Abstract
Chronic kidney disease (CKD) patients have high cardiovascular mortality and morbidity. The presence of traditional and CKD related risk factors results in exaggerated vascular calcification in these patients. Vascular calcification is associated with reduced large arterial compliance and thus impaired baroreflex sensitivity (BRS) resulting in augmented blood pressure (BP) variability and hampered BP regulation. Baroreflex plays a vital role in short term regulation of BP. This review discusses the normal baroreflex physiology, methods to assess baroreflex function, its determinants along with the prognostic significance of assessing BRS in CKD patients, available literature on BRS in CKD patients and the probable patho-physiology of baroreflex dysfunction in CKD.
Keywords: Large arterial compliance, Chronic kidney disease, Vascular calcification, Baroreflex sensitivity, Blood pressure variability
Core tip: Cardiovascular dysfunction is an important complication and risk factor of mortality and morbidity in chronic kidney disease (CKD). Baroreflex is a functional integrator of cardiovascular homeostasis. Derangement in baroreflex function is not only a manifestation of cardiovascular pathogenesis in general and in CKD but also contribute to ongoing etio-pathogenesis. The present review discusses the physiology and dysfunction in CKD in light of the available literature.
INTRODUCTION
Most common etiology of mortality and morbidity in chronic kidney disease (CKD) patients are cardiovascular events, rather than uremia itself. Interestingly, CKD is now recognized as an independent risk factor for cardiovascular disease[1,2]. Practice guidelines from the National Kidney Foundation 2002 recommend that CKD be considered a coronary artery disease risk equivalent[3].
Cardiovascular abnormalities in CKD includes both cardiomyopathy (left ventricular hypertrophy) and vasculopathy (arteriosclerosis and atherosclerosis) - which ultimately culminates to ischemic heart disease and cardiac failure[4,5] (Figure 1).
Figure 1.
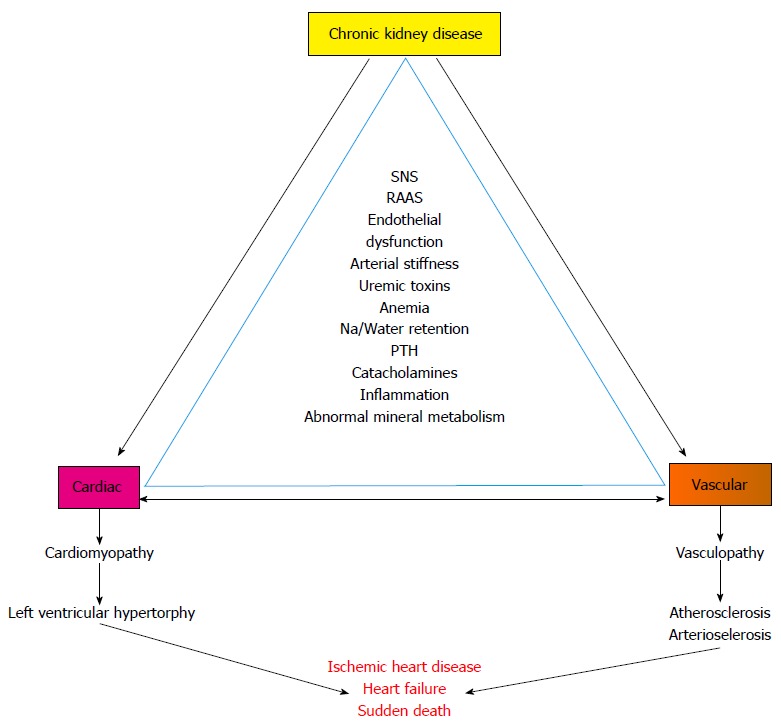
Cardiovascular abnormalities in chronic kidney disease. Depicts the association of chronic kidney disease related risk factors and cardiac and vascular abnormalities and outcomes in chronic kidney disease. SNS: Sympathetic nervous system; RAAS: Renin angiotensin aldosterone system; Na: Sodium; PTH: Parathyroid hormone.
Clinical presentation of cardiovascular disease in CKD includes hypertension, left ventricular hypertrophy, congestive heart failure, myocardial infarction and sudden death. Moreover in end stage renal disease (ERSD) patients, the prevalence of left ventricular hypertrophy and coronary artery disease are 75% and 40%, respectively. Death from cardiac causes is 10-20 times more common in patients with ESRD than in age matched segments of the general population and amounts for almost 30% to 50% of all death[4,6-8].
Mechanism of cardiovascular dysfunction
CKD is associated with both traditional and CKD related risk factors. Furthermore, the presence of added CKD related (non-traditional) risk factors in this population accounts for the exorbitant cardiovascular risk in these patients[9-14] as listed in Table 1 (Sarnak et al[15]).
Table 1.
Cardiovascular risk factors in chronic kidney disease
| Traditional risk factors | Non-traditional factors |
| Sympathetic hyperactivity | Albuminuria |
| Hyperhomocysteinemia | Inflammation |
| Hypertension | Oxidative stress |
| High LDL cholesterol | Anemia |
| Low HDL cholesterol | Abnormal calcium/phosphate metabolism |
| Diabetes | Extracellular fluid volume overload |
| Smoking | Electrolyte imbalance |
| Physical inactivity | Malnutrition |
| Menopause | Sleep disturbances |
| Family history of CVD | Endothelial dysfunction |
LDL: Low density lipoprotein; HDL: High density lipoprotein; CVD: Cardiovascular disease.
Cumulative effect of ensemble of these risk factors results ultimately to vascular calcification in CKD patients[9,10,16]. Central to the pathogenesis of cardiovascular dysfunction is vascular calcification[16-19]. Reviews are available discussing the mechanism of vascular calcification in CKD patients[16,20-29]. Vascular calcification results in stiffer arteries with reduced compliance[18,28]. Reduction in compliance of central arteries not only results in higher afterload and diminished perfusion of heart (London et al[30]) but also impaired baroreflex sensitivity (BRS)[31-34].
Baroreflex is a major regulatory mechanism for buffering short-term blood pressure (BP) fluctuations by modulating the heart rate and vascular tone. Baroreflex loop functioning is an important indicator of integrity of cardiovascular homeostatic regulation. Impaired baroreflex function results in loss of dampening of BP fluctuations and thus higher BP variabilities[34,35]. Higher blood pressure variability (BPV) has been associated with end-organ damage[36-38]. Previously a study by Kaur et al[34] proposed a model for showing the improvement in baroreflex function after renal transplantation (RT) in ESRD patients discussing the relationship between BRS, arterial stiffness and BPV and found that RT results in improvement in arterial stiffness followed by normalization in BRS and reduction in BPV. This highlights the significance of baroreflex function in CKD. The purpose of this review is to consolidate the published evidence on baroreflex physiology, methods of assessment, its determinants and dysfunction in CKD patients.
PHYSIOLOGY OF BARORECEPTOR REFLEX
Baroreceptor reflex (baroreflex) plays a significant role in the short term regulation of arterial BP. Pioneering works on animal models by Hering, Korner, Cowley, Guyton and others have clearly implicated its role in buffering arterial BP fluctuations induced by internal and external perturbations[39-41]. Evidence is currently accumulating in support of the hypothesized role of baroreceptors in long term regulation of arterial BP as well[42].
