Summary
Background
Many countries now offer support to teenage mothers to help them to achieve long-term socioeconomic stability and to give a successful start to their children. The Family Nurse Partnership (FNP) is a licensed intensive home-visiting intervention developed in the USA and introduced into practice in England that involves up to 64 structured home visits from early pregnancy until the child's second birthday by specially recruited and trained family nurses. We aimed to assess the effectiveness of giving the programme to teenage first-time mothers on infant and maternal outcomes up to 24 months after birth.
Methods
We did a pragmatic, non-blinded, randomised controlled, parallel-group trial in community midwifery settings at 18 partnerships between local authorities and primary and secondary care organisations in England. Eligible participants were nulliparous and aged 19 years or younger, and were recruited at less than 25 weeks' gestation. Field-based researchers randomly allocated mothers (1:1) via remote randomisation (telephone and web) to FNP plus usual care (publicly funded health and social care) or to usual care alone. Allocation was stratified by site and minimised by gestation (<16 weeks vs ≥16 weeks), smoking status (yes vs no), and preferred language of data collection (English vs non-English). Mothers and assessors (local researchers at baseline and 24 months' follow-up) were not masked to group allocation, but telephone interviewers were blinded. Primary endpoints were biomarker-calibrated self-reported tobacco use by the mother at late pregnancy, birthweight of the baby, the proportion of women with a second pregnancy within 24 months post-partum, and emergency attendances and hospital admissions for the child within 24 months post-partum. Analyses were by intention to treat. This trial is registered with ISRCTN, number ISRCTN23019866.
Findings
Between June 16, 2009, and July 28, 2010, we screened 3251 women. After enrolment, 823 women were randomly assigned to receive FNP and 822 to usual care. All follow-up data were retrieved by April 25, 2014. 304 (56%) of 547 women assigned to FNP and 306 (56%) of 545 assigned to usual care smoked at late pregnancy (adjusted odds ratio [AOR] 0·90, 97·5% CI 0·64–1·28). Mean birthweight of 742 babies with mothers assigned to FNP was 3217·4 g (SD 618·0), whereas birthweight of 768 babies assigned to usual care was 3197·5 g (SD 581·5; adjusted mean difference 20·75 g, 97·5% CI −47·73 to 89·23. 587 (81%) of 725 assessed children with mothers assigned to FNP and 577 (77%) of 753 assessed children assigned to usual care attended an emergency department or were admitted to hospital at least once before their second birthday (AOR 1·32, 97·5% CI 0·99–1·76). 426 (66%) of 643 assessed women assigned to FNP and 427 (66%) 646 assigned to usual care had a second pregnancy within 2 years (AOR 1·01, 0·77–1·33). At least one serious adverse event (mainly clinical events associated with pregnancy and infancy period) was reported for 310 (38%) of 808 participants (mother–child) in the usual care group and 357 (44%) of 810 in the FNP group, none of which were considered related to the intervention.
Interpretation
Adding FNP to the usually provided health and social care provided no additional short-term benefit to our primary outcomes. Programme continuation is not justified on the basis of available evidence, but could be reconsidered should supportive longer-term evidence emerge.
Funding
Department of Health Policy Research Programme.
Introduction
Individual, social, and economic circumstances faced by teenage mothers can challenge a successful start for children and interrupt mothers' long-term socioeconomic stability. Children of teenage mothers are more likely to have lower birthweight, not be breastfed, be at greater risk of accidents and early death, do worse educationally, have more emotional and behavioural problems, and become teenage parents themselves.1, 2, 3, 4 Intervention early in the lives of families with young mothers might enhance life chances for both mother and child.5, 6, 7, 8
Research in context.
Evidence before this study
We reviewed the existing trial evidence in May, 2008. Because intellectual property is owned by the University of Colorado, all previous trials were restricted to the three US evaluations (in Elmira [NY], Memphis [TN], and Denver [CO]). As is commonly found in home-visiting evaluations, each trial in the USA identified multiple outcomes and was not formally adjusted for multiple significance testing. The trials reported positive effects in prenatal health behaviours, birth outcomes, sensitive child care, child and adolescent functioning, and maternal lifecourse. Two outcomes with prior replicated positive effects by 24 months post-partum are changes in smoking and second pregnancy. In Elmira, the intervention reduced cigarettes smoked by four, and in Denver a reduction in cotinine was reported in mothers who received the intervention. In Memphis, nurse-visited mothers reported fewer second pregnancies by 24 months post-partum (36% vs 47%). In Denver, nurse-visited mothers reported fewer subsequent pregnancies by 24 months post-partum (29% vs 41%). In 2013, a trial of the intervention in the Netherlands reported lower rates of smoking in nurse-visited women in late pregnancy.
Added value of this study
Because the programme was adapted for use in England and used young age as an enrolment criteria, we would not regard our work as a simple replication study of previous trials, although the intervention remained a licensed programme. Rather, our trial provides data for the intervention as adapted for implementation in a UK setting and delivered in a substantially different publicly funded and configured health-care system. We show that adding the Family Nurse Partnership programme to usual care provided no additional short-term benefit for our selected primary outcomes (smoking in pregnancy, birthweight, emergency hospital attendance and admission for the child, and subsequent pregnancy). The study findings are the most directly applicable to practice and setting in the UK, where the programme is now provided in England under the operating framework for the NHS to about 16 000 concurrent women in parallel and subsequent to the trial period. The lack of benefit evidenced for changes in smoking during pregnancy and second pregnancies therefore limits the degree to which programme benefits shown elsewhere can be assumed in different health-care settings and to different service populations, even when objective programme fidelity from US trials was maintained.
Implications of all the available evidence
Continued provision of the Family Nurse Partnership programme cannot be supported on the basis of the trial evidence found for its effectiveness in the UK setting. Subsequent changes to the intervention itself, to usually provided care, or to the population targeted would justify re-examination. Similarly, any positive benefits observed through longer-term follow-up of the current trial cohort might shift the evidentiary balance in favour of the intervention and warrants continued evaluation of the trial cohort. Nevertheless, the overall level of unaddressed clinical need such as smoking in pregnancy (56%) and rate of subsequent pregnancies (66%) remains high in this population and a public health challenge that remains unresolved.
