Abstract
Fine-root production and turnover are important regulators of the biogeochemical cycles of ecosystems and key components of their response to global change. We present a nearly continuous 6-year record of fine-root production and mortality from minirhizotron analysis of a closed-canopy, deciduous sweetgum forest in a free-air CO2 enrichment experiment. Annual production of fine roots was more than doubled in plots with 550 ppm CO2 compared with plots in ambient air. This response was the primary component of the sustained 22% increase in net primary productivity. Annual fine-root mortality matched annual production, and the mean residence time of roots was not altered by elevated CO2, but peak fine-root standing crop in midsummer was significantly higher in CO2-enriched plots, especially deeper in the soil profile. The preferential allocation of additional carbon to fine roots, which have a fast turnover rate in this species, rather than to stemwood reduces the possibility of long-term enhancement by elevated CO2 of carbon sequestration in biomass. However, sequestration of some of the fine-root carbon in soil pools is not precluded, and there may be other benefits to the tree from a seasonally larger and deeper fine-root system. Root-system dynamics can explain differences among ecosystems in their response to elevated atmospheric CO2; hence, accurate assessments of carbon flux and storage in forests in a globally changing atmosphere must account for this unseen and difficult-to-measure component.
Researchers who attempt to quantify C pools and fluxes in terrestrial ecosystems often find it expedient to measure only the aboveground components despite the wide recognition that a substantial fraction of net primary production occurs belowground (1). Fine roots (ephemeral, nonwoody roots generally of diameters <1 mm) are important regulators of biogeochemical cycling (2), and their turnover is a key component of C sequestration in soil (3). Root production often is enhanced when the plants are grown in a CO2-enriched atmosphere (4, 5). However, the difficulty in measuring these roots, which cannot generally be seen, are not easily sampled, and can grow and die rapidly and unpredictably, means that they are left out of both retrospective (6) and prospective (7) studies of the potential for terrestrial ecosystems to sequester additional C in response to a changing atmosphere and climate. Fine-root production and distribution in forests exhibit high spatial and temporal variation, making their characterization especially difficult, and most of the experimental evidence on tree root responses to elevated CO2 comes from static or short-term observations that are strongly confounded with plant developmental trajectories (5). Free-air CO2 enrichment (FACE) experiments (8) provide an opportunity for longer-term observations under realistic conditions in fully developed forests stands.
We used minirhizotron observation tubes and video imaging (9, 10) to quantify fine-root production and mortality in a FACE experiment in an established deciduous forest. Minirhizotrons permit observations of the production and disappearance of individual fine roots over frequent sampling intervals without the repeated disturbance effects associated with coring techniques. Although the problem of high spatial variability remains (and the labor-intensive nature of the analysis precludes optimal replication), spatial and temporal variation are not confounded. We assumed that the root system in this closed-canopy forest stand had fully occupied the soil volume and was no longer expanding, and an initial hypothesis for the FACE experiment was that full soil occupancy would constrain the N cycle (5). Here we present fine-root dynamics for six growing seasons and evaluate their role in the integrated response of the forest to CO2 enrichment of the atmosphere.
Methods
Experimental Site. The experimental site is a planted sweetgum (Liquidambar styraciflua L.) forest stand, which was established in 1988 at the Oak Ridge National Environmental Research Park in Roane County, TN (35°54′N, 84°20′W). One-year-old, bare-rooted sweetgum seedlings were planted at a spacing of 2.3 × 1.2 m. Herbicide was used in 1989 and 1990 to control competition from weeds; no fertilizer has been added. The soil at the site, which is classified as an Aquic Hapludult, developed in alluvium washed from upland soils derived from a variety of rocks including dolomite, sandstone, and shale, has a silty clay loam texture, and is moderately well drained. The soil is slightly acidic (water pH, ≈5.5–6.0) with high base saturation largely dominated by exchangeable Ca. The mean annual temperature (1962–1993) is 13.9°C, and the mean annual precipitation is 1,371 mm, with a generally even distribution of precipitation throughout the year (11).
