Abstract
The study objective was to assess age-related changes in glutathione (GSH) adaptive response to cigarette smoke (CS) exposure. Older cigarette smokers show a decline (67%) in lung epithelial lining fluid (ELF) GSH and a 1.8-fold decreased GSH adaptive response to cigarette smoking with a concomitant elevation (47%) of exhaled nitric oxide compared with younger smokers. In order to isolate the changes in tissue GSH from other age-related effects, pharmacological inhibition of the rate limiting step in GSH synthesis was employed to examine the lung’s response to CS exposure in young mice. The γ-glutamylcysteine ligase inhibitor l-buthionine–sulfoximine (BSO) was administered in the drinking water (20 mM) to decrease by half the in vivo GSH levels to those found in aged mice and humans. Mice were then exposed to CS (3 h/day) for 5 or 15 days. Biochemical analysis of the ELF and lung tissue revealed an inhibition of the CS-induced GSH adaptive response by BSO with a concurrent increase in mixed protein–GSH disulfides indicating increased cysteine oxidation. The prevention of the GSH adaptive response led to an increase in pro-inflammatory cytokines present in the lung. Airspace enlargement is a hallmark of lung emphysema and was observed in mice treated with BSO and exposed to CS for as little as 15 days, whereas these types of changes normally take up to 6 months in this model. BSO treatment potentiated both lung elastase and matrix metalloproteinase activity in the CS group. These data suggest that age-related decline in the GSH adaptive response can markedly accelerate many of the factors thought to drive CS-induced emphysema.
Keywords: antioxidants, agents, inhalation toxicology, respiratory toxicology, oxidative injury, systems toxicology
Smoking cigarettes places an incredible oxidative burden on the lung, yet due to the endogenous protective mechanisms individuals can tolerate smoking for decades without serious ailments. Only ∼25% of chronic smokers are diagnosed with chronic obstructive pulmonary disease (COPD) around the age of 60 (Buist et al., 2007). COPD is a lung disease associated with smoking where nearly 90% of cases occur in current or former smokers (Snider, 1989). Despite the high occurrence of COPD in chronic smokers and decades of research, the biochemical changes that protect the lung prior to the development of lung disease are not well understood.
The underlying adaptive changes in the lung that fail in chronic smokers with the subsequent development of COPD are not well defined. Cigarette smoke (CS) is both a xenobiotic and oxidative challenge to the lung. The lung responds to this challenge with adaptive responses that include up regulation of glutathione (GSH) antioxidant defenses. Gene upregulation due to CS exposure occurs largely from nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activation. Nrf2 controls the expression of many xenobiotic and oxidant responsive genes including the rate-limiting step in de novo GSH synthesis, γ-glutamylcysteine ligase (EC 6.3.2.2) (GCL) (Motohashi and Yamamoto, 2004). The GSH adaptive response consists of a coordinated response between GSH synthesis, utilization, recycling, and transport into the lung epithelial lining fluid (ELF) (Gould and Day, 2011). Elevation in lung ELF GSH levels is thought to act as a defense mechanism to limit the damaging effects of chronic smoking.
Much of the CS-related research has focused on targeted gene knockout models (Churg et al., 2011a; Wright and Churg, 2010). This general approach gives insight into which genes are involved in protecting the lung against CS; however, they do not give any insight into how COPD develops. Many studies of gene-related targets have focused on enzymes or pathways related to antioxidant defense; most notable would be studies showing that Nrf2−/− mice are highly susceptible to CS-induced emphysema (Rangasamy et al., 2004). Nrf2 controls hundreds of different gene products (Ishii et al., 1999; Kong et al., 2001; Wild and Mulcahy, 2000) and many are antioxidant or anti-inflammatory in nature (Motohashi and Yamamoto, 2004). This makes it hard to differentiate between those that are important and those that are less important in protecting against CS. Others have taken an approach looking at single gene targets that are modified (either increased or decreased) in COPD patients (Bracke et al., 2006; Hwang et al., 2011; Mercado et al., 2011). While this approach has some connection with the disease in humans, it is unclear whether many of these markers are a cause of or consequence of the disease.
It is well established that aging is an independent risk factor for the development of COPD (Fukuchi, 2009). It is also known that some antioxidant defenses decline with age in both rodents and humans (Ross, 1969; Sekhar et al., 2011) and could contribute to increased inflammatory cytokines present in the airways and alveolar spaces. GSH is a robust and important antioxidant used by the lung to protect against damage (Gould and Day, 2011). GSH levels drop sharply in humans around the age of 45 (Jones et al., 2002) and this shortly proceeds the age at which COPD develops in chronic smokers. Previous studies in mice have shown that aging causes as much as a 50% fall in basal GSH levels in the ELF of the lung and aging also impairs GSH adaptive responses to CS resulting in increased inflammation and oxidative stress (Gould et al., 2010). This study confirmed these findings in human smokers and sought to model the lower GSH levels found in the aged mice utilizing young mice. The use of young mice isolates the involvement of GSH from other deficiencies known to occur with age. l-buthionine-sulfoximine (BSO) disrupts GSH synthesis by irreversible inhibition of GCL producing similar changes in ELF and tissue GSH levels seen in aged mice and humans (Watanabe et al., 2003). In addition, the BSO-treated young mice developed accelerated oxidative stress, inflammation, and airspace enlargement that are all hallmarks of CS-mediated COPD.
MATERIALS AND METHODS
Ethics statement
All human experiments were approved by the National Jewish Institutional Review Board. All animal experiments were approved by the National Jewish Health Animal Care and Use Committee.
Human subject studies
Healthy smoker and nonsmoker volunteers were recruited to participate in this research study with informed consent and approval by the National Jewish Health IRB. Inclusion criteria used in smoker subject recruitment included: (1) young adult subjects between the ages 18 and 30 years old and older subjects between the ages 50 and 70 years old with >3 pack years of smoking; (2) a post-bronchodilator FEV1 >60% predicted; (3) ability to perform and adhere with study protocol; and (4) had ability to provide informed consent. Exclusion criteria used in smoker subject recruitment included: (1) asthma demonstrated by positive methacholine challenge and >12% reversal of FEV1 with bronchodilator or other co-morbid lung disease other than COPD; (2) hypoxemia as defined by PaO2 < 55 mmHg or SpO2 < 88% of room air; (3) COPD exacerbation within the last 6 weeks; (4) an upper or lower respiratory tract infection within the last 6 weeks; (5) not currently smoking; (6) unstable angina, myocardial infarction, angioplasty/stenting, or bypass surgery within the last 6 months; (7) abnormalities of platelet count, prothrombin time, or partial thromboplastin time; and (8) pregnancy or nursing. Inclusion criteria used in normal healthy subject recruitment included: (1) young adult subjects between the ages of 18–30 years old and older subjects between the ages of 50 and 70 years old with no smoking in the last year and <1 pack/year total; (2) no respiratory or cardiac symptoms and no physician diagnosed cardiac or respiratory disease; (3) not taken medications within 24 h of testing; and (4) normal pulmonary function upon testing. Exclusion criteria used in healthy control subject recruitment included: (1) evidence on history, physical examination, or screening spirometry of lung disease or other disease that would affect participation in study; (2) a PC20 < 25 mg/ml; and (3) pregnancy or nursing.