Baroreceptors are stretch sensitive receptors located in the high pressure (high pressure baroreceptors) as well as low pressure (low pressure baroreceptors) areas of the circulatory system. High pressure arterial baroreceptors located in the Carotid sinus and Aortic arch (Sinoaortic baroreceptors) are considered to play a dominant role in the moment to moment regulation of arterial BP. Considering this fact, alterations in arterial baroreflex mechanisms have been implicated in clinical disorders characterized by abnormal fluctuations in BP imposed commonly by postural variations. This section of the review would cite and discuss literature relevant to understand the physiology of arterial baroreflex and the methods of assessment of arterial BRS in human subjects and patients.
Baroreflex arc
Sino-aortic baroreceptors provide the cardiovascular regulatory centres in the brainstem with a continuous stream of information on the beat to beat fluctuations in BP. These stretch sensitive receptors are encapsulated or free nerve endings located in the tunica adventitia of carotid sinus and aortic arch that respond to the changes in dimensions of the arterial wall produced by fluctuations in transmuralpressure. Afferent information from the receptors are relayed to brainstem nuclei through glossopharyngeal (afferents from carotid sinus) and vagus nerves (afferents from aortic arch) which act as the centre of the baroreflex arc (Figure 2). Baroreceptor inputs to brain stem primarily reach the nucleus tractus solitarius (NTS) located in the dorsal medulla which has intricate connections with the cardioinhibitory and vasomotor centers located in the caudal and rostral ventrolateral medulla (RVLM) and the nucleus ambiguus of vagus. RVLM projects to preganglionic sympathetic neurons located in the intermediolateral gray column of thoracic and lumbar spinal segments. Axons of the neurons in nucleus ambiguus project as pre-ganglionic parasympathetic supply to the heart. Figure 2 depicts the neuronal circuitry of the baroreflex arc.
Figure 2.
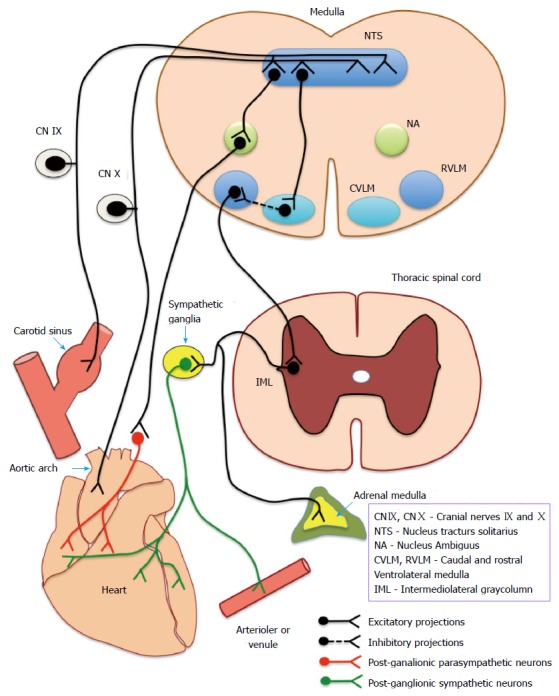
Neuronal circuitry of the baroreflex arc. Depicts the complete baroreflex arc - beginning from the baroreceptors (located in carotid sinus and aortic arch), afferents (IX and X cranial nerve) ascend to medullary centres and send efferents (sympathetic and parasympathetic) to end organs (heart and vasculature). CN IX and CN X: Cranial nerve IX and X; NTS: Nucleus tractus solitarius; NA: Nucleus ambiguous; CVLM: Caudal ventrolateral medulla; RVLM: Rostral ventrolateral medulla; IML: Intermediolateral gray column.
Baroreflex activation and effector responses
NTS continuously receives a tonic input from sino-aortic baroreceptor afferents which discharge in phase with the arterial pressure waveform. Within the operating range, the frequency of discharge in baroreceptor afferents responds to changes in both the magnitude and slope of arterial pressure waveform. A rise in systemic mean arterial and/or pulse pressure would lead to an increased discharge in the baroreceptor afferents phase-locked with the arterial pressure waveform. Increase in baroreceptor input to NTS initiates reciprocal changes in the efferent vago-sympathetic discharge leading to increased firing of cardioinhibitory vagal neurons innervating sino-atrial node and decreased firing of sympathetic neurons controlling heart and peripheral blood vessels. This would produce a decrease in heart rate mainly through the vagal limb and a decrease in cardiac contractility, peripheral vascular resistance and venous return through the sympathetic limb. All these changes will ultimately bring the BP down, close to its set point thereby instituting negative feedback control to establish circulatory homeostasis. Thus, baroreflex arc may be considered to operate through two physiologically antagonistic efferent pathways comprising of vagal and sympathetic fibres innervating heart and peripheral blood vessels. The vagal limb is quick to act with latencies as low as 200 ms to 600 ms in comparison to the sympathetic limb which takes more than 2 s to 3 s to produce any noticeable change in the cardiac contractility or peripheral resistance. This discrepancy is largely attributed to the obvious differences in cholinergic and adrenergic signal transduction mechanisms at the target cells.
DETERMINATION OF BRS - METHODOLOGICAL CONSIDERATIONS
Quantification of BRS has largely been part of experimental laboratory work in animal models and human subjects until recently when clinical investigations started revealing impaired BRS as a pathophysiological entity in cardiovascular disorders[43-45]. Moreover, BRS estimation has been attributed immense prognostic value in predicting cardiac mortality in the large multicentric autonomic tone and reflexes after myocardial infarction study[46]. Similar observations have also been reported in a group of patients with mild to moderate heart failure, signifying the role of BRS as a prognostic indicator in the risk stratification of patients[47].
From a physiological control system perspective, baroreceptor reflex is considered to operate in closed loop with open loop characteristics, i.e., changes in BP elicit appropriate heart rate responses through open loop negative feedback mechanisms which tend to buffer the initiating change in BP through a feedforward influence of heart rate on BP that closes the loop[48,49]. Majority of the BRS assessment protocols ignore the feedforward influence considering it as inconsequential and compute BRS as the feedback gain of the open loop[50,51].
Methodologically, BRS assessment strategies can be broadly categorized into (1) those based on artificially imposed changes in arterial BP or carotid sinus pressure including pharmacological methods, Valsalva maneuver and neck chamber techniques; (2) those based on analysis of spontaneous oscillations in BP and heart rate.
Methods based on artificially imposed changes in arterial BP or carotid sinus pressure
These methods use physiological maneuvers or pharmacological agents to impose changes in BP. The resulting baroreflex mediated changes in heart intervals are simultaneously acquired along with BP signal and subjected to appropriate analysis to derive various estimates of BRS.
Pharmacological method
Pharmacological method, also termed as the “Oxford technique” involves intravenous administration of graded bolus doses of a suitable vasoconstrictor agent to produce rise in BP that would lead to baroreflex induced bradycardia[51-53]. Phenylephrine, a pure alpha adrenoceptor agonist is the commonly preferred vasoconstrictor agent as it is considered to have minimal extravascular effects. Many investigators prefer to administer in addition, a vasodilator agent (sodium nitroprusside infusion or amyl nitrite by inhalation) to induce fall in BP to precipitate baroreflex mediated increase in heart rate to capture responses on either side of the setpoint. Beat to beat BP and ECG signals are simultaneously recorded during the periods when BP rises above and below the resting baseline values under the influence of the vasoactive agents. Consecutive systolic BP values are plotted against the simultaneously recorded RR intervals or pulse intervals with one beat delay to fit the linear regression line between the two variables. BRS is computed as the slope of this line and expressed in ms/mm of Hg. Despite being invasive, pharmacological method is the commonly employed method to estimate BRS for risk stratification of patients owing to its repeatability and accuracy.
