Abstract
The increase of adipose tissue mass associated with obesity is due in part to an increase in the number of adipocytes. This hyperplasia results from recruitment of pluripotent stem cells present in the vascular stroma of adipose tissue. A model cell culture system has been developed that recapitulates this process both ex vivo and in vivo. After treatment of pluripotent C3H10T1/2 stem cells with bone morphogenic protein 4 (BMP4) during proliferation followed by differentiation inducers at growth arrest, the cells synchronously enter S phase and undergo mitotic clonal expansion, a hallmark of preadipocyte differentiation. Upon exiting the cell cycle, these cells express adipocyte markers and acquire adipocyte characteristics at high frequency. C3H10T1/2 cells treated with BMP4 in cell culture and implanted s.c. into athymic mice develop into tissue indistinguishable from adipose tissue in normal fat depots. We interpret the findings as evidence that BMP4 is capable of triggering commitment of pluripotent C3H10T1/2 stem cells to the adipocyte lineage.
Keywords: adipose tissue, differentiation, bone morphogenic protein 4, mitotic clonal expansion, development
The increase in adipose tissue mass in obesity is due to both an increase in the number and size of adipocytes (1, 2). The increase in cell number is the result of recruitment of preadipocytes from a population of pluripotent stem cells that resides in the vascular stroma of adipose tissue (3). Stem cell commitment to the adipose lineage produces preadipocytes that, when appropriately induced, undergo mitotic clonal expansion and then differentiate into adipocytes (4). Resident pluripotent stem cells in the vascular stroma of adipose tissue and mesenchymal stem cells have the capacity to undergo commitment to several lineages including the adipose, muscle, bone, or cartilage lineages (3, 5–7). Increasing evidence indicates that commitment is triggered by factors/cytokines that activate expression of genes/proteins that direct entry of lineage-specific developmental programs (8). Identification of these genes/proteins should provide insight into the mechanisms by which pluripotent progenitors undergo commitment to the adipose lineage. Understanding these mechanisms may identify potential targets for therapeutic intervention, whereby progression of the events of the adipocyte hyperplasia of obesity can be prevented or, in the case of lipodystrophy, how these events can be enhanced.
Established preadipocyte cell lines, most notably the 3T3-L1 and 3T3-F442A lines, have proven to be faithful in vitro models for adipocyte differentiation in vivo (9–11). When implanted s.c. into athymic mice, 3T3-F442A (12, 13) and genetically modified 3T3-L1 preadipocytes (14) produce fat pads that are histologically/biochemically identical to host adipose tissue. We now know a great deal about the adipocyte differentiation program and the genes involved in this phase of adipocyte development. However, little is known about the “commitment” phase by which pluripotent stem cells are induced to commit to the adipocyte lineage.
The C3H10T1/2 cell line was established in 1973 from 14- to 17-day-old C3H mouse embryos (15). These cells display fibroblastic morphology in cell culture and are functionally similar to mesenchymal stem cells. Inhibiting methylation with 5-azacytidine in C3H10T1/2 cells produces cells that exhibit stable morphological and biochemical features of muscle, adipose, bone, or cartilage cells (16). It is believed that this phenotypic alteration is the result of activation of endogenous genes in response to blocking methylation. By using this approach, MyoD, a protein involved in commitment of C3H10T1/2 cells to the muscle lineage, was discovered (8).
In this article, we report that bone morphogenic protein 4 (BMP4), a member of the transforming growth factor type β superfamily, can induce commitment of C3H10T1/2 cells to preadipocytes that, when subjected to an adipocyte differentiation protocol, develop into cells of the adipocyte phenotype.
Materials and Methods
Differentiation of 3T3-F442A and 3T3-L1 Preadipocytes. The 3T3-F442A and 3T3-L1 preadipocytes were propagated and differentiated as described (12, 17).
