Abstract
Background
Chronic pain is common, costly, and leads to significant morbidity in older adults, yet we have limited data on medication safety. We sought to evaluate the association of incident High Risk Medication in the Elderly (HRME) with mortality, emergency department (ED) or hospital care among older adults with chronic pain.
Methods
A retrospective Veterans Health Administration cohort study was conducted examining older Veterans with chronic pain diagnoses and use of incident HRME (opioids, skeletal muscle relaxants, antihistamines, and psychotropics). Outcomes evaluated included all-cause mortality, ED visits, or inpatient hospital care. Descriptive statistics summarized variables for the overall cohort, the chronic pain cohort, and those with and without HRME. Separate generalized linear mixed-effect regression models were used to examine the association of incident HRME on each outcome, controlling for potential confounders.
Results
Among 1,807,404 Veterans who received VA care in 2005–2006, 584,066 (32.3%) had chronic pain; 45,945 Veterans with chronic pain (7.9%) had incident HRME exposure. The strongest significant associations of incident HRME were for: high-risk opioids with all-cause hospitalizations (OR 2.08, 95%CI 1.95–2.23); skeletal muscle relaxants with all-cause ED visits (OR 2.62, 95%CI 2.52–2.73) and mortality (OR 0.80, 95%CI 0.74–0.86); antihistamines with all-cause ED visits (OR 2.82 95%CI 2.72–2.95); and psychotropics with all-cause hospitalizations (OR 2.15, 95%CI 1.96–2.35).
Conclusions
Our data indicate that incident HRME is associated with clinically important adverse outcomes in older Veterans with chronic pain and highlight the importance of being judicious with prescribing certain classes of drugs in this vulnerable population.
Keywords: Pharmacoepidemiology, Adverse drug outcomes, Chronic pain, Aging
INTRODUCTION
Chronic pain is highly prevalent in all adult groups 1 with over 50% of the US population age 65 and older reporting bothersome pain in the last month 2. Among primary care patients in the Veterans Health Administration (VHA), up to 50% of male and 75% of female patients report chronic pain 3–5. Pain is associated with poorer self-reported health status, higher levels of emotional distress, decreased social and physical activities 3,6, and greater use of healthcare resources 7,8. While pain is common, costly, and leads to significant morbidity, older adults are not well represented in clinical trials 9; therefore, we have limited evidence to inform decisions especially with respect to medication safety in this population 10.
Older adults with chronic pain have high rates of analgesic use 11,12 and are also highly susceptible to adverse effects (ADEs) of analgesic treatments 10. A meta-analysis of observational studies found that the odds of being hospitalized for ADE-related conditions are four times higher for older compared with younger individuals. Up to 88% of ADE-related hospitalizations in older adults are potentially preventable 13. Compared with younger adults, older adults are twice as likely to present to emergency departments for ADE (over 177,000 emergency visits each year) and nearly seven times more likely to be hospitalized after an emergency visit 14. As highlighted by the Institute of Medicine15, preventing ADEs in older adults is a public health and patient safety priority.
The risk of ADEs increases in older adults due to a unique combination of age-related physiologic changes including altered drug absorption, decreased renal excretion, multimorbidity, polypharmacy, and functional impairments 10,16. Exposure to high-risk medications for the elderly (HRME), a Healthcare Effectiveness Data and Information Set (HEDIS) quality measure, was developed by the National Committee on Quality Assurance (NCQA) in 2006 to address this concern. The HEDIS HRME measure 17 was developed by an expert panel and includes drugs from the Beers criteria 18 for potentially inappropriate prescribing in the elderly that have been previously identified as being high risk for potentially severe outcomes19. This measure is now used to benchmark the quality of medication management in older adults enrolled in Medicare and other managed care plans20,21. Older adults with chronic pain may be at high risk for ADEs related to HRME such as opioids 22, skeletal muscle relaxants 23, antipsychotics 24, and sedating antihistamines, which are often prescribed in patients with pain although the evidence for their effectiveness and safety is lacking.
