Abstract
Introduction:
Biochemical confirmation (BC) of self-report is the gold standard of evidence for abstinence in smoking cessation research, but difficulty in obtaining samples may bias estimates of quit rates. Proxy confirmation (PC) has not been validated in cessation trials. We assessed the feasibility and validity of PC in a cessation trial for hospitalized smokers.
Methods:
We enrolled 402 daily cigarette smokers during a hospital admission. At enrollment, participants provided demographics, smoking history, and named proxies to confirm their smoking status at follow-up. Participants provided self-reported (SR) 7-day tobacco abstinence by telephone at 6 months post-discharge. SR quitters were asked to mail a saliva sample for BC. Incentives were offered for survey completion ($20) and returned samples ($50). We called proxies for all those with SR to obtain PC. Quit rates were calculated with missing data indicating smoking. We assessed associations of nonresponse with baseline characteristics using chi-squared tests and logistic regression. We calculated the sensitivity and specificity of PC in detecting smokers as determined by BC.
Results:
All patients named at least one proxy. Response rates were 82% for SR, 84% for PC, and 69% for BC. Observed participant characteristics were unrelated to provision of sample for BC. Estimated quit rates were 35% for SR, 27% for SR + PC, 21% for SR + BC and 27% for SR + BC or PC. Sensitivity of PC was not higher than SR (73% vs. 77%); specificity was lower (84% vs. 100%).
Conclusion:
PC was feasible but not superior to self-report in a cessation trial.
Introduction
Studies evaluating smoking cessation interventions require post-intervention assessment of participant smoking status. It is generally accepted that participants in such trials tend to over-report success in quitting, which necessitates some type of corroboration of self-reported abstinence.1,2 Biochemical verification of smoking status, usually in the form of expired carbon monoxide (CO), or cotinine, a nicotine metabolite, in blood, urine or saliva, has become the “gold standard” in smoking cessation research. However, to comply with a request for biochemical verification, participants must provide a sample by returning in person to the research setting or using a kit to collect a sample themselves and returning it by mail, a much greater burden than providing self-report, which can be given over the telephone. Because those who fail to provide samples are usually classified as smokers, studies employing biochemical verification probably underestimate successful quitting.
Proxy informants have been used as an alternative to self-report in population-based surveys.3–6 Investigators conducting surveys designate one household member to serve as a proxy for household members who are not present and report on their smoking status. The use of proxies in this context is intended to improve the speed and ease of data collection rather than ensure its accuracy. Validation studies of this method have shown proxies to be accurate when evaluated against self-report.7,8 The success of proxy informants in surveillance research has prompted the use of proxy verification to corroborate self-report in a few studies of smoking cessation interventions.9–11 However, there are important differences between the two types of studies that may render proxy informants less accurate in cessation research. Proxies face greater uncertainty in cessation research, where they are asked to report the smoking status of someone who is actively attempting to quit, compared to proxies in a population-based study where a recent change in smoking status is not expected. Further, the desire to quit may motivate cessation study participants to conceal their smoking from a proxy, and proxies themselves may be biased by optimism or loyalty to over-report quitting.
We assessed the feasibility and validity of using proxy informants to corroborate self-reported smoking status in a randomized controlled trial of a smoking cessation intervention. Our primary aim was to test the accuracy of proxy reports against the standard of biochemical verification. Our second aim was to examine possible bias in proxy reports, to which end we contacted proxies regardless of the participant’s self-reported smoking status. Finally, we explored associations between proxy accuracy and the proxy’s relationship to the participant.
