Abstract
For the first time, we investigated the relationship between postprandial dysmetabolism and the Peroxidation of Leukocytes Index Ratio (PLIR), a test that measures the resistance of leukocytes to exogenous oxidative stress and their functional capacity of oxidative burst upon activation. Following a blind, placebo controlled, randomized, crossover design, ten healthy subjects ingested, in two different occasions, a high fat and high carbohydrates meal with Snello cookie (HFHCM-S) or with control cookies (HFHCM-C). Snello cookie, a functional food covered by dark chocolate and containing glucomannan, inulin, fructooligosaccharides, and Bacillus coagulans strain GanedenBC30, significantly improved postprandial metabolic stress (insulin, glucose, and triglycerides) and reduced the postprandial increase of uric acid. HFHCM-S improved PLIR of lymphocytes, but not of monocytes and granulocytes. Both meals increased granulocytes' count and reduced the lipoperoxidation induced by both exogenous free radicals and reactive oxygen species (ROS) produced by oxidative burst. Our results suggest that the healthy status of the subjects could be a limitation of this pilot study for PLIR evaluation on cells that produce ROS by oxidative burst. In conclusion, the relationship between PLIR and postprandial dysmetabolism requires further investigations.
1. Introduction
Postprandial dysmetabolism has been linked to atherosclerosis and inflammation [1]. Therefore, fatty meal consumption represents a model of acute inflammatory response and has been applied to study the effect of antioxidant-rich foods, beverages, or nutritional supplements, but results are scarce and controversial [2, 3].
Despite the fact that the consumption of high fat and high carbohydrates meals (HFHCM) has been associated with oxidative stress and with a decline in antioxidant defences in plasma, increases in plasma nonenzymatic antioxidant capacity have been reported following HFHCM [4, 5]. Furthermore, in healthy subjects, both increased [6] and reduced [7] reactive oxygen species (ROS) generation were observed in peripheral blood mononuclear cells (PBMC) during the postprandial period.
In this context, although oxidative stress is involved in metabolic syndrome, decreases in oxidative burst of neutrophils occurred in some conditions, such as hypercholesterolaemia [8] and non-insulin-dependent diabetes mellitus (NIDDM) [9].
Based on the potential protective effects against the onset of metabolic syndrome [10], functional foods containing probiotics, prebiotics, and/or polyphenols were placed on the market. Improvement of metabolic profile, oxidative stress, and inflammation has been reported also for glucomannan [11, 12].
de Luis et al. [13] pointed out that one of the problems of dietetic therapy is the lack of patient adherence and suggested that one possibility to overcome this problem is to include functional cookies in the diet. In particular, significant decreases of cholesterol and C reactive protein were observed in obese patients after the consumption of an alpha linolenic acid, fructooligosaccharides (FOS), and inulin-enriched cookie [13]. Improvements of glycemic control and lipid profile have been reported after consumption of glucomannan-enriched biscuits in subjects with impaired glucose tolerance, reduced high density lipoprotein (HDL) cholesterol, elevated serum triglycerides, and moderate hypertension [14].
In this pilot study, we aimed to investigate the relationship between the improvement of postprandial dysmetabolism by a functional cookie, covered by dark chocolate and containing glucomannan, inulin, FOS, and Bacillus coagulans strain GanedenBC30 [15], and the Peroxidation of Leukocytes Index Ratio (PLIR), a test that measures the resistance of leukocytes to exogenous oxidative stress and their functional capacity of oxidative burst upon activation [16].
2. Methods
2.1. Subjects' Selection
We recruited 10 healthy subjects who volunteered in response to advertisements.
Selection of subjects was made according to the following criteria: being healthy, being aged between 25 and 50 years, and taking no drugs, supplements, probiotics, or functional foods. Exclusion criteria include smoking habits and adherence to special diets (vegetarian, vegan).
Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), and heart rate (HR) were measured. MEDScore [17, 18] was calculated by the MedDietScore Software [19], and physical activity was calculated according to the “Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire” (IPAQ) [20, 21].
2.2. Study Design and Meals' Composition
For 2 days prior to each feeding study, the subjects followed a low antioxidant and low purine diet (washout), by avoiding fresh fruit and vegetables with high antioxidants' content and their products (juices or soups), tea, cocoa, nuts, coffee and wine, meat, and fish. Subjects were asked to refrain from exercise 2 days before the study. Compliance with dietary instructions was evaluated through dietary records and all subjects ingested pasta (160 ± 40 g/day), white bread (160 ± 50 g/day), croissant or cookies (50 ± 10 g/day), eggs (1/2 days), cheese (70 ± 20 g/day), fresh cheese (150 ± 50 g/day), milk (200 ± 50 mL/d), only a fruit/day (apple or pear), and less than 60 g/d of vegetables (zucchini, endive, or fennel).