The objective of this study is to evaluate the association of incident HRME with mortality, emergency department (ED) or hospital care among older adults (65+) with chronic pain. To accomplish these objectives, data were used from a Department of Veterans Affairs (VA) nationwide cohort of older adults with chronic pain. We hypothesized that, after adjusting for potential confounders, older adults with chronic pain and incident HRME will be more likely than those without incident HRME to experience adverse outcomes after adjusting for potential confounders.
METHODS
For this population-based cohort study, we used data from the VA Health Care System administrative and clinical databases. These databases are the repositories of clinical data from more than 150 VA hospitals and 850 outpatient clinics. Details regarding this study cohort have been previously published 25,26.
Ethics Statement
The Institutional Review Board of the University of Texas Health Science Center at San Antonio approved this study and granted a waiver of informed consent.
Study Design, Setting, and Sample
The cohort consisted of Veterans 65 years of age and older on October 1, 2005, and who received VA care (n = 1,807,404) during Fiscal Year (FY) 2005 (October 1, 2004– September 30, 2005) and FY 2006 (October 1, 2005– September 30, 2006). From that population, the sample was restricted to Veterans who had diagnoses of chronic pain [neuropathic, nocireceptive, mixed] defined using ICD-9-CM codes 27 at least two times, 7 or more days apart during FY2004-2006 (Appendix 1). To examine incident use, individuals who had prior exposure to outpatient prescriptions for any HRME in FY05 were excluded from the analyses.
Data Sources
We used inpatient and outpatient demographic, healthcare utilization, and comorbidity data from the VA National Patient Care Database. Pharmacy data extracted from VA Pharmacy Benefits Management (PBM) dataset and vital status information obtained from the Vital Status file, were described in greater detail in prior publications 26. Encrypted patient identifiers linked information across these databases.
Outcomes
The outcome measures examined were all-cause mortality, ED visits, or inpatient hospital care. Outcomes were evaluated from initial HRME exposure to one year after the index date. Secondary outcomes were inpatient admissions or ED visits, due to falls or non-spine fractures. Both primary and secondary outcomes were selected based on clinical importance and as potential surrogate markers for falls and fractures.
Independent Variables
We evaluated the four types of most commonly 25 used HEDIS HRME: opioids, skeletal muscle relaxants, antihistamines, and psychotropics. Drug exposure was defined using the VA Product name, which identifies any dose or formulation of that drug (see Table 1). We identified incident HRME by drug category. We attempted to evaluate incident use of indomethacin and oral ketorolac, however, the sample size (n= 925) was too small to evaluate our outcomes of interest.
Table 1.
High Risk Medications in Older Adults with Chronic Pain
| Drug Group | Drugs Included | Concerns Regarding Use |
|---|---|---|
| Opioids | Propoxyphene, meperidine, pentazocine | Confusion, falls, fractures, dependency, withdrawal |
| Skeletal muscle relaxants | Methocarbamol, cyclobenzaprine, carisoprodol, chlorzoxazone, metaxalone, orphenadrine | Anticholinergic adverse effects, excessive sedation, and weakness; questionable effectiveness |
| Antihistamines | Diphendydramine, hydroxyzine, promethazine, cyproheptadine, dexchlorpheniramine, tripelennamine | Confusion and sedation, anticholinergic adverse effects |
| Psychotropics | Diazepam, chlordiazepoxide, flurazepam, Thioridazine, meprobamate, barbiturates | Excessive sedation, falls, central nervous system and extrapyrimidal side effects, dependency |
Covariates
We identified patient demographic characteristics (i.e., age, gender, race/ethnicity) between FY2004 and FY2006. Race/ethnicity was categorized as white, black, Hispanic (of any race), other, and missing. Missing demographic data on race/ethnicity are common in VA files; however, several years of data alleviate this problem. We examined socioeconomic status based on the VA means test; prior studies have shown that income under the VA poverty limit is associated with exposure to HRME 28–30.