Five 25-m-diameter plots were laid out in 1996, and FACE apparatus (8) was assembled in four of them; site disturbance was minimized during construction. When pretreatment measurements were made in 1997, the trees (≈90 per plot) were 12 m tall with an average diameter of 11 cm and a stand basal area of 28 cm2·m-2 (11). The trees were in a linear growth phase, leaf area index was 5.5 m2·m-2, and the canopy was no longer expanding (12). Exposure to elevated CO2 commenced in two plots in April 1998 and has continued during the growing season (April to November) since then. The average daytime CO2 concentrations during the 1998–2003 growing seasons were 528, 538, 546, 548, 552, and 549 ppm in the two CO2-enriched plots, including periods when the exposure system was not functioning, and 384–398 ppm in ambient plots. The standard deviation of 1-min averages in the CO2-enriched plots was 60 ppm. The site and experimental design has been fully described in ref. 11, and operating and meteorological conditions are documented at http://face.ornl.gov and http://cdiac.ornl.gov/programs/FACE/ornldata/ornldata.html.
Minirhizotron Analysis. Five minirhizotron tubes were installed in each FACE plot in July 1997. Cellulose acetate butyrate minirhizotron tubes (Bartz Technology, Santa Barbara, CA) were installed in each plot at a 60° angle from vertical and to a depth of 60 cm by using a hand auger (10). The tubes were stabilized with rebar and wrapped above the soil surface with black foam insulation, the upper ends sealed with a rubber stopper. Video images of the root systems were recorded beginning in February 1998 and continued biweekly during the growing seasons through November 2003. Video images were collected with a BTC-2 minirhizotron camera with a Smucker handle (Bartz Technology). Images were not collected during the winter because shrinkage of the tubes in cold soil precluded movement of the camera in the tubes. Each tube had 90 viewing windows (12.4 × 18.0 mm) for a total viewing area of 0.0201 m2. From 1998 to 2002, individual frames on the videotape were captured by using a Targa+ video board (True Vision, Indianapolis) and digitized by using roots software (Michigan State University, Lansing). In 2003, digital images were captured in the field by using the I-CAP system (Bartz Technology) and analyzed with rootracker software (Duke University, Durham, NC). The length and width of each root segment were measured, and the incremental growth, death, or disappearance was recorded. Root-length production, mortality, and standing crop were calculated as described in ref. 9. Roots were not included in mortality until they disappeared; although a few roots that were most likely dead were observed in the minirhizotron windows, they always disappeared quickly, and we equate “disappearance” with “mortality.”
Cohort analysis was used to estimate fine-root longevity. We measured survivorship on an annual basis for all roots produced between the first filming of the year to June 1 of that year. Roots present at the first filming were excluded, as were all roots >1.0 mm in diameter. Survival data were analyzed (13) and tested for significance by using Peto and Peto's log-rank test (with 1° of freedom and α = 0.05) for censored data, where not all individuals followed begin the treatment at the same time. Half-lives for the root cohorts were calculated from regressions of log-transformed data by year and CO2 treatment.
Soil Cores. Five soil cores were extracted from each plot in July 2002 by using a 10-cm-diameter hole saw. The cores were 15 cm deep and collected in 5-cm increments. Roots were picked from the cores first by hand, and then the soil was washed through a 590-μm sieve. Roots were sorted into diameter classes (<0.5 mm, 0.5–1.0 mm, 1.0–2.0 mm, and >2.0 mm). A subsample of different diameter classes from each plot and depth was removed and kept cold and moist; the remaining roots were oven-dried and weighed. The subsampled roots were laid out individually on a minirhizotrom tube in the laboratory and filmed and digitized to determine length by using the same techniques as used in the field. These roots were then dried and weighed, and specific root lengths (SRLs) (length/mass) were determined. Additional soil cores (two per plot) were collected in October 2003 to compare a direct measurement of fine-root mass with the estimate from minirhizotron analysis.
