1 from the corresponding raw values. For MGB and IC, the same procedures were used, except that selections were hand-drawn using a polygon tool to restrict sampling to targeted subdivisions (MGv, ventral; MGd, dorsal, ICc, central), taking care to exclude section edges, artifacts, and adjoining nuclei. Raw grayscale intensity was corrected against the corpus callosum, where background levels were relatively constant and ISH signals were absent. For all brain regions measured, the background-corrected values for VGluT1, VGluT2, and VGAT were normalized against the background-corrected GAPDH values of the same brain regions from the same brain, adapted from a study comparing methods for quantifying ISH images (Lazic 2009). Finally, for each age group, brain area, and gene, normalized grayscale intensity values for each condition were averaged over both hemispheres of all three brains. Note that measurements for VGAT in the MGB and VGluT1 in the IC were entered as null values into tables and plots, since there were typically no labeled cells. Analysis of variance (ANOVA) with Tukey post hoc testing was used to screen for significant differences in expression between ages for each brain area and gene, using p ≤ 0.05 as the significance threshold (Tables 3, 5).
Table 3.
Analysis of variance (ANOVA) comparing differences in expression levels across age (P7 to adult) by brain region and gene for RNAseq (mean normalized read counts) and ISH (normalized grayscale intensity) assays
| VGAT | VGluT1 | VGluT2 | |
|---|---|---|---|
| RNASeq | |||
| A1 | F(3,20) = 10.437, p < 0.001 | F(3,20) = 102.848, p < 0.001 | F(3,20) = 23.083, p < 0.001 |
| MGB | F(3,18) = 3.169, p = 0.050 | F(3,18) = 15.062, p < 0.001 | F(3,18) = 3.714, p = 0.031 |
| ISH | |||
| A1 | F(4,25) = 0.766, p = 0.557 | F(4,25) = 28.169, p < 0.001 | F(4,25) = 7.206, p = 0.001 |
| IC | F(4,25) = 1.905, p = 0.141 | F(4,25) = n/a | F(4,25) = 14.960, p < 0.001 |
| MGd | F(4,25) = n/a | F(4,25) = 10.343, p < 0.001 | F(4,25) = 5.211, p = 0.003 |
| MGv | F(4,25) = n/a | F(4,25) = 16.043, p < 0.001 | F(4,25) = 7.054, p = 0.001 |
F(x,y) = z, where x is degrees of freedom for between-groups comparison, y for within groups, and z is the F statistic. n/a denotes that no measurements were made due to absence of reactivity
Note that although we did not perform RNAseq on the IC, this structure was included in the ISH images and analyses. The rationale for inclusion of the IC data was that VGluT1 mRNA is not expressed in the IC and therefore not expected to contribute to VGluT1-ir terminals in the MGB. In contrast, many VGluT2+ and VGAT+ IC neurons do project to the MGB, where their proteins are expressed in terminals (Ito et al. 2009, 2011; Ito and Oliver 2010). At a minimum, demonstration that these genes are expressed in the IC at all ages supports the IHC data and broadens the context for discussion of the circuitry.
IHC image acquisition and analyses
IHC image acquisition
Widefield images and image montages of tissue sections were obtained with a Nikon 90i epifluorescence microscope and Hamamatsu Orca 4.0 CCD camera, controlled by Nikon Elements AR software. Using a 10× objective, composite images of each color channel (red, green, and far red) were acquired sequentially at full resolution. Montages were reconstructed from multiple composite images by the software (Figs. 14, 15, 16, 17; Supplementary Figs. S2–11, S26–27). Each montage comprised several hundred single images of each color channel. Prior to acquisition, exposure times were independently adjusted for each color channel using the RGB histograms to obtain balanced brightness across channels. To avoid over- or underexpo-sure and ensure uniformity of imaging, the color balancing was standardized using adult specimens, where staining density was typically the highest and held constant for the acquisition of images in the other age groups. Images were assembled in Adobe Illustrator CS6 (Adobe Systems, Inc.).
Confocal images were obtained using an Olympus Fluoview FV1000 laser scanning microscope, using a 60× oil immersion lens (NA—1.42). In A1, image stacks (1024 × 1024 pixels; 0.207 μm/pixel) were obtained at each of seven to eight sequential locations from L1 to the white matter using the mosaic acquisition function. In the MGB, all parameters were identical, except that stacks were obtained from one to two locations near the center of each division. Acquisition parameters were uniform across age groups (Figs. 19, 20, 21, 22, 23; Supplementary Figs. S12–25).
IHC image analyses
The purpose of the image analyses performed was to derive a graphical representation of the changing trajectories in immunoreactivity across layers of cortex and subdivisions of the MGB. Plots of the data pooled over two animals (4 hemispheres) are based on descriptive statistics (means, standard deviations), but statistical comparisons were not performed.
Estimates of the magnitude of immunoreactivity were performed in two different ways, based on separate analyses of 10× widefield and 60× confocal fluorescence images, respectively. Minor differences were observed in density estimates using these two methods, which we attribute to differences in background illumination obtained by the two methods of microscopy.
In the 10× widefield images, measurements were obtained from 8-bit grayscale channels of the composite RGB images using ImageJ software (NIH, nih.gov). The images of each protein marker occupied a different color channel and were measured independently. For cortex, two types of analyses were performed (Fig. 5): (1) grayscale intensity profiles that spanned all layers; and (2) laminar intensity profiles of individual cortical layers or sublayers. First, intensity profiles (e.g., Fig. 5, left panels) were obtained with a line tool oriented perpendicular to the cortical surface extending from the black space above the pia across all layers and into the white matter (Hackett et al. 2001). Tissue edge artifact, created by high-density staining of the pia, was cropped by setting the start of the profile to the mean density of layer 1a for each sample. The stepwise reduction in density at the white matter border marked the bottom of the profile and was cropped at this point. This procedure generated high-resolution profiles of between 1000 and 1500 pixels in length from the top of L1a to the bottom of L6 or the subplate. These raw grayscale profiles were normalized to minimize differences in tissue staining between samples by subtracting the average grayscale intensity of the white matter beneath A1 from the raw density value of each pixel in the profile. The relative grayscale intensities in Fig. 18 represent these normalized profiles averaged over profiles acquired from four hemispheres (two left, two right). The rationale for using adjoining white matter to normalize grayscale intensity was that axons were not labeled by the primary antibodies used, but background staining was present from nonspecific binding of the secondary antibodies, primarily to white matter tracks. Subtraction of the background evened out differences in staining intensity between tissue sections. Alternatively, using other brain areas for normalization confounds interpretation, because the vesicular transporter proteins are expressed to some degree in most brain areas, and their expression in any or all areas could vary with age.
Second, using a round sampling tool sized to be slightly smaller than the layer of interest, grayscale intensity measurements were taken from 12 samples across the width of A1 in each layer, avoiding its dorsal and ventral borders, and avoiding large blank spaces created by empty blood vessel profiles. These samples were normalized to the average white matter intensity, as for the radial profiles, then averaged to obtain the mean relative grayscale intensity of each layer (Fig. 5, right panels). Layers and sublayers were identified using the NeuN-labeled cells and preserved in the red color channel of each image. This multifluorescence approach enabled greater precision in the identification of layers compared to the matching of adjacent tissue sections stained for different markers. Note that we did not distinguish between L6 and the subplate in the radial profiles illustrated in Fig. 18 and avoided sampling from the subplate in the laminar intensity analyses. Some minor qualitative differences in immunoreactivity between L6 and subplate layers were noted, however, and discussed in the text with reference to higher-magnification images.
In confocal image stacks, immunoreactivity was measured based on the density of immunoreactive (-ir) puncta, adapting the approach of Coleman et al. (2010). RGB confocal image stacks were converted to 8-bit grayscale images at full resolution (1024 × 1024 pixels), where each color channel was confined to a single file, and each image file contained one confocal slice (0.74 μm/slice). For each protein marker, images were thresholded to reduce background and visually separate closely spaced puncta. For each marker, threshold was held constant across samples and set to the upper edge of the histogram for each color channel (typically 25–40 % of maximum intensity). This strict threshold criterion produced the greatest separation between puncta and minimized the counting of particles in which immunoreactivity was low or nonspecific. Estimates of puncta number and density were then calculated from each region of interest (ROI) using the Analyze Particles routine in ImageJ. The thresholded image was filtered to count ovoid puncta between 0.414 and 4.14 μm2. For VGluT2 staining of the MGv only, the inclusion range for puncta was 0.414–35 μm2. The range was expanded to include large VGluT2-ir puncta that were especially prominent in the P21 and adult brain (see “Results”). This expanded range was not used for VGluT1 or VGAT, because these puncta were typically small and inclusion of larger particle sizes would have permitted the counting of aggregates and other artifacts.
From each image stack, puncta counts were obtained from three single confocal slices (0.74 μm/slice) selected from planes where immunoreactivity was the most even (typically in the middle third of the stack). For each slice, an ROI was drawn using the polygon selection tool in ImageJ. The ROI was confined to a single cortical layer and restricted to the neuropil. That is, ROIs were drawn in a manner that excluded the empty profiles of blood vessels and somata. This additional step improved the reliability of measurements between slices, as the total area occupied by empty profiles varies between slices and cortical layers and, therefore, can skew the density calculations. To avoid counting the same particles more than once, ROIs were obtained from non-adjacent slices or from different regions in adjacent slices. The puncta densities (puncta/100 μm2) in each graph represent the average of three confocal slices.
At the light microscope level, it is difficult to resolve small axon terminals in immunostained material and to distinguish them from small particles that may be nonspecifically stained. Although we chose antibodies that produced the strongest signals with the least nonspecific labeling, some of the labeled particles may not be terminals. In the absence of verification by EM, it is conventional to use the name ‘puncta’ for labeled particles. We adopted this nomenclature, but observed that the levels of nonspecific labeling were both minimal and comparable across samples, which would not bias our results. Previous localization studies increased our confidence and confirmed that the vast majority of VGAT, VGluT1, and VGluT2-ir puncta are axon terminals (Chaudhry et al. 1998; Kaneko et al. 2002; Minelli et al. 2003a, b).
Note also that our impressions concerning differences in the sizes of labeled puncta (below) were limited to qualitative judgments of the confocal images. Those impressions must be validated by other methods, such as EM, as we did not attempt to measure terminal sizes from the confocal images.
Architectonic features of A1 and MGB
The gross anatomical and cytoarchitectonic features used to identify A1 and MGB divisions are illustrated in Fig. 1. Criteria for parcellation were based on reference to online (Allen Brain Atlas, Brain Maps.org) and published atlases (Franklin and Paxinos 2007), and other sources in which architectonic features of the AC and MGB were described (Anderson et al. 2009; Bartlett et al. 2000; Cruikshank et al. 2001; Hackett et al. 2011a; Linke 1999; Linke and Schwegler 2000; Llano and Sherman 2008; Winer et al. 1999). Briefly, in coronal sections, A1 is distinguished from the adjoining areas (dorsal and ventral) by a relatively broad L4, in which cell packing is higher than in L3 and L5 and there is reduced cell density in L5. This feature is visible by Nissl staining, NeuN IHC, GAPDH ISH, and VGluT1 ISH, which permit assessment of the cytoarchitecture. VGluT2-ir density is also higher in the L3b/4 band of A1 compared to surrounding areas. The MGv is larger in size than MGd, and primarily distinguished by higher cell density in MGv. These features are visible in the same histological preparations that permit assessment of the cytoarchitecture. The MGv also contains clusters of VGluT2-ir puncta, compared to more even distribution of smaller puncta in MGd. The medial (MGm) and suprageniculate (Sg) divisions are primarily distinguished by slightly larger neuron size and reduced cell density compared to MGv. Although cytochrome oxidase staining was not formally part of this study, we used this preparation in an earlier study (Hackett et al. 2011a). An image is included for reference in Fig. 1 that shows darker staining in A1 L4 and MGv.
Organization of images
Two sets of photographic images support the findings of the present study. The first set (Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16, 17, 19, 20, 21, 22, 23) is embedded within the main body of this manuscript. Each is a moderate-resolution version of the key widefield and confocal images. The second set of images is Supplementary (Figs. S1–S28), and referred to throughout the text. Links to full-resolution versions of all images are contained in Supplementary material 1. Many of these can be viewed through weblinks to high-resolution montages created with the Zoomify™ (Zoomify Inc., Aptos, CA, USA) plugin in Photoshop CS6 (Adobe Systems, Inc.). These images open in a browser window (requires updated Adobe Flash Player). Tools located at the bottom of the Zoomify window permit zoom and move functions, some of which may also be controlled using secondary mouse functions. Note that browser settings may need to be adjusted to allow viewing and that upload/zoom speeds vary.
Results
RNAseq and in situ hybridization
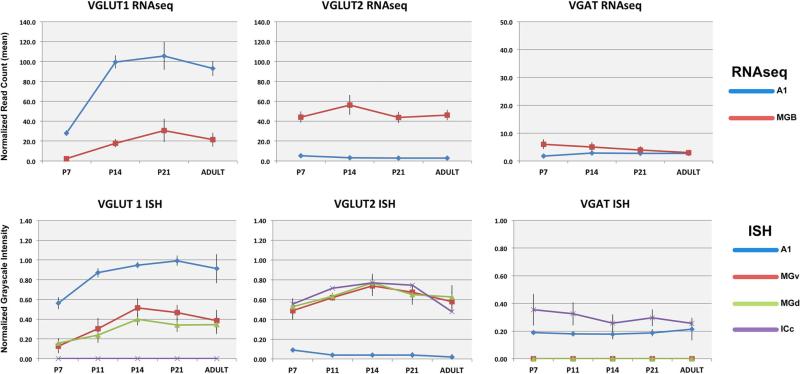
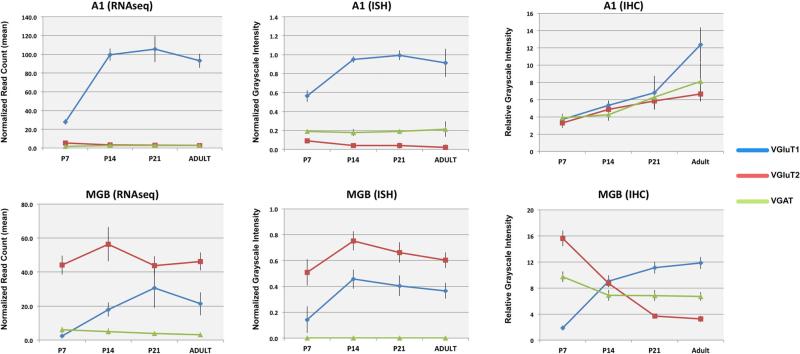
The data obtained for each gene and brain region are described separately in the text and figures below. Figures 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 contain ISH and FISH images for each gene and brain region as a function of postnatal age. Figure 13 contains graphical summaries of the ISH and RNAseq data analyses derived from the corresponding data in Tables 3, 4 and 5. Note that a P11 age group was not available for RNAseq analysis, but the absence of data from this time-point did not alter the conclusions of this study.
Fig. 2.
In situ hybridization and sample acquisition. a Single colorimetric ISH of VGluT1, VGluT2, VGAT, and GAPDH in adjacent sections from the same brain. b Triple FISH of a section from a different brain showing co-expression of VGluT1 (red), VGluT2 (white), and VGAT (green). c Photograph of a frozen brain during harvesting of samples from A1 and MGB for sequencing. The location of A1 within the AC is shown, along with a sketch of the 0.5 mm punch used to obtain samples. Note that the size and shape of the punch compresses tissue outside of the punched volume. The left MGB has been circumscribed prior to extraction. Scale bars 1 mm all panels. A1 primary auditory cortex area 1, AC auditory cortex, MGB medial geniculate body
Fig. 3.
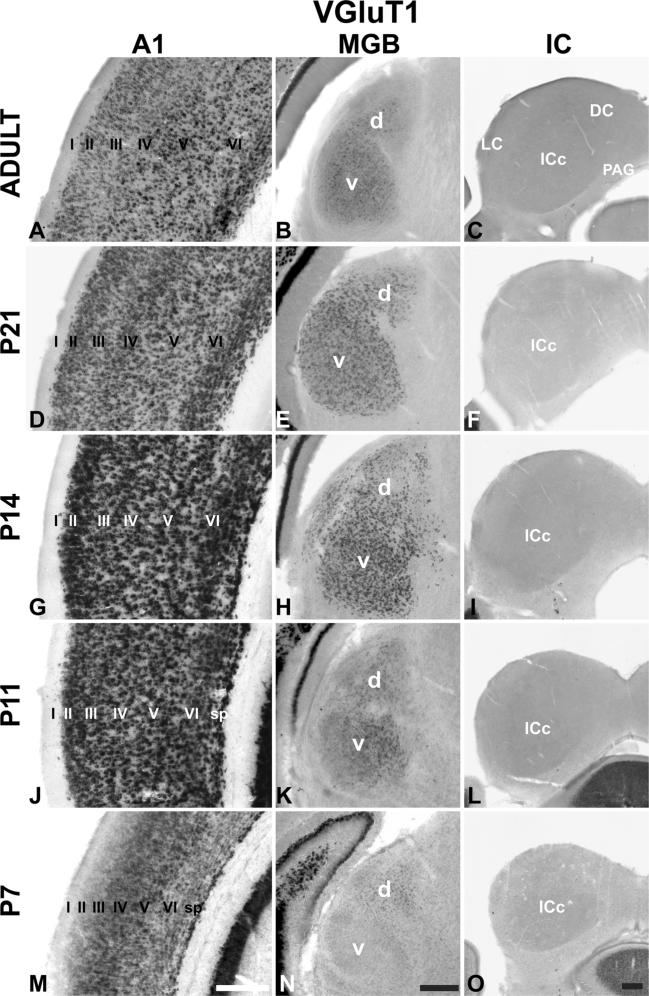
Single colorimetric ISH assay for VGluT1 in AC centered on A1 (left column), MGB (middle column), and IC (right column) at P7, P11, P14, P21, and adult. Coronal sections. Roman numerals indicate layers (sp subplate). Scale bars 250 μm in all panels. d dorsal division of MGB, v ventral division of MGB, DC dorsal cortex, IC inferior colliculus, ICc central nucleus, LC lateral cortex, PAG periaqueductal gray
Fig. 4.
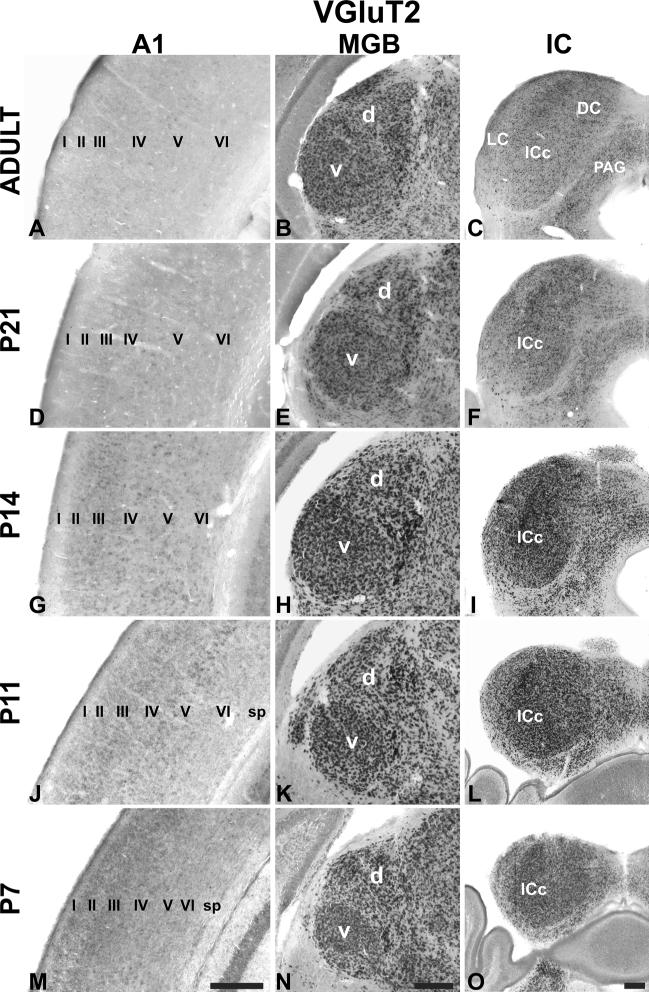
Single colorimetric ISH assay for VGluT2 ISH in AC centered on A1 (left column), MGB (middle column), and IC (right column) at P7, P11, P14, P21, and adult. Coronal sections. Roman numerals indicate layers (sp subplate). Scale bars 250 μm in all panels. d dorsal division of MGB, v ventral division of MGB, DC dorsal cortex, IC inferior colliculus, ICc central nucleus, LC lateral cortex, PAG periaqueductal gray
Fig. 5.
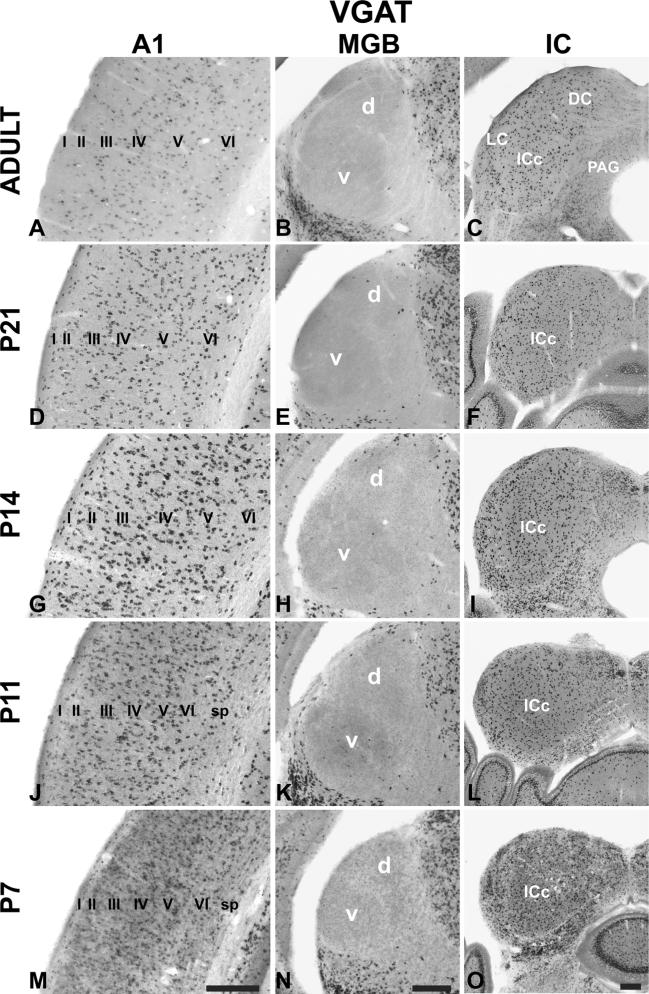
Single colorimetric ISH assay for VGAT ISH in AC centered on A1 (left column), MGB (middle column), and IC (right column) at P7, P11, P14, P21, and adult. Coronal sections. Roman numerals indicate layers (sp subplate). Scale bars 250 μm in all panels. d dorsal division of MGB, v ventral division of MGB, DC dorsal cortex, IC inferior colliculus, ICc central nucleus, LC lateral cortex, PAG periaqueductal gray
Fig. 6.
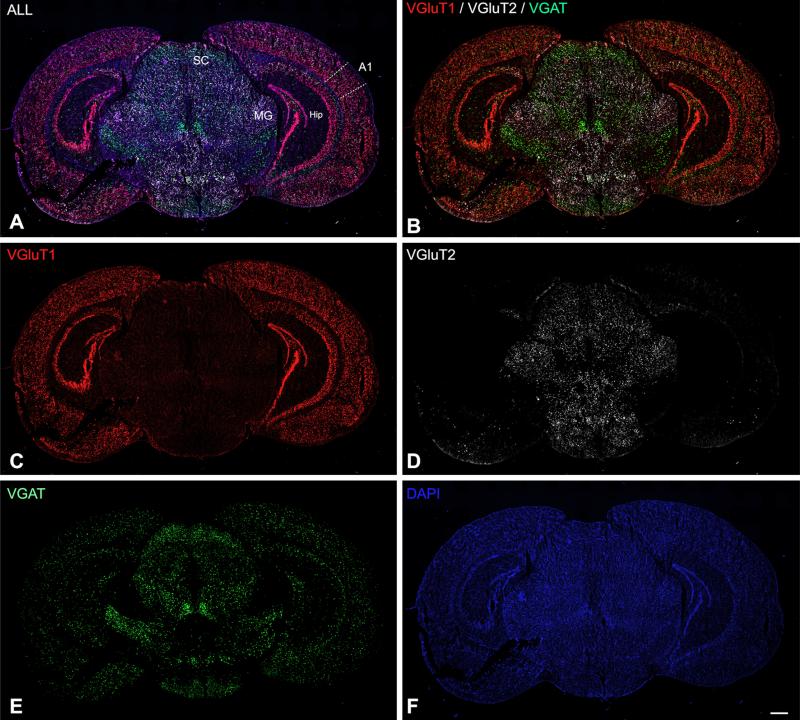
Triple FISH assay in coronal sections of adult mouse at the level of A1 and MGB. The low-magnification image montages obtained at 10× show combined (a) and single channel expression (b–f) for VGAT, VGluT1, and VGluT2 mRNA, counterstained by DAPI. SC superior colliculus, Hip hippocampus, MG medial geniculate body. Scale bars 500 μm in all panels
Fig. 7.