Greater disease burden -- defined by more physical and psychiatric comorbidities, prior health care utilization, and more prescribed medications -- place patients at higher risk for adverse outcomes 31,32. We used ICD-9-CM codes from VA inpatient and outpatient data (FY04-05) to identify individuals with physical and psychiatric conditions using the Selim comorbidity indices 33. The Selim measures consist of a continuous count of up to 30 physical disease diagnoses (including stroke, hypertension, diabetes, cardiovascular disease, peripheral vascular disease, osteoarthritis) and a count of up to six mental health diagnoses (including schizophrenia, bipolar disorder, depressive disorder, posttraumatic stress disorder, substance abuse disorder, and anxiety disorders). These comorbidity measures were selected because they were developed and validated in a VA population. We also examined prior healthcare utilization including any emergency department visits or hospital admissions, as well as the number of primary care visits in FY 2005. Finally, we included the number of unique medication classes received in FY 2005.
Statistical Analyses
Descriptive statistics were used to summarize our cohort. Chi-square tests were used to test the relationship between categorical variables and student’s t-test to test the relationship for continuous variables. We used separate generalized linear mixed-effect regression models to examine the association of the incident HRME on each of our [binary] outcomes with the primary medical center as a random effect after adjusting for the variables listed in Table 3.
Table 3.
Chronic Pain Cohort: Characteristics Among Subjects With and Without Incident HRME
| Characteristic | No Incident HRME (n = 538,121) n (%) | Incident HRME (n = 45,945) n (%) | P-value |
|---|---|---|---|
| Age groups | |||
| 65–74 | 234,643 (43.6) | 23,270 (50.7) | <0.001 |
| 75–84 | 250,906 (46.6) | 19,211 (41.8) | |
| 85+ | 52,573 (9.8) | 3,464 (7.5) | |
| Male | 527,637 (98.1) | 44,474 (96.8) | <0.001 |
| Race/Ethnicity | |||
| White | 370,212 (68.8) | 33,710 (73.4) | <0.001 |
| African American | 37,830 (7.0) | 4,362 (9.5) | |
| Hispanic | 17,646 (3.3) | 2,332 (5.1) | |
| Other/Missing | 112,433 (20.9) | 5541 (12.1) | |
| Under the poverty limit | 349,733 (65.0) | 36,617 (79.7) | |
| Selim Physical Comorbidity Index | |||
| 0–1 | 50,088 (9.3) | 2,751 (6.0) | <0.001 |
| 2–3 | 256,449 (47.7) | 17,016 (37.0) | |
| 4–5 | 168,587 (31.3) | 16,292 (35.5) | |
| 6+ | 62,997 (11.7) | 9,886 (21.5) | |
| Selim Psychiatric Comorbidity Index | |||
| 0 | 454,350 (84.4) | 34,405 (74.9) | <0.001 |
| 1 | 65,591 (12.2) | 8,288 (18.0) | |
| 2+ | 18,180 (3.4) | 3,252 (7.1) | |
| Number of unique drugs | |||
| 0–5 | 207,216 (28.5) | 8,869 (19.3) | <0.001 |
| 6–8 | 142,062 (26.4) | 9,976 (21.7) | |
| 9–11 | 94,365 (17.5) | 9,754 (21.2) | |
| 12+ | 94,478 (17.6) | 17,346 (37.8) | |
| Outcomes | |||
| Death within 1 year | 24,869 (4.6) | 3,817 (8.3) | <0.001 |
| ED visits | 88,616 (16.5) | 19,473 (42.4) | <0.001 |
| ED visits related to falls/fractures | 2,677 (.50) | 3,344 (0.57) | <0.001 |
| Hospitalization | 32,455 (6.0) | 8,311 (18.1) | <0.001 |
| Hospitalization related to falls/ fractures | 658 (0.1) | 164 (0.4) | <0.001 |
Statistical significance was defined as a two-tailed p value of ≤ 0.05. STATA 13 (College Station, Texas) was used for all analyses.
