Abstract
Curcumin exerts a protective effect in cerebral ischemia through its anti-oxidant and anti-inflammatory activities. γ-enolase is a glycolytic enzyme expressed in neurons that is known to exerts a neuroprotective effect. We investigated whether curcumin regulates γ-enolase expression in focal cerebral ischemic injury in rats. Middle cerebral artery occlusion (MCAO) was performed to induce focal cerebral ischemia. Adult male rats were injected intraperitoneally with either vehicle or curcumin (50 mg/kg) 1 h after MCAO and cerebral cortex tissues were isolated 24 h after MCAO. We found that MCAO-induced injury resulted in a reduction in γ-enolase expression in vehicle-treated animals using a proteomics approach. However, this reduction was attenuated in animals with MCAO treated with curcumin. Reverse-transcription PCR and Western blot analyses also showed that curcumin treatment prevented the MCAO injury-induced reduction in γ-enolase expression. The results of this study suggest that curcumin exerts its neuroprotective function in focal cerebral ischemia by regulating the expression of γ-enolase.
Keywords: γ-enolase, curcumin, neuroprotection
Enolases are glycolytic enzymes that participate in cellular activities such as cell growth and cell differentiation [1]. There are three enolase isoforms: α-, β-, and γ-enolase [2]. Among these, γ-enolase is known as neuronal-specific enolase (NSE) or enolase 2 because it is localized in nervous tissue. This isoform has neurotrophic and neuroprotective effects in a broad spectrum of neurons including neocortical neuron, mesencephalic neurons, and spinal cord cultures [3,4]. γ-enolase promotes the survival of neurons and protects neurons in a low-oxygen atmosphere [3,4].
Curcumin (C21H20O6) is a polyphenol component present in the roots of Curcuma longa Linn (Zingiberaceae) plants (turmeric) [5]. It is the most abundant curcuminoid in turmeric. Curcumin is bright-yellow in color and used as a food additive to coloring or spices. Moreover, curcumin has various antitumor, antioxidant, anti-amyloid, and anti-inflammatory effects [6,7,8,9]. Curcumin protects neuronal cells against middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia and counteracts oxidative stress resulting from traumatic brain injury [6,10]. Although curcumin exerts a neuroprotective effect in ischemic brain injury, little is known about how curcumin exerts its neuroprotective effects at the molecular level. We hypothesized that curcumin may regulate γ-enolase expression in ischemic brain injury. Thus, we investigated whether curcumin can regulates γ-enolase expression in focal cerebral ischemia.
Materials and Methods
Experimental animals and treatment
Male Sprague-Dawley rats (225-250 g, n=40) were obtained from Samtako (Animal Breeding Center, Osan, Korea) and were randomly divided into four groups as follow: vehicle+sham, curcumin+sham, vehicle+MCAO, and curcumin+MCAO (n=10 per group). The animals were kept 18-22℃ with a 12 h light/12 h dark cycle and had free access to a pellet diet. All experimental procedures minimized animal suffering and followed a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Gyeongsang National University (GNU-LA-021). A single dose of vehicle or curcumin (50 mg/kg) was given via intraperitoneal injection 1 h after the onset of MCAO as described previously [11,12]. A physiological saline solution including 1% dimethyl sulfoxide was used as the vehicle and curcumin (Sigma, St. Louis, MO, USA) was dissolved in this solution.
Middle cerebral artery occlusion
MCAO surgical operation was performed as described previously [13]. Briefly, animals were anesthetized with sodium pentobarbital (30 mg/kg) via intraperitoneal injection before MCAO. For the right MCAO surgical operation, the right common carotid artery, external carotid artery, and internal carotid artery were exposed through a midline incision. A 4/0 monofilament nylon suture with a rounded tip obtained by heating was inserted into the right external carotid artery and gently advanced into the internal carotid artery until the rounded tip blocked the origin of the middle cerebral artery. Body temperature was kept at approximately 37℃ with a heating pad during the surgical procedure and recovery. Sham-operated animals underwent the same surgical operation without insertion of monofilament nylon. At 24 h after the onset of permanent occlusion, animals were sacrificed and right cortex tissues were collected.