Results and Discussion
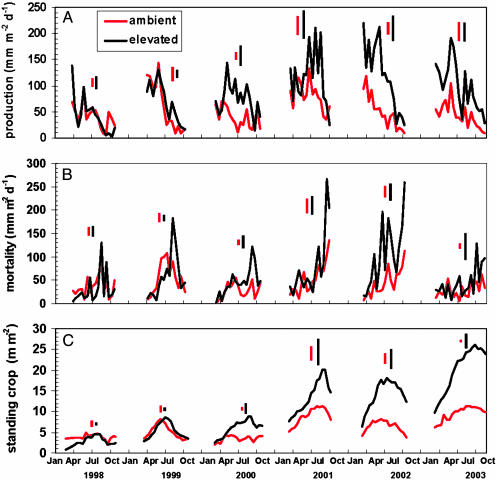
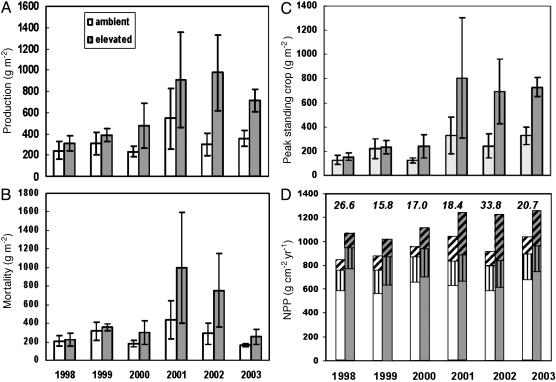
The seasonal pattern of fine-root production varied from year to year, and peaks of activity occurred throughout the growing season (Fig. 1A). Generally, root productivity was higher in March to June than in July to October. The large majority of root length (≈80%) observed in the minirhizotrons was in roots <0.5 mm in diameter, and <5% was in roots >1 mm in diameter. This pattern did not change with time or CO2 treatment. CO2 enrichment began to have an effect on root productivity during the third year, and the response was strongest in midsummer (June to August). The CO2 effect on annual production (Fig. 2A) was highly significant, with production 2.2-fold higher in CO2-enriched plots from 2000 to 2003. This large and significant effect of CO2 on fine-root productivity would not have been seen if the measurements had been maintained for only the first 2 years of the experiment. Mortality was highly variable, with peaks occurring throughout the year (Fig. 1B), but generally mortality was highest in late summer and fall. Annually, mortality (Fig. 2B) matched production in both ambient and elevated CO2.
Fig. 1.
Seasonal dynamics of fine roots were determined by analysis of minirhizotron images collected biweekly during the growing seasons from February 1998 to November 2003. (A) Production during an observation interval was calculated as the length of new roots plus increases in length of existing roots. (B) Mortality is the disappearance of roots during an interval, with corrections made for roots that subsequently reappear. (C) Standing crop is the total length of root visible; changes in standing crop generally reflect production minus mortality. Data are expressed as the length of root per square meter of observation window. Pooled SEs for each year are shown.
Fig. 2.
Fine roots comprised an increasing fraction of NPP in CO2-enriched trees. Annual budgets of production (A), mortality (B), and maximum standing crop (C) were expressed in grams of dry matter per square meter of ground. Increases in root mass between the last measurement in the fall and the first measurement in the spring of the next year were attributed to production in the spring, whereas decreases were attributed to mortality in the fall. Data are the means ± SE of three ambient or two elevated CO2 plots. Analysis of variance indicated the main effect of CO2 on production, mortality, and peak standing crop to be significant at P < 0.013, P < 0.068, and P < 0.010, respectively. Probability levels for the effect of year were P < 0.157 (production), P < 0.057 (mortality), and P < 0.028 (standing crop); CO2–years interaction was never significant (P > 0.20). (D) The contribution of fine-root production to total annual NPP (grams of C per square meter of ground area). NPP was calculated through allometric relationships, leaf litter trap collections, and the data in A, following methods described in ref. 20. Conversion of dry matter units to C units was based on measured C concentration values of 47.1% in wood (open), 46.3% in leaf litter (vertical hatching), and 39.6% in fine roots (diagonal hatching). The percentage increase in NPP is shown above the bars. The effects of CO2 on leaf litter C mass and NPP are statistically significant (P < 0.001 and P < 0.002, respectively), but, excluding 1998, there is no statistically significant difference in wood C mass production.
The net result of seasonal patterns of productivity and mortality is the fine-root standing crop (Fig. 1C). Peak standing crop in ambient CO2 varied from June to early September in different years, but the peak in elevated CO2 always occurred later by 15–42 days. The effect of CO2 on peak standing-root mass was highly significant (Fig. 2C). Root turnover, the fraction of the population that is replaced during a year, was calculated as annual production divided by the maximum standing crop (14). Averaged over the 6 years of observation, root turnover was 1.7 years-1, corresponding to mean residence time (MRT) of 0.62 years, and this was unaffected by CO2 treatment. An independent estimate of MRT from cohort analysis of roots <1.0 mm in diameter ranged from 0.81–1.4 years and did not vary with CO2 treatment. In a third approach, MRT of C in sweetgum roots in elevated CO2 plots was calculated to be 1.25 years based on the replacement of pretreatment roots with new roots with the distinct 13C signature of the added CO2 (3). The absence of an effect of CO2 on turnover rate from the first two methods indicates that the increase in root mortality and concomitant input of root C into the soil was a direct result of increased root production and not an alteration of root physiology.