RESULTS
Baseline Characteristics
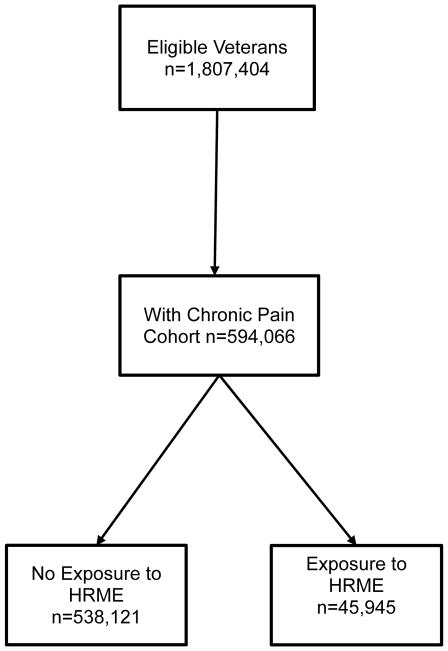
Of the 1,807,404 eligible veterans identified in FY 2006, 584,066 met inclusion criteria for chronic pain and were included in this cohort (see Figure 1). Table 2 shows the difference between the chronic pain cohort and the rest of the cohort. The chronic pain group is more likely to be 85 years of age or older and has a greater number of physical and psychiatric comorbid conditions. Among those with chronic pain, 7.9% had exposure to incident HRME compared with 4% exposure to incident HRME in the non-pain group.
Figure 1.
Derivation of study cohort
Table 2.
Description of Chronic Pain Group vs. Those Without Chronic Pain
| Characteristic | Chronic Pain (n =584,066) n (%) | Without Chronic Pain (n =1,223,338) | P-value |
|---|---|---|---|
| Age groups | |||
| 65–74 | 257,913 (44.2) | 561,992 (45.9) | <0.001 |
| 75–84 | 270,116 (46.3) | 558,343 (45.6) | |
| 85+ | 56,037 (9.6) | 103,003 (8.4) | |
| Male | 572,111 (98.0) | 1,206,167 (98.6) | <0.001 |
| Race/Ethnicity | |||
| White | 403,922 (69.2) | 788,732 (64.5) | <0.001 |
| African American | 42,192 (7.2) | 70,691 (5.8) | |
| Hispanic | 19,978 (3.4) | 36,309 (3.0) | |
| Other/Missing | 117,974 (20.2) | 327,606 (26.8) | |
| Below the poverty limit | 386,350 (66.2) | 673,324 (55.0) | <0.001 |
| Selim Physical Comorbidity Index | |||
| 0–1 | 52,839 (9.1) | 461,005 (37.7) | <0.001 |
| 2–3 | 273,465 (46.8) | 583,124 (47.7) | |
| 4–5 | 184,879 (31.7) | 151,606 (12.4) | |
| 6+ | 72,883 (12.5) | 27,603 (2.3) | |
| Selim Psychiatric Comorbidity Index | |||
| 0 | 488,755 (83.7) | 1,089,177 (89.0) | <0.001 |
| 1 | 73,879 (12.7) | 109,909 (9.0) | |
| 2+ | 21,432 (3.7) | 24,252 (2.0) | |
| Number of unique drugs | |||
| 0–5 | 216,085 (37.0) | 662,815 (54.2) | <0.001 |
| 6–8 | 152,038 (26.0) | 313,917 (25.7) | |
| 9–11 | 104,119 (17.8) | 150,710 (12.3) | |
| 12+ | 111,824 (19.2) | 95,896 (7.8) | |
| Incident HRME | 45,945 (7.9) | 48,739 (4.0) | <0.001 |
In the chronic pain cohort, nearly half (44.2%) were between 65 and 74 years, 46.3% were between 75 and 84 years, and 9.6% were 85 years of age and older. The chronic pain cohort was primarily male (98.0%), white (69.2%), and nearly half had 2–3 physical comorbidities (46.8%). The majority had no psychiatric comorbidities (83.7%). During the 1-year study period, 28,686 (4.9%) died, 40,766 (7.0%) had one or more hospital admissions, and 108,089 (18.5%) received emergency care.