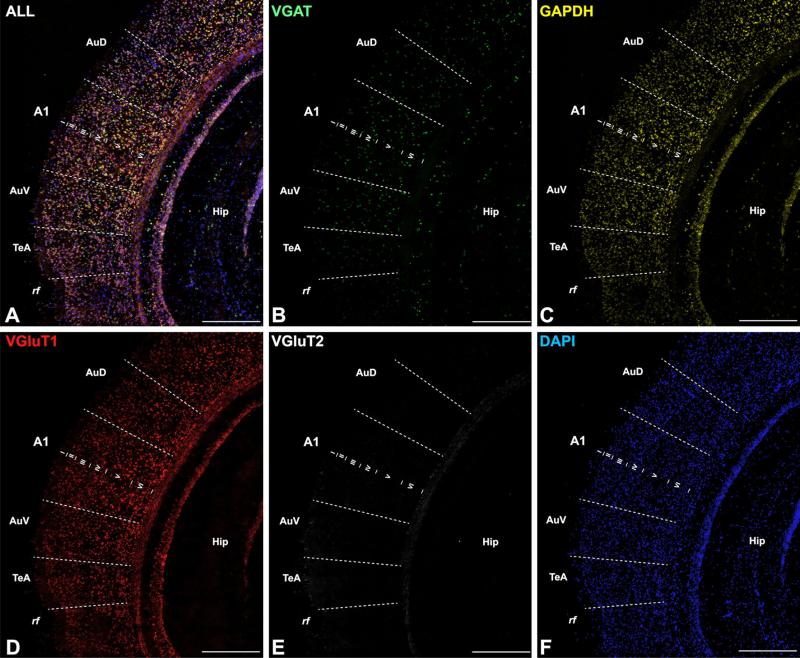
Quadruple FISH assay in a coronal section of adult mouse showing A1 and surrounding areas of auditory cortex. The image montages obtained at 40× in a–f show combined or single channel expression for VGAT, GAPDH, VGluT1, and VGluT2 mRNA, counterstained by DAPI. Scale bars 500 μm in all panels. A1 primary auditory cortex area 1, AuD dorsal auditory cortex, AuV ventral auditory cortex, rf rhinal fissure, TeA temporal cortex area A
Fig. 8.
Quadruple FISH assay in a coronal section of adult mouse centered on A1 (from Fig. 7). a The image montages obtained at 40× in a show combined expression for VGAT, GAPDH, VGluT1, and VGluT2 mRNA, counterstained by DAPI. Subpanels 1–8 show 100× image stacks taken at sites in different layers of A1, as indicated in the composite image (left). b–g Higher-resolution examples of transcript labeling from subpanel 4 shown separately for each gene. Scale bars a 250 μm, all other panels 20 μm
Fig. 9.
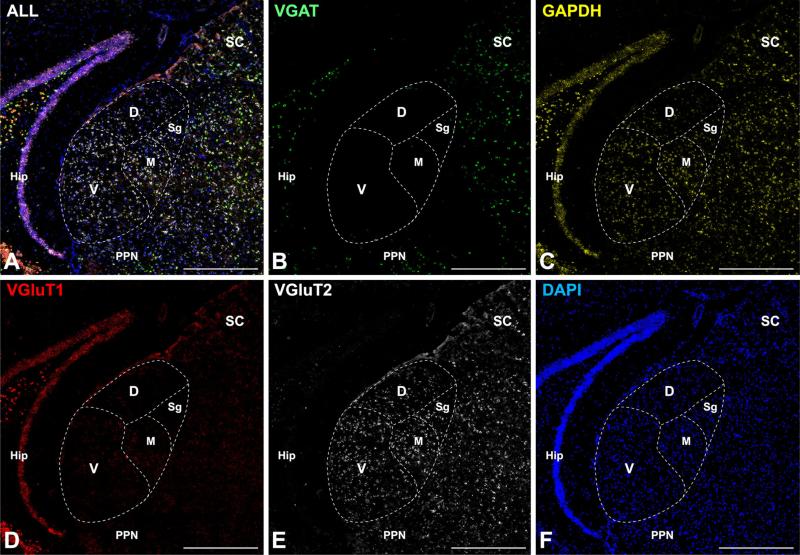
Quadruple FISH assay in a coronal section of adult mouse showing MGB and surrounding regions. The image montages obtained at 40× in a–f show combined or single channel expression for VGAT, GAPDH, VGluT1, and VGluT2 mRNA, counterstained by DAPI. Scale bars 500 μm in all panels. D dorsal division of MGB, Hip hippocampus, M medial division of MGB, PPN peripeduncular nucleus, V ventral division of MGB, SC superior colliculus, Sg suprageniculate
Fig. 10.
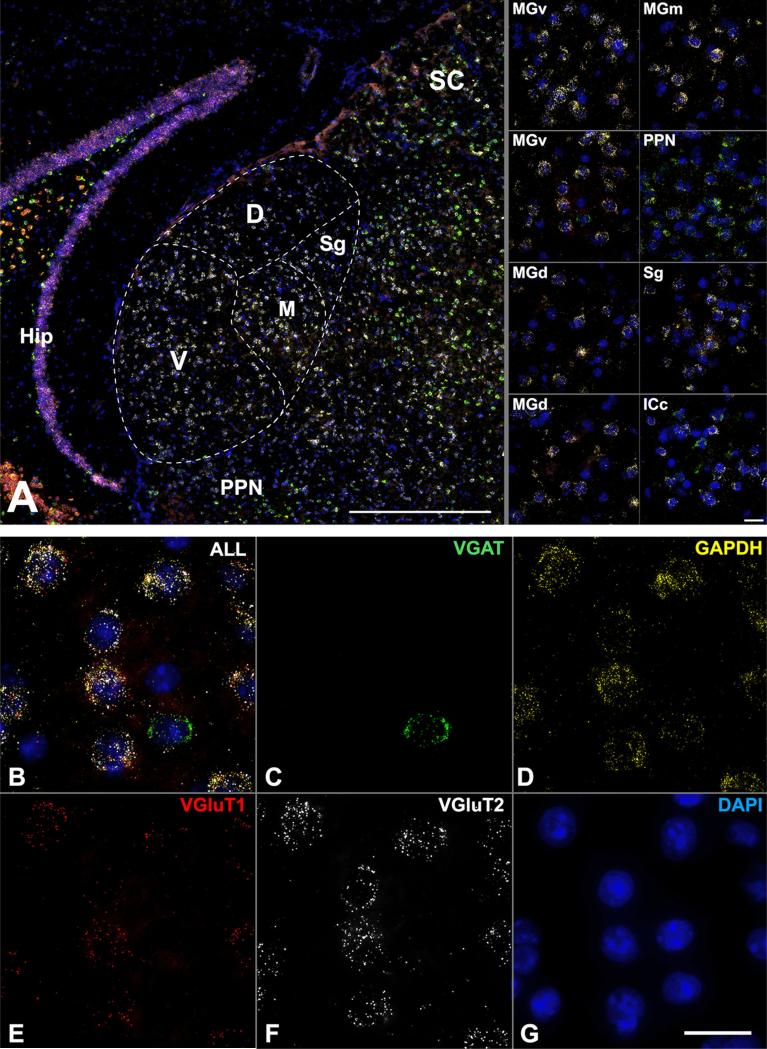
Quadruple FISH assay in a coronal section of adult mouse MGB (from Fig. 9). a The image montages obtained at 40× show combined expression for VGAT, GAPDH, VGluT1, and VGluT2 mRNA, counterstained by DAPI. Subpanels (right) show 100× image stacks from MGB divisions, adjoining nuclei, and the central nucleus of the inferior colliculus (ICc). b–g Higher-resolution examples of transcript labeling from MGv shown separately for each gene. Scale bars a 250 μm, all other panels 20 μm. D and MGd dorsal division of MGB, Hip hippocampus, ICc central nucleus of inferior colliculus, M and MGm medial division of MGB, PPN peripeduncular nucleus, V ventral division of MGB, SC superior colliculus, Sg suprageniculate
Fig. 11.
Triple FISH assays in the mouse auditory cortex at P7, P14 and adult. Panels show combined or single channel expression for VGAT (green), VGluT2 (white), and VGluT1 (red). a Adult AC centered on A1. Row 1 low-magnification images (10×) of each gene shown separately and all three combined (Merge). Row 2 higher magnification (40×) of L1–3. Note low levels of VGluT2 in all layers. b, c Same as a, but at P14 and P7. Note elevated expression of VGluT2 in L2–3 at P7 and co-localization with VGluT1. Scale bars: top panels 10×, 200 μm; other panels 40×, 100 μm. A1 primary auditory cortex area 1, Hip hippocampus
Fig. 12.
Triple FISH assays in the mouse MGB at P7, P14 and adult. Panels show combined or single channel expression for VGAT (green), VGluT2 (white), and VGluT1 (red). a Adult MGB. Row 1 low-magnification images (10×) of each gene shown separately and all three combined (Merge). Note absence of VGAT in MGB. Row 2 40× images in the MGv (v) to show co-localization of VGluT1 and VGluT2. b Same as a, but at P14. Note co-localization of VGluT1 and VGluT2 at this age. c Same as in b and c, but at P7. Note very low levels of VGluT1 expression in the MGd shown at 40× in the bottom panels (VGluT1 absent in MGv) at this age and co-localization with VGluT2. Scale bars: top panels 10×, 200 μm; other panels 40×, 100 μm. D dorsal division of MGB, Hip hippocampus, M medial division of MGB, PPN peripeduncular nucleus, V ventral division of MGB
Fig. 13.
Graphical summaries of gene expression obtained by analyses of RNAseq and ISH measurements. Top Mean normalized read counts from RNAseq for VGluT1, VGluT2, and VGAT in A1 (blue lines) and MGB (red lines). Bottom Mean relative optical density of ISH for the same genes in A1 (blue), MGv (red), and MGd (green). In all plots, error bars denote standard deviation. For each data series, significant differences in expression levels between each age and all others (p ≤ 0.05) are listed in Tables 4 (RNAseq) and 5 (ISH). Note that RNAseq samples contained both MGv and MGd (i.e., MGB). A1 primary auditory cortex area 1, ICc inferior colliculus central nucleus, MGB medial geniculate body, MGd dorsal division of MGB, MGv ventral division of MGB
Table 4.
Summary statistics for RNAseq sample comparisons
| VGluT1 |
VGluT2 |
VGAT |
||||
|---|---|---|---|---|---|---|
| A1 | MGB | A1 | MGB | A1 | MGB | |
| Adult (Ad) | ||||||
| Mean | 93.00 | 21.30 | 2.82 | 46.25 | 2.67 | 3.01 |
| SD | 7.45 | 6.81 | 0.27 | 5.29 | 0.22 | 0.87 |
| p < 0.05 | P7 | P7 | P7 | None | P7 | None |
| P21 | ||||||
| Mean | 105.61 | 30.69 | 2.85 | 43.77 | 2.75 | 3.88 |
| SD | 14.03 | 11.72 | 0.25 | 5.63 | 0.35 | 1.34 |
| p < 0.05 | P7 | P7, P14 | P7 | None | P7 | None |
| P14 | ||||||
| Mean | 99.59 | 17.91 | 3.23 | 56.38 | 2.81 | 5.01 |
| SD | 6.61 | 4.15 | 0.28 | 10.06 | 0.61 | 1.69 |
| p < 0.05 | P7 | P7, P21 | P7 | None | P7 | None |
| P7 | ||||||
| Mean | 27.91 | 2.35 | 5.37 | 44.14 | 1.70 | 6.02 |
| SD | 2.88 | 0.87 | 1.16 | 5.56 | 0.30 | 1.75 |
| p < 0.05 | All | All | All | None | All | None |
For each gene, the mean normalized read count and standard deviation (SD) are listed by postnatal age and brain region. For each condition, comparisons with all other age groups that reached significance are listed by age (i.e., P7, P14, P21, Ad, or All). Significance determined by Tukey post hoc comparisons (p < 0.05)
n/a no measurements available, none no significant differences with any other age group
Table 5.
Summary statistics for ISH sample comparisons
| VGluT1 |
VGluT2 |
VGAT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | MGv | MGd | IC | A1 | MGv | MGd | IC | A1 | MGv | MGd | IC | |
| Adult (Ad) | ||||||||||||
| Mean | 0.91 | 0.39 | 0.35 | 0.0 | 0.02 | 0.58 | 0.63 | 0.48 | 0.21 | 0.00 | 0.00 | 0.26 |
| SD | 0.15 | 0.11 | 0.10 | 0.0 | 0.03 | 0.11 | 0.12 | 0.13 | 0.08 | 0.00 | 0.00 | 0.02 |
| p < 0.05 | P7 | P7 | P7 | n/a | P7 | P14 | None | P11/14/21 | None | n/a | n/a | None |
| P21 | ||||||||||||
| Mean | 0.99 | 0.47 | 0.34 | 0.0 | 0.04 | 0.67 | 0.65 | 0.74 | 0.19 | 0.00 | 0.00 | 0.30 |
| SD | 0.05 | 0.08 | 0.07 | 0.0 | 0.03 | 0.08 | 0.10 | 0.10 | 0.03 | 0.00 | 0.00 | 0.06 |
| p < 0.05 | P7 | P7/11 | P7 | n/a | P7 | P7 | None | P7/Ad | None | n/a | n/a | None |
| P14 | ||||||||||||
| Mean | 0.95 | 0.51 | 0.40 | 0.0 | 0.04 | 0.74 | 0.77 | 0.77 | 0.18 | 0.00 | 0.00 | 0.26 |
| SD | 0.03 | 0.10 | 0.06 | 0.0 | 0.02 | 0.10 | 0.09 | 0.04 | 0.04 | 0.00 | 0.00 | 0.06 |
| p < 0.05 | P7 | P7/11 | P7/11 | n/a | P7 | P7/Ad | P7 | P7/Ad | None | n/a | n/a | None |
| P11 | ||||||||||||
| Mean | 0.87 | 0.30 | 0.24 | 0.0 | 0.04 | 0.62 | 0.63 | 0.72 | 0.18 | 0.00 | 0.00 | 0.32 |
| SD | 0.05 | 0.11 | 0.08 | 0.0 | 0.02 | 0.03 | 0.03 | 0.05 | 0.01 | 0.00 | 0.00 | 0.08 |
| p < 0.05 | P7 | P7/14/21 | P14 | n/a | P7 | None | None | P7/Ad | None | n/a | n/a | None |
| P7 | ||||||||||||
| Mean | 0.56 | 0.13 | 0.16 | 0.0 | 0.09 | 0.49 | 0.53 | 0.56 | 0.19 | 0.00 | 0.00 | 0.35 |
| SD | 0.06 | 0.07 | 0.05 | 0.0 | 0.02 | 0.09 | 0.08 | 0.06 | 0.02 | 0.00 | 0.00 | 0.11 |
| p < 0.05 | All | All | P14/21/Ad | n/a | All | P14/21 | P14 | P11/14/21 | None | n/a | n/a | None |
For each gene, the mean normalized read count and standard deviation (SD) is listed as a function of postnatal age and brain region. For each condition, comparisons with all other age groups that reached significance are listed by age (i.e., P7, P11, P14, P21, Ad, or All). Significance determined by Tukey post hoc comparisons (p < 0.05)
n/a no measurements available, none no significant differences with any other age group
Expression of VGluT1 mRNA
At all ages, VGluT1 mRNA was expressed in A1 and MGB, but not the IC (Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13; Tables 3, 4, 5). Expression levels, derived from analyses of RNAseq and ISH assays (Fig. 13), were greater in A1 than MGB, and the spatial expression patterns in the tissue varied by location within each region. In A1, VGluT1+ cells were densely packed in L2–6. At P7, expression was concentrated in L3b–5, but by P11 there was little visible difference between layers, except L1 where VGluT1+ cells were rarely observed (Fig. 3). Expression in A1 increased most rapidly between P7 and P14 and then remained stable through adulthood. Overall in the MGB, VGluT1 levels were lowest at P7. At this age, VGluT1+ cells were concentrated in the MGd, as very few MGv cells were VGluT1+. By P11, VGluT1+ cells were more numerous in the MGv, especially in its medial half. At P14, VGluT1+ neurons were broadly distributed throughout both divisions. Average expression levels increased significantly between P7 and P14 (ISH) or P21 (RNAseq) and then declined slightly into adulthood.
Although not a focus of the present study, VGluT1+ transcripts were also present at modest levels in MGm and Sg neurons, but expression in the adjoining nuclei tended to be low or absent (see also the high magnification panels in Fig. 10a). VGluT1+ cells were relatively sparse in the posterior pole of the MGB at all ages (see Storace et al. 2012).
Expression of VGluT2 mRNA
In contrast to VGluT1, VGluT2 expression was highest in the MGB and IC (Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13; Tables 3, 4, 5). In A1, where VGluT2 levels were relatively low across the age range, expression was slightly, but significantly, elevated at P7 (Figs. 4, 13). This was attributed to a minor concentration of labeled transcripts in L2–3 that rapidly declined after P7. By adulthood, when VGluT2 transcripts were sparse in L2–3, nominal VGluT2 expression in A1 was found in some of the larger cell bodies in L5. In the MGB, VGluT2 expression was already quite strong by P7 in all divisions of the MGB, including MGm and Sg. Expression levels increased significantly until P14, then declined slightly in a manner similar to VGluT1. Expression trajectories and levels were nearly identical for the MGv and MGd. Finally, VGluT2 expression was robust in all divisions of the IC at all ages (Fig. 4).
Expression of VGAT mRNA
Compared with VGluT1 and VGluT2, the expression of VGAT was relatively low at all ages in A1 and MGB (Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13; Tables 3, 4, 5) (note the change in scale between graphs of VGAT and VGluT1/2). In A1, VGAT+ cells were found in all layers at all ages (Fig. 5). Compared to VGluT1, the VGAT+ neurons were much less densely packed, which likely accounts for the relatively low average expression levels (Fig. 13). There were no significant changes in overall expression across the age range, although RNAseq revealed a small, but significant increase in A1 from P7 to P14. VGAT mRNA expression in the MGB remained at background levels, occasioned by the rare discovery of a VGAT+ neuron in the MGd or MGv (e.g., Fig. 10c). However, RNAseq revealed low levels of VGAT+ transcripts in the MGB. We suspect that this was due to the inclusion of cells from adjacent nuclei (e.g., posterior lateral, PoL; peripeduncular, PPN), where VGAT+ neurons are abundant. Although not quantified by cell counts, our impression from the density measurements (see Fig. 13) was that VGAT+-labeled cell density in the IC decreased from P7 to adult in a relatively steady manner. This was similar to A1, where cell density also diminished. In both cases, these qualitative changes were minimized in the grayscale density measures by the normalization.
Transcript Colocalization
Multiplexed FISH assays (Figs. 6, 7, 8, 9, 10, 11, 12) were conducted to confirm anatomical features observed in the single-gene colorimetric ISH assays and to reveal co-localization of transcripts in the same neurons. Figures 6, 7, 8, 9 and 10 show the expression of GAPDH, VGAT, VGluT1, VGluT2, and DAPI in adult A1 and MGB at low and high (100×) magnification. High-resolution versions of these images can be viewed using links to their Zoomify™ versions (Supplementary material 1, Supplementary figures). In Figs. 8 and 10, the composite images in panel a are the same as, or derived from, panel a in Figs. 7 and 9, respectively. Transcript labeling for each gene was punctate and intermingled in the cytoplasm in close proximity to the DAPI-labeled nucleus. Some nuclei, possibly glia, lacked significant accumulations of puncta in the surrounding cytoplasm. Puncta associated with different genes were easily distinguishable by color, and specificity of the multiplex FISH assays was exceptionally high. We observed no instances in which labeled puncta were positive for more than one marker. Images of the positive and negative controls further confirm the specificity of both the probes and fluorescent tags (Supplementary Figure 1).
GAPDH (yellow) was present in all neurons and was therefore co-localized in neurons that also contained VGAT, VGluT1, or VGluT2 transcripts. Although not quantified, we observed that GAPDH expression levels (puncta number) varied between neurons, but no anatomically relevant pattern was evident (e.g., cell type, layer, nuclear division).
VGAT+ neurons (green) were located in all layers of cortex at every age, but rarely in MGv, MGd, MGm, or Sg. An example of a rare VGAT+ neuron in the MGv is illustrated in Fig. 10b, c. The paucity of GABAergic neurons in the MGB is a particular feature of rodents and some bat species (Winer and Larue 1996). In contrast, VGAT+ neurons were widespread in the peripeduncular nucleus (PPN) and adjoining posterior group of nuclei that border the MGB ventrally and medially, as well as the superior and inferior colliculus (Figs. 9, 10). These nuclei also contained large numbers of VGluT2+ neurons. Although highly interspersed in the same brain regions, VGAT+ transcripts did not co-localize in neurons that contained either VGluT1 or VGluT2 transcripts in A1 or MGB at any age.
VGluT1 (red) and VGluT2 (white) transcripts were frequently co-localized in the same A1 and MGB neurons (Figs. 6, 7, 8, 9, 10, 11, 12). Overall, VGluT1 transcripts dominated in A1, while expression of VGluT2 was strongest in the MGB. Most of the VGluT2+ MGB neurons also expressed VGluT1, but VGluT2+ transcripts in A1 were relatively sparse, especially in the infragranular layers and in adults. In addition, co-expression patterns were age dependent, reflecting changing expression levels during maturation. As observed in the colorimetric assays, VGluT1 expression in A1 at P7 was relatively low in L2–6, with a minor concentration in L3b–5 (Fig. 11c). VGluT2 expression in A1 at P7 was concentrated in L2–3, and in some L5 neurons, as also noted in the single ISH assays. Co-localization of both transcripts was therefore more widespread in L2–3 neurons at this age. By P14, VGluT2 expression in L2–3 declined, and therefore co-localization with VGluT1 was less pronounced (Fig. 11b). By adulthood, VGluT2 levels reached a minimum in L2–3, and co-localization with VGluT1 was only visible at high magnification due to the low number of VGluT2 transcripts (Figs. 8, 11c). In the MGB, VGluT2 expression was strong at all ages (Figs. 9, 10, 12). At P7, VGluT1 mRNA was nearly absent from the MGv and very weak in the MGd, and so co-localization of VGluT1 and VGluT2 was limited to a few cells in the MGd at this age (Fig. 12c). By P14, however, VGluT1 and VGluT2 co-localization was widespread among MGv and MGd neurons and grew strong through adulthood (Figs. 9, 10, 12a, b).
Immunohistochemistry
In this section, the illustrations, analyses, and descriptions proceed from lower to higher levels of magnification to reveal different aspects of the immunohistochemical expression patterns. Descriptions focus on A1 and MGB, with limited reference to other brain areas for comparison.
