Abstract
Background
Genetic factors may play a role in the susceptibility of Ischemic stroke (IS). Previous studies have shown that Tumour necrosis factor-α (TNF-α) gene polymorphisms were associated with the risk of IS in multiple ethnicities. The present case–control study tested the hypothesis that genetic polymorphisms of the TNF-α gene may affect the risk of IS in North Indian population. We investigated the association of four single nucleotide polymorphisms (− 308G/A, + 488G/A, − 857C/T and -1031 T/C) within TNF-α gene promoter and their haplotypes with the risk of IS.
Methods
IS was classified using the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification. Genotyping was performed for 250 IS patients and 250 age- and sex-matched IS free controls by using SNaPshot technique. Multivariate logistic regression was used to control the confounding effects of demographic and risk factor variables. Haplotype analyses were done by using PHASE software and Linkage disequilibrium (LD) analyses were done by using Haploview version 4.2 software.
Results
An independent association between TNF-α + 488G/A (OR = 2.59; 95%CI 1.46 to 4.60; p = 0.001) and -857C/T (OR = 1.77; 95%CI 1.01 to 3.11; p < 0.04) and risk of IS was observed under dominant model. However, no significant association between -308G/A and -1031 T/C gene polymorphisms and risk of IS was observed. Haplotype analysis showed that A308-G488-C857-T1031 haplotypes were significantly associated with the increased risk of IS [OR = 1.66; 95%CI 1.02 to 2.71; p = 0.003]. Strong linkage disequilibrium (LD) was observed for + 488G/A and -857C/T (D’ = 0.41, r2 = 0.004).
Conclusions
Two SNPs (+ 488G/A and -857C/T) of TNF-α gene and their haplotypes are significantly associated with the risk of IS in the population enrolled from North India. Our findings indicate that polymorphisms and haplotypes of TNF-α gene may be used as a genetic marker for identifying individuals at increased risk for developing IS.
Keywords: Ischemic stroke, Inflammatory gene, Single nucleotide polymorphisms, Tumor necrosis factor-alpha, Cytokine
1. Introduction
Ischemic stroke (IS) is a complex multifactorial disease which accounts for 80–85% of stroke and its pathophysiology is regulated by a combination of lifestyle, environmental and unclear genetic risk factors (Bevan and Markus, 2011). Recent data suggested that inflammatory processes are involved in the pathogenesis of IS. Several frequent polymorphisms have been identified in the Tumour necrosis factor-α (TNF-α) gene (Carr et al., 2002, Matarin et al., 2009, Hansson, 2005, Flex et al., 2004, Hollegaard and Bidwell, 2006). TNF-α is one of the main pro-inflammatory cytokines and plays a central role in initiating and regulating the inflammatory response (Zaremba, 2000).
Human TNF-α gene is located on chromosome 6p21.3 which consists of four small exons and encodes protein of 233 amino acid residue (Nedwin et al., 1985). TNF-α increases capillary permeability, activates endothelium, and causes a significant neutrophil adherence and accumulation in capillaries and small blood vessels. TNF-α also exacerbates ischemic brain injury and increases the infarct size by various mechanisms that include thrombus formation, release of endothelin 1 and nitric oxide (potent vaso-active agents), promotion of leukocyte adhesion, and infiltration in addition to blood-barrier breakdown and tissue swelling (Feuerstein et al., 1994, Feuerstein et al., 1998, Barone et al., 1997, Liu et al., 1994, Maemura et al., 1992, Pinto et al., 2006; Tuttolomondo et al., 2014, Tuttolomondo et al., 2015). TNF-α regulates the inflammatory response and activates blood coagulation and therefore is an important candidate gene for stroke (Bazzoni and Beutler, 1996). Genetic screening has revealed four polymorphic regions (− 308G/A, + 488G/A, − 857C/T and -1031 T/C) in the promoter region of TNF-α gene. A number of studies have shown the association of − 308 G/A polymorphism with stroke. However, the results have not been consistent across population. The A allele, which has been associated with elevated TNF levels (Wilson et al., 1997), was found to be protective in Korean adults with IS (Um and Kim, 2004). On the other hand, it conferred an increased risk of IS in younger Italian patients (Rubattu et al., 2005). Patients with high TNF-α level might be at an increased risk of developing thrombotic complications because of the effect of this cytokine on the endothelium.
Only single study conducted in South Indian population by Munshi et al. (2011) reported that + 488G/A polymorphism in TNF-α gene is an important risk factor for IS. Limited number of studies are available for the association between TNF-α (-857C/T and -1031 T/C) gene polymorphisms with the risk of stroke. As per our knowledge, no information is available from North Indian population on the association between these four SNPs with the risk of IS. Hence this study was undertaken to investigate the association of (− 308G/A, + 488G/A, − 857C/T and -1031 T/C) polymorphisms in TNF-α gene with risk of IS.
2. Materials and methods
2.1. Subjects
The present case–control study was a hospital based study and was completed in one and a half years (October 2013 to April 2015). The study was conducted in the Department of Neurology, All India Institute of Medical Sciences (AIIMS), New Delhi in collaboration with Institute of Genomics and Integrative Biology (IGIB), New Delhi. A total of 250 patients were recruited in the study after radiologic confirmation of IS by computed tomography (CT) or magnetic resonance imaging (MRI) scans of the brain. All patients had clinical signs consistent with the World Health Organization (WHO) definition of stroke. A control group comprising of 250 age and sex matched individuals was recruited from volunteers and healthy persons accompanying the patients in the general outpatient department (OPD) and was assessed by questionnaire for verifying stroke free status (QVSFS) (Jones et al., 2001). Written informed consent was obtained from all the subjects before the collection of information and blood samples. Patients with a history of transient ischemic attack, fever, rheumatologic disease, autoimmune disease, any acute or chronic infection, CT/MRI proven hemorrhagic stroke, and a history of regular immunosuppressive or analgesic therapies were excluded. The study was approved by the Local Institutional Ethics Committee.
