Abstract
Objectives This research examined whether individual and family-level factors during the transition from late childhood to early adolescence protected individuals from an increased risk of poor glycemic control across time, which is a predictor of future diabetes-related complications (i.e., health resilience). Methods This longitudinal, multisite study included 239 patients with type 1 diabetes and their caregivers. Glycemic control was based on hemoglobin A1c. Individual and family-level factors included: demographic variables, youth behavioral regulation, adherence (frequency of blood glucose monitoring), diabetes self-management, level of parental support for diabetes autonomy, level of youth mastery and responsibility for diabetes management, and diabetes-related family conflict. Results Longitudinal mixed-effects logistic regression indicated that testing blood glucose more frequently, better self-management, and less diabetes-related family conflict were indicators of health resilience. Conclusions Multiple individual and family-level factors predicted risk for future health complications. Future research should develop interventions targeting specific individual and family-level factors to sustain glycemic control within recommended targets, which reduces the risk of developing future health complications during the transition to adolescence and adulthood.
Keywords: diabetes, health promotion and prevention, longitudinal research, resilience
Resilience is defined as achieving a positive outcome despite exposure to one or more adverse events or other significant risk factors (Hilliard, Harris, & Weissberg-Benchell, 2012; Luthar, Cicchetti, & Becker, 2000). Research on resilience in children and adolescents has generally focused on psychological outcomes rather than health outcomes. Resilience research in pediatric chronic illness is no exception, including research on resilience in pediatric type 1 diabetes (Hilliard et al., 2012). However, health outcomes are extremely important, especially for children and adolescents with chronic conditions. There are extraordinary individual differences in pediatric health outcomes; ranging from a relative absence of health problems and a decreased risk for developing future health problems (defined as health resilience) to a high level of morbidity and future health complications.
It is well-documented that intensive diabetes self-management and adherence to the prescribed treatment regimen during childhood and adolescence yields long-term health benefits into adulthood (Silverstein et al., 2005). Chronically elevated glycemic control during childhood and adolescence puts individuals at an increased risk for developing diabetes-related complications in adulthood (e.g., cardiovascular problems; problems with eyes, kidneys, and peripheral and autonomic nervous systems; etc.) (DCCT Research Group, 1994; Nathan et al., 2005, 2009; Silverstein et al., 2005). It is critical to identify factors that contribute to resilient health outcomes in pediatric type 1 diabetes (i.e., glycemic control within the American Diabetes Association [ADA] recommendations), which can inform the development of interventions to reduce risk of health complications. Hilliard et al. (2012) recommended that models of health resilience in pediatric type 1 diabetes utilize glycemic control as a dichotomized health outcome (e.g., “health resilience” or glycemic control within ADA recommendations versus glycemic control above ADA recommendations) to determine what potential risk factors, assets, and protective processes predict health resilience.
A significant limitation of previous research investigating pediatric health resilience is the absence of scientifically valid, clinically relevant measures to identify resilient health outcomes relatively early in development (Hilliard et al., 2012). Type 1 diabetes is a model condition in which to study individual differences in resilient health outcomes based on the availability of an objective, valid biomarker of glycemic control: hemoglobin A1c (HbA1c), which is predictive of risk for serious health complications (Hilliard et al., 2012; Rohan et al., 2014). Previous research investigating subgroups or trajectories of glycemic control in adolescents with type 1 diabetes found subgroups demonstrating varying levels of glycemic control over time: subgroups that demonstrated optimal levels of glycemic control, and subgroups that demonstrated moderately elevated to chronically elevated glycemic control. Furthermore, individual and family-level factors predicted these group trajectories of glycemic control (Helgeson et al., 2010; Hilliard, Wu, Rausch, Dolan, & Hood, 2013; King et al., 2012; Luyckx & Seiffge-Krenke, 2009; Rohan et al., 2014). Research in pediatric type 1 diabetes that extends the scientific understanding of influences on resilient health outcomes has been limited by the primary focus on studying the prediction of mean glycemic control or group-based trajectories. Glycemic control lends itself to a person-centered approach for examining health resilience by using pre-determined thresholds for resilient (i.e., HbA1c ≤ 7.5%) versus not resilient (i.e., HbA1c > 7.5%) health outcomes.
Type 1 diabetes is a difficult and stressful chronic condition to manage for patients and their families, especially for patients transitioning from childhood to early adolescence. The complex treatment demands in the context of other life demands increases risk for psychosocial difficulties and decreased quality of life (Hilliard et al., 2012). Health status that appeared to be resilient at school age often deteriorates during adolescence and elevates risk for future diabetes-related health complications (Helgeson et al., 2010; Rohan et al., 2014). Deteriorations in health status could be related to a number of factors including but not limited to: increased adolescent independence, decreased parental involvement in diabetes management, less adaptive self-management behaviors, decreased adherence, and increased family conflict, including diabetes-related conflict (Anderson et al., 2009; Helgeson, Siminerio, Escobar, & Becker, 2009; Helgeson et al., 2010; Hilliard et al., 2012, 2013; Ingerski, Anderson, Dolan, & Hood, 2010; Miller-Johnson et al., 1994; Moran et al., 2002; Rohan et al., 2014; Weissberg-Benchell, Goodman, Lomaglio, & Zebracki, 2007; Wu et al., 2014; Wysocki, 1993). Although management of type 1 diabetes is a burden for many pediatric patients and families, many of these patients achieve healthy outcomes (i.e., optimal glycemic control).
Hilliard and colleagues (2012) described an excellent model of diabetes resilience that included individual and family-level factors predictive of health resilience or achieving glycemic control levels within ADA recommendations (i.e., ≤7.5% for pediatric patients). Previous research has indicated that decreased diabetes knowledge and deficits in behavioral regulation are predictive of deteriorations in glycemic control (Hilliard et al., 2012; Holmes et al., 2006; Levine et al., 2001; Miller et al., 2013). Increased family conflict, poor diabetes self-management, less frequent blood sugar checking, and poor mastery of diabetes management tasks are also predictive of deteriorations in glycemic control (Helgeson, Honcharuk, Becker, Escobar, & Siminerio, 2011; Helgeson et al., 2009, 2010; Hood, Peterson, Rohan, & Drotar, 2009; Rausch et al., 2012; Rohan et al., 2014; Wysocki, Meinhold, et al., 1996). Demographic and medical characteristics (e.g., age, ethnicity/race, insulin therapy regimens, family structure, and gender) are also associated with health resilience (Hilliard et al., 2012; Whittemore, Jaser, Guo, & Grey, 2010).