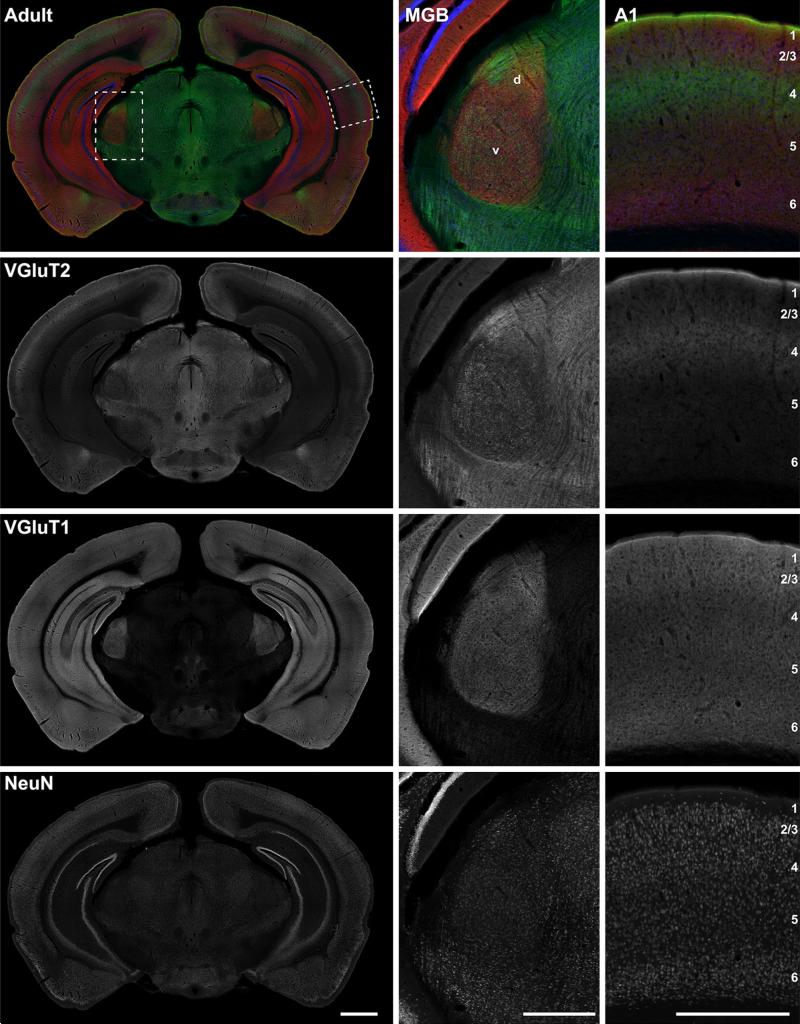
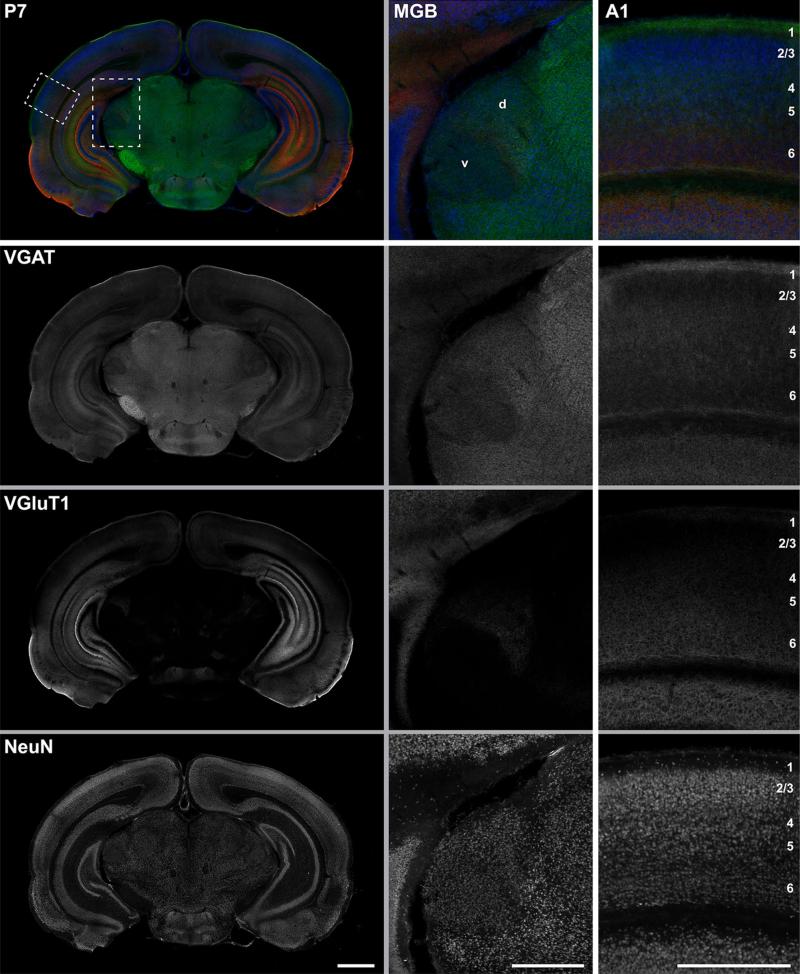
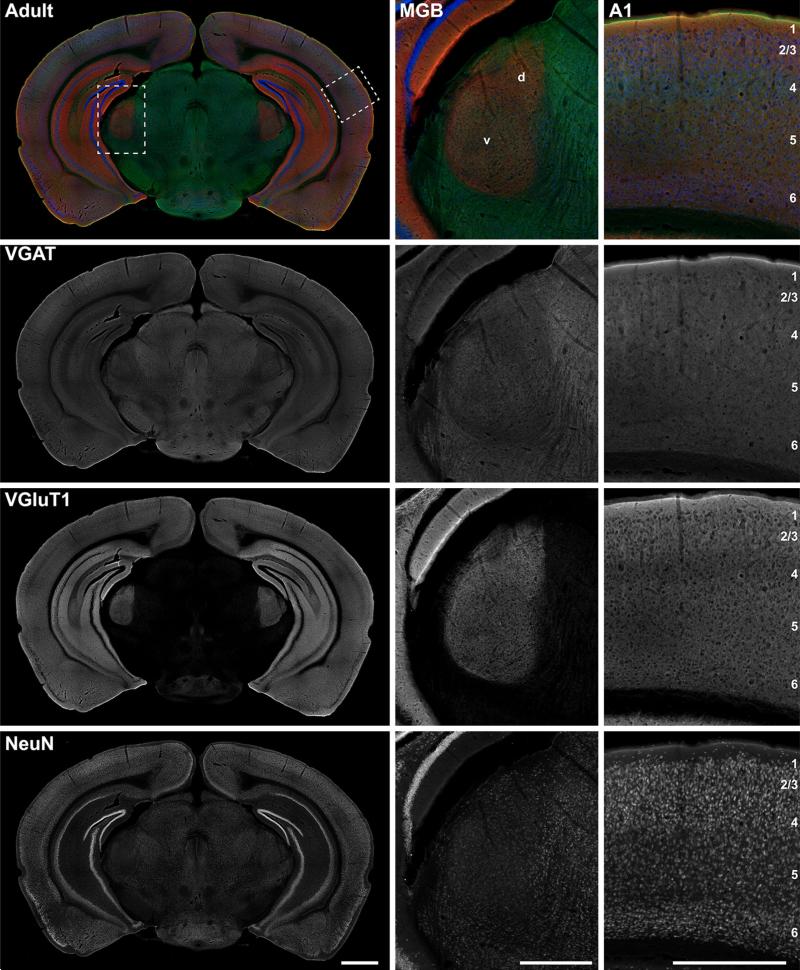
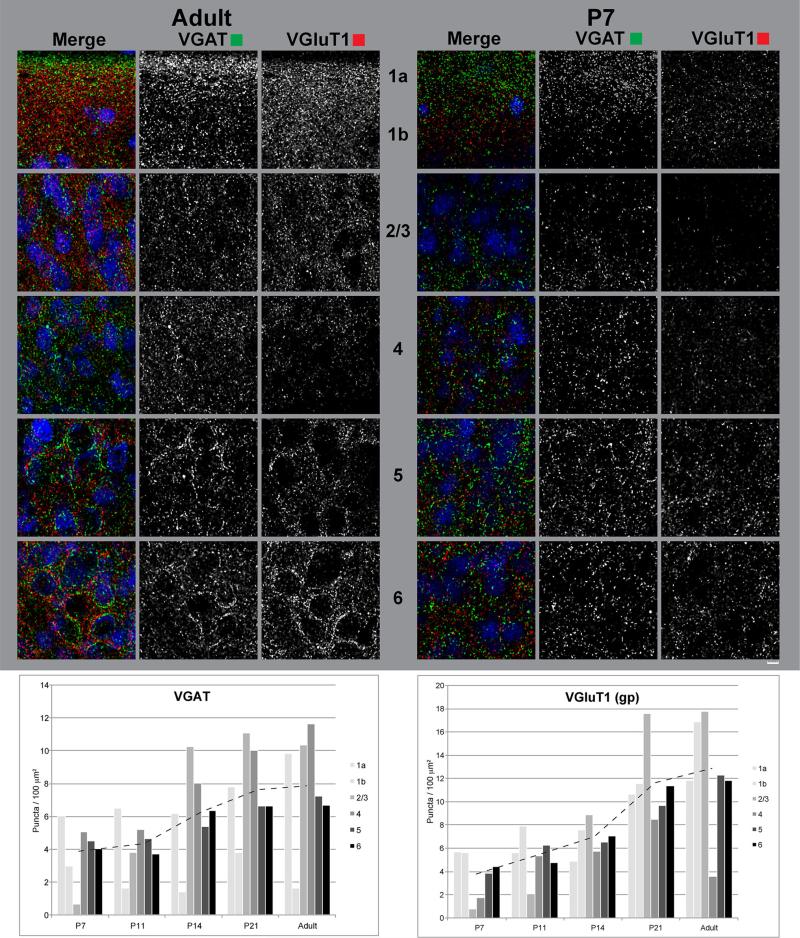
Figures 14, 15, 16, 17 are widefield image montages of single coronal sections through A1 and the MGB at each age (Figs. 14, 15: VGluT2 + VGluT1 + NeuN; Figs. 16, 17: VGAT ? VGluT1 ? NeuN). The merged color images at the top of each image set are separated into grayscale color channels in the panels below, where each channel corresponds to one protein marker. The left column contains images of the full section. Rectangular insets correspond to images of the MGB and A1 in the middle and right columns, respectively. Image brightness was held constant across these images. Accordingly, signal intensity for some images is quite low, reflecting weak immunore-activity of that marker (e.g., VGluT1 in Figs. 14, 16 at P7). Image brightness was held constant to maintain the proper impression of relative immunoreactivity between ages and brain regions. Plots of the relative grayscale intensity values are summarized in Fig. 18.
Fig. 14.
Coronal section at the level of A1 and MGB showing immunoreactivity for VGluT2, VGluT1rb, and NeuN at P7. Top the merged RGB images show VGluT2 (green), VGluT1 (red), and NeuN (blue) reactivity in the same tissue section. The left column contains an image of the full section. Rectangular insets correspond to midpower images of MGB and A1 in the middle and right columns. Below the RGB composites in panels below the top row are separated into grayscale color channels, where each channel corresponds to one protein marker. Scale bars: left column 1 mm; middle and right column 500 μm. d dorsal division of MGB, v ventral division of MGB
Fig. 15.
Coronal section at the level of A1 and MGB showing immunoreactivity for VGluT2, VGluT1rb, and NeuN in adult. Top the merged RGB images show VGluT2 (green), VGluT1 (red), and NeuN (blue) reactivity in the same tissue section. The left column contains an image of the full section. Rectangular insets correspond to midpower images of MGB and A1 in the middle and right columns. Below the RGB composites in panels below the top row are separated into grayscale color channels, where each channel corresponds to one protein marker. Scale bars: left column 1 mm; middle and right column 500 μm. d dorsal division of MGB, v ventral division of MGB
Fig. 16.
Coronal section at the level of A1 and MGB showing immunoreactivity for VGAT, VGluT1gp, and NeuN at P7. Top the merged RGB images show VGAT (green), VGluT1 (red), and NeuN (blue) reactivity in the same tissue section. The left column contains an image of the full section. Rectangular insets correspond to midpower images of MGB and A1 in the middle and right columns. Below The RGB composites in panels below the top row are separated into grayscale color channels, where each channel corresponds to one protein marker. Scale bars: left column 1 mm; middle and right column 500 μm. d dorsal division of MGB, v ventral division of MGB
Fig. 17.
Coronal section at the level of A1 and MGB showing immunoreactivity for VGAT, VGluT1 gp, and NeuN in adult. Top the merged RGB images show VGAT (green), VGluT1 (red), and NeuN (blue) reactivity in the same tissue section. The left column contains an image of the full section. Rectangular insets correspond to midpower images of MGB and A1 in the middle and right columns. Below the RGB composites in panels below the top row are separated into grayscale color channels, where each channel corresponds to one protein marker. Scale bars: left column 1 mm; middle and right column 500 μm. d dorsal division of MGB, v ventral division of MGB
Fig. 18.
Relative grayscale intensity measurements of VGluT1gp, VGluT2, and VGAT immunoreactivity in A1. Left laminar intensity profiles from L1a to L6 at each age. Right mean relative grayscale intensity measurements (and standard deviations) of immunoreactivity by layer or sublayer (layers 1a–6)
Confocal image stacks, comparing A1 and the MGB at P7 and adult, are depicted in Figs. 19, 20, 21, 22, 23 for each set of markers (A1: Figs. 19, 20; MGB: Figs. 21, 22). Puncta counts for all ages are graphically summarized below each image set. Figure 23 shows VGluT2-ir puncta in the MGB at high power to show the terminal size at P7 and adult.
Fig. 19.
Confocal images and puncta counts for VGluT2, VGluT1rb, and NeuN in A1 at P7 and adult. Top single confocal slices from each layer of A1, showing immunoreactivity for VGluT2 and VGluT1. Bottom average puncta density of each protein by layer and age group. Scale bar 20 μm (links to Zoomify™ images for all ages contained in Supp. Figs. S12–S16)
Fig. 20.
Confocal images and puncta counts for VGAT, VGluT1gp, and NeuN in A1 at P7 and adult. Top single confocal slices from each layer of A1, showing immunoreactivity for VGluT2 and VGluT1. Bottom average puncta density of each protein by layer and age group. Scale bar 20 μm (links to Zoomify™ images for all ages contained in Supp. Figs. S19–S23)
Fig. 21.
Confocal images and puncta counts for VGluT2, VGluT1rb, and NeuN in MGB at P7 and adult. Top single confocal slices from each layer of A1, showing immunoreactivity for VGluT2 and VGluT1. Bottom average puncta density of each protein by layer and age group. Scale bar 20 μm. MGv ventral division of MGB, MGd dorsal division of MGB (links to Zoomify™ images for all ages contained in Supp. Figs. S17–S18)
Fig. 22.
Confocal images and puncta counts for VGAT, VGluT1gp, and NeuN in MGB at P7 and adult. Top single confocal slices from each layer of A1, showing immunoreactivity for VGluT2 and VGluT1. Bottom average puncta density of each protein by layer and age group. Scale bar 20 μm. MGv ventral division of MGB, MGd dorsal division of MGB (links to Zoomify™ images for all ages contained in Supp. Figs. S24–S25)
Fig. 23.
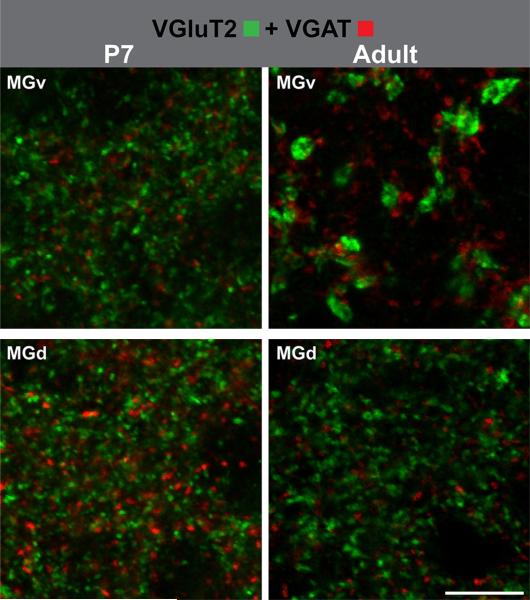
High-magnification confocal maximum intensity projection from five consecutive slices to show details of VGluT2 (green) and VGAT (red) puncta labeling in MGv and MGd at P7 and adult. Scale 20 μm. MGv ventral division of MGB, MGd dorsal division of MGB
VGluT1, VGluT2, and VGAT immunoreactivity: general observations
Immunolabeling of the vesicular transporters was typically punctate and presumed to be contained within putative axon terminals (see Figs. 19, 20, 21, 22, 23; Supp. Figs. S12–25). Labeling was not found within the cytoplasmic domains of neuronal somata or dendrites. Some differences in the size of labeled puncta were evident (but not measured), and in some regions (e.g., MGB) puncta morphology changed with age (details below). We did not find convincing evidence that VGluT2, VGluT1, or VGAT localized to the same puncta within A1 or MGB at any age, suggesting that a given terminal does not normally contain more than one vesicular transporter (at least not at levels detectable by our methods). Because our observations were limited to the light microscope level of inspection, however, we cannot rule out possible co-expression in terminals where expression levels were below the resolution or detection of our approach (e.g., Fig. 26). Generally, the age-related trends visible at low power were also observed at high-power. However, some minor discrepancies are visible, presumably due to differences in illumination (fluorescence excitation) between 10× widefield and 60× confocal.
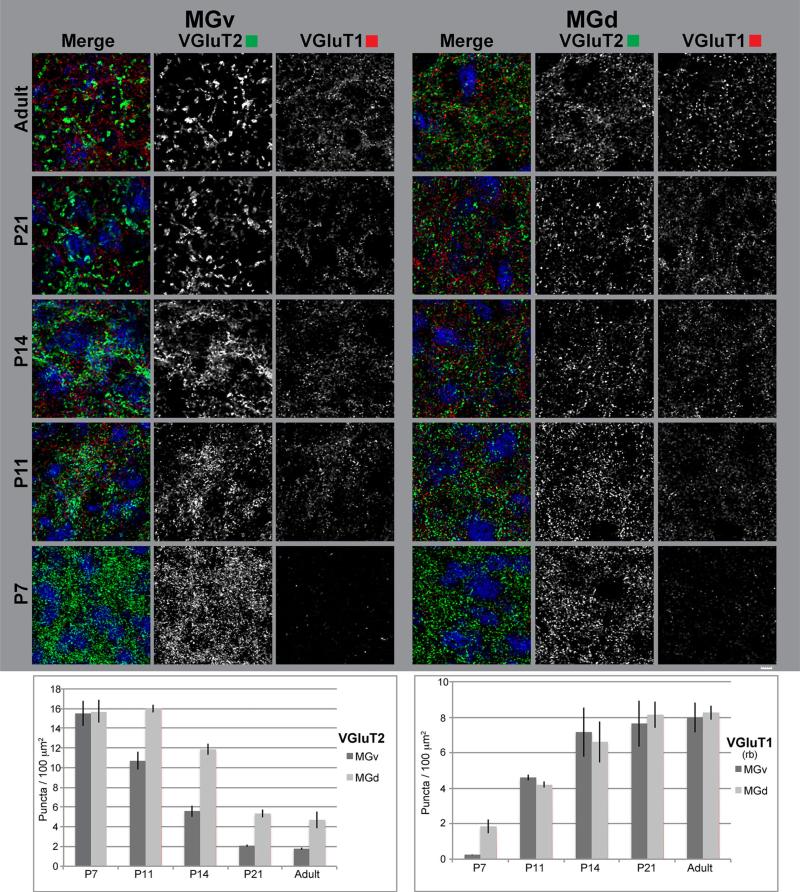
VGluT2 immunoreactivity in A1 and the MGB
In A1, VGluT2-ir puncta, presumably reflecting mainly TC projections, were present in all layers at all ages, with prominent peaks in L1a and L4 (Figs. 14, 15, 18, 19; Supplementary Figs. S2–6, S12–16). In L5b/6a, a slightly elevated band of VGluT2-ir was visible, but at levels well below L1a and L4, with immunoreactivity reaching a minimum in L6b. The L5b/6a band contained strings of beaded (en passant) VGluT2 puncta (varicosities) that were rare in L6b. The axonal segments joining puncta were not as clearly delineated by the antibody that was used in the final images/analyses, which generated highly punctate labeling of terminal endings (Table 2). However, in preliminary testing of antibodies, the interconnected strings of en passant terminals were clearly visible with one antibody, in particular (Millipore MAB5504). This antibody was excluded from the final assays due to problems associated with reliability in mouse, although it was successfully used in our earlier study of macaque monkey auditory cortex, where the en passant configuration was robust in L5b/6a (Hackett and de la Mothe 2009). Thus, punctate labeling of presumptive TC terminals was observed in varied concentrations in all layers of A1, but in L5b/6a some of the terminals were en passant.
Laminar density profiles in A1 obtained from low-power images were highly overlapping at P7 and P11, and also at P21 and adult, with the greatest increases in terminal density taking place prior to P21, especially in L4 (Fig. 18). Confocal images and puncta counts (Fig. 19) were generally consistent with these trends, indicating that laminar patterns were well established by P7, although immunoreactivity continued to increase through P21. VGluT2-ir terminals were rather uniformly dispersed between cell somata where they likely make contact with dendritic processes (Richardson et al. 2009). Somatic contacts appeared to be rare.
In the MGB, VGluT2-ir puncta, primarily reflecting tT projections, were present in the ventral (MGv) and dorsal (MGd) divisions at all ages (Figs. 14, 15, 18, 20; Supplementary Figs. S2–6, S17–18). Puncta were densely packed in the neuropil of both divisions. In the MGv, VGluT2-ir puncta became concentrated in patch-like formations from P11 onward, producing a lobulated appearance. In contrast to A1, VGluT2-ir decreased in both divisions after P7, with minimal change between P21 and adult (Fig. 20). The reduction was most pronounced in the MGv; so that, by maturity, VGluT2-ir in the MGd was higher than in the MGv. The observations can be explained by reduced numbers of VGluT2-ir puncta, accompanied by an increase in their size (Figs. 20, 23). Qualitatively, the puncta at P7 and P11 were relatively small and densely packed. At P21 and in the adult, most were medium to large in size and were more widely dispersed. A transitional stage was discernible at P14, when small and large puncta were intermixed and weak labeling of distal axons was visible. In the MGd, puncta density also decreased after P7, though puncta size did not appear to change appreciably and puncta counts remained higher than in the MGv. Large VGluT2-ir terminals were rare in the MGd.
VGluT1 immunoreactivity in A1 and the MGB
In A1, VGluT1-ir puncta, primarily reflecting CC projections, were present in all layers of cortex (Figs. 14, 15, 16, 17, 18, 19, 20, 21, 22, 23; Supplementary Figs. S2–16, S19–23).
Compared with VGluT2, laminar density profiles (Fig. 18) lacked prominent peaks, as VGluT1-ir puncta were more uniformly distributed across layers. One exception was in L1; whereas VGluT2 and VGAT were concentrated in L1a and VGluT1-ir puncta were more evenly distributed across L1a and L1b (Figs. 18, 19, 20). A second exception was a relative reduction in L4 immunoreactivity (most prominent after P11). This “negative peak” complemented the positive VGluT2 peak found in L4 (Figs. 18, 19, 20).
Several age-related changes were observed in A1. Overall, VGluT1-ir puncta density increased from P7 to adult, especially prior to P21, but the changes were not uniform across brain regions or cortical layers. Most notable were the changes in L2–4. At P7, immunoreactivity was relatively low in L2/3 and L4, bounded by modest peaks in L1 and L5/6 (Fig. 18). The dip in L2/3 was largely resolved by P14, but the negative peak in L4 persisted through adulthood, becoming more prominent as surrounding layers matured. Another age-related change of interest was an apparent increase in the presence of VGluT1-ir terminals in close proximity to cell somata, most notably in L5–6a (Figs. 19, 20; Supplementary Figs. S12–16, S19–23). In these layers, as the overall numbers of VGluT1-ir terminals increased, the prominence of such terminals also increased. In Figs. 19, 20, note the clustering of VGAT and VGluT1 around somatic profiles in the adult, but not at P7. We found no clear examples of VGluT1 in close apposition to somata at P7 or P11. At P14, as VGluT1 levels were becoming more robust across layers in cortex, VGluT1-ir puncta were in relatively close proximity to a few somata, forming weak outlines. At P21 and in the adult, puncta with a perisomatic arrangement were common and most obvious around L5 pyramidal neurons (see “Discussion” for explanation).
In the MGB, VGluT1-ir puncta, primarily reflecting CT terminals, were evenly distributed in the MGv and MGd, although the density may be slightly higher in MGd (Figs. 14, 15, 16, 17, 18, 21, 22; Supplementary Figs. S2–11, S17–18, S24–25). The expression density was low at P7 and then increased markedly through P14, especially in the MGv, with more gradual increases thereafter. The two VGluT1 antibodies yielded somewhat different appearances, but the same overall trajectories. VGluT1-ir puncta varied somewhat in size (qualitative observation), but were typically small relative to either VGluT2 or VGAT puncta, with no obvious change in either MGB division with age (Figs. 21, 22). We looked for, but only found a few potential examples of larger puncta that might correspond to giant terminals of L5 CT projections. Because it is almost certain that such CT terminals in the MGd are VGluT1-ir, their rarity may reflect a methodological or anatomical peculiarity associated with VGluT1. However, larger VGluT1-ir puncta were visible in the posterior medial nucleus bordering the MGB medially (not shown), suggesting that antibody labeling can delineate at least some changes in terminal size. In any case, the developmental increase in VGluT1-ir puncta density contrasts sharply with the decrease of VGluT2 in MGv and also differed from A1, where the expression of both increased with age. Finally, in both A1 and MGB, we found that maturation of VGluT1 immunoreactivity lagged behind that of VGluT2 and VGAT.
