Abstract
Although Autism Spectrum Disorder (ASD) is generally a lifelong disability, a minority of individuals with ASD overcome their symptoms to such a degree that they are generally indistinguishable from their typically-developing peers. That is, they have achieved an Optimal Outcome (OO). The question addressed by the current study is whether this normalized behavior reflects normalized brain functioning, or alternatively, the action of compensatory systems. Either possibility is plausible, as most participants with OO received years of intensive therapy that could alter brain networks to align with typical function or work around ASD-related neural dysfunction. Individuals ages 8 to 21 years with high-functioning ASD (n = 23), OO (n = 16), or typical development (TD; n = 20) completed a functional MRI scan while performing a sentence comprehension task. Results indicated similar activations in frontal and temporal regions (left middle frontal, left supramarginal, and right superior temporal gyri) and posterior cingulate in OO and ASD groups, where both differed from the TD group. Furthermore, the OO group showed heightened “compensatory” activation in numerous left- and right-lateralized regions (left precentral/postcentral gyri, right precentral gyrus, left inferior parietal lobule, right supramarginal gyrus, left superior temporal/parahippocampal gyrus, left middle occipital gyrus) and cerebellum, relative to both ASD and TD groups. Behaviorally normalized language abilities in OO individuals appear to utilize atypical brain networks, with increased recruitment of language-specific as well as right homologue and other systems. Early intensive learning and experience may normalize behavioral language performance in OO, but some brain regions involved in language processing may continue to display characteristics that are more similar to ASD than typical development, while others show characteristics not like ASD or typical development.
Keywords: Autism, Optimal outcomes, Language, fMRI
Highlights
-
•
fMRI study of "optimal outcome" (OO) youth with no symptoms of autism spectrum disorder.
-
•
Results show “compensatory” language activation in some areas in OO.
-
•
OO youth also had some “residual ASD” patterns of activation (OO, ASD > TD).
-
•
There was no evidence of areas of normalized brain function (OO, TD ≠ ASD).
-
•
Early treatment may normalize behavior but not brain in some individuals with ASD.
1. Introduction
Recent research on individuals with autism spectrum disorder (ASD) indicates a somewhat surprising trajectory in long-term outcomes — the possibility of behavioral normalization from ASD. While there are few studies of this phenomenon to date, from three to 25% of children appear to lose their diagnosis and enter the normal range of cognitive, adaptive and social skills (Helt et al., 2008), here called an ‘optimal outcome’ (OO). A recent longitudinal study reported that eight of 85 children (9%), seen initially at age two and again at 19 years, had attained “very positive outcomes” (Anderson et al., 2014). We described a group of 34 similar individuals, comparing them to a group of children with current high-functioning ASD (the “HFA” group) and a group with a history of typical development (TD) (Fein et al., 2013). While the OO children met criteria for ASD early in development, they had lost all symptoms of ASD and were functioning socially within the normal range. Another study of OO found that such individuals exhibited one of the most well-replicated markers of ASD in early development (Redcay and Courchesne, 2005): head circumferences that were significantly enlarged at 10–25 months (Mraz et al., 2009). Studies of language processing in younger samples of OO children (ages 5 to 9) generally find only subtle difficulties with pragmatic and semantic language, but intact grammatical abilities (Kelley et al., 2006), findings which seem to hold at ages 9–14 (Kelley et al., 2010). A comprehensive evaluation of language skills in the current OO sample confirmed normalization of essentially all basic language functions (Tyson et al., 2014), including a measure of subtle pragmatic function (Irvine, Eigsti and Fein, in review). Although the factors involved in determining which individuals are likely to experience OO are not fully explained, one report indicates that they are likely to receive earlier, more intense intervention (specifically, applied behavior analysis, or ABA), and to have above average cognitive abilities (Orinstein et al., 2014), although, clearly, many individuals with autism receive comparable early ABA and do not reach an optimal outcome.
Youth with OO appear behaviorally more or less indistinguishable from typically-developing youth, but an important question remains unanswered: To what degree does normative language performance reflect normative brain function? For most individuals, many aspects of language are a left-lateralized function. Particularly critical brain regions for meaningful sentence comprehension include, broadly, middle and inferior temporal cortex; pars orbitalis; bilateral superior temporal sulci; Heschl's gyrus, in dorsal temporal lobe and containing primary auditory cortex; and inferior parietal lobule, which contains the angular gyrus (Dichter, 2012, Price, 2010). Studies of language processing in ASD have shown atypical activations during language processing, including impaired functional connectivity (Catarino et al., 2011, Kleinhans et al., 2008, Verly et al., 2014), abnormal lateralization (Boddaert et al., 2003, Eigsti et al., 2012, Grezes et al., 2009, Groen et al., 2010, Hesling et al., 2010), and some recruitment of brain regions not typically involved in language (e.g., Catarino et al., 2011, Knaus et al., 2010, Mizuno et al., 2011, Redcay and Courchesne, 2008). Thus, fMRI studies of language in ASD show both atypical regions of activation during language processing, as well as atypical activation of typical language-implicated brain regions.
An early paper by Mundy and Crowson (1997) hypothesized that behavior intervention in ASD at an early age will normalize brain function. Under such a “neural normalization” model, neural systems should look nearly identical in individuals with TD and OO, analogous to normalization of brain activations in successfully-treated dyslexia (Aylward et al., 2003, Simos et al., 2002) or aphasia (Saur and Hartwigsen, 2012). Though there are no such fMRI studies of OO to date, a normalization pattern was reported in an EEG study of toddlers who completed intensive early intervention (Dawson et al., 2012).
Alternatively, early intensive behavioral treatment may work, not by normalizing processing pathways, but by teaching relevant skills until alternative neural pathways are recruited to achieve “compensation” (Koegel and Frea, 1993). If this holds, neural activation in OO would look substantially different from TD, and furthermore, would differ from HFA, where critical skills may have been learned less effectively; this pattern is dubbed “neural compensation.” Compensatory activation has been observed in aging (Ansado et al., 2013), where processing inefficiencies cause the aging brain to recruit more neural resources in the same or different regions to achieve computational output equivalent to that of a younger brain; and in successfully remediated dyslexia in adults, with findings of normalization of activity in the left hemisphere as well as compensatory right hemisphere (language homologue) activation (Eden et al., 2004). Results showing atypical asymmetry in language-related white-matter structure in young children with HFA, as a function of language abilities, further support such a model (Joseph et al., 2014).
A third potential pattern, “residual ASD,” describes brain activation in OO that resembles that seen in individuals with ASD. It is possible that while some brain systems under some conditions would have normalized or show compensatory activation, others will still show a pattern of activation more reflective of the ASD history of these individuals, suggesting that they continue to reflect the individual's history of ASD.
The purpose of the current study was to describe the neural processes implicated in a language comprehension task. Specifically, we chose a sentence comprehension paradigm previously studied in adults with ASD (Kana et al., 2006), because this task was likely to activate classic language processing regions, enabling us to examine the neural underpinnings of language comprehension where that domain was previously impaired but, in the OO sample, had fully normalized at a behavioral level. This task also draws on relative strengths (in visuospatial processing) in ASD. Although adults with ASD show behaviorally normal task performance in some studies, there is evidence of differential neural activation in some studies, such that parietal and occipital brain regions associated with visual imagery were activated while participants read sentences with low visual “demand”; in contrast, TD controls activated those regions only for sentences with high imagistic content (Kana et al., 2006; see also Sahyoun et al., 2010). We asked whether individuals with OO, who show fully typical behavioral language abilities, especially on standardized tests (Troyb et al., 2013), might show distinctive neural activity while comprehending written sentences and further, whether such a pattern would indicate neural normalization, neural compensation, or residual ASD patterns during sentence comprehension.