Few studies have focused on understanding the specific longitudinal factors that contribute to health resilience in type 1 diabetes during the transition from late childhood to early adolescence, which is a difficult time for many pediatric type 1 diabetes patients, as they are seeking greater autonomy, but may not be ready to assume full responsibility for diabetes management. It is imperative to examine specific demographic and medical characteristics and individual and family-level factors that could be utilized as markers of risk so that clinicians can initiate preventative interventions at the cusp of adolescence to reduce pediatric patients risk for future health complications (Fogel & Weissberg-Benchell, 2010). Unfortunately, relatively few studies with sufficiently large sample sizes have used prospective methods to assess the health resilience status of children with type 1 diabetes during the transition to adolescence.
To address these needs, the present study evaluated several individual and family-level factors that might contribute to resilient health status based on objective methods in a prospective study design in a large multisite sample of children undergoing the transition to adolescence. Given that achieving optimal glycemic control is the primary goal of diabetes management, research investigating the prospective relationship between individual and family-level factors and health resilience is an extremely important area of research. Early adolescence is an especially important time in the development of health resilience (Hilliard et al., 2012). The development and practice of protective health behaviors and physiological processes will likely contribute to improved future health outcomes (i.e., health resilience). The current study utilized the health resilience model described by Hilliard et al. (2012) as a framework to test a predictive model of health resilience, which included a number of demographic and medical characteristics, individual factors, and family-level factors related to glycemic control and increased risk for development of future health complications. To our knowledge, this is the first study of health resilience that examines which demographic and medical factors (e.g., patient age, gender, duration of diabetes, type of insulin regimen, ethnicity and race, one- versus two-parent household), individual factors (e.g., adolescent behavioral regulation, adolescent mastery of diabetes management and responsibility for diabetes management, quality of diabetes self- management, and adherence to diabetes treatment, e.g., frequency of blood glucose testing), and family-level factors (e.g., family conflict, parental support of diabetes-related autonomy, and appropriate allocation of responsibility for diabetes management) predicted resilient versus not resilient health outcomes. It was hypothesized that positive behavioral regulation, more adaptive diabetes self-management, more frequent blood sugar checking, and mastery of and responsibility for diabetes management tasks, along with parental support of autonomy, family involvement in diabetes management, and low levels of diabetes-related family conflict, would predict health resilience as defined by glycemic control within the ranges recommended by the ADA (i.e., HbA1c ≤ 7.5%) (Silverstein et al., 2005).
Methods
Study Design and Participants
Data were collected as part of a three-year longitudinal, multisite, observational study. This is the first study that has focused on the prediction of health resilience using longitudinal individual and family-level predictors. Four other studies were published using the three-year outcomes from this data set. These studies focused on paternal involvement in diabetes management, parent–child interactions when discussing diabetes management tasks, adolescent autonomy and relationship to adherence, and trajectories of glycemic control (Hilliard et al., 2014; Iskander, Rohan, Pendley, Delamater, & Drotar, 2014; Rohan et al., 2014; Wu et al., 2014). Participants were pediatric patients diagnosed with type 1 diabetes and their maternal caregivers who were followed at three pediatric medical centers in the United States. Patients and their maternal caregivers completed all questionnaire measures at baseline and then again at yearly intervals from baseline to three years. Patients and their primary caregivers were provided with incentives at baseline and at yearly study visits. Institutional review boards at each site approved the study. Demographic and medical characteristics for the whole sample, and those with resilient versus at-risk HbA1c levels are provided in Table I.
Table I.
Descriptive Statistics for Glycemic Control, Covariates, and Nonsignificant Predictors: Resilient Versus At-Risk Patients
| Baseline | 12 months |
24 months |
36 months |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health resilience | Sample | Resilient | At risk | Sample | Resilient | At risk | Sample | Resilient | At risk | Sample | Resilient | At risk |
| Mean glycemic control | 8.20 ± 1.37 | 6.88 ± 0.33 | 8.8 ± 1.26 | 8.31 ± 1.38 | 6.85 ± 0.36 | 8.81 ± 1.23 | 8.51 ± 1.41 | 6.83 ± 0.38 | 9.05 ± 1.22 | 8.77 ± 1.60 | 6.88 ± 0.33 | 9.21 ± 1.45 |
| Glycemic control range, n | 6–7% | 6–7%, 73 | 8–17%, 164 | 6–14% | 6–7%, 59 | 8–14%, 164 | 6–13% | 6–7%, 52 | 8–13%, 172 | 6–16% | 6–7%, 42 | 8–16%, 179 |
| Covariates | Sample | Resilient | At risk | Sample | Resilient | At risk | Sample | Resilient | At risk | Sample | Resilient | At risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 10.54 ± 0.94 | 10.34 ± 0.93 | 10.61 ± 1.07 | 11.59 ± 0.97 | 11.53 ± 1.04 | 11.66 ± 1.02 | 12.62 ± 0.96 | 12.44 ± 1.00 | 12.67 ± 1.01 | 13.62 ± 0.97 | 13.43 ± 1.02 | 13.68 ± 1.05 |
| Duration | 4.41 ± 2.46 | 4.33 ± 2.29 | 4.44 ± 2.56 | 5.43 ± 2.49 | 5.12 ± 2.30 | 5.50 ± 2.55 | 6.46 ± 2.43 | 6.49 ± 2.32 | 6.47 ± 2.46 | 7.48 ± 2.43 | 7.30 ± 2.02 | 7.53 ± 2.52 |