2.2. Clinical examination
A detailed history and clinical evaluation was carried out by neurologist. IS was categorized using the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification (Meschia, 2002). The National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS) and Barthel Index (BI) scores were used for the determination of clinical severity and independency. At six months, disability and functional independence was assessed telephonically by mRS and BI.
2.3. Definition of variables
Definitions of variables were modified from the study (Feigin et al., 1998) and are as follows: Hypertension: Subjects will be considered to have hypertension if they either have the diagnosis of hypertension or treated for hypertension before the stroke or reference date. In addition, if a control will have no recorded blood pressure before the reference date, but diastolic pressure of 90 mmHg or more or a systolic pressure of 140 mmHg or more on two or more occasions during the study evaluation, he or she will be considered to have hypertension. Diabetes: if a subject will have the diagnosis documented by a physician in the medical record or if fasting blood sugar level will be > 126 mg/dl, Dyslipidemia: if they either will have the diagnosis of dyslipidemia or treated for dyslipidemia. Smoker: Person will be defined as regular smoker if a person smoking ≥ 1 cigarettes daily, Bidis, Cigar for proceeding > 3 months. Body Mass Index (BMI): BMI will be calculated by weight in kilograms divided by the square of height in meters. Family history of Stroke: A positive family history of stroke will be considered if a subject's first-degree relative (parent or sibling) had a stroke. Socioeconomic Status: It was classified into two classes based on four items, mainly two wheeler, refrigerator, computer or car. Low – not possessing any of the four, High: possessing either two- wheeler or refrigerator or computer or car. Occupational behaviour: It comprised of Sedentary or sitting occupations (mostly sitting e.g. shopkeeper, clerk, etc.), Moderate physical work (involves walking e.g. salesman, nurses, housework etc), Heavy physical work (carrying, lifting e.g. labourer, coolie etc.). Physical activity: Physical activity was defined if a person engaged in morning or evening walk/running/jogging/swimming/cycling at least half an hour in four days or more in a week (Kumar et al., 2014, Kumar et al., 2015).
2.4. DNA isolation and genotyping
Single time one teaspoon (4 ml) venous blood samples were taken from IS patients and controls in a tube containing ethylene diamine tetra acetic acid (EDTA). Genomic DNA was isolated from whole blood through standard phenol-chloroform method. The primers were designed for the four selected SNPs using the Primer3 online tool, (http://bioinfo.ut.ee/primer3-0.4.0/). The TNF-α regions were amplified in T-100 thermal cycler (Bio-Rad) using the primer sequences and conditions for Polymerase Chain Reaction (PCR) are listed in Table 1. Genotyping was performed on 3130xl automated DNA sequencer (Applied Biosystems) using the SNaPshot method.
Table 1.
List of primer sequences and PCR conditions used for TNF- α gene polymorphisms.
| SNPs | rsID | Primers | Annealing (°C) | Amplicon Size (bp) |
|---|---|---|---|---|
| − 308G/A | rs1800629 | F.P- 5-AGGCAATAGGTTTTGAGGGCCAT-3 R.P- 5-TCCTCCCTGCTCCGATTCCG-3 S.P- 5- CAATAGGTTTTGAGGGGCATG -3 |
55 | 107 |
| + 488G/A | rs1800610 | F.P- 5-GCCAGACATCCTGTCTCTCC-3 R.P- 5-CAGAGGGAAGAGGTGAGTGC-3 S.P – 5- TGCATCCCCGTCTTTCTCCA -3 |
60 | 220 |
| − 857C/T | rs1799724 | F.P- 5-GGCTCTGAGGAATGGGTTAC-3 R.P- 5-CCTCTACATGGCCCTGTCTAC-3 S.P- 5- GTATGGGGACCCCCCCTTAA -3 |
56.5 | 127 |
| − 1031 T/C | rs1799964 | F.P- 5-TATGTGATGGACTCACCAGGT-3 R.P- 5-CCTCTACATGGCCCTGTCTT-3 S.P- 5- CAAAGGAGAAGCTGAGAAGA -3 |
63 | 264 |
Abbreviations: F.P-Forward primer; R.P-Reverse Primer; S.P-SNaPshot Primer; bp-base pair.
2.5. Statistical analysis
The chi-square test was used to determine whether the allelic frequencies were in accordance with Hardy-Weinberg equilibrium (HWE). The conditional logistic regression analysis was used to estimate Odds Ratio (OR) and 95% confidence intervals (CIs) for the strength of association between TNF-α gene polymorphisms and risk of IS. Multivariate logistic regression was used to control the confounding effects of demographic and risk factor variables. Tests were considered significant at p < 0.05. Data was analyzed using the STATA, version 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). The linkage disequilibrium (LD) analyses were performed using HaploView 4.2 software (Barrett et al., 2005) and haplotype analyses were done by PHASE software. The threshold value of the frequencies of the haplotypes included in the analysis was set to 2%.
3. Results
After screening 389 stroke cases, 250 IS cases were included in the study. For the control group, 321 people were screened and 250 age and sex matched controls were recruited for the study. The mean age of IS patients was 52.8 ± 12.5 years and control group was 50.9 ± 12.7 years and both groups consisted of 203 males and 47 females. The clinical characteristics of IS patients and controls are presented in Table 2. The risk factors examined such as history of hypertension (cases 58.4% vs controls 16.8%), diabetes (cases 31.6% vs controls 10.4%), smoking (cases 38.8% vs controls 26.8%), alcohol intake (cases 32.4% vs controls 22.4%) and dyslipidemia (cases 22.8% vs controls 5.6%) were found significantly more often in cases than in controls (p < 0.05). Out of 250 cases, 157 (62.8%) cases were recruited from the outpatient department (OPD) and 93 (37.2%) cases were recruited from the inpatient department (IPD). 240 cases (96.0%) completed full 6 month telephonic follow-up, 7 patients (2.8%) died, 2 patients (0.4%) had a recurrence of ischemic stroke and 10 (4.0%) were lost to follow-up. The mean and standard deviation (S.D) was 12.55 ± 13.42 for the NIHSS at admission, 3.06 ± 1.05 for mRS and 63.56 ± 16.69 for BI at discharge. After telephonic follow up at 6 months, the mean and S.D. was 1.30 ± 1.16 for mRS and 85.31 ± 15.26 for BI.