Following a blind, placebo controlled, randomized, crossover design, subjects were allocated into Group A (n = 5) (HFHCM + control cookies) (HFHCM-C) or Group B (n = 5) (HFHCM + Snello cookie) (HFHCM-S). Panna cotta with caramel and control cookies were purchased from the supermarket. Snello cookie, a functional food commercially available in Italy, covered by dark chocolate and containing glucomannan (2.4 g/48 g), inulin (2.3 g/48 g), FOS (0.2 g/48 g), and Bacillus coagulans strain GanedenBC30 (3 × 106 UFC/g), was provided by Nutripharma S.r.l.
After 12 days, subjects followed again 2 days of washout and 14 days after the first test the groups were crossed over to the alternative cookies. The macronutrient composition of the two meals, given as breakfast with 500 mL of water, is depicted in Table 1.
Table 1.
Macronutrient composition of the two meals.
| Kcal | Lipids (saturated) | Proteins | Carbohydrates (sugars) | |
|---|---|---|---|---|
| HFHCM-C (total) | 831.4 | 40.9 (29.6) | 6.9 | 105.7 (63.3) |
| Panna cotta with caramel (240 g) | 647.4 | 34.3 (27.4) | 4.3 | 77.5 (54.5) |
| Control cookies (40 g) | 184 | 6.6 (2.2) | 2.6 | 28.2 (8.8) |
|
| ||||
| HFHCM-S (total) | 846.4 | 40.6 (31.2) | 6.8 | 108.8 (65.0) |
| Panna cotta with caramel (240 g) | 647.4 | 34.3 (27.4) | 4.3 | 77.5 (54.5) |
| Snello cookie (48 g) | 199 | 6.3 (3.8) | 2.5 | 31.3 (10.5) |
C: control cookies; HFHCM: high fat and high carbohydrates meals; S: Snello cookie.
In patients with coronary artery disease (CAD) [22] or type 2 diabetes (T2D) [23], 2-hour breakfast tests [22, 23] revealed the improving effects of lipid lowering (fibrate) [22] or oral hypoglycaemic (mitiglinide) [23] drugs on the phorbol ester-activated leukocyte ROS production [22], plasma malondialdehyde (MDA), oxidized low density lipoproteins (oxLDL), plasma total radical-trapping antioxidant parameter (TRAP), and inflammatory cytokines [23]. Notwithstanding the above, the Ethics Committee approved a 3-hour test meal considering that ROS generation by polymorphonuclear (PMN) cells reached a peak 3 hours after meal in healthy subjects [6].
On the day of the study, after an overnight fast, venous blood samples were collected before (T0) and 30 minutes (T0.5), 2 hours (T2), and 3 hours (T3) after meal.
2.3. Clinical Markers
Blood was collected in Silicone-Coated tubes. The serum was stored at −80°C.
Serum levels of triglycerides (TG), glucose (GLU), and uric acid (UA) were quantified enzymatically using colorimetric kits (Sentinel CH. SpA, Italy). Plasma insulin was measured with an enzyme-linked immunosorbent assay (ELISA) kit (Li StarFish S.r.l., Italy).
2.4. PLIR Method
Blood was collected in EDTA tubes. After red blood cells' lysis and 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (C11-BODIPY, Invitrogen, final concentration 1 μM) staining, leukocytes were treated as previously described [24] with phorbol 12-myristate 13-acetate (PMA, Sigma, final concentration 1 μg/mL), 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH, Sigma, final concentration 10 mM), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, Sigma, final concentration 10 μM), PMA 1 μg/mL + Trolox 10 μM, or AAPH 10 mM + Trolox 10 μM. After 30 min at 37°C, cells were stored in ice, to stop reactions, and rapidly analyzed on an Accuri C6 BD cytometer.
