Data resource basics
The calculation of robust lifetime cardiovascular disease (CVD) risk estimates requires specific types of data with several features: (i) substantial person-years of follow-up are needed for the years of mid life through advanced age (typically 95 years-old), the critical years over which these estimates are applied; (ii) given the low prevalence of optimal risk factor levels in the USA, a large sample size is needed to generate stable lifetime risk estimates when stratified by risk factor burden; and (iii) near 100% follow-up for vital status is essential in order to account for effects of competing risks of death which become substantial when estimating risk beyond the short-term (10-year) time frame. Therefore, the Lifetime Risk Pooling Project (LRPP) was designed as an individual-level pooled dataset from 20 US community-based cardiovascular disease cohorts. Cohorts were selected if they represented community-based samples, included at least one baseline examination with direct measurements of physiological or anthropometric variables, and included at least 5 years of follow-up for fatal or nonfatal CVD events with high-quality CVD endpoint assessment. Studies were funded by the National Heart, Lung, and Blood Institute, National Institute of Aging, US Department of Health and Human Services and the Centers for Disease Control and Prevention.
Data resource area and population coverage
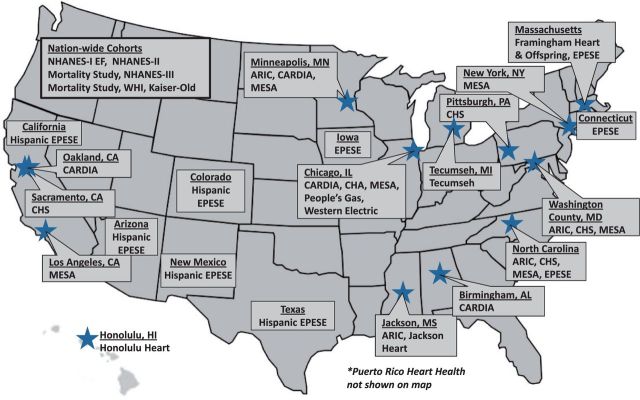
All participants included in the current iteration of the LRPP are residents of the USA or the Commonwealth of Puerto Rico. We are continuously updating and expanding the dataset with new examination data from cohorts already included and adding data from other cohorts as well. Figure 1 shows a detailed description of the location of all field centres and examination sites for the community-based cohorts included in the LRPP. Longitudinal follow-up data from three National Health and Nutrition Examination Surveys (NHANES I, NHANES II and NHANES III) are included in the dataset as well. NHANES uses a complex multi-staged probability sampling design to derive a representative sample of the US civilian non-institutionalized population. The demographics and enrolment details of individuals included in the LRPP dataset are presented in Table 1. The majority of cohort participants are non-institutionalized US-born Caucasians. Among the minority groups represented in the LRPP dataset, most are African Americans with the remaining groups including Japanese Americans, Chinese Americans, and Latinos/Hispanics. Because of the study design for the included cohorts, the majority of data come from participants who were ≥45 years old at the time of baseline examination.
Figure 1.
Geographical locations of cohorts included in the Lifetime Risk Pooling Project. Locations (city and state) of the examination sites for each cohort study included in the LRPP. If statewide data were used without a specific examination site, city is not listed. Nationwide cohorts are listed separately in box on top left. Puerto Rico Heart Health is not shown on map for space considerations. ARIC, Atherosclerosis Risk in Communities Study; CHS, Cardiovascular Health Study; CHA, Chicago Heart Association Detection Project in Industry; CARDIA, Coronary Artery Risk Development in Young Adults; EPESE, Established Populations for Epidemiologic Studies of the Elderly; Kaiser Old, Kaiser Permanente Study of the Oldest Old; NHANES I EF, National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study; MESA, Multi-Ethnic Study of Atherosclerosis; WHI, Women’s Health Initiative.
Table 1.