VGAT immunoreactivity in A1 and the MGB
In A1, the distribution of VGAT-ir puncta, reflecting GABAergic interneuron terminals, was relatively even across most layers (Figs. 16, 17, 18, 20; Supp. Figs. S7–11, S19–23), with a couple of exceptions. First, a prominent positive peak was found in L1a at all ages, matching the VGluT2 peak in L1a and contrasting with the even distribution of VGluT1 in L1a and L1b (Fig. 18). Second, at P21 and adulthood, there was also a relatively broad but shallow positive peak centered over L3/4, reflecting a somewhat higher density of puncta in the thalamorecipient layers. VGAT density increased steadily from P7 to adulthood, increasing most rapidly in the superficial layers (L1–3). The prevalence of perisomatic VGAT-ir puncta increased with age in A1 and apparently in tandem with closely apposed VGluT1-ir puncta (Fig. 20). Zooming in on the Supplementary figures at all ages shows more clearly that these terminals were rare at P7 and P11, and started to become more numerous by P14, especially in L5–6a, where perisomatic terminal density was most obvious. At P21, perisomatic labeling in L5–6a was comparable that found in the adult, but below adult levels in L2/3. Thus, it appears that the maturation of this feature continues after P21, along with a gradual increase in VGAT-ir puncta in all layers. The subplate, visible through about P14, contained a slightly higher concentration of VGAT-ir puncta, but these measurements were not included in the quantification of L6 (see Supplementary confocal images).
In the MGB, VGAT-ir puncta, which reflect both tT and TRN terminals, were fairly uniformly distributed in the MGv and MGd, with a somewhat higher density of terminals in the MGd at all ages (Figs. 16, 17, 22; Supp. Figs. S7–11, S24–25). At low magnification (Figs. 16, 17), VGAT-ir puncta in the MGv appeared concentrated in irregular patches, similar to those observed in VGluT2 preparations, and a general increase in the brightness of labeling resulted in an increase in grayscale density over time. The confocal images (Fig. 22; Supp. Figs. S7–11, S24–25) showed that VGAT-ir puncta were present at P7 in both divisions, and at slightly higher levels than for older animals. In the MGv, puncta density decreased modestly from P7 to P11 and then remained fairly stable. In the MGd, puncta density decreased modestly from P7 to about P14 and then on very slightly through adulthood. Unlike VGluT2 in the MGv, no obvious or widespread changes in terminal morphology were evident that might be correlated with the decrease in VGAT puncta density, although our qualitative impression was that a subpopulation of larger terminals persists through to the adult stage while the numbers of smaller puncta diminish, suggesting some pruning of less developed terminals.
Co-localization of immunoreactive puncta
We looked for, but did not find, any clear instances in which VGluT1-ir and VGluT2-ir puncta were co-localized in presumptive terminals of any layer of A1 or MGB division at any age. Similarly, VGAT-ir puncta were never found to co-localize with either VGluT1 or VGluT2 in any location. We cannot rule out the possibility that very low expression levels were not detectable by our methods. As elaborated below, these results indicate that although VGluT1 and VGluT2 mRNA were frequently co-expressed in the same neurons of A1 and MGB, the transporter proteins are not co-localized at detectable levels in the same axon terminals. The results favor the possibility (also beyond the methods used in this study) that if VGluT1 transporter proteins are present in TC axon terminals at significant levels, they are sorted to different axon terminals than VGluT2, in which case they would not be distinguishable from VGluT1-ir CC terminations by immunohistochemistry alone. Similarly, if VGluT2 transporters are normally contained in the axon terminals of any cortical neurons, they are present at relatively low levels, or sorted to different terminals than VGluT1, or both (see “Co-expression of VGluT1 and VGluT2” and “Discussion”).
Correspondence between gene and protein expression
In Fig. 24, the data from Figs. 12 and 16 were replotted to enable comparisons of the RNAseq, ISH, and IHC data. P11 data were omitted from these graphs to facilitate comparisons with RNAseq at this age (P11 samples were not obtained for sequencing). The RNAseq and ISH graphs show a fairly close correspondence in the data obtained by the two mRNA assays. The IHC data were generally comparable, but several interesting differences were observed. In A1, immunoreactivity increased steadily for all three protein markers from P7 through adulthood. In contrast, gene expression increased through P14 for VGluT1 with a slight decline in adulthood, and remained flat across the age range for VGluT2 and VGAT. In the MGB, VGluT1 mRNA levels climbed through P14/P21, then declined slightly, but VGluT1-ir grew steadily. VGluT2 mRNA levels in MGB also peaked at P14, with only a slight decline in adulthood, whereas VGluT2-ir showed a sharp decline from P7 to P21. VGAT-ir also dropped from P7 to P14. As discussed above, these differences partly reflect differences in subcellular localization of mRNA transcripts (somatic) and proteins (terminals), but may also reflect differences in the regulation of transcription or translation during postnatal maturation.
Fig. 24.
Graphical summaries of VGluT1, VGluT2, and VGAT expression in A1 and MGB obtained by analyses of RNAseq, ISH, and IHC assays. The data from Figs. 13, 19, 20, 21 and 22 were replotted to facilitate comparison between conditions and methods as a function of brain region and age. Top Primary auditory cortex (A1) at P7, P14, P21, and adult. Bottom medial geniculate body (MGB) at the same ages. Other conventions as in Fig. 13. Note the general correspondence between RNAseq and ISH data and differences for some IHC conditions (e.g., VGluT2 in MGB)
Discussion
In the central auditory pathways, characterization of VGluT1, VGluT2, and VGAT localization and function is ongoing (Barroso-Chinea et al. 2007; Hackett and de la Mothe 2009; Hackett et al. 2011b; Ito et al. 2009, 2011; Ito and Oliver 2010; Nahmani and Erisir 2005; Rovo et al. 2012; Storace et al. 2012). In most structures, expression of at least one of these transporters has been studied in adult animals, but systematic studies of their maturation over the weeks surrounding the onset of hearing are just beginning to emerge.
The main purpose of this study was to characterize postnatal changes in the expression of the vesicular transporters for glutamate (VGluT1, VGluT2) and GABA (VGAT) in A1 and the MGB over the period surrounding the onset of hearing. By profiling the gene (RNAseq, ISH) and protein (IHC) expression patterns, we obtained data from populations of projecting neurons (mRNA transcripts in neuronal somata) and their targets (immunoreactive axon terminations). Accordingly, the combined results reflect maturational features of the corticocortical (CC) corticothalamic (CT), thalamocortical (TC), tectothalamic (tT), and thalamic reticular nucleus (TRN) systems of projections in the auditory forebrain. Overall, significant changes were observed in the expression of all three transporters between P7 and adult, but the rate and direction of change were not uniform between markers or brain regions (summarized in Figs. 24, 25). As discussed below, these differences suggest variable trajectories of maturation between pathways and establish a baseline to which related anatomical features can be compared (e.g., postsynaptic receptors, ion channels, other neuromodulators, specific neuronal subpopulations, etc.). The data also draw attention to the presynaptic machinery related to neurotransmission and its role in activity-dependent homeostatic changes that accompany critical periods of experience-dependent brain development.
Fig. 25.
Summary of vesicular transporter gene and protein expression in A1, MGB, and IC at P7 and adult for VGluT1, VGluT2, and VGAT. Schematic drawings illustrate relative expression of each marker in cortical layers of A1, MGd (d), MGv (v), Rt, and IC (ICc and adjacent divisions). Gene expression levels of neuronal somata (triangles, circles, ovals) in each structure are indicated by grayscale shading (black highest expression). Protein expression levels are indicated by grayscale shading by cortical layer or subcortical region. For IC and Rt, relative immunoreactivity at P7 and adult were based on qualitative impressions (see Supplementary Figures 26 and 27). Major connections between structures are indicated by arrows. Solid arrows corticocortical (CC), thalamocortical (TC), and tectothalamic (tT) projections. Dashed arrows corticothalamic (CT), corticotectal (Ct), and reticulothalamic (RT) projections. Brackets from L1 to L6 indicate that TC projections are found in all layers. For VGluT2, grayscale shading density by layer is correlated with TC terminal density. Question marks (?) denote connections for which the vesicular transporter involved is not known or is speculative (i.e., VGluT1 TC; VGluT2 CC). Asterisk in the VGAT panel denotes that CC* projections of VGAT are primarily local (some long-range projections exist)
Regional expression patterns of VGluT1, VGluT2, and VGAT
Generally, the regional expression patterns of VGluT1 and VGluT2 in the forebrain are complementary, while VGAT is relatively more evenly distributed. This can be instantly appreciated in viewing the low-magnification images in this report (e.g., Figs. 2, 15) and the higher-resolution image libraries that depict immunoreactivity from P7 to adult across the entire brain for all three markers (Supplementary Figures 26, 27). The regional distinctions are most distinct for mRNA. VGluT1 mRNA is widely expressed by most, if not all, glutamatergic neurons in the neocortex, but is only found in a few subcortical structures—mainly the sensory relay nuclei in the thalamus and the striatum, whereas, VGluT2 mRNA is widely expressed by glutamatergic neurons in the thalamus and brainstem, but is present at relatively low levels in the cortex (Balaram et al. 2011; Barroso-Chinea et al. 2007; Bryant et al. 2012; De Gois et al. 2005; Fremeau et al. 2001, 2004; Graziano et al. 2008; Hackett et al. 2011b; Herzog 2001; Hur and Zaborszky 2005; Kaneko et al. 2002; Nahmani and Erisir 2005; Rovo et al. 2012). In contrast, VGluT1 and VGluT2 protein expression is more overlapping in both cortex and thalamus, because VGluT1 is contained in CC and CT projections (axon terminals), and VGluT2 is expressed in tT and TC projections. Compared to the glutamate transporters, VGAT mRNA is widely expressed by cortical and subcortical neurons. Since most (but not all) of their projections are local, VGAT immunoreactivity also tends to be local; thus there is a much stronger correspondence in the regional expression patterns of VGAT mRNA and protein.
As for the auditory forebrain, the major glutamatergic and GABAergic projection systems in the P7 and adult mouse are summarized in Fig. 25. In these schematics, VGluT1, VGluT2 and VGAT expression in axon terminals (protein) or neuronal somata (mRNA transcripts) are depicted separately. Minor projections and intrinsic connections were omitted for clarity.
In A1, VGluT1 mRNA appears to be expressed by all glutamatergic neurons. Accordingly, VGluT1-ir is also high, primarily reflecting CC connections among auditory and other cortical areas, but possibly also the terminals of VGluT1+ subpopulations in the MGB (see Storace et al. 2012 and “Transcript Co-localization”, above). VGluT2 expression is very low in auditory cortex, although subsets of neurons with detectable transcript levels are present in most areas, with the weakest expression in the infragranular layers (De Gois et al. 2005; Graziano et al. 2008; Hackett et al. 2011b; Ito and Oliver 2010). As observed in the present study, VGluT2 transcripts in A1 appear to always be contained in neurons that express VGluT1, but not all VGluT1+ neurons express VGluT2. Little is known about the neuronal subpopulations that express both genes in cortex, and it remains unclear whether VGluT2 transporter proteins are normally present in any of their axon terminations (but see below). Thus, most of the VGluT2-ir terminals in cortex are presumed to represent TC projections. In A1, these puncta are found in all layers (as depicted in Fig. 25), but form a prominent band centered on L4. VGAT+ neurons are broadly distributed in all layers of A1, with the fewest number in L1. Most of the VGAT-ir terminals are derived locally, resulting in dense immunoreactivity in most layers, except for L1b (Fig. 25).
In the MGB, nearly all neurons express VGluT2 mRNA, but a subset also expresses VGluT1, at least in rodents (Barroso-Chinea et al. 2007; Ito et al. 2011; Storace et al. 2012). VGluT1 mRNA appears to be rather sparse in the MGB of adult primates (Hackett et al. 2011b), however, reflecting a possible species difference in the chemoarchitecture of these circuits. VGluT2-ir in the MGB primarily reflects the inputs from VGluT2+ neurons in the IC, since VGluT1 transcripts are rare in any division of the IC, as observed in the present and previous studies (Ito et al. 2011; Ito and Oliver 2010). VGluT1-ir in the MGB is also strong, reflecting CT inputs from VGluT1+ neurons in the infragranular layers of auditory cortex. Since many MGB neurons coexpress VGluT1 and VGluT2 mRNA, both transporter proteins could be co-localized to the same terminals or trafficked to different TC terminals. These details (discussed below) remain unknown. Most of the GABAergic inputs to the MGB arise from the thalamic reticular nucleus (TRN) and the IC (Bartlett and Smith 1999; Bartlett et al. 2000; Geis and Borst 2013; Ito et al. 2009, 2011; Mellott et al. 2014a, b; Montero and Scott 1983; Peruzzi et al. 1997; Winer et al. 1996). The TRN neurons receive axon collaterals from both the TC and CT systems of projections, with the exception of the CT axons that originate in L5 (Crabtree 1998; Kimura et al. 2005). In contrast, GABA, GAD, or VGAT mRNA expression within MGB neurons is very rare in mice and rats, as observed in the present and previous studies (Ito et al. 2011; Winer and Larue 1996; Yuge et al. 2011). Thus, a network of intrinsic GABAergic projections is absent from the MGB in these species and inhibitory influences on activity are almost entirely external.
Maturation of VGluT1 and VGluT2 in the auditory forebrain
Overall, vesicular glutamate and GABA transporter expression changed significantly between P7 and adulthood (Fig. 25). The greatest changes occurred prior to P21, encompassing a 2-week period before and after ear canal opening (~P11–P13). Yet, each projection system matured at slightly different rates.
In the IC and MGB, VGluT2 mRNA expression was well established by P7, with little change thereafter, accompanied by strong VGluT2-ir terminal labeling in the MGB and in L4 of A1. These findings suggest that the potential for VGluT2-mediated transmission in tT and TC projections is well established by P7, in agreement with descriptions of TC transmission prior to the onset of hearing (Barkat et al. 2011). Note also that TC projections to A1 are not limited to L4, but are distributed across most layers in different concentrations. This indicates that TC information reaches neurons in several layers within a narrow window of time. In comparison, VGluT1 mRNA expression was quite low at P7 in MGB, especially in MGv, and concentrated in L2–3 in A1. VGluT1-ir was also very low in MGB and formed minor peaks in L1a/b and L5/6 of A1. Together, these patterns indicate that VGluT2-mediated tT and TC transmission is established well in advance of VGluT1-mediated CC and CT signaling, which lags behind until after the onset of hearing at P11. These maturational trends correspond well with prior studies of sensory cortex and thalamus in mice and rats (Boulland et al. 2004; De Gois et al. 2005; Liguz-Lecznar and Skangiel-Kramska 2007; Minelli et al. 2003b; Nakamura et al. 2005). One interesting difference between modalities is that VGluT1 and VGluT2 expression reaches adult levels by about P14 in S1. This is approximately 1 week faster than in A1 and V1 (Minelli et al. 2003b; Nakamura et al. 2005), suggesting that VGluT1 and VGluT2 expression is linked to the onset of sensation, which can vary between different sensory modalities.
Looking more closely at these maturational trends in A1 and MGB, several observations are of interest, as they may signal important differences in the development of specific pathways.
The first concerns maturation of the CT projections from A1 to MGB. CT inputs to the MGB primarily arise from neurons in L5 and 6 of auditory cortex (Bartlett 2013; Lee 2013). CT neurons in L6 project to the MGv and MGd, ending in small terminals on distal dendrites (Bajo et al. 1995; Bartlett et al. 2000; Llano and Sherman 2008; Ojima 1994; Rouiller and Welker 1991; Smith et al. 2007). In contrast, CT projections from L5 are relatively sparse, have large terminal boutons, and predominantly target the MGd. Neurons in both L5 and 6 are almost exclusively VGluT1+ at all ages, indicating that CT terminals in the MGB should also be VGluT1-ir. Although VGluT1 mRNA expression was relatively low at P7 in A1, maturation in L5 was a bit ahead of L6. Further, VGluT1 mRNA and protein expression was strongly biased toward the MGd at P7, becoming more evenly distributed between the MGv and MGd at later ages. Taken together, the gene and protein expression findings suggest that the L5–MGd CT projections may be active before and mature earlier than the L6 CT projection to the MGv/MGd. These anatomical data support and refine our earlier report that CT axons were biased toward the dorsal subdivision prior to hearing onset but more evenly distributed thereafter (Torii et al. 2013). Note also that over the same period, VGluT2 mRNA expression was relatively strong in both divisions of the MGB, then VGluT2-ir puncta density decreased, especially after P11, accompanied by an increase in puncta size in MGv (discussed below). Thus, ear canal opening (~P11–13) was followed by distinct patterns of VGluT1 and VGluT2 expression in the MGv and MGd.
Second, the laminar expression patterns of VGluT1 mRNA and VGluT1-ir in A1 are partially complementary. VGluT1-ir puncta were concentrated in L1 and L5/6 at P7 and gradually increased in the intervening layers over development. In contrast, VGluT1+ mRNA was most strongly expressed in L3b–5 at P7, but was weaker in L2 and L6. This difference was largely gone by P11, when VGluT1+ expression became uniform across L2–6. One possible interpretation is that VGluT1-mediated glutamate release by neurons in L3–5 onto targets in L1 and L5/6 matures a bit ahead of the VGluT1+ projections to other layers prior to the onset of hearing. Alternatively, or additionally, the VGluT1-ir band of terminals in L1 at P7 and later might contain some VGluT1-ir inputs from the MGd (or other nuclei), since modest levels of VGluT1 mRNA are already present in MGd neurons at P7. These patterns raise intriguing questions about laminar patterns of signaling within A1 before and after hearing onset.
Third, VGluT2 mRNA levels decreased from P7 to adult in A1, reaching very low levels at maturity. The ISH and FISH assays showed that expression at younger ages was largely concentrated in L2–3. These laminar patterns and their developmental time course are comparable to those observed by De Gois et al. (2005), and also compare well with Allen Brain Atlas data at comparable developmental ages. It is not entirely clear why VGluT2 levels in L2/3 neurons should be elevated at P7, but the answer may be related to activity-dependent regulation of vesicular transporter expression (discussed below), where the relative levels of VGluT1 and VGluT2 are subject to activity-dependent modification. In addition, the downstream targets of the subpopulations of cells that express VGluT2 (and are co-localized with VGluT1) are also unknown, at present. We are thus led to speculate whether these neurons might have different response properties, and whether the VGluT1 and VGluT2 proteins might be trafficked to the same or different terminals.
Maturation of VGAT expression in the auditory forebrain
Like the glutamatergic system, the GABAergic network in the cortex is in flux for several weeks after birth (Ben-Ari et al. 2004, 2012; Ciceri et al. 2013; Espinosa and Stryker 2012; Gelman and Marin 2010; Griffen and Maffei 2014), resulting in a gradual increase in inhibitory strength (Chattopadhyaya et al. 2004; Li et al. 2012; Maffei and Turrigiano 2008). At the synaptic level, much emphasis has been placed on the documentation of changes in GABA receptor subunit expression, which vary over time by brain region and even cell type, and contribute to the maturation of inhibitory synapses (Ben-Ari et al. 1994, 2012; Bosman et al. 2002; Davis et al. 2000; Fritschy et al. 1994; Heinen et al. 2004; Hensch 2005; Hornung and Fritschy 1996; Huang 2009; Laurie et al. 1992; Maffei and Turrigiano 2008; Mohler 2006; Peden et al. 2008; Takesian et al. 2010).
Overall, the time course of these postsynaptic changes parallels the maturational trajectory of presynaptic VGAT-ir observed in the present and prior studies. Although VGAT mRNA levels did not change significantly from P7 to adult in either A1 or MGB, we found that VGAT-ir in A1 increased steadily from P7, leveling off at around P21. The difference in gene and protein expression suggests that translation of VGAT is differentially regulated, perhaps in a manner that reflects the onset of activity. For example, the trajectory of VGAT-ir maturation in A1 closely matches the findings of Boulland and Chaudhry (2012), who studied the structural and functional properties of VGAT-ir puncta during postnatal development in several brain areas. In the cortex, hippocampus, and thalamus they observed an increase in VGAT expression during the first three post-natal weeks, in tandem with an increase in the expression of markers related to GABA synthesis and neurotrans-mission (e.g., GAD). In rats Minelli et al. (2003b) found that VGAT levels in S1 increased gradually from P0, reaching 50 % of adult levels between P5 and P10, and adult levels by about P15. The laminar patterns of terminal labeling were comparable to the present study. They also noted that VGAT expression through P5 was slightly ahead of the synaptogenesis of inhibitory contacts, and suggested that at this stage it may contribute to the transition to mature forms of GABA release at synapses. For example, Takayama and Inoue (2010) tracked the expression of the potassium chloride co-transporter 2 (KCC2), GABA, and VGAT proteins in mouse barrel cortex from P0 to adult. KCC2 and VGAT expression followed a similar time course. VGAT puncta were present in L1a at birth, with little change after P5. Puncta appeared in L5/6 after P3, L4 after P5, L2/3 after P7, and were relatively homogeneous across layers by P14. Functionally, the onset of KCC2 expression is correlated with the point at which GABA binding causes hyperpolarization—rather than depolarization—of the postsynaptic cell membrane (Blaesse et al. 2006; Lee et al. 2005; Rivera et al. 2005). Their data suggested that by about P10, GABA is an inhibitory transmitter for most cortical neurons in mice. Compared to S1, then, the time course of VGAT maturation in A1 is delayed. This is likely related to the delayed onset of sensory transmission in the auditory system, which is minimal until after ear canal opening (P11–13). It is not clear whether the shift to inhibition in GABAergic neurons is also delayed in auditory cortex, relative to S1. Pursuing this in future studies would be of interest.
Of additional interest is that the onset of GABA-mediated inhibition in cortex is delayed relative to subcortical nuclei in the thalamus and brainstem (Kullmann and Kandler 2001; Milenkovic et al. 2007; Perreault et al. 2003; Venkataraman and Bartlett 2013; Ziburkus et al. 2003), suggesting that the time course varies between regions. In the MGB, where VGAT-ir terminals originate primarily from the TRN and IC, there was a slight decline in the numbers of VGAT-ir puncta between P7 and P14, but immunoreactivity was otherwise stable after P7. This suggests that the possibility that GABAergic circuitry in the MGB matures ahead of A1. Comparable data on VGAT-ir from other sensory systems are rather limited. Singh et al. (2012) studied GAD67 and VGluT2 expression in the dorsal lateral geniculate nucleus (dLGN) from P3 to adulthood in mice. In their study, GAD67-ir terminal density increased from P7 to P14, but size remained constant. Using an antibody for GABA, Bickford et al. (2010) found little immunoreactivity in the dorsal LGN (dLGN) at P7, but strong labeling by P14. This anatomical change was supported by electrophysiological measurements. These trends match our qualitative impressions of VGAT-ir in LGN from P7 to adult (see Supplementary Figure 27). As for terminal size, there was no obvious maturational change in the MGB, as found in the dLGN (Singh et al. 2012). On average, the size of GABAergic terminals in the MGB were qualitatively smaller than excitatory (VGluT2-ir) terminals from the IC, but there does not appear to be a definitive way to distinguish GABAergic terminal populations from the thalamic reticular nucleus (TRN) and IC based on morphology (Bartlett et al. 2000). Their delineation would be of interest for future anatomical studies, and may serve to support recent neurophysiological studies that have focused on maturation of excitatory and inhibitory properties in the MGB during postnatal development (Venkataraman and Bartlett 2013, 2014).
Co-expression of VGluT1 and VGluT2
An important observation of the present study was that co-localization of VGluT1 and VGluT2 transcripts within the same neurons was common in A1 and MGB of juvenile and adult animals. Throughout the brain, VGluT1 and VGluT2 mRNA expression is generally complementary between regions (e.g., cortical versus subcortical); however, co-expression (within a region) and co-localization (within the same cellular compartment, such as somata or terminals) have been reported in some structures in adult animals. Barroso-Chinea et al. (2007) conducted single and dual expression assays of VGluT1 and VGluT2 mRNA throughout the thalamus of adult rats. In the MGB and all other sensory relay nuclei (e.g., lateral geniculate nucleus, ventroposterior nucleus), most if not all neurons contained both transcripts. Frequent co-localization was also reported in the MGB (and most other auditory brainstem nuclei) of adult mice and rats by Ito et al. (2011), with the greatest numbers of co-expressing neurons in the MGv (see also Storace et al. 2012).