Generation of BMP4-Conditioned Medium and Induction of Commitment of C3H10T1/2 Stem Cells to the Adipocyte Lineage. A BMP4 expression vector was constructed by cloning the full-length BMP4 cDNA into the expression vector pCEP4. A BMP4-expressing cell line was generated by stable transfection of 293T cells with this construct and selection with hygromycin B. The expression of BMP4 was confirmed by RT-PCR. To generate BMP4-conditioned medium, this cell line was propagated and fed with fresh medium when they reached 70–80% confluence. After 24 h, the medium was filtered with a 0.22-μm filter and stored at -80°C.
To induce commitment, C3H10T1/2 stem cells were plated at low density, cultured in DMEM containing 10% calf serum mixed with BMP4-conditioned medium in 1:1 (vol/vol) ratio, or in DMEM containing 10% calf serum with different concentrations of purified recombinant BMP4 or BMP2 (R & D Systems). After the cells reached postconfluence, they were induced to differentiate by using the standard 3T3-L1 differentiation protocol described above. Expression of adipocyte markers was assessed by immunoblotting cell extracts, prepared on day 6, with Abs against CCAAT enhancer-binding protein (C/EBPα), peroxisome proliferator activator (PPARγ), and 422/aP2. Accumulation of cytoplasmic triglyceride in these cells was detected by staining with Oil Red O on day 8.
Immunoblotting. To follow changes in the levels of cyclin A and adipocyte markers (C/EBPα, PPARγ, or 422/aP2), 2-day post-confluent (day 0) 3T3-L1 or 3T3-F442A preadipocytes or C3H10T1/2 stem cells (treated or not with BMP4) were induced to differentiate as described (12, 17). At various times thereafter, cell monolayers were washed once with cold PBS (pH 7.4) and then scraped into lysis buffer containing 1% SDS and 60 mM Tris·HCl (pH 6.8). Lysates were heated at 100°C for 10 min and clarified by centrifugation, and equal amounts of protein were subjected to SDS/PAGE and immunoblotted with Abs against cyclin A, C/EBPα, PPARγ, or 422/aP2. C/EBPα and 422/aP2 Abs were prepared in this laboratory (18); PPARγ Ab was provided by Mitchell Lazar (University of Pennsylvania, Philadelphia), and cyclin A Ab was purchased from Santa Cruz Biotechnology.
s.c. Implantation, Excision of Fat Pads, and Histology. C3H10T1/2 stem cells were treated or not with BMP4 at 50 ng/ml and grown to near confluence, trypsinized, and suspended in DMEM containing 10% calf serum. After centrifugation, cell pellets were resuspended in FBS and injected s.c. (3 × 107 cells per site) with a 17-gauge needle at the sternum of BALB/c athymic mice (Charles River Breeding Laboratories). Mice were housed in microisolator cages. At 4 wk the mice were killed by cervical dislocation, and the fat pads derived from the implanted cells and epididymal fat pads were excised and fixed in neutral-buffered formalin (Baxter Scientific Products, McGaw Park, IL). For light microscopy, fat pads derived from implanted preadipocytes and epididymal fat pads were paraffin-embedded after 24 h of fixation in buffered formalin. Paraffin tissue sections (4 μm) were stained with hematoxylin and eosin for histological analysis.
Results
BMP4 Induces Commitment of C3H10T1/2 Stem Cells to the Adipocyte Lineage. Initially, a subline of C3H10T1/2 cells was selected that exhibited virtually no commitment to the adipocyte lineage when subjected to the standard adipocyte differentiation protocol (17). Unlike 3T3-L1 preadipocytes, C3H10T1/2 cells do not differentiate into adipocytes with the differentiation protocol, as indicated by failure to express adipocyte markers proteins (Fig. 1A) or cytoplasmic triglyceride (Fig. 1B). The C3H10T1/2 subline was used for studies described below.
Fig. 1.