| Therapy | ||||||||||||
| Injection | 109 (45.6) | 20 (18.3%) | 89 (81.7%) | 76 (33.6) | 12 (15.8%) | 64 (84.2%) | 67 (29.8) | 7 (10.6%) | 59 (89.4%) | 68 (30.6) | 6 (8.8%) | 62 (91.2%) |
| Pump | 130 (54.4) | 53 (41.4%) | 75 (58.6%) | 150 (66.4) | 47 (32.0%) | 100 (68.0%) | 154 (68.4) | 43 (27.9%) | 111 (72.1%) | 153 (68.9) | 36 (23.7%) | 116 (76.3%) |
| Gender | ||||||||||||
| Male | 109 (45.61) | 36 (33.6%) | 71 (66.4%) | 103 (45.6) | 31 (30.1%) | 72 (69.9%) | 104 (46.2) | 25 (24.3%) | 78 (75.7%) | 101 (45.5) | 21 (21.0%) | 79 (79.0%) |
| Female | 130 (54.39) | 37 (28.5%) | 93 (71.5%) | 123 (54.4) | 28 (23.3%) | 92 (76.7%) | 121 (53.8) | 27 (22.3%) | 94 (77.7%) | 121 (54.5) | 21 (17.4%) | 100 (82.6%) |
| Ethnicity | ||||||||||||
| Non-Hispanic, Caucasian | 179 (74.9) | 63 (35.6%) | 114 (64.4%) | 171 (75.7) | 50 (29.4%) | 120 (70.6%) | 170 (75.6) | 43 (25.3%) | 127 (74.7%) | 170 (76.6) | 38 (22.5%) | 131 (77.5%) |
| Non-Hispanic, Minority | 27 (11.3) | 4 (14.8%) | 23 (85.2%) | 26 (11.5) | 5 (20.8%) | 19 (79.2%) | 25 (11.1) | 4 (16.7%) | 20 (83.3%) | 23 (10.4) | 2 (8.7%) | 21 (91.3%) |
| Hispanic | 33 (13.8) | 6 (18.2%) | 27 (81.8%) | 29 (12.8) | 4 (13.8%) | 25 (86.2%) | 30 (13.3) | 5 (16.7%) | 25 (83.3%) | 29 (13.1) | 2 (6.9%) | 27 (93.1%) |
| Household structure | ||||||||||||
| One Parent | 51 (21.3) | 7 (13.7%) | 44 (86.3%) | 46 (20.4) | 6 (13.0%) | 40 (87.0%) | 47 (20.9) | 6 (12.8%) | 41 (87.2%) | 46 (20.7) | 2 (4.3%) | 44 (95.7%) |
| Two Parent | 188 (78.7) | 66 (35.5%) | 120 (64.5%) | 180 (79.6) | 53 (29.9%) | 124 (70.1%) | 178 (79.1) | 46 (26.0%) | 131 (74.0%) | 176 (79.3) | 40 (22.9%) | 135 (77.1%) |
Note. Resilient defined as having HbA1c within ADA recommendations (≤7.5%) at that time point. At risk defined as having HbA1c above ADA recommendations (>7.5%) at that time point.
Eligibility Criteria
Potentially eligible patients were identified by medical staff and approached by research staff who explained the study procedures and verified eligibility. Participants were eligible for study participation if they were ages 9-11 (at recruitment), diagnosed with type 1 diabetes for at least one year, spoke English, not involved in foster care at baseline, and had no known plans to relocate during the study. Patients who were diagnosed with a comorbid chronic condition requiring burdensome treatment (e.g., cystic fibrosis) or diagnosed with an intellectual or developmental disability that impacted completion of study procedures were ineligible to participate. Informed consent was obtained from a legal guardian. Children 11 years and older completed written assent, and verbal assent was obtained from children younger than 11 years.
Of the 361 eligible patients who were approached, 240 (66.5%) consented and participated. Reasons for nonparticipation included: being too busy (n = 54), no transportation (n = 3), and other (n = 64). After enrollment, one child was diagnosed with monogenic diabetes of the young; the child no longer required insulin, and was removed from subsequent data analyses. Overall attrition across three years was 4.2% (n = 10). Reasons for attrition included: no longer interested in research or too busy/overwhelmed to participate (n = 4), relocated or changed to an endocrinologist not affiliated with the hospital (n = 2), or lost to follow-up and dropped from the study (n = 4). In addition to attrition, missing data included families that did not complete a yearly study visit. There were no significant differences (p > 0.05) in demographic or medical characteristics between those who participated in the one-, two-, and three-year follow-ups and those who did not complete the one- (n = 13), two- (n = 14), or three-year (n = 7) study visits.
Measures: Primary Outcome
Glycemic Control: Hemoglobin A1c (HbA1c). Blood samples were obtained every six months by a finger stick, yielding a total of seven samples across the three years. Samples were analyzed by one central laboratory using the TOSOH-G7 method (reference range 4.0–6.0%). Glycemic control recommendations provided by the ADA are in place to minimize risk for future diabetes-related complications (<7.5% for pediatric patients) (Silverstein et al., 2005). There is strong evidence that chronically elevated glycemic control levels above ADA recommendations increase a pediatric patient’s risk for future diabetes-related complications in adulthood (DCCT Research Group, 2002). In the present study, health resilience was measured at each time point using glycemic control values that were collected across the three years. “Resilience” was defined as an HbA1c value within ADA recommendations for pediatric patients (i.e., ≤7.5%). If a patient received an HbA1c value above ADA recommendations, the HbA1c value was categorized as “at risk or not resilient.”
Measures: Predictors of Health Resilience
Executive Functioning: Behavioral Regulation. Adolescent behavioral regulation was measured using the 86-item parent-reported Behavior Rating Inventory of Executive Functioning (BRIEF), which assessed parent perceptions of adolescent’s behavioral regulation and metacognitive abilities (Gioia, Isquith, Guy, & Kenworthy, 2000). In the current study, the Behavioral Regulation Index was examined, which assessed parent perceptions of the adolescent’s ability to shift cognitive set and moderate emotions and behaviors through emotional control. Standardized t-scores of 65 or higher are considered clinically significant indicating deficits in behavioral regulation. Reliability of the BRIEF has been established for both clinical and normative samples, and validity has been documented with other measures of behavioral and attentional functioning (Gioia et al., 2000). In the present sample, internal consistency (α) from baseline to 36 months was .96–.98.