Demographics and enrolment details of cohorts included in the Lifetime Risk Pooling Project
| Cohort | Enrolment period | Enrolment sample size* (N) | Sex | Age at enrolment (years) | Race/ethnicity |
|---|---|---|---|---|---|
| Framingham Heart | 1948 | 5209 | Men and women | 30–62 | White |
| Western Electric | 1957–58 | 2107 | Men | 40–59 | White |
| People’s Gas | 1958–59 | 3005 | Men | 25–59 | White, African American |
| Tecumseh Study | 1959–60 | 8641 | Men and women | >16 | White |
| Honolulu Heart Program | 1965–68 | 8006 | Men | 28–62 | Japanese American |
| Puerto Rico Heart Health | 1965 | 9824 | Men | 45–64 | Puerto Rican |
| CHA | 1967–73 | 39522 | Men and women | >18 | White, African American |
| Framingham Offspring | 1971 | 5124 | Men and women | 5–70 | White |
| NHANES I EF | 1971–75 | 14407 | Men and women | 25–74 | White, African American |
| Kaiser Old | 1971, 1980 | 5990 | Men and women | 65–95 | White, African American, Hispanic and other |
| NHANES II Mortality | 1976–80 | 9250 | Men and women | 30–74 | White, African American |
| EPESE | 1981–87 | 14458 | Men and women | >65 | White, African American |
| CARDIA | 1985–86 | 5115 | Men and women | 18–30 | White, African American |
| ARIC | 1987–89 | 15792 | Men and women | 45–64 | White, African American |
| NHANES III Mortality | 1988–94 | 20050 | Men and women | 17–90 | White, African American, Mexican American |
| CHS | 1989–90 | 5887 | Men and women | >65 | White, African American |
| 1992 (supplement) | |||||
| WHI | 1993–98 | 93676 | Women | 50–79 | White, African American, Hispanic and other |
| Hispanic EPESE | 1993–94 | 3050 | Men and women | >65 | Mexican American |
| MESA | 2000–02 | 6814 | Men and women | 45–84 | White, African American, Asian, Hispanic |
| Jackson Heart | 2000–04 | 3676 | Men and women | 35–85 | African American |
CHA, Chicago Heart Association Detection Project in Industry; NHANES I EF, National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study; Kaiser Old, Kaiser Permanente Study of the Oldest Old; NHANES II Mortality, National Health and Nutrition Examination Survey II Mortality Study; EPESE, Established Populations for Epidemiologic Studies of the Elderly; CARDIA, Coronary Artery Risk Development in Young Adults; ARIC, Atherosclerosis Risk in Communities Study; NHANES III Mortality, National Health and Nutrition Examination Survey III Mortality Study; CHS, Cardiovascular Health Study; WHI, Women’s Health Initiative; Hispanic EPESE, Hispanic Established Populations for Epidemiologic Studies of the Elderly; MESA, Multi-Ethnic Study of Atherosclerosis.
*Size of sample included in LRPP dataset.
Survey frequency
The era and frequency of examinations vary by cohort. Figure 2A demonstrates the calendar years of data collection and the range of years in which examinations were performed. The earliest year of data collection was 1948 (inception of the Framingham Heart Study) and the most recent 2010. Much of the data included in the LRPP were collected from 1965 through 1995, during an era prior to the widespread use of preventive statin drug therapies. The duration of follow-up and examination frequency are shown in Figure 2B. The longest interval of follow-up, 59 years, comes from the Framingham Heart Study. Examination frequency is variable, with annual examinations in Cardiovascular Health Study, longer intervals between examinations in many cohorts and only single baseline examination in several studies. Cardiovascular outcomes data were typically collected at exams and using surveillance techniques (e.g. National Death Index etc., see Table 3).
Figure 2.