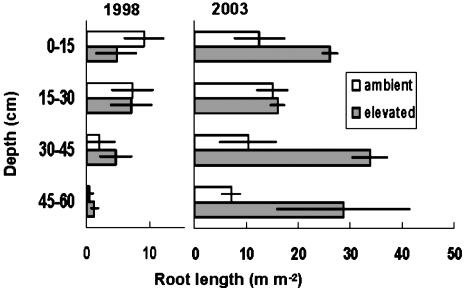
Elevated CO2 can increase standing root crop by accelerating whole-plant development, as in a young, developing tree stand (15), through a specific change in plant allocation or indirectly through its effects on transpiration and soil moisture (16). Separation of these often confounded mechanisms can be difficult, but it is important for prediction of long-term responses in fully developed stands (5). Our initial assumption was that the sweetgum trees had fully occupied the site. This assumption was verified above ground in that peak leaf area index did not increase from 1998 to 2003 (12), but the root system may have still been expanding during this period. Peak root length (Fig. 1C) and mass (Fig. 2C) in ambient CO2 were 150% greater in 2003 than in 1998, although the trend through time was not statistically significant (R2 = 0.19, P = 0.07). However, there was little change in peak length during the last 3 years in either ambient or elevated CO2, so the effect of CO2 cannot be ascribed simply to an acceleration of ontogeny. Indirect effects of CO2 through soil moisture can be discounted. Although stomatal conductance of upper canopy leaves was usually lower in elevated CO2, canopy conductance and stand-level evapotranspiration were little affected (17). Hence, soil moisture was not significantly different between plots. Our results suggest that CO2 enrichment significantly increased peak-standing root crop by altering allocation such that the potential for root occupancy of the soil volume was increased. This response was manifested especially in the deeper distribution of roots in the soil profile (Fig. 3). In 1998 most of the root length was in the upper soil (40% in the top 15 cm and 79% in the top 30 cm). Five years later 63% of the root length was still at 0–30 cm depth in ambient CO2, but the root distribution was distinctly different in elevated CO2 plots. CO2 enrichment increased root length in the upper profile, but the largest increases occurred in deeper soil: 3-fold more length at 30–45 cm and 4-fold more at 45–60 cm. This response, although statistically significant, was highly variable: 3 of the 10 minirhizotron tubes in elevated CO2 accounted for 90% of the root length deeper than 45 cm. Soil cores collected in October 2003 confirmed the presence of fine roots at 60 cm, but not enough cores were collected to verify the effect of CO2 on depth distribution given the high spatial variability. High variability in root distribution is not surprising and cannot be dismissed as a sampling artifact: morphological plasticity of root systems, including spatially explicit root proliferation, is considered a major mechanism whereby plants cope with the heterogeneity of soil resources (2, 18). Other reports of effects of CO2 on root distribution include an upward shift in a grassland ecosystem (16), a downward shift in a developing Populus stand (15), and stimulation of both surface and deep roots in a scrub oak system (19).
Fig. 3.
Distribution of fine-root length by depth in soil at the time of peak standing-root length in 1998 and 2003. Data are the means ± SE of three ambient and two elevated plots, based on analyses of five minirhizotron tubes per plot and expressed as length per square meter of observation window. CO2 had a significant effect on root length at 0–15 cm (P < 0.035), 30–45 cm (P < 0.001), and 45–60 cm (P < 0.013) but did not at 15–30 cm. The patterns in intervening years were intermediate to those shown here.
The mass of fine roots produced in a year was a significant fraction (11–34%) of net primary productivity (NPP) (Fig. 2D). Converting minirhizotron data to units comparable with aboveground production data is problematic, requiring assumptions about the volume of soil observed (10) and SRL of roots that vary over a wide range. Root length (meters per square meter viewing area) was converted to length per unit land area by dividing by the 0.002-m effective depth of field (10) and multiplying by 0.6 m3 of soil per m2 of land area to account for the soil volume observed to a depth of 60 cm. This value was then expressed as grams of root per square meter by dividing by the SRLs specific to each plot and year. SRL of roots extracted from soil cores did not vary significantly between FACE plots, but it was very different for the different diameter classes: 15.5 m·g-1 for roots <0.5 mm in diameter, 4.4 m·g-1 for roots 0.5–1.0 mm in diameter, and 1.5 m·g-1 for roots 1.0–2.0 mm in diameter. A composite SRL was calculated for each plot and year based on the diameter distribution when the standing crop was maximum. The average standing crop calculated from minirhizotron data corresponded closely with that measured directly in soil cores collected at the same time, lending confidence to our scaling assumptions. The mass of roots in soil cores (0–15 cm in depth) collected in July 2002 and averaged across all plots was 103 g·m-2, compared with 95 g·m-2 calculated from minirhizotron data for the same date and depth. Fine-root mass in cores collected in October 2003 was 228 g·m-2 (0–30 cm in depth), compared with 239 g·m-2 from minirhizotron data.