2. Methods and materials
2.1. Participants
Sixteen individuals with optimal outcomes from ASD (OO), 23 high-functioning individuals with a current ASD diagnosis (HFA), and 20 typically developing (TD) peers completed an fMRI scan and cognitive testing. Participants (ages 8–21 years) comprised a subset of the groups described in 2013 (Fein et al., 2013) (Table 1). All participants in the behavioral study (Fein et al., 2013) were invited to participate in the current study, unless they met the MRI-based exclusion criteria (metal in the body, etc.) Groups were matched on age, handedness, gender, and nonverbal IQ (all p's > .50). OO and TD groups had significantly higher VIQ scores than the HFA group, but all scores were within the normal range (1.5 SD of 100) as participation in the full study required FSIQ > 85. All participants were native monolingual speakers of English. Twenty-one participants had a comorbid diagnosis (primarily ADHD or anxiety disorders), and 11 participants were taking medication (see Table 1 for details). Study procedures were approved by the Institutional Review Boards of University of Connecticut, Institute of Living/Hartford Hospital, Children's Hospital of Philadelphia, and Queens University.
Table 1.
Demographic information for participants with high functioning ASD (HFA), optimal outcomes (OO), and typical development (TD).
| HFA n = 23 |
OO n = 16 |
TD n = 20 |
F/χ2 | p | t value for tests at near significance | |
|---|---|---|---|---|---|---|
| Age | 13.9 (3.3); 8–20 |
13.7 (3.6); 8–21 |
13.3 (2.8); 9–21 |
0.08 | 0.92 | |
| Gender (M:F) | 22:1 | 15:1 | 17:3 | 1.71 | 0.43 | |
| Handedness (R:L) | 18:2 | 16:0 | 17:3 | 2.50 | .29 | |
| ADOS-Social | 6.70 (2.6) 4–13 |
1.25 (1.81) 0–7 |
.55 (.76) 0–2 |
63.43 | < .001 | HFA/OO: < .001 HFA/TD: < .001 |
| ADOS-Communication | 3.87 (1.25) 2–6 |
.50 (.89) 0–3 |
.40 (.60) 0–2 |
86.36 | < .001 | HFA/OO: < .001 HFA/TD: < .001 |
| ADOS-repetitive behaviors | .83 (.89) 0–3 |
.31 (1.01) 0–4 |
.05 (.22) 0–1 |
5.58 | .006 | HFA/OO: 0.05 HFA/TD: .002 |
| CELF-core language | 98 (7); 70–126 |
114 (9); 94–126 |
119 (6); 108–129 | 16.48 | < .001 | HFA/OO: < .001 HFA/TD: < .001 |
| VIQ | 105 (15); 81–142 | 115 (13); 91–136 | 112 (12); 93–138 | 3.12 | 0.05 | HFA/OO: 0.02 HFA/TD: 0.08 |
| NVIQ | 113 (8); 99–127 |
114 (11); 92–129 | 110 (11); 89–134 | 0.87 | 0.43 | |
| Medicationsa | 8 subjects (fluoxetine, sertraline, bupropion, methylphenidate, depakote, lisdexamfetamine, atomoxetine, guanfacine, risperidone, quetiapine, clonidine) | 3 subjects (concerta, atomoxetine, fluoxetine, lisdexamfetamine, risperidone) | none | 14.12 | .007 | HFA/TD: 0.001 OO/TD: 0.06 |
| Co-morbid diagnoses | 12 subjects (ADHD, ODD, CD, Tic, OCD, specific and social phobia, GAD, depression) | 5 subjects (ADHD, specific phobia,depression) | 4 subjects (ADHD, OCD, ODD) | 4.60 | 0.10 |
Note. Data are reported as M (SD); range. ADOS = Autism Diagnostic Observation Schedule Lord et al., 1994. VIQ = WASI Verbal IQ, NVIQ = Nonverbal IQ Wechsler (1999) . Also note that while groups differed in standardized language (CELF) scores, with significantly lower scores in the HFA group relative to both TD and OO groups (which did not differ), scores were not included as covariates in analyses. This statistical correction would be likely to reduce power to detect group differences in brain activity that were hypothesized to underlie these behavioral differences [see also Dennis et al., 2009 for relevant discussion].
Medication information was not available for some participants (n = 7, 2 and 2, for HFA, OO, TD groups, respectively).
Diagnostic assignment with the DSM-IV criteria was determined via clinical consensus by experienced clinicians using data from the Autism Diagnostic Interview—Revised (ADI-R; Lord et al., 1994), the Autism Diagnostic Observation Schedule—Generic (ADOS-G; Lord et al., 1994), and clinical observation. Participants in the OO group had a documented ASD diagnosis (with early language delay) before the age of 5 from a physician or psychologist specializing in autism. They could not meet current criteria for any ASD according to the ADOS. Participants in the HFA group had a current diagnosis of ASD, according to the ADOS. Full inclusion and exclusion criteria, along with recruitment information, are detailed in Fein et al. (2013).
2.2. Clinical assessment
The ADOS was administered to evaluate ASD diagnostic criteria; 20% were double-coded by raters naïve to group status, with high inter-rater reliability (86%). Cognitive abilities were assessed using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Semantic and syntactic aspects of language were assessed using the Core Language score from the Clinical Evaluation of Language Fundamentals (Semel et al., 2003).
2.3. Neuroimaging procedures
MRI data were collected on a 3T Siemens Allegra scanner at the Olin Neuropsychiatry Research Center. After MRI safety screening and training in a mock scanner, participants were placed on the bed of the scanner and donned MRI-compatible noise-blocking headphones and earplugs. Head motion was restricted using custom-built cushions inside the head coil. Localizer images were acquired for use in prescribing the functional image volumes. A echo planar image (EPI) gradient-echo pulse sequence was used to measure task-elicited BOLD signal change (TR/TE 1500/28 ms, flip angle 65°, FOV 24 × 24 cm, 64 × 64 matrix, 3.4 × 3.4 mm in plane resolution, 5 mm effective slice thickness, 30 slices). Each of two fMRI sessions acquired 225 images. The first 6 images during which T1 effects stabilized were discarded.
During scanning, participants completed the task described by Kana et al. (2006); stimulus materials were provided by Kana. They read short declarative statements displayed for up to 4 s until a response was made, designed to have imageable content that was low (“Addition, subtraction and multiplication are all math skills”) or high (“Sometimes fluffy clouds can look just like round cotton balls”). Participants made a true/false judgment about each statement via response device button press. Experimental sessions included 12 low-imagery, 12 high-imagery, and 24 control trials presented in pseudo-random order against a “null event” fixation cross that served as an implicit baseline for statistical modeling. Following Kana et al., control trials comprised “LLLLLL” or “RRRRRR,” prompting one of two possible button presses; this control condition was designed to elicit a similar motor response, and to involve similar visual processing of letters, but not to elicit semantic or other linguistic processing. Although the Kana et al. paradigm employed a block rather than event-related design, task materials were comparable (the wording of several items was modified for this younger age-group).
fMRI data processing included correction for head motion using rigid body transformation using INRIAlign (Freire et al., 2002) followed by slice-timing acquisition temporal difference correction, spatial normalization to the EPI.mnc template in MNI stereotactic space, and 8 mm smoothing in SPM8. Mean translation and rotation values did not differ among groups when evaluated by ANOVA. Only three subjects had translation values > 1 voxel length. These datasets were retained because they were simple movement spikes that were “censored” from single-subject activation GLM models by including a separate covariate for each timepoint.