Valsalva’s maneuver
Valsalva’s maneuver is one of the earliest known physiological maneuvers used to study the baroreflex function in humans. Performance of the maneuver involves forced expiration against a closed or partly open glottis to raise the intrathoracic and intraabdominal pressures with secondary hemodynamic effects. The maneuver physiologically imposes fall in BP due to decreased venous return during phase II and rise in BP during phases IV due to uninterrupted venous return to an already stimulated heart. The corresponding baroreflex mediated RR interval changes are acquired simultaneously with the beat to beat BP values. A linear regression analysis is commonly performed between consecutive systolic BP values and corresponding RR intervals with one beat delay during phase IV to derive BRS (also known as cardiovagal gain) as the slope of the fitted line[51,54]. Estimation of BRS by Valsalva’s manoeuvre has been reported to be non-selective for arterial baroreflex as it also engages other low pressure baroreceptors into action[55].
Neck chamber technique
The neck chamber technique[56,57] produces activation or deactivation of carotid baroreceptors through a graded application of negative or positive pneumatic pressure around the neck region. Negative neck pressure increases the carotid sinus transmural pressure leading to increased stretching of its wall and afferent baroreceptor firing. This would induce a fall in systemic arterial pressure consequent to baroreflex mediated changes in heart rate, cardiac contractility, peripheral resistance and venous return. BRS is computed by linear regression analysis using the transmural carotid sinus pressure and RR interval data acquired during the phases of manipulation. Neck chamber technique is the only method which selectively estimates the carotid BRS. However, it has been sparingly used in clinical investigations with most of the available literature citing its use relate to studies conducted in association with experimental laboratory work.
Methods based on analysis of spontaneous oscillations in BP and heart intervals
Since 1980s, with the invention and widespread use of non-invasive beat to beat BP monitors based on the volume clamp principle of “Penaz”, there has been tremendous progress in the development of purely non-invasive methods of BRS assessment. This newer generation of techniques employs computer based analysis of spontaneous oscillations in the BP coupled with reflex changes in heart rate to derive estimates of BRS. The spontaneous BRS estimation methods can be broadly categorised into time domain and frequency domain methods.
Time domain methods
Time domain methods analyse the time series data of beat to beat BP and heart intervals to estimate BRS. In the “sequence method” proposed by Parati et al[50,51,58], the algorithm automatically searches and identifies “sequences” in which BP shows a continuous increase (or decrease) for at least three consecutive beats that is accompanied by lengthening (or shortening) of consecutive RR intervals with zero to two beats delay. Sequence method considers the associated RR interval changes as baroreflex mediated response to the spontaneously emerging ascending or descending pressure ramps. A linear regression analysis between the BP and RR interval variables will derive the slope of the best fit line as estimated BRS.
Frequency domain methods
The spectral or frequency domain methods are based on the principle that, spontaneous oscillations in BP centered around a particular frequency will lead to baroreflex mediated oscillations in heart interval in the same frequency. The ratio of the powers of the oscillations estimated by autoregressive or other methods in a particular frequency band or the modulus of the transfer function relating BP with heart interval oscillations are computed as the BRS estimates[50,51]. Oscillations in two frequency bands are usually being taken for the computations; a low frequency band centered around 0.1 Hz (ranging from 0.04 to 0.15 Hz) and a high frequency band of respiratory origin ranging from 0.15 to 0.4 Hz. One of the commonly used spectral methods estimates BRS as the root-squared ratio between heart interval and systolic pressure powers calculated in the LF band (α LF) or in the HF band (α HF). These spectral indices are considered to be valid when the linear correlation (coherence) between BP and heart rate oscillations in the specified frequency bands are sufficiently high. The transfer function method proposed by Robbe et al[59], computes BRS as the modulus or gain of the transfer function between variations in BP and heart interval in a specified frequency band. Transfer function is usually computed for both low frequency and high frequency bands deriving two different estimates of BRS named HLF and HHF respectively. Other spectral methods for estimating BRS include describing the spontaneous oscillations in BP and heart intervals using mathematical models and deriving BRS using the model coefficients.
Choice of the appropriate method of BRS assessment in clinical setting
Despite being invasive, pharmacological method is the most preferred technique for BRS estimation by most clinical investigators owing to its repeatability across different populations of patients. With the advent of non-invasive beat to beat BP monitors, impetus on the usage of spontaneous methods as replacement for the invasive pharmacological method has been steadily growing. Sequence method is considered to be the physiological replica of pharmacological method since both the techniques analyse heart interval responses to ascending or descending pressure ramps originating spontaneously or in response to vasoactive agents. However, many investigators believe spectral indices to give better and reliable estimates of BRS comparable to that obtained by invasive pharmacological methods[59-61]. Choice of the most appropriate spontaneous method of BRS estimation is dependent on experimental factors and stationarity of the BP and heart interval signals. Reliable estimation of BRS by spectral methods is guaranteed only if the blood pressure and heart rate signals are stationary during the selected window of analysis. Sequence method is preferred over spectral indices if the stationarity of the signals cannot be ensured[50,51]. Majority of the initial reports on baroreflex functions in CKD patients have employed pharmacological method[62-65] to estimate BRS while a few have also used Valsalva maneuver[66]. Spontaneous sequence and spectral methods have also been utilized in the studies conducted in the recent past[34,67].
DETERMINANTS OF BRS
Factors determining BRS can broadly be categorized as demographic and physiological, as reported by multiple studies conducted in healthy subjects and patients using both invasive and non-invasive methods. Age, gender, systolic and diastolic BP, resting heart rate and body mass index have been reported as the major determinants of BRS[68,69]. The relationship between age and BRS was observed to be physiologically linked through age related changes in carotid distensibility[70-73]. Age related decline in carotid distensibility tends to minimise the diameter changes associated with arterial pressure fluctuations, thereby reducing the transduction abilities of sino-aortic baroreceptors. This has been corroborated by direct estimation of carotid distensibility coefficients and its correlation with BRS as quantified by pharmacological method in healthy human subjects[74]. Central arterial stiffness as measured by aortic pulse wave velocity has been reported to be an independent predictor of BRS by the Rotterdam cohort study conducted in 2083 elderly subjects[75]. Reduction in arterial distensibility associated with stiffening of the central arteries and a consequent fall in BRS is one of the possible mechanisms implicated in baroreflex dysfunction in CKD patients.
PROGNOSTIC SIGNIFICANCE OF BAROREFLEX ASSESSMENT IN CKD
BRS is emerging as a cardinal prognostic risk factor in CKD patients. Johansson et al[76] studied BRS in hypertensive CKD patients and then followed them up prospectively for 41 +/- 15 mo and found that 69 patients died during the follow-up. Cardiovascular diseases and uremia resulted in the majority of deaths (60% and 20%, respectively), while sudden cardiac death occurred in 15 patients. Reduced BRS was found to be an independent predictor of sudden cardiac death (RR = 0.29; 95%CI: 0.09-0.86 for an increase of one standard deviation in BRS, P = 0.022). The authors concluded that BRS may convey important prognostic information that will have clinical implications for patients with CKD.