Table 2.
Demographic and risk factor variables for ischemic stroke (IS) patients and control subjects.
| Characteristics | Controls (N = 250) n (%) | Ischemic stroke (N = 250) n (%) | Crude OR [95% CI], p value | *Adjusted OR [95% CI], p value |
|---|---|---|---|---|
| Age in years (Mean + S.D) | 52.83 ± 12.59 | 50.97 ± 12.70 | Matched | |
| Male/Female, n | 203/47 | 203/47 | ||
| LVD | - | 107 (42.8) | ||
| SVD | - | 83 (33.2) | ||
| CE | - | 26 (10.4) | ||
| ODE | - | 22 (8.8) | ||
| UDE | - | 12 (4.8) | ||
| NIHSS at admission (Mean + S.D) | - | 12.55 ± 13.42 | ||
| BI score at discharge (Mean + S.D) | - | 63.56 ± 16.69 | ||
| BI score at 6 months (Mean + S.D) | - | 85.51 ± 15.26 | ||
| mRS score at discharge (Mean + S.D) | - | 3.06 ± 1.05 | ||
| mRS score at 6 months (Mean + S.D) | - | 1.3 ± 1.16 | ||
| Hypertension | 42 (16.8) | 146 (58.4) | 8.4 [4.8 to 14.6],<0.0001 | 6.2 [3.2 to 12], < 0.0001 |
| Diabetes | 26 (10.4) | 79 (31.6) | 3.5 [2.1 to 5.7], < 0.0001 | 2.1 [1.1 to 4.2],0.02 |
| Dyslipidemia | 14(5.6) | 57(22.8) | 5.2 [2.6 to10.4], < 0.0001 | 2.4 [1.0 to 5.7],0.04 |
| Smoking | 67 (26.8) | 97 (38.8) | 1.7 [1.1 to 2.5],0.005 | 1.1 [0.6to1.9],0.69 |
| Alcohol | 56 (22.4) | 81 (32.4) | 1.8 [1.1 to 2.8],0.008 | 1.8 [0.9 to 3.5],0.05 |
| Myocardial Infarction | 4 (1.6) | 17 (6.8) | 5.3 [1.5 to 18.3],0.008 | 1.8 [0.4 to 7.1],0.36 |
| Migraine with Aura | 8 (3.2) | 10 (4) | 1.2 [0.4 to 3.1],0.63 | 1.6 [0.4 to 5.8],0.40 |
| Migraine without Aura | 4 (1.6) | 5 (2) | 1.2 [0.3 to 4.6],0.33 | 1.3 [0.1 to 10.9],0.78 |
| Low Socioeconomic Status | 14 (5.6) | 66 (26.4) | 5.0 [2.7 to 9.0], < 0.0001 | 7.5 [3.0 to18.2],<0.0001 |
| High BMI | 89 (35.6) | 77 (30.8) | 0.7 [0.4 to 1.0],0.11 | 0.8 [0.4 to1.4],0.50 |
| Sedentary Life Style | 106 (42.4) | 127 (50.8) | 1.4 [1.0 to 2.1],0.04 | 1.1 [0.6 to1.9],0.6 |
| Physical activity | 136 (54.4) | 107 (42.8) | 0.6 [0.4 to 0.8], 0.009 | 0.7 [0.4 to 1.3],0.36 |
| Family history of stroke | 9 (3.6) | 32 (12.8) | 3.5 [1.6 to 7.4],0.001 | 6.8 [2.2 to 20.9],0.001 |
| Family history of diabetes | 28 (11.2) | 48 (19.2) | 1.8 [1.1 to 3.1],0.015 | 3.1 [1.4 to 6.9],0.004 |
| Family history of hypertension | 34 (13.6) | 61 (24.4) | 2.0 [1.2 to 3.1],0.003 | 1.8 [0.9 to 3.4],0.06 |
| Family history of heart Attack | 14 (5.6) | 19 (7.6) | 1.4 [0.6 to 3.1],0.33 | 1.7[0.5 to 5.9],0.38 |
Conditional Logistic Regression Analysis.
*Adjusted variables include Hypertension, Diabetes, Dyslipidemia, Smoking, Family History of stroke, Alcohol, Sedentary Life Style and Low socioeconomic status.
Abbreviations: BMI- Body Mass Index; OR- Odds Ratio; CI- Confidence Interval; SD- Standard Deviation;LVD-Large Vessel Disease;SVD-Small Vessel disease;CE-Cardioembolic;ODE-Other determined etiology; UDE-Undetermined etiology.
All genotype and allelic frequencies were in HWE in both IS patients and controls. Genetic analysis for TNF-α (− 308G/A, + 488G/A, − 857C/T and -1031 T/C) gene polymorphisms were conducted for all 250 IS cases and 250 age-sex matched controls and are summarized in Table 3. Adjusted conditional logistic regression analysis showed an independent association of TNF-α + 488G/A (OR 2.59; 95%CI-1.46 to 4.60; P = 0.001) and -857C/T (OR 1.77; 95%CI 1.01 to 3.11; P < 0.04) with the risk of IS under dominant model. However, no significant association was observed for -308G/A and -1031 T/C gene polymorphisms with the risk of IS. After further analysis based on TOAST classification, we observed significant association between TNF-α -308G/A gene polymorphism and risk of IS under dominant (OR 4.57; 95%CI 1.39 to 15.0; P = 0.01) and allelic (OR 2.67; 95%CI 1.19 to 5.95; P = 0.01) models for others (Stroke due to determined + undetermined etiology) subtype of IS.
Table 3.