2.4. Statistical analyses
Reaction time and true/false accuracy for groups in High versus Low Imagery were examined using repeated-measures ANOVA. Voxelwise signal change of fMRI data for each condition was estimated using a canonical hemodynamic response model in SPM8. One-sample t tests characterized brain activation to each condition. Because activation profiles and reaction times differed for Control versus Low and High Imagery conditions, we evaluated activation relative to the unmodeled implicit baseline for all conditions. We then treated the Control condition as a separate, usefully informative condition of interest that allowed us to determine if group differences occurred in the absence of language processing demands. Accordingly, we used three separate SPM8 one-way ANOVA models to test group difference predictions, each having age as a covariate to control for developmental differences in brain activation. Statistical inference used a p < .05 clusterwise significance level, following Monte Carlo simulation that determined the extent of contiguous voxels needed to survive statistical corrections for searching the whole brain (voxelwise entry p < .005, t = 2.66). For the regions showing overall group differences in regional activation, we extracted peak BOLD signal change estimates. Using these estimates, we conducted pairwise group t tests (two-tailed p < .05 corrected to .016667 for 3 comparisons) to characterize patterns that reflected our hypothesized profiles of normalization, residual ASD, or compensation (e.g., compensation would be OO regional activation > HFA and TD).
3. Results
3.1. Behavioral analyses
Sentence comprehension accuracy showed no main effect of group, F(2,51) = 0.58, p = .56, and no group × condition interaction, F(2,51) = 0.09, p = .91. There was a significant main effect of High/Low Imagery condition, F(1,51) = 31.24, p < .001, ή2p = .38, with better accuracy on the Low (M = .82) than the High Imagery condition (M = .75). Reaction time (RT) similarly showed no main effect of group, F(2,51) = 1.99, p = .15, and no group × High/Low Imagery condition interaction, F(2,51) = 0.32, p = .73. There was a significant main effect of imagery, F(1,51) = 19.87, p < .001, ή2p = .28, such that for all groups, RT was faster in the Low Imagery condition (Low = 3.27 s, High = 3.56 s). Thus, there were no group differences in behavioral indices of task performance; all participants showed the fastest and most accurate performance in the Low Imagery condition [Accuracy M (SD) in High Imagery, for HFA, OO, and TD groups, respectively: .732 (.11); .771 (.11); and .748 (.12); Accuracy, Low Imagery: .813 (.08); .839 (.09); .821 (.11)]. There was no speed/accuracy trade-off [RT in High Imagery, M (SD) for HFA, OO, and TD groups, respectively: 3508 (517); 3347 (606); and 3606 (978); RT, Low Imagery: 3243 (649); 3111 (723); 3458 (890)]. These data are consistent with behavioral performance on standardized assessments of language (Tyson et al., 2014) and academic skills (Troyb et al., 2013), which indicate that participants with OO and with HFA had behaviorally typical sentence comprehension.
3.2. MRI results: main effects of condition
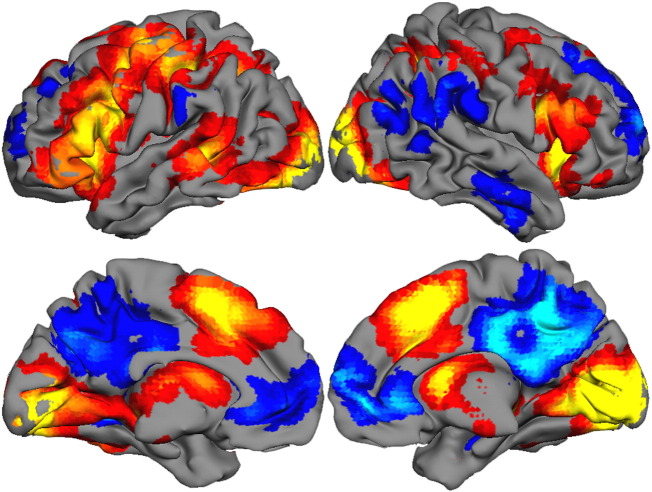
The comprehension task engaged a broad bi-hemispheric network across all groups, with particularly strong responses in the “easier” Low Imagery condition. Table 2 lists activated brain regions (or, “deactivated,” in the case of the default mode network) for all three task conditions. Table 3 identifies regions showing a significant difference between High and Low Imagery conditions; regions that correspond with activation main effects are noted. Because activation profiles for Low and High Imagery were similar, activation maps were collapsed for visualization purposes and rendered on a representative brain (Fig. 1). Activations were found for sensory-motor regions, particularly on the left, in anterior and posterior language areas, and in visual areas; deactivation can be seen in the medial default network. While regions of activation largely replicated those reported by Kana et al.(2006), many regions unexpectedly showed higher activation amplitude for Low Imagery trials.
Table 2.
Brain regions showing signal change to Language Imagery fMRI task stimuli in the entire sample (p < .001 clusterwise significance using Monte Carlo simulation to determine the extent of contiguous voxels needed to survive statistical corrections for searching the whole brain volume with an entry threshold p < .005, t = 2.66). For activation main effects, negative t scores indicate negative BOLD signal change (i.e., “deactivation”).
| “Activation” main effect |
||||||
|---|---|---|---|---|---|---|
| Low Imagery |
High Imagery |
Control Stimuli |
||||
| Brain region | Peak x, y, z | Peak t | Peak x, y, z | Peak t | Peak x, y, z | Peak t |
| Positive BOLD signal change | ||||||
| Midline cingulate and medial/superior frontal gyri (BA 32/6/8) | − 6, 8, 55 | 11.76 | − 6, 11, 55 | 10.36 | − 9, − 1, 52 | 7.61 |
| Left precentral gyrus | − 42, 2, 46 | 9.45 | − 36, − 4, 61 | 8.79 | ||
| Right middle/precentral gyri (BA 6) | 33, − 4, 55 | 4.47 | 27, − 1, 46 | 5.36 | 33, − 7, 58 | 4.13 |
| Left inferior frontal/middle gyri (BA 9/44) | − 45, 14, 25 | 11.09 | − 45, 8, 28 | 13.07 | − 45, 2, 25 | 3.90 |
| Right inferior frontal gyrus (BA 44) | 48, 11, 22 | 4.87 | 51, 11, 25 | 8.00 | 51, 11, 13 | 4.48 |
| Left inferior frontal gyrus | − 51, 14, 7 | 9.98 | − 51, 11, 10 | 9.66 | ||
| Left anterior insular cortex | − 30, 23, 1 | 11.53 | − 33, 17, − 2 | 10.34 | − 36, 11, 10 | 4.61 |
| Right anterior insular cortex | 33, 23, − 2 | 10.99 | 33, 23, − 2 | 10.54 | 39, 17, − 2 | 3.63 |
| Right middle frontal gyrus (BA 11) | 33, 35, − 17 | 3.38 | 24, 44, − 14 | 3.55 | 27, 50, − 11 | 4.65 |
| Left postcentral/precentral gyri (BA 3/1/6) | − 36, − 16, 61 | 12.33 | − 36, − 19, 58 | 7.77 | − 36, − 19, 61 | 10.08 |
| Left superior/inferior parietal lobule (BA 7/40) | − 27, − 61, 46 | 7.53 | − 27, − 61, 46 | 8.07 | ||
| Right inferior parietal lobule (BA 40) | 45, − 37, 52 | 3.82 | 45, − 37, 46 | 6.98 | 48, − 37, 46 | 6.22 |