Diabetes Self-Management
The Diabetes Self-Management Profile (DSMP) is a 25-item structured interview, which was administered to mothers and assessed adolescent’s diabetes-related self-management behaviors (e.g., exercise, hypoglycemia management, diet, blood glucose monitoring, and insulin administration) across the previous 3 months (Harris et al., 2000). The DSMP included yes/no items and 3- to 5-point Likert scale items. Higher total scores reflected better self-management behaviors. The DSMP has demonstrated adequate validity and reliability in previous studies (Harris et al., 2000). In the current study, internal consistency (α) for the total DSMP score across three years was .66–.71 for mothers.
Treatment Adherence: Blood Glucose Monitoring (BGM) Frequency
BGM results for the two weeks prior to each study visit were downloaded from the patient’s blood glucose meter(s). If a meter was unavailable, the information was obtained from the patient’s logbook (baseline, 17%; 1 year, 17%; 2 years, 15%; 3 years, 11%). Average number of blood glucose monitoring checks per day was calculated for the 2 weeks prior to data collection. BGM frequency is a well-validated measure of treatment adherence for prediction of glycemic control in pediatric type 1 diabetes (Hood et al., 2009).
Mastery of and Responsibility for Diabetes Management Tasks
Adolescent mastery of diabetes management tasks and responsibility for diabetes management was assessed using parent-reported modified versions of the 28-item Continuous Subcutaneous Insulin Infusion (CSII)-Use Survey (Weissberg-Benchell et al., 2007) or the 38-item Diabetes Independence Survey: Injection (Wysocki, Meinhold, et al., 1996). Mastery of diabetes management was assessed using yes/no items. Total scores ranged from 0% (no mastery) to 100% (total mastery). Parents also rated who was responsible for completing diabetes management tasks: parent (1), shared (2), or youth (3). An average responsibility score was calculated by averaging the scores across all of the items. Higher mean scores indicated that the child demonstrated increased responsibility for diabetes management tasks. This measure has demonstrated adequate validity and reliability in previous studies (Weissberg-Benchell et al., 2007; Wysocki, Meinhold, et al., 1996). Internal consistency (α) across three years was .94–.95 (injection) and .88–.91 (pump).
Parent Support of Autonomy in Diabetes Management
Parental support for autonomy in diabetes management was assessed using maternal reports of a six-item modified version of the Diabetes-Specific Parental Support for Adolescent’s Autonomy Scale, which has demonstrated adequate validity and reliability in previous studies (Hanna, Dimeglio, & Fortenberry, 2005). Items were rated on a 5-point Likert scale (none of the time to all of the time). Total scores ranged from 0 to 24, with higher scores indicating higher levels of parent support for youth autonomy. Internal consistency (α) across three years was .68–.78 for maternal caregivers.
Family Conflict
The Diabetes Family Conflict Scale – Revised (Hood, Butler, Anderson, & Laffel, 2007) is a 19-item self-report measure completed by mothers and summarized the level of diabetes-specific family conflict. All items were rated on a 3-point Likert scale (never argue, sometimes argue, always argue). Total scores ranged from 19 to 57, with higher scores representing higher levels of diabetes-related family conflict. This measure has demonstrated adequate validity and reliability in previous studies (Hood et al., 2007). Internal consistency (α) across three years was .85–.87 for maternal caregivers.
Measures: Covariates
Clinically relevant demographic and medical factors (e.g., age, gender, ethnicity/race, diagnosis duration, therapy regimen, household structure) that could potentially protect individuals from problematic glycemic control (Helgeson et al., 2010; Hilliard et al., 2012; Whittemore et al., 2010) were included in the predictive models as covariates.
Approach to Statistical Analysis
It was hypothesized that more frequent blood sugar monitoring, more adaptive self-management, greater mastery of diabetes management tasks, more adaptive behavioral regulation, higher parental support for diabetes management, appropriate responsibility for diabetes management, and lower diabetes-related family conflict would predict health resilience. A longitudinal binomial logistic regression model was used to examine whether health resilience as measured by a glycemic control value within ADA recommendations (≤7.5%) versus above ADA recommendations (>7.5%) could be predicted by clinically relevant demographic and medical characteristics, child behavior, and family functioning.
Health resilience was dichotomized at each time point and was categorized as “resilient” versus “not resilient: at risk for future diabetes-related complications.” Clinically relevant demographic and medical characteristics were entered as covariates. Correlations within and between predictors was controlled for by using autoregressive models.
Results
Glycemic Control Over Time: Resilient Versus At-Risk Patients
As shown in Table I, glycemic control for the patients categorized as resilient ranged from 6% to 7% across the three years. The patients categorized as “not resilient” had much higher HbA1c levels, ranging from 8% to 17% across the three years.
Maternal-Reported Model of Health Resilience
As hypothesized, health resilience (i.e., having HbA1c levels within ADA recommendations) was significantly predicted by several clinically relevant demographic and medical characteristics, child behavior characteristics, and family functioning. Those variables that significantly predicted health resilience are shown in Figure 1. Those variables that were not significant predictors of health resilience are shown in Figure 2. The descriptive statistics of the model tested and logistic regression results are provided in Tables I and II. Patients who used an insulin pump (OR: 2.07) were significantly more likely to demonstrate health resilience relative to patients who used insulin injections. On the other hand, health resilience was not significantly predicted by patient age, gender, duration of type 1 diabetes, ethnicity/race, or one- versus two-parent families.