Reduced BRS is also associated with hemodialysis related hypotension, which results in significant mortality in hemodialysis patients as they are unable to counteract dialysis induced volume depletion[66,77]. Chesterton et al[31], reviewed the importance of assessment of BRS in CKD patients, especially its relevance in prediction of vasomotor instability during dialysis. The authors inferred from literature that there are demonstrable pathological alterations in CKD, contributing to structural and functional changes in the cardiovascular system that may result in both haemodynamic instability and cardiovascular mortality. Understanding the associations between conventional markers of haemodynamic instability and BRS (as a measure of autonomic function) will allow early and better risk stratification, prevention and managementin CKD patients.
EVIDENCE OF IMPAIRED BRS IN CKD
Baroreceptor reflex control, as studied by BRS is reduced in CKD patients and worsenswith the disease severity (Table 2). Studies have also compared BRS of patients on different treatment modality of CKD - hemodialysis, peritoneal dialysis and RT with inconsistent results. Although the literature on BRS assessment in CKD is scarce, but most existing studies suggest that dialysis fails to improve BRS in CKD patients while renal transplant undoubtedly improves it. Few studies have also examined the correlation of BRS with vascular compliance and autonomic parameters in-order to understand the pathophysiology of baroreflex dysfunction in CKD patients. Although this still remains to be studied in further details.
Table 2.
Baroreflex sensitivity in chronic kidney disease
| Ref. | Number of patients Study design | Method of BRS assessment | Results |
| Pickering et al[65] | 32 patients on HD serially studied | Intra-venous bolus of phenylephrine | BRS was found to be low |
| HD improved reflex sensitivity over the long term, but did not have any consistent immediate effect | |||
| Lazarus et al[64] | 13 patients on HD and 5 controls | Intra-venous angiotensin and inhaled amyl nitrite | BRS lower in patients than controls for both pressor and depressor stimuli |
| Cross- sectional | |||
| Tomiyama et al[78] | 22 non-dialysed patients and controls | Intra-venous bolus of phenylephrine and inhaled amyl nitrite | Lower BRS in patients as compared to controls |
| Agarwal et al[62] | Cross- sectional | Intra-venous bolus of phenylephrine | Lower BRS in patients |
| 25 non-dialyzed patients and 8 controls | 8 patients restudied after HD, BRS lower in hypotension-prone vs normotensive group | ||
| 8 patients reassessed after 6.6 +/- 1.0 wk of hemodialysis | 12 patients restudied after RT, BRS improved | ||
| 12 patients were restudied 24 +/- 4.0 wk after renal transplantation | |||
| Gerhardt et al[67] | 20 patients of HD, RT and controls each | Sequence analysis | Reduced BRS in CKD vs Controls |
| Cross-sectional | Similar BRS in RT and controls | ||
| Gao et al[79] | 17 ESRD patients and 29 controls | Sequence analysis | BRS was 62% lower in ESRD than controls |
| Cross-sectional | |||
| Johansson et al[80] | 216 hypertensive CKD patients with 43 age-matched controls | Spontaneous method | BRS was reduced by 51% in CKD patients as compared with controls |
| Greater reductions in BRS noted in diabetic vs non-diabetic patients | |||
| Chan et al[32] | 10 hypertensive ESRD patients receiving conventional hemodialysis were studied before and 2 mo after conversion to nocturnal hemodialysis | Spontaneous method | Improvement in BRS by nocturnal HD as compared to conventional HD |
| Assessed BRS along with total arterial compliance | Increases in BRS correlated with increases in total arterial compliance | ||
| Bavanandan et al[81] | 105 non-dialysis CKD patients | Spontaneous method | Nondialysis dependent CKD patients have impaired BRS |
| Baseline and follow-up of 42 mo | BRS is related to decreasing GFR | ||
| Studied relationship with increasing degrees of uremia | A trend towards poorer prognosis in patients with impaired BRS | ||
| Recorded primary (death, dialysis, transplantation) and secondary (fatal and nonfatal cardiovascular events) outcome measures | |||
| Studinger et al[33] | Juvenile study group with 14 HD patients, 14 RT and 14 controls | Pharmacological and spontaneous method | BRS was markedly reduced in HD as compared to controls |
| BRS with HRV and carotid artery stiffness | Carotid artery stiffness was higher in HD than controls and was inversely related to BRS | ||
| HRV was also compromised in HD, and was directly related to BRS | |||
| No significant differences in any of these variables between RT and controls | |||
| Decreased baroreflex function in juvenile HD is partly due to loss of carotid artery elasticity and partly due to impaired heart rate variability. Renal transplantation may partly prevent impairment or improve compromised baroreflex function in young patients with ESRD | |||
| Chesterton et al[31] | 40 HD patients | Spontaneous method | Reduced BRS in HD patients |
| Assessed BRS with arterial calcification and arterial stiffness indices | Reduced BRS is associated with increased vascular calcification and arterial stiffness | ||
| Lacy et al[82] | 55 non-dialysis non-diabetic CKD patients | Spectral method | BRS reduced as renal disease severity increases Reduced GFR was correlated with increased PWV and decreased cardiac BRS |
| BRS relationship with arterial stiffness and GFR | |||
| Non-dialysis non-diabetic CKD patients with decreasing GFR have reduced cardiac BRS and increased large artery stiffness | |||
| Rubinger et al[35] | 52 HD, 44 RT and 41 controls | Spontaneous method | In HD patients, BPV was increased, while HRV and BRS were markedly decreased as compared to controls |
| 16 patients before and after transplant | RT was associated with normalization of BPV at short term (≤ 1 yr) and long term and with improvement of HRV at a long-term (> 1 yr) follow-up. After RT baroreceptor indices were significantly increased and returned to values similar to those of the control | ||
| BRS with HRV and BPV | |||
| Chesterton et al[77] | 34 chronic HD | Spontaneous method | Impaired BRS predicts intra-dialytic hypotension |
| Cross-sectional | |||
| Relation with intra-dialytic hypotension | |||
| Kaur et al[34] | 23 ESRD patients studied prospectively before and at 3 and 6 mo after RT | Spontaneous method | RT normalizes BRS in ESRD patients by 6 mo which follows the improvement in the central arterial stiffness |
| BRS with central arterial stiffness and HRV and BPV |
HD: Hemodialysis; RT: Renal transplantation; CKD: Chronic kidney disease; ESRD: End stage renal disease; GFR: Glomerular filtration rate; HRV: Heart rate variability; BPV: Blood pressure variability; BRS: Baroreflex sensitivity.
CONCEPTUAL MODEL EXPLAINING THE REDUCED BRS IN CKD
We have previously seen in the earlier section (Figure 2) the complete baroreflex arc. Conceptually a defect anywhere in this loop could result in impaired BRS in CKD.
Till now different schools of thoughts have been categorized to summarize the defect in baroreflex function in CKD: (1) Vascular vs Neural debate; (2) Structural vs functional mechanisms.
As a matter of fact, none of these contemplations are full-proof and mutually exclusive. There exists a grey area of overlap of these factors resulting in baroreflex dysfunction.
In the next section, we will discuss the limited evidence available to possibly speculate the patho-physiology of reduction in BRS in CKD patients.
PATHOPHYSIOLOGY OF BAROREFLEX DYSFUNCTION IN CKD
Vascular vs neural
The baroreflex arc is integral to the short-term regulation of BP and is under autonomic regulation. A change in BP results in an alteration in transmural stretch within the baro-sensitive central arteries. This causes activation of the baroreceptors (level 1 in Figure 3) located within the adventitia of arterial wall. Modified firing from these receptors is transmitted via the afferent nerves (level 2 in Figure 3) to the central autonomic centre (level 3 in Figure 3). Sympathetic and parasympathetic systems (level 4 in Figure 3) influencing vessels and heart (level 5 in Figure 3) constitute the efferent response. Thus, a change in BP results in a corresponding change in the RR interval and vessel tone, restoring BP to normal limits.