| Right superior parietal lobule (BA 7) | 30, − 61, 46 | 6.47 | 27, − 61, 46 | 8.36 | ||
| Left postcentral gyrus | − 48, − 22, 22 | 5.95 | − 48, − 22, 25 | 3.18 | − 54, − 19, 22 | 7.29 |
| Left posterior middle temporal gyrus (BA 22) | − 51, − 40, 1 | 9.79 | − 54, − 40, 4 | 8.05 | ||
| Right posterior hippocampus/parahippocampus | 24, − 28, 1 | 6.08 | 24, − 28, 1 | 6.25 | ||
| Left lingual gyrus | − 6, − 61, 1 | 7.71 | − 15, − 55, − 2 | 5.05 | − 15, − 58, − 5 | 3.34 |
| Left lingual gyrus | − 6, − 88, − 5 | 12.70 | − 6, − 94, − 5 | 11.39 | − 6, − 85, − 8 | 9.02 |
| Right lingual gyrus | 9, − 94, 1 | 13.52 | 9, − 94, 1 | 13.56 | ||
| Left middle occipital gyrus | − 27, − 100, 4 | 11.97 | − 21, − 100, − 2 | 13.07 | ||
| Right middle occipital gyrus | 27, − 97, − 2 | 12.57 | 27, − 97, − 2 | 13.68 | ||
| Left thalamus | − 12, − 16, 10 | 9.62 | − 9, − 13, 7 | 8.29 | − 15, − 22, 10 | 5.44 |
| Right thalamus | 12, − 10, 7 | 7.15 | 12, − 10, 7 | 5.47 | ||
| Right midbrain | 0, − 16, − 23 | 2.96 | 6, − 16, − 14 | 3.14 | ||
| Left anterior cerebellum | 21, − 55, − 29 | 6.90 | 18, − 55, − 26 | 5.64 | 18, − 55, − 26 | 8.86 |
| Right posterior cerebellum | 30, − 70, − 29 | 6.48 | 30, − 67, − 32 | 6.78 | ||
| Negative signal change | ||||||
| Left middle/superior frontal gyrus (BA 8) | − 33, 35, 46 | − 4.00 | − 27, 35, 49 | − 3.98 | ||
| Right middle/superior frontal gyrus (BA 8) | 27, 38, 46 | − 3.95 | 24, 32, 52 | − 6.32 | ||
| Anterior cingulate gyrus (rostral) | 0, 32, − 2 | − 5.59 | − 3, 38, − 5 | − 7.51 | ||
| Medial frontal gyrus | 0, 62, 13 | − 6.20 | 0, 62, 13 | − 8.90 | − 3, 65, 16 | − 6.33 |
| Medial frontal gyrus | 0, 50, − 2 | − 8.13 | − 3, 53, − 5 | − 2.84 | ||
| Right medial frontal gyrus | 21, 65, 16 | − 4.65 | 18, 65, 19 | − 6.07 | ||
| Right supramarginal gyrus | 63, − 49, 29 | − 4.82 | 60, − 61, 28 | − 6.63 | ||
| Cingulate gyrus | 6, − 25, 46 | − 5.81 | 0, − 22, 34 | − 7.66 | ||
| Right cingulate gyrus (BA 31) | 9, − 49, 46 | − 7.28 | 9, − 58, 31 | 9.27 | ||
| Left precuneus | − 12, − 67, 34 | − 7.25 | − 9, − 64, 31 | − 9.69 | − 6, − 55, 10 | − 6.78 |
| Right precuneus | 15, − 64, 34 | − 6.73 | ||||
| Right posterior middle temporal gyrus | 54, − 70, 16 | − 2.89 | ||||
| Right middle/inferior temporal gyri | 42, − 10, − 20 | − 4.17 | 57, − 7, − 20 | − 5.80 | 57, − 7, − 17 | − 3.66 |
Table 3.
Brain regions showing differences between High and Low Imagery fMRI task stimuli (p < .001 clusterwise significance using Monte Carlo simulation to determine the extent of contiguous voxels needed to survive statistical corrections for searching the whole brain volume with an entry threshold p < .005, t = 2.66). For negative-going BOLD signal change (e.g., “default mode” network regions), the Low > High represents arithmetic differences, e.g., regions marked Low > High indicate a “greater deactivation” to stimuli.
| High Imagery vs. Low Imagery |
|||
|---|---|---|---|
| Brain region | Peak x, y, z | Peak t | Direction |
| Positive BOLD signal change | |||
| Left middle/superior frontal gyri (BA 6) | − 21, 2, 55 | 5.33 | High > Low |
| Left inferior frontal/middle gyri (BA 9/44)a | − 48, 5, 25 | 7.42 | High > Low |
| Right inferior frontal gyrus (BA 44)a | 54, 11, 19 | 5.35 | High > Low |
| Left inferior/middle frontal gyri (BA 46) | − 48, 35, 10 | 5.96 | High > Low |
| Right inferior frontal gyrus (BA 46) | 48, 38, 7 | 4.47 | High > Low |
| Left inferior parietal lobule/postcentral gyrus | − 39, − 43, 49 | 6.66 | High > Low |
| Right inferior parietal lobule/postcentral gyrus | 57, − 31, 52 | 6.92 | High > Low |
| Right inferior parietal lobule (BA 40)a | 57, − 31, 52 | 6.92 | High > Low |
| Left middle temporal/fusiform gyri | − 48, − 61, − 5 | 9.24 | High > Low |
| Right middle/inferior temporal/fusiform gyri | 51, − 61, − 11 | 4.96 | High > Low |
| Left anterior insular cortexa | − 42, 26, − 8 | − 4.41 | Low > High |
| Left postcentral/precentral gyri (BA 3/1/6)a | − 39, − 16, 61 | − 4.05 | Low > High |
| Left postcentral gyrusa | − 51, − 25, 19 | − 5.05 | Low > High |
| Right postcentral gyrus/inferior parietal lobule (BA 40/43) | 57, − 25, 16 | − 3.86 | Low > High |
| Left superior/inferior parietal lobule (BA 7/40)a | − 45, − 64, 31 | − 8.14 | Low > High |
| Right inferior parietal lobule/angular gyrus | 48, − 64, 34 | − 7.80 | Low > High |
| Right superior parietal lobule (BA 7)a | 48, − 64, 34 | − 7.80 | Low > High |
| Left posterior middle temporal gyrus (BA 22)a | − 60, − 37, − 5 | − 6.51 | Low > High |
| Left middle/inferior temporal gyri | − 57, − 4, − 29 | 7.89 | Low > High |
| Left lingual gyrusa | − 9, − 76, − 8 | − 3.56 | Low > High |
| Left lingual gyrusa | − 3, − 82, − 2 | − 3.66 | Low > High |
| Left thalamusa | − 15, − 7, 7 | − 3.71 | Low > High |
| Right thalamusa | 0, − 7, 10 | − 3.93 | Low > High |
| Left putamen/lentiform nucleus | − 21, − 7, − 5 | − 4.48 | Low > High |
| Right putamen/lentiform nucleus | 24, − 1, − 5 | − 3.04 | Low > High |
| Negative signal change | |||
| Left middle/superior frontal gyrus (BA 8)a | − 30, 20, 49 | − 5.52 | Low > High |
| Right middle/superior frontal gyrus (BA 8)a | 15, 38, 46 | − 3.91 | Low > High |
| Medial frontal gyrusa | − 6, 62, 19 | − 7.87 | Low > High |
| Right medial frontal gyrusa | 15, 62, 7 | − 6.30 | Low > High |
| Right supramarginal gyrusa | 48, − 64, 34 | − 7.80 | Low > High |
| Right cingulate gyrus (BA 31)a | − 6, − 40, 34 | − 9.37 | Low > High |
| Left precuneusa | − 6, − 61, 22 | − 10.66 | Low > High |
| Right middle/inferior temporal gyria | 63, − 7, − 20 | − 5.29 | Low > High |
Indicates a brain region that showed main effect of BOLD signal change to Low, High, or Control stimuli.
Fig. 1.
Main effect of activation to Language Imagery Task sentence stimuli, averaged across study groups, shown on renderings from an atlas-based reconstruction of cortical regions. Given the similarity of activation patterns, activation also was collapsed across High and Low Imagery conditions. The image is thresholded at the t equivalent of p < .001 clusterwise corrections for multiple comparisons.