2-Dimensional gel electrophoresis
A proteomics technique approach was performed as previously described [14]. Briefly, right cortex tissues were homogenized in lysis buffer (8M urea, 4% CHAPS, ampholytes, and 40 mM Tris-HCl), the lysates were centrifuged at 16,000 g for 20 min at 4℃, and the supernatants were collected. Protein concentration was determined with the Bradford assay (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol. IPG gel strips (range pH 4-7 and pH 6-9, 17 cm, Bio-Rad) were incubated in rehydration buffer (8 M urea, 2% CHAPS, 20mM DTT, 0.5% IPG buffer, and bromophenol blue) for 13 h at room temperature. Proteins extracts (50 µg) were loaded on IPG strips (pH 4-7 and 6-9) via a sample cup and subjected to first dimension isoelectric focusing (IEF) using an Ettan IPGphor 3 (GE Healthcare, Uppsala, Sweden) as follow: l,250 V (15 min), 10,000 V (3 h), and then 10,000-50,000 V. After first dimension separation, strips were incubated in equilibration buffer (6 M urea, 30% glycerol, 2% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl, bromophenol blue) containing DTT and iodoacetamide. Strips were then loaded onto gradient gels (7.5-17.5%) and second-dimension electrophoresis was performed using Protein-II XI electrophoresis equipment (Bio-Rad) at 5 mA for 2 h followed by 10 mA at 10℃ until the bromophenol blue dye migrated off the bottom of the gel.
Silver staining, image analysis and protein identification
Gels were then fixed in a fixation solution (12% acetic acid, 50% methanol) for 2 h and stained with silver stain solution (0.2% silver nitrate, 0.75 mL/L formaldehyde). Silver-stained gels were scanned using an Agfar ARCUS 1200™ scanner (Agfar-Gevaert, Mortsel, BEL). PDQuest 2-D analysis software (Bio-Rad) was used to determine which protein spots were differentially expressed among the different groups. Gel particles of identified protein spots were subjected to trypsin digestion and the resultant peptides were extracted using extraction buffer (5% trifluoroacetic acid in 50% acetonitrile). Extracted peptides were dried and analyzed with a Voyager-DE℃ STR biospectrometry workstation (Applied Biosystems, Foster City, CA, USA) for MALDI-TOF mass spectrometric analysis. Database searches were performed using MSFit and ProFound programs. SWISS-PROT and NCBI were used as reference protein sequence databases.
Reverse-transcription PCR
For reverse-transcription PCR, total RNA was extracted using Trizol reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). Total RNA (1 µg) was reverse transcribed for PCR using the Superscript III. First-Strand System (Invitrogen) according to the manufacturer's instructions. Primer sequences for γ-enolase were 5'-TGGATCTCCATACTGCCAAAG-3' (forward) and 5'-CCAACTCCTCTTCAATCCTCAT-3' (reverse). Primer sequences for β-actin were 5'-GGGTC AGAAGGACTCCTACG-3' (forward) and 5'-GGTCTC AAACATGATCTGGG-3' (reverse). Thirty cycles of amplification were performed as follows: melting at 95℃ for 30 sec, annealing at 52.5℃ for 30 sec, and extension at 72℃ for 30 sec. PCR products were loaded on 1% agarose gels and visualized under UV light.
Western blot analysis
Western blot analysis was performed as previously described method [14]. Right cerebral cortices were dissolved in lysis buffer (1M Tris-HCI, 5M sodium chloride, 0.5% sodium deoxycholate, 10% sodium dodecyl sulfate, 1% sodium azide, 10% NP-40) containing 10 mM leupeptin and 200 µM phenylmethylsulfonyl fluoride. After sonication and centrifugation, protein concentration was determined using a bicinchoninic acid (BCA) kit (Pierce, Rockford, IL, USA) according to the manufacturer's protocol. An equal amount of protein (30 µg) per sample was electrophoresed on 10% SDS-polyacrylamide gels and then proteins were transferred to poly-vinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). To minimize nonspecific antibody binding, the membranes were blocked with skim milk for 1 h at room temperature. Membranes were washed in Tris-buffered saline containing 0.1% Tween-20 and then incubated with the following antibodies: rabbit polyclonal anti-rat γ-enolase (diluted 1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse monoclonal anti-rat actin (diluted 1:1000, Santa Cruz Biotechnology). Membrane were then incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000; Pierce). An enhanced chemiluminescence Western blot analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, USA) was used to detect immunoreactivity reaction according to the manufacturer's protocol. SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, USA) were used to analyze band intensities.