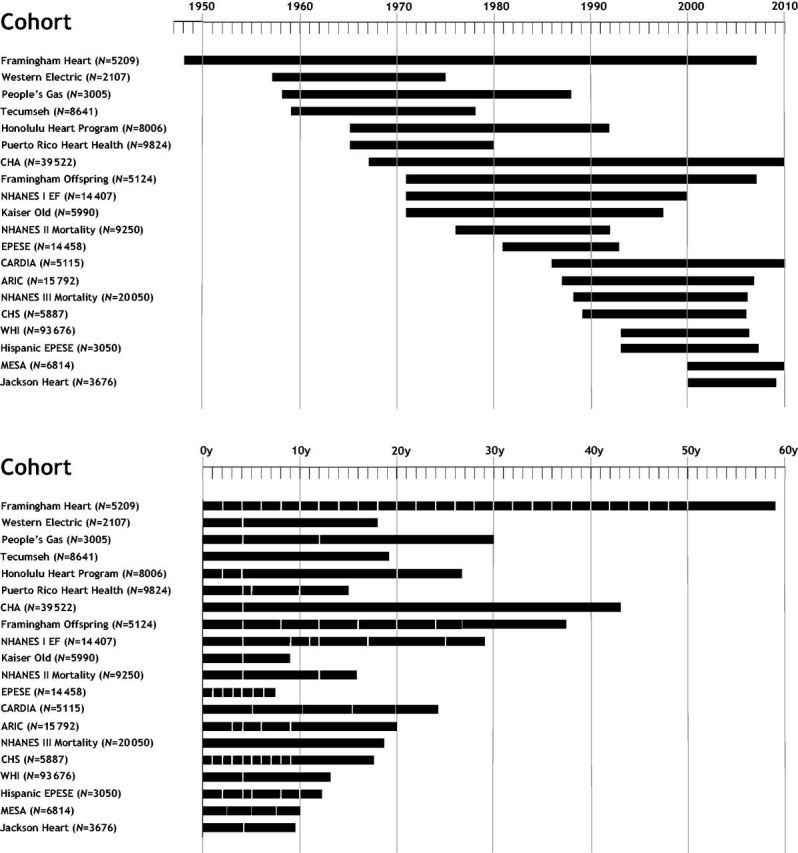
(a) Follow-up time for each cohort included in the Lifetime Risk Pooling Project. Length of follow-up from initial year of enrolment to last year of follow-up data for each cohort. CHA, Chicago Heart Association Detection Project in Industry; NHANES I EF, National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study; Kaiser Old, Kaiser Permanente Study of the Oldest Old; EPESE, Established Populations for Epidemiologic Studies of the Elderly; CARDIA, Coronary Artery Risk Development in Young Adults; ARIC, Atherosclerosis Risk in Communities Study; CHS, Cardiovascular Health Study; WHI, Women’s Health Initiative; MESA, Multi-Ethnic Study of Atherosclerosis. (b) Follow-up time for each cohort included in the Lifetime Risk Pooling Project. Cumulative years of follow-up for each cohort with sampling frequencies (depicted by vertical white bars). Cohort abbreviations as outlined above in figure 2a; y, years.
Table 3.
Outcomes assessed in Lifetime Risk Pooling Project cohorts
| Cohort | Outcome Ascertainment | Nonfatal MI | Angina | Ischaemic stroke | Haemorrhagic stroke | AFib Aflutter | Sudden death | PAD | HF | CHD death | CVD death | All-cause death | Non-CVD death | Cancer death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Framingham Heart | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Western Electric | ICD-8 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| People’s Gas | ICD-8 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Tecumseh Study | ICD-9 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Honolulu Heart Program | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Puerto Rico Heart Health* | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| CHA | ICD-8&9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Framingham Offspring | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| NHANES I EF | ICD-9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Kaiser Old | Chart review and ICD-8&9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| NHANES II Mortality | ICD-9 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| EPESE | ICD-9 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| CARDIA | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| ARIC | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| NHANES III Mortality | ICD-10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| CHS | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| WHI | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hispanic EPESE | ICD-9 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| MESA | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Jackson Heart | Adjudicated | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
MI, myocardial infarction; Afib, atrial fibrillation; Aflutter, atrial flutter; PAD, peripheral arterial disease; HF, heart failure; CHD, coronary heart disease; CVD, cardiovascular disease; CHA, Chicago Heart Association Detection Project in Industry; NHANES I EF, National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study; Kaiser Old, Kaiser Permanente Study of the Oldest Old; NHANES II Mortality, National Health and Nutrition Examination Survey II Mortality Study; EPESE, Established Populations for Epidemiologic Studies of the Elderly; CARDIA, Coronary Artery Risk Development in Young Adults; ARIC, Atherosclerosis Risk in Communities Study; NHANES III Mortality, National Health and Nutrition Examination Survey III Mortality Study; CHS, Cardiovascular Health Study; WHI, Women’s Health Initiative; Hispanic EPESE, Hispanic Established Populations for Epidemiologic Studies of the Elderly; MESA, Multi-Ethnic Study of Atherosclerosis.