Table 3 lists baseline descriptive characteristics of the chronic pain cohort for those who did and did not receive incident HRME in FY2006 (n=584,066). Osteoarthritis, low back pain, and diabetes were the most frequently reported comorbid conditions regardless of HRME status. The frequencies of outcomes (mortality, ED, and hospitalizations) by incident HRME exposure are shown in Table 3.
In the chronic pain cohort among those who received incident HRME (n=45,945), the incident drug exposure by class of drug was: high-risk opioid use (n=3,609, 7.9%), muscle relaxant use (n=6,932, 15.1%), antihistamine use (n=6,544, 14.2%), and psychotropic use (n=2,252, 4.9%).
Results of Multivariable Regression Models
Table 4 summarizes results for the association between incident HRME drugs and the specified outcomes. We found statistically significant associations of incident HRME with high-risk opioids for all adverse outcomes, especially all-cause hospitalizations (OR 2.08, 95%CI 1.95–2.23), and ED visits related to falls or fracture (OR 2.15, 95%CI 1.78–2.62). The association between exposure to skeletal muscle relaxants and all adverse outcomes was statistically significant, except for hospitalizations related to falls or fracture; the strongest association was for all-cause ED visits (OR 2.62, 95%CI 2.52–2.73). While the association between incident exposure to antihistamines was statistically significant with all adverse outcomes, the strongest was for all-cause ED visits (OR 2.82 95%CI 2.72–2.95). The association between incident exposure to psychotropic medications and adverse outcomes was statistically significant, except for hospitalizations related to falls or fractures (OR 1.09, 95%CI 0.56–2.12). Curiously, exposure to muscle relaxants had a significant association with lower all-cause mortality (OR 0.80, 95%CI 0.74–0.86).
Table 4.
Multilevel Regression Models that Examined the Associations Between Incident HRME Drug Class and Outcomes in Chronic Pain Cohort
| All-Cause Mortality | ED | ED-falls/fractures | Hospitalization | Hospitalization- falls/fracture | |
|---|---|---|---|---|---|
| Adjusted OR (95% CI) | |||||
| Opioids | 1.13 (1.03–1.24) | 1.94 (1.84–2.06) | 2.15 (1.78–2.62) | 2.08 (1.95–2.23) | 2.04 (1.39–3.00) |
| Skeletal muscle relaxants | 0.80 (0.74–0.86) | 2.62 (2.52–2.73) | 1.81 (1.57–2.09) | 1.56 (1.48–1.65) | 1.10 (0.77–1.58) |
| Antihistamines | 1.78 (1.68–1.89) | 2.83 (2.72–2.95) | 1.60 (1.39–1.84) | 2.22 (2.12–2.32) | 2.03 (1.57–2.62) |
| Psychotropics | 1.15 (1.01–1.32) | 2.02 (1.87–2.19) | 1.47 (1.11–1.96) | 2.15 (1.96–2.35) | 1.09 (0.56–2.12) |
DISCUSSION
Our study is the first, to our knowledge, to describe the associations of incident HRME with potentially ADE-related acute care in a population-based sample of older Veterans with chronic pain. Prior work from this study found much lower incident HRME exposure in the full cohort of older VA patients. These findings are relevant because they come from a nationally representative sample, allowing for extrapolation to the entire older veteran population and potentially other populations of older men in the United States. Further, our results highlight the importance of being judicious with prescribing certain classes of drugs in older adults with chronic pain.
The associations between incident HRME and ED/hospital visits are consistent with findings from Albert et al. 34 who reported prevalent HRME use with nearly a 2-fold increase in hospital admissions in a retiree cohort receiving employer-based drug benefits 34. However, Albert et al did not specifically evaluate a chronic pain population and examined prevalent HRME rather than incident HRME, which may result in misclassification and potentially underestimate this association.