Several home-visiting interventions have been introduced to support families, with evidence of their benefit mostly reported from the USA.9 Programmes typically address multiple maternal and child outcomes and can improve birth outcomes, cognitive and socioeconomic development, use of preventive health care, and reduce potential abuse (eg, injuries, ingestions, and emergency department attendances) and maltreatment.10, 11, 12, 13
In England, the Healthy Child Programme (HCP) forms the universal offer of clinical and public health for children and families during pregnancy to adulthood.14 Specific programmes to support children growing up in disadvantaged circumstances have included area-based Sure Start local programmes and the more targeted Sure Start Plus schemes for pregnant teenage girls.15, 16, 17 In 2006, the UK Government announced its intention to test the Family Nurse Partnership (FNP) programme, University of Colorado, Denver, CO, USA, as an intensive preventive home-visiting service. FNP was initially adapted from the US Nurse Family Partnership programme, one of 12 programmes meeting criteria for an evidence-based model.13, 18, 19, 20, 21 FNP is a fee-based programme available only under licence, with the intellectual property owned by the University of Colorado who retain authority for approving any alterations. Three US evaluations (in Elmira [NY], Memphis [TN], and Denver [CO]) have taken place, as well as one in the Netherlands, with positive results for mothers and babies.18, 20, 21, 22
For roll-out in England, an implementation evaluation in ten sites involving first-time pregnant women aged up to 23 years at enrolment preceded our trial and reported evaluation results (pregnancy and post-partum period) focused on feasibility and acceptability from 2008.23, 24, 25 As the third phase of a model of international replication (after adaptation and pilot testing phases), we aimed to establish the effectiveness of FNP when delivered in a broadly based, publicly funded, health-care setting.
Methods
Study design and participants
We did a pragmatic, open-label, individually randomised controlled trial to compare usual care (through primary-care public health and social care services) plus FNP (FNP group) to usually provided health and social care alone (usual care group). The protocol has been published.26 The 18 study sites were partnerships of primary and secondary local National Health Service (NHS) organisations and local authorities. Sites applied to the Department for Health to deliver FNP and additionally to participate in the trial with selection against set eligibility criteria (appendix).
To be eligible, women had to be nulliparous, aged 19 years or younger, living within the catchment area of a local FNP team, of less than 25 weeks' gestation, and able to provide consent and speak English. Women expecting multiple births and those with a previous pregnancy ending in miscarriage, stillbirth, or termination were eligible. Women planning to have their child adopted or to move outside of the FNP catchment area for longer than 3 months were not eligible. Women were identified and approached via local maternity services and recruited usually at their home by locally based researchers. The study was approved by the Wales NHS Research Ethics Committee (09/MRE09/08) and received governance approval from all participating NHS sites. All women provided written informed consent.
Randomisation and masking
Women were randomly assigned to FNP or usual care, with randomisation stratified by site and minimised by gestation (<16 weeks vs ≥16 weeks), smoking (yes vs no), and preferred language of data collection (English vs non-English) and weighted towards minimising the imbalance in trial groups with probability 0·8. The allocation programme was created by a programmer at the Bristol Randomised Trials Collaboration, a registered Clinical Trials Unit, and allocation was concealed using a remote computer-based system, accessible via telephone and internet by the recruiting researcher. Mothers and field-based researchers (even if also assessors) were not masked to group allocation, but assessors collecting data by computer-assisted telephone interview were masked to allocation.
Procedures
FNP involves up to 64 structured home visits by specially recruited and trained family nurses (appendix). Developed in the USA for first-time pregnant women, it was adapted under licence for delivery in England from early pregnancy until children were 2 years old. FNP aims to affect risks and protective factors within prenatal health-related behaviours, sensitive and competent care-giving, and early parental lifecourse. Core specialist training for nurses includes motivational interviewing and the adoption of a guiding autonomy-supportive communication style with clients.
All participants (both groups) were eligible to receive usually provided publicly funded health and social care. This included the HCP (universally offered screening, education, immunisation, and support from birth to the child's second birthday) delivered by either family nurses (FNP group) or specialist community public health nurses (usual care group), and maternity care appropriate to clinical need.
Routine data were collected by field-based researchers from maternity units, by direct data download by a trial statistician from the Health and Social Care Information Centre (HSCIC), by field-based researchers or practice staff from primary care centres, from the Abortions Statistics Manager at the Department of Health for abortion statistics, and from COVER (Coverage Of Vaccination Evaluated Rapidly) contacts directly from primary health-care authorities and used to obtain information about birthweight, emergency department attendances and admissions and second pregnancies, as well as for some secondary outcomes. Additionally, information about emergency department attendance and admissions, and second pregnancies was collected by maternal report. Information about tobacco use was collected by self-report and from urine samples. Self-report data for secondary outcomes (appendix) were collected at baseline (before allocation) and 24 months post-partum by local researchers using a face-to-face structured computer-assisted personal interview; interviewers were not blinded to allocation but independent of service provision. Office-based researchers who were blind to allocation used computer-assisted telephone interviews to collect self-reported data at late pregnancy (34–36 weeks' gestation), and 6, 12 and 18 months post-partum.
Urine samples for cotinine assay were obtained during the baseline interview and at late pregnancy (34–36 weeks' gestation). At both timepoints women were categorised as non-smokers if they reported not smoking in the 3 days before interview and had a urinary cotinine concentration lower than 100 ng/mL. When only baseline cotinine concentrations were available, women reporting not smoking at late pregnancy and who were classified as either accurate or over-reporters of tobacco use (from comparing baseline self-report and cotinine concentrations; appendix) were also categorised as non-smokers. All other women were categorised as smokers in the primary analysis.