The additional C taken up and converted to organic matter by trees in CO2-enriched plots (average 22% increase in NPP) was allocated primarily to fine-root production (Fig. 2D). After the first year of treatment, there was no significant increase in aboveground woody dry matter production (stem growth). Because fine-root turnover is much faster than turnover of woody stems, the preferential allocation to fine roots should significantly reduce the potential for additional C sequestration in trees in elevated CO2 (20). However, sequestration of some of that C in the forest remains a possibility. As fine roots die, their C enters the soil system where there is the potential for movement into long-lived organic matter pools. Although the efflux of CO2 from the soil surface has been higher in the CO2-enriched plots (21), the response is inconsistent and smaller than the increase in fine-root production and mortality. Soil analysis indicates that there is increased accumulation of new C in CO2-enriched plots (E. Hanlon and W. M. Post, personal communication), particularly in microaggregate fractions (at 0–5 cm in depth) that facilitate movement of C into pools with long residence times (J. Jastrow, personal communication). It may become especially important that the greatest increases in root production in elevated CO2 occur in deeper soil, where sequestration into longer-lived pools may be more likely (22). Construction of a complete C budget for the stand is a clear need and a formidable challenge.
These results contrast with those from the similar FACE experiment in a loblolly pine stand (23). There, the enhancement in NPP was allocated primarily to aboveground stem increment. The C in pine fine roots has a 3-fold-higher MRT than in sweetgum roots (3) (similar to the difference in MRT of the evergreen vs. deciduous canopy); therefore, annual fine-root production and mortality rates are much lower than in our sweetgum stand, and the pine root system is less responsive to elevated CO2 (24). There has been no evidence for increases in soil C content in the pine stand (25). Hence, the inherent difference in fine-root dynamics between these two contrasting tree species is a key ecosystem attribute controlling how C flux and storage respond to rising CO2.
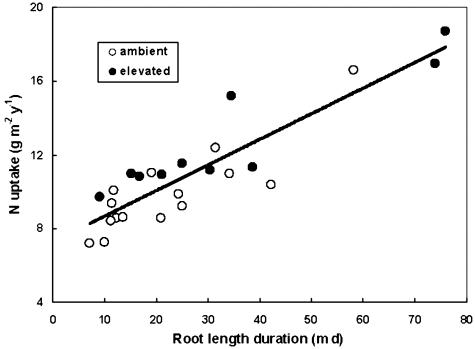
The substantial response of fine-root productivity to CO2 enrichment in the sweetgum stand may be a mechanism for increasing N uptake to meet the increased demand associated with additional C uptake in elevated CO2. Long-term effects of elevated CO2 on tree growth and C sequestration in forests are highly dependent on the availability and cycling of N (7, 26), and N availability is considered a major factor regulating fine-root production in elevated CO2 (27). In grasslands, microbial N processes that control the supply of N to plants have been responsive to elevated CO2, with N availability increasing because of an indirect effect of CO2 on soil moisture (28) or declining because of reduced quality of litter inputs (29). Microbial N cycling and N availability have not responded to elevated CO2 in this or other forest stands (30); therefore, the N needed to meet the requirement for increased NPP must be met through increased access to soil N. To assess this possibility, we calculated fine-root-length duration as an integrative measure of the capacity for N uptake by the root system during the growing season. N uptake has been linearly associated with fine-root-length duration (Fig. 4) in support of the premise that the fine-root response is important in the interaction between C and N cycles. Our hypothesis that full soil occupancy in this forest stand would constrain the N cycle is not supported. Elevated CO2 increased the capacity of roots to occupy the soil volume, and there was a concomitant increase in N uptake. However, increased fine-root production may represent a futile mechanism for improving N nutrition in this forest stand. The increased C supply in CO2-enriched plots was used to support more root production, but the resulting increase in N uptake has so far supported only increased root production and not whole-tree N nutrition or aboveground growth (31).