Subjects underwent bronchoalveolar lavage (BAL) where the bronchoalveolar lavage fluid (BALF) was spun down and cell-free supernatants were used to determine total GSH and urea levels as described below. In addition, subjects provided a blood sample by venous puncture that was used to determine plasma urea levels. BALF GSH levels were converted to ELF levels by using the urea dilution method (Chinard, 1992). Exhaled nitric oxide (eNO) on-line measurements were obtained on all human subjects using a rapid response chemiluminescent analyzer (model 280, Sievers Instrument) as previously described (Silkoff et al., 1999).
Animals and CS exposure
Two-month-old male C57B/6 mice (Jackson laboratory) were exposed to CS from Kentucky reference cigarettes (3R4F, University of Kentucky) for 3 h/day for 5 or 15 days using a TE-10 smoking system (Teague Enterprises) as previously reported (Kariya et al., 2008). The mice that were exposure for 15 days were rested over the weekends. Briefly, an FTC method of puffing cigarettes for 2 s once a minute at a volume of 35 cm3 was utilized to generate side stream and mainstream CS. The TE-10 smoking system generated an environmental tobacco smoke by mixing mainstream and side stream CS. This CS was diluted with air to maintain a total suspended particulate matter average of 80 mg/m3 and carbon monoxide levels below 300 ppm. Mice were anesthetized with pentobarbital and euthanized by cardiac exsanguination 16 h after CS exposure. BAL was performed by cannulation of the trachea and introducing and gently aspirating 2 consecutive 750 µL rinses of cold isotonic 50 mM potassium phosphate solution, pH 7, using a 1-ml syringe. The returned volumes were measured and pooled as the BAL fluid. BAL cells were removed from the BAL fluid by centrifugation at 2000 × g for 10 min.
Treatment with l-BSO
Mice were administered BSO (Sigma) in the drinking water at a final concentration of 20 mM for 7 days prior to the CS exposure and continually throughout the CS exposure. This BSO exposure has been previously reported to decrease in vivo GSH levels around 50% in mice (Sun et al., 1985). Two sets of studies were performed that looked at BSO effects after 5 or 15 days of CS exposure. BSO has been shown to be stable in drinking water for at least 14 days without significant loss (Watanabe et al., 2003); therefore, to prevent any loss during the experiment the BSO supplemented drinking water was changed at day 10 for the 15-day exposure time.
Measurement of GSH, protein bound GSH (PSSG), and γ-GCL activity
Total GSH was measured spectrophotometrically in the BALF and lung tissues as previously described (Gould et al., 2009). Briefly, samples or standards were incubated in the presence of 5,5′-dithio-bis(2-nitrobenzoic acid), GSH reductase and the reaction started with NADPH. The absorbance was read at 412 nm for 5 min on a platereader (Spectromax 340PC, Molecular Devices). BALF measurements are corrected by a dilution factor obtained by the urea method (BUN, Teco diagnostics) (Chinard, 1992). Tissue values are normalized to protein content.
The measurement of protein bound GSH (PSSG) was performed as previously described (Han and Han, 1994; Priora et al., 2010) with the following modifications. Briefly, lung tissue lysate proteins were precipitated with chilled 3.3% meta-phosphoric acid (MPA) and collected by centrifugation at 20 000× g for 10 min. Protein pellet was resuspended in 1% MPA and washed 6 times to remove free GSH and GSSG. The protein pellet was then resuspended in PBS and PSSG was reduced with 2 mM tris(2-carboxyethyl)phosphine for 30 min. Proteins were pelleted with 7.2% sulfosalicylic acid and removed by centrifugation at 20 000× g for 20 min. GSH was quantified in the supernatant fraction using HPLC with electrochemical detection as previously described (Brechbuhl et al., 2010).
GCL activity was measured in lung homogenates as previously described (White et al., 2003) with the following modifications. Briefly, lung lysates were split into 2 groups where 1 group was used for baseline and 1 group for reaction measurements. The reaction buffer contained 10 mM ATP, 5 mM l-glutamic acid, 0.5 mM EDTA, 5 mM sodium borate, 0.5 mM serine, 10 mM MgCl2, and reaction was started with 0.5 mM l-cysteine. The l-cysteine was not added to baseline group during the 10-min incubation time. The reaction was stopped after 10 min with 50 mM sulfosalicylic acid and precipitated proteins removed by centrifugation. Supernatant GSH was derivatized with 10 mM 2,3-napthalenedicarboxoaldehyde (NDA) at pH 12.5 for 30 min in the dark. Fluorescence intensity was measured at excitation wavelength of 455 nm and emission wavelength of 528 nm with a fluorescence platereader (Synergy 2, BioTek). GCL activity was calculated as the difference in GSH concentration between the reaction and baseline samples per minute normalized to the protein content.
Cytokine analysis
Cytokines were measured in lung tissue homogenates using a mouse pro-inflammatory multiplex 7-plex assay kit according to the manufactures recommendations (Mesoscale Discovery) utilizing a Sector Imager 2400 (Mesoscale Discovery). Cytokines measured and lower levels of detection were IL-1β (0.75 pg/ml), IL-12p70 (35 pg/ml), IFN-γ (0.38 pg/ml), IL-6 (4.5 pg/ml), KC (3.3 pg/ml), IL-10 (11 pg/ml), and TNF-α (0.85 pg/ml). Only IL-1β and KC were detectable in lung tissue homogenates above their lower level of detection of the assay. Cytokine concentrations were estimated based on an 8-point standard curve of known concentrations.
Elastase and matrix metalloproteinase (MMP) lung tissue activities
Elastase activity in lung lysates was measured using the EnzCheck elastase assay (Invitrogen). Briefly, lung elastase-like activity was determined using BODIPY-FL-labeled DQ elastin conjugate that upon enzymatic digestion yields highly fluorescent fragments that were quantitated over 20 min at 37°C. Activity is expressed as change in relative fluorescent units at excitation wavelength of 455 nm and emission wavelength of 528 nm (Synergy 2, BioTek) per hour normalized to the protein content.
MMP activity in lung lysates was measured using the methoxycoumarin conjugated Mca-PLGL-Dpa-AR-NH2 MMP peptide substrate (R&D Systems). This is a good substrate for MMP1, 2, 7, 8, 9, 12, 13, 14, 15, 16, and cathepsin D and E. Activity is expressed as change in relative fluorescent units at excitation wavelength of 320 nm and emission wavelength of 405 nm (Synergy 2, BioTek) per hour normalized to the protein content.
Histology
A separate set of 6 mice/group was used for lung histology. Lungs were intubated and fixed with 10% buffered formalin/0.2% glutaraldehyde at a pressure of 20 cm of H2O. Fixed lung tissue volume was measured by water displacement and random lung slices were taken and embedded in paraffin blocks and sections were stained with H&E. Mean linear intercept is a commonly used parameter to measure the mean-free distance in the air spaces that is elevated in emphysema. A total of 10 randomly selected fields per mouse lung under 100× magnification were evaluated using grids overlays created by an automated lung imaging system (Cast, Olympus, Denmark). The mean linear intercept (Lm) was derived from the total length of all the lines divided by the number of intercepts, and averaged from each of the 10 fields taken as previously described (Vlahovic et al., 1999).