To measure the number of contacts between women and support workers in both groups, mothers reported access to health care during the trial (including visits from community midwives and health visitors) and family nurses reported contact with their clients.
Participants were able to withdraw at any time and had the right to withdraw use of data already collected. Women who wanted to discontinue the intervention were offered the opportunity to still provide follow-up data. All participants who had not formally withdrawn were asked if they would complete the 24 month post-partum assessment, even if they had previously not responded to data collection in preceding waves of follow-up. Women were regarded as mandatory withdrawals should miscarriage, death, or adoption of the child occur. Adverse events notified to the trials office by standard reporting template were reviewed by a senior clinical researcher (JS) to establish relatedness and severity.
Outcomes
Primary outcomes were tobacco use at late pregnancy (34–36 weeks' gestation), birthweight, emergency attendances and hospital admissions for the infant within 24 months of birth, and the proportion of women with a second pregnancy within 24 months post-partum (appendix). Secondary prespecified outcomes were collected for many measures of pregnancy and birth, child health and development, and parental lifecourse from late pregnancy up to 24 months post-partum; a full list is in the appendix and the published protocol.26 Adverse events were reported via standard template and were categorised as an adverse event, serious adverse event, and expected serious adverse event (appendix).
Statistical analysis
We estimated effect sizes detectable given the number of available sites, recruitment period, and eligibility, consent, and follow-up rates. We calculated that a sample of 1418 women was needed to provide at least 90% power at the two-sided 2·5% α level to detect differences between trial groups of 10% (30% vs 40%) in proportion having any emergency attendance or hospital admission, and of 7·5% (12·5% vs 20·0%) in proportions of women with a second pregnancy by 24 months post-partum (ie, one child and one maternal outcome). We allowed for a pregnancy loss of 1·5% on the basis of clinically informed estimates (>16 week miscarriage rate of around 1%, terminations for fetal anomalies 0·02%, and a stillbirth rate of 0·5%) in our calculations. We expected to obtain follow-up data for three primary outcomes for 90% of participants using medical records. Therefore, we aimed to recruit 1600 pregnant women. We chose a 2·5% α level to allow for multiple primary outcomes within each individual population (ie, two primary outcomes each for mother and child).
All analyses were done by intention to treat without imputation, with outcome values compared between groups using mixed-effects three-level regression models to adjust for site as a stratification variable and to allow for clustering by a family nurse in the intervention group. When clustering was negligible in the FNP group at level of family nurse, as assessed by the Family Nurse level variance component from the three-level model, the simpler two-level model was used. Models were also adjusted for minimisation variables. For continuous outcomes, we fitted a linear-regression model and presented results as difference in adjusted means (FNP minus usual care). Estimates were obtained using restricted maximum likelihood. For binary outcomes a logistic model was used and the result presented as adjusted odds ratios (ORs) comparing the odds of an event in FNP compared with usual care group. Count data were analysed using a Poisson multilevel model or a negative binomial model if overdispersion was evident.
Where applicable, an analysis of covariance model was used with baseline measurement as a covariate. Where data were collected over several timepoints, a repeated measures model (using a generalised linear mixed model) was used with timepoints (6, 12, 18, and 24 months) nested within participants (nested within family nurse and site) and included an interaction term for time and trial group to investigate any divergent or convergent pattern in outcomes. The global interaction effect was tested and was in all cases non-significant, and the interaction and time term were both dropped from the model. Both the intercepts and slopes of participants' measures were allowed to vary randomly when possible. Various hierarchies were investigated, and the Akaike information criterion (AIC) used to establish the best fitting model.
We estimated costs per participant (including all health-related costs and intervention costs) from the perspective of the NHS and personal social services and discounted where appropriate. We did multiple imputation to allow for missing data and sensitivity analysis to assess robustness of results from this and other assumptions.
We assessed intervention delivery against FNP-specified fidelity goals including gestation at programme enrolment, number of valid visits received, attrition rates, visit duration, and coverage of planned visit content (appendix). Additional qualitative and quantitative substudies were also done to map usual care and assess use of motivational interviewing in routine FNP consultations, programme clients' experience, and perspectives of professionals (health visitors, midwives, and family nurses), but these are reported separately.
Analyses were done by two statisticians (RC-J and ZESR) using SPSS version 20 and Stata version 13. An independent data monitoring committee, reporting to an independent steering committee, oversaw the study. The trial is registered at the ISRCTN registry, number ISRCTN23019866.
Role of the funding source
The Department of Health (England) commissioned and funded the study, managed the grant application process, and acted as sponsor. The funder required the inclusion of specified policy-relevant primary outcomes and determined the number of study sites, but played no role in finalising study design, data collection, data analysis, data interpretation, or report writing. The corresponding author had full access to all study data, vouches for data accuracy and completeness, and had final responsibility for the decision to submit for publication.
Results
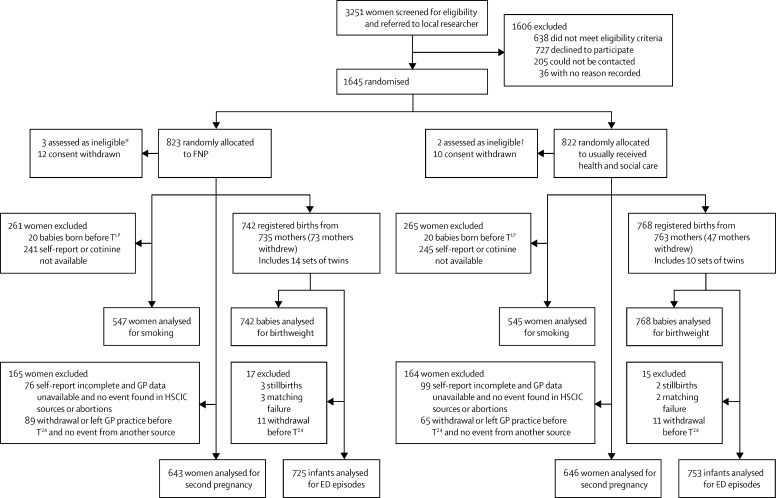
Between June 16, 2009, and July 28, 2010, 3251 women were screened (figure). 1645 women were recruited, with five subsequently excluded due to non-eligibility. 22 women who chose to withdraw also removed consent for use of their data. 121 women had withdrawn by late pregnancy and a further 72 subsequently, either electively or mandatorily, hence 215 women withdrew in total. Baseline characteristics were well balanced between trial groups (table 1). Numbers entered for analysis differed according to timing of planned assessment and data source (figure). 24 month post-partum participant assessments were completed by April 24, 2013, and HSCIC data retrieved by April 25, 2014.