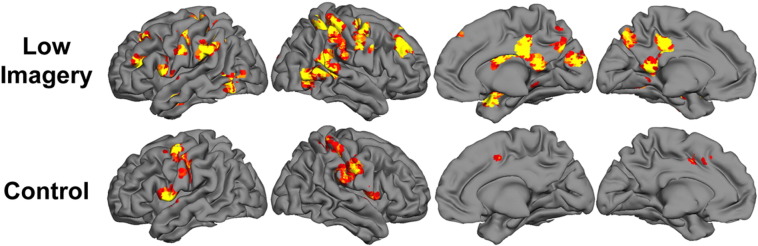
3.3. MRI results: group differences
Group differences were most prominent in the Low Imagery condition (Table 4, Fig. 2), with significant group differences for a number of brain regions. There was no evidence for neural normalization. As detailed below, some regions showed “residual-ASD” activation, such that both OO and HFA groups had significantly greater activations than the TD group, but did not differ from each other. The majority of group differences followed a neural compensation pattern, in which the OO group showed heightened activation relative to both HFA and TD groups.
Table 4.
Brain regions where activation to Low Imagery sentences differed by study group (in ANOVA analysis with p < .05 clusterwise significance using Monte Carlo simulation to determine the extent of contiguous voxels needed to survive statistical corrections for searching the whole brain volume with an entry threshold p < .025, F = 3.95). Regions are ordered by group difference pattern and anatomical location.
| Brain region | Peak x, y, z | Peak t | Direction |
|---|---|---|---|
| Compensation | |||
| Left precentral/postcentral gyri (BA 4/3) | − 51, − 13, 52 | 6.03 | OO > HFA,TD |
| Right precentral/middle frontal gyri (BA 6/4) | 45, − 7 58 | 7.33 | OO > HFA,TD |
| Left precentral/inferior frontal/superior temporal gyri (BA 44) | − 57, 8, 10 | 6.84 | OO > HFA,TD |
| Left postcentral/inferior parietal lobule/precuneus | − 33, − 31, 67 | 5.15 | OO > HFA,TD |
| Right supramarginal gyrus (BA 40) | 48, − 40, 58 | 9.37 | OO > HFA,TD |
| Left superior temporal gyrus (BA 38) | − 36, 5, − 20 | 6.42 | OO > HFA,TD |
| Right superior temporal/parahippocampal gyri (BA 38) | 36, 8, − 23 | 8.10 | OO > HFA,TD |
| Left middle occipital/middle and inferior temporal gyri (posterior) (BA 37/19) | − 45, − 64, − 5 | 6.22 | OO > HFA,TD |
| Left anterior cerebellum | − 21, − 61, − 20 | 9.96 | OO > HFA,TD |
| Right anterior cerebellum | 15, − 58, − 20 | 7.51 | OO > HFA,TD |
| Left posterior cerebellum | − 42, − 61, − 32 | 9.87 | OO > HFA,TD |
| Right posterior cerebellum | 21, − 70, − 35 | 7.28 | OO > HFA,TD |
| Right middle/superior frontal gyri (BA 9/8/10) | 36, 38, 34 | 9.21 | OO > HFA > TD |
| Right supramarginal gyrus | 51, − 43, 25 | 9.43 | OO > HFA > TD |
| Residual ASD | |||
| Left middle frontal gyrus (BA 8/9/46) | − 30, 29, 40 | 7.16 | OO,HFA > TD |
| Left supramarginal/angular gyri (BA 40) | − 42, − 52, 40 | 7.73 | OO,HFA > TD |
| Left supramarginal/angular gyri (BA 40) | − 57, − 34, 28 | 8.77 | OO,HFA > TD |
| Posterior cingulate gyrus (BA 31) | 6, − 28, 31 | 11.48 | OO,HFA > TD |
| Right superior/middle temporal gyri (posterior) (BA 22/37/40) | 63, − 58, 10 | 8.61 | OO,HFA > TD |
Fig. 2.
Study group differences to Low Imagery sentences and Control stimuli from SPM8 one-way ANOVA, shown on renderings from an atlas-based reconstruction of cortical regions. The images are thresholded at the t equivalent of p < .05 clusterwise corrections for multiple comparisons (p < .025, F = 3.95 entry-level threshold).
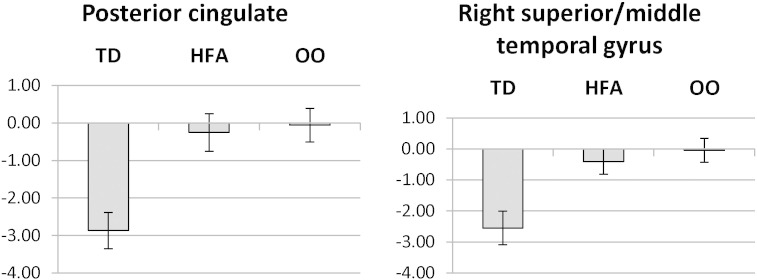
3.4. Residual-ASD
There were several regions showing residual ASD-like activation. These included regions of left dorsolateral prefrontal cortex, left inferior parietal lobule (supramarginal gyrus), bilateral posterior cingulate gyrus, and right superior/middle temporal gyrus (the right-hemisphere homologue of Wernicke's area). Sample graphs depicting the extent of activations are shown in Fig. 3.
Fig. 3.
Examples of residual-ASD activation patterns for Low Imagery stimuli.
Note: In each region, TD activation differs significantly from OO and HFA activations, which do not differ.
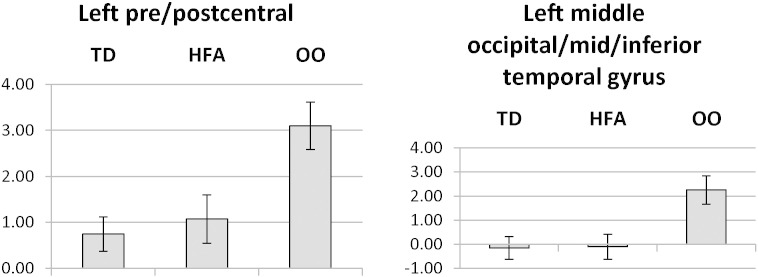
3.5. Neural compensation
There were numerous regions showing compensation (Table 4), including motor and supplementary motor regions of the right hemisphere, right middle and superior frontal gyrus, right supramarginal gyrus, right superior temporal and parahippocampal gyrus, left precentral and inferior temporal gyrus, left precuneus, left superior temporal gyrus, left occipital gyrus, and several regions of both anterior and posterior cerebellum (Fig. 4). A figure illustrating both compensation and residual-ASD patterning regions is shown in Supplemental Fig. S2.
Fig. 4.
Examples of compensatory activation patterns for Low Imagery stimuli.
Note: In each region, OO activation differs significantly from HFA and TD activations.
Although none of the group differences to High Imagery survived corrections for multiple comparisons, some few differences were seen using more liberal clusterwise entry-level thresholds (see Supplemental Table 1).
Of the few group differences for Control stimuli, most overlapped with Low Imagery findings (e.g., left operculum BA 44/55 Broca's region, right precentral, postcentral, and surpramarginal gyrus; these data are available in Supplemental Table 2 and Fig. 2).
4. Discussion
This study aimed to distinguish between two possible neural mechanisms for recovered behavioral function in the OO group: 1) essentially normative brain activations reflecting normalization of neural processing, possibly due to intensive early intervention; versus 2) the recruitment of alternative processing pathways sculpted by the early acquisition of foundational skills or exposure to language via drills. A third possibility was that of residual ASD-like brain activations. Somewhat surprisingly, there was almost no evidence for normalization; there were no regions in the OO group in which brain activity differed from the HFA group, and did not differ from the TD group. Instead, the bulk of group differences pointed to the recruitment of compensatory neural networks for language comprehension, with heightened brain activation underlying language processing for the OO group only.