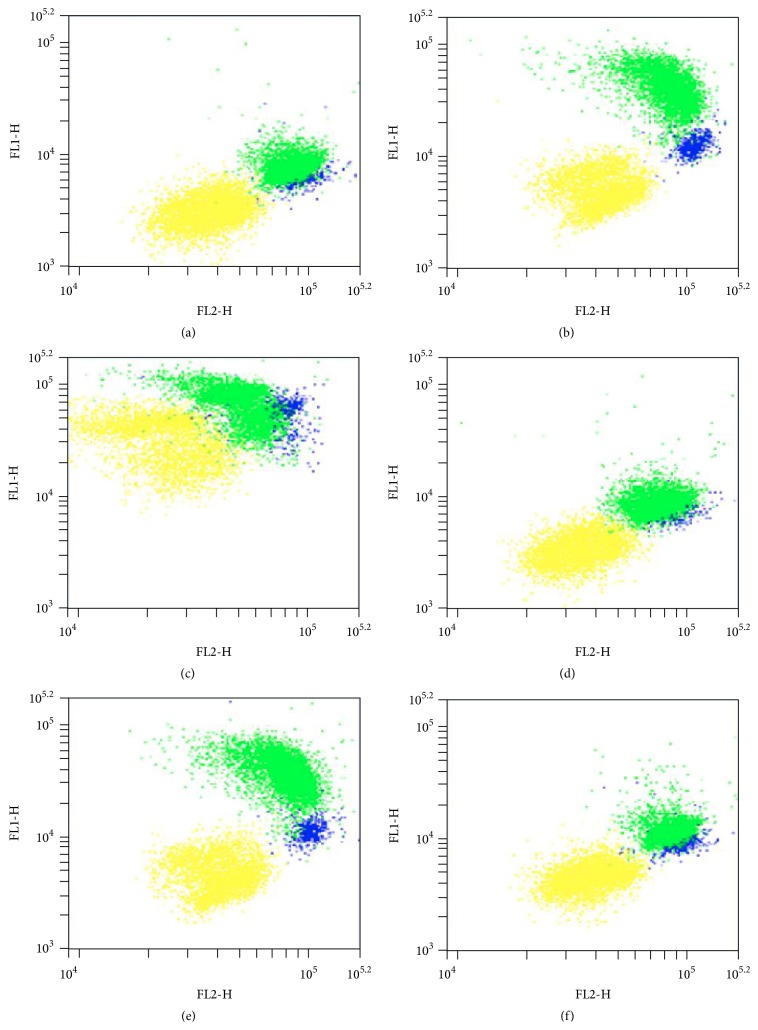
C11-BODIPY, used in the PLIR method, modifies its fluorescence from red (FL2) to green (FL1) as a result of oxidation [25]. Treatment with AAPH or PMA changed the C11-BODIPY fluorescence in a different manner compared to unstimulated cells (Figure 1(a)), showing that oxidative burst induced ROS production only in activated cells (Figure 1(b)), while all cells were sensitive to exogenous (AAPH) ROS injury (Figure 1(c)). Trolox did not affect neither baseline levels of fluorescence (Figure 1(d)) nor the PMA-induced change in fluorescence of monocytes and granulocytes (Figure 1(e)) but decreased the AAPH-induced change in fluorescence of lymphocytes, monocytes, and granulocytes (Figure 1(f)).
Figure 1.
Typical dot plots of C11-BODIPY red (FL2) and green (FL1, oxidized) fluorescence of lymphocytes (yellow), monocytes (blue), and granulocytes (green) in unstimulated (UNST) samples (a) and after treatment with phorbol 12-myristate 13-acetate (PMA, (b)), 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH, (c)), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, (d)), PMA + Trolox (e), or AAPH + Trolox (f).
Both FL1 and FL2 are higher in monocytes and granulocytes compared to lymphocytes in unstimulated samples (Figure 1(a)). Therefore, in order to normalize for cell incorporation of the probe into membrane, data acquired on the cytometer were exported in FCS format and analyzed by FCS Express software (De Novo Software) to calculate the ratio of oxidation of the probe C11-BODIPY (FL1/FL2). This ratio, being independent of the concentration of the probe, has been used to calculate PLIR, applying the previously described [16, 24] formula:
| (1) |
PLIR is a functional index that measures the ratio between the resistance to exogenous (Trolox μM equivalents AAPH) and resistance to endogenous (Trolox μM equivalents PMA) ROS injury [16]. Although PLIR is independent of the baseline levels of oxidation, this functional index is sensitive to the difference between leukocytes isolated from fresh and stored blood [24].
Also Side Scatter (SS) was recorded and leukocytes' count was measured as previously described [26].