All four of the HRME drug categories evaluated in this study are frequently prescribed (with the exception of propoxyphene which is no longer available) and often considered benign by both prescribers and patients. Surprisingly, we found that skeletal muscle relaxants were associated with a reduced risk of mortality. It is possible that physicians prescribe muscle relaxants to the most functional patients, or those who request these medications to maintain an active lifestyle, however, underlying mechanisms for this association is unclear. Both skeletal muscle relaxants and antihistamines were associated with significantly higher odds of potentially ADE-related acute care particularly ER visits. Given the relative paucity of data with regard to medication safety (as well as potential efficacy) in older adults, our results, for all four classes of incident HRME, provide evidence for prescribing these medications with caution in older Veterans with chronic pain.
The challenge that clinicians face is choosing a medication that will reduce the pain symptoms, safely, in older populations. Chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) is not recommended in older adults for well-known/established gastrointestinal, renal, and cardiovascular toxicity 35,36. Capturing NSAID use is a recognized challenge since this class of medications is often purchased over the counter and not always through the VA pharmacy. Our small sample size for those receiving indomethacin and oral ketorolac precluded evaluation of our outcomes of interest. Adjuvant therapies, such as tricyclic antidepressants, for example, are often tried, however, limited evidence for its safety and efficacy exists specifically in older adults, and the tertiary tricyclic antidepressants (including amitriptyline) are not included in the HEDIS measure. Given known (and ongoing research that is uncovering) adverse effects of pharmacologic therapies, further emphasis on non-pharmacological approaches (i.e. physical therapy, cognitive behavioral therapy, transcutaneous electrical nerve stimulation, mindfulness, meditation, relaxation, guided affective imagery, biofeedback, prayer, and music therapy) is encouraged 7,27,36. Ultimately, future research must focus on determining tolerable and safe combinations of pharmacological and non-pharmacological management approaches that are effective and sustainable in older adults with chronic pain.
Important strengths of our study include large population-based sample of Veterans who receive care from an integrated health care system and the use of EMR data that has been shown to enhance ADE detection 37. Another important strength to highlight is the use of Selim measures of comorbidity to provide a more comprehensive assessment of disease burden (especially the psychiatric component). This is particularly important given the known relationship between chronic pain and psychiatric conditions 38.
This study has several limitations. The outcomes selected were potentially caused by ADEs however we did not validate the likelihood of these encounters being ADE- related by manually reviewing each medical record. Another potential source of underestimation of these problems is our inability to capture potentially ADE-related acute care that occurred outside the VA system. While we evaluated high-risk opioids in this analysis, propoxyphene (on the list of included opioids) has since been removed from the market. Many patients who had been receiving this opioid could have been transitioned to another opioid not listed in Table 1; these opioids (for example, oxycodone) are not included in the HEDIS HRME or Beer’s criteria, and therefore, not the focus of this paper. Despite this, our results do show increased adverse outcomes in chronic pain patients using this class of medication. We would anticipate, based on class effect, that given the wide-spread use of opioids such as oxycodone, the outcomes we report for opioids are potentially an underestimate. This should be evaluated in future research. The sample consisted mostly of community dwelling older male veterans so the results may not be extrapolated to older men who reside in long term care facilities, older females, or younger populations.
CONCLUSIONS
Our data indicate that incident HRME is associated with clinically important adverse outcomes in older Veterans with chronic pain. A better understanding of the outcomes associated with incident HRME in an older chronic pain population is a critical step towards making providers more aware of potentially harmful effects of medications. Further research is needed to develop intervention measures to reduce exposure to these high-risk medications. These are all necessary steps towards proposing effective, safe and cost conscious management approaches for older adults with chronic pain.
Supplementary Material
Acknowledgments
The authors would like to thank Joseph T. Hanlon, PharmD, MS for his input on this manuscript.
Funding: Dr. Makris was supported by the Rheumatology Research Foundation/ASP Junior Career Development Award in Geriatric Medicine, an NIA GEMSSTAR (R03AG040653) and the Center for Translational Medicine, NIH/NCATS Grants (KL2TR001103 and UL1TR001105).