Effect of treatment of C3H10T1/2 stem cells with BMP4-conditioned medium on their capacity to differentiate into adipocytes. (A) The 2-day postconfluent C3H10T1/2 stem cells and 3T3-L1 preadipocytes were induced (or not) by using our standard differentiation protocol, and the expression of adipocyte markers (C/EBPα, PPARγ, and 422/aP2) were detected on days 0 and 2 by immunoblotting. (B) C3H10T1/2 stem cells cultured with or without BMP4-conditioned medium were induced (or not) to differentiate by using our differentiation protocol, and the accumulation of cytoplasmic triglyceride was detected with Oil Red O staining (Upper) on day 8. This was compared to 3T3-L1 cells (Lower). (C) C3H10T1/2 stem cells cultured with or without BMP4-conditioned medium were induced to differentiate with the standard 3T3-L1 differentiation protocol, and the expression of adipocyte markers (C/EBPα, PPARγ, and 422/aP2) was detected with cell extract made on day 6.
Based on earlier reports (19),§ we tested the possibility that BMP4 can induce C3H10T1/2 cells to commit to the adipocyte lineage. To generate BMP4-conditioned medium, human embryonic kidney 293T cells were stably transfected with a BMP4 expression vector under control of the cytomegalovirus promoter. Proliferating C3H10T1/2 cells were treated with BMP4-conditioned media from preconfluent BMP4-expressing human embryonic kidney 293T cells. None of the cells treated in this manner acquired the adipocyte phenotype as indicated by the failure to express adipocyte protein markers (Fig. 1C) or cytoplasmic triglyceride (Fig. 1B). However, treatment of C3H10T1/2 cells with BMP4-conditioned medium during proliferation (until 2 days postconfluent), followed by the standard differentiation protocol, caused >90% of the cells to acquire the adipocyte phenotype. Cells treated in this manner accumulated cytoplasmic trigylceride (Fig. 1B) and expressed C/EBPα, PPARγ, and 422/aP2 (Fig. 1C) at a level comparable with that of fully differentiated 3T3-L1 adipocytes (Fig. 1 A and B). These observations suggested that BMP4 can induce commitment of C3H10T1/2 cells to preadipocyte-like cells that have the capacity to differentiate into adipocytes when exposed to differentiation inducers.
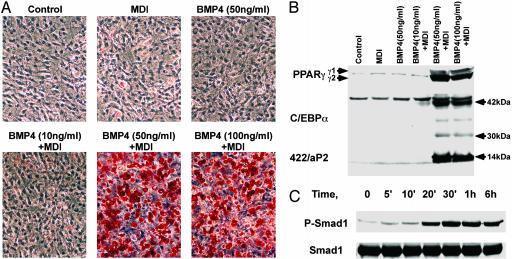
To verify that commitment of C3H10T1/2 cells to the adipocyte lineage is specific to BMP4 and not to an unknown factor(s) secreted by the human embryonic kidney 293T cells, purified recombinant BMP4 was tested. Thus, C3H10T1/2 cells, plated at low density and cultured in 10% calf serum, were treated or not with purified BMP4 at levels of 10, 50, or 100 ng/ml, until cells achieved growth arrest, i.e., 2 days after reaching confluence, and then were subjected to the preadipocyte differentiation protocol. The earlier observation that treatment with BMP4-conditioned medium alone, i.e., in the absence of differentiation inducers, did not produce the adipocyte phenotype was confirmed (Fig. 2 A and B). However, when the cells were treated with BMP4 followed by differentiation inducers, ≥90% of the cells differentiated into adipocytes by day 8 after induction of differentiation (Fig. 2 A and B), a rate and extent of differentiation comparable with that of 3T3-L1 preadipocytes treated only with differentiation inducers (Fig. 1B). Thus, only C3H10T1/2 cells treated with 50 or 100 ng/ml exhibited Oil Red O staining and expression of adipocyte protein markers, both levels of BMP4 treatment giving similar results; BMP4 at 10 ng/ml had little or no effect. Based on the results with purified BMP4, as well as those with BMP4-conditioned media, we conclude that BMP4 induces commitment of C3H10T1/2 cells to become preadipocytes that are programmed to differentiate when subjected to differentiation inducers.