Fig. 4.
Relationship between N uptake and root-length duration. N uptake per plot for each year (1998–2002) was calculated as the N content (dry matter production times N concentration) of woody increment, leaf litter, and annual fine-root production plus net foliar leaching (31). Root-length duration is the area under the plots of fine-root standing crop (Fig. 1C). Regression line: N uptake = 5.74 × root-length duration - 36.3; R2 = 0.80.
The CO2-induced increase in fine-root standing crop in summer could also be an important mechanism for conferring increased resistance to late-season droughts. Because leaf area index of this stand did not respond to CO2 enrichment (12), there was a large increase in the ratio of fine-root length to leaf area. This result implies a reduction in hydraulic resistance at the rhizosphere, making more soil water available (32). The stimulation of root growth in deeper soil could be particularly important in buffering trees against seasonal droughts. However, we have not observed any interactions between CO2 and drought in this stand.
The potential for CO2 enhancement of root productivity mandates that analyses of ecosystem responses to atmospheric change take a whole-ecosystem approach. Analyses based solely on aboveground production will possibly miss a significant fraction of C and underestimate the potential of the ecosystem for additional C storage. Deciduous forest ecosystems with highly dynamic root systems will exhibit different relationships between C uptake, allocation, storage, and cycling than forests with less dynamic root systems, and generalizations about the potential for forests to sequester additional C and provide a negative feedback to the rising atmospheric concentration must account for these differences. Although current methods for quantifying root dynamics are cumbersome and not appropriate for documenting compliance to C sequestration management (33), methodological limitations should not be an excuse for ignoring this critical ecosystem response to global change.
Acknowledgments
We thank Stan Wullschleger, Roser Matamala, Julie Jastrow, and four anonymous reviewers for helpful comments on the manuscript. This work was supported by the Oak Ridge Institute for Science Education (C.D.R.), Summer Undergraduate Research Experience of the Department of Energy Global Change Education Program (N.E.M.), and the Department of Energy's Office of Science, Biological, and Environmental Research. Oak Ridge National Laboratory is managed by UT-Battelle for the Department of Energy (Contract DE-AC05-00OR22725).
Abbreviations: FACE, free-air CO2 enrichment; MRT, mean residence time; NPP, net primary productivity; SRL, specific root length.
References
- 1.Jackson, R. B., Mooney, H. A. & Schulze, E.-D. (1997) Proc. Natl. Acad. Sci. USA 94, 7362-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pregitzer, K. S., DeForest, J. L., Burton, A. J., Allen, M. F., Ruess, R. W. & Hendrick, R. L. (2002) Ecol. Monogr. 72, 293-309. [Google Scholar]
- 3.Matamala, R., Gonzàlez-Meler, M. A., Jastrow, J. D., Norby, R. J. & Schlesinger, W. H. (2003) Science 302, 1385-1387. [DOI] [PubMed] [Google Scholar]
- 4.Rogers, H. H., Runion, G. B. & Krupa, S. V. (1994) Environ. Pollut. 83, 155-189. [DOI] [PubMed] [Google Scholar]
- 5.Norby, R. J., Wullschleger, S. D., Gunderson, C. A., Johnson, D. W. & Ceulemans, R. (1999) Plant Cell Environ. 22, 683-714. [Google Scholar]