Figure.
Trial profile
FNP=Family-Nurse Partnership. ED=emergency department. GP=general practitioner. HSCIC=Health & Social Care Information Centre. TLP=late pregnancy timepoint. *Two women registered with GP outside study area, one not pregnant at first scan. †One woman not Gillick competent, one woman registered with GP outside study area.
Table 1.
Sociodemographic characteristics at baseline
| FNP group (n=808) | Usual care group (n=810) | ||
|---|---|---|---|
| Age (years) | 17·9 (17·0–18·8) | 17·9 (16·9–18·8) | |
| Gestation | |||
| 16 weeks' or more | 330/807 (41%) | 328/810 (40%) | |
| Less than 16 weeks' | 477/807 (59%) | 482/810 (59%) | |
| Smoker* | |||
| No | 331/759 (44%) | 324/766 (42%) | |
| Yes | 428/759 (56%) | 442/766 (58%) | |
| Ethnic origin | |||
| White | 711 (88%) | 714 (88%) | |
| Mixed | 47 (6%) | 42 (5%) | |
| Asian | 16 (2%) | 11 (1%) | |
| Black | 31 (4%) | 40 (5%) | |
| Other | 3 (<1%) | 3 (<1%) | |
| Language usually spoken at home by participants | |||
| English only | 768 (95%) | 775 (96%) | |
| English and other language | 39 (5%) | 33 (4%) | |
| Other language or languages only | 1 (<1%) | 2 (<1%) | |
| Relationship status with baby's father | |||
| Married | 9 (1%) | 11 (1%) | |
| Separated | 79 (10%) | 86 (11%) | |
| Closely involved or boyfriend | 613 (76%) | 609 (75%) | |
| Just friends | 107 (13%) | 104 (13%) | |
| Living with parents | |||
| Both | 181 (22%) | 171 (21%) | |
| One | 318 (39%) | 349 (43%) | |
| Neither | 309 (38%) | 290 (36%) | |
| Living with father of baby | |||
| Yes | 184/736 (25%) | 184/744 (25%) | |
| No | 552/736 (75%) | 560/744 (75%) | |
| Not in education employment or training (NEET) status† | |||
| Yes | 333/695 (48%) | 330/685 (48%) | |
| No | 362/695 (52%) | 355/685 (52%) | |
| Has a paid job | |||
| Yes | 174 (21%) | 164 (20%) | |
| No | 634 (78%) | 646 (80%) | |
| In receipt of government welfare payments | |||
| Yes | 301/808 (37%) | 283/808 (35%) | |
| No | 507/808 (63%) | 525/808 (65%) | |
| Ever been homeless | |||
| Yes | 144 (18%) | 170 (21%) | |
| No | 664 (82%) | 640 (79%) | |
| Highest parental qualification | |||
| Up to post-graduate | 108/805 (13%) | 111/810 (14%) | |
| Up to A-level‡ | 172/805 (21%) | 176/810 (22%) | |
| Overseas or other qualifications | 79/805 (10%) | 80/810 (10%) | |
| None of these | 130/805 (16%) | 129/810 (16%) | |
| Do not know | 316/805 (39%) | 314/810 (39%) | |
| IMD score§ | 38·3 (24·9–52·4)‖ | 38·2 (25·5–51·6)** | |
Data are n (%), n/N (%), or median (IQR). FNP=Family Nurse Partnership programme plus usual care. IMD=Index of Multiple Deprivation.
Cotinine-calibrated smoking status at baseline.
NEET status applicable only to those older than 16 years at end of previous academic year.
High school diploma equivalent.
Higher IMD score indicates greater deprivation.
N=802.
N=804.
The proportion of mothers who smoked at late pregnancy did not differ between women assigned to FNP (56%) and those assigned to usual care (56%) for those with a calibrated smoking score (adjusted OR 0·90, 97·5% CI 0·64–1·28; table 2). This finding was robust to sensitivity analyses, which included only women with complete self-report data and recorded cotinine concentrations at both baseline and follow-up (n=870; appendix). Reported number of cigarettes smoked per day at late pregnancy did not differ between groups for women (n=610) classified at baseline as smokers (adjusted difference in means 0·12 cigarettes, 97·5% CI −0·73 to 0·97; table 2). Mean birthweights of babies were 3217·4 g (SD 618·0 g) for babies of women assigned to FNP and 3197·5 g (581·5 g) for those of women assigned to usual care, an adjusted difference of 20·75 g (97·5% CI −47·73 to 89·23). The proportion of women with a second pregnancy within 24 months of their first child's birth did not differ between the FNP (66%) and usual care (66%) groups, with an adjusted OR of 1·01 (97·5% CI 0·77–1·33; table 2). Sensitivity analyses for second pregnancy are in the appendix. Rates of emergency attendances or hospital admissions within 24 months of birth included in the analysis were 81% for FNP and 77% usual care group (OR 1·32, 97·5% CI 0·99–1·76). No differential effects due to maternal age, deprivation, participation in employment, education or training, or basic life skills were found for any primary outcome in planned subgroup analyses.
Table 2.