Statistical analysis
All data are represented as the mean ± standard error of the mean. A 2-way analysis of variance was performed with a Holm-Sidak correction for multiple comparisons post-test using Prism 6 software (GraphPad), groups that differ significantly (P < .05) are delineated by different letters.
RESULTS
Loss of Chronic CS-Induced Adaptive ELF GSH Response With Age in Humans
Previous studies by our group have demonstrated a loss of CS-induced adaptive GSH responses with age in mice (Gould et al., 2010). This observation was followed up in young and older human cigarette smokers. Demographics of the human subjects are described in Table 1. Young subjects were defined as 18–30 year olds and older subjects were defined as 50–70 year olds. Cigarette smoking history among the subjects varied from 3 to 5 pack years in young smokers to 35–50 pack years in old smokers. There were no differences in the % predicted forced expiratory volumes over 1 min (FEV1) among the cohorts. ELF steady-state levels of total GSH were determined in young and older subjects and between smokers and nonsmokers (Fig. 1A). A similar decline in ELF GSH levels was observed with age as previously reported for mice where older nonsmoker subjects had a 67% decrease compared with young nonsmoker subjects. Young smokers exhibited a robust response to cigarette smoking with a 3-fold increase in ELF GSH levels. Older smokers exhibited ELF GSH levels comparable to young nonsmokers but had a 1.8-fold decrease in ELF GSH adaptive responses compared with young smokers.
TABLE 1.
Human Subject Demographics
| Young NonSmokers | Young Smokers | Older Nonsmokers | Older Smokers | P Value | |
|---|---|---|---|---|---|
| Age (years) | 25 ± 1 a | 27 ± 1a | 57 ± 2b | 57 ± 4b | .0001 |
| Gender (M/F) | 1/2 | 2/1 | 2/1 | 3/0 | – |
| Pack years | N/A | 4 ± 0.4a | N/A | 38 ± 6b | .014 |
| FEV1 (liter) | 3.7 ± 0.2 | 3.8 ± 0.3 | 3.2 ± 0.7 | 3.5 ± 0.3 | ns |
| % predicted FEV1 | 95 ± 6 | 94 ± 6 | 95 ± 5 | 97 ± 12 | ns |
| FVC (liter) | 4.7 ± 0.6 | 4.5 ± 0.3 | 4.9 ± 0.9 | 5.0 ± 0.1 | ns |
| % predicted FVC | 101 ± 5 | 91 ± 6 | 112 ± 7 | 108 ± 7 | ns |
| FEV1/FVC | 0.80 ± 0.05a | 0.86 ± 0.02a | 0.66 ± 0.03b | 0.70 ± 0.05b | .024 |
Data are expressed as the mean ± SD and groups that differ significantly (P < .05) are delineated by different letters.
N/A: not applicable
FIG. 1.
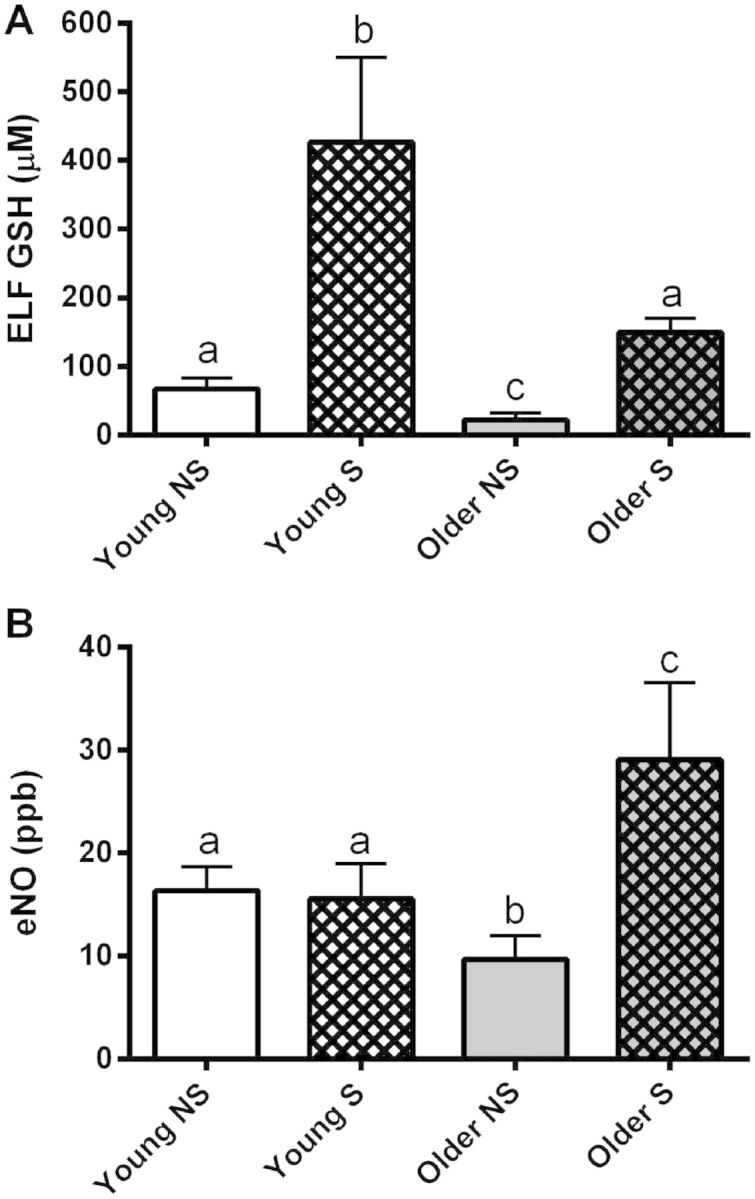
A, Age-related decline in basal and cigarette smoker ELF GSH adaptive responses in smokers (S) with comparison to nonsmokers (NS); B, Concomitant increase in eNO in old smokers. Young subjects are 18–30 years old and older subjects are 45–65 years old. Bars with different letters are statistically different from one another (P < .05) (n = 3).
Elevated eNO levels have been used clinically in asthma as a measure of airway inflammation (Barnes et al., 2010; Silkoff et al., 1998). eNO was assessed in young and older smokers and nonsmokers (Fig. 1B). Both young smokers and nonsmokers had similar eNO levels that differed from the older smokers and nonsmokers. However, the older nonsmokers had about a 40% decrease in their eNO levels compared with young subjects, and older smokers had about an 80% increase in their eNO levels compared with young smokers.