Methods
Participants and Setting
The participants were 402 smokers who enrolled in a randomized controlled trial of a smoking cessation intervention during an admission at Massachusetts General Hospital in Boston, Massachusetts between July 2010 and April 2012. The study, which is described in detail elsewhere,12,13 compared an intervention which offered post-discharge care consisting of 90 days of cessation medication and telephone counseling support to standard care (referral at discharge to the state quitline). Patients are identified as smokers on admission to Massachusetts General Hospital and automatically referred to the inpatient Tobacco Treatment Service. Counselors offer bedside cessation counseling and cessation medication recommendations. Patients were eligible for the trial if they had received cessation counseling, were daily cigarette smokers, age 18 or older, interested in quitting, willing to accept cessation medication at discharge and could be reached by telephone. The first five participants were recruited during a pilot phase and were all allocated to the intervention arm. The remaining 397 were randomized. Both groups were included in the analyses.
Procedure
At enrollment, participants completed a baseline survey that included demographic information and smoking history. Contact information was collected, including two alternates, who would be called for updated contact information in the event that we were unable to reach the participant for follow-up. Participants were also asked to name three people who would be contacted after the follow-up survey to confirm their self-reported smoking status. The proxy name, telephone number, and relationship to the participant were recorded. The same person could serve as both alternate and proxy at the participant’s discretion.
Self-Reported Smoking Status
Participants were contacted by telephone at 3 and 6 months after hospital discharge to complete a brief survey that included smoking status. Abstinence was defined as no use of any form of tobacco or e-cigarettes for the previous 7 days. Participants received incentives of $20 for each survey completed.
Proxy Confirmation of Smoking Status
After each completed survey, a proxy was contacted and asked if the participant had smoked within the past 7 days. For participants who provided multiple proxies, we attempted to reach the proxies one at a time, in the order specified by the participant at enrollment, until one proxy was reached or at least three attempts were made to each proxy. Failure to reach a proxy, responses of “don’t know,” and refusals were interpreted as evidence that the participant was smoking. Proxies were asked what their relationship to the participant was (spouse/partner, other relative, or friend), whether they lived with the participant (yes or no), and when they last saw the participant (past week, past month, or >30 days ago). Proxies did not receive any incentive for participation.
Biochemical Verification of Smoking Status
Participants who reported being abstinent were asked to provide a saliva sample for verification. Saliva sample collection was accomplished by mailing the participant a kit along with a prepaid mailer for returning the sample. Those who continued to use nicotine replacement therapy at follow-up were asked to return to the hospital to provide an expired CO sample. Participants received incentives of $50 for each sample received. At both 3 and 6 months, the sample was requested at the end of the survey and a kit was mailed or an appointment made to collect a CO sample. At the 3-month follow-up, no further effort was made to obtain the sample after the initial request. The primary outcome of the trial was biochemically confirmed abstinence at 6 months and therefore a much more extensive effort was made to obtain the sample, including reminder phone calls, repeated mailings of kits, and home visits, for up to 60 days after the survey. We considered saliva cotinine less than or equal to 10ng/ml or CO less than 9 ppm to be evidence of abstinence. Participant failure to provide a sample was interpreted as biochemical evidence of smoking.
Statistical Analysis
We calculated quit rates four ways: (1) using self-report only; (2) self-report with proxy confirmation; (3) self-report with biochemical confirmation; and (4) self-report confirmed biochemically if available or by proxy if not. In calculating the proxy- and biochemically confirmed rates, we assumed self-reports of smoking to be true, and confirmation was required only for self-reported abstinence. We interpreted nonresponse of participants and proxies and failure to provide a sample as evidence of current smoking, and included all participants except those known to be deceased at follow-up (3 months: N = 6; 6 months: N = 8). We compared rates of agreement between the methods using chi-squared tests. We validated proxy confirmation in an analysis limited to survey respondents only because we sought proxy reports only after obtaining self-report. We evaluated proxy confirmation against biochemically confirmed self-report at the 6-month follow-up. For each method of detecting smokers (self-report, proxy-report, and self-report with proxy confirmation), we calculated sensitivity (the percent of true smokers who were detected) and specificity (the percent of those classified as smokers who were true smokers), as well as the percent of participants correctly classified using biochemically confirmed quit status as the gold standard. We present kappa scores for the agreement between participants and proxies and between proxy- and biochemically confirmed smoking status. We assessed the association between the characteristics of the proxy reached for confirmation (relationship to participant, whether the proxy lives with the participant, and time since the proxy last saw the participant) with chi-squared tests and logistic regression to test for trends in ordinal variables.