2.5. Statistics
Two-Way Repeated Measures Analysis of Variance (Two-Factor Repetition ANOVA), with cookies and time as within-subject factors, was performed. Student-Newman-Keuls post hoc analysis (All Pairwise Multiple Comparison Procedure) was used to isolate differences between groups.
All statistical evaluations were performed using the SigmaStat and Sigmaplot software (Jandel Scientific, Inc.).
3. Results
3.1. Characteristics of Subjects
Based on the exclusion criteria, ten subjects (6 men and 4 women), with a mean age of 36.0 ± 2.9 years and a mean body mass index of 23.3 ± 1.4, were recruited. Volunteers had a mean homeostasis model assessment of insulin resistance (HOMA-IR) of 1.6 ± 0.3 and were normotensive (SBP: 122.9 ± 3.1 mmHg, DBP: 75.9 ± 2.1 mmHg, and HR: 73 ± 2.5 beats/min).
Subjects had a MEDScore of 35.0/55 ± 1.9 (63.6% adherence's level to Mediterranean diet) [19] and a moderate physical activity (1089 ± 180 MET-minutes/week) [21].
3.2. Clinical Markers
Statistical analysis revealed a normal distribution for all markers (Normality Test Shapiro-Wilk passed: GLU: p > 0.8; INS: p > 0.5; TG: p > 0.8; UA: p > 0.9).
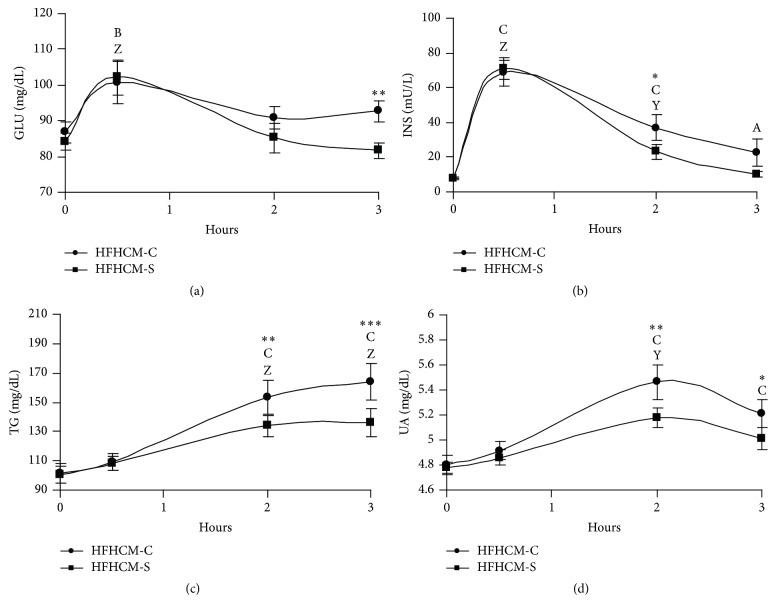
The glucose and insulin time courses reflected the postprandial load for healthy people. Both glucose and insulin levels peaked 30 min after meal ingestion (Figures 2(a) and 2(b)).
Figure 2.
Line plots showing the serum levels as means ± standard errors (n = 10) in plasma glucose (GLU, (a)), insulin (INS, (b)), triglycerides (TG, (c)), and uric acid (UA, (d)), following high fat and carbohydrates meal ingestion with control (HFHCM-C) or Snello (HFHCM-S) cookies. Two-Way Repeated Measures ANOVA followed by Student-Newman-Keuls post hoc analysis. A: p < 0.05; B: p < 0.01; C: p < 0.001, single time point versus before meal intake within HFHCM-C; Y: p < 0.01; Z: p < 0.001, single time point versus before meal intake within HFHCM-S; ∗ p < 0.05; ∗∗ p < 0.01; ∗∗∗ p < 0.001, HFHCM-S versus HFHCM-C within time.
Although glucose and insulin levels began to decrease within one hour with both HFHCM-C and HFHCM-S, they returned to baseline values only with the latter (Figures 2(a) and 2(b)). In particular, glucose and insulin values were significantly higher after HFHCM-C compared to HFHCM-S at 3 hours (HFHCM-S versus HFHCM-C: p < 0.01; Figure 1(a)) and 2 hours (HFHCM-S versus HFHCM-C: p < 0.05; Figure 2(b)), respectively. Furthermore, insulin remained significantly above preingestion values for 3 hours only after HFHCM-C (p < 0.05 versus baseline; Figure 2(b)).