During postnatal development in rodents, the results of two studies are highly relevant to the present investigation. Danik et al. (2005) studied the co-localization of VGluT1 and VGluT2 mRNA in dissociated neurons from the hippocampus, cortex, and cerebellum (Danik et al. 2005). Overall, VGluT1 mRNA levels increased with age, while VGluT2 levels decreased. At P14, about 80 % of neurons that expressed VGluT1 mRNA also expressed VGluT2. Co-expression dropped to about 60 % by P60, still reflecting a sizable majority. De Gois et al. (2005) demonstrated widespread co-localization of VGluT1 and VGluT2 mRNA in pyramidal neurons and L4 neuronal subtypes in both primary cell cultures and in vivo tissue sections. Somata containing both transcripts increased in numbers through P10 in L2–4 and also L5–6 in the auditory and parietal cortices of rats. VGluT1 mRNA levels also increased through P14, but then became stable, whereas VGluT2 mRNA levels decreased through at least P21, but remained at detectable levels. By adulthood, neurons expressing only VGluT1 mRNA predominated, VGluT1/2 co-expressing neurons were a significant minority, and cells containing only VGluT2 were rare. Thus, most of the cells in cortex that expressed VGluT2, also expressed VGluT1.
Given the widespread co-expression of VGluT1 and VGluT2 mRNA, one might predict frequent co-localization of their proteins in axon terminals (e.g., see Fig. 26). However, evidence for this has been inconsistent. Typically, VGluT1 and VGluT2 proteins in adult animals are localized in discrete populations of axon terminals that overlap, but do not co-localize (Bragina et al. 2007). In the present study, we found only rare examples of terminals that were candidates for the co-localization of VGluT1 and VGluT2 proteins in any layer of cortex at any postnatal age. In contrast, in rat primary neocortical cell cultures, De Gois et al. (2005) found that VGluT1 and VGluT2 were co-localized to many (~10 %) terminals between 5 and 21 days in vitro. Similarly, Nakamura et al. (2005) found that VGluT1 and VGluT2 were frequently co-localized in terminals in L4 barrel cortex between P5 and P10, especially in barrel hollows, but rarely in adult brains. Liguz-Lecanar and Skangiel-Kramska (2007) also noted co-localization in terminals within barrels prior to adulthood (Liguz-Lecznar and Skangiel-Kramska 2007). These combined results suggest that co-localization might be a unique and transient feature that occurs primarily during the first two postnatal weeks. The reduced levels of VGluT2 mRNA at later postnatal stages and adulthood may result in lower protein levels and explain why co-localization of VGluT1 and VGluT2 transcripts is rarely found in the sensory cortex of older juvenile and mature animals.
Fig. 26.
Schematic diagrams depicting possible variations in the trafficking and expression of VGluT1 and VGluT2 transporters in TC (MGB to AC) and CC (AC to OTHER) projections. In all examples, VGluT1 and VGluT2 mRNA is co-expressed in somata, but relative levels differ by brain region. a Only VGluT2 is expressed on vesicles in TC axon terminals, and VGluT1 is expressed on vesicles in all CC terminals. b VGluT1 and VGluT2 are sorted to different terminals. c VGluT1 and VGluT2 are co-expressed in TC and CC axon terminals; d VGluT2 or VGluT1 is expressed alone in some terminals, but co-expressed in others. Note that many additional configurations are possible. Sorting may vary by cortical layer or target cell type and may be modified by sensory experience or other manipulations. Note also that variations in trafficking may apply to other molecules expressed in these neurons (for further discussion, see “Co-expression of VGluT1 and VGluT2”). MGB medial geniculate body, AC auditory cortex
The presence of both proteins in neurons or cortical terminals at any stage of development raises intriguing questions about its functional importance and has potentially profound implications for the organization and plasticity of cortical circuitry. First, why should VGluT1 and VGluT2 transporters ever be co-localized, and under what circumstances are expression levels up- or down-regulated? Clues may be found by returning to the study by De Gois et al. (2005), in which expression levels of VGluT1, VGluT2, and VGAT varied dynamically with pharmacological modulation of the local excitatory and inhibitory environment. Treatment of cultures with bicuculline (BIC) or gabazine over 48 h led to decreased expression of VGluT1 mRNA (in somata) and protein (in terminals), but increases in VGAT and VGluT2. Conversely, application of tetrodotoxin (TTX) increased VGluT1 levels, but decreased VGAT and VGluT2. These findings indicate that prolonged changes in activity levels lead to bidirectional and opposite regulation of VGluT1 and VGluT2/VGAT. The authors suggested that the numbers of VGluT1, VGluT2, or VGAT molecules within a terminal are a critical factor in regulating vesicle filling with glutamate or GABA. Although it has not been determined whether VGluT1 and VGluT2 proteins are located on the same or different populations of synaptic vesicles, the increase in VGluT2 expression after BIC treatment may compensate or substitute for the reduction in VGluT1. Because co-expression occurs in only a subpopulation of terminals, VGluT2 would be selectively increased in a subset of terminals after prolonged activity reduction. This shift in the VGluT1/VGluT2 balance could prevent a reduction in glutamate release and/or increase glutamate release probabilities from these terminals (Edwards 2007; Erickson et al. 2006; Fei et al. 2008; Fremeau et al. 2004; Omote et al. 2011; Takamori et al. 2006; Wilson et al. 2005; Wojcik et al. 2004). Thus, it appears that VGluT1, VGluT2, and VGAT levels can be dynamically altered by the experimental manipulation of activity and also change naturally during development. These results imply that dual expression confers important functionality in both developing and mature animals, and that activity-dependent mechanisms regulate the likelihood of co-expression. For example, overall VGluT2 levels—as well as the probability of co-expression with VGluT1—could be increased under conditions of reduced sensory input occurring either early in development, when cochlear thresholds are still immature, or later in life as a consequence of hearing loss. These presynaptic changes are but a few of the many pre- and postsynaptic homeostatic mechanisms of plasticity that alter the synaptic strength of excitatory and inhibitory circuits, suggesting the need for further study (Coleman et al. 2010; De Gois et al. 2005; Dorrn et al. 2010; Lazarevic et al. 2013; Nahmani and Erisir 2005; Sun et al. 2010; Turrigiano 2012; Wang and Maffei 2014; Wilson et al. 2005; Zhang et al. 2011).
Second, what does the apparent absence of significant co-expression of VGluT1 and VGluT2 transporter proteins in axon terminals in normal, mature brains suggest about the organization of TC and CC circuitry in the auditory forebrain? Although VGluT1 and VGluT2 transcripts were commonly co-localized in neurons located in supragranular layers of A1 and principal divisions of the MGB, we did not find evidence that these transporter proteins were co-localized in presumptive terminals of any A1 layer or MGB division at any age. It remains possible that our methods were unable to detect low levels of immunoreactivity in puncta, but widespread or significant co-expression can be ruled out, as the resolution of confocal microscopy is sufficient to reveal co-localization at those levels (Samano et al. 2006). Thus, for A1, the present results (combined with the studies reviewed above) favor the conclusion that if VGluT1 transporter proteins are present in TC axon terminals at significant levels, then VGluT1 and VGluT2 are usually sorted to different axon terminals, in which case they would not be distinguishable from VGluT1-ir CC puncta by immunohistochemistry alone. That is, VGluT1-ir CC and TC terminals could be interspersed in any layer in which TC projections terminate. Similarly, if VGluT2 transporters are normally contained in the axon terminals of any cortical neurons, then they are present at very low levels, or sorted to different terminals than VGluT1, or both. Based on retrograde tracing studies in rat auditory cortex, Storace et al. (2012) found evidence supporting differential expression or co-expression of VGluT1 and VGluT2 transporters in the projections from MGB to different AC areas. Although the present findings were not consistent with significant terminal co-localization in TC projections to A1, the possibility that the transporters are sorted to different terminal populations remains open. Definitive answers may require higher-resolution inspection of terminals in different layers of AC areas that can reliably detect low levels of one or both transporters (e.g., electron microscopy of double-labeled tissue sections).
Several hypothetical configurations are illustrated for consideration in Fig. 26, based on growing evidence that neurons in several brain regions can synthesize and segregate neurotransmitters, their synthesizing enzymes, or associated vesicular transporters to different populations of synaptic vesicles in the same terminals, or sort these molecules to different axon processes (El Mestikawy et al. 2011; Samano et al. 2012; Vaaga et al. 2014). This makes it possible for terminals to co-release (or co-transmit) neurotransmitters onto the same or different targets (Saunders et al. 2015). In addition, the sorting of these molecules is also subject to plasticity (see Samano et al. 2012), which may also contribute to observations of activity-dependent changes in expression levels (De Gois et al. 2005). While presently unknown for TC and CC projections in the auditory forebrain, these findings suggest that some neurons may possess the machinery needed to route transmitters and other molecules to different terminal processes in a dynamic manner.
Although the potential significance of these structural details may not be immediately obvious, they have potentially profound implications for the nature of excita-tory neurotransmission in TC and CC circuits. VGluT2 has been associated with a higher probability of glutamate release than VGluT1 in some brain regions (Blakely and Edwards 2012; Hnasko and Edwards 2012; Kaneko and Fujiyama 2002; Santos et al. 2009; Varoqui et al. 2002). In addition, the number of transporters located on each vesicle has an impact on vesicle filling, quantal size, and the amount of neurotransmitter released (Blakely and Edwards 2012; Edwards 2007; Erickson et al. 2006; Fei et al. 2008; Hnasko and Edwards 2012; Omote et al. 2011; Santos et al. 2009; Takamori et al. 2006; Wilson et al. 2005; Wojcik et al. 2004). Given these differences in the synaptic properties associated with VGluT1 and VGluT2, rather different influences could be conferred upon a variety of postsynaptic targets. For example, if VGluT1 and VGluT2 were segregated to different terminals, their impact on postsynaptic activity in a location would likely be different than if the transporters are co-localized to some or all of those terminals. In addition, since the branches of TC axons end in terminals that are distributed over layers 1–6 in different concentrations, VGluT1 and VGluT2 may also be differentially sorted to (or co-localized within) terminals in a laminar-specific and even cell-specific manner. Such details are currently unknown, but many configurations are possible, each associated with different functional outcomes (see Storace et al. 2012). Further, since transporter expression levels and terminal sorting may be altered through activity-dependent regulation, synaptic properties could shift with changes in the expression of VGluT1 and VGluT2. Thus, while the activity of a TC terminal that contains low levels of VGluT1 and high levels of VGluT2 might not be functionally distinguishable from one in which VGluT1 is entirely absent, the relative levels of either transporter are subject to plastic modifications that could lead to functionally significant changes in that terminal. Clearly, this is fertile ground for further exploration.
Morphological changes in VGluT2-ir puncta in the MGv
One of the more interesting and novel findings of the present study was that the decrease in VGluT2 puncta density after about P11 was accompanied by a substantial increase in puncta size in the MGv, but not MGd. The time course of these morphological changes was closely tied to the onset of hearing, suggesting the involvement of activity-dependent mechanisms. Although this has not been previously described in the MGB, comparable and even more dramatic changes in synaptic morphology associated with the onset of hearing or hearing loss have been identified in the auditory brainstem after the onset of hearing (e.g., endbulb and calyx of of Held) (Yu and Goodrich 2014). The collective findings indicate that acoustic stimulation is required for these morphological changes to develop during a critical period of maturation and that these changes are necessary to support the physiological demands in these circuits (i.e., rapid and reliable synaptic transmission at high rates). The molecular mechanisms responsible are still being worked out.
In addition to the auditory brainstem, comparable changes in VGluT2 terminal density and morphology have also been observed in the thalamic relay nuclei of other sensory systems. Nakamura et al. (2005) found that decreased VGluT2 density with age in the ventroposterior nucleus (VP) was concurrent with a visible increase in puncta size. Although the MGB was not specifically mentioned, low-power images of VGluT2-ir (their figures 2 and 8) suggest that immunoreactivity was evenly and densely distributed in the MGB at P7and then declined thereafter, accompanied by the appearance of clusters of larger terminals by P14. In the dLGN, Bickford et al. (2010) studied axon terminal development of mice at P7, P14, and adult. At P7, the terminals were uniformly small in size and more numerous. By P14 (onset of vision is P12– P14), the terminals were fewer in number and ranged more widely in size from small to large. These patterns were stable through adulthood. Singh et al. (2012) studied GAD67 and VGluT2 expression in the dLGN from P3 to adulthood in mice. Their results were nearly identical to our MGv findings. VGluT2-ir terminal density in the dLGN dropped with age, and terminal size increased. GAD67-ir terminal density also increased, but the size remained constant. In that study, fibroblast growth factor 22 was identified as a factor contributing to the formation and maturation of the VGluT2 terminals. Although we did not specifically analyze puncta size in LGN and VP, the development of large VGluT2-ir terminals can be clearly seen in our image library that shows immunoreactivity from P7 to adult across the rostral–caudal range of the mouse brain (see Supplementary Figure 26).
These morphological changes are linked to specific activity-dependent synaptic refinements in these systems. Before eye opening around P12, up to 20 retinal ganglion cell (RGC) axons make synaptic contact with each LGN neuron. Within about 2 weeks, the number of contacts declines to between one and three, but these synapses are some 50 times stronger (Chen and Regehr 2000; Jaubert-Miazza et al. 2005). This pruning and synaptic strengthening is inhibited by TTX blockade of retinal activity (Hooks and Chen 2006). In the medial ventroposterior nucleus (VPM), a similar series of events has been observed (Arsenault and Zhang 2006; Wang and Zhang 2008). At P7–P9, about eight lemniscal axons innervate each VPM neuron. This number decreases through P16– P17, when most neurons are innervated by only one or two lemniscal neurons. This ratio is stable thereafter. As in the dLGN, synapse elimination is accompanied by increased strength of the remaining synapses. Sensory deprivation by whisker plucking at this age interferes with synapse elimination and the normal increase in synaptic strength. Comparable anatomical studies have not been conducted in the MGB, but analogous processes are likely to be found. Thus, the available data suggest that the transition from smaller to larger VGluT2-ir terminals is a feature common to the principal thalamic sensory nuclei and is closely tied to the onset of effective sensory stimulation in each system.
Although activity-dependent mechanisms are likely responsible for the morphological changes in MGv, it is not clear why VGluT2-ir terminals in MGd and VGluT1-ir terminals in either division are unaffected. In part, the differences may reflect basic organizational features of the pathways involved. The MGv and MGd have distinct connections, cellular morphology, neurochemical profiles, and neurophysiological response properties (Bartlett 2013; Lee 2013). The MGv primarily receives subcortical inputs from VGluT2+ neurons in the ipsilateral central nucleus of the inferior colliculus (ICc). These tT terminations range from small to rather large in size (Bartlett and Smith 1999; Bartlett et al. 2000; Pallas and Sur 1994). Subcortical input sources to the MGd are more diffuse and include the dorsal and external (lateral) divisions of the IC, tegmentum, and sagulum. The tT terminations in the MGd are also VGluT2-ir, but tend to be smaller in size, on average, compared to those that target the MGv (Bartlett et al. 2000). Overlaid on the ascending projections to each division are dense CT inputs from VGluT1+ neurons in cortex, which also exhibit distinct patterns in the MGv and MGd. Neurons in L6 of A1 project to the MGv and MGd, ending in small terminals on distal dendrites (Bajo et al. 1995; Bartlett et al. 2000; Llano and Sherman 2008; Ojima 1994; Rouiller and Welker 1991; Smith et al. 2007). By contrast, layer 5 CT projections are more sparse, feature large terminal boutons, and predominantly target the MGd. Thus, it appears that the morphological changes observed in the MGv are limited to the ICc–MGv projection system, in support of the physiological roles mediated by that neuronal subpopulation.
Delayed formation of perisomatic axon terminals
One additional age-related change of interest in our data was an increase in either the numbers or visibility of VGAT and VGluT1-ir puncta in close proximity to cell somata in L2–6. The clustering of VGAT, and a few VGluT1, puncta around somatic profiles began at around P14, especially in L5/6, and their prevalence increased slowly through P21 into adulthood. We found no clear examples of such terminals in our material at P7 or P11 (Figs. 19, 20; Supp. Figs. S2–3, S7–8, S12–13, S19–20), suggesting that they formed, or became immunoreactive, after the onset of hearing.
Perisomatic GABAergic terminals are an established feature of the inhibitory network in the cortex (DeFelipe 1997; Freund et al. 1983; Kisvarday 1992; Somogyi et al. 1983a, b; Tamas et al. 1997) and typical of fast-spiking parvalbumin expressing basket cells. Thus, it is of interest that this particular type of GABAergic contact does not become prominent in juvenile cortex until after VGAT-ir puncta are already rather abundant in the neuropil (Packer et al. 2013). The developmental trajectory appears to differ between sensory regions. In S1 and hippocampus of mice, perisomatic VGAT contacts form early around P7, with continued maturation through about P21 (Boulland and Chaudhry 2012; Takayama and Inoue 2010). In the primary visual cortex of mice, Chattopadhyaya et al. (2004) found that the numbers of GABAergic perisomatic synapses increased over an extended period of postnatal development that began after eye opening at P14 and continued until about the fifth postnatal week. In addition, the proliferation could be slowed by visual deprivation through about P28, which parallels the onset and closure of the critical period for ocular dominance plasticity (Gordon and Stryker 1996). The anatomical sequence in the visual cortex is more consistent with our findings in A1, as we began to notice perisomatic VGAT-ir terminals only after P11–13, when the ear canals open. Given that proliferation of these terminals does not commence until after the onset of hearing (vision), the process appears to be triggered by experience, and may therefore play some role in the opening and closing of critical periods. GABA-mediated inhibition has a major impact on auditory response properties, such as frequency tuning and temporal precision (Chang et al. 2005; de Villers-Sidani et al. 2008; Dorrn et al. 2010; Tan and Wehr 2009; Tan et al. 2004; Wehr and Zador 2003; Wu et al. 2006, 2008; Zhang et al. 2003), and so it is expected that maturation of these physiological features would be correlated with structural changes of this kind.
In contrast to GABAergic puncta, glutamatergic terminals do not commonly make synaptic contacts with somata or proximal dendrites, although they may be tightly packed around somata in the neuropil (DeFelipe 1997; Freund et al. 1983; Kisvarday 1992; Somogyi et al. 1983a, b; Tamas et al. 1997). We found no examples of perisomatic puncta that were VGluT2-ir, consistent with recent observations in mouse A1, where TC contacts were primarily clustered on proximal dendrites close to the soma, but not in contact with it (Richardson et al. 2009). However, we did found that numerous VGluT1-ir terminals in close proximity to neurons with pyramidal cell profiles had the appearance of perisomatic contacts, sometimes positioned directly next to perisomatic VGAT-ir puncta. Fortunately, this feature has been explored previously and may serve to guide interpretation of our data. Alonso-Nanclares et al. (2004) studied such VGluT1-ir terminals around neurons in L2/3 and L5 in light and electron microscope preparations from the parietal cortex in adult rats. They found that these terminals were apposed to the cell membrane and frequently adjacent to symmetric (inhibitory) axon terminals that made synaptic contact with the somatic membrane. However, the VGluT1 terminals made asymmetric contacts only with dendritic shafts and spines, and not somata. The functional significance of this unusual arrangement is not known, but the authors suggested that glutamate spillover from the VGluT1 synapse may influence GABAergic activity locally. In any case, the appearance of terminals in contact with or in close proximity to the somatic membrane appears to be a maturational feature that occurs in parallel for both VGluT1 and VGAT, and may contribute to balanced excitation and inhibition in these developing circuits (Dorrn et al. 2010; Sun et al. 2010).
Comments on the precocious maturation of layer 1
Much remains to be learned about the roles of TC and CC inputs to L1, and whether those functions change during postnatal development. Given that VGluT2 and VGAT immunoreactive puncta were prominent in L1a by P7, their impact on activity in A1 prior to the onset of hearing is likely significant. Even VGluT1, which had relatively low expression in L2–4 at P7, formed a significant band of terminals in L1a/b at P7. Although L1 contains few neurons, all GABAergic, it is a prominent site of convergence for the TC, CC, and subcortical projections of neurons from local and distant sources. VGluT2-ir terminals in A1, which are concentrated in L1a, arise from the MGv, but also include inputs from secondary and multisensory thalamic nuclei, such as the MGm, Sg, or posterior lateral/medial nuclei (MGd also projects to L1, but of non-primary areas). VGluT1-ir terminals are presumed to reflect CC inputs, although it is not known whether VGluT1 transporters may be present in TC projections of MGB neurons that express VGluT1 and VGluT2 mRNA (see “Co-expression of VGluT1 and VGluT2”). In their study of A1 in cats, Huang and Winer (2000) noted that some of the thickest TC axons were found after injections of the MGm. Upon reaching L1, these axons (up to 6 μm in diameter) ran horizontally in LIa over long distances and appeared to come from the large magnocellular neurons unique to the MGm (Mitani et al. 1987; Niimi et al. 1984). These may correspond to the same class of large-diameter axons found in the macaque monkey, which were found to arise from calbindin immunoreactive neurons in the MGm (Hashikawa et al. 1995). Locally, within columns, the axons of subpopulations of VGluT1+ cells in L2–4 reach L1 (Callaway 2002; Ojima et al. 1991; Wallace et al. 1991b; Wozny and Williams 2011) and blend with convergent CC inputs from both hemispheres. Among the targets of the CC and TC terminations are the apical dendrites of pyramidal and interneuron subpopulations whose somata are located in L2–5 (Code and Winer 1985; Feldmeyer et al. 2005; Lubke and Feldmeyer 2007; Markram 1997; Mitani et al. 1985; Prieto and Winer 1999; Sousa-Pinto 1973; Winer 1984, 1985). The apical dendrites of L2/3 pyramidal neurons are concentrated in L1a, whereas the tufts of L5 neurons are evenly distributed throughout L1 (Smith et al. 2010; Vogt and Peters 1981). Finally, over-lying this dense matrix are VGAT-ir terminals concentrated in L1a, as well as dopaminergic, noradrenergic, cholinergic and serotonergic projections to L1a/b (Avendano et al. 1996; Campbell et al. 1987; Edeline 2003; Mesulam and Geula 1992; Wallace et al. 1991a). Their influences on activity are poorly understood, but likely to be significant.
The impressive blend of inputs to L1 from multiple sources is substantial in scope, even from an early stage of development (Konig et al. 1975), and therefore positioned to modulate the activity of excitatory and inhibitory neurons from L1–5. As an example, Cruikshank et al. (2012) found that TC inputs to L1 could transmit synaptic signals sufficient to drive interneurons in L1, with associated inhibitory effects on L2/3 pyramidal neurons. The effect of a given TC input to L1, therefore, can have significant impact on activity well beyond L1. In addition, Ma et al. (2013) found that the numbers of different neuronal sub-types increased from P2 to P14 and that the response properties of those neurons evolved substantially over this period. With respect to hearing, it is reasonable to consider how the impact of TC and CC inputs to L1 differs before and after the onset of hearing, as the driving and modula-tory influences appear to vary considerably across this period.
Maturation of vesicular transporters and the onset of hearing
Neurophysiological studies in altricial mammals such as mice, rats, and gerbils have shown that many basic response features are adult-like in A1 at the onset of hearing, presumably reflecting intrinsically generated genetic and electrical signals (Gurung and Fritzsch 2004; Tritsch et al. 2010). These include tonotopy (de Villers-Sidani et al. 2007), binaural matching of frequency tuning (Polley et al. 2013), and the laminar and topographic organization of feedforward TC functional response profiles (Barkat et al. 2011). One notable exception can be found in the cortical sensitivity to airborne tone bursts, which increases 100-fold (i.e., 40 dB) between P11 and P14 in accordance with the commensurate maturation of ear canal patency and cochlear biomechanics that also unfold during this brief period (Adise et al. 2014; Mikaelian et al. 1965; Mills and Rubel 1998). However, the dynamic expression levels of VGluT1, VGluT2, and VGAT in conjunction with changes in the intrinsic membrane properties (Metherate and Cruikshank 1999; Oswald and Reyes 2011) and ligand-gated receptor composition (Hsieh et al. 2002; Venkataraman and Bartlett 2013, 2014) in the auditory forebrain between P11 and P14 suggest that the rapid maturation of sound-evoked responses measured in A1 and MGB of intact animals during the first days of hearing may reflect a combination of peripheral and central changes.
Second-order auditory representational features, which are extracted either from the monaural signal over time (e.g., frequency modulation rate or amplitude modulation depth) or from dichotic features (e.g., interaural level difference) mature over a comparatively protracted time course in A1 (Brown and Harrison 2010; Carrasco et al. 2013; Insanally et al. 2010; Polley et al. 2013; Razak and Fuzessery 2007; Rosen et al. 2010; Sarro et al. 2011; Trujillo et al. 2013). Here too, the ongoing maturation of VGluT1, VGluT2, and VGAT considered alongside the delayed development of cortical inhibitory tone (Chang et al. 2005; Dorrn et al. 2010; Oswald and Reyes 2011) and spine protrusion density (Barkat et al. 2011; Schachtele et al. 2011) may provide a biological correlate to support these features. Looking forward, one major challenge for future research will be to understand whether and how changes in these critical biomarkers of auditory forebrain development mechanistically relate to the postnatal maturation of sound features that reach an adult-like state by the end of the second postnatal week (e.g., tonotopy, binaural frequency matching, threshold) versus those that do not reach maturity until the third postnatal week or longer (e.g., frequency modulation direction tuning, amplitude modulation depth encoding, or ipsilateral interaural level difference sensitivity).