Figure 3.
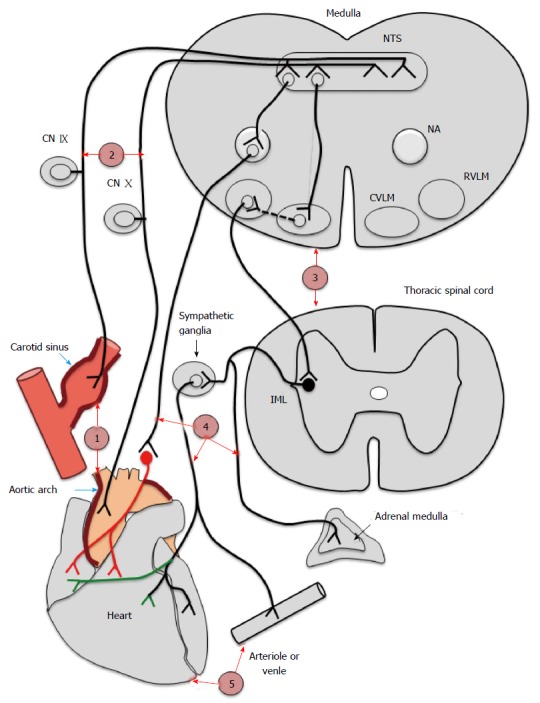
Probable levels of defect in baroreflex arc in chronic kidney disease. Depicts the different probable levels of defect in chronic kidney disease. Level 1 represents the baroreceptors affected by calcification of central arteries. Level 2, 3, 4 and 5 represents afferents (IX and X nerves), centres, efferents and endorgans (heart and vessels) respectively. CN IX and CN X: Cranial nerve IX and X; NTS: Nucleus tractus solitarius; NA: Nucleus ambiguous; CVLM: Caudal ventrolateral medulla; RVLM: Rostral ventrolateral medulla; IML: Intermediolateral gray column.
BRS is well recognized as a composite marker of the overall integrity of the baroreflex arc[31]. BRS is therefore determined by the mechanical properties of the arterial wall which constitutes the vascular component, and the parasympathetic and sympathetic nervous system forming the neural component.
Chesterton et al[31] found that BRS is impaired in CKD which explains the development of intra-dialytic hypotension (IDH) in these patients. IDH is associated with increased mortality in hemodilaysis (HD) patients. Additionally, they investigated the link between vascular calcification (measure of arterial structure), arterial stiffness (measure of arterial function) and BRS in chronic HD patients and concluded that there is a positive association between vascular calcification and BRS. Thus the impaired BRS observed in CKD patients could be due to the excessive vascular calcification observed in them.
In concordance Kaur et al[34] studied the reversibility of arterial stiffness indices along with BRS before, at 3 mo and 6 mo after RT in-order to understand the temporal connection between these parameters. They reported the normalization of BRS in ESRD patients by 6 mo which followed the early improvement in arterial stiffness.
On similar lines, Boutouyrie et al[83] also theoretically categorized the baroreflex loop into vascular compartment which includes the wall stretch component (receptor level) and neural comprising of afferent, centre and efferent arc of baroreflex. Notably, baroreceptors embedded in the adventitia of central arteries are sensitive only to vessel wall stretch and not directly to intravascular pressure. Pressure changes inside the vascular lumen need to get translated as vessel wall stretch to get sensed by baroreceptors. This pressure to stretch conversion is dependent on arterial compliance and thus, stiffness of large arteries become a crucial determinant of the vascular component of baroreflex[74,84]. CKD is associated with both vascular remodelling and autonomic dysfunction. The authors commented on a previous study[34] and discussed that questions still remain regarding how transplantation improves baroreflex - is it through amelioration of arterial properties or neural components or/and a relative contribution of both.
This puzzle remains unresolved till date. Most available data is suggestive of a probable defect at level 1 that is the sensing by baroreceptors itself. Although there exists data regarding dysfunction at other sites also in human and animal studies - level 2 - afferents[85,86], level 3 - centre[86,87], level 4 - efferents[85,88,89] and level 5 - end organ[90-92] in CKD.
By studying in detail the large artery and neural parts components of baroreflex arc, studies in future may help in understanding this concept further.
Structural vs functional modulation of the arterial baroreflex
Large artery structural changes are considered to be the predominant mechanism responsible for decreased BRS[74]. There is an emerging concept of the role of “functional mechanisms” responsible for altered baroreflex function which could be either at the level of peripheral sensory endings and/or at the central nervous system.
Structure of the central arteries determines the deformation and thus the strain of baroreceptor endings with changes in blood pressure[93,94]. That is the reason for structural changes in the large arteries and increased arterial stiffness being considered the cardinal mechanism responsible for the reduced BRS and resetting of baroreceptors in hypertension, atherosclerosis, and aging.
The current concept focuses on the functional mechanisms and thus the postulate that baroreceptor activity is not merely a manifestation of associated vascular strain.
Studies have identified various mechanisms involved in the modulation of the baroreflex arc. These are referred to as functional factors to differentiate them from structural changes. Based on their site of action, functional factors are categorized into two: (1) peripheral sensory mechanisms involving barorceptors and or sensory afferents; and (2) central mechanisms involving the neural areas coupling the afferent sensory stimuli to efferent autonomic responses[95].
Chapleau et al[96] studied the role of functional mechanism in baroreflex alteration in hypertensives and aged people. They examined on both cultured baroreceptor nodose neurons and isolated carotid sinus preparation of dogs and rabbits.
In their study[96], they found that peripheral sensory mechanisms include: (1) Lack of endogenous PGI2 and increase in free radicals and platelet aggregation which result in deranged baroreflex function in chronic hypertension and atherosclerosis; (2) Stretch activated channel and transient outward K current which are responsible for mechanoelectrical transduction and adaptation of baroreceptors respectively; and (3) Na-K pump inhibition, which occurs with fall in arterial pressure and leads to prompt (within minutes) reversal of chronic baroreceptor resetting in chronic hypertensive rabbits. The rapidity of response rules out structural change and could be due to functional change.
In their study[96], they have also commented on central mechanisms which include: (1) Loss of inhibition of sympathetic system and inefficient coupling of afferent stimuli to efferent response which could be attributed to reducedcentral arterial compliance and rapid frequency of baroreceptor discharge. It has been seen that 3 and a low frequency (< 3 Hz) of baroreceptor discharges sustain the reflex inhibition of sympathetic system; and (2) Defect in neural centres mediating the baroreflex arc. Authors have suggested that this may be the chief cause of the reduction in baroreflex functioning with aging.
Notionally, chronic kidney patients might have a similar structural and functional defect and functional changes may precede the structural changes unlike the present-day postulation but this concept has not been studied in CKD patients yet.
CONCLUSION
CKD patients have high cardiovascular mortality and morbidity. Baroreceptor function assessment is an independent predictor of cardiovascular risk. There are different methodological techniques and determinants of BRS. The underlying patho-physiology of baroreflex dysfunction is still unclear but probable defect seems to be central arterial stiffness in CKD patients resulting in dampened firing by baroreceptors (receptor level defect).