*Puerto Rico Heart Health: nonfatal events only for the first 8 years of follow-up.
Measures
Details of CVD exposures (behavioural and physiological risk factors) and CVD outcomes of interest are provided in Tables 2 and 3. Briefly, age, sex, race and smoking status were self-reported across all LRPP cohorts. Cohort clinic staff measured blood pressure with a sphygmomanometer using standard methods. Details of lipid fraction measurements are provided in Table 2. All cohorts include at least one total cholesterol measurement, obtained in the fasting or non-fasting state. Fasting measurements of standard lipid fractions [triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C)] are available in nine of the LRPP cohorts. Lipid-lowering medication use, a diagnosis of diabetes and/or use of diabetes medications were determined by self-report as well. Serum glucose measurements were obtained in 12 cohorts. A select number of cohorts provide information on diet, physical activity and alcohol consumption. In its current form, genotypic data are not included in the LRPP dataset.
Table 2.
Exposure variables in Lifetime Risk Pooling Project cohorts
| Cohort | BP | BP meds | Total cholesterol | LDL-C | HDL-C | TG | Lipid meds | BMI | Fasting blood glucose | DM Meds | Smoking | ABI | Alcohol use | Physical activity | Dietary intake |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Framingham Heart | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Western Electric | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| People’s Gas | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Tecumseh Study | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Honolulu Heart Program | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Puerto Rico Heart Health | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| CHA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Framingham Offspring | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| NHANES I EF | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Kaiser Old | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| NHANES II Mortality | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| EPESE | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| CARDIA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| ARIC | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| NHANES III Mortality | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| CHS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| WHI | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Hispanic EPESE | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| MESA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Jackson Heart | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
BP, blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; BMI, body mass index; DM, diabetes mellitus; ABI, ankle brachial index; CHA, Chicago Heart Association Detection Project in Industry; NHANES I EF, National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study; Kaiser Old, Kaiser Permanente Study of the Oldest Old; NHANES II Mortality, National Health and Nutrition Examination Survey II Mortality Study; EPESE, Established Populations for Epidemiologic Studies of the Elderly; CARDIA, Coronary Artery Risk Development in Young Adults; ARIC, Atherosclerosis Risk in Communities Study; NHANES III Mortality, National Health and Nutrition Examination Survey III Mortality Study; CHS, Cardiovascular Health Study; WHI, Women’s Health Initiative; Hispanic EPESE, Hispanic Established Populations for Epidemiologic Studies of the Elderly; MESA, Multi-Ethnic Study of Atherosclerosis.
Since exposures of interest are measured differently across cohorts, we employ several techniques to harmonize data and minimize bias that could be introduced by systematic differences in measurement techniques across cohorts. First, we attempt to convert health behaviours to a common unit of measurement. For example, when alcohol consumption is a covariate of interest, we convert different units of measurement (ounces, millilitres) and frequencies (per day, per week) to a common unit of ‘drinks per day’. If this is not possible, such behaviour data are not pooled. Second, for a continuous variable that is measured differently across cohorts (e.g. cholesterol), we compare the distribution of the measure across cohorts to assess for differences in accuracy and/or precision in the measure. We then convert the continuous measure to a cohort-specific Z-score and perform sensitivity analyses using the Z-score value of the variable. Third, we adjust each analysis for cohort, which may help attenuate any confounding due to measurement differences across cohorts.