Genotype and allelic frequencies of TNF-α (− 308G/A, + 488G/A, − 857C/T and -1031 T/C) gene polymorphisms in IS patients and controls.
| Polymorphisms | LVD N = 107 |
SVD N = 83 |
CE N = 26 |
Others N = 34 |
IS N = 250 |
Controls N = 250 |
||
|---|---|---|---|---|---|---|---|---|
| G308A | Genotype | GG, n (%) | 93 (86.9) | 75 (90.3) | 23 (88.5) | 27 (79.4) | 218 (86.8) | 225 (90) |
| GA, n (%) | 14 (13) | 7 (8.4) | 3 (11.5) | 5 (14.7) | 29 (11.6) | 23 (9.2) | ||
| AA, n (%) | 0 | 1 (1.2) | 0 | 2 (5.8) | 3 (1.2) | 2 (0.8) | ||
| Allele | G, n (%) | 200 (93.4) | 157 (94.5) | 49 (94.2) | 59 (86.7) | 465 (93) | 473 (94.6) | |
| A, n(%) | 14 (6.6) | 9 (5.5) | 3 (5.8) | 9 (13.2) | 35 (7) | 27 (5.4) | ||
| Dominant GA + GG vs. AA | Adjusted OR (95% CI), P value | 1.40 (0.59–3.35), 0.43 | 1.04 (0.37–2.88), 0.93 | 1.59 (0.35–7.29), 0.54 | 4.57 (1.39–15.0), 0.01 | 1.42 (0.73–2.76), 0.3 | ||
| Unadjusted OR (95% CI), P value | 1.35 (0.67–2.72), 0.39 | 0.96 (0.41–2.21), 0.92 | 1.17 (0.32–4.18), 0.80 | 2.33 (0.92–5.90), 0.07 | 1.36 (0.75–2.47), 0.29 | |||
| Recessive AA vs. GA + GG | Adjusted OR (95% CI), P value | NE | 4.94 (0.41–58.79), 0.20 | NE | NE | 4.15 (0.63–27.30), 0.13 | ||
| Unadjusted OR (95% CI), P value | NE | 1.51 (0.13–16.89), 0.73 | NE | 1.75 (1.05–56.93), 0.04 | 1.49 (0.25–8.97), 0.65 | |||
| Allelic A vs. G | OR (95% CI), P value | 1.22 (0.62–2.38), 0.54 | 1.00(0.46–2.18), 0.56 | 1.07 (0.31–3.66), 0.55 | 2.67(1.19–5.95), 0.01 | 1.31 (0.78–2.21) ,0.29 |
||
| Polymorphisms | LVD N = 107 |
SVD N = 83 |
CE N = 26 |
Others N = 34 |
IS N = 250 |
Controls N = 250 |
||
|---|---|---|---|---|---|---|---|---|
| G488A | Genotype | GG, n (%) | 68 (63.5) | 59 (71) | 18 (69.2) | 17 (50) | 162 (64.8) | 192 (76.8) |
| GA, n (%) | 36 (33.6) | 22 (26.5) | 8 (30.8) | 16 (47) | 82 (32.8) | 55 (22) | ||
| AA, n (%) | 3 (2.8) | 2 (2.4) | 0 | 1 (3) | 6 (2.4) | 3 (1.2) | ||
| Allele | G (%) | 172 (80.4) | 140 (84.3) | 44 (84.6) | 50 (73.5) | 406 (81.2) | 439 (87.7) | |
| A (%) | 42 (19.6) | 26 (15.7) | 8 (15.4) | 18 (26.4) | 94 (18.8) | 61 (12.2) | ||
| Dominant (GG + GA vs. AA) | Adjusted OR (95% CI), P value | 2.23 (1.20–4.14), 0.01 | 2.33 (1.17–4.65), 0.01 | 1.89 (0.65–5.50), 0.23 | 4.88 (1.90–12.52), 0.01 | 2.59 (1.46–4.60), 0.001 | ||
| Unadjusted OR (95% CI), P value | 1.89 (1.16–3.10), 0.01 | 1.34 (0.77–2.35), 0.29 | 1.47 (0.60–3.55), 0.39 | 3.31 (1.58–6.89), 0.001 | 1.78 (1.20–2.66), 0.004 | |||
| Recessive (GG vs. AA + GA) | Adjusted OR (95% CI), P value | 2.12 (0.22–19.93), 0.50 | 1.61 (0.14–18.29), 0.70 | NE | 0.58 (0.03–10.91), 0.72 | 1.75 (0.29–10.33), 0.53 | ||
| Unadjusted OR (95% CI), P value | 2.37 (0.47–11.96), 0.29 | 2.03 (0.33–12.380, 0.44 | NE | 2.49 (0.25–24.69), 0.43 | 2.00 (0.50–7.99), 0.32 | |||
| Allelic G vs. A | OR (95% CI), P value | 1.75 (1.14–2.70), 0.009 | 1.33 (0.81–2.19), 0.25 | 1.30 (0.58–2.91), 0.50 | 2.59 (1.41–4.72), < 0.001 | 1.66 (1.17–2.36), 0.003 |
||
| Polymorphisms | LVD N = 107 |
SVD N = 83 |
CE N = 26 |
Others N = 34 |
IS N = 250 |
Controls N = 250 |
||
|---|---|---|---|---|---|---|---|---|
| C857T | Genotype | CC, n (%) | 71 (66.3) | 59 (71) | 18 (69.2) | 22 (64.7) | 170 (68) | 172 (68.8) |
| CT, n (%) | 29 (27.1) | 21 (25.3) | 7 (26.9) | 10 (29.4) | 67 (26.8) | 68 (27.2) | ||