Footnotes
Conflict-of-interest statement: The above-mentioned authors of this manuscript hereby declare that they do not have any conflict-of-interest (including but not limited to commercial, personal, political, intellectual, or religious interests) related to the work submitted herein.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 13, 2015
First decision: September 17, 2015
Article in press: November 25, 2015
P- Reviewer: Ohashi N S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
References
- 1.McCullough PA, Rocher LR, Nistala R, Whaley-Connell A. Chronic kidney disease as a cardiovascular risk state and considerations for the use of statins. J Clin Lipidol. 2008;2:318–327. doi: 10.1016/j.jacl.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Shishehbor MH, Oliveira LP, Lauer MS, Sprecher DL, Wolski K, Cho L, Hoogwerf BJ, Hazen SL. Emerging cardiovascular risk factors that account for a significant portion of attributable mortality risk in chronic kidney disease. Am J Cardiol. 2008;101:1741–1746. doi: 10.1016/j.amjcard.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606–1615. doi: 10.1681/ASN.V1071606. [DOI] [PubMed] [Google Scholar]
- 5.Shamseddin MK, Parfrey PS. Sudden cardiac death in chronic kidney disease: epidemiology and prevention. Nat Rev Nephrol. 2011;7:145–154. doi: 10.1038/nrneph.2010.191. [DOI] [PubMed] [Google Scholar]
- 6.Excerpts from the United States Renal Data Systems 2002 annual report: Atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003;41:v–ix, S7-S254. doi: 10.1016/s0272-6386(03)80001-x. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- 9.Anavekar NS, Pfeffer MA. Cardiovascular risk in chronic kidney disease. Kidney Int Suppl. 2004;(92):S11–S15. doi: 10.1111/j.1523-1755.2004.09203.x. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Mallamaci F, Tripepi G. Novel cardiovascular risk factors in end-stage renal disease. J Am Soc Nephrol. 2004;15 Suppl 1:S77–S80. doi: 10.1097/01.asn.0000093240.84097.fe. [DOI] [PubMed] [Google Scholar]
- 11.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 12.Hruska K, Mathew S, Lund R, Fang Y, Sugatani T. Cardiovascular risk factors in chronic kidney disease: does phosphate qualify? Kidney Int Suppl. 2011;(121):S9–S13. doi: 10.1038/ki.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanayakkara PW, Gaillard CA. Vascular disease and chronic renal failure: new insights. Neth J Med. 2010;68:5–14. [PubMed] [Google Scholar]
- 14.Shoji T, Abe T, Matsuo H, Egusa G, Yamasaki Y, Kashihara N, Shirai K, Kashiwagi A. Chronic kidney disease, dyslipidemia, and atherosclerosis. J Atheroscler Thromb. 2012;19:299–315. doi: 10.5551/jat.10454. [DOI] [PubMed] [Google Scholar]
- 15.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 16.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 17.London GM. Cardiovascular calcifications in uremic patients: clinical impact on cardiovascular function. J Am Soc Nephrol. 2003;14:S305–S309. doi: 10.1097/01.asn.0000081664.65772.eb. [DOI] [PubMed] [Google Scholar]
- 18.Ketteler M, Gross ML, Ritz E. Calcification and cardiovascular problems in renal failure. Kidney Int Suppl. 2005;(94):S120–S127. doi: 10.1111/j.1523-1755.2005.09428.x. [DOI] [PubMed] [Google Scholar]
- 19.Bhan I, Thadhani R. Vascular calcification and ESRD: a hard target. Clin J Am Soc Nephrol. 2009;4 Suppl 1:S102–S105. doi: 10.2215/CJN.04800709. [DOI] [PubMed] [Google Scholar]
- 20.Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. 2001;60:472–479. doi: 10.1046/j.1523-1755.2001.060002472.x. [DOI] [PubMed] [Google Scholar]
- 21.Floege J, Ketteler M. Vascular calcification in patients with end-stage renal disease. Nephrol Dial Transplant. 2004;19 Suppl 5:V59–V66. doi: 10.1093/ndt/gfh1058. [DOI] [PubMed] [Google Scholar]
- 22.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 23.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 24.Cozzolino M, Mazzaferro S, Pugliese F, Brancaccio D. Vascular calcification and uremia: what do we know? Am J Nephrol. 2008;28:339–346. doi: 10.1159/000111827. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 26.Stompór T. An overview of the pathophysiology of vascular calcification in chronic kidney disease. Perit Dial Int. 2007;27 Suppl 2:S215–S222. [PubMed] [Google Scholar]
- 27.Cozzolino M, Brancaccio D, Gallieni M, Slatopolsky E. Pathogenesis of vascular calcification in chronic kidney disease. Kidney Int. 2005;68:429–436. doi: 10.1111/j.1523-1755.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- 28.Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL, Goldsmith DJ. Vascular calcification in chronic kidney disease. Clin Sci (Lond) 2010;119:111–121. doi: 10.1042/CS20090631. [DOI] [PubMed] [Google Scholar]
- 29.Shroff R, Long DA, Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179–189. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 30.London GM, Marchais SJ, Metivier F, Guerin AP. Cardiovascular risk in end-stage renal disease: vascular aspects. Nephrol Dial Transplant. 2000;15 Suppl 5:97–104. doi: 10.1093/ndt/15.suppl_5.97. [DOI] [PubMed] [Google Scholar]
- 31.Chesterton LJ, Sigrist MK, Bennett T, Taal MW, McIntyre CW. Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness. Nephrol Dial Transplant. 2005;20:1140–1147. doi: 10.1093/ndt/gfh808. [DOI] [PubMed] [Google Scholar]
- 32.Chan CT, Jain V, Picton P, Pierratos A, Floras JS. Nocturnal hemodialysis increases arterial baroreflex sensitivity and compliance and normalizes blood pressure of hypertensive patients with end-stage renal disease. Kidney Int. 2005;68:338–344. doi: 10.1111/j.1523-1755.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 33.Studinger P, Lénárd Z, Mersich B, Reusz GS, Kollai M. Determinants of baroreflex function in juvenile end-stage renal disease. Kidney Int. 2006;69:2236–2242. doi: 10.1038/sj.ki.5000307. [DOI] [PubMed] [Google Scholar]
- 34.Kaur M, Chandran D, Lal C, Bhowmik D, Jaryal AK, Deepak KK, Agarwal SK. Renal transplantation normalizes baroreflex sensitivity through improvement in central arterial stiffness. Nephrol Dial Transplant. 2013;28:2645–2655. doi: 10.1093/ndt/gft099. [DOI] [PubMed] [Google Scholar]
- 35.Rubinger D, Backenroth R, Sapoznikov D. Restoration of baroreflex function in patients with end-stage renal disease after renal transplantation. Nephrol Dial Transplant. 2009;24:1305–1313. doi: 10.1093/ndt/gfn732. [DOI] [PubMed] [Google Scholar]
- 36.Zhang QQ, Zhang XJ, Chang BB, Qiu BY, Zhang Y, Li J, Zeng Z. [Blood pressure variability correlates with target-organ damage in elderly patients with hypertension] Sichuan Daxue Xubao Yixueban. 2011;42:252–255. [PubMed] [Google Scholar]