Results
The participants were 53 years old on average, approximately half were female, the majority were white, and nearly half had some education beyond high school (Table 1). All participants named at least one proxy, but only about half named the three that were requested. The mean number of proxies named was 2.33 (SD: 0.73). Approximately half of the participants named a spouse or partner. Seven percent of the participants named friends only without including a spouse, partner, or other relative.
Table 1.
Participant Baseline Characteristics by 6-Month Follow-Up Response Category
| Characteristic | Total | Follow-up response category | ||||
|---|---|---|---|---|---|---|
| Not reached for survey | Self-reported smoking status at follow-up | |||||
| Smoker | Quit | |||||
| Total | Did not provide sample | Provided sample | ||||
| N | 402 | 78 | 185 | 139 | 44 | 95 |
| Percent (95% CI) | 19 (15.8 to 23.6) | 46 (41.1 to 50.9) | 35 (30.1 to 39.4) | 11 (8.2 to 14.4) | 24 (19.7 to 28.1) | |
| Age, mean (SD) | 53 (12.0) | 50 (12.1) | 52 (11.4) | 55 (12.4) | 53 (13.8) | 55 (11.7) |
| Gender, N (%) | ||||||
| Female | 210 (52) | 47 (22) | 101 (48) | 62 (30) | 25 (12) | 37 (18) |
| Male | 192 (48) | 31(16) | 84 (44) | 77 (40) | 19 (10) | 58 (30) |
| Race, N (%) | ||||||
| White | 343 (86) | 67 (19) | 160 (47) | 116 (34) | 37 (11) | 79 (23) |
| Black | 21 (5) | 3 (14) | 10 (48) | 8 (38) | 3 (14) | 4(24) |
| Other/unknown | 38 (9) | 8 (21) | 15 (39) | 15 (40) | 4 (11) | 11 (29) |
| Education, N (%) | ||||||
| High school diploma or less | 208 (52) | 45 (22) | 91 (44) | 72 (35) | 21 (10) | 51 (25) |
| Some college or more | 193 (48) | 33 (17) | 94 (49) | 66 (34) | 23 (12) | 43 (22) |
| Baseline CPD, median (IQR) | 16 (10–20) | 17 (10–20) | 20 (10–20) | 15 (9–20) | 15 (10–20) | 15 (9–20) |
| Lives with a smoker, N (%) | ||||||
| Yes | 167 (42) | 31 (19) | 85 (51) | 51 (31) | 19 (11) | 32 (19) |
| No | 231 (58) | 46 (20) | 98 (42) | 87 (38) | 25 (11) | 62 (27) |
| Smokefree home, N (%) | ||||||
| Yes | 152 (39) | 35 (23) | 62 (41) | 55 (36) | 22 (14) | 33 (22) |
| No | 240 (61) | 41 (17) | 119 (50) | 80 (33) | 21 (9) | 59 (25) |
| Closest proxy named, N (%) | ||||||
| Spouse/partner | 188 (47) | 35 (19) | 79 (42) | 74 (40) | 22 (12) | 52 (28) |
| Relative | 185 (46) | 32 (17) | 95 (51) | 58 (31) | 20 (11) | 38 (21) |
| Friend | 29 (7) | 11 (38) | 11 (38) | 7 (24) | 2 (7) | 5 (17) |
| Number of proxies named, N (%) | ||||||
| 1 | 62 (15) | 14 (23) | 34 (55) | 14 (23) | 4 (6) | 10 (16) |
| 2 | 148 (37) | 27 (18) | 69 (46) | 52 (35) | 15 (10) | 37 (25) |
| 3 | 192 (48) | 37 (19) | 82 (42) | 73 (38) | 25 (13) | 48 (25) |
| Study arm, N (%) | ||||||
| Control | 199 (50) | 43 (22) | 100 (50) | 56 (28) | 21 (11) | 35 (18) |
| Intervention | 203 (50) | 35 (17) | 85 (42) | 83 (41) | 23 (11) | 60 (30) |
CI = confidence interval; CPD = cigarettes per day; IQR = interquartile range.