Primary outcome results
| FNP group | Usual care group | Total | Unadjusted risk difference (97·5% CI) | Adjusted*intervention effect (97·5% CI) | p value | |||
|---|---|---|---|---|---|---|---|---|
| Maternal outcomes | ||||||||
| Total mothers | 808 | 810 | 1618 | .. | .. | .. | ||
| Smoking at late pregnancy† | ||||||||
| Women who smoked | 304/547 (56%) | 306/545 (56%) | 610/1092 (56%) | −0·6% (−7·3 to 6·2) | 0·90‡ (0·64 to 1·28) | 0·51 | ||
| Unadjusted mean cigarettes smoked per day | 8·8 (5·6) | 8·4 (5·9) | 8·6 (5·8) | .. | 0·12§ (−0·73 to 0·97) | 0·75 | ||
| Women who did not smoke | 243/547 (44%) | 239/545 (44%) | 482/1092 (44%) | .. | .. | .. | ||
| Pregnancies within 24 months¶ | ||||||||
| Women who were recorded as pregnant within 24 months | 426/643 (66%) | 427/646 (66%) | 853/1289 (66%) | 0·2% (−5·8 to 6·1) | 1·01‡ (0·77 to 1·33) | 0·92 | ||
| Women not pregnant within 24 months | 217/643 (34%) | 219/646 (34%) | 436/1289 (34%) | .. | .. | .. | ||
| Child outcomes | ||||||||
| Total babies | 742 | 768 | 1510 | .. | .. | .. | ||
| Birthweight | ||||||||
| Unadjusted mean birthweight (g) | 3217·4 (618·0) | 3197·5 (581·5) | 3207·3 (599·6) | .. | 20·75§ (−47·73 to 89·23) | 0·50 | ||
| At least one emergency attendance or admission within 24 months of birth‖ | ||||||||
| Emergency attendance or admission | 587/725 (81%) | 577/753 (77%) | 1164/1478 (79%) | 4·3% (0·2 to 8·5) | 1·32‡ (0·99 to 1·76) | 0·03 | ||
| No emergency attendance or admission | 138 (19%) | 176 (23%) | 314 (21%) | |||||
Data are n (%), n/N (%), or mean (SD), unless otherwise specified. FNP group=Family Nurse Partnership programme plus usual care.
Adjusted for stratification (site) and minimisation variables (gestational age and smoking status at recruitment, and first or preferred language); one participant did not have two minimisation variables recorded and was excluded from all analyses.
Data were missing for 526 women: 421 not interviewed at late pregnancy, 40 not asked smoking questions as participant had given birth, and 65 had incomplete self-report or missing cotinine at baseline.
Adjusted odds ratio for FNP versus usual care.
Adjusted difference in means for FNP minus usual care.
Data were missing for 329 women: 154 were missing due to incomplete follow-up (withdrawals or left the general practitioner practice before 2 years no subsequent pregnancy flagged), 175 where both maternal self-report and primary care records (taken as the most reliable sources) were missing and no subsequent pregnancy was flagged in any of the other data sources (inpatients, outpatients, abortions).
Data were missing for 32 children: five children could not be linked to Health and Social Care Information Centre data; the remaining 27 were withdrawals that had no event recorded up to point of withdrawal.
Some secondary outcomes suggested small positive impacts of the FNP (appendix). These were intention-to-breastfeed, maternally reported child cognitive development (at 24 months only), language development using a modified maternal-reported assessment (at 12 and 18 months) and using a standardised assessment (the Early Language Milestone; at 24 months), levels of social support, partner-relationship quality, and general self-efficacy. Rates of child safeguarding concerns documented in primary care records were higher for FNP clients. There were no other differences found (for more detail see appendix).
The mean number of visits of community midwives between baseline and 24 months post-partum were roughly the same between groups (10·40 [SD 5.34] for the FNP group [n=459] vs 10·68 [SD 5·25] for the usual care group [n=422]). Mean number of visits from health visitors were nearly double for those assigned to usual care (8·60 [13·74] for the FNP group [n=363] vs 16·25 [12·15] for the usual care group [n=321]). The FNP group (n=709) received 39·28 (15·19) FNP nurse visits (the usual care group [n=10] received 0·45 [4·26] because of enrolment to the FNP group in error).
A full-sample base case analysis (782 in FNP group vs 786 in usual care group) with use of multiple imputation showed an incremental cost for FNP of £1993 per participant. Although individual types of resource use were similar across trial groups, intervention delivery costs (FNP calls and visits) accounted for the substantial incremental cost of FNP. Sensitivity analysis that included complete cases only (217 in FNP group vs 186 in usual care group) suggested the incremental cost of the FNP was £4670 (95% CI 3322–6017).
The sample was similar to other populations of women offered FNP in non-trial sites in England, and in trial sites after the closure of the trial (appendix). Attrition at key assessments (24 month interview, smoking) resulted in mostly small differences in women not included in analysis between groups (appendix). The delivery of FNP assessed against programme fidelity goals is described in the appendix.
The proportion of participants with at least one serious adverse event (mainly clinical events associated with pregnancy and infancy period) was similar (310 [38%] of 808 mother–child dyads in the usual care group vs 357 [44%] of 810 participants in the FNP group) and no events were considered related to the intervention.
Discussion
In this randomised controlled, pragmatic trial, we show no evidence of benefit from FNP for smoking cessation, birthweight, rates of second pregnancies, and emergency hospital visits for the child (table 2). Between-group differences were shown in a few secondary outcomes, of which there were a large number. FNP was delivered mostly in-line with FNP fidelity goals and as it could be provided in usual local NHS settings. In the absence of evidence of benefit, the programme cannot be considered cost effective for the primary outcomes of most relevance to FNP—ie, smoking cessation and second pregnancies.