Outcomes of interest are provided in Table 3. Events were ascertained using each cohort’s specific protocol. Many cohorts used linkage to the National Death Index for underlying cause of death from death certificate data, whereas others adjudicated cause of death by review of medical records and/or autopsies by study investigators. Vital status is nearly 100% complete across all cohorts included in the LRPP dataset and adjudicated as CVD, non-CVD and cancer-related across 18 of 20 cohorts in the LRPP dataset. We have performed external validation of cancer-related deaths within the LRPP by demonstrating that lifetime risk estimates for cancer death using the LRPP are similar to those generated by the nationally representative Surveillance Epidemiology and End Results (SEER) programme of the National Cancer Institute.1 Fatal and non-fatal myocardial infarction events have been adjudicated in 14 of 20 cohorts, using standardized clinical criteria that consisted of at least two of the following: electrocardiographic changes consistent with myocardial infarction; biomarker elevations seen with myocardial necrosis; and/or typical chest pain or ICD-8/ICD-9 codes. Angina was adjudicated in 11 of 20 cohorts. One half of the cohorts adjudicated stroke (haemorrhagic and ischaemic) using clinical criteria (new neurological deficit persisting >24 h consistent with central neurologicla injury without other cause), and/or with ICD-8, ICD-9 codes. Ischaemic and haemorrhagic stroke were differentiated by neuroimaging or autopsy findings when available. Incident atrial fibrillation data from 7 of 20 cohorts are included in the dataset. Incident heart failure, determined by clinical criteria and/or ICD-8, and ICD-9 codes, is available in 13 of the 20 cohorts in the dataset. Given the high variation in validity for recording heart failure using administrative data, we would recommend either restricting analyses to those cohorts that used adjudication criteria to ascertain heart failure events, or adjusting analyses for cohorts that ascertained heart failure by administrative data.2
Data resource use
The predecessor to the majority of atherosclerotic CVD events, arterial atherosclerosis, originates early in life and progresses insidiously over decades before becoming clinically manifest typically in middle to older age. Avoidance of adverse levels of the traditional risk factors, even within the ‘clinically normal’ range, such as elevated blood pressure, elevated BMI, elevated total cholesterol, smoking and diabetes, can substantially reduce CVD risk. Given the gradual progression of atherosclerotic CVD, large-scale data are needed to follow the effect of CVD risk factors and patterns of CVD events across the life course, especially in smaller subgroups, such as African Americans or those who have all-optimal levels of risk factors in young adulthood or middle age. Until recently, there was a paucity of data on long-term CVD risks and patterns of CVD risk across the adult life course. We created the Cardiovascular Disease Lifetime Risk Pooling Project, a pooled dataset with decades of follow-up time, to describe patterns of CVD risk across the life course and to quantify lifetime risk estimates for CVD. We believe that an improved ability to describe CVD risk across the life course can improve risk communication between patients and clinicians, identify new populations at risk for CVD who may benefit from prevention efforts, predict the future population burden of disease and help public heath professionals identify strategies to reduce the burden of CVD.
The large sample size and follow-up intervals across all cohorts included in the LRPP make it an ideal dataset to explore the natural history of patterns of CVD risk development across the life course. Furthermore, due to the detailed, high-quality risk factor and endpoint phenotyping of the cohorts included in the dataset, researchers can explore risks associated with rare risk factor phenotypes or explore uncommon outcomes in a prospective context.