Data analysis
All data are expressed as mean±S.E.M. Results from each group were compared by two-way analysis of variance (ANOVA) followed by a post-hoc Scheffe's test. Differences were considered significant when P<0.05.
Results
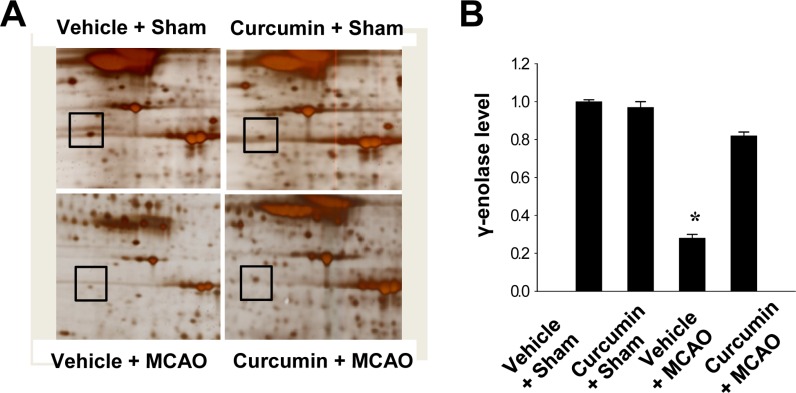
We found that expression of γ-enolase differed between control MCAO animals and those treated with curcumin after MCAO. Expression levels of γ-enolase were lower in the cerebral cortices of vehicle+MCAO animals than sham-operated animals. However, this decrease in γ-enolase expression was prevented in the curcumin+MCAO animals. Expression levels of γ-enolase were similar between vehicle+sham and curcumin +sham animals. Levels of γ-enolase levels were 0.28±0.02 and 0.82±0.02 in the vehicle+MCAO and curcumin +MCAO groups, respectively. Moreover, levels of γ-enolase were 1.01±0.02 and 0.97±0.02 in the vehicle+sham and curcumin+sham groups, respectively (Figure 1).
Figure 1. γ-Enolase protein spots identified by MALDI-TOF in the cerebral cortices from vehicle+sham, curcumin+sham, vehicle+middle cerebral artery occlusion (MCAO), and curcumin+MCAO animals. Squares indicate the protein spots. The intensity of spots was measured using PDQuest software. The ratio of intensity is described as spots intensity of these animals to spots intensity of sham+vehicle animals. Data are shown as mean±S.E.M. *P<0.05.
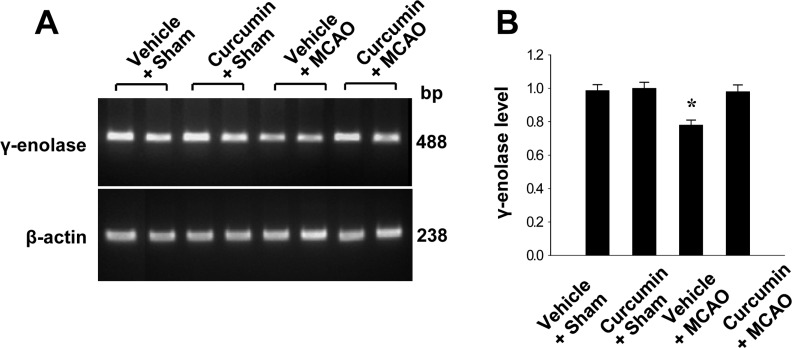
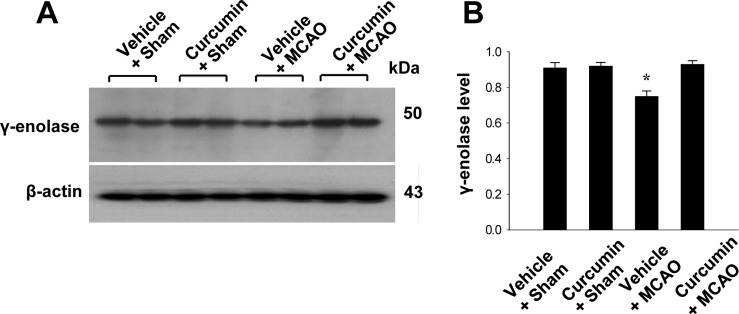
Reverse transverse-PCR and Western blot analyses were used to further investigate these results. γ-Enolase transcript levels were decreased in the cerebral cortices of vehicle+MCAO animals, while curcumin treatment prevented these decrease. Transcript levels of γ-enolase were 0.78±0.03 and 0.98±0.04 in the vehicle+MCAO and curcumin+MCAO groups, respectively. Transcript levels of γ-enolase were similar between vehicle+sham and curcumin+sham animals (Figure 2). Moreover, γ-enolase protein levels were reduced in the cerebral cortices of vehicle+MCAO animals compared to sham-operated animals. However, this reduction in γ-enolase protein expression was prevented in curcumin+MCAO animals (Figure 3). γ-Enolase protein levels were expressed at similar levels in two sham-operated animal groups. γ-enolase protein levels were 0.75±0.03 and 0.93±0.02 in the vehicle+MCAO and curcumin+MCAO groups, respectively (Figure 3).