| TT, n (%) | 7 (6.5) | 3 (3.6) | 1 (3.8) | 2 (5.8) | 13 (5.2) | 10 (4) | ||
| Allele | C (%) | 171 (79.9) | 139 (83.7) | 43 (82.6) | 54 (79.4) | 407 (81.4) | 412 (82.4) | |
| T (%) | 43 (20.1) | 27 (16.3) | 9 (17.4) | 14 (20.5) | 93 (18.6) | 88 (17.6) | ||
| Dominant CC + CT vs. TT | Adjusted OR (95% CI), P value | 1.57 90.86–2.86), 0.13 | 1.74 (90.89–3.36), 0.10 | 1.51 (0.53–4.29), 0.43 | 1.52 (0.61–3.82), 0.36 | 1.77 (1.01–3.11), 0.04 | ||
| Unadjusted OR (95% CI), P value | 1.11 (0.69–1.81), 0.65 | 0.89 (0.52–1.54), 0.69 | 0.98 (0.40–2.35), 0.l9 | 1.20 (0.56–2.55), 0.63 | 1.03 (0.71–1.51), 0.84 | |||
| Recessive TT vs. CT + CC | Adjusted OR (95% CI), P value | 2.32 (0.69–7.73), 0.17 | 1.17 (0.25–5.28), 0.83 | 0.76 (0.07–8.01), 0.82 | 3.14 (0.53–18.47), 0.20 | 1.70 (0.62–4.60), 0.29 | ||
| Unadjusted OR (95% CI), P value | 1.68 (0.62–4.53), 0.30 | 0.90 (0.24–3.35), 0.87 | 0.96 (0.11–7.81), 0.11 | 1.50 (0.31–7.15), 0.61 | 1.33 (0.56–3.16), 0.51 | |||
| Allelic T vs. C | OR (95% CI), P value | 1.17 (0.78–1.76), 0.43 | 0.90 (0.56–1.45), 0.68 | 0.97 (0.46–2.08), 0.56 | 1.21 (0.64–2.28), 0.54 | 1.06 (0.77–1.47), 0.68 |
||
| Polymorphisms | LVD N = 107 |
SVD N = 83 |
CE N = 26 |
Others N = 34 |
IS N = 250 |
Controls N = 250 |
||
|---|---|---|---|---|---|---|---|---|
| T1031C | Genotype | TT, n (%) | 64 (59.8) | 39 (46.9) | 13 (50) | 18 (52.9) | 134 (53.6) | 121 (48.4) |
| TC, n (%) | 34 (31.7) | 26 (31.3) | 12 (46.1) | 13 (38.2) | 35 (38) | 115 (46) | ||
| CC, n (%) | 9 (8.4) | 8 (9.6) | 1 (3.8) | 3 (8.8) | 21 (8.4) | 14 (5.6) | ||
| Allele | T (%) | 162 (75.7) | 114 (68.6) | 38 (73) | 49 (72) | 363 (72.6) | 357 (71.4) | |
| C (%) | 52 (24.2) | 52 (31.3) | 14 (27) | 19 (28) | 137 (27.4) | 143 (28.6) | ||
| Dominant (TT + TC vs. CC) | Adjusted OR (95% CI), P value | 0.54 (0.31–0.96), 0.03 | 1.09 (0.60–1.99), 0.76 | 0.96 (0.36–2.54), 0.94 | 0.83 (0.35–1.97), 0.67 | 0.54 (0.31–0.91), 0.02 | ||
| Unadjusted OR (95% CI), P value | 0.63 (0.39–0.99), 0.04 | 1.05 (0.64–1.74), 0.82 | 0.93 (0.41–2.10), 0.87 | 0.83 (0.40–1.70), 0.62 | 0.81 (0.57–1.15), 0.25 | |||
| Recessive CC vs. TC + TT | Adjusted OR (95% CI), P value | 1.94 (0.69–5.45), 0.20 | 2.46 (0.84–7.16), 0.09 | 0.66 (0.05–8.53), 0.75 | 2.31 (0.48–11.10), 0.29 | 1.60 (0.70–3.64), 0.26 | ||
| Unadjusted OR (95% CI), P value | 1.54 (0.64–3.69), 0.32 | 1.79 (0.72–4.45), 0.20 | 0.67 (0.08–5.34), 0.70 | 1.63 (0.44–5.99) 0.46 | 1.50 (0.76–2.94), 0.24 | |||
| Allelic C vs. T | OR (95% CI), P value | 0.80 (0.55–1.15), 0.23 | 1.13 (0.77–1.60), 0.56 | 0.91 (0.48–1.74), 0.79 | 0.96 (0.55–1.70), 0.92 | 0.94 (0.71–1.24), 0.67 | ||
Abbreviations: LVD- large vessel stroke; SVD-small vessel stroke; CE-cardioembolic stroke; others includes- stroke due to undetermined aetiology + other determined aetiology; IS-Ischemic Stroke; NE- Not Estimable; OR- Odds Ratio; CI- Confidence Interval.
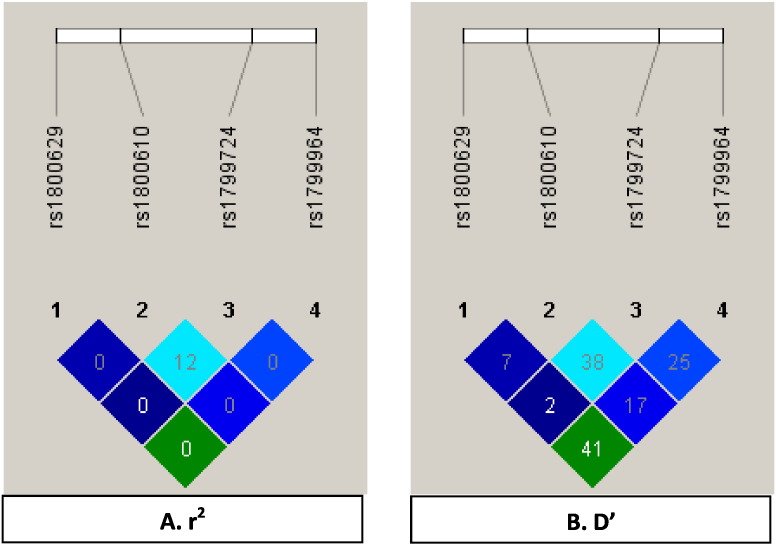
Haplotype analysis showed that A308-G488-C857-T1031 haplotypes were significantly associated with the increased risk of IS [OR 1.66; 95%CI 1.02 to 2.71; P = 0.003] (Table 4). Strong linkage disequilibrium (D’ = 0.41, r2 = 0.004) was detected between two SNPs (+ 488G/A and -857C/T) in the TNF-α gene (Fig. 1).