The massive sample size of the dataset with follow-up intervals that span 7 decades create a cohort that provides follow-up information across 40–50 years from mid life to the end of the natural life course. Our group has used the LRPP dataset to describe competing-risk-adjusted risks for CVD events from midlife (index ages 45 years and older) through age 95 years in the context of traditional risk factor burden at different index ages of interest, stratified by White or African American race and by sex. These data demonstrated substantial lifetime risks for atherosclerotic CVD and a strong and consistent association between risk factor levels and aggregate risk factor burden with long-term CVD event rates across different cohorts.3 Furthermore, our group has quantified the duration of healthy longevity and, due to the virtually 100% vital status follow-up, we are able to quantify the duration of survival after an incident CVD event by risk factor burden in mid life. When compared with individuals with more than two major risk factors, individuals with all optimal risk factors at age 45 years lived on average up to 14 years longer free of all forms of CVD, and appear to have had a shorter post-event survival, suggesting a ‘compression of morbidity’4 from CVD in individuals who maintain optimal risk factors through mid life.5
The high-quality, in person, phenotyping of risk factors during serial clinic visits over long follow-up periods also allows for exploration of patterns in risk factor development over time and the associations between different risk factor patterns (notably blood pressure) and cardiovascular events later in life. For example, our group has quantified the long-term CVD risks associated with blood pressure and blood pressure change in mid life by race and sex. Allen et al. observed that individuals who maintained normal blood pressure levels, or decreased their blood pressure to normal levels in mid life, were at lower CVD risk than individuals who developed higher blood pressures from ages 45–55 years. Similar types of analyses, using different risk factor characteristics and endpoints of interest, are possible with this dataset.6
Because of near complete vital status follow-up, the LRPP allows for examination of CVD risks in the context of competing risks for death. Using novel statistical techniques and data from three of the cohorts included in the LRPP, Feinstein et al. demonstrated an earlier onset of CVD events in African Americans than was seen in White cohort participants. However, when competing risks for non-cardiovascular death were accounted for, young African American men were actually at lower risks for incident CVD than White men of the same age group.7 Similarly, Huffman et al. demonstrated that lifetime risks for incident heart failure in African American men were less than White men because of higher competing risks of non-CVD death among African Americans.8
The detailed risk factor phenotype data available allow examination of the associations between common and uncommon cardiovascular risk factor characteristics and cardiovascular outcomes. Furthermore, many of the cohorts included in the LRPP contain detailed data on demographics and health behaviours (diet, exercise, alcohol consumption) that can be accounted for and adjusted in multivariable equations. For example, data from six cohorts included in the LRPP were used to generate 10–20-year risks for CHD events across a broad range of HDL-C values, including in individuals with unusually high (>80 mg/dl) levels of HDL cholesterol. Our analysis suggests a plateau effect in terms of CHD risk reduction for men and women with HDL-C >80 mg/dl. Heretofore detailed, multivariable adjusted estimates of CVD risk were unavailable for individuals with very high levels of HDL-C.9
Strengths and weaknesses
The primary strength of the LRPP is the large sample size and very long follow-up intervals that allow investigators to examine the natural history of CVD risk and CVD risk determinants across the adult life course. Furthermore, the exposure and outcome data are of very high quality. CVD risk factors are determined by in-person examinations and outcomes are adjudicated by physician committee or derived from the National Death Index of Medical Claims. The LRPP dataset allows researchers to use data from participants in specific cohorts containing specific exposures and outcomes of interest to answer relevant a priori research questions. Likewise, data from other cohorts can be added to the LRPP to expand the LRPP to include more data representative of the early life course, or to improve the representation of minorities.