These included a set of regions classically associated with language processing such as left precentral temporal gyrus (BA 44), or Broca's area, and left superior temporal gyrus (STG; BA 38), often activated in combinatorial semantics tasks (e.g., for processing sentences relative to unrelated strings of words; see Visser et al., 2010) or acoustic familiarity with a word (Leech et al., 2009), as well as brain regions with extensive links to aspects of language processing. Right supramarginal gyrus (BA 40) is associated with phonological (as opposed to purely semantic) processing (Price et al., 1997) during reading, with the comprehension of complex verbal material (Klepousniotou et al., 2014), and in phonological processing (Hartwigsen et al., 2010), and right superior temporal/parahippocampal gyri (BA 38), for which activation is associated with the processing of verbal context (including irony, Akimoto et al., 2014). Left middle occipital/middle and inferior temporal gyrus (BA 37/19) is extensively implicated in reading (Pugh et al., 2008). Its heightened activation may reflect increased reliance on mental imagery (D'Esposito et al., 1997).
Other regions showing heightened OO activation are more strongly linked to non-language abilities, but occasionally have been found relevant to language. For instance, left postcentral/inferior parietal lobule/precuneus is associated with self-reflective functioning, and theory of mind processing (Saxe et al., 2004), and less centrally with prosodic processing (Eigsti et al., 2012). Right precentral/middle frontal gyri (BA 6/4), and left precentral gyrus (BA 4) are associated with motor control. However, ventral aspects of BA 4 have been found to be involved in word retrieval and covert articulation (Price, 2010). There also was compensatory activation in right middle/superior frontal gyri (BA 9/8/10), often associated with complex motor planning tasks. BA 8 in particular includes the frontal eye fields, known to be involved in logical inference-making (Monti et al., 2009) and the handling of uncertainty (Volz et al., 2005). Finally, there was heightened activation in many regions of cerebellum, including both left and right anterior cerebellum, previously described as showing atypical connectivity to frontal regions in ASD (Hodge et al., 2010). The right region forms part of a network that shares substrates with overt speech and may represent an inner speech pathway that increases activity with greater working memory demands (Marvel and Desmond, 2012). There was also compensatory activation in both left and right posterior cerebellum, most classically associated with motor control; clinical studies of aphasia, mutism, agraphia, and cerebellar lesions have also suggested that left posterior cerebellum is also implicated in verbal fluency, semantic processing, and metalinguistic skills (De Smet et al., 2013).
These regions of increased activity in the OO group during language processing are consistent with fMRI literature which suggests that less efficient processing is associated with greater “tissue use” (Hare et al., 2008; though c.f. Poldrack, 2015). The lack of behavioral group differences both reinforces the idea that OO required more effortful top-down control for sentence comprehension, and also suggests that groups did not differ in attention to or engagement with the task. fMRI-measured brain activation group differences were found primarily in the Low Imagery condition, which could suggest that even for stimuli that might otherwise be thought to be simple or low in processing demands, participants in the OO group process such stimuli with significantly greater effort. Alternatively, the relative lack of group differences in the High Imagery condition could reflect use of widely different cognitive strategies. This idea is based on the fact that Low Imagery judgments tended to involve simple factual knowledge, such as when telephones were invented. In contrast, High Imagery judgments such as whether a hippo or an elephant is big enough to crush a car, may have required more evaluative comparisons. As such, they may have elicited more heterogeneity in task strategies and, thus, fewer reliable group differences. Consistent with this, participants were slower and less accurate in High Imagery behavioral performance, partially consistent with a previous study of high-functioning adult ASDs using a comparable task (Kana et al., 2006).
A second set of results suggested residual-ASD patterns of activation, in that brain activity as participants engaged in this language comprehension task showed considerable overlap between ASD and OO groups and, in all cases, heightened activity relative to a TD comparison group. These regions included those implicated in cognitive control, such as left middle frontal gyrus (BA 9/8/46), part of dorsolateral prefrontal cortex. This region is heavily implicated in executive functions (Gogtay et al., 2004) and also contains von Economo neurons, an evolutionarily late group of spindle neurons showing distinctive neural shape/size and thought to be recruited by social processes (Fajardo et al., 2008). Also showing residual-ASD activations was posterior cingulate gyrus (BA 31). This region is engaged for emotional decision-making and linking of behavioral outcomes to emotional responses (Posner et al., 2007). There are reports that individuals with ASD show differential connectivity of posterior cingulate and language regions (specifically Wernicke's area; Nielsen et al., 2014). A third region showing residual-ASD patterning was left supramarginal/angular gyri (BA 40; IPL). This region is massively connected with auditory, visual, and somatosensory cortices, and contains multimodal neurons that respond to specific classes of stimuli. It is connected via large fiber bundles to Broca's and Wernicke's areas. As such, IPL is critically involved in comprehending multiple properties of words — their sound, their appearance, their function, etc. It also is involved in concept formation and abstract semantic representations, including the selection of competing alternatives in semantic memory (Kan and Thompson-Schill, 2004). This region is activated in many language tasks, including reading (Buchsbaum and D'Esposito, 2009, Liebenthal et al., 2013, Paulesu et al., 1993, Tourville et al., 2008). Interestingly, results indicated deactivation of left supramarginal gyrus for the TD group, a somewhat surprising result in a reading comprehension task; this may be deactivation related to task demands, as has been reported recently (Harrison et al., 2011). Finally, the HFA and OO groups showed heightened activation of right superior/middle temporal gyrus (BA 22/37/40), which includes the right homologue of Wernicke's area, shown to be associated with prosodic processing (Ross and Monnot, 2008).
There were differences between current results and those of Kana et al.(2006). Specifically, while the regions of activation reported in Kana et al. were somewhat replicated in the current findings, with main effects of group, the current results failed to replicate the finding of heightened use of visual regions (left lingual gyrus, left IPS) in a high-functioning ASD group during Low Imagery sentence comprehension. Rather, the data here suggested similar activations in those areas across all three groups, with the exception of compensatory activation in the OO group in the left middle occipital gyrus region. Somewhat parallel to Kana's results, there were Group × Condition interactions; in every case, the groups differed for the Low Imagery condition, with HFA and OO groups showing greater activation. This difference in findings could reflect the use of an event-related design in the present study (rather than a block design); another possible contributory factor is subject age, as participants in the current study were significantly younger. If so, the visual cortex results reported in Kana et al. (2006) could reflect compensatory processing in ASD that has taken hold over a longer developmental period. This possibility, while intriguing, is speculative and requires additional research. It is also possible that this younger generation of HFA and OO participants received earlier and more targeted intervention.
Study limitations include the cross-sectional rather than longitudinal design, making it impossible to ascertain that current differences were causally implicated in the optimal outcomes. There is evidence that the OO sample was similar to the HFA sample early in development (i.e., that they had ASD of generally similar severity; Eigsti and Fein, 2013, Mraz et al., 2009). As such, the current results provide a strong impetus for pursuing longitudinal research with this population. A second limitation is that our three groups differed in CELF core language score (and VIQ; see note, Table 1). It would be difficult to recruit an ASD sample that is not lower in VIQ in comparison to the OO sample; indeed, this difference likely reflects, in part, the reduction in ASD symptomatology. While it is a strength that groups performed similarly on the language task, group differences at the neural level may have been more apparent in the context of a behavioral task which also elicited performance differences. Thirdly, while the groups did not differ in age, there was a very broad age range (eight to 21 years) for participants; it was difficult to assemble this very particular subset of a diagnostic group. Future studies will be able to target age as an additional predictor variable, in looking for changes in OO groups that are a function of development (e.g., examining whether older participants show less compensation and a trend towards normalization); unfortunately, the sample size in the current study precluded such an analysis. An additional limitation relates to the behavioral task (based on Kana et al.); the materials were generously shared by that research team, but there is little information about the validation of the high and low visual imagery sentences. Finally, while our OO and HFA groups differed in their intervention histories (described in detail in Orinstein et al., 2014), it was not possible to explicitly link activations in this study to individual details regarding interventions (because of the complexity of those histories). As such, while we hypothesize that it is the provision of early intervention, and specifically ABA intervention, that contributed to the compensatory activations reported here, the current results cannot conclusively confirm such a relationship.