Synaptic plasticity, critical periods, and vesicular transporters
The maturation of pre- and postsynaptic signaling machinery supports the progressive refinement of sensory feature representations but also establishes the timing of critical periods during which experience can shape the organization of functional circuits in the sensory cortex (Barkat et al. 2011; Insanally et al. 2009; Polley et al. 2013; Popescu and Polley 2010; Speechley et al. 2007; Takesian and Hensch 2013). Prolonged deprivation of afferent activity can lead to homeostatic adjustments in synaptic strength that serve to balance excitation and inhibition in affected circuits (Maffei et al. 2012). The adjustments are mediated by local structural changes in both pre- and postsynaptic structures, involving modifications at the transcript and protein levels (Batish et al. 2012; Cajigas et al. 2010, 2012; De Gois et al. 2005; Desai et al. 2002; Edwards 2007; Erickson et al. 2006; Holt and Schuman 2013; Lazarevic et al. 2011, 2013; Muller and Davis 2012; Perez-Otano and Ehlers 2005; Turrigiano and Nelson 2004; Vitureira et al. 2012; Wilson et al. 2005).
It is also well established that structural remodeling of axons, spines, and synapses is related to various forms of plasticity in cortical circuits (Bosch et al. 2014; Butz et al. 2009; De Roo et al. 2008; Gogolla et al. 2007; Holtmaat and Svoboda 2009; Yamahachi et al. 2009; Zito et al. 2009). The gross changes in axon terminal density and size that we observed in A1 and the MGB from P7 to adulthood could provide a small window into the numerous structural changes taking place at the synaptic level during this period. As an example, Coleman et al. (2010) studied modifications of VGluT2-ir thalamocortical synapses in L4 of the mouse visual cortex (V1). Synapse density and size were reduced after brief monocular deprivation lasting 3 days, but recovered by 7 days. The synaptic changes peaked at 3 days, corresponding to maximal depression of responses to the deprived eye (Blais et al. 2008). In contrast, monocular inactivation with TTX for 17–22 h had no effect on VGluT2-ir synapses. These results suggest that the diminished retinal activity in the deprived animals had a different impact on TC synapses compared to the absence of retinal activity in the TTX group, and that the structural changes varied with time (Nahmani and Erisir 2005).
Although presynaptic mechanisms are more often associated with short-term synaptic dynamics (Blackman et al. 2013; Fioravante and Regehr 2011), the Coleman study also draws attention to the possible involvement of presynaptic structures in longer-term homeostatic changes. In the presynaptic realm, there is tremendous heterogeneity in the proteins that regulate neurotransmitter release probability, which is an important determinant of synaptic strength (Cajigas et al. 2012; Lazarevic et al. 2013; Turrigiano 2012). Some of the mechanisms involved contribute to balancing glutamatergic and GABAergic signaling at the synapse (Burrone et al. 2002; Dickman et al. 2012; Thiagarajan et al. 2005; Tokuoka and Goda 2008; Wierenga et al. 2006). A variety of evidence indicates that the vesicular transporter proteins may be involved in scaling presynaptic GABA or glutamate storage and release through alterations in their levels on vesicles, as discussed earlier (De Gois et al. 2005; Erickson et al. 2006; Wilson et al. 2005; Wojcik et al. 2004).
With respect to mechanisms of plasticity, these results are intriguing since they imply that the different types of vesicular transport proteins are regulated independently, even in response to identical changes in activity. These differences, in turn, may reflect specific interactions with other presynaptic proteins associated with neurotransmitter release. Bragina et al. (2007, 2010), for example, studied co-expression of VGluT1, VGluT2, and VGAT with several protein families involved in vesicle fusion and exocytosis, trafficking, and neurotransmitter release (e.g., synapsin, synaptophysin, synaptosomal-associated protein, synaptogyrin, vesicle-associated membrane protein, and syntaxin) (Bragina et al. 2007, 2010). The degree to which each of these proteins co-localized with VGluT1, VGluT2, and VGAT was distinctive. Thus, while there is significant heterogeneity in the molecular machinery that governs neurotransmitter release presynaptically, there is also specificity. Further, to the extent that the co-expression of these proteins is constant, the expression of VGluT1, VGluT2, and VGAT could be a useful index of associated structural modifications that occur during development, aging, and pathology.
Correspondence between gene and protein expression assays
In the present study, VGluT1, VGluT2, and VGAT mRNA levels derived from ISH and RNAseq assays were comparable across brain areas and developmental ages. In some instances (e.g., VGluT2 in MGv, VGAT in A1), however, gene and protein expression trajectories were not always well correlated. One factor that could account for these differences is that the vesicular transporter proteins and transcripts are typically localized in separate neuronal compartments and often different brain regions (e.g., cortex vs. thalamus). As summarized in Figs. 25 and 26, the mRNA transcripts of these genes are largely confined to the somatic cytoplasm, whereas their proteins are trafficked to presynaptic axon terminals, which may be local or distant from the soma (Chaudhry et al. 1998; Kaneko et al. 2002; Melone et al. 2005; Minelli et al. 2003a, b). Accordingly, neurons synthesizing VGluT1 mRNA predominate in cortical areas, and the transporter protein can be found in the axon terminals of their CC, CT, and Ct targets (Rovo et al. 2012). VGluT2+ neurons are concentrated in subcortical brain areas, and the transporter protein is expressed in their thalamocortical (TC), tectothalamic (tT), and subcortical projections. VGAT mRNA appears to be expressed by all GABAergic neurons in cortical and subcortical regions, whereas expression of the protein in axon terminals may reflect both local and distant projections (Chaudhry et al. 1998; Dumoulin et al. 1999; Frahm et al. 2006; Henny and Jones 2006; Ito et al. 2011; Minelli et al. 2003a; Wang et al. Wang et al. 2009). Thus, differential localization of mRNA transcripts and their corresponding proteins is one reason why gene and protein expression levels may not be correlated within a given brain region and must be factored into the interpretation, as above.
Secondly, differing trajectories for gene and protein expression suggest that transcription and translation are regulated differently during postnatal development and in a manner that is also region specific. Numerous recent studies have demonstrated rather convincingly that gene and protein expression levels are only modestly correlated, implying that indices of mRNA abundance do not reliably predict protein concentrations (Ghazalpour et al. 2011; Nie et al. 2007; Park et al. 2009; Vogel and Marcotte 2012). Although methodological factors have been noted, these poor correlations are linked to fundamental differences in the regulation of transcription and translation for many genes. In the case of VGluT1 and VGluT2, for example, their transcription and translation can be differentially regulated by other proteins (e.g., BDNF and MeCP2) in a level-dependent manner that varies with postnatal age (Melo et al. 2013; Nguyen et al. 2012) (see Supplementary Fig. S28). In addition, epigenetic factors and noncoding RNA can also regulate gene and protein expression differentially through multiple mechanisms that may change during development (Earls et al. 2014; Elramah et al. 2014). This variability underscores the importance of a multimodal approach to profiling gene and protein expression in neural circuits to account for such differences and more accurately describe their structural elements.
Species differences in vesicular transporter expression
In comparing the results of the present study to our prior studies of the auditory and visual systems in primates (Balaram et al. 2011; Balaram et al. 2013; Hackett et al. 2011b), we found significant differences in the expression of both VGluT1 and VGluT2. In sensory cortex of the adult monkey, VGluT2 mRNA levels are present at moderate levels in most pyramidal neurons in L2–4, punctuated by strong expression in larger pyramidal neurons of L3b. Expression in L5/6 is very weak in primates. In adult rodents, VGluT2 levels are relatively low across layers. In the MGB and LGN, VGluT1 mRNA is strongly expressed in mice, but not in primates. Conversely, VGAT is expressed in MGB of primates, but not rodents. We have noticed species differences for several other genes in preliminary studies, in agreement with a growing number of reports (Mashiko et al. 2012; Nehme et al. 2012; Van der Zee and Keijser 2011; Watakabe 2009; Zeng et al. 2012). Detailed documentation of the differences in gene expression between model species is clearly needed, but in its absence we must be vigilant to consider the potential for differences in the interpretation gene and protein profiling results.
Summary and future directions
The data contained in this study establish a broad baseline for further study of the changes in excitatory and inhibitory circuitry that occur during postnatal development in the auditory forebrain. The data also draw attention to the presynaptic machinery involved in neurotransmission, as well as homeostatic mechanisms that operate in development, aging, and hearing loss. Building on this foundation, future studies could reveal additional features that are likely to impact auditory-related activity during maturation.
First, given that gene and protein expression levels may be differentially regulated in both developing and adult animals, it will be important to identify and characterize the factors involved. Among these factors are the non-coding regulatory sequences (e.g., micro-RNA, long non-coding RNA) associated with the expression of each of these genes, as they would expand our understanding of the factors that govern changes in their expression during postnatal development before and after the onset of hearing (Elramah et al. 2014; Gao 2010; Olde Loohuis et al. 2012; Schratt 2009). Our current models of developmental plasticity and activity-dependent alterations in sensory processing lack these details, but would certainly be improved by their inclusion.
Second, in studies of developing sensory cortex, much emphasis has been placed on the maturation of pre- and postsynaptic glutamatergic (Barth and Malenka 2001; Blundon et al. 2011; Chun et al. 2013; Jiang et al. 2006; Lu et al. 2001; Walz et al. 2010; Wang et al. 2012b; Yanagisawa et al. 2004) and GABA receptors (Ben-Ari et al. 1994, 2012; Bosman et al. 2002; Davis et al. 2000; Fritschy et al. 1994; Heinen et al. 2004; Hensch 2005; Hornung and Fritschy 1996; Huang 2009; Laurie et al. 1992; Maffei and Turrigiano 2008; Mohler 2006; Peden et al. 2008; Takesian et al. 2010). Changes in the amount and subunit composition of ligand-gated receptors are thought to be causally related to the timing of developmental windows that govern the translation of afferent activity patterns into long-term modification of synapses and representational maps (Kotak et al. 2013; Takesian et al. 2010; Venkataraman and Bartlett 2013). However, the developmental trajectories of all receptor types have not been fully documented in the auditory forebrain. The present findings indicate that their developmental trajectories will vary by brain region, cortical layer, and even cell type (see also Hackett et al. 2015). In addition, an important complement to the glutamatergic and GABAergic networks are the neuromodulatory systems (cholinergic, noradrenergic, and monoaminergic), whose projections converge in A1 and MGB from various sources. Integration of these patterns would provide a useful synthesis of the changing relationships between excitatory, inhibitory, and modulatory systems in adult and developing animals.
A third line of inquiry concerns the apparent absence of VGluT1 and VGluT2 co-localization in TC and CC terminals from P7 to adult. The two main possibilities are: (1) VGluT1 and VGluT2 are expressed at nominal levels in TC and CC projections, respectively (or not at all); or (2) VGluT1 and VGluT2 are sorted to different axon terminals (Fig. 26). Many configurations are possible. Given their importance for understanding normal patterns of excitatory neurotransmission in auditory cortex, a thorough understanding of these relationships should be pursued. A closely related subject is that glutamate (and GABA) transporter expression and trafficking are subject to plastic modification and may therefore be linked to homeostatic adjustments at the synaptic level. It would be of interest to document whether and how expression (or co-expression) and trafficking may be altered, and whether critical period windows are modified by plastic changes in the magnitude and sites of expression.
A fourth topic concerns the morphological changes in axon terminals that accompanied the onset of hearing. In A1, perisomatic VGAT and VGluT1 terminals became prominent after the onset of hearing. What functionality is conferred to cortical pyramidal cells as these terminals coalesce around the action potential initiation zone? What is the nature of the interaction between the VGAT terminals that form somatic synapses and the closely apposed VGluT1 terminals that do not? In MGB, we observed changes in the numbers and size of VGluT2 terminals during the period of pruning and morphological change between P7 and adulthood. Does the increase in VGluT2 terminal size in MGv follow a decrease in the number of terminals that synapse with MGv neurons, as in the the VPM and LGN? What are the molecular mechanisms involved, and how might these events be impacted by alterations in auditory input? In addition, why did terminal size remain unchanged in the MGd and how might that impact the activity of MGd neurons as a function of age?
Fifth, the laminar patterns of VGluT1 maturation in A1 are of some interest, as expression in the middle layers (L2–4) lagged behind L1 and L5/6. The VGluT1 projections to each layer may reflect several different input sources, including local and distant cortical areas, inter-hemispheric projections, and even thalamic sources. Inputs to the middle layers also include subplate neurons, which are largely depleted from primary sensory areas by the onset of hearing (Kanold 2004; Kanold and Luhmann 2010; Viswanathan et al. 2012). Presumably, these are VGluT1-ir but protein expression levels in the middle layers are very low before P14. A related question concerns the nature of the relationship between TC inputs and CC inputs as they mature. TC inputs to L1a and L4 are established well before hearing onset, but the CC projections develop later. How do those differential trajectories impact activity in A1?
Finally, the present study demonstrates that a multi-modal sequencing-driven approach to neurochemical profiling is both efficient and highly advantageous. On the front end, RNA sequencing is a powerful tool that can be used to reveal all of the genes that are highly expressed in a brain region or cell population of interest, or those that may be changing with time, experience, or other manipulation (see Hackett et al. 2015). Sequencing also provides a means to explore relationships among a set of functionally related genes, as well as make testable predictions about their regulation by other coding and non-coding genes or proteins. In that sense, the present study supports the viability and utility of a sequencing-driven approach to transcriptomic and proteomic profiling of neural circuitry and architecture. Upon identification of target genes from the sequencing data, targeted ISH and IHC can be subsequently employed to reveal their spatial expression patterns in intact tissue sections, where natural anatomical features are preserved. Multiplexed ISH and IHC using fluorescent probes permit detailed evaluation of co-expression and co-localization of several genes and proteins in cells or regions of interest. Documentation of the subpopulations that express one or more genes (or proteins) is an important endeavor in the neurochemical profiling of neuronal circuitry in auditory pathways, with implications for functional studies where genetic specificity is important (e.g., optogenetics). Together, this type of deep structural profiling provides a unique window into the functional subsystems that comprise a given pathway and can foster improved hypotheses about function.
Supplementary Material
Acknowledgments
Special thanks to Tia Hughes and Cara Sutcliffe in the VANTAGE core at Vanderbilt University for expert assistance with RNA isolation and sample quality assessment; Dr. Holli Hutcheson Dilks in the VANTAGE core for design and supervision of RNA sequencing; Dr. Yan Guo in the VANGARD core for expertise and implementation of bioinformatics analyses. The authors gratefully acknowledge the support of NIH/NIDCD grants K18 DC012527 to T.A.H. and R01 DC009836 to D.P.
Abbreviations
- A1
Primary auditory cortex, area 1
- AC
Auditory cortex
- AuD
Auditory cortex, dorsal area
- AuV
Auditory cortex, ventral area
- CC
Corticocortical connections
- CT
Corticothalamic connections
- Ct
Corticotectal connections
- D
Medial geniculate body, dorsal division
- DAPI
4′,6-Diamidino-2-phenylindole
- DC
Inferior colliculus, dorsal cortex
- dLGN
Lateral geniculate nucleus, dorsal division
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IC
Inferior colliculus
- ICc
Inferior colliculus, central nucleus
- IHC
Immunohistochemistry
- ISH
In situ hybridization
- Hip
Hippocampus
- LC
Inferior colliculus, lateral cortex
- M
Medial geniculate body, medial division
- MGB
Medial geniculate body
- MGd
Medial geniculate body, dorsal division
- MGm
Medial geniculate body, medial division
- MGv
Medial geniculate body, ventral division
- NeuN
Neuron-specific RNA-binding protein, Fox-3
- PAG
Periaqueductal gray
- PP
Thalamus, peripeduncular nucleus
- rf
Rhinal fissure
- Rt
Reticular nucleus
- RT
Reticulothalamic connections
- SC
Superior colliculus
- Sg
Thalamus, suprageniculate nucleus
- TC
Thalamocortical connections
- TRN
Thalamic reticular nucleus
- tT
Tectothalamic connections
- TeA
Temporal cortex area A
- V
Medial geniculate body, ventral division
- VGluT1
Vesicular glutamate transporter 1
- VGluT2
Vesicular glutamate transporter 2
- VGAT
Vesicular GABA/glycine transporter
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-015-1062-3) contains supplementary material, which is available to authorized users.
References
- Adise S, Saliu A, Maldonado N, Khatri V, Cardoso L, Rodriguez-Contreras A. Effect of maternal care on hearing onset induced by developmental changes in the auditory periphery. J Neurosci. 2014;34:4528–4533. doi: 10.1523/JNEUROSCI.4188-13.2014. doi:10.1523/JNEUROSCI.4188-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Nanclares L, Minelli A, Melone M, Edwards RH, Defelipe J, Conti F. Perisomatic glutamatergic axon terminals: a novel feature of cortical synaptology revealed by vesicular glutamate transporter 1 immunostaining. Neuroscience. 2004;123:547–556. doi: 10.1016/j.neuroscience.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2014 doi: 10.1093/bioinformatics/btu638. doi:10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LA, Christianson GB, Linden JF. Mouse auditory cortex differs from visual and somatosensory cortices in the laminar distribution of cytochrome oxidase and acetylcholinesterase. Brain Res. 2009;1252:130–142. doi: 10.1016/j.brainres.2008.11.037. doi:10.1016/j.brainres.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Arellano JI, Guadiana SM, Breunig JJ, Rakic P, Sarkisian MR. Development and distribution of neuronal cilia in mouse neocortex. J Comp Neurol. 2012;520:848–873. doi: 10.1002/cne.22793. doi:10.1002/cne.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault D, Zhang ZW. Developmental remodelling of the lemniscal synapse in the ventral basal thalamus of the mouse. J Physiol. 2006;573:121–132. doi: 10.1113/jphysiol.2006.106542. doi:10.1113/jphysiol.2006.106542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendano C, Umbriaco D, Dykes RW, Descarries L. Acetylcholine innervation of sensory and motor neocortical areas in adult cat: a choline acetyltransferase immunohistochemical study. J Chem Neuroanat. 1996;11:113–130. doi: 10.1016/0891-0618(96)00132-9. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Rouiller EM, Welker E, Clarke S, Villa AE, de Ribaupierre Y, de Ribaupierre F. Morphology and spatial distribution of corticothalamic terminals originating from the cat auditory cortex. Hear Res. 1995;83:161–174. doi: 10.1016/0378-5955(94)00199-z. [DOI] [PubMed] [Google Scholar]
- Balaram P, Hackett T, Kaas JH. VGLUT1 mRNA and protein expression in the visual system of prosimian galagos (Otolemur garnetti). Eye and Brain. 2011;3:81–98. doi: 10.2147/EB.S23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaram P, Hackett TA, Kaas JH. Differential expression of vesicular glutamate transporters 1 and 2 may identify distinct modes of glutamatergic transmission in the macaque visual system. J Chem Neuroanat. 2013;50–51:21–38. doi: 10.1016/j.jchemneu.2013.02.007. doi:10.1016/j.jchemneu.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1194. doi: 10.1038/nn.2882. doi:10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Perez-Manso M, Erro E, Tunon T, Lanciego JL. Expression of the mRNAs encoding for the vesicular glutamate transporters 1 and 2 in the rat thalamus. J Comp Neurol. 2007;501:703–715. doi: 10.1002/cne.21265. [DOI] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. doi:10.1038/85070. [DOI] [PubMed] [Google Scholar]
- Bartlett EL. The organization and physiology of the auditory thalamus and its role in processing acoustic features important for speech perception. Brain Lang. 2013;126:29–48. doi: 10.1016/j.bandl.2013.03.003. doi:10.1016/j.bandl.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Anatomic, intrinsic, and synaptic properties of dorsal and ventral division neurons in rat medial geniculate body. J Neurophysiol. 1999;81:1999–2016. doi: 10.1152/jn.1999.81.5.1999. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Stark JM, Guillery RW, Smith PH. Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body. Neuroscience. 2000;100:811–828. doi: 10.1016/s0306-4522(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Batish M, van den Bogaard P, Kramer FR, Tyagi S. Neuronal mRNAs travel singly into dendrites. Proc Natl Acad Sci USA. 2012;109:4645–4650. doi: 10.1073/pnas.1111226109. doi:10.1073/pnas.1111226109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL. gamma-Aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. doi:10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. doi:10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. doi:10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C, Guido W. Synaptic development of the mouse dorsal lateral geniculate nucleus. J Comp Neurol. 2010;518(5):622–635. doi: 10.1002/cne.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman AV, Abrahamsson T, Costa RP, Lalanne T, Sjostrom PJ. Target-cell-specific short-term plasticity in local circuits. Front Synaptic Neurosci. 2013;5:11. doi: 10.3389/fnsyn.2013.00011. doi:10.3389/fnsyn.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, et al. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci. 2006;26:10407–10419. doi: 10.1523/JNEUROSCI.3257-06.2006. doi:10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais BS, Frenkel MY, Kuindersma SR, Muhammad R, Shouval HZ, Cooper LN, Bear MF. Recovery from monocular deprivation using binocular deprivation. J Neurophysiol. 2008;100:2217–2224. doi: 10.1152/jn.90411.2008. doi:10.1152/jn.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harbor Perspect Biol. 2012 doi: 10.1101/cshperspect.a005595. doi:10.1101/cshperspect.a005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundon JA, Bayazitov IT, Zakharenko SS. Presynaptic gating of postsynaptically expressed plasticity at mature thalamocortical synapses. J Neurosci. 2011;31:16012–16025. doi: 10.1523/JNEUROSCI.3281-11.2011. doi:10.1523/JNEUROSCI.3281-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–459. doi: 10.1016/j.neuron.2014.03.021. doi:10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 2002;545:169–181. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulland JL, Chaudhry FA. Ontogenetic changes in the distribution of the vesicular GABA transporter VGAT correlate with the excitation/inhibition shift of GABA action. Neurochem Int. 2012;61:506–516. doi: 10.1016/j.neuint.2012.03.018. doi:10.1016/j.neuint.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Boulland JL, et al. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. doi:10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- Bragina L, Candiracci C, Barbaresi P, Giovedi S, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex. Neuroscience. 2007;146:1829–1840. doi: 10.1016/j.neuroscience.2007.02.060. doi:10.1016/j.neuroscience.2007.02.060. [DOI] [PubMed] [Google Scholar]
- Bragina L, Giovedi S, Barbaresi P, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex: analysis of synaptogyrin, vesicle-associated membrane protein, and syntaxin. Neuroscience. 2010;165:934–943. doi: 10.1016/j.neuroscience.2009.11.009. doi:10.1016/j.neuroscience.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Brown TA, Harrison RV. Postnatal development of neuronal responses to frequency-modulated tones in chinchilla auditory cortex. Brain Res. 2010;1309:29–39. doi: 10.1016/j.brainres.2009.10.053. doi:10.1016/j.brainres.2009.10.053. [DOI] [PubMed] [Google Scholar]