- 37.Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep. 2012;14:421–431. doi: 10.1007/s11906-012-0290-7. [DOI] [PubMed] [Google Scholar]
- 38.Ryu J, Cha RH, Kim DK, Lee JH, Yoon SA, Ryu DR, Oh JE, Kim S, Han SY, Lee EY, et al. The clinical association of the blood pressure variability with the target organ damage in hypertensive patients with chronic kidney disease. J Korean Med Sci. 2014;29:957–964. doi: 10.3346/jkms.2014.29.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cowley AW, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- 40.Korner PI. The effect of section of the carotid sinus and aortic nerves on the cardiac output of the rabbit. J Physiol. 1965;180:266–278. doi: 10.1113/jphysiol.1965.sp007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheffers IJ, Kroon AA, de Leeuw PW. Carotid baroreflex activation: past, present, and future. Curr Hypertens Rep. 2010;12:61–66. doi: 10.1007/s11906-009-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thrasher TN. Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R819–R827. doi: 10.1152/ajpregu.00813.2004. [DOI] [PubMed] [Google Scholar]
- 43.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92:3206–3211. doi: 10.1161/01.cir.92.11.3206. [DOI] [PubMed] [Google Scholar]
- 44.Bristow JD, Honour AJ, Pickering GW, Sleight P, Smyth HS. Diminished baroreflex sensitivity in high blood pressure. Circulation. 1969;39:48–54. doi: 10.1161/01.cir.39.1.48. [DOI] [PubMed] [Google Scholar]
- 45.Osculati G, Grassi G, Giannattasio C, Seravalle G, Valagussa F, Zanchetti A, Mancia G. Early alterations of the baroreceptor control of heart rate in patients with acute myocardial infarction. Circulation. 1990;81:939–948. doi: 10.1161/01.cir.81.3.939. [DOI] [PubMed] [Google Scholar]
- 46.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 47.Osterziel KJ, Hänlein D, Willenbrock R, Eichhorn C, Luft F, Dietz R. Baroreflex sensitivity and cardiovascular mortality in patients with mild to moderate heart failure. Br Heart J. 1995;73:517–522. doi: 10.1136/hrt.73.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamiya A, Kawada T, Sugimachi M. Systems physiology of the baroreflex during orthostatic stress: from animals to humans. Front Physiol. 2014;5:256. doi: 10.3389/fphys.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.La Rovere MT. Baroreflex Sensitivity Assessment - Latest Advances and Strategies. 2011. Available from: http: //www.radcliffecardiology.com/articles/baroreflex-sensitivity-assessment-latest-advances-and-strategies.
- 50.Parati G, Saul JP, Castiglioni P. Assessing arterial baroreflex control of heart rate: new perspectives. J Hypertens. 2004;22:1259–1263. doi: 10.1097/01.hjh.0000125469.35523.32. [DOI] [PubMed] [Google Scholar]
- 51.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191–207. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.James MA, Robinson TG, Panerai RB, Potter JF. Arterial baroreceptor-cardiac reflex sensitivity in the elderly. Hypertension. 1996;28:953–960. doi: 10.1161/01.hyp.28.6.953. [DOI] [PubMed] [Google Scholar]
- 53.Pitzalis MV, Mastropasqua F, Passantino A, Massari F, Ligurgo L, Forleo C, Balducci C, Lombardi F, Rizzon P. Comparison between noninvasive indices of baroreceptor sensitivity and the phenylephrine method in post-myocardial infarction patients. Circulation. 1998;97:1362–1367. doi: 10.1161/01.cir.97.14.1362. [DOI] [PubMed] [Google Scholar]
- 54.Palmero HA, Caeiro TF, Iosa DJ, Bas J. Baroreceptor reflex sensitivity index derived from Phase 4 of te Valsalva maneuver. Hypertension. 1981;3:II–134-II-137. doi: 10.1161/01.hyp.3.6_pt_2.ii-134. [DOI] [PubMed] [Google Scholar]
- 55.Hunt BE, Farquhar WB. Nonlinearities and asymmetries of the human cardiovagal baroreflex. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1339–R1346. doi: 10.1152/ajpregu.00038.2004. [DOI] [PubMed] [Google Scholar]
- 56.Cooper VL, Hainsworth R. Carotid baroreflex testing using the neck collar device. Clin Auton Res. 2009;19:102–112. doi: 10.1007/s10286-009-0518-z. [DOI] [PubMed] [Google Scholar]
- 57.Båth E, Lindblad LE, Wallin BG. Effects of dynamic and static neck suction on muscle nerve sympathetic activity, heart rate and blood pressure in man. J Physiol. 1981;311:551–564. doi: 10.1113/jphysiol.1981.sp013604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12:214–222. doi: 10.1161/01.hyp.12.2.214. [DOI] [PubMed] [Google Scholar]
- 59.Robbe HW, Mulder LJ, Rüddel H, Langewitz WA, Veldman JB, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension. 1987;10:538–543. doi: 10.1161/01.hyp.10.5.538. [DOI] [PubMed] [Google Scholar]
- 60.Pagani M, Somers V, Furlan R, Dell’Orto S, Conway J, Baselli G, Cerutti S, Sleight P, Malliani A. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension. 1988;12:600–610. doi: 10.1161/01.hyp.12.6.600. [DOI] [PubMed] [Google Scholar]
- 61.Watkins LL, Grossman P, Sherwood A. Noninvasive assessment of baroreflex control in borderline hypertension. Comparison with the phenylephrine method. Hypertension. 1996;28:238–243. doi: 10.1161/01.hyp.28.2.238. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal A, Anand IS, Sakhuja V, Chugh KS. Effect of dialysis and renal transplantation on autonomic dysfunction in chronic renal failure. Kidney Int. 1991;40:489–495. doi: 10.1038/ki.1991.236. [DOI] [PubMed] [Google Scholar]
- 63.Bondia A, Tabernero JM, Macias JF, Martin-Luengo C. Autonomic nervous system in haemodialysis. Nephrol Dial Transplant. 1988;3:174–180. [PubMed] [Google Scholar]
- 64.Lazarus JM, Hampers CL, Lowrie EG, Merrill JP. Baroreceptor activity in normotensive and hypertensive uremic patients. Circulation. 1973;47:1015–1021. doi: 10.1161/01.cir.47.5.1015. [DOI] [PubMed] [Google Scholar]
- 65.Pickering TG, Gribbin B, Oliver DO. Baroreflex sensitivity in patients on long-term haemodialysis. Clin Sci. 1972;43:645–657. doi: 10.1042/cs0430645. [DOI] [PubMed] [Google Scholar]
- 66.Heber ME, Lahiri A, Thompson D, Raftery EB. Baroreceptor, not left ventricular, dysfunction is the cause of hemodialysis hypotension. Clin Nephrol. 1989;32:79–86. [PubMed] [Google Scholar]
- 67.Gerhardt U, Riedasch M, Steinmetz M, Hohage H. Kidney transplantation improves baroreceptor sensitivity. Int J Cardiol. 1999;70:233–239. doi: 10.1016/s0167-5273(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 68.Laitinen T, Hartikainen J, Vanninen E, Niskanen L, Geelen G, Länsimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol (1985) 1998;84:576–583. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- 69.Kardos A, Watterich G, de Menezes R, Csanády M, Casadei B, Rudas L. Determinants of spontaneous baroreflex sensitivity in a healthy working population. Hypertension. 2001;37:911–916. doi: 10.1161/01.hyp.37.3.911. [DOI] [PubMed] [Google Scholar]