In conclusion, the OO groups shows heightened and ASD-like activation of brain regions often implicated in language comprehension and prosody, in cognitive control and in using motivation in decision-making, and also in the right-lateralized regions of brain that, on the left, are often associated with language. This is consistent with a body of previous work showing less left-lateralized, more bilateral, and more right-lateralized activation during language tasks in ASD (Knaus et al., 2008, Knaus et al., 2010), and suggests that the OO group retains this somewhat atypical ‘signature’ of language processing. These results support the presence of the diagnosis in the OO group. A parallel is found in studies of neurofunctional reorganization in healthy aging. There is evidence that, to maintain the same level of behavioral performance, older individuals display compensatory activation of right-lateralized homologues for typically left-lateralized cognitive process (Ansado et al., 2013). Reorganization during aging may serve as an analogue to the developmental plasticity observed in OO, in that the recruitment of right-homologue tissue facilitates behaviorally normal language. These findings highlight the impressive and clinically meaningful plasticity of neural circuits underlying language and have important implications for exactly how rigorous, early interventions for ASD-diagnosed children may work.
Financial disclosures
The authors have no financial conflicts of interest to report.
Acknowledgments
The authors are very grateful to the participants and their families, to Dr. Lynn Brennan and Harriet Levin for their help with recruitment, to Drs. Molly Helt, Michael Rosenthal, and Kathryn Tyson for their help in testing the children, and to many invaluable undergraduate research assistants. We also gratefully acknowledge our funding: NationalInstitutes of HealthR01 MH076189. Portions of this manuscript were previously reported in Eigsti et al. (2013, May).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.11.014.
Appendix A. Supplementary data
Supplementary material.
References
- Akimoto Y., Sugiura M., Yomogida Y., Miyauchi C.M., Miyazawa S., Kawashima R. Irony comprehension: social conceptual knowledge and emotional response. Hum. Brain Mapp. 2014;35(4):1167–1178. doi: 10.1002/hbm.22242. (Epub 22013 Feb 22213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.K., Liang J.W., Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry. 2014;55(5):485–494. doi: 10.1111/jcpp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansado J., Marsolais Y., Methqal I., Alary F., Joanette Y. The adaptive aging brain: evidence from the preservation of communication abilities with age. Eur. J. Neurosci. 2013;37(12):1887–1895. doi: 10.1111/ejn.12252. [DOI] [PubMed] [Google Scholar]
- Aylward E.H., Richards T.L., Berninger V.W., Nagy W.E., Field K.M., Grimme C.…Cramer S.C. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Boddaert N., Belin P., Chabane N., Poline J.B., Barthelemy C., Mouren-Simeoni M.C.…Zilbovicius M. Perception of complex sounds: abnormal pattern of cortical activation in autism. Am. J. Psychiatr. 2003;160(11):2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- Buchsbaum B., D'Esposito M. Repetition suppression and reactivation in auditory–verbal short-term recognition memory. Cereb. Cortex. 2009;19:1474–1485. doi: 10.1093/cercor/bhn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino A., Luke L., Waldman S., Andrade A., Fletcher P.C., Ring H. An fMRI investigation of detection of semantic incongruities in autistic spectrum conditions. Eur. J. Neurosci. 2011;33(3):558–567. doi: 10.1111/j.1460-9568.2010.07503.x. (Epub 02010 Dec 07529) [DOI] [PubMed] [Google Scholar]
- Dawson G., Jones E.J., Merkle K., Venema K., Lowy R., Faja S.…Webb S.J. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(11):1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Francis D.J., Cirino P.T., Schachar R., Barnes M.A., Fletcher J.M. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J. Int. Neuropsychol. Soc. 2009;15:1–13. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet H.J., Paquier P., Verhoeven J., Marien P. The cerebellum: its role in language and related cognitive and affective functions. Brain Lang. 2013;127(3):334–342. doi: 10.1016/j.bandl.2012.11.001. (Epub 2013 Jan 1017) [DOI] [PubMed] [Google Scholar]
- D'Esposito M., Detre J.A., Aguirre G.K., Stallcup M., Alsop D.C., Tippet L.J., Farah M.J. A functional MRI study of mental image generation. Neuropsychologia. 1997;35(5):725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Dichter G.S. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin. Neurosci. 2012;14(3):319–351. doi: 10.31887/DCNS.2012.14.3/gdichter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden G.F., Jones K.M., Cappell K., Gareau L., Wood F.B., Zeffiro T.A.…Flowers D.L. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44(3):411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Eigsti I.M., Fein D.A. More is less: pitch discrimination and language delays in children with optimal outcomes from autism. Autism Res. 2013;6(6):605–613. doi: 10.1002/aur.1324. (Epub 2013 Aug 1008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti I.M., Schuh J.M., Mencl E., Schultz R.T., Paul R. The neural underpinnings of prosody in autism. Child Neuropsychol. 2012;18(6):600–617. doi: 10.1080/09297049.2011.639757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti I.M., Stevens M., Schultz R., Naigles L.R., Kelley E., Orinstein A., Fein D.A.… . Paper Presented at the International Meeting for Autism Research (IMFAR-13), San Sebastian, Spain. 2013. Oral presentation: neural activation to sentences in individuals with high-functioning autism, typical development, and autism spectrum disorder optimal outcome. (May) [Google Scholar]
- Fajardo C., Escobar M.I., Buritica E., Arteaga G., Umbarila J., Casanova M.F., Pimienta H. Von Economo neurons are present in the dorsolateral (dysgranular) prefrontal cortex of humans. Neurosci. Lett. 2008;435(3):215–218. doi: 10.1016/j.neulet.2008.02.048. (Epub 2008 Mar 1014) [DOI] [PubMed] [Google Scholar]
- Fein D., Barton M., Eigsti I.M., Kelley E., Naigles L.R., Schultz R.T., Tyson K.… Optimal outcome in individuals with a history of autism. J. Child Psychol. Psychiatry. 2013;54(2):195–205. doi: 10.1111/jcpp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire L., Roche A., Mangin J. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans. Med. Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M.… Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. ([doi] 0402680101 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J., Wicker B., Berthoz S., de Gelder B. A failure to grasp the affective meaning of actions in autism spectrum disorder subjects. Neuropsychologia. 2009;47(8–9):1816–1825. doi: 10.1016/j.neuropsychologia.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Groen W.B., Tesink C., Petersson K.M., van Berkum J., van der Gaag R.J., Hagoort P., Buitelaar J.K. Semantic, factual, and social language comprehension in adolescents with autism: an FMRI study. Cereb. Cortex. 2010;20(8):1937–1945. doi: 10.1093/cercor/bhp264. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go–nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Contreras-Rodriguez O., Soriano-Mas C., Lopez-Sola M., Deus J.…Menchon J.M.… Task-induced deactivation from rest extends beyond the default mode brain network. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022964. (Epub 0022011 Jul 0022929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G., Baumgaertner A., Price C.J., Koehnke M., Ulmer S., Siebner H.R. Phonological decisions require both the left and right supramarginal gyri. Proc. Natl. Acad. Sci. 2010;107(38):16494–16499. doi: 10.1073/pnas.1008121107. (Epub 1008122010 Aug 1008121131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helt M., Kelley E., Kinsbourne M., Pandey J., Boorstein H., Herbert M., Fein D. Can children with autism recover? If so, how? Neuropsychol. Rev. 2008;18(4):339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Hesling I., Dilharreguy B., Peppe S., Amirault M., Bouvard M., Allard M. The integration of prosodic speech in high functioning autism: a preliminary fMRI study. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S.M., Makris N., Kennedy D.N., Caviness V.S., Jr., Howard J., McGrath L., Harris G.J.… Cerebellum, language, and cognition in autism and specific language impairment. J. Autism Dev. Disord. 2010;40(3):300–316. doi: 10.1007/s10803-009-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R.M., Fricker Z., Fenoglio A., Lindgren K.A., Knaus T.A., Tager-Flusberg H. Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging Behav. 2014;8(1):60–72. doi: 10.1007/s11682-013-9245-0. [DOI] [PubMed] [Google Scholar]