Some short-term effects in the US trials were most evident in more vulnerable women (eg, with multiple risk factors or low psychological resources) at enrolment.18, 21 In the Dutch VoorZorg trial22 a two-stage selection process included women with multiple risk factors. In England, young maternal age was chosen as an easily measurable proxy for low income and its associated long-term adverse child outcomes.27 This programme criterion might have resulted in greater heterogeneity and comparatively less disadvantage than in previous studies. Although the absence of any interactions suggests that broad FNP enrolment criteria did not mask intervention effects in subgroups defined by a-priori markers of vulnerability, differential effect by subgroup could be explored further—we did not power this study to detect such effects.28
Unlike women in the US settings in which the intervention originated, teenage mothers in England can access many statutory supportive health and social services, including community based family doctors, midwives, and public-health nurses, and, in most trial sites, specialist teenage pregnancy midwives. Obstetric antenatal care, child surveillance, and access to emergency medicine were provided for usual care group participants in the US trials of NFP; however, in this UK-based trial, the extent of care provision accessible to the usual care group might have diluted any effect of FNP. Although our data do not quantify duration of health-care contact episodes, the overall rate of reported community midwifery contact was similar between groups. Women assigned to usual care saw health visitors eight more times than did those in the FNP group, but women assigned to FNP had had an average of 39 specialist nurse visits, each lasting on average longer than 1 h. Therefore, it is not likely that an equivalent level of health care was provided to women in the usual care group and that can itself explain a reduction in level of intervention effect. Further exploration of differential health-care provision (either usual care or FNP care) to the most vulnerable woman in our sample (who have previously been found to derive particular benefit most from FNP) could explain the relative absence of effects in our trial.
The intervention was originally developed by David Olds as the Nurse Family Partnership and adapted for use in England under licence by the Family Nurse Partnership National Unit, originally located within the Department of Health. The Department of Health commissioned the trial as the sole experimental and independent evaluation of the programme. The programme specification is in part summarised by the programme's core model elements and fidelity goals. Our assessment against these targets shows that the intervention has been delivered as intended, giving us confidence in its validity.
Effective planning of subsequent pregnancies should enable parents to complete education, manage work, and provide more focused parental nurture and guidance for their children.29, 30, 31 We did not find subsequent pregnancies to be more effectively planned in our study, reducing the potential to modify maltreatment and unintentional injury. The benefits of the intervention on maltreatment comes from the Elmira and Memphis studies,18, 20 evidenced through effect on injury and ingestions that resulted in emergency department visits, hospital admission, and length of hospital stay.32 Higher rates of safeguarding initiation for FNP-group children in our study might have resulted from surveillance bias, with higher levels of health-care contact and nurses adopting a lower threshold for intervention. Similarly, the higher presentation rates for emergency secondary care for children of mothers in the FNP group might reflect lower maternal thresholds for accessing care influenced by the FNP. Admissions rates for injuries and ingestions might be a more direct assessment of maltreatment compared with overall presentation to emergency departments. However, there remained no evidence of a difference between groups when we focused on this outcome (appendix). Only longer-term follow-up can establish whether any preventive effect exists.
Rates for all-cause attendances and admissions rates of emergency department episodes were higher than we anticipated. Factors such as changes to out-of-hours services for family doctors and difficulties in securing appointments might have contributed to such rates, although mothers reported frequent access overall to primary care for both themselves and for their child.
The Building Blocks trial was independent of programme delivery and adequately powered to detect intervention effects by the child's second birthday. Routine data sources enabled high levels of ascertainment for three of the primary outcomes. Previous US trials of the intervention were single-centre studies (one semi-rural, two urban), involved a small number of nurses delivering the intervention (eg, ten in Denver, CO, USA), and led by the intervention developers. By contrast, our trial was led independently of the 131 family nurses working in locally managed teams delivering the intervention in 18 trial sites across England. Our trial therefore represents a more pragmatic evaluation of FNP compared with previous trials, which had a greater emphasis on efficacy.
Due to the number of comparisons, conclusions must be drawn cautiously about secondary outcomes for which differences were shown, such as in language. Aside from safeguarding concerns, we show no evidence for differences between the two groups for maltreatment, prenatal health behaviour, and poor birth outcomes, all expected predictors of child neurodevelopment by the FNP model.33 Similarly, language and cognitive delays should be reduced by sensitive and competent caregiving. We did find small improvements in maternal self-efficacy, social support, and relationship quality, which would be consistent with mothers providing such care. We show no evidence of differences in maternal–child interaction or parental-role strain between groups, although these were the only assessments of parenting that we did. The observed development benefits were maternally reported and might be subject to reporting bias, but the relative importance of language development and the reduction in language delay that has been previously observed by age 2 years (in the Denver trial) would warrant further assessment in an older age group.
In commissioning the trial, the Department of Health Research Programme that funded this study specified inclusion of prenatal tobacco use, childhood injuries requiring emergency department attendance or admission, and interbirth interval as primary outcome domains that were considered on the basis of previous trials to be modifiable by FNP. Other primary outcomes, including birthweight, were also recommended by the meeting of an advisory committee to the funders as part of the development of the commissioning brief and included input from the representatives of the programme. Therefore, we included birthweight as a policy-relevant and readily measurable outcome that was applicable to all trial participants. It is identified as an outcome addressed by the programme (eg, on the current FNP website), although we would recognise that only the Elmira efficacy study has shown differences for the subgroup of younger women (as summarised in the logic model). We selected all emergency episodes as a primary outcome because we were confident that it could be reliably ascertained (ie, episodes of care more likely to be recorded than not, whereas reason for visit less accurately recorded), although episodes for injuries and ingestions were included as key secondary outcomes. An episode rate for all attendances can include both addressable and non-addressable reasons, hence the importance of the outcomes that focus on injury and ingestions alone. Events specific to injuries and ingestions therefore represent outcomes of most relevance to FNP, and clearer markers of programme impact. Focusing on injury and ingestion episodes alone in primary care, attendance of accident and emergency department, and hospital admission still did not show any group difference. Although length of stay has also been taken as an indicator of maltreatment, differences might exist between health-care settings in the UK and the USA that affects its interpretation. Length of stay is dependent not only on injury severity but also time and day of attendance, management required, and the availability of appropriate senior staff including anaesthetists to do relevant investigations. These considerations might distance length of stay from factors such as amounts of supervision, causative factors, or intent to injure. Although this was not recommended at commissioning, nor included as a trial outcome, it can be further explored in secondary analyses. Transparent reporting in this paper of all a-priori outcomes allows for further assessment of the relative benefits of the programme by policy makers and service commissioners.