BSO Prevents CS-Induced GSH Adaptive Response in the Lung
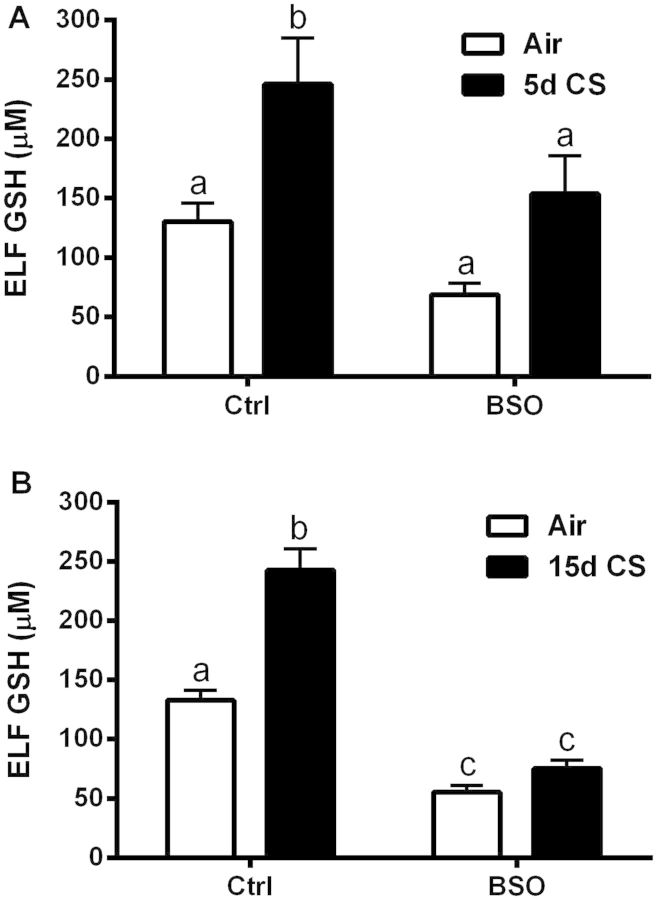
Based on previous reports, the in vivo depletion of GSH with 20 mM BSO delivered in tap water stabilizes within 7 days resulting in GSH depletion of the BALF to roughly half of the basal levels (Sun et al., 1985). The lung maintains a basal concentration of GSH in the ELF at 100 µM, while the BSO treatment reduced basal levels of ELF GSH to roughly 50% for both the shorter 5 days CS exposure (Fig. 2A) and the longer 15 days CS exposure (Fig. 2B). Furthermore, congruent with previous studies (Gould et al., 2011b), ELF GSH was significantly increased in response to both the 5 and 15 days CS exposures while BSO blocked CS-induced GSH adaptive responses.
FIG. 2.
l-BSO prevents CS-induced ELF GSH adaptive response. ELF GSH levels in mice exposed to CS for 5 (A) or 15 days (B) after treatment with tap water or BSO (20 mM) supplemented tap water. Bars with different letters are statistically different from one another (P < .05) (n = 5–10).
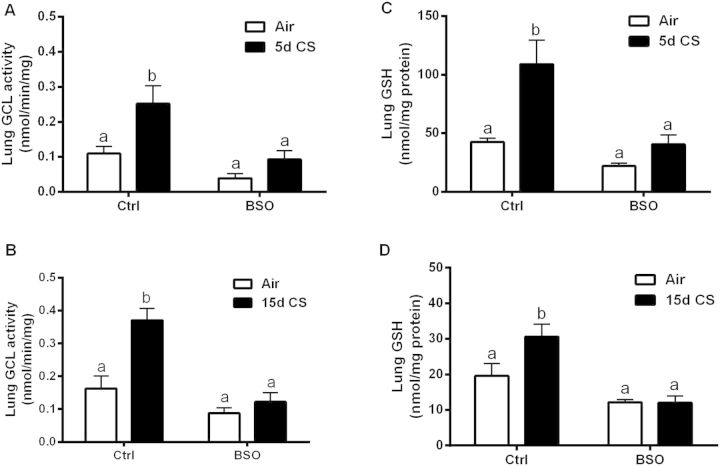
The lung epithelium is responsible for the transport of GSH into the apically located ELF. GSH in the ELF is the first line of defense against the large number of reactive species found in CS. The rate limiting step in GSH synthesis is carried out by GCL (Davis et al., 1973) and GCL tissue activity is an indicator of the effectiveness of BSO inhibition. CS increased the activity of GCL during the 5 (Fig. 3A) and 15 days (Fig. 3B) exposures, while BSO prevented the CS-induced increase in GCL activity. CS produced a significant increase in lung tissue GSH levels with both the 5 (Fig. 3C) and 15 days (Fig. 3D) exposures. Interestingly, BSO was much more effective at inhibiting the CS-induced increase in both GSH levels and GCL activity in the longer 15 days exposure as compared with the shorter 5 days exposure.
FIG. 3.
l-BSO inhibits CS-induced adaptive response in the lung. GCL activity levels in the tissue of mice treated with or without BSO and exposed to filtered air (open bars) or CS (closed bars) for 5 (A) or 15 days (B). Resulting lung GSH levels in both 5 (C) and 15 days (D) CS exposures. Bars with different letters are statistically different from one another (P < .05) (n = 5–10).
CS-Induced Protein Oxidation Is Potentiated by GSH Depletion
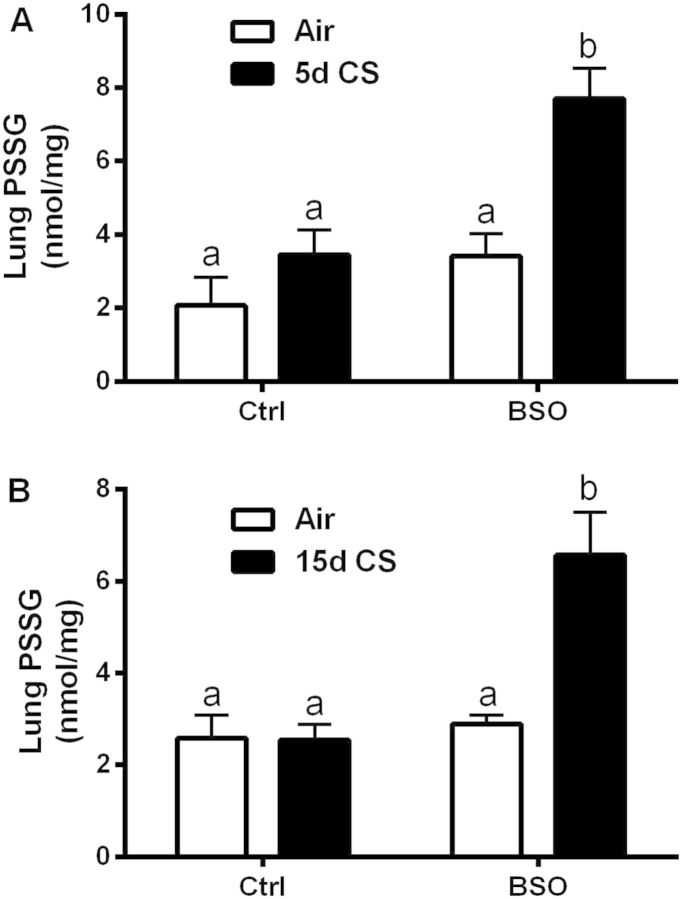
A primary function of GSH is to maintain redox homeostasis that minimizes the oxidation of critical cysteine residues in cellular proteins. There are a number of proteins that have critical functional cysteine residues that must be maintained in the reduced form for protein functionality and increased oxidation can result in loss of protein function (Wang et al., 2011). GSH can form mixed disulfides with proteins (PSSG), effectively creating a pool of protein bound GSH. Under normal conditions, the pool of PSSG is in low abundance. Lung tissue PSSG levels did not increase over the basal levels in either a 5 (Fig. 4A) or 15 days (Fig. 4B) exposure to CS alone. BSO treatment potentiated the levels of PSSG with CS in both 5- and 15-day exposures. Interestingly, levels of PSSG are not different with BSO alone, indicating that this pool of GSH is relatively insensitive to GSH depletion.