Footnotes
The report has not been published elsewhere, nor is it under consideration elsewhere.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Center for Translational Medicine, UT Southwestern Medical Center and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
References
- 1.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009 Feb 9;169(3):251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: Findings from the 2011 National Health and Aging Trends Study. PAIN®. 2013;154(12):2649–2657. doi: 10.1016/j.pain.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans' reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system. Journal of rehabilitation research and development. 2003 Sep-Oct;40(5):371–379. doi: 10.1682/jrrd.2003.09.0371. [DOI] [PubMed] [Google Scholar]
- 4.Haskell SG, Heapy A, Reid MC, Papas RK, Kerns RD. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Womens Health (Larchmt) 2006 Sep;15(7):862–869. doi: 10.1089/jwh.2006.15.862. [DOI] [PubMed] [Google Scholar]
- 5.Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. Journal of pain and symptom management. 2002 Feb;23(2):131–137. doi: 10.1016/s0885-3924(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 6.Haskell SG, Papas RK, Heapy A, Reid MC, Kerns RD. The association of sexual trauma with persistent pain in a sample of women veterans receiving primary care. Pain Med. 2008 Sep;9(6):710–717. doi: 10.1111/j.1526-4637.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age and ageing. 2013 Mar;42( Suppl 1):i1–57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 8.Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Medical care research and review : MCRR. 2003 Sep;60(3 Suppl):40S–53S. doi: 10.1177/1077558703256724. [DOI] [PubMed] [Google Scholar]
- 9.Paeck T, Ferreira ML, Sun C, Lin CW, Tiedemann A, Maher CG. Are older adults missing from low back pain clinical trials? - A systematic review and meta-analysis. Arthritis care & research. 2013 Dec 10; doi: 10.1002/acr.22261. [DOI] [PubMed] [Google Scholar]
- 10.Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011 Sep;12(9):1336–1357. doi: 10.1111/j.1526-4637.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008 Sep 15;138(3):507–513. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. American journal of public health. 2010 Dec;100(12):2541–2547. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharmacy world & science : PWS. 2002 Apr;24(2):46–54. doi: 10.1023/a:1015570104121. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) [accessed June 21, 2012];Adults and older adults adverse drug events: Medication Safety Program. Available at http://www.cdc.gov/MedicationSafety/Adult_AdverseDrugEvents.html.
- 15.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington DC: National Academic Press; 2011. [PubMed] [Google Scholar]
- 16.Field TS, Gurwitz JH, Harrold LR, et al. Risk factors for adverse drug events among older adults in the ambulatory setting. J Am Geriatr Soc. 2004 Aug;52(8):1349–1354. doi: 10.1111/j.1532-5415.2004.52367.x. [DOI] [PubMed] [Google Scholar]
- 17.High Risk Medications in the Elderly. National Committee on Quality Assurance; [Accessed June 21, 2012]. [online]. Available at http://www.ncqa.org/portals/0/hedisqm/HEDIS2008/Vol2/NDC/Table%20DAE-A.doc. [Google Scholar]
- 18.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003 Dec 8–22;163(22):2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 19.Zhan C, Sangl J, Bierman AS, et al. Potentially inappropriate medication use in the community-dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey. JAMA. 2001 Dec 12;286(22):2823–2829. doi: 10.1001/jama.286.22.2823. [DOI] [PubMed] [Google Scholar]
- 20.The state of health care quality: value, variation and vulnerable populations 2009. National Committee for Quality Assurance; [Accessed August 6, 2010]. (online). Available at http://www.ncqa.org/Portals/0/Newsroom/SOHC/SOHC_2009.pdf. [Google Scholar]
- 21.Use of High Risk Medications in the Elderly. National Committee on Quality Assurance; [Accessed October 24, 2005]. (online). Available at http://www.ncqa.org/portals/0/hedisqm/HEDIS2008/Vol2/NDC/Table%20DAE-A.doc. [Google Scholar]