Fig. 2.
Effect of treatment of C3H10T1/2 stem cells with recombinant BMP4 on their capacity to differentiate into adipocytes. (A) C3H10T1/2 stem cells were cultured without or with purified recombinant BMP4 at 10, 50, and 100 ng/ml until postconfluent and then induced to differentiate with our standard differentiation protocol. The accumulation of cytoplasmic triglyceride was detected by Oil Red O staining on day 8, and at which point the cells were photographed. (B) C3H10T1/2 stem cells were treated as above and the expression of adipocyte markers (C/EBPα, PPARγ, and 422/aP2) was detected with cell extract made on day 6. (C) C3H10T1/2 stem cells were treated with 50 ng/ml BMP4 at ≈30% confluency; whole cell extracts were made at times indicated, and Western blotting was performed with Abs against Smad1 (Santa Cruz Biotechnology) or phospho-Smad1 (Cell Signaling Technology, Beverly, MA).
BMP4 is known to function by interacting with a cell-surface receptor that phosphorylates members of the Smad family of transcription factors causing translocation to the nucleus. Smad1, a known down-stream target of BMP4 (20), is constitutively expressed by C3H10T1/2 cells and is rapidly (≤20 min) phosphorylated after BMP4 treatment, the phosphorylated state persisting for at least 6 h (Fig. 2C). These results verify that the BMP4–Smad1-signaling system is functional in C3H10T1/2 cells.
BMP2 has been reported to promote adipocyte differentiation of the C3H10T1/2 cell line (19). Therefore, we examined the possibility that BMP2 can commit C3H10T1/2 cells to the adipocyte lineage. C3H10T1/2 cells were treated with BMP2 or BMP4 at 50 ng/ml until postconfluence and then were subjected to the differentiation protocol. Whereas BMP4 induced commitment, BMP2 did not. Thus, BMP2 followed by differentiation inducers failed to cause accumulation of cytoplasmic triglyceride or the expression of 422/aP2 protein, an adipocyte-specific marker (Fig. 3).
Fig. 3.
Effect of recombinant BMP2 on commitment of C3H10T1/2 stem cells to the adipocyte lineage. (A) C3H10T1/2 stem cells were treated with or without BMP2 or BMP4 until postconfluence, and then induced to differentiate with the standard differentiation protocol. The accumulation of cytoplasmic triglyceride was detected by Oil Red O staining on day 8 and photographed. (B) The expression of the adipocyte marker 422/aP2 was detected with cell extract made on day 6.
C3H10T1/2 Cells Previously Exposed to BMP4 Synchronously Undergo Mitotic Clonal Expansion upon Treatment with Differentiation Inducers. When induced to differentiate, growth-arrested 3T3-L1 preadipocytes synchronously reenter the cell cycle and undergo mitotic clonal expansion, a required event for adipocyte differentiation (4, 21, 22). The question was raised whether C3H10T1/2 cells, after commitment with BMP4, undergo mitotic clonal expansion when induced to differentiate. As shown in Fig. 4A, growth-arrested C3H10T1/2 stem cells treated only with differentiation inducers did not exhibit a significant increase in cell number. This result was anticipated because differentiation inducers alone did not promote adipogenesis in these cells (Fig. 2 A and B). Similarly, treatment of C3H10T1/2 cells with BMP4 alone caused only a small, i.e., ≈1.5-fold, increase in cell number (Fig. 4A). However, when first treated with BMP4 followed by differentiation inducers, cell number increased dramatically by ≈3-fold (Fig. 4A). It can be concluded that committed C3H10T1/2 stem cells undergo mitotic clonal expansion when subjected to the differentiation protocol.
Fig. 4.