Table 4.
Frequencies and association of Tumor Necrosis Factor Alpha (− 308G/A, + 488G/A, − 857C/T and -1031 T/C) haplotypes in IS patients and controls.
| Haplotypes | IS cases n (%) |
Controls n (%) |
Odds Ratio (95% CI) | P value |
|---|---|---|---|---|
| G308-G488-C857-T1031 | 248 (49.6) | 253 (50.6) | Reference | |
| G308-G488-C857-C1031 | 87 (17.2) | 112 (22.4) | 0.56 (0.56–1.10) | 0.16 |
| G308-G488-T857-T1031 | 31 (6.2) | 26 (5.2) | 1.21 (0.70–2.10) | 0.69 |
| G308-G488-T857-C1031 | 31 (6.2) | 26 (5.2) | 1.21 (0.70–2.10) | 0.69 |
| G308-A488-C857-T1031 | 13 (2.6) | 23 (4.6) | 0.57 (0.28–1.16) | 0.12 |
| G308-A488-C857-C1031 | 6 (1.2) | 3 (0.6) | 2.04 (0.50–8.24) | 0.31 |
| G308-A488-T857-T1031 | 49 (9.8) | 30 (6) | 1.66 (1.02–2.71) | 0.03 |
| A308-G488-C857-T1031 | 35 (7) | 27 (5.4) | 1.32 (0.77–2.25) | 0.30 |
| Total | 500 | 500 | ||
Fig. 1.
LD plots of the four SNPs (− 308G/A, + 488G/A, − 857C/T and -1031 T/C) of TNF-α gene in North Indian population. The values in the squares are the pair-wise calculations of r2 (A) or D’ (B). The squares with the “0″ indicate r2 = 0 (i.e., No LD between a pair of SNPs). The square with the “41″ indicate D’ = 0.41 (i.e., medium LD between a pair of SNPs).
4. Discussion
The present study was the first study from North India which revealed that TNF-α (+ 488G/A and -857C/T) gene polymorphisms and their haplotypes were significantly associated with increased risk of IS. Case–control genetic association studies are being used for studying the genetic basis of complex multifactorial diseases. The TNF-α gene represents a strong candidate gene for the pathogenesis of stroke. In fact, TNF is known to play several pro-inflammatory and pro-coagulant effects on endothelium and, therefore, to expose vascular segments to local inflammation, thrombosis and hemorrhage (Terry et al., 1999, Mark et al., 2001, Hallenbeck, 2002). Its contributory role to stroke initiation and progression has been the topic of recent investigations. In this regard, the experimental evidence has clearly documented a critical role of TNF-stimulation on the increased sensitivity to induction of brain ischemia and hemorrhage (Sirén et al., 2001, Pinto et al., 2006; Tuttolomondo et al., 2014, Tuttolomondo et al., 2015). The previous evidence in the favour of a relationship of TNF-α gene with occurrence of IS in humans has been reported in different populations as documented by the previous meta-analyses published by (Pereira et al., 2007) and (Gu et al., 2013) suggest that TNF-α -308G/A polymorphism might be a protective factor for IS in adult Asian population.
Recently several Genome Wide Association Studies (GWAS) for stroke have been reported, (Kubo et al., 2007, Ikram et al., 2009, Matarín et al., 2007, Matarin et al., 2009, Yamada et al., 2009, Gretarsdottir et al., 2008) but most of these study's populations were of European origin and they did not detect the association of -308G/A gene polymorphism in TNF-α gene with risk of stroke. Another study published by Cui et al. (2012) showed a significant association between –308G/A polymorphism and risk of stroke (OR 1.34; 95% CI 1.02 to 1.77) and did not show any association for -857C/T and -1031 T/C gene polymorphism with IS risk. TNF-α + 488G/A was found to be an important risk factor for ischemic stroke in South Indian population (Munshi et al., 2011). Our present study suggests a significant association between + 488G/A and -857C/T gene polymorphisms in TNF-α gene but shows no significant association between -308G/A and -1031 T/C gene polymorphisms with IS risk.
The results of our present case–control study provide more convincing evidence of the association between TNF-α gene polymorphisms and risk of IS after adjusting the confounding variables including hypertension, alcohol, diabetes, dyslipidemia, family history of stroke, sedentary life style and low socioeconomic status. A high degree of LD was observed between the two SNPs (+ 488G/A and -857C/T) in our study. The study results published by (Banerjee et al., 2008, Munshi et al., 2011, Sultana et al., 2011, Tong et al., 2010) showed the protective role of TNF-α -308G/A gene polymorphism with the risk of IS. Our study results are in accordance with the study published by (Wawrzynek et al., 2014) showing non-significant association with the risk of IS in Caucasians living in Poland. Our findings suggest significant association of TNF-α -308G/A gene polymorphism with others subtype (Stroke due to determined + undetermined etiology) of IS. Tuttolomondo et al. (2012) showed no differences in the genotype and allelic distributions (Tuttolomondo et al., 2012). The most studied and interesting aspects of -308A/G polymorphism remain unexplained: there are many discrepancies between the results. However, the cause of this is not clear. Differences in the ethnicity of the studied population may be taken as one of the possibilities.
However, there were a few limitations in our study. Firstly, the study was conducted in a single hospital and the participants might not have been the representatives from other areas. Therefore, further large sample size and multicentric studies are needed to confirm our findings. Secondly, we did not evaluate the plasma level of TNF-α in IS patients and controls. Despite these limitations, our study provides strong evidence for the association between TNF-α (+ 488G/A and -857C/T) gene polymorphisms and risk of IS.