- 22.Pergolizzi J, Böger RH, Budd K, et al. Opioids and the Management of Chronic Severe Pain in the Elderly: Consensus Statement of an International Expert Panel with Focus on the Six Clinically Most Often Used World Health Organization step III Opioids (Buprenorphine, Fentanyl, Hydromorphone, Methadone, Morphine, Oxycodone) Pain Pract. 2008;8(4):287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 23.van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine (Phila Pa 1976) 2003 Sep 1;28(17):1978–1992. doi: 10.1097/01.BRS.0000090503.38830.AD. [DOI] [PubMed] [Google Scholar]
- 24.Merskey H, Hester RA. The treatment of chronic pain with psychotropic drugs. Postgraduate medical journal. 1972 Oct;48(564):594–598. doi: 10.1136/pgmj.48.564.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugh MJ, Marcum ZA, Copeland LA, et al. The quality of quality measures: HEDIS(R) quality measures for medication management in the elderly and outcomes associated with new exposure. Drugs & aging. 2013 Aug;30(8):645–654. doi: 10.1007/s40266-013-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh MJ, Hanlon JT, Wang CP, et al. Trends in use of high-risk medications for older veterans: 2004 to 2006. J Am Geriatr Soc. 2011 Oct;59(10):1891–1898. doi: 10.1111/j.1532-5415.2011.03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persons APoPPiO. The management of persistent pain in older persons. J Am Geriatr Soc. 2002 Jun;50(6 Suppl):S205–224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 28.Pugh MJ, Starner CI, Amuan ME, et al. Exposure to potentially harmful drug-disease interactions in older community-dwelling veterans based on the Healthcare Effectiveness Data and Information Set quality measure: who is at risk? J Am Geriatr Soc. 2011 Sep;59(9):1673–1678. doi: 10.1111/j.1532-5415.2011.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VA Information Resource Center. Medical SAS® inpatient dataset FY2009: VIReC research user guide. Hines (IL): VIReC; [Accessed December 2013]. 2011. [online]; Available from URL: http://www.virec.research.va.gov/DataSourcesName/Medical-SAS-Datasets/MedSAS-Inpt-RUG/MedSAS-RUG-Inpt.htm. [Google Scholar]
- 30.VA Information Resource Center. VHA Medical SAS® outpatient datasets and inpatient encounters dataset FY2009: VIReC Research User Guide. Washington, DC: VA Information Resource Center; 2011. [Accessed December 2013]. [online]; Available from URL: http://www.virec.research.va.gov/DataSourcesName/Medical-SAS-Datasets/MedSAS-Outpt-RUG/MedSAS-RUG-Outpt.htm. [Google Scholar]
- 31.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011 Nov 24;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine; Washington DNAP, editor To err is human: Building a safer health system. [Google Scholar]
- 33.Selim AJ, Fincke G, Ren XS, et al. Comorbidity assessments based on patient report: results from the Veterans Health Study. The Journal of ambulatory care management. 2004 Jul-Sep;27(3):281–295. doi: 10.1097/00004479-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Albert SM, Colombi A, Hanlon J. Potentially inappropriate medications and risk of hospitalization in retirees: analysis of a US retiree health claims database. Drugs & aging. 2010 May;27(5):407–415. doi: 10.2165/11315990-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age and Ageing. 2013 Mar;42:I1–I57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 36.AGS Panel on Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. An Update. J Am Geriatr Soc. 2009;57:1331–46. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 37.Nebeker JR, Hoffman JM, Weir CR, Bennett CL, Hurdle JF. High rates of adverse drug events in a highly computerized hospital. Arch Intern Med. 2005 May 23;165(10):1111–1116. doi: 10.1001/archinte.165.10.1111. [DOI] [PubMed] [Google Scholar]
- 38.Reid MC, Williams CS, Gill TM. The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. J Am Geriatr Soc. 2003 Aug;51(8):1092–1098. doi: 10.1046/j.1532-5415.2003.51357.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.