In conclusion, we show substantial additional cost, no benefit for policy relevant main outcomes, and some advantage for a few secondary outcomes for mother and child when adding FNP to existing health service provision in England. Evidence for benefit for child development outcomes would mainly arise in children after the age of 2 years, requiring longer-term follow-up for this outcome.
Acknowledgments
Acknowledgments
This is an independent report commissioned and funded by the Policy Research Programme in the Department of Health (reference 006/0060). The views expressed are not necessarily those of the Department of Health. The South East Wales Trials Unit (SEWTU) is funded by the Wales Assembly Government through Health and Care Research Wales and the authors gratefully acknowledge SEWTU's contribution to study implementation. We thank all the women who participated in the study, the local professionals who facilitated recruitment and study implementation, and the family nurses who delivered the intervention. We acknowledge all other contributors to the study who are listed in full in the published full study report. We thank the trial steering committee independent members: Ann Louise Kinmonth (Chair), Silvia van den Heijkant, Pamela Park, Stavros Petrou, Rachel Tonkin; and the data monitoring committee independent members: Gordon Taylor (Chair), Lucy Akhtar, Sara Kenyon. We would like to pay special tribute to the late Professor Paul Wainwright who was the initial Chair of the Data Monitoring Committee. We thank the stakeholder involvement work package members: Joyce Kenkre (lead), Lily Bidmead, Kamila Hawthorne, Lesley Lowes, with contributions from members of the Books & Babies Group, and the Young Mums Groups. The trial administrators were Jackie Swain, Katy Addison, and Rhys Thomas.
Contributors
MR was the chief investigator and acts as overall guarantor for the study. JS was senior clinical researcher. All authors contributed to study design. KP was lead for maternal-reported outcomes. AK was lead for maltreatment assessment. All authors were responsible for study management. CCB chaired the trial management group. DT was lead for economics. SC was lead for the process evaluation. JK was lead for stakeholder involvement. EO-J was the trial manager, GM was the data manager, and M-JB was the process evaluation researcher. KB, BCM, GR, SR, and ES undertook the economic analyses. GM was responsible for data cleaning. AAM was the lead for the statistical analysis and RC-J and ZESR designed and undertook the statistical analysis. CCB, AK, JK, AAM, KP, and DT were co-applicants with MR on the original funding application. All authors interpreted the results of the analysis. GM drew the figure. RC-J was responsible for producing the tables. MR drafted the study report. All authors reviewed manuscript drafts, revised for important intellectual content, and approved the final version.
Local investigators
Sue Gibson, Anne Lewis, Andrea Anderson, Melvyn Dunstall, Julia Love, Linda Saynor, Alison Cook, Helen Ross-McGill, John Forde, Stephen Keay, Sarah Cooper, Sue Knowles, Anne Musgrave, Angela Oxley, Julia Savage, Chris Tully, Christine Davey, Stephen Lindow, Kate Brintworth, Yinglen Butt, Amanda Williams, Jane Mischenko, Julie Scarfe, Jan McColgan, Helen Scholfield, Trish Devey, Leroy Edozien, Gbemisola Okunoye, Eileen Stringer, Vanda Wellock, Sharon Toyer, Donna Darbyshire, Jenny Henry, Sarah St-Pierre, Liz Glenister, Barbara Hills, Nina Khazaezadeh, Diane Jones, Kim Hinshaw, Sonia Stewart, Deanne Gibbs, Karen Plumb, Lin Gostling, and David Shakespeare.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Kuh D, Hardy R, Langenberg C, Richards M, Wadsworth ME. Mortality in adults aged 26-54 years related to socioeconomic conditions in childhood and adulthood: post war birth cohort study. BMJ. 2002;325:1076–1080. doi: 10.1136/bmj.325.7372.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Power C, Matthews S. Origins of health inequalities in a national population sample. Lancet. 1997;350:1584–1589. doi: 10.1016/S0140-6736(97)07474-6. [DOI] [PubMed] [Google Scholar]
- 3.Evans GW, English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- 4.Lissau I, Sorensen TIA. Parental neglect during childhood and increased risk of obesity in young adulthood. Lancet. 1994;343:324–327. doi: 10.1016/s0140-6736(94)91163-0. [DOI] [PubMed] [Google Scholar]
- 5.Paranjothy S, Broughton H, Adappa R, Fone D. Teenage pregnancy: who suffers? Arch Dis Child. 2009;94:239–245. doi: 10.1136/adc.2007.115915. [DOI] [PubMed] [Google Scholar]
- 6.Harden A, Brunton G, Fletcher A, Oakley A, Burchett H, Backhans M. Young people, pregnancy and social exclusion: a systematic synthesis of research evidence to identify effective, appropriate and promising approaches for prevention and support. EPPI-Centre, Social Science Research Unit, Institute of Education, University of London; London: 2006. [Google Scholar]
- 7.Turley RNL. Are children of young mothers disadvantaged because of their mother's age or family background? Child Dev. 2003;74:465–474. doi: 10.1111/1467-8624.7402010. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay RE. Developmental origins of disruptive behaviour problems: the ‘original sin’ hypothesis, epigenetics and their consequences for prevention. J Child Psychol Psychiatry. 2010;51:341–367. doi: 10.1111/j.1469-7610.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services Home-visiting grants & grantees. http://mchb.hrsa.gov/programs/homevisiting/grants.html (accessed April 24, 2015).