FIG. 4.
Decreased GSH levels results in increased protein oxidation. Levels of mixed protein–GSH disulfides (PSSG) were quantified in both the 5 (A) and 15 days (B) CS exposure groups. Bars with different letters are statistically different from one another (P < .05) (n = 5–10).
CS-Induced Lung Inflammation Is Potentiated by GSH Depletion
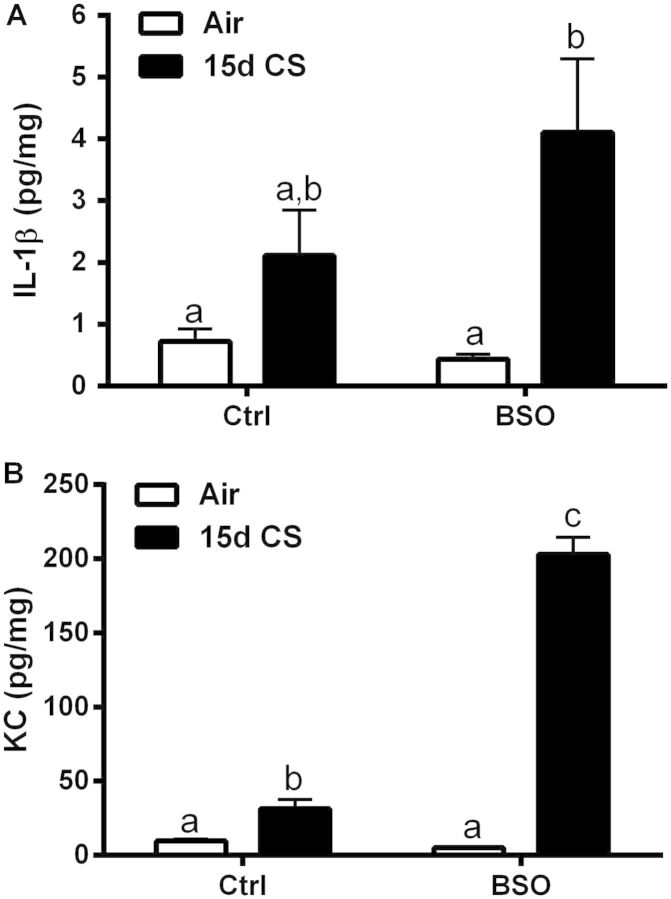
Persistent low-grade lung inflammation is a hallmark of COPD even after the cessation of smoking. This indicates dysfunction in the regulatory mechanisms that control inflammatory cytokine release in COPD patients. Recently, decreased GSH levels have been shown to exaggerate the inflammatory cytokine response to CS, while increased GSH levels have been shown to mediate the suppression of the cytokine levels (Gould et al., 2010, 2011a). Consistent with these reports, the BSO model of CS exposure showed increased levels of inflammatory cytokines present in the lungs of mice treated with BSO and exposed to CS for 15 days (Figs. 5A and 5B). Only IL-1β and KC were detectable in lung tissue of the panel of 7 cytokines assessed. There was a trend for IL-1β levels to be increased in the CS exposed group which was augmented 94% in the BSO/CS group. There was a significant 2.6-fold increase in lung tissue KC levels with CS exposure that was significantly potentiated over 20-fold in the BSO/CS group. BSO treatment alone had no effect on lung tissue IL1-β or KC levels compared with air controls. These data indicate that GSH plays a role in modulating basal cytokine tone and that diminished GSH adaptive responses can contribute to the exaggerated inflammation.
FIG. 5.
l-BSO potentiates CS-induced inflammatory cytokines in the lung. A panel of inflammatory cytokines (IL-1β, KC, INF-γ, IL-12p70, IL-6, TNF-α, and IL-10) in lung tissue was analyzed after 15 days CS exposures. Only IL-1β and KC (B) levels were above the level of detection. Bars with different letters are statistically different from one another (P < .05) (n = 5–10).
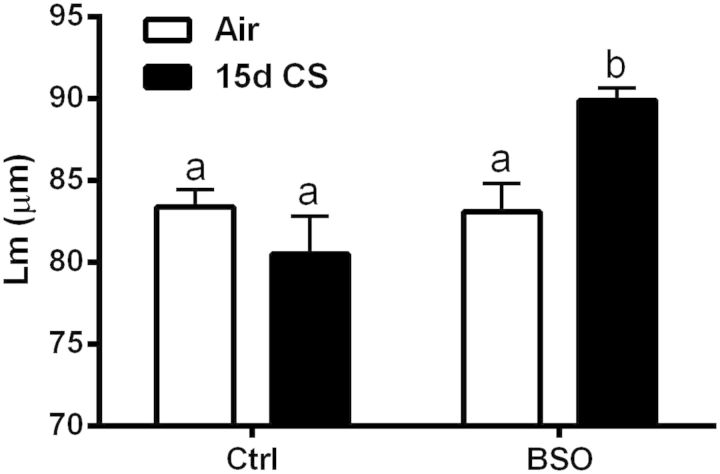
GSH Depletion Induces Airspace Enlargement in CS Exposed Mice
A primary outcome measure of CS-induced lung damage is changes in lung morphometry, as measured by increases in the mean linear intercept (Lm). Histological analysis of the lung revealed essentially no pathology in the alveolar spaces of the filtered air controls, BSO-treated mice or mice exposed to CS for 15 days (data not shown). However, upon examination of the BSO-treated mice exposed to 15 days CS exposure there is evidence of mild enlargement of the alveolar spaces as determined by mean linear intercept (Lm) measurements showing a significant increase in the Lm in the BSO + CS mice, with no changes in any of the other groups (Fig. 6).
FIG. 6.
GSH depletion drives CS-induced alveolar enlargement. Quantification of the alveolar enlargement using mean liner intercept (Lm) measurements were performed in the filtered air group, l-BSO group, CS only group, and the BSO/CS interaction group. Bars with different letters are statistically different from one another (P < .05) (n = 6).