Mitotic clonal expansion of BMP4-committed C3H10T1/2 stem cells upon induction with differentiation inducers. (A) C3H10T1/2 stem cells were cultured with or without 50 ng/ml BMP4 until postconfluence and then induced to differentiate with 3T3-L1 standard differentiation protocol. Cells were counted on day 6 and plotted. (B) Committed C3H10T1/2 (by BMP4 at 50 ng/ml) stem cells and 3T3-L1 and 3T3-F442A preadipocytes were induced to differentiate, and the expression level of cyclin A was followed by Western blotting.
At the outset of mitotic clonal expansion, 3T3-L1 preadipocytes reenter the cell cycle and synchronously traverse the G1-S checkpoint, which occurs ≈14 h after treatment with differentiation inducers (18). At this point in the differentiation program, 3T3-L1 preadipocytes (and 3T3-F442A preadipocytes) express cyclin A, a marker for S phase entry (Fig. 4B). Previous studies have shown that labeled thymidine incorporation into DNA, expression of Cdk2 and down-regulation of p27/kip also occur at this point (4, 21). To determine whether committed C3H10T1/2 cells exhibit similar synchrony at the G1-S checkpoint, the kinetics of expression of cyclin A, a key marker of S phase entry, was assessed after treatment with differentiation inducers. As shown in Fig. 4B, the expression of cyclin A occurred synchronously in C3H10T1/2 cells between 12 and 16 h after induction of differentiation, mirroring the responses of 3T3-L1 and 3T3-F442A preadipocytes. Thus, C3H10T1/2 stem cells, previously committed with BMP4, also exhibit this characteristic feature of preadipocytes.
C3H10T1/2 Stem Cells, Committed with BMP4 in Cell Culture, Give Rise to Adipose Tissue When Implanted into Athymic Mice. To verify the cell culture findings described above in a more physiological context, we applied an approach used previously in which 3T3 preadipocytes harboring a marker gene, were implanted s.c. into athymic mice (12). These cells developed into tissue indistinguishable from adipose tissue from the same animal (12). Like normal adipose tissue, the tissue derived from the implanted cells became highly vascularized, innervated, and responded to hormones administered to the mice (12). Thus, C3H10T1/2 stem cells were first treated with BMP4 in cell culture as described in Fig. 2. Approximately 30 million of the BMP4-committed stem cells were then implanted s.c. into athymic mice adjacent to the sternum, a site normally devoid of adipose tissue. As illustrated in Fig. 5, after 4 wk, the implanted C3H10T1/2 cells that had been treated with BMP4 in cell culture developed into tissue indistinguishable from epididymal adipose tissue of the same animal. In contrast, implanted C3H10T1/2 cells that had not been treated with BMP4 in cell culture failed to develop into mature unilocular adipocytes by 4 wk. Nevertheless, it appears that these cells had begun to exhibit some small cytoplasmic fat droplets. These cells, although delayed in their development into adipocytes, may have undergone limited commitment because of BMP4 and/or other factors present in the tissue milieu at the site of implantation. In the same experiment, positive controls, i.e., 3T3-F442A preadipocytes, also developed into adipose-like tissue. These findings thus verify that C3H10T1/2 stem cells committed with BMP4 in cell culture have the capacity to differentiate into adipocytes and to produce adipose tissue in the intact animal.
Fig. 5.
Effect of previous treatment of C3H10T1/2 stem cells in cell culture on their capacity to generate adipose tissue when s.c. implanted into athymic mice. C3H10T1/2 stem cells were treated with or without 50 ng/ml BMP4 until near confluency and implanted into athymic mice. Later (4 wk), the fat pads derived from the implanted cells were analyzed with hematoxylin/eosin staining and photographed. The 3T3-F442A preadipocytes serve as a positive control, and the development of adipose tissue from these implanted cells were compared to endogeneous epididymal fat pads.