- 6.Caspersen, J. P., Pacala, S. W., Jenkins, J. C., Hurtt, G. C., Moorcroft, P. R. & Birdsey, R. A. (2000) Science 290, 1148-1151. [DOI] [PubMed] [Google Scholar]
- 7.Oren, R., Ellsworth, D. S., Johnsen, K. H., Phillips, N., Ewers, B. E., Maier, C., Schäfer, K. V. R., McCarthy, H., Hendrey, G., McNulty, S. G. & Katul, G. G. (2001) Nature 411, 469-472. [DOI] [PubMed] [Google Scholar]
- 8.Hendrey, G. R., Ellsworth, D. S., Lewin, K. F. & Nagy, J. (1999) Glob. Change Biol. 5, 293-309. [Google Scholar]
- 9.Hendrick, R. L. & Pregitzer, K. S. (1993) Can. J. For. Res. 23, 2507-2520. [Google Scholar]
- 10.Johnson, M. G., Tingey, D. T., Phillips, D. L. & Storm, M. J. (2001) Environ. Exp. Bot. 45, 263-289. [DOI] [PubMed] [Google Scholar]
- 11.Norby, R. J., Todd, D. E., Fults, J. & Johnson, D. W. (2001) New Phytol. 150, 477-487. [Google Scholar]
- 12.Norby, R. J., Sholtis, J. D., Gunderson, C. A. & Jawdy, S. S. (2003) Oecologia 136, 574-584. [DOI] [PubMed] [Google Scholar]
- 13.Pyke, D. A. & Thompson, J. N. (1986) Ecology 67, 240-245. [Google Scholar]
- 14.Dahlman, R. C. & Kucera, C. L. (1965) Ecology 46, 84-89. [Google Scholar]
- 15.Lukac, M., Calfapietra, C. & Godbold, D. L. (2003) Glob. Change Biol. 9, 838-848. [Google Scholar]
- 16.Arnone, J. A., Zaller, J. G., Spehn, E. M., Niklaus, P. A., Wells, C. E. & Körner, C. (2000) New Phytol. 147, 73-86. [Google Scholar]
- 17.Wullschleger, S. D. & Norby, R. J. (2001) New Phytol. 150, 489-498. [Google Scholar]
- 18.Hodge, A. (2004) New Phytol. 162, 9-24. [Google Scholar]
- 19.Day, F. P., Weber, E. P., Hinkle, C. R. & Drake, B. G. (1996) Glob. Change Biol. 2, 143-148. [Google Scholar]
- 20.Norby, R. J., Hanson, P. J., O'Neill, E. G., Tschaplinski, T. J., Weltzin, J. F., Hansen, R. T., Cheng, W., Wullschleger, S. D., Gunderson, C. A., Edwards, N. T. & Johnson, D. W. (2002) Ecol. Appl. 12, 1261-1266. [Google Scholar]
- 21.King, J. S., Hanson, P. J., Bernhardt, E., DeAngelis, P., Norby, R. J. & Pregitzer, K. S. (2004) Glob. Change Biol. 10, 1027-1042. [Google Scholar]
- 22.Gill, R. A. & Burke, I. C. (2002) Plant Soil 241, 233-242. [Google Scholar]
- 23.Hamilton, J. G., DeLucia, E. H., George, K., Naidu, S. L., Finzi, A. C. & Schlesinger, W. H. (2002) Oecologia 131, 250-260. [DOI] [PubMed] [Google Scholar]
- 24.Matamala, R. & Schlesinger, W. H. (2000) Glob. Change Biol. 6, 967-979. [Google Scholar]
- 25.Schlesinger, W. H. & Lichter, J. (2001) Nature 411, 466-469. [DOI] [PubMed] [Google Scholar]
- 26.Hungate, B. A., Dukes, J. S., Shaw, M. R., Luo, Y. & Field, C. B. (2003) Science 302, 1512-1513. [DOI] [PubMed] [Google Scholar]
- 27.Pregitzer K. S., Zak, D. R., Curtis, P. S., Kubiske, M. E., Teeri, J. A. & Vogel, C. S. (1995) New Phytol. 129, 579-585. [DOI] [PubMed] [Google Scholar]
- 28.Hungate, B. A., Chapin, F. S., Zhong, H., Holland, E. A. & Field, C. B. (1997) Oecologia 109, 149-153. [DOI] [PubMed] [Google Scholar]
- 29.Gill, R. A., Polley, H. W., Johnson, H. B., Anderson, L. J., Maherali, H. & Jackson, R. B. (2002) Nature 417, 279-282. [DOI] [PubMed] [Google Scholar]
- 30.Zak, D. R., Holmes, W. E., Finzi, A. C., Norby, R. J. & Schlesinger, W. H. (2003) Ecol. Appl. 13, 1508-1514. [Google Scholar]
- 31.Johnson, D. W., Cheng, W., Joslin, J. D., Norby, R. J., Edwards, N. T. & Todd, D. E., Jr. (2004) Biogeochemistry, in press.
- 32.Sperry, J. S., Hacke, U. G., Oren, R. & Comstock, J. P. (2002) Plant Cell Environ. 25, 251-263. [DOI] [PubMed] [Google Scholar]
- 33.Brown, S. (2002) Environ. Pollut. 116, 363-372. [DOI] [PubMed] [Google Scholar]