Response Rates
At 3 months, 336 participants were reached for a follow-up survey response rate of 85% (336/396, excluding six known deaths). The response rate dropped slightly at 6 months to 82% (324/394, excluding eight known deaths). Proxies were reached for 81% (273/336) of respondents at 3 months and 84% (272/324) at 6 months (Table 2). Biochemical samples (saliva or expired CO) were provided by 40% (67/166) of self-reported quitters at 3 months. At 6 months, when a much more robust effort was made to obtain biochemical samples, this rate was much higher (69%, 96/139). Among the 43/139 who did not provide a sample are seven respondents who had refused to provide a sample and 11 who reported being quit when surveyed but subsequently acknowledged having relapsed and did not provide a sample.
Table 2.
Response and Quit Rates
| Measure | Follow-up | |
|---|---|---|
| 3 months | 6 months | |
| Response rates | ||
| Survey | 85% (336/396) | 82% (324/394) |
| Proxy | 81% (273/336) | 84% (272/324) |
| Biochemical sample | 40% (67/166) | 69% (96/139) |
| Quit rates | ||
| Self-report only | 42% (166/396) | 35% (139/394) |
| Self-report, proxy-confirmed | 27% (110/396) | 27% (105/394) |
| Self-report, biochemically confirmed | 13% (52/396) | 21% (83/394) |
| Self-report, confirmed biochemically or by proxy if sample not provided | 26% (104/396) | 27% (106/394) |
Table 1 presents the distribution of participant baseline characteristics by 6-month follow-up response category: not reached, self-reported smoker, or self-reported quitter. The latter category is further divided by whether the participant provided a sample for biochemical verification. Overall, 19% of participants were not reached for the survey. Those not reached for the survey were younger than those who responded (50.1 vs. 53.3 years old, P = .0349). Those naming only friends as proxies were more likely to be nonresponders than those naming spouses or partners (38% vs. 19%, P = .031). Not being reached for the survey was not associated with the other characteristics in Table 1. Eleven percent of participants reported being quit but did not provide a biochemical sample for confirmation. This response category was not associated with any of the baseline characteristics considered. A total of 30% of participants were classified as smoking because they did not respond to the survey or did not provide a sample for biochemical verification.
Estimated Quit Rates
Quit rates were estimated including all participants (except for those known to be deceased) and interpreting nonresponse as evidence of smoking. Self-reported quit rates dropped from 42% at 3 months to 35% at 6 months (P = .055). Proxy-confirmed quit rates were 27% at both follow-ups. Contrary to self-report, biochemically confirmed quit rates increased from 13% at 3 months to 21% at 6 months (P = .007). Biochemical disconfirmation rates (lie rates), calculated among participants who provided samples only, were 21% (14/67) at 3 months and 14% (13/96) at 6 months (P = .214).