Discussion
The increase of adipose tissue mass that accompanies obesity is due both to an increase in adipocyte number (hyperplasia) and size (1, 2). Presumably, this hyperplasia is the result of recruitment of preadipocytes from a population of pluripotent stem cells in the vascular stroma of adipose tissue (3), followed by the mitotic clonal expansion that occurs during preadipocyte differentiation (4). These stem cells have the capacity to undergo commitment, not only to the adipose lineage, but also the muscle, bone, or cartilage lineages (16). Our findings show that treatment of C3H10T1/2 stem cells with BMP4 gives rise to preadipocyte-like cells that, when appropriately induced, undergo mitotic clonal expansion and differentiation into adipocytes. Thus, the adipocyte development can be resolved into and studied in cell culture in at least three distinct steps: (i) adipocyte lineage commitment, (ii) mitotic clonal expansion, and (iii) terminal differentiation into adipocytes. Although much is now known about the differentiation of preadipocytes to adipocytes, there is a paucity of information on the commitment of stem cells to the adipose lineage. In this article, we demonstrate that commitment to the adipocyte lineage and subsequent adipogenic hyperplasia can be mimicked and studied with the established C3H10T1/2 stem cell line both in cell culture and in an in vivo model system.
Sporadic reports in the literature have suggested that treatment of C3H10T1/2 pluripotent stem cells can trigger commitment/differentiation into adipocytes (19).§ We have subcloned a derivative of C3H10T1/2 cells that is unable to undergo adipogenesis by treatment with BMP4 alone or by differentiation inducers alone. Thus, these cells do not accumulate cytoplasmic triglyceride nor do they express adipocyte marker proteins after exposure to BMP4 or differentiation inducers alone (Figs. 1 and 2). However, treatment of C3H10T1/2 cells with BMP4 during proliferation, followed by our standard adipocyte differentiation protocol, leads to differentiation into adipocytes, as evidenced by accumulation of cytoplasmic triglyceride and expression of adipocyte protein markers (Figs. 1 and 2). We conclude that BMP4 causes the cells to undergo lineage commitment into preadipocytes that can undergo terminal differentiation to adipocytes upon subsequent exposure to differentiation inducers. Moreover, this process can be recapitulated in an in vivo context. Thus, when C3H10T1/2 cells are first treated with BMP4 in culture and then implanted s.c. into athymic mice, the implanted cells develop into tissue indistinguishable from adipose tissue in normal fat depots of the same animal (Fig. 5).
Once differentiation of growth-arrested 3T3-L1 preadipocytes has been induced, a cascade of events is initiated including the synchronous reentry of the cell cycle for two rounds of mitosis, referred to as mitotic clonal expansion (4, 21). Recent evidence indicates that this process is required for terminal adipocyte differentiation (4, 21). We found that the C3H10T1/2 subline undergoes a similar process of mitotic clonal expansion upon treatment with differentiation inducers but only after the cells have been committed with BMP4 (Fig. 4B). We suggest that in combination, commitment and mitotic clonal expansion, account for the increase of adipocyte number associated with obesity.
Many of the key players in mitotic clonal expansion and terminal differentiation have been characterized by using the 3T3-L1 and 3T3-F442A preadipocyte cell lines established by Green et al. (23–25). Little is known, however, about the factors/genes that trigger the commitment process. The C3H10T1/2 stem cell subline and the approaches described in this article should prove useful in future studies on pluripotent stem cell commitment to the adipose lineage.
Acknowledgments
We thank Kathleen Anuzis for excellent technical assistance. This research was supported by National Institutes of Health Research Grant DK-000627 (to M.D.L.), National Institutes of Health KO Award DK-61355 (to Q.-Q.T.), and National Institutes of Health National Research Service Award DK61840 (to T.C.O.).
Abbreviations: BMP, bone morphogenic protein; C/EBP, ccaat enhancer-binding protein; PPAR, peroxisome proliferator activator.
Footnotes
Butterwith, S. C., Wilkie, R. S. & Clinton, M. (1996) Biochem. Soc. Trans. 24, 163S (abstr.).