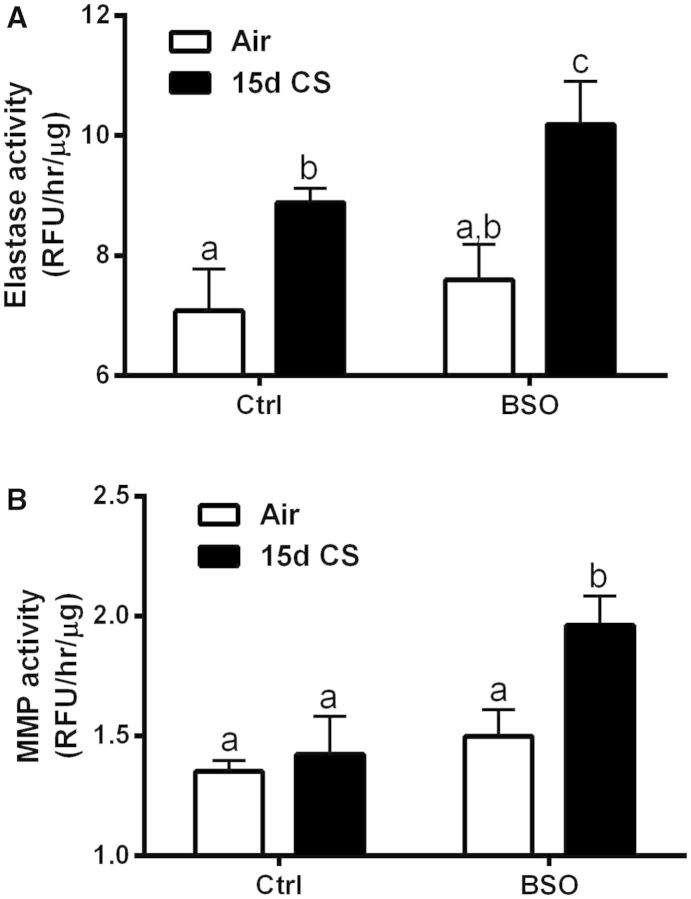
Proteases have been utilized in their purified forms to induce enlargements in alveolar spaces (Kaplan et al., 1973) and increases in the activities of various MMP isoforms and elastase have been linked to the development of COPD (Churg et al., 2011b). To further support our findings of increased Lm changes in the 15 days BSO/CS group, 2 protease markers of elastase and MMP activity were measured. Lung elastase activity was increased with CS in both the untreated and BSO-treated mice (Fig. 7A); however, BSO treatment had a potentiating effect on the elastase activity in both the air and CS exposed mice. On the other hand, MMP activity was only increased in the BSO/CS exposure group (Fig. 7B). This further indicates that an imbalance in proteases exists in this model and taken in conjunction with the changes in Lm measurements indicates that this imbalance favors the degradation and destruction of the alveolar surface area even with a relatively short CS exposure.
FIG. 7.
GSH depletion drives extracellular matrix breakdown. Potentiation of CS induced increases in lung elastase (A) and MMP (B) activity by l-BSO depletion of GSH. Bars with different letters are statistically different from one another (P < .05) (n = 5–10).
DISCUSSION
Insights into the pathophysiologic development of COPD are difficult to study due to the lag time between chronic exposures and the development of lung disease. This study has demonstrated that young human smokers have a robust steady-state increase in their ELF GSH levels which is greatly diminished in older smokers and mirrors what has been reported in animal models of CS exposure (Gould et al., 2010). This study also characterizes a mouse model in which changes in lung morphology, ie, increases in alveolar spaces, have occurred with relatively short exposures to CS once the endogenous GSH adaptive mechanisms are compromised as observed in aged animals (Gould et al., 2010). The pathogenesis of COPD is typically characterized by 3 main factors; oxidative stress, protease imbalance, and inflammation (Fischer et al., 2011). The model presented herein has evidence of all 3 pathogenic trends seen in COPD.
The process of aging is an independent risk factor for COPD (Karrasch et al., 2008). A number of processes are dysfunctional in the aged lung, include mucociliary clearance, inflammation, and immunosenescence (Lowery et al., 2013) that could be contributing factors in developing COPD. There are even theories that airspace enlargement is a natural process and would be a common phenotype if we lived longer lifespans (Ito and Barnes, 2009). This concept has been further elaborated upon with the view that COPD may be a form of accelerated lung aging that involves a number of process that included chronic ROS and mitochondrial dysfunction (Mercado et al., 2015; Thannickal et al., 2015). Aging is also associated with a decrease in GSH levels by as much as half in rodents (Gould et al., 2010) and man (Jones et al., 2002). The present studies suggest that steady-state ELF GSH levels are diminished with age and older smokers have impaired ELF GSH adaptive responses to CS with corresponding increases in inflammation as illustrated by elevated eNO levels. The present studies corroborate other reports of decreases in airway GSH levels (Drost et al., 2005) and altered systemic GSH/GSSG redox status in COPD patients with exacerbations (Nicks et al., 2011). Our study is limited by a small sample size and needs to be confirmed in a larger study looking at direct temporal changes in response to smoking a cigarette. Our data may implicate a compromised endogenous GSH adaptive mechanism in some smokers that may no longer afford the protection needed to prevent lung damage with continued smoking.
The aim of this study was to model the effect of aging on the GSH adaptive response to CS exposure irrespective of other age-associated factors. One can achieve this by using young mice and alter GSH by either pharmacologic or genetic approaches. Both GCLM and GCLC knockouts have been generated, but GCLC knockouts are embryonic lethal. A limitation of the genetic GCLM knockout is a compensatory upregulation of other genes which can compensate for the low GSH levels (Haque et al., 2010; Johansson et al., 2010). Thus, we have used a potent and specific inhibitor of GCL, BSO, to decrease GSH levels. The use of a pharmacological inhibitor allows one to titrate the dose and time to achieve different degrees of inhibition and consequently different GSH levels. The dose and time of BSO to titrate a 50% decline in lung GSH levels was chosen to model the diminished GSH levels observed in aged mice and humans. Although our focus was on the pulmonary system, BSO has been shown to decrease other tissues to levels similar to those reported in aged mice. It is very likely that extrapulmonary effects of altered steady-state levels of GSH may have important impact on the lung’s ability to respond to oxidative challenge. The resulting BSO model of aging provides the perspective of lower GSH adaptive responses, but without other associated effects of aging like decreased metabolism, protein synthesis, and gene expression (Guo et al., 1993).
There is ample evidence of defective cellular processes that have been observed with COPD. However, it is unclear if these are the result or a cause of the disease. While it is extremely difficult to answer this question, present data suggest that changes in GSH levels may occur in advance of COPD. One of the most interesting facets of COPD is that not every smoker develops COPD and it is only estimated that around 25% of smokers develop COPD (Lokke et al., 2006). Polymorphisms in the GCLC gene, responsible for the catalytically active subunit for GSH synthesis, have been shown to accelerate lung function decline (Siedlinski et al., 2008). One could speculate that it is the rate of decline which puts an individual smoker at increased risk for the development of COPD. This is supported by the changes in GSH that are reported in COPD subjects with exacerbations (Drost et al., 2005; Nicks et al., 2011). The BSO model exhibits a GSH decline that is very abrupt and rapid. BSO also disrupted the lung’s ability to upregulate GSH through GSH adaptive mechanisms that is also important in preventing major lung damage from chronic cigarette smoking. This inability to respond to environmental agents is exhibited in the current model with the blockade of the GSH adaptive response, but it has also been seen in other models of inhalation toxicity. Mice that are heterozygous for the GCLM deficiency were found to have normal GSH levels but an impaired response to diesel exhaust particulates which resulted in increased sensitivity (Weldy et al., 2011). Taken together these findings suggest that it is both GSH levels and the ability to increase those levels in response to stimuli that are important aspects for protecting the lung against the damaging effects of CS. The formation of PSSG alone can indicate either a pathological or adaptive mechanism. PSSG can function to protect susceptible cysteine residues from oxidative inactivation, although presumably when acting as an adaptive mechanism enzymatic function could be negatively affected. The increased lung protein glutathionylation (PSSG) seen in the BSO/CS model differs from previously reported studies in young mice exposed to CS for 4 weeks that reported a decrease in lung PSSG levels (Kuipers et al., 2012b). This discrepancy may be due to differences in the smoke exposures or the importance of basal GSH levels for glutaredoxin (Grx) activity which regulates steady-state PSSG levels. It is interesting that our BSO/CS model produced similar changes in PSSG as seen in Grx KO mice exposed to CS (Kuipers et al., 2012a). Whether CS-induced PSSG formation is a pathological consequence of the shifting oxidative environment or it is an adaptive mechanism in the lung is not clear.