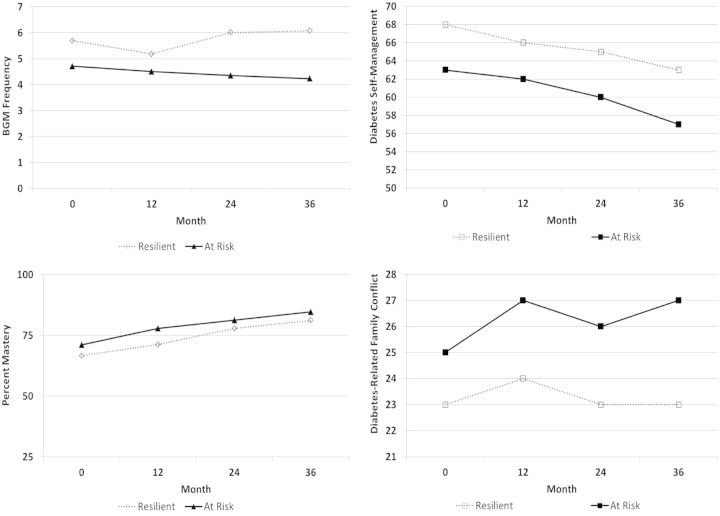
Figure 1.
Family conflict, diabetes self-management, blood glucose monitoring frequency, and mastery of diabetes management tasks from baseline to three years for resilient versus at-risk patients. Note. All predictors are significant.
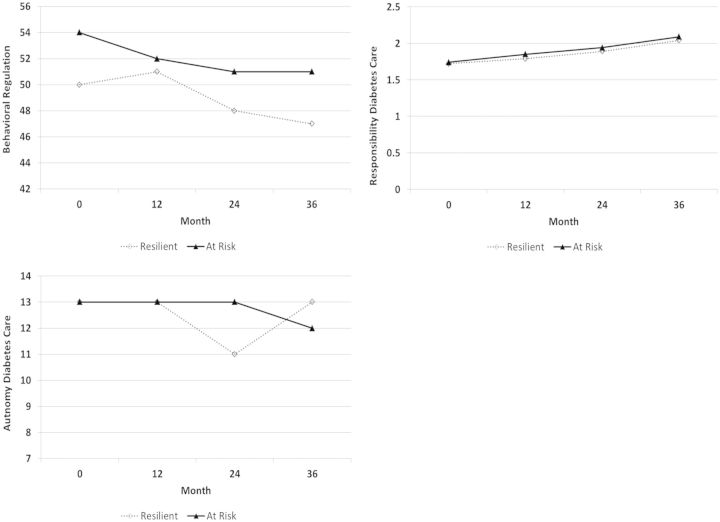
Figure 2.
Behavioral regulation, autonomy for diabetes care, and responsibility for diabetes care from baseline to three years for resilient versus at-risk patients. Note. These were nonsignificant predictors.
Table II.
Logistic Regression Models Predicting Resilient and At-Risk Diabetes-Related Health Outcomes
| Maternal-reported predictors |
||||
|---|---|---|---|---|
| Variable | χ2 | Odds ratio | 95% CI | p |
| Time | 0.06 | 1.00 | 0.97–1.02 | 0.81 |
| Gender (male vs. female) | 0.27 | 1.14 | 0.69–1.89 | 0.60 |
| Age | 0.08 | 0.96 | 0.74–1.25 | 0.78 |
| Duration | 0.13 | 1.02 | 0.92–1.13 | 0.72 |
| Therapy (pump vs. injection) | 6.45 | 2.07 | 1.18–3.61 | 0.01 |
| Ethnicity/Race (1 vs. 3) | 0.18 | 1.23 | 0.47–3.26 | 0.67 |
| Ethnicity/Race (2 vs. 3) | 0.00 | 0.99 | 0.29–3.39 | 0.99 |
| Household Structure (2 vs. 1 parent) | 2.67 | 1.95 | 0.88–4.36 | 0.10 |
| Diabetes self-management | 8.58 | 1.04 | 1.07 | <0.01 |
| BGM frequency | 12.47 | 1.19 | 1.08–1.32 | <0.01 |
| Behavioral Regulation (Parent) | 1.24 | 1.01 | 0.99–1.04 | 0.27 |
| Mastery (Diabetes management; parent) | 5.52 | 0.98 | 0.97–1.00 | 0.02 |
| Responsibility (Diabetes management; parent) | 0.34 | 1.33 | 0.51–3.46 | 0.56 |
| Support for Autonomy (Diabetes management) | 0.10 | 1.01 | 0.97–1.05 | 0.75 |
| Conflict | 20.10 | 0.87 | 0.81–0.92 | <0.01 |
Note. Ethnicity/Race: 1 = non-Hispanic Caucasian; 2 = non-Hispanic minority; 3 = Hispanic.
Significant predictors of health resilience are bolded.
More frequent blood sugar checking (OR: 1.19), more adaptive self-management per mother report (OR: 1.04), lower perceived mastery of diabetes management tasks per mother report (OR: 0.98), and lower levels of diabetes-related family conflict per mother report (OR: 0.87) increased odds for health resilience (i.e., achieving glycemic control levels within ADA recommendations). Contrary to hypotheses, maternal-reported responsibility and support for autonomy related to diabetes management did not significantly predict health resilience. Patients who were identified as resilient were reportedly less responsible for diabetes management tasks relative to their peers who were identified as at risk. On the other hand, parental support for autonomy was relatively similar for resilient and at-risk patients. Mother reported behavioral regulation did not significantly predict health resilience.
Discussion
To our knowledge, this study provided the first test of a predictive model of health resilience (i.e., achieving glycemic control values within ADA recommendations) in a large sample of pediatric patients transitioning from childhood to early adolescence. Our findings extended on previous research by identifying individual and family-level factors which predicted health resilience (i.e., maintaining recommended target levels of glycemic control or HbA1c ≤ 7.5%) across three years even after controlling for various demographic and medical characteristics. Those patients identified as health resilient achieved glycemic control well within ADA recommendations ranging from 6 to 7% across three years compared with at-risk peers whose glycemic control ranged from 8 to 17% across the same period. Health resilience or a decreased risk for developing future diabetes-related complications was significantly predicted by insulin regimen, more frequent blood sugar checking, more adaptive self-management skills (per mother report), appropriate mastery of diabetes management tasks (per mother report), and low levels of diabetes-related family conflict (per mother report).