There are acknowledged limitations. Cohort data included in the dataset are obtained from observations made from the late 1940s through 2010. Given the secular trends in risk factors and development of medical therapies for prevention of CVD, the possibility of birth cohort effects are substantial. However, although substantial secular trends in CVD risk factors has been observed over the past 60 years, the associations between risk factors and CVD events has been remarkably consistent until very recently when widespread antihypertensive and statin use for primary prevention may be altering the ‘natural history’ of these associations. Regardless, we are careful to pool data only after similar patterns of association between exposures and outcomes are observed across different cohorts; furthermore, multivariable models are adjusted for cohort.3
The LRPP contains data from US domestic cohorts exclusively. Given the high prevalence of CVD risk factors, this may limit the generalizability of these findings to other international or global populations. However, LRPP investigators are interested in expanding the cohort to include data from other nations, in an effort to increase the generalizability of the findings and the diversity of the sample included in the dataset.
Unfortunately, African Americans and other US minority groups were not well-represented in community-based CVD cohort studies until the mid to late 1980s. LRPP investigators are currently working to update and expand the dataset to increase the representation of minority groups (African American, Latino/Hispanic, Asian American and American Indian/Alaska Native populations) in the LRPP dataset.
Data resource access
Details regarding access to LRPP data for research are available on our website [http://www.preventivemedicine.northwestern.edu]. Enquiries regarding use of the LRPP for specific research studies are welcomed. For further information, please contact John T Wilkins at the Department of Preventive Medicine, Northwestern University Feinberg School of Medicine [j-wilkins@northwestern.edu].
Profile in a nutshell
The Cardiovascular Disease Lifetime Risk Pooling Project (LRPP) was set up to quantify long-term risks for cardiovascular disease (CVD) and to examine patterns of CVD development over the adult life course.
The LRPP is an individual-level pooled dataset of demographics, CVD risk factorsand CVD events from 20 distinct US community-based cohorts. It includes over 225 000 unique individuals with approximately 3–800 000 person*years of follow-up data collected over seven decades (1948–2010).
The LRPP dataset includes extensive information (including many repeated measures) on traditional risk factors such as age, sex, race, height, weight, blood pressure, traditional lipid fractions, medication use, fasting glucose, diabetes status, health behaviours and smoking status. All risk factors were measured in person using standardized methods during examination visits. CVD outcomes in the LRPP dataset include: non-fatal myocardial infarction, coronary heart disease death, stroke, incident heart failure, CVD death, cancer death and total mortality. Outcomes were ascertained using the National Death Index, Medicare claims and/or direct adjudication of medical records by trained physicians.
The sample size and follow-up of individuals included in the LRPP allow for an examination of patterns in CVD events and risk factors across the life course and can be used to calculate short-term and lifetime risk estimates for CVD events. Such information can estimate population burden of CVD, identify new populations at risk for CVD and examine patterns of CVD in the context of competing risks for other causes of death.
Funding
The LRPP was supported in its inception by the National Institutes of Health/National Heart, Lung, and Blood Institute [grant number R21 HL085375]. It is currently supported by funds from the Northwestern University Feinberg School of Medicine.
Conflict of interest: M.D.H. receives grant support from the National Heart, Lung and Blood Institute (NIH), National Cancer Institute (NIH), and World Heart Federation (via unrestricted educational grants from Astra Zeneca and BoehringerIngelheim).
References
- 1.Gawron A, Hou L, Ning H, Berry JD, Lloyd-Jones DM. Lifetime risk for cancer death by sex and smoking status: the lifetime risk pooling project. Cancer Causes Control 2012;23:1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One 2014;9:e104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med 1980;303:130–35. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012;308:1795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen N, Berry JD, Ning H, Van Horn L, Dyer A, Lloyd-Jones DM. Impact of blood pressure and blood pressure change during middle age on the remaining lifetime risk for cardiovascular disease: The Cardiovascular Lifetime Risk Pooling Project. Circulation 2012;125:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: The Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis. Circulation 2012;126:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among White and Black Americans. J Am Coll Cardiol 2013;61:1510–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkins JT, Ning H, Stone NJ, et al. Coronary heart disease risks associated with high levels of HDL cholesterol. J Am Heart Assoc 2014;3:e000519. [DOI] [PMC free article] [PubMed] [Google Scholar]