Validation of Proxy Confirmation at 6 Months
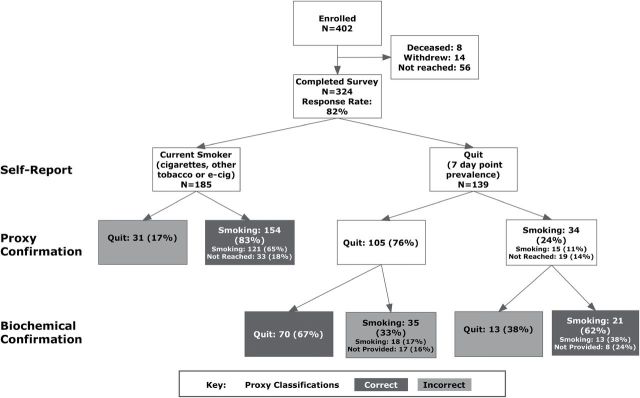
Classification of respondents by self-report, proxy, and biochemical sample is depicted in Figure 1. Proxies reported that 42% (136/324) of respondents were quit, a rate similar to respondent self-report (43%, 139/324). Overall, proxies correctly classified 76% [(154 + 70 + 21)/324] of respondents when measured against biochemically confirmed self-report. Correct classification was much more common when proxies said participants were smoking. Ninety-three percent [(154 + 21)/(154 + 34)] of those classified as smoking by proxies were current smokers based on self-report or biochemical confirmation while only 51% [70/(31 + 105)] of those classified as quit by proxies were confirmed abstinent by saliva or expired CO. The sensitivity of proxy report alone in detecting smoking was 73% [(154 + 21)/(185 + 35 + 21)] and specificity was 84% [70/(70 + 13)]. When proxy classifications were used only to confirm self-reported abstinence, they were 65% [(70 + 21)/139] correct. When self-report of smoking was combined with the proxy report (ie, a participant was classified as smoking if smoking was reported by the participant or the proxy), sensitivity increased to 85% [(185 + 21)/(185 + 35+ 21)], with specificity unchanged at 84% [70/(70 + 13)]. In comparison, the sensitivity of self-report alone was 77% [185/(185 + 35 + 21)]. Specificity of self-report of smoking is assumed to be 100%.
Figure 1.
Smoking status at 6-month follow-up by self-report, proxy, and biochemical verification.
For 52 of the 324 respondents, no proxy could be reached, and we interpreted the missing proxy reports as evidence of smoking. To assess the impact of this assumption, we examined the self-reported and biochemically confirmed smoking status in these respondents and the effect of excluding them on the sensitivity of the proxy report in detecting smoking. The majority of these respondents were smoking both by self-report (33/52, 63%) and by biochemical confirmation (42/52, 81%, Table 3). Because our assumption classifies them all as smokers, one would expect it to increase sensitivity in detecting smokers and decrease specificity compared to an analysis that excluded missing proxy reports. Considering self-reported smoking status as the standard, if we include the 52 missing proxy reports (N = 324), sensitivity was 83% [(121 + 33)/185], specificity was 76% (105/139), and 80% [(121 + 33 + 105)/324] were correctly classified. Excluding the missing reports yielded lower sensitivity [80%, 121/(121 + 31)], higher specificity [88%, 105/(15 + 105)], and an increase in the percent correctly classified [83%, (121 + 105)/272]. However, the number of respondents correctly classified was greater when including the missing reports than excluding them (259 vs. 226). We repeated this comparison using biochemically confirmed smoking status as the standard. When compared to an analysis including the missing reports, excluding them again yielded lower sensitivity (67% vs. 73%) and higher specificity (96% vs. 84%). The percent correctly classified was essentially unchanged (75% vs. 76%) and the number correctly classified was higher when including the missing reports than excluding them (245 vs. 203).
Table 3.
Proxy Report Outcome by Self-Reported and Biochemically Confirmed Smoking Status
| Smoking status | Proxy report | |||
|---|---|---|---|---|
| Smoking | Quit | Not reached | Total | |
| Self-reported | ||||
| Smoking | 121 | 31 | 33 | 185 |
| Quit | 15 | 105 | 19 | 139 |
| Biochemically confirmed | ||||
| Smoking | 133 | 66 | 42 | 241 |
| Quit | 3 | 70 | 10 | 83 |
| Total | 136 | 136 | 52 | 324 |
Agreement of Proxy Report With Self-Report and Biochemically Confirmed Smoking Status by Proxy Characteristics
At the 6-month follow-up, proxy characteristics were obtained from the 272 proxies who were reached. Thirty percent of proxies reached were spouse/partners, 51% were other relatives, and 19% were friends of the participant. Forty-three percent lived with the participant, and 85% had seen the participant within the past week. Agreement between participants and proxies who were reached was substantial (226/272, Kappa: .66) but dropped when failure to reach the proxy was considered evidence of smoking (259/324, Kappa: .59). Agreement between proxies and biochemical confirmation was less strong and did not vary whether calculated for only those participants whose proxies were reached (203/272, Kappa: .49) or all participants (245/324, Kappa: .47)
We examined the association between proxy characteristics and the concordance of proxy report with participant self-report and with biochemically confirmed smoking status considering only the 272 proxies reached at the 6-month follow-up survey (Table 4). Agreement between participants and proxies was higher when the proxy was closer to the participant (P = .022 for trend), 91% for partner or spouse, 81% for other relatives, and 76% for friends, but was not associated with whether the proxy lived with the participant (P = .247) or how long it had been since the proxy had seen the participant (P = .548 for trend). None of these characteristics were associated with whether the proxy agreed with the biochemically confirmed smoking status.