References
- 1.Hirsch, J. & Batchelor, B. (1976) Clin. Endocrinol. Metab. 5, 299-311. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd, P. R., Gnudi, L., Tozzo, E., Yang, H., Leach, F. & Kahn, B. B. (1993) J. Biol. Chem. 268, 22243-22246. [PubMed] [Google Scholar]
- 3.Yu, Z. K., Wright, J. T. & Hausman, G. J. (1997) Obes. Res. 5, 9-15. [DOI] [PubMed] [Google Scholar]
- 4.Tang, Q.-Q., Otto, T. C. & Lane, M. D. (2003) Proc. Natl. Acad. Sci. USA 100, 44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplan, A. (1991) J. Orthop. Res. 9, 641-650. [DOI] [PubMed] [Google Scholar]
- 6.Caplan, A. (1994) Clin. Plastic Surg. 21, 429-435. [PubMed] [Google Scholar]
- 7.Young, H. E, Mancini, M. L., Wright, R. P., Smith, J. C., Black, A. C., Jr., Reagan, C. R. & Lucas, P. A. (1995) Dev. Dyn. 202, 137-144. [DOI] [PubMed] [Google Scholar]
- 8.Davis, R. L., Weintraub, H. & Lassar, A. B. (1987) Cell 51, 987-1000. [DOI] [PubMed] [Google Scholar]
- 9.Cornelius, P., MacDougald, O. A. & Lane, M. D. (1994) Annu. Rev. Nutr. 14, 99-129. [DOI] [PubMed] [Google Scholar]
- 10.MacDougald, O. A. & Lane, M. D. (1995) Annu. Rev. Biochem. 64, 345-373. [DOI] [PubMed] [Google Scholar]
- 11.Mandrup, S. M. & Lane, M. D. (1997) J. Biol. Chem. 272, 5367-5370. [DOI] [PubMed] [Google Scholar]
- 12.Mandrup, S., Loftus, T. M., MacDougald, O. A., Kuhajda, F. & Lane, M. D. (1997) Proc. Natl. Acad. Sci. USA 94, 4300-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, H. & Kehinde, O. (1979) J. Cell Physiol. 101, 169-172. [DOI] [PubMed] [Google Scholar]
- 14.Ross, S. E., Hemati, N., Longo, K. A., Bennett, C. N., Lucas, P. C., Erickson, R. L. & MacDougald, O. A. (2000) Science 289, 950-953. [DOI] [PubMed] [Google Scholar]
- 15.Reznikoff, K. A., Brankow, D. W. & Heidelberger, C. (1973) Cancer Res. 33, 3231-3238. [PubMed] [Google Scholar]
- 16.Pinney, D. & Emerson, C., Jr. (1989) Environ. Health Perspect. 80, 221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Student, A. K., Hsu, R. Y. & Lane, M. D. (1980) J. Biol. Chem. 255, 4745-4750. [PubMed] [Google Scholar]
- 18.Tang, Q.-Q. & Lane, M. D. (1999) Genes Dev. 13, 2231-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahrens, M., Ankenbauer, T., Schroder, D., Hollnagel, A., Mayer, H. & Gross, G. (1993) DNA Cell Biol. 12, 871-880. [DOI] [PubMed] [Google Scholar]
- 20.Kretzschmar, M., Liu, F., Hata, A., Doody, J. & Massague, J. (1997) Genes Dev. 11, 984-995. [DOI] [PubMed] [Google Scholar]
- 21.Patel, Y. M. & Lane, M. D. (2000) J. Biol. Chem. 275, 17653-17660. [DOI] [PubMed] [Google Scholar]
- 22.Yeh, W.-C., Cao, Z., Classon, M. & McKnight, S. L. (1995) Genes Dev. 9, 168-181. [DOI] [PubMed] [Google Scholar]
- 23.Green, H. & Meuth, M. (1974) Cell 3, 127-133. [DOI] [PubMed] [Google Scholar]
- 24.Green, H. & Kehinde, O. (1974) Cell 1, 113-116. [Google Scholar]
- 25.Green, H. & Kehinde, O. (1975) Cell 5, 19-27. [DOI] [PubMed] [Google Scholar]