Similar to previous research, self-management and adherence emerged as significant predictors of health resilience (i.e., achieving glycemic control within ADA-recommended values). Diabetes self-management behaviors were higher in the resilient group compared with the at-risk group, suggesting that those patients who were identified as resilient were more likely to present with more adaptive diabetes self-management compared with at-risk peers. Blood glucose monitoring frequency for both resilient and at-risk patients was relatively stable over the three years, and averaged about five or more checks per day for patients achieving HbA1c values within ADA recommendations versus four or fewer checks per day for patients identified as at risk or achieving glycemic control above ADA recommendations. This finding is consistent with other findings linking adherence, self-management, and mean levels or trajectories of glycemic control (Helgeson et al., 2010; Hilliard et al., 2013; King et al., 2012; Luyckx & Seiffge-Krenke, 2009; Rohan et al., 2014).
In the present study, lower maternal-reported diabetes-specific family conflict was predictive of health resilience, which is similar to previous research investigating mean glycemic control and trajectories of glycemic control (Helgeson et al., 2010; Hilliard et al., 2013; Ingerski et al., 2010; King et al., 2012; Luyckx & Seiffge-Krenke, 2009; Rohan et al., 2014). Contrary to hypotheses, patients identified as less resilient or at risk for future diabetes-related complications reportedly demonstrated greater mastery of diabetes management tasks relative to peers who were identified as resilient. This finding suggests that patients in the resilient group likely had parents who were more involved in their daily diabetes management tasks relative to peers who were identified as being less resilient or having an increased risk for future diabetes-related complications. Prior research indicated that both parents and health care providers perceive that older children have increased knowledge and skills to assume diabetes management and hence provide adolescents with increased responsibility and less parental monitoring (Hanna et al., 2005; Iannotti et al., 2006; Wysocki, Taylor, et al., 1996). Our findings suggest that although patients are transitioning from childhood to early adolescence and might be perceived as having increased knowledge of diabetes management, it is still important for parents to be appropriately involved in diabetes management while at the same time minimizing and managing diabetes-related family conflict (Whittemore et al., 2010).
Although derived subgroups or trajectories can be helpful for identifying subgroups within pediatric samples and hence used for risk stratification purposes, the present study builds on this previous research by utilizing a clinically meaningful metric that is collected in routine medical visits. During routine medical visits, patients could be asked to complete short questionnaires to identify potential risk factors for elevated glycemic control (e.g., poor diabetes self-management, increased diabetes-related family conflict, etc.) to assist clinicians in identifying the need for additional supports (e.g., certified diabetes educator, social work, nutrition, psychologists, etc.). Utilizing a clinically meaningful metric (e.g., within or above ADA recommendations) and identification of risk is an applicable method for tailoring empirically supported interventions to individuals at different levels of risk, which will save resources and result in more efficient allocation of more time-intensive interventions. Those patients who are doing well with diabetes management and glycemic control can continue with routine medical follow-up and psychosocial supports as needed.
Our findings have other important implications for both clinical management of type 1 diabetes and research that focuses on preventative interventions to reduce the risk for future diabetes-related complications. Our findings indicated that psychosocial factors may serve as important targets during the transition to adolescence to reduce pediatric patients’ risk of developing future diabetes-related complications that are related to chronically elevated glycemic control. These findings also indicated that the type and the intensity of intervention can be targeted to these clinically relevant, diabetes- specific, potentially modifiable individual and family factors. Several demographic and medical characteristics should be considered when intervening. Puberty could trigger insulin resistance and reduce glycemic control (Moran et al., 2002). Duration of type 1 diabetes could potentially impact diabetes management. It is true that adolescents diagnosed at a very young age may feel less stress than those diagnosed later in life. However, there is emerging evidence that longer duration of type 1 diabetes diagnosis could be attributed to a phenomenon referred to as “diabetes burnout” and result in deteriorations in self-management, adherence, and glycemic control (Hood et al., 2009). Females were also more likely to have an increased risk for future diabetes-related complications relative to male peers, which could be related to females increased risk for eating disorders and blood glucose dysregulation associated with menstrual cycles (Maharaj, Rodin, Olmsted, Connolly, & Daneman, 2003; Whittemore et al., 2010). Although there were no ethnicity differences observed in the current study, previous research has indicated that Hispanic patients might have better glycemic control relative to non-Hispanic minority peers. Hispanic patients might present with better glycemic control relative to non-Hispanic peers for a number of reasons, such as cultural differences in parenting, family structure (e.g., increased paternal involvement), and more adaptive self-management behaviors (e.g., diet, increased parental monitoring and support) (Whittemore et al., 2010). Future research is needed to investigate the specific factors that might contribute to improved glycemic control for Hispanic patients diagnosed with type 1 diabetes.
Hilliard and colleagues (2012) recommended utilizing interventions that target and enhance modifiable protective processes (e.g., diabetes-related competence, treatment adherence, positive family communication). Previous research has provided strong evidence that family-based interventions involving pediatric patients and their parents improved treatment adherence and ameliorated family conflict (Butler et al., 2008; Harris et al., 2008; Wysocki et al., 2008). These interventions seemed to be most powerful at improving glycemic control when they targeted specific areas of problem-solving (e.g., how to respond to specific blood sugar values; identification of barriers and facilitators of treatment adherence; Butler et al., 2008; Harris et al., 2008; Wysocki et al., 2008). The present study also indicated the need to keep parents engaged in treatment while at the same time support child and adolescent autonomy in treatment. It is possible that families high in conflict may decrease support earlier because they are unable to sustain support with high diabetes-related conflict. Anderson, Brackett, Ho, and Laffel (1999) suggested that a low-intensity intervention delivered during routine medical visits for diabetes positively influenced pediatric patients diabetes management, enhanced parent–adolescent communication, and increased parental involvement in diabetes care while maintaining and promoting adolescent involvement in their own care, and decreasing diabetes-related family conflict. Such interventions might be most appropriate and realistic for those adolescents who demonstrate elevated risk for future diabetes-related complications or worse glycemic control over time. Those with optimal glycemic control could receive preventative interventions at the point of care. Those at highest risk may require more intensive interventions such as multi-systemic therapy (Ellis et al., 2012).