Table 4.
Agreement of Proxy Report with Participant Self-Report and Biochemically Confirmed Abstinence by Proxy Characteristic
| Proxy characteristic | N | Agreement of proxy report | |
|---|---|---|---|
| With participant self-report, N (%) | With biochemically confirmed abstinence, N (%) | ||
| Relationshipa | |||
| Partner/spouse | 78 | 71 (91) | 61 (78) |
| Other relative | 134 | 108 (81) | 102 (76) |
| Friend | 50 | 38 (76) | 35 (70) |
| P b | .022 | .561 | |
| Lives with participantc | |||
| No | 149 | 120 (81) | 110 (74) |
| Yes | 114 | 98 (86) | 88 (77) |
| P | .247 | .530 | |
| Last saw participant | |||
| Past week | 230 | 194 (84) | 170 (74) |
| Past month | 25 | 17 (68) | 19 (76) |
| >30 days ago | 17 | 15 (88) | 14 (82) |
| P b | .548 | .732 | |
| Total | 272 | 226 (83) | 203 (75) |
aUnknown for 10 proxies.
bLogistic regression test for trend.
cUnknown for nine proxies.
As a sensitivity analysis, we considered the performance of a “best proxy,” one who was a spouse/partner, who lived with the participant and had seen the participant in the week prior to being contacted for a proxy report. Ten of the 78 spouse/partner proxies did not live with the participant. The remaining 68 met all three criteria. This group was more likely than the other proxies to agree with the participant (91% vs. 80%, P = .033), but not more likely to agree with the biochemically confirmed smoking status (88% vs. 83%, P = .504).
Discussion
We assessed the use of proxies named by study participants to confirm smoking status in the context of a randomized controlled trial of a smoking cessation intervention. This method proved feasible, in that all participants named at least one proxy and the proxies were reached at the same rate as the participants themselves. The use of proxy reports to confirm self-reported quitting yielded quit rates that were lower than self-report alone, and higher than biochemically confirmed self-report. This is desirable if we assume that rates based on self-report overestimate quitting while those entailing biochemical confirmation underestimate quitting. However, comparisons of proxy report to biochemical confirmation on an individual basis showed that proxies were correct in only three-quarters of their reports and the sensitivity of proxies in detecting smoking was slightly lower than that of the participants themselves. Proxy reports of smoking were more likely to be correct than reports of abstinence. In our analyses, we interpreted proxy nonresponse as a proxy report of smoking. The performance of proxy reports in detecting smoking was not improved by limiting analyses to respondents for whom a proxy was reached.
We are aware of only one other cessation study that compared proxy reports to both self-report and biochemical verification.14 In that study, biochemical confirmation was obtained for participants whose self-report of abstinence was confirmed by a proxy. Proxy reports of abstinence were biochemically confirmed in 82% (14/17) of cases at 6 months after enrollment. This rate is substantially higher than in the present study, in which 67% [70/(70 + 35)] of proxy-confirmed reports of abstinence were confirmed biochemically. This difference may be attributable to the fact the participants in the other study were newly diagnosed with cardiovascular disease and most were already quit at enrollment.