- 10.Sweet MA, Appelbaum MI. Is home-visiting an effective strategy? A meta-analytic review of home-visiting programs for families with young children. Child Dev. 2004;75:1435–1456. doi: 10.1111/j.1467-8624.2004.00750.x. [DOI] [PubMed] [Google Scholar]
- 11.Filene JH, Kaminski JW, Valle LA, Cachat P. Components associated with home-visiting program outcomes: a meta-analysis. Pediatrics. 2013;132(suppl 2):S100–S109. doi: 10.1542/peds.2013-1021H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock S, Konrad S, Watson E, Nickel D, Muhajarine N. Effectiveness of home-visiting programs on child outcomes: a systematic review. BMC Public Health. 2013;13:17. doi: 10.1186/1471-2458-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avellar SA, Supplee LH. Effectiveness of home-visiting in improving child health and reducing child maltreatment. Pediatrics. 2013;132(Suppl 2):S90–S99. doi: 10.1542/peds.2013-1021G. [DOI] [PubMed] [Google Scholar]
- 14.Health Do . Healthy Child Programme. Pregnancy and the first five years of life. Department of Health; London: 2009. [Google Scholar]
- 15.Melhuish E, Belsky J, Barnes J. Evaluation and the value of Sure Start. Arch Dise Child. 2010;95:159–161. doi: 10.1136/adc.2009.161018. [DOI] [PubMed] [Google Scholar]
- 16.Melhuish E, Belsky J, Leyland AH, Barnes J, for the National Evaluation of Sure Start Research Team Effects of fully-established Sure Start Local Programmes on 3-year-old children and their families living in England: a quasi-experimental observational study. Lancet. 2008;372:1641–1647. doi: 10.1016/S0140-6736(08)61687-6. [DOI] [PubMed] [Google Scholar]
- 17.Melhuish E, Belsky J, MacPherson K, Cullis A. The quality of group childcare settings used by 3-4 year old children in Sure Start Local Programme areas and the relationship with child outcomes. Department of Education; London: 2010. [Google Scholar]
- 18.Olds DL, Henderson CR, Jr, Chamberlin R, Tatelbaum R. Preventing child abuse and neglect: a randomized trial of nurse home visitation. Pediatrics. 1986;78:65–78. [PubMed] [Google Scholar]
- 19.Olds DL, Henderson CR, Jr, Tatelbaum R, Chamberlin R. Improving the delivery of prenatal care and outcomes of pregnancy: a randomized trial of nurse home visitation. Pediatrics. 1986;77:16–28. [PubMed] [Google Scholar]
- 20.Kitzman H, Olds DL, Henderson CR., Jr Effect of prenatal and infancy home visitation by nurses on pregnancy outcomes, childhood injuries, and repeated childbearing. A randomized controlled trial. JAMA. 1997;278:644–652. [PubMed] [Google Scholar]
- 21.Olds DL, Robinson J, O'Brien R. Home-visiting by paraprofessionals and by nurses: a randomized, controlled trial. Pediatrics. 2002;110:486–496. doi: 10.1542/peds.110.3.486. [DOI] [PubMed] [Google Scholar]
- 22.Mejdoubi J, van den Heijkant SC, van Leerdam FJ, Crone M, Crijnen A, HiraSing RA. Effects of nurse home visitation on cigarette smoking, pregnancy outcomes and breastfeeding: a randomized controlled trial. Midwifery. 2014;30:688–695. doi: 10.1016/j.midw.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Barnes J, Ball M, Meadows P, Belsky J. Nurse-Family Partnership programme: first year pilot sites implementation in England—pregnancy and the post-partum period. Birkbeck, University of London; London: 2008. [Google Scholar]
- 24.Barnes J, Ball M, Meadows P, Belsky J, Team FNPIR. Nurse-Family Partnership programme: second year pilot sites implementation in England—the infancy period. Birkbeck, University of London; London: 2009. [Google Scholar]
- 25.Barnes J, Ball M, Meadows P. The Family-Nurse Partnership Programme in England: Wave 1 implementation in toddlerhood & a comparison between Waves 1 and 2a of implementation in pregnancy and infancy. London: Institute for the Study of Children, Families and Social Issues. University of London; Birkbeck: 2011. [Google Scholar]
- 26.Owen-Jones E, Bekkers M-J, Butler CC. The effectiveness and cost-effectiveness of the Family Nurse Partnership home-visiting programme for first time teenage mothers in England: a protocol for the Building Blocks randomised controlled trial. BMC Pediatrics. 2013;13:114. doi: 10.1186/1471-2431-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall D, Hall S. The “Family - Nurse Partnership”: developing an instrument for identification, assessment and recruitment of clients. Research report DCSF-RW022. Department of Children, Schools and Families; London: 2007. [Google Scholar]
- 28.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. AHRQ Technical Reviews. Criteria for distinguishing effectiveness from efficacy trials in systematic reviews. Agency for Healthcare Research and Quality; Rockville, MD: 2006. [PubMed] [Google Scholar]
- 29.Olds DL. The nurse-family partnership: an evidence-based preventive intervention. Infant Ment Health J. 2006;27:5–25. doi: 10.1002/imhj.20077. [DOI] [PubMed] [Google Scholar]
- 30.Furstenberg FFJ, Brooks-Gunn J, Morgan SP. Adolescent mothers and their children in later life. Fam Plan Persp. 1987;19:142–151. [PubMed] [Google Scholar]
- 31.Tygart CE. Juvenile-delinquency and number of children in a family—some empirical and theoretical updates. Youth Soc. 1991;22:525–536. [Google Scholar]
- 32.Zielinksi DS, Eckenrode J, Olds DL. Nurse home visitation and the prevention of child maltreatment: impact on the timing of official reports. Dev Psychopathol. 2009;21:441–453. doi: 10.1017/S0954579409000248. [DOI] [PubMed] [Google Scholar]
- 33.Olds DL. Prenatal and infancy home visiting by nurses: from randomized trials to community replication. Prev Sci. 2002;3:153–172. doi: 10.1023/a:1019990432161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.