The present study has limitations that should be considered when interpreting these findings and designing future research. The present study included a homogenous age range of children and adolescents at baseline (ages: 9–11). It will be important to determine whether the factors predictive of health resilience in this sample of early adolescents are sustained throughout adolescence and young adulthood. Additionally, although the present study included more Hispanic youth than many previous research studies, the current sample was majority Caucasian, higher socioeconomic status patients and families. The generalizability to more diverse patient samples needs to be established. Relevant factors that were not included in our predictive model that could have influenced health resilience and should be considered in future research are anxiety, depression, memory, peer relationships, quality of parent–child relationship, etc. In the current study, we were interested in specific factors that predicted health resilience. Future research should investigate whether behavioral resilience factors (self-management variables and treatment adherence) mediated the relationship between individual and family-level factors and health resilience (Hilliard et al., 2012). Future research should develop interventions that target modifiable risk factors in an effort to reduce the potential risk of short- and long-term health complications as children transition from childhood to adolescence to adulthood (e.g., social skills groups for females with type 1 diabetes, risk models for stratifying which patients could benefit from more intensive interventions, etc.). Non-modifiable risk factors (e.g., age, gender, duration of diabetes, ethnicity/race, etc.) could also be used to stratify or determine which groups would benefit most from intervention. Identifying these children before they reach adolescence could potentially prevent the development of future health complications as well as address the modifiable factors that contribute to risk of developing future diabetes-related complications.
Acknowledgments
The HbA1c data were analyzed by the Diabetes Diagnostic Laboratory (DDL) at the University of Missouri Columbia Health Sciences Center. Data collection and management of this study were facilitated by a talented group of research assistants, including Claire Peterson, Michelle Eakin, Danielle Rosnov, Daniela Fernandez, Jennifer Hernandez, Andrea Perry, Matthew Maley, Megan Derosier, Megan Miller, and Katharina Wetterau March.
Funding
The work reported in this article was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (1R01 DK069486). This work was also supported by the National Cancer Institute at the National Institutes of Health (NRSA Pre-Doctoral Training Fellowship: 1F31CA168307 to J.M.R.).
Conflicts of interest: None declared.
References
- Anderson B. J., Brackett J., Ho J., Laffel L. (1999). An office-based intervention to maintain parent-adolescent teamwork in diabetes management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care , 22, 713–721. [DOI] [PubMed] [Google Scholar]
- Anderson B. J., Holmbeck G., Iannotti R. J., McKay S. V., Lochrie A., Volkening L. K., Laffel L. (2009). Dyadic measures of the parent–child relationship during the transition to adolescence and glycemic control in children with type 1 diabetes. Families, Systems, & Health , .27, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. A., Zuehlke J. B., Tovar A., Volkening L. K., Anderson B. J., Laffel L. (2008). The impact of modifiable family factors on glycemic control among youth with type 1 diabetes. Pediatric Diabetes , 9(4 Pt 2), 373–381. [DOI] [PubMed] [Google Scholar]
- DCCT Research Group. (1994). Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Journal of Pediatrics , 125, 177–188. [DOI] [PubMed] [Google Scholar]
- DCCT Research Group. (2002). Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA , 287, 2563–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. A., Naar-King S., Chen X., Moltz K., Cunningham P. B., Idalski-Carcone A. (2012). Multisystemic therapy compared to telephone support for youth with poorly controlled diabetes: Findings from a randomized controlled trial. Annals of Behavioral Medicine, 44, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel N. R., Weissberg-Benchell J. (2010). Preventing poor psychological and health outcomes in pediatric type 1 diabetes. Current Diabetes Reports , 10, 436–443. [DOI] [PubMed] [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C., Kenworthy L. (2000). Behavior rating inventory of executive function. Child Neuropsychol , 6, 235–238. [DOI] [PubMed] [Google Scholar]
- Hanna K. M., Dimeglio L. A., Fortenberry J. D. (2005). Parent and adolescent versions of the diabetes-specific parental support for adolescents' autonomy scale: Development and initial testing. Journal of Pediatric Psychology , 30, 257–271. [DOI] [PubMed] [Google Scholar]
- Harris M. A., Antal H., Oelbaum R., Burkloh L. M., White N. H., Wysocki T. (2008). Good intentions gone awry: Assessing parental miscarried helping in diabetes. Families, Systems, & Health, 26, 393–403. [Google Scholar]
- Harris M. A., Wysocki T., Sadler M., Wilkinson K., Harvey L. M., Buckloh L. M., Mauras N., White N. H. (2000). Validation of a structured interview for the assessment of diabetes self-management. Diabetes Care , 23, 1301–1304. [DOI] [PubMed] [Google Scholar]
- Helgeson V. S., Honcharuk E., Becker D., Escobar O., Siminerio L. (2011). A focus on blood glucose monitoring: Relation to glycemic control and determinants of frequency. Pediatric Diabetes , 12, 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson V. S., Siminerio L., Escobar O., Becker D. (2009). Predictors of metabolic control among adolescents with diabetes: A 4-year longitudinal study. Journal of Pediatric Psychology , 34, 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson V. S., Snyder P. R., Seltman H., Escobar O., Becker D., Siminerio L. (2010). Brief Report: Trajectories of glycemic control over early to middle adolescence. Journal of Pediatric Psychology , 35, 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M., Harris M., Weissberg-Benchell J. (2012). Diabetes resilience: A model of risk and protection in type 1 diabetes. Current Diabetes Reports , 12, 739–748. [DOI] [PubMed] [Google Scholar]
- Hilliard M. E., Rohan J. M., Rausch J. R., Delamater A., Pendley J. S., Drotar D. (2014). Patterns and predictors of paternal involvement in early adolescents’ type 1 diabetes management over 3 years. Journal of Pediatric Psychology , 39, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., Wu Y. P., Rausch J., Dolan L. M., Hood K. K. (2013). Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. The Journal of Adolescent Health , 52, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C. S., Chen R., Streisand R., Marschall D. E., Souter S., Swift E. E., Peterson C. C. (2006). Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. Journal of Pediatric Psychology , 31, 770–784. [DOI] [PubMed] [Google Scholar]