We examined three factors that might influence the accuracy of proxy reports: the proxy’s relationship to the participant, whether the proxy lived with the participant, and how long ago the proxy had last seen the participant at the time of the report. Relationship to the participant was the only factor significantly associated with concordance between proxy and patient, with participants agreeing with 91% of reports from their spouses or partners and only 76% of reports from friends. None of the factors were associated with biochemical confirmation of proxy reports. Three cessation interventions9–11 that have employed proxies have relied on the participant’s spouse or partner, based on the assumption that this person would be the best informant. Our results do not support that assumption. It is also the case that limiting proxies to spouse/partners would not have been feasible in our study because half of our participants did not name a spouse/partner as proxy.
We estimated quit rates at 3 and 6 months after enrollment, by self-report only, with proxy confirmation and with biochemical confirmation. The self-reported quit rate dropped from 3 to 6 months, as is commonly seen in intervention trials, and we can assume this reflects a true increase in smoking prevalence. However, the proxy-confirmed quit rate remained the same, demonstrating a problematic insensitivity to change. It is also concerning that the biochemically confirmed rate actually rose.
Biochemically confirmed quit rates are lower than self-reported quit rates because some participants provide samples that disconfirm their claim of abstinence but also because some quitters simply fail to provide a sample. As a result, quit rates are biased downward to the extent that participants are truly quit but unmotivated or unable to provide a sample. There is potential for further bias if this group differs from the other participants in any way that is relevant to the intervention under study. We looked for, but did not find, associations between failure to provide a sample and a range of characteristics including study arm, demographics, and smoking history.
Investigators typically seek to eliminate this source of potential bias by motivating participants with financial incentives and making it easier for them to provide samples, by offering home visits, for example. These methods may be effective in reaching those who are truly quit. At 3 months, participants in the present study who reported abstinence were offered a financial incentive to provide a sample and were sent a sample collection kit, but no further effort was made to obtain the sample. At 6 months, the same financial incentive was offered but a much more intensive effort was made to obtain the sample (multiple reminders, repeat mailings of kits, offers of home or clinic visits). We found that the sample response rate rose from 40% at 3 months to 69% at 6 months. This increase was not associated with a higher disconfirmation rate; instead, it fell from 21% to 14%, suggesting that the greater effort increased the response rate among the truly abstinent without encouraging more participants who were truly smoking to send samples. It is possible that the financial incentive alone was sufficiently motivating for that subset of smokers who would falsely claim to be quit and further entreaties did not increase their response rate.
It is likely that no amount of investigator effort will be sufficient to obtain 100% compliance with requests for biochemical verification of smoking status. Bryant et al.15 obtained self-reported smoking status from participants in an in-person interview and immediately afterward asked them to provide an expired CO measurement. The request for the CO measurement was made only after self-report was given, and no financial incentive was offered. Overall, 14% of participants declined, including 10% of those who had already reported they were current smokers. This suggests that some participants are reluctant to provide a biochemical sample, even if there is no inconvenience involved and the result has already been disclosed. This finding is especially noteworthy given the sample requested was breath which participants probably regard as less invasive than the saliva, blood or urine needed for cotinine assessment.
Limitations
We assessed the use of proxy confirmation of smoking status in a randomized controlled trial of a smoking cessation intervention. It is likely that motivation of participants to quit or to have others believe they have quit was influenced by study procedures and the circumstance of their enrollment (during a hospitalization). The generalizability of our results is diminished to the extent that this influence is unique to our study. Our participants were recruited from a single large hospital in the northeastern United States that serves a largely white population. This may limit the generalizability of our findings to settings that are geographically or demographically similar to ours. Our ability to assess the validity of the proxy reports depended on a chain of responses: first the participant had to respond to the survey, then the proxy had to respond to our request for confirmation, and finally, participants had to respond to our request for samples. Our response rates compare favorably to similar studies, but we were faced with missing data at each step. In our analyses, we assumed that nonresponse was evidence of smoking, but this is not necessarily true.