- Bryant KL, Suwyn C, Reding KM, Smiley JF, Hackett TA, Preuss TM. Evidence for ape and human specializations in geniculostriate projections from VGLUT2 immunohistochemistry. Brain Behav Evol. 2012;80:210–221. doi: 10.1159/000341135. doi:10.1159/000341135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. doi:10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Buttini M, et al. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz M, Worgotter F, van Ooyen A. Activity-dependent structural plasticity. Brain Res Rev. 2009;60:287–305. doi: 10.1016/j.brainresrev.2008.12.023. doi:10.1016/j.brainresrev.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Cajigas IJ, Will T, Schuman EM. Protein homeostasis and synaptic plasticity. EMBO J. 2010;29:2746–2752. doi: 10.1038/emboj.2010.173. doi:10.1038/emboj.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajigas IJ, Tushev G, Will TJ, tom Dieck S, Fuerst N, Schuman EM. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron. 2012;74:453–466. doi: 10.1016/j.neuron.2012.02.036. doi:10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. Cell type specificity of local cortical connections. J Neurocytol. 2002;31:231–237. doi: 10.1023/a:1024165824469. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Lewis DA, Foote SL, Morrison JH. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta-hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987;261:209–220. doi: 10.1002/cne.902610204. doi:10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- Carrasco MM, Trujillo M, Razak K. Development of response selectivity in the mouse auditory cortex. Hear Res. 2013;296:107–120. doi: 10.1016/j.heares.2012.11.020. doi:10.1016/j.heares.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci USA. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. doi:10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. doi:10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Chun S, Bayazitov IT, Blundon JA, Zakharenko SS. Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex. J Neurosci. 2013;33:7345–7357. doi: 10.1523/JNEUROSCI.4500-12.2013. doi:10.1523/JNEUROSCI.4500-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marin O. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci. 2013;16:1199–1210. doi: 10.1038/nn.3485. doi:10.1038/nn.3485. [DOI] [PubMed] [Google Scholar]
- Code RA, Winer JA. Commissural neurons in layer III of cat primary auditory cortex (AI): pyramidal and non-pyramidal cell input. J Comp Neurol. 1985;242:485–510. doi: 10.1002/cne.902420404. [DOI] [PubMed] [Google Scholar]
- Coleman JE, Nahmani M, Gavornik JP, Haslinger R, Heynen AJ, Erisir A, Bear MF. Rapid structural remodeling of thalamocortical synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. J Neurosci. 2010;30:9670–9682. doi: 10.1523/JNEUROSCI.1248-10.2010. doi:10.1523/JNEUROSCI.1248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JW. Organization in the auditory sector of the cat's thalamic reticular nucleus. J Comp Neurol. 1998;390:167–182. [PubMed] [Google Scholar]
- Cruikshank SJ, Killackey HP, Metherate R. Parvalbumin and calbindin are differentially distributed within primary and secondary subregions of the mouse auditory forebrain. Neuroscience. 2001;105:553–569. doi: 10.1016/s0306-4522(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, Elmaleh M, Connors BW. Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci. 2012;32:17813–17823. doi: 10.1523/JNEUROSCI.3231-12.2012. doi:10.1523/JNEUROSCI.3231-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danik M, Cassoly E, Manseau F, Sotty F, Mouginot D, Williams S. Frequent coexpression of the vesicular glutamate transporter 1 and 2 genes, as well as coexpression with genes for choline acetyltransferase or glutamic acid decarboxylase in neurons of rat brain. J Neurosci Res. 2005;81:506–521. doi: 10.1002/jnr.20500. doi:10.1002/jnr.20500. [DOI] [PubMed] [Google Scholar]
- Darnell RB. RNA protein interaction in neurons. Annu Rev Neurosci. 2013;36:243–270. doi: 10.1146/annurev-neuro-062912-114322. doi:10.1146/annurev-neuro-062912-114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Penschuck S, Fritschy JM, McCarthy MM. Developmental switch in the expression of GABA(A) receptor subunits alpha(1) and alpha(2) in the hypothalamus and limbic system of the rat. Brain Res Dev Brain Res. 2000;119:127–138. doi: 10.1016/s0165-3806(99)00150-9. [DOI] [PubMed] [Google Scholar]
- De Gois S, et al. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. doi:10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. doi:10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. doi:10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Simpson KL, Lu YF, Lin RC, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci. 2008;11:957–965. doi: 10.1038/nn.2144. doi:10.1038/nn.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. doi:10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Tong A, Davis GW. Snapin is critical for presynaptic homeostatic plasticity. J Neurosci. 2012;32:8716–8724. doi: 10.1523/JNEUROSCI.5465-11.2012. doi:10.1523/JNEUROSCI.5465-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. doi:10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudanova I, Tabuchi K, Rohlmann A, Sudhof TC, Missler M. Deletion of alpha-neurexins does not cause a major impairment of axonal pathfinding or synapse formation. J Comp Neurol. 2007;502:261–274. doi: 10.1002/cne.21305. doi:10.1002/cne.21305. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, et al. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci. 1999;112(Pt 6):811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- Earls LR, Westmoreland JJ, Zakharenko SS. Non-coding RNA regulation of synaptic plasticity and memory: implications for aging. Ageing Res Rev. 2014 doi: 10.1016/j.arr.2014.03.004. doi:10.1016/j.arr.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM. The thalamo-cortical auditory receptive fields: regulation by the states of vigilance, learning and the neuromodulatory systems. Exp Brain Res. 2003;153:554–572. doi: 10.1007/s00221-003-1608-0. doi:10.1007/s00221-003-1608-0. [DOI] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. doi:10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. doi:10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- Elramah S, Landry M, Favereaux A. MicroRNAs regulate neuronal plasticity and are involved in pain mechanisms. Front Cell Neurosci. 2014;8:31. doi: 10.3389/fncel.2014.00031. doi:10.3389/fncel.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, De Gois S, Varoqui H, Schafer MK, Weihe E. Activity-dependent regulation of vesicular glutamate and GABA transporters: a means to scale quantal size. Neurochem Int. 2006;48:643–649. doi: 10.1016/j.neuint.2005.12.029. doi:10.1016/j.neuint.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. doi:10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CF, Horwitz MS, Hobbs MV, Oldstone MB. Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J Exp Med. 1996;184:2371–2384. doi: 10.1084/jem.184.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Grygoruk A, Brooks ES, Chen A, Krantz DE. Trafficking of vesicular neurotransmitter transporters. Traffic. 2008;9:1425–1436. doi: 10.1111/j.1600-0854.2008.00771.x. doi:10.1111/j.1600-0854.2008.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Roth A, Sakmann B. Monosynaptic connections between pairs of spiny stellate cells in layer 4 and pyramidal cells in layer 5A indicate that lemniscal and paralemniscal afferent pathways converge in the infragranular somatosensory cortex. J Neurosci. 2005;25:3423–3431. doi: 10.1523/JNEUROSCI.5227-04.2005. doi:10.1523/JNEUROSCI.5227-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol. 2011;21:269–274. doi: 10.1016/j.conb.2011.02.003. doi:10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Alexander ML, Threlkeld SW. Early neural disruption and auditory processing outcomes in rodent models: implications for developmental language disability. Front Syst Neurosci. 2013;7:58. doi: 10.3389/fnsys.2013.00058. doi:10.3389/fnsys.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm C, Siegel G, Grass S, Witte OW. Stable expression of the vesicular GABA transporter following photothrombotic infarct in rat brain. Neuroscience. 2006;140:865–877. doi: 10.1016/j.neuroscience.2006.02.045. doi:10.1016/j.neuroscience.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Franklin BJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd edn. Academic Press; New York: 2007. [Google Scholar]
- Fremeau RT, Jr, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Freund TF, Martin KA, Smith AD, Somogyi P. Glutamate decarboxylase-immunoreactive terminals of Golgi-impregnated axoaxonic cells and of presumed basket cells in synaptic contact with pyramidal neurons of the cat's visual cortex. J Comp Neurol. 1983;221:263–278. doi: 10.1002/cne.902210303. doi:10.1002/cne.902210303. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Jones BJ. Development of auditory cortical synaptic receptive fields. Neurosci Biobehav Rev. 2011;35:2105–2113. doi: 10.1016/j.neubiorev.2011.02.006. doi:10.1016/j.neubiorev.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Santamaria V, Alvarado JC, Gabaldon-Ull MC, Manuel Juiz J. Upregulation of insulin-like growth factor and interleukin 1beta occurs in neurons but not in glial cells in the cochlear nucleus following cochlear ablation. J Comp Neurol. 2013;521:3478–3499. doi: 10.1002/cne.23362. doi:10.1002/cne.23362. [DOI] [PubMed] [Google Scholar]
- Gao FB. Context-dependent functions of specific microRNAs in neuronal development. Neural Dev. 2010;5:25. doi: 10.1186/1749-8104-5-25. doi:10.1186/1749-8104-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis HR, Borst JG. Large GABAergic neurons form a distinct subclass within the mouse dorsal cortex of the inferior colliculus with respect to intrinsic properties, synaptic inputs, sound responses, and projections. J Comp Neurol. 2013;521:189–202. doi: 10.1002/cne.23170. doi:10.1002/cne.23170. [DOI] [PubMed] [Google Scholar]
- Gelman DM, Marin O. Generation of interneuron diversity in the mouse cerebral cortex. Eur J Neurosci. 2010;31:2136–2141. doi: 10.1111/j.1460-9568.2010.07267.x. doi:10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- Ghazalpour A, et al. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011;7:e1001393. doi: 10.1371/journal.pgen.1001393. doi:10.1371/journal.pgen.1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Curr Opin Neurobiol. 2007;17:516–524. doi: 10.1016/j.conb.2007.09.002. doi:10.1016/j.conb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gomez-Nieto R, Rubio ME. A bushy cell network in the rat ventral cochlear nucleus. J Comp Neurol. 2009;516:241–263. doi: 10.1002/cne.22139. doi:10.1002/cne.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol. 2008;507:1258–1276. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- Griffen TC, Maffei A. GABAergic synapses: their plasticity and role in sensory cortex. Frontiers in cellular neuroscience. 2014;8:91. doi: 10.3389/fncel.2014.00091. doi:10.3389/fncel.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Ye F, Sheng Q, Clark T, Samuels DC. Three-stage quality control strategies for DNA re-sequencing data. Brief Bioinform. 2013 doi: 10.1093/bib/bbt069. doi:10.1093/bib/bbt069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, et al. Multi-perspective quality control of Illumina exome sequencing data using QC3. Genomics. 2014a;103:323–328. doi: 10.1016/j.ygeno.2014.03.006. doi:10.1016/j.ygeno.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zhao S, Ye F, Sheng Q, Shyr Y. MultiRankSeq: multiperspective approach for RNAseq differential expression analysis and quality control. Biomed Res Int. 2014b;2014:248090. doi: 10.1155/2014/248090. doi:10.1155/2014/248090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung B, Fritzsch B. Time course of embryonic midbrain and thalamic auditory connection development in mice as revealed by carbocyanine dye tracing. J Comp Neurol. 2004;479:309–327. doi: 10.1002/cne.20328. doi:10.1002/cne.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, de la Mothe LA. Regional and laminar distribution of the vesicular glutamate transporter, VGluT2, in the macaque monkey auditory cortex. J Chem Neuroanat. 2009;38:106–116. doi: 10.1016/j.jchemneu.2009.05.002. doi:10.1016/j.jchemneu.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Preuss TM, Kaas JH. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol. 2001;441:197–222. doi: 10.1002/cne.1407. doi:10.1002/cne.1407. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Barkat TR, O'Brien BM, Hensch TK, Polley DB. Linking topography to tonotopy in the mouse auditory thalamocortical circuit. J Neurosci. 2011a;31:2983–2995. doi: 10.1523/JNEUROSCI.5333-10.2011. doi:10.1523/JNEUROSCI.5333-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Takahata T, Balaram P. VGLUT1 and VGLUT2 mRNA expression in the primate auditory pathway. Hear Res. 2011b;274:129–141. doi: 10.1016/j.heares.2010.11.001. doi:10.1016/j.heares.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, de la Mothe LA, Camalier CR, Falchier A, Lakatos P, Kajikawa Y, Schroeder CE. Feedforward and feedback projections of caudal belt and parabelt areas of auditory cortex: refining the hierarchical model. Front Neurosci. 2014;8:72. doi: 10.3389/fnins.2014.00072. doi:10.3389/fnins.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Guo Y, Clause A, Hackett NJ, Garbett K, Zhang P, Polley DB, Mirnics K. Transcriptional maturation of the mouse auditory forebrain. BMC Genomics. 2015 doi: 10.1186/s12864-015-1709-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa T, Molinari M, Rausell E, Jones EG. Patchy and laminar terminations of medial geniculate axons in monkey auditory cortex. J Comp Neurol. 1995;362:195–208. doi: 10.1002/cne.903620204. [DOI] [PubMed] [Google Scholar]
- Heinen K, et al. GABAA receptor maturation in relation to eye opening in the rat visual cortex. Neuroscience. 2004;124:161–171. doi: 10.1016/j.neuroscience.2003.11.004. doi:10.1016/j.neuroscience.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Innervation of orexin/hypocretin neurons by GABAergic, glutamatergic or cholinergic basal forebrain terminals evidenced by immunostaining for presynaptic vesicular transporter and postsynaptic scaffolding proteins. J Comp Neurol. 2006;499:645–661. doi: 10.1002/cne.21131. doi:10.1002/cne.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. doi:10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Herzog E, et al. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181, 20015807. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Takamori S, Jahn R, Brose N, Wojcik SM. Synaptic and vesicular co-localization of the glutamate transporters VGLUT1 and VGLUT2 in the mouse hippocampus. J Neurochem. 2006;99:1011–1018. doi: 10.1111/j.1471-4159.2006.04144.x. doi:10.1111/j.1471-4159.2006.04144.x. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. doi:10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Schuman EM. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron. 2013;80:648–657. doi: 10.1016/j.neuron.2013.10.036. doi:10.1016/j.neuron.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. doi:10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. doi:10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hornung JP, Fritschy JM. Developmental profile of GABAA-receptors in the marmoset monkey: expression of distinct subtypes in pre- and postnatal brain. J Comp Neurol. 1996;367:413–430. doi: 10.1002/(SICI)1096-9861(19960408)367:3<413::AID-CNE7>3.0.CO;2-8. doi:10.1002/(SICI)1096-9861(19960408)367:3<413:AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Chen Y, Leslie FM, Metherate R. Postnatal development of NR2A and NR2B mRNA expression in rat auditory cortex and thalamus. J Assoc Res Otolaryngol. 2002;3:479–487. doi: 10.1007/s10162-002-2052-8. doi:10.1007/s10162-002-2052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. doi:10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol. 2000;427:302–331. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected]. J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Ina A, et al. Cajal-Retzius cells and subplate neurons differentially express vesicular glutamate transporters 1 and 2 during development of mouse cortex. Eur J Neurosci. 2007;26:615–623. doi: 10.1111/j.1460-9568.2007.05703.x. doi:10.1111/j.1460-9568.2007.05703.x. [DOI] [PubMed] [Google Scholar]
- Insanally MN, Kover H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. doi:10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insanally MN, Albanna BF, Bao S. Pulsed noise experience disrupts complex sound representations. J Neurophysiol. 2010;103:2611–2617. doi: 10.1152/jn.00872.2009. doi:10.1152/jn.00872.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Oliver DL. Origins of glutamatergic terminals in the inferior colliculus identified by retrograde transport and expression of VGLUT1 and VGLUT2 genes. Front Neuroanat. 2010;4:1–11. doi: 10.3389/fnana.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Bishop DC, Oliver DL. Two classes of GABAergic neurons in the inferior colliculus. J Neurosci. 2009;29:13860–13869. doi: 10.1523/JNEUROSCI.3454-09.2009. doi:10.1523/JNEUROSCI.3454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Bishop DC, Oliver DL. Expression of glutamate and inhibitory amino acid vesicular transporters in the rodent auditory brainstem. J Comp Neurol. 2011;519:316–340. doi: 10.1002/cne.22521. doi:10.1002/cne.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci. 2005;22:661–676. doi: 10.1017/S0952523805225154. doi:10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Jiang J, Suppiramaniam V, Wooten MW. Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity. Neuro-Signals. 2006;15:266–282. doi: 10.1159/000105517. doi:10.1159/000105517. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kanold PO. Transient microcircuits formed by subplate neurons and their role in functional development of thalamocortical connections. NeuroReport. 2004;15:2149–2153. doi: 10.1097/00001756-200410050-00001. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. doi:10.1146/annurevneuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Keating P, King AJ. Developmental plasticity of spatial hearing following asymmetric hearing loss: context-dependent cue integration and its clinical implications. Front Syst Neurosci. 2013;7:123. doi: 10.3389/fnsys.2013.00123. doi:10.3389/fnsys.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. doi:10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. doi:10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Okamoto K, Tamai Y. Topography of projections from the primary and non-primary auditory cortical areas to the medial geniculate body and thalamic reticular nucleus in the rat. Neuroscience. 2005 doi: 10.1016/j.neuroscience.2005.06.089. [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF. GABAergic networks of basket cells in the visual cortex. Prog Brain Res. 1992;90:385–405. doi: 10.1016/s0079-6123(08)63623-7. [DOI] [PubMed] [Google Scholar]
- Konig N, Roch G, Marty R. The onset of synaptogenesis in rat temporal cortex. Anat Embryol (Berl) 1975;148:73–87. doi: 10.1007/BF00315564. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. doi:10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, MacKenzie PC, Sanes DH. Rescue of inhibitory synapse strength following developmental hearing loss. PLoS ONE. 2013;8:e53438. doi: 10.1371/journal.pone.0053438. doi:10.1371/journal.pone.0053438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15:552–562. doi: 10.1093/cercor/bhh156. doi:10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- Kral A, Hubka P, Heid S, Tillein J. Single-sided deafness leads to unilateral aural preference within an early sensitive period. Brain. 2013;136:180–193. doi: 10.1093/brain/aws305. doi:10.1093/brain/aws305. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. doi:10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann PH, Kandler K. Glycinergic/GABAergic synapses in the lateral superior olive are excitatory in neonatal C57Bl/6J mice. Brain Res Dev Brain Res. 2001;131:143–147. S0165380601002711. doi: 10.1016/s0165-3806(01)00271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Schone C, Heine M, Gundelfinger ED, Fejtova A. Extensive remodeling of the presynaptic cytomatrix upon homeostatic adaptation to network activity silencing. J Neurosci. 2011;31:10189–10200. doi: 10.1523/JNEUROSCI.2088-11.2011. doi:10.1523/JNEUROSCI.2088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Pothula S, Andres-Alonso M, Fejtova A. Molecular mechanisms driving homeostatic plasticity of neurotransmitter release. Front Cell Neurosci. 2013;7:244. doi: 10.3389/fncel.2013.00244. doi:10.3389/fncel.2013.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE. Statistical evaluation of methods for quantifying gene expression by autoradiography in histological sections. BMC Neurosci. 2009;10:5. doi: 10.1186/1471-2202-10-5. doi:10.1186/1471-2202-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC. Thalamic and cortical pathways supporting auditory processing. Brain Lang. 2013;126:22–28. doi: 10.1016/j.bandl.2012.05.004. doi:10.1016/j.bandl.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chen CX, Liu YJ, Aizenman E, Kandler K. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur J Neurosci. 2005;21:2593–2599. doi: 10.1111/j.1460-9568.2005.04084.x. doi:10.1111/j.1460-9568.2005.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort S, Gray AC, Turrigiano GG. Long-term inhibitory plasticity in visual cortical layer 4 switches sign at the opening of the critical period. Proc Natl Acad Sci USA. 2013;110:E4540–4547. doi: 10.1073/pnas.1319571110. doi:10.1073/pnas.1319571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. doi:10.1146/annurevneuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Li YT, Ma WP, Pan CJ, Zhang LI, Tao HW. Broadening of cortical inhibition mediates developmental sharpening of orientation selectivity. J Neurosci. 2012;32:3981–3991. doi: 10.1523/JNEUROSCI.5514-11.2012. doi:10.1523/JNEUROSCI.5514-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters VGLUT1 and VGLUT2 in the developing mouse barrel cortex. Int J Dev Neurosci. 2007;25:107–114. doi: 10.1016/j.ijdevneu.2006.12.005. doi:10.1016/j.ijdevneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Linke R. Differential projection patterns of superior and inferior collicular neurons onto posterior paralaminar nuclei of the thalamus surrounding the medial geniculate body in the rat. Eur J Neurosci. 1999;11:187–203. doi: 10.1046/j.1460-9568.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Linke R, Schwegler H. Convergent and complementary projections of the caudal paralaminar thalamic nuclei to rat temporal and insular cortex. Cereb Cortex. 2000;10:753–771. doi: 10.1093/cercor/10.8.753. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Evidence for nonreciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems. J Comp Neurol. 2008;507:1209–1227. doi: 10.1002/cne.21602. doi:10.1002/cne.21602. [DOI] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Lubke J, Feldmeyer D. Excitatory signal flow and connectivity in a cortical column: focus on barrel cortex Brain structure & function. 2007;212:3–17. doi: 10.1007/s00429-007-0144-2. doi:10.1007/s00429-007-0144-2. [DOI] [PubMed] [Google Scholar]
- Ma J, Yao XH, Fu Y, Yu YC. Development of layer 1 neurons in the mouse neocortex. Cereb Cortex. 2013 doi: 10.1093/cercor/bht114. doi:10.1093/cercor/bht114. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. doi:10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- Maffei A, Bucher D, Fontanini A. Homeostatic plasticity in the nervous system. Neural Plasticity. 2012;2012:913472. doi: 10.1155/2012/913472. doi:10.1155/2012/913472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H. A network of tufted layer 5 pyramidal neurons. Cereb Cortex. 1997;7:523–533. doi: 10.1093/cercor/7.6.523. [DOI] [PubMed] [Google Scholar]
- Martin CA, Krantz DE. Drosophila melanogaster as a genetic model system to study neurotransmitter transporters. Neurochem Int. 2014;73:71–88. doi: 10.1016/j.neuint.2014.03.015. doi:10.1016/j.neuint.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko H, et al. Comparative anatomy of marmoset and mouse cortex from genomic expression. J Neurosci. 2012;32:5039–5053. doi: 10.1523/JNEUROSCI.4788-11.2012. doi:10.1523/JNEUROSCI.4788-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott JG, Foster NL, Nakamoto KT, Motts SD, Schofield BR. Distribution of GABAergic cells in the inferior colliculus that project to the thalamus. Front Neuroanat. 2014a;8:17. doi: 10.3389/fnana.2014.00017. doi:10. 3389/fnana.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott JG, Foster NL, Ohl AP, Schofield BR. Excitatory and inhibitory projections in parallel pathways from the inferior colliculus to the auditory thalamus. Front Neuroanat. 2014b;8:124. doi: 10.3389/fnana.2014.00124. doi:10.3389/fnana.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo CV, Mele M, Curcio M, Comprido D, Silva CG, Duarte CB. BDNF regulates the expression and distribution of vesicular glutamate transporters in cultured hippocampal neurons. PLoS One. 2013;8:e53793. doi: 10.1371/journal.pone.0053793. doi:10.1371/journal.pone.0053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone M, Burette A, Weinberg RJ. Light microscopic identification and immunocytochemical characterization of glutamatergic synapses in brain sections. J Comp Neurol. 2005;492:495–509. doi: 10.1002/cne.20743. doi:10.1002/cne.20743. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Overlap between acetylcholinesterase-rich and choline acetyltransferase-positive (cholinergic) axons in human cerebral cortex. Brain Res. 1992;577:112–120. doi: 10.1016/0006-8993(92)90543-i. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cruikshank SJ. Thalamocortical inputs trigger a propagating envelope of gamma-band activity in auditory cortex in vitro. Exp Brain Res. 1999;126:160–174. doi: 10.1007/s002210050726. [DOI] [PubMed] [Google Scholar]
- Michalski D, et al. Region-specific expression of vesicular glutamate and GABA transporters under various ischaemic conditions in mouse forebrain and retina. Neuroscience. 2013;231:328–344. doi: 10.1016/j.neuroscience.2012.11.046. doi:10.1016/j.neuroscience.2012.11.046. [DOI] [PubMed] [Google Scholar]
- Mikaelian D, Alford BR, Ruben RJ. Cochlear potentials and 8 nerve action potentials in normal and genetically deaf mice. Ann Otol Rhinol Laryngol. 1965;74:146–157. doi: 10.1177/000348946507400113. [DOI] [PubMed] [Google Scholar]
- Mikhaylova M, et al. Cellular distribution of the NMDA-receptor activated synapto-nuclear messenger Jacob in the rat brain. Brain Struct Funct. 2014;219:843–860. doi: 10.1007/s00429-013-0539-1. doi:10.1007/s00429-013-0539-1. [DOI] [PubMed] [Google Scholar]
- Milenkovic I, Witte M, Turecek R, Heinrich M, Reinert T, Rubsamen R. Development of chloride-mediated inhibition in neurons of the anteroventral cochlear nucleus of gerbil (Meriones unguiculatus). J Neurophysiol. 2007;98:1634–1644. doi: 10.1152/jn.01150.2006. doi:10.1152/jn.01150.2006. [DOI] [PubMed] [Google Scholar]
- Mills DM, Rubel EW. Development of the base of the cochlea: place code shift in the gerbil. Hear Res. 1998;122:82–96. doi: 10.1016/s0378-5955(98)00079-3. [DOI] [PubMed] [Google Scholar]
- Minelli A, Alonso-Nanclares L, Edwards RH, DeFelipe J, Conti F. Postnatal development of the vesicular GABA transporter in rat cerebral cortex. Neuroscience. 2003a;117:337–346. doi: 10.1016/s0306-4522(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Minelli A, Edwards RH, Manzoni T, Conti F. Postnatal development of the glutamate vesicular transporter VGLUT1 in rat cerebral cortex. Brain Res Dev Brain Res. 2003b;140:309–314. doi: 10.1016/s0165-3806(02)00617-x. [DOI] [PubMed] [Google Scholar]