- Kan I.P., Thompson-Schill S.L. Selection from perceptual and conceptual representations. Cogn. Affect. Behav. Neurosci. 2004;4(4):466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley E., Paul J.J., Fein D., Naigles L.R. Residual language deficits in optimal outcome children with a history of autism. J. Autism Dev. Disord. 2006;36(6):807–828. doi: 10.1007/s10803-006-0111-4. [DOI] [PubMed] [Google Scholar]
- Kelley E., Naigles L.R., Fein D.A. An in-depth examination of optimal outcome children with a history of autism spectrum disorders. Res. Autism Spectr. Disord. 2010;4:526–538. [Google Scholar]
- Kleinhans N.M., Muller R.A., Cohen D.N., Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepousniotou E., Gracco V.L., Pike G.B. Pathways to lexical ambiguity: fMRI evidence for bilateral fronto-parietal involvement in language processing. Brain Lang. 2014;131:56–64. doi: 10.1016/j.bandl.2013.06.002. (Epub 2013 Nov 1011) [DOI] [PubMed] [Google Scholar]
- Knaus T.A., Silver A.M., Lindgren K.A., Hadjikhani N., Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. J. Int. Neuropsychol. Soc. 2008;14(6):967–979. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus T.A., Silver A.M., Kennedy M., Lindgren K.A., Dominick K.C., Siegel J., Tager-Flusberg H. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 2010;112(2):113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel R.I., Frea W.D. Treatment of social behavior in autism through the modification of pivotal social skills. J. Appl. Behav. Anal. 1993;26:369–377. doi: 10.1901/jaba.1993.26-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Holt L.L., Devlin J.T., Dick F. Expertise with artificial nonspeech sounds recruits speech-sensitive cortical regions. J. Neurosci. 2009;29(16):5234–5239. doi: 10.1523/JNEUROSCI.5758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenthal E., Sabri M., Beardsley S.A., Mangalathu-Arumana J., Desai A. Neural dynamics of phonological processing in the dorsal auditory stream. J. Neurosci. 2013;33(39):15414–15424. doi: 10.1523/JNEUROSCI.1511-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., LeCouteur A. Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Marvel C.L., Desmond J.E. From storage to manipulation: how the neural correlates of verbal working memory reflect varying demands on inner speech. Brain Lang. 2012;120(1):42–51. doi: 10.1016/j.bandl.2011.08.005. (Epub 2011 Sep 1011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A., Liu Y., Williams D.L., Keller T.A., Minshew N.J., Just M.A. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134(Pt 8):2422–2435. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M.M., Parsons L.M., Osherson D.N. The boundaries of language and thought in deductive inference. Proc. Natl. Acad. Sci. 2009;106(30):12554–12559. doi: 10.1073/pnas.0902422106. (Epub 0902422009 Jul 0902422116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz K.D., Dixon J., Dumont-Mathieu T., Fein D. Accelerated head and body growth in infants later diagnosed with autism spectrum disorders: a comparative study of optimal outcome children. J. Child Neurol. 2009;24(7):833–845. doi: 10.1177/0883073808331345. [DOI] [PubMed] [Google Scholar]
- Mundy P., Crowson M. Joint attention and early social communication: implications for research on intervention with autism. J. Autism Dev. Disord. 1997;27:653–676. doi: 10.1023/a:1025802832021. [DOI] [PubMed] [Google Scholar]
- Nielsen J.A., Zielinski B.A., Fletcher P.T., Alexander A.L., Lange N., Bigler E.D.…Anderson J.S. Abnormal lateralization of functional connectivity between language and default mode regions in autism. Mol. Autism. 2014;5(1):8. doi: 10.1186/2040-2392-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orinstein A., Helt M., Troyb E., Tyson K., Barton M.L., Eigsti I.M.…Fein D.A. Intervention history of children and adolescents with high-functioning autism and optimal outcomes. J. Dev. Behav. Pediatr. 2014;35(4):247–256. doi: 10.1097/DBP.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E., Frith C., Frackowiak R. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Is “efficiency” a useful concept in cognitive neuroscience? Dev. Cogn. Neurosci. 2015;11:12–17. doi: 10.1016/j.dcn.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn. Affect. Behav. Neurosci. 2007;7(4):391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Price C.J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price C.J., Moore C.J., Humphreys G.W., Wise R.J. Segregating semantic from phonological processes during reading. J. Cogn. Neurosci. 1997;9(6):727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Frost S.J., Sandak R., Landi N., Rueckl J.G., Constable R.T.…Mencl E. Effects of stimulus difficulty and repetition on printed word identification: an fMRI comparison of Non-Impaired and Reading Disabled Adolescent cohorts. J. Cogn. Neurosci. 2008;20(7):1146–1160. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol. Psychiatry. 2005;58(1):1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Redcay E., Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol. Psychiatry. 2008;64(7):589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E.D., Monnot M. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain Lang. 2008;104(1):51–74. doi: 10.1016/j.bandl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Sahyoun C.P., Belliveau J.W., Soulieres I., Schwartz S., Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. (Epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D., Hartwigsen G. Neurobiology of language recovery after stroke: lessons from neuroimaging studies. Arch. Phys. Med. Rehabil. 2012;93(1 Suppl.):S15–S25. doi: 10.1016/j.apmr.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Saxe R., Carey S., Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annu. Rev. Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Semel E., Wiig E.H., Secord W.A. 4th ed. Harcourt Assessment, Inc.; San Antonio, TX: 2003. Clinical Evaluation of Language Fundamentals. [Google Scholar]
- Simos P.G., Fletcher J.M., Bergman E., Breier J.I., Foorman B.R., Castillo E.M.…Papanicolaou A.C. Dyslexia-specific brain activation becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Tourville J., Reilly K., Guenther F. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39:1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyb E., Orinstein A., Tyson K., Helt M., Eigsti I.M., Stevens M., Fein D. Academic abilities in children and adolescents with a history of autism spectrum disorders who have achieved optimal outcomes. Autism. 2013;18(3):233–243. doi: 10.1177/1362361312473519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson K., Kelley E., Fein D., Orinstein A., Troyb E., Barton M., Rosenthal M.… Language and verbal memory in individuals with a history of autism spectrum disorders who have achieved optimal outcomes. J. Autism Dev. Disord. 2014;44(3):648–663. doi: 10.1007/s10803-013-1921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly M., Verhoeven J., Zink I., Mantini D., Peeters R., Deprez S., Sunaert S.… Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. NeuroImage. 2014;4:374–382. doi: 10.1016/j.nicl.2014.01.008. (eCollection 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M., Jefferies E., Lambon Ralph M.A. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J. Cogn. Neurosci. 2010;22(6):1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Volz K.G., Schubotz R.I., von Cramon D.Y. Variants of uncertainty in decision-making and their neural correlates. Brain Res. Bull. 2005;67(5):403–412. doi: 10.1016/j.brainresbull.2005.06.011. (Epub 2005 Jul 2007) [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1 ed. Pearson Psychological Corporation; New York: 1999. Manual for the Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.