Figure 2. Reverse transcription-PCR analysis of γ-enolase in the cerebral cortices from vehicle+sham, curcumin+sham, vehicle+middle cerebral artery occlusion (MCAO), and curcumin+MCAO animals. Each lane represents an individual experimental animal. Densitometric analysis is represented as intensity of γ-enolase to intensity of actin. Data (n=5) are represented as mean±S.E.M. *P<0.05.
Figure 3. Western blot analysis of γ-enolase in the cerebral cortices from vehicle+sham, curcumin+sham, vehicle+middle cerebral artery occlusion (MCAO), and curcumin+MCAO animals. Each lane represents an individual experimental animal. Densitometric analysis is represented as intensity of g-enolase to intensity of actin. Data (n=5) are represented as mean±S.E.M. *P<0.05.
Discussion
Curcumin exerts a powerful neuroprotective effect on focal cerebral ischemia in rats by anti-apoptotic mechanisms [11,12]. Curcumin treatment improves neurological scores and reduces infarct volume against cerebral ischemic injury [12]. Moreover, curcumin protects neurons against cerebral ischemia through suppression of the inflammatory response and oxidative damage [15,16]. The results of this study demonstrated that MCAO injury decreased the expression of γ-enolase, whereas curcumin treatment attenuated this decrease. γ-enolase has neurotrophic and neuroprotective effects on cultured neurons from embryonic rat brains [4]. Moreover, γ-enolase promotes neurite regeneration and neuron survival [1,3,4]. Thus, γ-enolase is considered to be a neuronal survival factors in the central nervous system [17]. We previously reported decreased expression of γ-enolase in focal cerebral ischemia [14]. This study clearly showed that γ-enolase expression is decreased in cerebral ischemic injury, whereas curcumin treatment prevents the injury-induced decrease. We confirmed these results using reverse transverse-PCR and Western blot analyses. Our results clearly show that curcumin regulates γ-enolase expression in an experimental models of cerebral ischemia. Thus, our findings suggest that curcumin maintains the level of γ-enolase expression against MCAO-induced ischemic injury and that this maintenance of γ-enolaseexpression contributes to the neuroprotective effect of curcumin.
γ-Enolase is known to promotes neurite outgrowth and cell survival by activation of PI3K/Akt and MAPK/ERK signalling pathways [18]. Curcumin exerts a neuroprotective effect in cortical neuron against oxygen and glucose deprivation through a PI3K/Akt dependent pathway [19]. Moreover, curcumin ameliorates oxygen and glucose deprivation-induced blood brain barrier dysfunction in brain microvascular endothelial cells via the heme oxygenase-1 pathway [20]. Therefore, curcumin regulates various proteins to protect cells against oxidative stress. Although the relationship between γ-enolase and curcumin is not yet well established, preservation of γ-enolase by curcumin during MCAO contributes to the neuroprotective effect of curcumin. Further studies are required to elucidate how curcumin regulates γ-enolase expression. In conclusion, our findings indicate that curcumin modulates γ-enolase expression during focal cerebral ischemia, and that regulation of γ-enolase by curcumin contributes to curcumin's neuroprotective effects.