- Mitani A, Shimokouchi M, Itoh K, Nomura S, Kudo M, Mizuno N. Morphology and laminar organization of electrophysiologically identified neurons in the primary auditory cortex in the cat. J Comp Neurol. 1985;235:430–447. doi: 10.1002/cne.902350403. [DOI] [PubMed] [Google Scholar]
- Mitani A, Itoh K, Mizuno N. Distribution and size of thalamic neurons projecting to layer I of the auditory cortical fields of the cat compared to those projecting to layer IV. J Comp Neurol. 1987;257:105–121. doi: 10.1002/cne.902570108. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. doi:10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Montero VM, Scott GL. Ultrastructural identification of satellite interneurons in the rat dorsal lateral geniculate nucleus. Arch Biol Med Exp. 1983;16:343–360. [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Schnupp JW, King AJ. Acoustic factors govern developmental sharpening of spatial tuning in the auditory cortex. Nat Neurosci. 2003;6:981–988. doi: 10.1038/nn1108. doi:10.1038/nn1108. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Versnel H, King AJ. Development of contralateral and ipsilateral frequency representations in ferret primary auditory cortex. Eur J Neurosci. 2006;23:780–792. doi: 10.1111/j.1460-9568.2006.04609.x. doi:10.1111/j.1460-9568.2006.04609.x. [DOI] [PubMed] [Google Scholar]
- Muller M, Davis GW. Transsynaptic control of presynaptic Ca(2)(+) influx achieves homeostatic potentiation of neurotransmitter release. Curr Biol. 2012;22:1102–1108. doi: 10.1016/j.cub.2012.04.018. doi:10.1016/j.cub.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. VGluT2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol. 2005;484:458–473. doi: 10.1002/cne.20505. [DOI] [PubMed] [Google Scholar]
- Nahmani M, Turrigiano GG. Adult cortical plasticity following injury: recapitulation of critical period mechanisms? Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.04.029. doi:10.1016/j.neuroscience.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol. 2005;492:263–288. doi: 10.1002/cne.20705. doi:10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- Nehme B, Henry M, Mouginot D, Drolet G. The expression pattern of the Na(+) sensor, Na(X) in the hydromineral homeostatic network: a comparative study between the rat and mouse. Front Neuroanat. 2012;6:26. doi: 10.3389/fnana.2012.00026. doi:10.3389/fnana.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MV, et al. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J Neurosci. 2012;32:10021–10034. doi: 10.1523/JNEUROSCI.1316-12.2012. doi:10.1523/JNEUROSCI.1316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L, Wu G, Culley DE, Scholten JC, Zhang W. Integrative analysis of transcriptomic and proteomic data: challenges, solutions and applications. Crit Rev Biotechnol. 2007;27:63–75. doi: 10.1080/07388550701334212. doi:10.1080/07388550701334212. [DOI] [PubMed] [Google Scholar]
- Niimi K, Ono K, Kusunose M. Projections of the medial geniculate nucleus to layer 1 of the auditory cortex in the cat traced with horseradish peroxidase. Neurosci Lett. 1984;45:223–228. doi: 10.1016/0304-3940(84)90103-4. [DOI] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex. 1994;4:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Ojima H, Honda CN, Jones EG. Patterns of axon collateralization of identified supragranular pyramidal neurons in the cat auditory cortex. Cereb Cortex. 1991;1:80–94. doi: 10.1093/cercor/1.1.80. [DOI] [PubMed] [Google Scholar]
- Olde Loohuis NF, Kos A, Martens GJ, Van Bokhoven H, Nadif Kasri N, Aschrafi A. MicroRNA networks direct neuronal development and plasticity. Cell Mol Life Sci. 2012;69:89–102. doi: 10.1007/s00018-011-0788-1. doi:10.1007/s00018-011-0788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote H, Miyaji T, Juge N, Moriyama Y. Vesicular neurotransmitter transporter: bioenergetics and regulation of glutamate transport. Biochemistry. 2011;50:5558–5565. doi: 10.1021/bi200567k. doi:10.1021/bi200567k. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J Neurophysiol. 2008;99:2998–3008. doi: 10.1152/jn.01160.2007. doi:10.1152/jn.01160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Development of inhibitory timescales in auditory cortex. Cereb Cortex. 2011;21:1351–1361. doi: 10.1093/cercor/bhq214. doi:10.1093/cercor/bhq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, McConnell DJ, Fino E, Yuste R. Axo-dendritic overlap and laminar projection can explain interneuron connectivity to pyramidal cells. Cereb Cortex. 2013;23:2790–2802. doi: 10.1093/cercor/bhs210. doi:10.1093/cercor/bhs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas SL, Sur M. Morphology of retinal axon arbors induced to arborize in a novel target, the medial geniculate nucleus. II. Comparison with axons from the inferior colliculus. J Comp Neurol. 1994;349:363–376. doi: 10.1002/cne.903490304. doi:10.1002/cne.903490304. [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Fritschy JM, Yanagawa Y, Obata K, Sassoe-Pognetto M. GABAergic phenotype of periglomerular cells in the rodent olfactory bulb. J Comp Neurol. 2007;502:990–1002. doi: 10.1002/cne.21356. doi:10.1002/cne.21356. [DOI] [PubMed] [Google Scholar]
- Park CC, Petyuk VA, Qian WJ, Smith RD, Smith DJ. Dual spatial maps of transcript and protein abundance in the mouse brain. Expert Rev Proteomics. 2009;6:243–249. doi: 10.1586/epr.09.46. doi:10.1586/epr.09.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden DR, et al. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J Physiol. 2008;586:965–987. doi: 10.1113/jphysiol.2007.145375. doi:10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perederiy JV, Luikart BW, Washburn EK, Schnell E, Westbrook GL. Neural injury alters proliferation and integration of adult-generated neurons in the dentate gyrus. J Neurosci. 2013;33:4754–4767. doi: 10.1523/JNEUROSCI.4785-12.2013. doi:10.1523/JNEUROSCI.4785-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. doi:10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Qin Y, Heggelund P, Zhu JJ. Postnatal development of GABAergic signalling in the rat lateral geniculate nucleus: presynaptic dendritic mechanisms. J Physiol. 2003;546:137–148. doi: 10.1113/jphysiol.2002.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, et al. Distribution of vesicular glutamate transporters 1 and 2 in the rat spinal cord, with a note on the spinocervical tract. J Comp Neurol. 2006;497:683–701. doi: 10.1002/cne.20987. doi:10.1002/cne.20987. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J Neurosci. 1997;17:3766–3777. doi: 10.1523/JNEUROSCI.17-10-03766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Thompson JH, Guo W. Brief hearing loss disrupts binaural integration during two early critical periods of auditory cortex development. Nat Commun. 2013;4:2547. doi: 10.1038/ncomms3547. doi:10.1038/ncomms3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. doi:10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto JJ, Winer JA. Layer VI in cat primary auditory cortex: Golgi study and sublaminar origins of projection neurons. J Comp Neurol. 1999;404:332–358. doi: 10.1002/(sici)1096-9861(19990215)404:3<332::aid-cne5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol. 2006;499:231–243. doi: 10.1002/cne.21099. doi:10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Development of inhibitory mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. J Neurosci. 2007;27:1769–1781. doi: 10.1523/JNEUROSCI.3851-06.2007. doi:10.1523/JNEUROSCI.3851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007;30:119–125. doi: 10.1016/j.tins.2007.01.004. doi:10.1016/j.tins.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Blundon JA, Bayazitov IT, Zakharenko SS. Connectivity patterns revealed by mapping of active inputs on dendrites of thalamorecipient neurons in the auditory cortex. J Neurosci. 2009;29:6406–6417. doi: 10.1523/JNEUROSCI.0258-09.2009. doi:10.1523/JNEUROSCI.0258-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K + -Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. doi:10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MJ, Semple MN, Sanes DH. Exploiting development to evaluate auditory encoding of amplitude modulation. J Neurosci. 2010;30:15509–15520. doi: 10.1523/JNEUROSCI.3340-10.2010. doi:10.1523/JNEUROSCI.3340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. Morphology of corticothalamic terminals arising from the auditory cortex of the rat: a Phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Hear Res. 1991;56:179–190. doi: 10.1016/0378-5955(91)90168-9. [DOI] [PubMed] [Google Scholar]
- Rovo Z, Ulbert I, Acsady L. Drivers of the primate thalamus. J Neurosci. 2012;32:17894–17908. doi: 10.1523/JNEUROSCI.2815-12.2012. doi:10.1523/JNEUROSCI.2815-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samano C, Zetina ME, Marin MA, Cifuentes F, Morales MA. Choline acetyl transferase and neuropeptide immunoreactivities are colocalized in somata, but preferentially localized in distinct axon fibers and boutons of cat sympathetic preganglionic neurons. Synapse. 2006;60:295–306. doi: 10.1002/syn.20300. doi:10.1002/syn.20300. [DOI] [PubMed] [Google Scholar]
- Samano C, Cifuentes F, Morales MA. Neurotransmitter segregation: functional and plastic implications. Prog Neurobiol. 2012;97:277–287. doi: 10.1016/j.pneurobio.2012.04.004. doi:10.1016/j.pneurobio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr Opin Neurobiol. 2009;19:188–199. doi: 10.1016/j.conb.2009.05.014. doi:10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Kotak VC. Developmental plasticity of auditory cortical inhibitory synapses. Hear Res. 2011;279:140–148. doi: 10.1016/j.heares.2011.03.015. doi:10.1016/j.heares.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Woolley SM. A behavioral framework to guide research on central auditory development and plasticity. Neuron. 2011;72:912–929. doi: 10.1016/j.neuron.2011.12.005. doi:10.1016/j.neuron.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MS, Li H, Voglmaier SM. Synaptic vesicle protein trafficking at the glutamate synapse. Neuroscience. 2009;158:189–203. doi: 10.1016/j.neuroscience.2008.03.029. doi:10.1016/j.neuroscience.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Rosen MJ, Sanes DH. Taking advantage of behavioral changes during development and training to assess sensory coding mechanisms. Ann N Y Acad Sci. 2011;1225:142–154. doi: 10.1111/j.1749-6632.2011.06023.x. doi:10.1111/j.1749-6632.2011.06023.x. [DOI] [PubMed] [Google Scholar]
- Saunders A, et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature. 2015 doi: 10.1038/nature14179. doi:10.1038/nature14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele SJ, Losh J, Dailey ME, Green SH. Spine formation and maturation in the developing rat auditory cortex. J Comp Neurol. 2011;519:3327–3345. doi: 10.1002/cne.22728. doi:10.1002/cne.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–849. doi: 10.1038/nrn2763. doi:10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. doi:10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva A, Jansen HT. Neuroanatomical plasticity in the gonadotropin-releasing hormone system of the ewe: seasonal variation in glutamatergic and gamma-aminobutyric acidergic afferents. J Comp Neurol. 2009;515:615–628. doi: 10.1002/cne.22087. doi:10.1002/cne.22087. [DOI] [PubMed] [Google Scholar]
- Siembab VC, Smith CA, Zagoraiou L, Berrocal MC, Mentis GZ, Alvarez FJ. Target selection of proprioceptive and motor axon synapses on neonatal V1-derived Ia inhibitory interneurons and Renshaw cells. J Comp Neurol. 2010;518:4675–4701. doi: 10.1002/cne.22441. doi:10.1002/cne.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Su J, Brooks J, Terauchi A, Umemori H, Fox MA. Fibroblast growth factor 22 contributes to the development of retinal nerve terminals in the dorsal lateral geniculate nucleus. Front Mol Neurosci. 2012;4:61. doi: 10.3389/fnmol.2011.00061. doi:10.3389/fnmol.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Bartlett EL, Kowalkowski A. Cortical and collicular inputs to cells in the rat paralaminar thalamic nuclei adjacent to the medial geniculate body. J Neurophysiol. 2007;98:681–695. doi: 10.1152/jn.00235.2007. doi:10.1152/jn.00235.2007. [DOI] [PubMed] [Google Scholar]
- Smith PH, Manning KA, Uhlrich DJ. Evaluation of inputs to rat primary auditory cortex from the suprageniculate nucleus and extrastriate visual cortex. J Comp Neurol. 2010;518:3679–3700. doi: 10.1002/cne.22411. doi:10.1002/cne.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Freund TF, Wu JY, Smith AD. The section-Golgi impregnation procedure. 2. Immunocytochemical demonstration of glutamate decarboxylase in Golgi-impregnated neurons and in their afferent synaptic boutons in the visual cortex of the cat. Neuroscience. 1983a;9:475–490. doi: 10.1016/0306-4522(83)90167-7. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Smith AD, Nunzi MG, Gorio A, Takagi H, Wu JY. Glutamate decarboxylase immunoreactivity in the hippocampus of the cat: distribution of immunoreactive synaptic terminals with special reference to the axon initial segment of pyramidal neurons. J Neurosci. 1983b;3:1450–1468. doi: 10.1523/JNEUROSCI.03-07-01450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Pinto A. The structure of the first auditory cortex (A I) in the cat. I. Light microscopic observations on its organization. Arch Ital Biol. 1973;111:112–137. [PubMed] [Google Scholar]
- Speechley WJ, Hogsden JL, Dringenberg HC. Continuous white noise exposure during and after auditory critical period differentially alters bidirectional thalamocortical plasticity in rat auditory cortex in vivo. Eur J Neurosci. 2007;26:2576–2584. doi: 10.1111/j.1460-9568.2007.05857.x. doi:10.1111/j.1460-9568.2007.05857.x. [DOI] [PubMed] [Google Scholar]
- Storace DA, Higgins NC, Chikar JA, Oliver DL, Read HL. Gene expression identifies distinct ascending glutamatergic pathways to frequency-organized auditory cortex in the rat brain. J Neurosci. 2012;32:15759–15768. doi: 10.1523/JNEUROSCI.1310-12.2012. doi:10.1523/JNEUROSCI.1310-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YJ, et al. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–931. doi: 10.1038/nature09079. doi:10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. doi:10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Takayama C, Inoue Y. Developmental localization of potassium chloride co-transporter 2 (KCC2), GABA and vesicular GABA transporter (VGAT) in the postnatal mouse somatosensory cortex. Neurosci Res. 2010;67:137–148. doi: 10.1016/j.neures.2010.02.010. doi:10.1016/j.neures.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. doi:10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurol. 2009;4:331–349. doi: 10.2217/FNL.09.5. doi:10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Presynaptic GABA(B) receptors regulate experience-dependent development of inhibitory short-term plasticity. J Neurosci. 2010;30:2716–2727. doi: 10.1523/JNEUROSCI.3903-09.2010. doi:10.1523/JNEUROSCI.3903-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J Neurosci. 1997;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AY, Wehr M. Balanced tone-evoked synaptic excitation and inhibition in mouse auditory cortex. Neuroscience. 2009;163:1302–1315. doi: 10.1016/j.neuroscience.2009.07.032. doi:10.1016/j.neuroscience.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Tan AY, Zhang LI, Merzenich MM, Schreiner CE. Toneevoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol. 2004;92:630–643. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. doi:10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Tokuoka H, Goda Y. Activity-dependent coordination of presynaptic release probability and postsynaptic GluR2 abundance at single synapses. Proc Natl Acad Sci USA. 2008;105:14656–14661. doi: 10.1073/pnas.0805705105. doi:10.1073/pnas.0805705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Hackett TA, Rakic P, Levitt P, Polley DB. EphA signaling impacts development of topographic connectivity in auditory corticofugal systems. Cereb Cortex. 2013;23:775–785. doi: 10.1093/cercor/bhs066. doi:10.1093/cercor/bhs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima M, et al. Preferential localization of neural cell recognition molecule NB-2 in developing glutamatergic neurons in the rat auditory brainstem. J Comp Neurol. 2009;513:349–362. doi: 10.1002/cne.21972. doi:10.1002/cne.21972. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Rodriguez-Contreras A, Crins TT, Wang HC, Borst JG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci. 2010;13:1050–1052. doi: 10.1038/nn.2604. doi:10.1038/nn.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo M, Carrasco MM, Razak K. Response properties underlying selectivity for the rate of frequency modulated sweeps in the auditory cortex of the mouse. Hear Res. 2013;298:80–92. doi: 10.1016/j.heares.2012.12.013. doi:10.1016/j.heares.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. doi:10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harbor Perspect Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. doi:10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. doi:10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflamm. 2014;11:82. doi: 10.1186/1742-2094-11-82. doi:10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaga CE, Borisovska M, Westbrook GL. Dual-transmitter neurons: functional implications of co-release and co-transmission. Curr Opin Neurobiol. 2014;29:25–32. doi: 10.1016/j.conb.2014.04.010. doi:10.1016/j.conb.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee EA, Keijser JN. Localization of pre- and postsynaptic cholinergic markers in rodent forebrain: a brief history and comparison of rat and mouse. Behav Brain Res. 2011;221:356–366. doi: 10.1016/j.bbr.2010.11.051. doi:10.1016/j.bbr.2010.11.051. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman Y, Bartlett EL. Postnatal development of synaptic properties of the GABAergic projection from the inferior colliculus to the auditory thalamus. J Neurophysiol. 2013;109:2866–2882. doi: 10.1152/jn.00021.2013. doi:10.1152/jn.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman Y, Bartlett EL. Postnatal development of auditory central evoked responses and thalamic cellular properties. Dev Neurobiol. 2014;74:541–555. doi: 10.1002/dneu.22148. doi:10.1002/dneu.22148. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys. J Comp Neurol. 2011;519:989–1005. doi: 10.1002/cne.22563. doi:10.1002/cne.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S, Bandyopadhyay S, Kao JP, Kanold PO. Changing microcircuits in the subplate of the developing cortex. J Neurosci. 2012;32:1589–1601. doi: 10.1523/JNEUROSCI.4748-11.2012. doi:10.1523/JNEUROSCI.4748-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitureira N, Letellier M, Goda Y. Homeostatic synaptic plasticity: from single synapses to neural circuits. Curr Opin Neurobiol. 2012;22:516–521. doi: 10.1016/j.conb.2011.09.006. doi:10.1016/j.conb.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. doi:10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Peters A. Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J Comp Neurol. 1981;195:603–625. doi: 10.1002/cne.901950406. doi:10.1002/cne.901950406. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Kitzes LM, Jones EG. Chemoarchitectonic organization of the cat primary auditory cortex. Exp Brain Res. 1991a;86:518–526. doi: 10.1007/BF00230525. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Kitzes LM, Jones EG. Intrinsic inter- and intralaminar connections and their relationship to the tonotopic map in cat primary auditory cortex. Exp Brain Res. 1991b;86:527–544. doi: 10.1007/BF00230526. [DOI] [PubMed] [Google Scholar]
- Walz C, Elssner-Beyer B, Schubert D, Gottmann K. Properties of glutamatergic synapses in immature layer Vb pyramidal neurons: coupling of pre- and postsynaptic maturational states. Exp Brain Res. 2010;200:169–182. doi: 10.1007/s00221-009-2051-7. doi:10.1007/s00221-009-2051-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Maffei A. Inhibitory plasticity dictates the sign of plasticity at excitatory synapses. J Neurosci. 2014;34:1083–1093. doi: 10.1523/JNEUROSCI.4711-13.2014. doi:10.1523/JNEUROSCI.4711-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang ZW. A critical window for experience-dependent plasticity at whisker sensory relay synapse in the thalamus. J Neurosci. 2008;28:13621–13628. doi: 10.1523/JNEUROSCI.4785-08.2008. doi:10.1523/JNEUROSCI.4785-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Fluorescent labeling of both GABAergic and glycinergic neurons in vesicular GABA transporter (VGAT)-venus transgenic mouse. Neuroscience. 2009;164:1031–1043. doi: 10.1016/j.neuroscience.2009.09.010. doi:10.1016/j.neuroscience.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Wang F, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012a;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. doi:10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fontanini A, Maffei A. Experience-dependent switch in sign and mechanisms for plasticity in layer 4 of primary visual cortex. J Neurosci. 2012b;32:10562–10573. doi: 10.1523/JNEUROSCI.0622-12.2012. doi:10.1523/JNEUROSCI.0622-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A. Comparative molecular neuroanatomy of mammalian neocortex: what can gene expression tell us about areas and layers? Dev Growth Differ. 2009;51:343–354. doi: 10.1111/j.1440-169X.2008.01085.x. doi:10.1111/j.1440-169X.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Whitton JP, Polley DB. Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: a comparison of basic and clinical studies. J Assoc Res Otolaryngol. 2011;12:535–547. doi: 10.1007/s10162-011-0271-6. doi:10.1007/s10162-011-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Walsh MF, Turrigiano GG. Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol. 2006;96:2127–2133. doi: 10.1152/jn.00107.2006. doi:10.1152/jn.00107.2006. [DOI] [PubMed] [Google Scholar]
- Wilson NR, et al. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. doi:10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Broser PJ, Kuner T, Bruno RM. Experience-induced plasticity of thalamocortical axons in both juveniles and adults. J Comp Neurol. 2010;518:4629–4648. doi: 10.1002/cne.22483. doi:10.1002/cne.22483. [DOI] [PubMed] [Google Scholar]
- Winer JA. Identification and structure of neurons in the medial geniculate body projecting to primary auditory cortex (AI) in the cat. Neuroscience. 1984;13:395–413. doi: 10.1016/0306-4522(84)90239-2. [DOI] [PubMed] [Google Scholar]
- Winer JA. Structure of layer II in cat primary auditory cortex (AI). J Comp Neurol. 1985;238:10–37. doi: 10.1002/cne.902380103. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT. Evolution of GABAergic circuitry in the mammalian medial geniculate body. Proc Natl Acad Sci USA. 1996;93:3083–3087. doi: 10.1073/pnas.93.7.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Saint Marie RL, Larue DT, Oliver DL. GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proc Natl Acad Sci USA. 1996;93:8005–8010. doi: 10.1073/pnas.93.15.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Sally SL, Larue DT, Kelly JB. Origins of medial geniculate body projections to physiologically defined zones of rat primary auditory cortex. Hear Res. 1999;130:42–61. doi: 10.1016/s0378-5955(98)00217-2. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, et al. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci USA. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. doi:10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Hartig W, Groenewegen HJ, Voorn P. Density gradients of vesicular glutamate- and GABA transporter-immunoreactive boutons in calbindin and mu-opioid receptor-defined compartments in the rat striatum. J Comp Neurol. 2012;520:2123–2142. doi: 10.1002/cne.23031. doi:10.1002/cne.23031. [DOI] [PubMed] [Google Scholar]
- Wozny C, Williams SR. Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb Cortex. 2011;21:1818–1826. doi: 10.1093/cercor/bhq257. doi:10.1093/cercor/bhq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GK, Li P, Tao HW, Zhang LI. Nonmonotonic synaptic excitation and imbalanced inhibition underlying cortical intensity tuning. Neuron. 2006;52:705–715. doi: 10.1016/j.neuron.2006.10.009. doi:10.1016/j.neuron.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GK, Arbuckle R, Liu BH, Tao HW, Zhang LI. Lateral sharpening of cortical frequency tuning by approximately balanced inhibition. Neuron. 2008;58:132–143. doi: 10.1016/j.neuron.2008.01.035. doi:10.1016/j.neuron.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahachi H, Marik SA, McManus JN, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–729. doi: 10.1016/j.neuron.2009.11.026. doi:10.1016/j.neuron.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T, Tsumoto T, Kimura F. Transiently higher release probability during critical period at thalamocortical synapses in the mouse barrel cortex: relevance to differential short-term plasticity of AMPA and NMDA EPSCs and possible involvement of silent synapses. Eur J Neurosci. 2004;20:3006–3018. doi: 10.1111/j.1460-9568.2004.03756.x. doi:10.1111/j.1460-9568.2004.03756.x. [DOI] [PubMed] [Google Scholar]
- Yu WM, Goodrich LV. Morphological and physiological development of auditory synapses. Hear Res. 2014 doi: 10.1016/j.heares.2014.01.007. doi:10.1016/j.heares.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuge K, et al. Region-specific gene expression in early postnatal mouse thalamus. J Comp Neurol. 2011;519:544–561. doi: 10.1002/cne.22532. doi:10.1002/cne.22532. [DOI] [PubMed] [Google Scholar]
- Zeng H, et al. Large-scale cellular-resolution gene profiling in human neocortex reveals species-specific molecular signatures. Cell. 2012;149:483–496. doi: 10.1016/j.cell.2012.02.052. doi:10.1016/j.cell.2012.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jiao YY, Sun QQ. Developmental maturation of excitation and inhibition balance in principal neurons across four layers of somatosensory cortex. Neuroscience. 2011;174:10–25. doi: 10.1016/j.neuroscience.2010.11.045. doi:10.1016/j.neuroscience.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Guo Y, Sheng Q, Shyr Y. Advanced heat map and clustering analysis using heatmap3. Biomed Res Int. 2014;2014:986048. doi: 10.1155/2014/986048. doi:10.1155/2014/986048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vesicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500:777–787. doi: 10.1002/cne.21208. doi:10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Lo FS, Guido W. Nature of inhibitory postsynaptic activity in developing relay cells of the lateral geniculate nucleus. J Neurophysiol. 2003;90:1063–1070. doi: 10.1152/jn.00178.2003. doi:10.1152/jn.00178.2003. [DOI] [PubMed] [Google Scholar]
- Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. doi:10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.