5. Conclusion
Two SNPs (+ 488G/A and -857C/T) of TNF-α gene and their haplotypes are significantly associated with the risk of IS in the population enrolled from North India. Our findings indicate that polymorphisms and haplotypes of TNF-α gene may be used as a genetic marker for identifying individuals at increased risk for developing IS.
Conflict of Interest
The authors have declared that no competing interests exist.
Funding Source
Institute of Genomics and Integrative Biology, New Delhi.
Acknowledgements
We thank all patients and controls participants for providing blood samples.
References
- Banerjee I., Gupta V., Ahmed T., Faizaan M., Agarwal P., Ganesh S. Inflammatory system gene polymorphism and the risk of stroke: a case–control study in an Indian population. Brain Res. Bull. 2008;75:158–165. doi: 10.1016/j.brainresbull.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Barone F.C., Arvin B., White R.F., Miller A., Webb C.L., Willette R.N., Lysko P.G., Feuerstein G.Z. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke J. Cereb. Circ. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinforma. Oxf. Engl. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bazzoni F., Beutler B. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- Bevan S., Markus H.S. Genetics of common polygenic ischaemic stroke: current understanding and future challenges. Stroke Res. Treat. 2011;2011:179061. doi: 10.4061/2011/179061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr F.J., McBride M.W., Carswell H.V.O., Graham D., Strahorn P., Clark J.S., Charchar F.J., Dominiczak A.F. Genetic aspects of stroke: human and experimental studies. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2002;22:767–773. doi: 10.1097/00004647-200207000-00001. [DOI] [PubMed] [Google Scholar]
- Cui G., Wang H., Li R., Zhang L., Li Z., Wang Y., Hui R., Ding H., Wang D.W. Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke. J. Neuroinflammation. 2012;9:235. doi: 10.1186/1742-2094-9-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin V.L., Wiebers D.O., Nikitin Y.P., O'Fallon W.M., Whisnant J.P. Risk factors for ischemic stroke in a Russian community: a population-based case–control study. Stroke J. Cereb. Circ. 1998;29:34–39. doi: 10.1161/01.str.29.1.34. [DOI] [PubMed] [Google Scholar]
- Feuerstein G.Z., Liu T., Barone F.C. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc. Brain Metab. Rev. 1994;6:341–360. [PubMed] [Google Scholar]
- Feuerstein G., Wang X., Barone F.C. Cytokines in brain ischemia–the role of TNF alpha. Cell. Mol. Neurobiol. 1998;18:695–701. doi: 10.1023/A:1020690004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flex A., Gaetani E., Papaleo P., Straface G., Proia A.S., Pecorini G., Tondi P., Pola P., Pola R. Proinflammatory genetic profiles in subjects with history of ischemic stroke. Stroke J. Cereb. Circ. 2004;35:2270–2275. doi: 10.1161/01.STR.0000140740.19421.fe. [DOI] [PubMed] [Google Scholar]
- Gretarsdottir S., Thorleifsson G., Manolescu A., Styrkarsdottir U., Helgadottir A., Gschwendtner A., Kostulas K., Kuhlenbäumer G., Bevan S., Jonsdottir T. Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann. Neurol. 2008;64:402–409. doi: 10.1002/ana.21480. [DOI] [PubMed] [Google Scholar]
- Gu L., Wu G., Long J., Su L., Yan Y., Chen Q., Xie J., Hu Y. The role of TNF-α 308G > a polymorphism in the risk for ischemic stroke. Am. J. Med. Sci. 2013;345:227–233. doi: 10.1097/MAJ.0b013e31825f92da. [DOI] [PubMed] [Google Scholar]
- Hallenbeck J.M. The many faces of tumor necrosis factor in stroke. Nat. Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hollegaard M.V., Bidwell J.L. Cytokine gene polymorphism in human disease: on-line databases, supplement 3. Genes Immun. 2006;7:269–276. doi: 10.1038/sj.gene.6364301. [DOI] [PubMed] [Google Scholar]
- Ikram M.A., Seshadri S., Bis J.C., Fornage M., DeStefano A.L., Aulchenko Y.S., Debette S., Lumley T., Folsom A.R., van den Herik E.G. Genomewide association studies of stroke. N. Engl. J. Med. 2009;360:1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W.J., Williams L.S., Meschia J.F. Validating the questionnaire for verifying stroke-free status (QVSFS) by neurological history and examination. Stroke J. Cereb. Circ. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- Kubo M., Hata J., Ninomiya T., Matsuda K., Yonemoto K., Nakano T., Matsushita T., Yamazaki K., Ohnishi Y., Saito S. A nonsynonymous SNP in PRKCH (protein kinase C eta) increases the risk of cerebral infarction. Nat. Genet. 2007;39:212–217. doi: 10.1038/ng1945. [DOI] [PubMed] [Google Scholar]
- Kumar A., Prasad M., Kathuria P. Sitting occupations are an independent risk factor for ischemic stroke in north Indian population. Int. J. Neurosci. 2014;124:748–754. doi: 10.3109/00207454.2013.879130. [DOI] [PubMed] [Google Scholar]
- Kumar A., Prasad M., Kathuria P., Nair P., Pandit A.K., Sahu J.K., Prasad K. Low socioeconomic status is an independent risk factor for ischemic stroke: a case–control study in north Indian population. Neuroepidemiology. 2015;44:138–143. doi: 10.1159/000374118. [DOI] [PubMed] [Google Scholar]
- Liu T., Clark R.K., McDonnell P.C., Young P.R., White R.F., Barone F.C., Feuerstein G.Z. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke J. Cereb. Circ. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Maemura K., Kurihara H., Morita T., Oh-hashi Y., Yazaki Y. Production of endothelin-1 in vascular endothelial cells is regulated by factors associated with vascular injury. Gerontology. 1992;38(Suppl 1):29–35. doi: 10.1159/000213360. [DOI] [PubMed] [Google Scholar]