Conclusions
Confirmation of smoking status by proxy was feasible, but we were unable to demonstrate the validity of proxy reports in the context of a smoking cessation intervention study. Given the substantial disconfirmation rates we observed, proxy-confirmation is not an adequate substitute for biochemical verification. We found no evidence linking participant characteristics to failure to obtain samples for biochemical verification, but the quit rates estimated with this method may be more sensitive to the effort expended to achieve a satisfactory response rate than to the underlying true quit rate.
Funding
This work was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (RC1 HL099668 and K24 HL004440). SJJ’s time was supported in part by the US Department of Veterans Affairs Clinical Sciences Research and Development Service career development award (1IK2CX000918-01A1). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Declaration of Interests
NAR has received royalties from UpToDate, Inc. and has been an unpaid consultant for Pfizer regarding smoking cessation. DEL has been a paid consultant to CVS Inc. to provide expertise on tobacco policy.
Acknowledgments
We are indebted to the dedicated counselors of the Tobacco Treatment Service, Caitlin McCann, Nancy McCleary, Kathy McKool, and Jean Mizer, for conducting preliminary screening of participants. We also grateful for the assistance of our study participants and their family and friends who served as proxies.
References
- 1. Noonan D, Jiang Y, Duffy SA. Utility of biochemical verification of tobacco cessation in the department of veterans affairs. Addict Behav. 2013;38(3):1792–1795. 10.1016/j.addbeh.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 3. McLaughlin JK, Dietz MS, Mehl ES, Blot WJ. Reliability of surrogate information on cigarette smoking by type of informant. Am J Epidemiol. 1987;126(1):144–146. [DOI] [PubMed] [Google Scholar]
- 4. Gilpin EA, Pierce JP, Cavin SW, et al. Estimates of population smoking prevalence: self-vs proxy reports of smoking status. Am J Public Health. 1994;84(10):1576–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hyland A, Cummings KM, Lynn WR, Corle D, Giffen CA. Effect of proxy-reported smoking status on population estimates of smoking prevalence. Am J Epidemiol. 1997;145(8):746–751. [DOI] [PubMed] [Google Scholar]
- 6. Navarro AM. Smoking status by proxy and self report: rate of agreement in different ethnic groups. Tob Control. 1999;8(2):182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111(1):23–41. [DOI] [PubMed] [Google Scholar]
- 8. Chen Y, Rennie D, Dosnan J. The reliability of cigarette consumption reports by spousal proxies. Am J Public Health. 1995;85(12):1711–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: a randomized trial. Am J Med. 2003;114(7):555–562. S0002934303000810. [DOI] [PubMed] [Google Scholar]
- 10. Carmody TP, Duncan C, Simon JA, et al. Hypnosis for smoking cessation: a randomized trial. Nicotine Tob Res. 2008;10(5):811–818. 10.1080/14622200802023833. [DOI] [PubMed] [Google Scholar]
- 11. Simon JA, Duncan C, Huggins J, Solkowitz S, Carmody TP. Sustained-release bupropion for hospital-based smoking cessation: a randomized trial. Nicotine Tob Res. 2009;11(6):663–669. 10.1093/ntr/ntp047. [DOI] [PubMed] [Google Scholar]
- 12. Japuntich SJ, Regan S, Viana J, et al. Comparative effectiveness of post-discharge interventions for hospitalized smokers: study protocol for a randomized controlled trial. Trials. 2012;13:124. 10.1186/1745-6215-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728. 10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson DK, Wallston KA, King JE, Smith MS, Heim C. Validation of smoking abstinence in newly diagnosed cardiovascular patients. Addict Behav. 1993;18(4):421–429. [DOI] [PubMed] [Google Scholar]
- 15. Bryant J, Bonevski B, Paul C, Lecathelinais C. Assessing smoking status in disadvantaged populations: is computer administered self report an accurate and acceptable measure? BMC Med Res Methodol. 2011;11:153. 10.1186/1471-2288-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]