- Hood K., Peterson C., Rohan J., Drotar D. (2009). Association between adherence and glycemic control in pediatric type 1 diabetes: A meta- analysis. Pediatrics , 124, 1171–1179. [DOI] [PubMed] [Google Scholar]
- Hood K. K., Butler D. A., Anderson B. J., Laffel L. M. B. (2007). Updated and revised diabetes family conflict scale. Diabetes Care , 30, 1764–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti R. J., Schneider S., Nansel T. R., Haynie D. L., Plotnick L. P., Clark L. M., Sobel D. O., Simons-Morton B. (2006). Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. Journal of Developmental and Behavioral Pediatrics , 27, 98–105. [DOI] [PubMed] [Google Scholar]
- Ingerski L. M., Anderson B. J., Dolan L. M., Hood K. K. (2010). Blood glucose monitoring and glycemic control in adolescence: Contribution of diabetes-specific responsibility and family conflict. Journal of Adolescent Health , 47, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskander J. M., Rohan J. M., Pendley J. S., Delamater A., Drotar D. (2014). A 3-year prospective study of parent–child communication in early adolescents with type 1 diabetes: Relationship to adherence and glycemic control. Journal of Pediatric Psychology, 40, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. S., Berg C. A., Butner J., Drew L. M., Foster C., Donaldson D., Murray M., Swinyard M., Wiebe D. J. (2012). Longitudinal trajectories of metabolic control across adolescence: Associations with parental involvement, adolescents' psychosocial maturity, and health care utilization. Journal of Adolescent Health , 50, 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. S., Anderson B. J., Butler D. A., Antisdel J. E., Brackett J., Laffel L. (2001). Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. The Journal of pediatrics , 139, 197–203. [DOI] [PubMed] [Google Scholar]
- Luthar S. S., Cicchetti D., Becker B. (2000). The construct of resilience: A critical evaluation and guidelines for future work. Child Development , 71, 543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx K., Seiffge-Krenke I. (2009). Continuity and change in glycemic control trajectories from adolescence to emerging adulthood relationships with family climate and self-concept in type 1 diabetes. Diabetes Care , 32, 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj S., Rodin G., Olmsted M., Connolly J., Daneman D. (2003). Eating disturbances in girls with diabetes: The contribution of adolescent self-concept, maternal weight and shape concerns and mother-daughter relationships. Psychological Medicine , 33, 525–539. [DOI] [PubMed] [Google Scholar]
- Miller-Johnson S., Emery R. E., Marvin R. S., Clarke W., Lovinger R., Martin M. (1994). Parent-child relationships and the management of insulin-dependent diabetes mellitus. Journal of Consulting and Clinical Psychology , 62, 603–610. [DOI] [PubMed] [Google Scholar]
- Miller M., Rohan J. M., Delamater A., Pendley J. S., Dolan L., Reeves G., Drotar D. (2013). Changes in executive functioning and self- management in adolescents with type 1 diabetes: A growth curve analysis. Journal of Pediatric Psychology , 38, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A., Jacobs D.R., Jr, Steinberger J., Cohen P., Hong C.P., Prineas R., Sinaiko A.R. (2002). Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. Journal of Clinical Endocrinology and Metabolism , 87, 4817–4820. [DOI] [PubMed] [Google Scholar]
- Nathan D. M., Cleary P. A., Backlund J., Genuth S. M., Lachin J. M., Orchard T. J., Raskin P., Zinman B.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. (2005). Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New England Journal of Medicine , 353, 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. M., Zinman B., Cleary P. A., Backlund J. Y. C., Genuth S., Miller R., Orchard T. J. (2009). Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: The diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Archives of Internal Medicine , 169, 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch J. R., Hood K. K., Delamater A., Shroff Pendley J., Rohan J. M., Reeves G., Dolan L., Drotar D. (2012). Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care , 35, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan J. M., Rausch J. R., Pendley J. S., Delamater A. M., Dolan L., Reeves G., Drotar D. (2014). Identification and prediction of group-based glycemic control trajectories during the transition to adolescence. Health Psychology, 33, 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J., Klingensmith G., Copeland K., Plotnick L., Kaufman F., Laffel L., Deeb L., Grey M., Anderson B., Holzmeister L. A. (2005). Care of children and adolescents with type 1 diabetes A statement of the American Diabetes Association. Diabetes Care , 28, 186–212. [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J., Goodman S. S., Lomaglio J. A., Zebracki K. (2007). The use of continuous subcutaneous insulin infusion (CSII): Parental and professional perceptions of self-care mastery and autonomy in children and adolescents. Journal of Pediatric Psychology , 32, 1196–1202. [DOI] [PubMed] [Google Scholar]
- Whittemore R., Jaser S., Guo J., Grey M. (2010). A conceptual model of childhood adaptation to type 1 diabetes. Nursing Outlook , 58, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. P., Rausch J., Rohan J. M., Hood K. K., Pendley J. S., Delamater A., Drotar D. (2014). Autonomy support and responsibility-sharing predict blood glucose monitoring frequency among youth with diabetes. Health Psychology, 33, 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T. (1993). Associations among teen-parent relationships, metabolic control, and adjustment to diabetes in adolescents. Journal of Pediatric Psychology , 18, 441–452. [DOI] [PubMed] [Google Scholar]
- Wysocki T., Harris M. A., Buckloh L. M., Mertlich D., Lochrie A. S., Taylor A., Sadler M., White N. H. (2008). Randomized, controlled trial of behavioral family systems therapy for diabetes: Maintenance and generalization of effects on parent-adolescent communication. Behavior Therapy , 39, 33–46. [DOI] [PubMed] [Google Scholar]
- Wysocki T., Meinhold P. A., Taylor A., Hough B. S., Barnard M. U., Clarke W. L., Bellando B. J., Bourgeois M. J. (1996). Psychometric properties and normative data for the diabetes independence suvey. The Diabetes Educator, 22, 587–591. [DOI] [PubMed] [Google Scholar]
- Wysocki T., Taylor A., Hough B. S., Linscheid T. R., Yeates K. O., Naglieri J. A. (1996). Deviation from developmentally appropriate self-care autonomy: Association with diabetes outcomes. Diabetes Care , 19, 119–125. [DOI] [PubMed] [Google Scholar]