The BSO model also provides a uniquely sensitive model to CS exposure, which actually necessitated a lower CS particulate concentration during the exposure (∼80 mg/m3), in comparison to the upward trend in the literature of vastly higher levels of smoke (∼300 mg/m3) to achieve evidence of lung inflammation and airspace enlargement. The other common occurrence with CS-induced pathology is simply the amount of time it takes for changes in the lung to develop. Typical CS exposures needed to develop increases in Lm are generally greater than 6 months (∼120 days of high CS particulate exposure), versus our model which has taken only 15 days, a reduction by at least 87%. This is the first reported instance of changes in lung morphology under such an acute exposure to CS without direct damaging agents (LPS, NO, H2S, or proteases) administered to accelerate the alveolar destruction. Furthermore, one could theoretically utilize such a model to examine the biochemical changes in the lung that occur in the time leading up to lung pathology without the need for excessively large sets of animals. Our model is a paradigm shift to examining the changes in the lung that lead to COPD and potentially a more suitable avenue for understanding the disease development and testing of pharmaceutical approaches to delay or prevent COPD development.
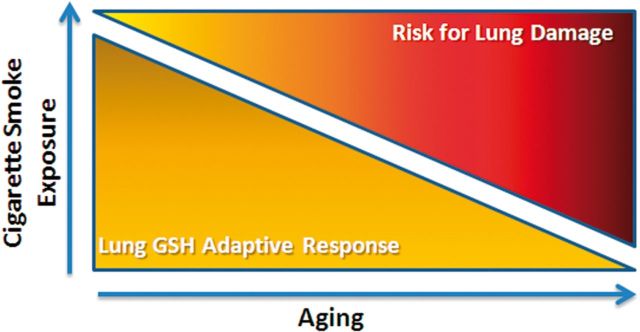
The relatively short time in which alveolar enlargement has occurred suggests that the same might hold true with the development or progression of COPD. People can normally smoke for decades without any major diseases developing, but as they get older the incidence of smoker-related diseases and deaths climbs dramatically (Fukuchi, 2009). It may be that a secondary process, such as aging, changes the threshold which disrupts the endogenous protective mechanisms and contributes to the development of COPD (Fig. 8). Furthermore, our data would indicate that once those endogenous protective mechanisms are affected, pathology can occur quite rapidly and may be used to identify individuals that are at risk for development of COPD.
FIG. 8.
Schematic depicting how loss of GSH adaptive responses increases the risk of lung injury due to CS exposure.
FUNDING
Flight Attendant Medical Research Institute (FMARI) grant (H.W.C.) and a NIH R01 HL084469 grant (B.J.D.).
REFERENCES
- Barnes P. J., Dweik R. A., Gelb A. F., Gibson P. G., George S. C., Grasemann H., Pavord I. D., Ratjen F., Silkoff P. E., Taylor D. R., et al. (2010). Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 138, 682–692. [DOI] [PubMed] [Google Scholar]
- Bracke K. R., D’Hulst A. I., Maes T., Moerloose K. B., Demedts I. K., Lebecque S., Joos G. F., Brusselle G. G. (2006). Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J. Immunol. 177, 4350–4359. [DOI] [PubMed] [Google Scholar]
- Brechbuhl H. M., Gould N., Kachadourian R., Riekhof W. R., Voelker D. R., Day B. J. (2010). Glutathione transport is a unique function of the ATP-binding cassette protein ABCG2. J. Biol. Chem. 285, 16582–16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist A. S., McBurnie M. A., Vollmer W. M., Gillespie S., Burney P., Mannino D. M., Menezes A. M., Sullivan S. D., Lee T. A., Weiss K. B., et al. (2007). International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 370, 741–750. [DOI] [PubMed] [Google Scholar]
- Chinard F. P. (1992). Quantitative assessment of epithelial lining fluid in the lung. Am. J. Physiol. 263, L617–L618. [DOI] [PubMed] [Google Scholar]
- Churg A., Sin D. D., Wright J. L. (2011a). Everything prevents emphysema: are animal models of cigarette smoke-induced COPD any use? Am. J. Respir. Cell Mol. Biol. 45, 1111–1115. [DOI] [PubMed] [Google Scholar]
- Churg A., Zhou S., Wright J. L. (2011b). Matrix metalloproteases in COPD. Eur. Respir. J. 39, 197–209. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Balinsky J. B., Harington J. S., Shepherd J. B. (1973). Assay, purification, properties and mechanism of action of gamma-glutamylcysteine synthetase from the liver of the rat and Xenopus laevis. Biochem. J. 133, 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost E. M., Skwarski K. M., Sauleda J., Soler N., Roca J., Agusti A., MacNee W. (2005). Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 60, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B. M., Pavlisko E., Voynow J. A. (2011). Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int. J. Chron. Obstruct. Pulmon. Dis. 6, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi Y. (2009). The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc. Am. Thorac. Soc. 6, 570–572. [DOI] [PubMed] [Google Scholar]
- Gould N. S., Day B. J. (2011). Targeting maladaptive glutathione responses in lung disease. Biochem. Pharmacol. 81, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould N. S., Min E., Day B. J. (2011a). Macropinocytosis of extracellular glutathione ameliorates tumor necrosis factor alpha release in activated macrophages. PLoS One 6, e25704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould N. S., Min E., Gauthier S., Chu H. W., Martin R., Day B. J. (2010). Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am. J. Respir. Crit. Care Med. 182, 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould N. S., Min E., Gauthier S., Martin R. J., Day B. J. (2011b). Lung glutathione adaptive responses to cigarette smoke exposure. Respir. Res. 12, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould N. S., White C. W., Day B. J. (2009). A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. J. Pharmacol. Exp. Ther. 328, 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Wang M., Tian G., Burger J., Gochfeld M., Yang C. S. (1993). Age- and gender-related variations in the activities of drug-metabolizing and antioxidant enzymes in the white-footed mouse (Peromyscus leucopus). Growth Dev. Aging 57, 85–100. [PubMed] [Google Scholar]
- Han J. C., Han G. Y. (1994). A procedure for quantitative determination of tris(2-carboxyethyl)phosphine, an odorless reducing agent more stable and effective than dithiothreitol. Anal. Biochem. 220, 5–10. [DOI] [PubMed] [Google Scholar]
- Haque J. A., McMahan R. S., Campbell J. S., Shimizu-Albergine M., Wilson A. M., Botta D., Bammler T. K., Beyer R. P., Montine T. J., Yeh M. M., et al. (2010). Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice. Lab. Invest. 90, 1704–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. W., Rajendrasozhan S., Yao H., Chung S., Sundar I. K., Huyck H. L., Pryhuber G. S., Kinnula V. L., Rahman I. (2011). FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J. Immunol. 187, 987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Itoh K., Sato H., Bannai S. (1999). Oxidative stress-inducible proteins in macrophages. Free Radic. Res. 31, 351–355. [DOI] [PubMed] [Google Scholar]