- Mark K.S., Trickler W.J., Miller D.W. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J. Pharmacol. Exp. Ther. 2001;297:1051–1058. [PubMed] [Google Scholar]
- Matarín M., Brown W.M., Scholz S., Simón-Sánchez J., Fung H.-C., Hernandez D., Gibbs J.R., De Vrieze F.W., Crews C., Britton A. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol. 2007;6:414–420. doi: 10.1016/S1474-4422(07)70081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarin M., Brown W.M., Dena H., Britton A., De Vrieze F.W., Brott T.G., Brown R.D., Worrall B.B., Case L.D., Chanock S.J. Candidate gene polymorphisms for ischemic stroke. Stroke J. Cereb. Circ. 2009;40:3436–3442. doi: 10.1161/STROKEAHA.109.558015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschia J.F. Subtyping in ischemic stroke genetic research. J. Stroke Cerebrovasc. Dis. 2002;11:208–219. doi: 10.1053/jscd.2002.129599. [DOI] [PubMed] [Google Scholar]
- Munshi A., Rajeshwar K., Kaul S., Al-Hazzani A., Alshatwi A.A., Shafi G., Balakrishna N., Jyothy A. Association of tumor necrosis factor-α and matrix metalloproteinase-3 gene variants with stroke. Eur. J. Neurol. 2011;18:1053–1059. doi: 10.1111/j.1468-1331.2010.03334.x. [DOI] [PubMed] [Google Scholar]
- Nedwin G.E., Svedersky L.P., Bringman T.S., Palladino M.A., Goeddel D.V. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J. Immunol. Baltim. Md. 1985;1950(135):2492–2497. [PubMed] [Google Scholar]
- Pereira T.V., Rudnicki M., Franco R.F., Pereira A.C., Krieger J.E. Effect of the G-308A polymorphism of the tumor necrosis factor alpha gene on the risk of ischemic heart disease and ischemic stroke: a meta-analysis. Am. Heart J. 2007;153:821–830. doi: 10.1016/j.ahj.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Pinto A., Tuttolomondo A., Di Raimondo D., Fernandez P., Licata G. Risk factors profile and clinical outcome of ischemic stroke patients admitted in a department of internal medicine and classified by TOAST classification. Int. Angiol. J. Int. Union Angiol. 2006;25:261–267. [PubMed] [Google Scholar]
- Rubattu S., Speranza R., Ferrari M., Evangelista A., Beccia M., Stanzione R., Assenza G.E., Volpe M., Rasura M. A role of TNF-alpha gene variant on juvenile ischemic stroke: a case–control study. Eur. J. Neurol. 2005;12:989–993. doi: 10.1111/j.1468-1331.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- Sirén A.L., McCarron R., Wang L., Garcia-Pinto P., Ruetzler C., Martin D., Hallenbeck J.M. Proinflammatory cytokine expression contributes to brain injury provoked by chronic monocyte activation. Mol. Med. Camb. Mass. 2001;7:219–229. [PMC free article] [PubMed] [Google Scholar]
- Sultana S., Kolla V.K., Jeedigunta Y., Penagaluru P.K., Joshi S., Rani P.U., Reddy P.P. Tumour necrosis factor alpha and interleukin 10 gene polymorphisms and the risk of ischemic stroke in south Indian population. J. Genet. 2011;90:361–364. doi: 10.1007/s12041-011-0079-5. [DOI] [PubMed] [Google Scholar]
- Terry C.M., Clikeman J.A., Hoidal J.R., Callahan K.S. TNF-alpha and IL-1alpha induce heme oxygenase-1 via protein kinase C, Ca2 +, and phospholipase A2 in endothelial cells. Am. J. Physiol. 1999;276:H1493–H1501. doi: 10.1152/ajpheart.1999.276.5.H1493. [DOI] [PubMed] [Google Scholar]
- Tong Y., Geng Y., Xu J., Wang Z., Zhang Y., Lin L., Zhang R., Deng P., Li Y., Hou W. The role of functional polymorphisms of the TNF-alpha gene promoter in the risk of ischemic stroke in Chinese Han and Uyghur populations: two case–control studies. Clin. Chim. Acta Int. J. Clin. Chem. 2010;411:1291–1295. doi: 10.1016/j.cca.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A., Di Raimondo D., Forte G.I., Casuccio A., Vaccarino L., Scola L., Pecoraro R., Serio A., Clemente G., Arnao V. Single nucleotide polymorphisms (SNPs) of pro-inflammatory/anti-inflammatory and thrombotic/fibrinolytic genes in patients with acute ischemic stroke in relation to TOAST subtype. Cytokine. 2012;58:398–405. doi: 10.1016/j.cyto.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A., Pecoraro R., Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Des. Devel. Ther. 2014;8:2221–2238. doi: 10.2147/DDDT.S67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttolomondo A., Pecoraro R., Casuccio A., Di Raimondo D., Buttà C., Clemente G., Della Corte V., Guggino G., Arnao V., Maida C. Peripheral frequency of CD4 + CD28- cells in acute ischemic stroke: relationship with stroke subtype and severity markers. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J.-Y., Kim H.-M. Tumor necrosis factor alpha gene polymorphism is associated with cerebral infarction. Brain Res. Mol. Brain Res. 2004;122:99–102. doi: 10.1016/j.molbrainres.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Wawrzynek A., Dobiała J., Wender M., Kozubski W., Michałowska-Wender G. TNFα gene G-308A polymorphism and the risk of ischemic stroke. Neurol. Neurochir. Pol. 2014;48:387–390. doi: 10.1016/j.pjnns.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Wilson A.G., Symons J.A., McDowell T.L., McDevitt H.O., Duff G.W. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Fuku N., Tanaka M., Aoyagi Y., Sawabe M., Metoki N., Yoshida H., Satoh K., Kato K., Watanabe S. Identification of CELSR1 as a susceptibility gene for ischemic stroke in Japanese individuals by a genome-wide association study. Atherosclerosis. 2009;207:144–149. doi: 10.1016/j.atherosclerosis.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Zaremba J. Contribution of tumor necrosis factor alpha to the pathogenesis of stroke. Folia Morphol. (Warsz) 2000;59:137–143. [PubMed] [Google Scholar]