- 70.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001;281:H284–H289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 71.Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation. 2001;104:1627–1632. doi: 10.1161/hc3901.096670. [DOI] [PubMed] [Google Scholar]
- 72.Lábrová R, Honzíková N, Maderová E, Vysocanová P, Nováková Z, Závodná E, Fiser B, Semrád B. Age-dependent relationship between the carotid intima-media thickness, baroreflex sensitivity, and the inter-beat interval in normotensive and hypertensive subjects. Physiol Res. 2005;54:593–600. [PubMed] [Google Scholar]
- 73.Kaushal P, Taylor JA. Inter-relations among declines in arterial distensibility, baroreflex function and respiratory sinus arrhythmia. J Am Coll Cardiol. 2002;39:1524–1530. doi: 10.1016/s0735-1097(02)01787-4. [DOI] [PubMed] [Google Scholar]
- 74.Bonyhay I, Jokkel G, Kollai M. Relation between baroreflex sensitivity and carotid artery elasticity in healthy humans. Am J Physiol. 1996;271:H1139–H1144. doi: 10.1152/ajpheart.1996.271.3.H1139. [DOI] [PubMed] [Google Scholar]
- 75.Mattace-Raso FU, van den Meiracker AH, Bos WJ, van der Cammen TJ, Westerhof BE, Elias-Smale S, Reneman RS, Hoeks AP, Hofman A, Witteman JC. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25:1421–1426. doi: 10.1097/HJH.0b013e32811d6a07. [DOI] [PubMed] [Google Scholar]
- 76.Johansson M, Gao SA, Friberg P, Annerstedt M, Carlström J, Ivarsson T, Jensen G, Ljungman S, Mathillas O, Nielsen FD, et al. Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J Hypertens. 2007;25:163–168. doi: 10.1097/01.hjh.0000254377.18983.eb. [DOI] [PubMed] [Google Scholar]
- 77.Chesterton LJ, Selby NM, Burton JO, Fialova J, Chan C, McIntyre CW. Categorization of the hemodynamic response to hemodialysis: the importance of baroreflex sensitivity. Hemodial Int. 2010;14:18–28. doi: 10.1111/j.1542-4758.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- 78.Tomiyama O, Shiigai T, Ideura T, Tomita K, Mito Y, Shinohara S, Takeuchi J. Baroreflex sensitivity in renal failure. Clin Sci (Lond) 1980;58:21–27. doi: 10.1042/cs0580021. [DOI] [PubMed] [Google Scholar]
- 79.Gao SA, Johansson M, Hammarén A, Nordberg M, Friberg P. Reproducibility of methods for assessing baroreflex sensitivity and temporal QT variability in end-stage renal disease and healthy subjects. Clin Auton Res. 2005;15:21–28. doi: 10.1007/s10286-005-0224-4. [DOI] [PubMed] [Google Scholar]
- 80.Johansson M, Gao SA, Friberg P, Annerstedt M, Bergström G, Carlström J, Ivarsson T, Jensen G, Ljungman S, Mathillas O, et al. Reduced baroreflex effectiveness index in hypertensive patients with chronic renal failure. Am J Hypertens. 2005;18:995–1000; discussion 1016. doi: 10.1016/j.amjhyper.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Bavanandan S, Ajayi S, Fentum B, Paul SK, Carr SJ, Robinson TG. Cardiac baroreceptor sensitivity: a prognostic marker in predialysis chronic kidney disease patients? Kidney Int. 2005;67:1019–1027. doi: 10.1111/j.1523-1755.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 82.Lacy P, Carr SJ, O’Brien D, Fentum B, Williams B, Paul SK, Robinson TG. Reduced glomerular filtration rate in pre-dialysis non-diabetic chronic kidney disease patients is associated with impaired baroreceptor sensitivity and reduced vascular compliance. Clin Sci (Lond) 2006;110:101–108. doi: 10.1042/CS20050192. [DOI] [PubMed] [Google Scholar]
- 83.Boutouyrie P, Zanoli L, Briet M, Karras A, Delahousse M. Baroreflex sensitivity after kidney transplantation: arterial or neural improvement? Nephrol Dial Transplant. 2013;28:2401–2403. doi: 10.1093/ndt/gft341. [DOI] [PubMed] [Google Scholar]
- 84.Hunt BE, Fahy L, Farquhar WB, Taylor JA. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension. 2001;37:1362–1368. doi: 10.1161/01.hyp.37.6.1362. [DOI] [PubMed] [Google Scholar]
- 85.Rostand SG, Brunzell JD, Cannon RO, Victor RG. Cardiovascular complications in renal failure. J Am Soc Nephrol. 1991;2:1053–1062. doi: 10.1681/ASN.V261053. [DOI] [PubMed] [Google Scholar]
- 86.Salman IM, Hildreth CM, Ameer OZ, Phillips JK. Differential contribution of afferent and central pathways to the development of baroreflex dysfunction in chronic kidney disease. Hypertension. 2014;63:804–810. doi: 10.1161/HYPERTENSIONAHA.113.02110. [DOI] [PubMed] [Google Scholar]
- 87.Salman IM, Phillips JK, Ameer OZ, Hildreth CM. Abnormal central control underlies impaired baroreflex control of heart rate and sympathetic nerve activity in female Lewis polycystic kidney rats. J Hypertens. 2015;33:1418–1428. doi: 10.1097/HJH.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 88.Zoccali C, Ciccarelli M, Mallamaci F, Maggiore Q. Parasympathetic function in haemodialysis patients. Nephron. 1986;44:351–354. doi: 10.1159/000184018. [DOI] [PubMed] [Google Scholar]
- 89.Converse RL, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 90.Botey A, Gaya J, Montoliu J, Torras A, Rivera F, López-Pedret J, Revert L. Postsynaptic adrenergic unresponsiveness in hypotensive haemodialysis patients. Proc Eur Dial Transplant Assoc. 1981;18:586–591. [PubMed] [Google Scholar]
- 91.Rascher W, Schömig A, Kreye VA, Ritz E. Diminished vascular response to noradrenaline in experimental chronic uremia. Kidney Int. 1982;21:20–27. doi: 10.1038/ki.1982.4. [DOI] [PubMed] [Google Scholar]
- 92.Leineweber K, Heinroth-Hoffmann I, Pönicke K, Abraham G, Osten B, Brodde OE. Cardiac beta-adrenoceptor desensitization due to increased beta-adrenoceptor kinase activity in chronic uremia. J Am Soc Nephrol. 2002;13:117–124. doi: 10.1681/ASN.V131117. [DOI] [PubMed] [Google Scholar]
- 93.Andresen MC, Kuraoka S, Brown AM. Baroreceptor function and changes in strain sensitivity in normotensive and spontaneously hypertensive rats. Circ Res. 1980;47:821–828. doi: 10.1161/01.res.47.6.821. [DOI] [PubMed] [Google Scholar]
- 94.Coleridge HM, Coleridge JC, Poore ER, Roberts AM, Schultz HD. Aortic wall properties and baroreceptor behaviour at normal arterial pressure and in acute hypertensive resetting in dogs. J Physiol. 1984;350:309–326. doi: 10.1113/jphysiol.1984.sp015203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chapleau MW, Hajduczok G, Abboud FM. Pulsatile activation of baroreceptors causes central facilitation of baroreflex. Am J Physiol. 1989;256:H1735–H1741. doi: 10.1152/ajpheart.1989.256.6.H1735. [DOI] [PubMed] [Google Scholar]
- 96.Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension. 1995;26:341–347. doi: 10.1161/01.hyp.26.2.341. [DOI] [PubMed] [Google Scholar]