- Ito K., Barnes P. J. (2009). COPD as a disease of accelerated lung aging. Rev. Port. Pneumol. 15, 743–746. [DOI] [PubMed] [Google Scholar]
- Johansson E., Wesselkamper S. C., Shertzer H. G., Leikauf G. D., Dalton T. P., Chen Y. (2010). Glutathione deficient C57BL/6J mice are not sensitized to ozone-induced lung injury. Biochem. Biophys. Res. Commun. 396, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. P., Mody V. C., Jr, ., Carlson J. L., Lynn M. J., Sternberg P., Jr (2002). Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic. Biol. Med. 33, 1290–1300. [DOI] [PubMed] [Google Scholar]
- Kaplan P. D., Kuhn C., Pierce J. A. (1973). The induction of emphysema with elastase. I. The evolution of the lesion and the influence of serum. J. Lab. Clin. Med. 82, 349–356. [PubMed] [Google Scholar]
- Kariya C., Chu H. W., Huang J., Leitner H., Martin R. J., Day B. J. (2008). Mycoplasma pneumoniae infection and environmental tobacco smoke inhibit lung glutathione adaptive responses and increase oxidative stress. Infect. Immun. 76, 4455–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrasch S., Holz O., Jorres R. A. (2008). Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir. Med. 102, 1215–1230. [DOI] [PubMed] [Google Scholar]
- Kong A. N., Owuor E., Yu R., Hebbar V., Chen C., Hu R., Mandlekar S. (2001). Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metab. Rev. 33, 255–271. [DOI] [PubMed] [Google Scholar]
- Kuipers I., Bracke K. R., Brusselle G. G., Aesif S. W., Krijgsman R., Arts I. C., Wouters E. F., Reynaert N. L. (2012a). Altered cigarette smoke-induced lung inflammation due to ablation of Grx1. PLoS One 7, e38984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers I., Bracke K. R., Brusselle G. G., Wouters E. F., Reynaert N. L. (2012b). Smoke decreases reversible oxidations S-glutathionylation and S-nitrosylation in mice. Free Radic. Res. 46, 164–173. [DOI] [PubMed] [Google Scholar]
- Lokke A., Lange P., Scharling H., Fabricius P., Vestbo J. (2006). Developing COPD: a 25 year follow up study of the general population. Thorax 61, 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery E. M., Brubaker A. L., Kuhlmann E., Kovacs E. J. (2013). The aging lung. Clin. Interv. Aging 8, 1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado N., Ito K., Barnes P. J. (2015). Accelerated ageing of the lung in COPD: new concepts. Thorax 70, 482–489. [DOI] [PubMed] [Google Scholar]
- Mercado N., Thimmulappa R., Thomas C. M., Fenwick P. S., Chana K. K., Donnelly L. E., Biswal S., Ito K., Barnes P. J. (2011). Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 406, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H., Yamamoto M. (2004). Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends. Mol. Med. 10, 549–557. [DOI] [PubMed] [Google Scholar]
- Nicks M. E., O’Brien M. M., Bowler R. P. (2011). Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD 8, 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priora R., Coppo L., Salzano S., Di Simplicio P., Ghezzi P. (2010). Measurement of mixed disulfides including glutathionylated proteins. Methods Enzymol. 473, 149–159. [DOI] [PubMed] [Google Scholar]
- Rangasamy T., Cho C. Y., Thimmulappa R. K., Zhen L., Srisuma S. S., Kensler T. W., Yamamoto M., Petrache I., Tuder R. M., Biswal S. (2004). Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 114, 1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. H. (1969). Aging, nutrition and hepatic enzyme activity patterns in the rat. J. Nutr. 97(Suppl 1), 565. [DOI] [PubMed] [Google Scholar]
- Sekhar R. V., Patel S. G., Guthikonda A. P., Reid M., Balasubramanyam A., Taffet G. E., Jahoor F. (2011). Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 94, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlinski M., Postma D. S., van Diemen C. C., Blokstra A., Smit H. A., Boezen H. M. (2008). Lung function loss, smoking, vitamin C intake, and polymorphisms of the glutamate-cysteine ligase genes. Am. J. Respir. Crit. Care Med. 178, 13–19. [DOI] [PubMed] [Google Scholar]
- Silkoff P. E., McClean P. A., Slutsky A. S., Caramori M., Chapman K. R., Gutierrez C., Zamel N. (1998). Exhaled nitric oxide and bronchial reactivity during and after inhaled beclomethasone in mild asthma. J. Asthma. 35, 473–479. [DOI] [PubMed] [Google Scholar]
- Silkoff P. E., Stevens A., Pak J., Bucher-Bartelson B., Martin R. J. (1999). A method for the standardized offline collection of exhaled nitric oxide. Chest 116, 754–759. [DOI] [PubMed] [Google Scholar]
- Snider G. L. (1989). Chronic obstructive pulmonary disease: risk factors, pathophysiology and pathogenesis. Annu. Rev. Med. 40, 411–429. [DOI] [PubMed] [Google Scholar]
- Sun J. D., Ragsdale S. S., Benson J. M., Henderson R. F. (1985). Effects of the long-term depletion of reduced glutathione in mice administered L-buthionine-S,R-sulfoximine. Fund. Appl. Toxicol. 5, 913–919. [DOI] [PubMed] [Google Scholar]
- Thannickal V. J., Murthy M., Balch W. E., Chandel N. S., Meiners S., Eickelberg O., Selman M., Pardo A., White E. S., Levy B. D., et al. (2015). Blue journal conference. Aging and susceptibility to lung disease. Am. J. Respir. Crit. Care Med. 191, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahovic G., Russell M. L., Mercer R. R., Crapo J. D. (1999). Cellular and connective tissue changes in alveolar septal walls in emphysema. Am. J. Respir. Crit. Care Med. 160, 2086–2092. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang J., Yi J. (2011). Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxid. Redox. Signal. 16, 649–657. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Sagisaka H., Arakawa S., Shibaya Y., Watanabe M., Igarashi I., Tanaka K., Totsuka S., Takasaki W., Manabe S. (2003). A novel model of continuous depletion of glutathione in mice treated with l-buthionine (S,R)-sulfoximine. J. Toxicol. Sci. 28, 455–469. [DOI] [PubMed] [Google Scholar]
- Weldy C. S., White C. C., Wilkerson H. W., Larson T. V., Stewart J. A., Gill S. E., Parks W. C., Kavanagh T. J. (2011). Heterozygosity in the glutathione synthesis gene Gclm increases sensitivity to diesel exhaust particulate induced lung inflammation in mice. Inhal. Toxicol. 23, 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. C., Viernes H., Krejsa C. M., Botta D., Kavanagh T. J. (2003). Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal. Biochem. 318, 175–180. [DOI] [PubMed] [Google Scholar]
- Wild A. C., Mulcahy R. T. (2000). Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic. Res. 32, 281–301. [DOI] [PubMed] [Google Scholar]
- Wright J. L., Churg A. (2010). Animal models of cigarette smoke-induced chronic obstructive pulmonary disease. Expert. Rev. Respir. Med. 4, 723–734. [DOI] [PubMed] [Google Scholar]