γ-Enolase is known to promotes neurite outgrowth and cell survival by activation of PI3K/Akt and MAPK/ERK signalling pathways [18]. Curcumin exerts a neuroprotective effect in cortical neuron against oxygen and glucose deprivation through a PI3K/Akt dependent pathway [19]. Furthermore, a previous study demonstrated that curcumin increases brain derived neurotrophic factor, TrkB, and phosphatidylinositide 3-kinases (PI3K) protein expressions in hippocampal injury [20]. They demonstrated that curcumin mediated the neuroprotection against brain injury and the promotion neural regeneration, which the underlying mechanism is involved in activating derived neurotrophic factor and TrkB pathway [20]. Moreover, curcumin ameliorates oxygen and glucose deprivation-induced blood brain barrier dysfunction in brain microvascular endothelial cells via the heme oxygenase-1 pathway [21]. Therefore, curcumin regulates various proteins to protect cells against oxidative stress.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2007300).
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Schmechel DE, Brightman MW, Marangos PJ. Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res. 1980;190(1):195–214. doi: 10.1016/0006-8993(80)91169-5. [DOI] [PubMed] [Google Scholar]
- 2.Rider CC, Taylor CB. Enolase isoenzymes in rat tissues. Electrophoretic, chromatographic, immunological and kinetic properties. Biochim Biophys Acta. 1974;365(1):285–300. doi: 10.1016/0005-2795(74)90273-6. [DOI] [PubMed] [Google Scholar]
- 3.Hattori T, Ohsawa K, Mizuno Y, Kato K, Kohsaka S. Synthetic peptide corresponding to 30 amino acids of the C-terminal of neuron-specific enolase promotes survival of neocortical neurons in culture. Biochem Biophys Res Commun. 1994;202(1):25–30. doi: 10.1006/bbrc.1994.1888. [DOI] [PubMed] [Google Scholar]
- 4.Hattori T, Takei N, Mizuno Y, Kato K, Kohsaka S. Neurotrophic and neuroprotective effects of neuron-specific enolase on cultured neurons from embryonic rat brain. Neurosci Res. 1995;21(3):191–198. doi: 10.1016/0168-0102(94)00849-b. [DOI] [PubMed] [Google Scholar]
- 5.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57(1):1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 6.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197(2):309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Guo L, Xing Y, Pan R, Jiang M, Gong Z, Lin L, Wang J, Xiong G, Dong J. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS One. 2013;8(8):e70565. doi: 10.1371/journal.pone.0070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82(1):138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 9.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 10.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74(8):969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–232. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010;35(3):374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 13.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 14.Gim SA, Koh PO. Ferulic acid prevents the injury-induced decrease of γ-enolase expression in brain tissue and HT22 cells. Lab Anim Res. 2014;30(1):8–13. doi: 10.5625/lar.2014.30.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002;46(3):273–279. doi: 10.1016/s1043-6618(02)00123-8. [DOI] [PubMed] [Google Scholar]
- 16.Liu ZJ, Liu W, Liu L, Xiao C, Wang Y, Jiao JS. Curcumin Protects Neuron against Cerebral Ischemia-Induced Inflammation through Improving PPAR-Gamma Function. Evid Based Complement Alternat Med. 2013;2013:470975. doi: 10.1155/2013/470975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takei N, Kondo J, Nagaike K, Ohsawa K, Kato K, Kohsaka S. Neuronal survival factor from bovine brain is identical to neuron-specific enolase. J Neurochem. 1991;57(4):1178–1184. doi: 10.1111/j.1471-4159.1991.tb08277.x. [DOI] [PubMed] [Google Scholar]
- 18.Hafner A, Obermajer N, Kos J. γ-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem J. 2012;443(2):439–450. doi: 10.1042/BJ20111351. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen Y, Zhao J, Zhao Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One. 2013;8(3):e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Song S, Li J, Liang T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson's disease rat. Pathol Res Pract. 2014;210(6):357–362. doi: 10.1016/j.prp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang YF, Gu YT, Qin GH, Zhong L, Meng YN. Curcumin ameliorates the permeability of the blood-brain barrier during hypoxia by upregulating heme oxygenase-1 expression in brain microvascular endothelial cells. J Mol Neurosci. 2013;51(2):344–351. doi: 10.1007/s12031-013-9989-4. [DOI] [PubMed] [Google Scholar]