With respect to lipid metabolism, both HFHCM-C and HFHCM-S induced lipaemia at 2 and 3 hours; however, TG increase was significantly lower after HFHCM-S compared to HFHCM-C (HFHCM-S versus HFHCM-C: p < 0.01 within 2 hours, p < 0.001 within 3 hours; Figure 2(c)). Besides, HFHCM ingestion caused a significant increase in the endogenous antioxidant UA at 2 hours, but the latter was lower with HFHCM-S compared to HFHCM-C (within 2 hours: p < 0.01, within 3 hours: p < 0.05; Figure 2(d)). Furthermore, only HFHCM-C increased UA at 3 hours (p < 0.001 versus baseline; Figure 2(d)).
3.3. PLIR, Count, and Scatter of Leukocytes
Statistical analysis revealed a normal distribution for L, M, and G populations (Normality Test Shapiro-Wilk passed: L: p > 0.3; M: p > 0.2; G: p > 0.3).
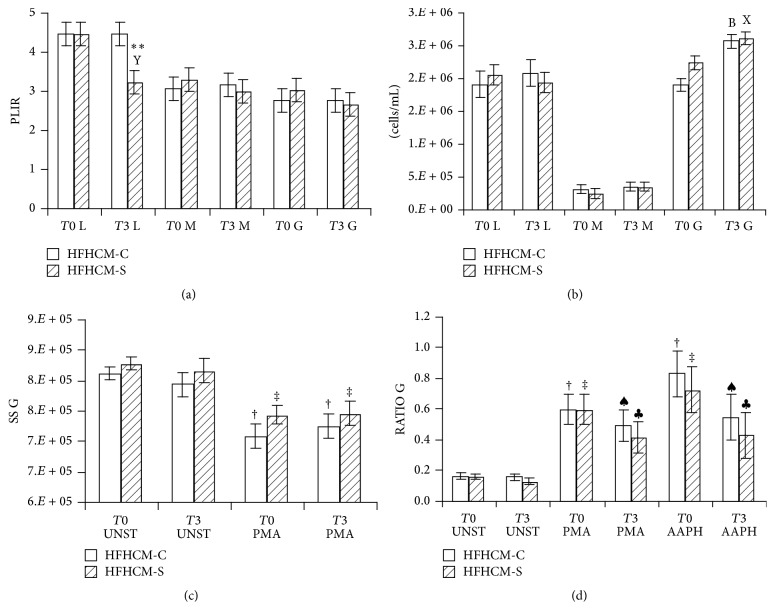
HFHCM-S, but not HFHCM-C, significantly decreased PLIR of lymphocytes at 3 hours (p < 0.01 versus baseline; HFHCM-S versus HFHCM-C: p < 0.01 within 3 hours; Figure 3(a)), whereas a nonsignificant decrease was observed for PLIR of monocytes and granulocytes after HFHCM-S (Figure 3(a)). In particular, considering the major components of PLIR affected by treatment, compared to baseline, the AAPH-induced (exogenous) oxidation of lymphocytes appeared significantly lower after HFHCM-S (difference of means RATIO AAPH versus RATIO UNST: at baseline 0.68, p < 0.001, at 3 hours 0.29, p < 0.01).
Figure 3.
Vertical bars showing the values as means ± standard errors (n = 10) of PLIR (a), leukocytes count (b), Side Scatter of granulocytes (c), and RATIO of fluorescence (FL1/FL2) of granulocytes (d), following high fat and carbohydrates meal ingestion with control (HFHCM-C) or Snello (HFHCM-S) cookies. L: lymphocytes; M: monocytes; G: granulocytes. Two-way Repeated Measures ANOVA followed by Student-Newman-Keuls post hoc analysis. B: p < 0.01, 3 hours (T3) versus before (T0) meal intake within HFHCM-C; X: p < 0.05; Y: p < 0.01, 3 hours (T3) versus before (T0) meal intake within HFHCM-S; ∗∗ p < 0.01, HFHCM-S versus HFHCM-C within time; ♠ p < 0.01; † p < 0.001, treatment (PMA: phorbol 12-myristate 13-acetate; AAPH: 2,2′-azobis(2-methylpropionamidine) dihydrochloride) versus unstimulated (UNST) samples within HFHCM-C; ♣ p < 0.01; ‡ p < 0.001, treatment versus unstimulated (UNST) samples within HFHCM-S.
We analyzed also leukocytes' count and scatter after meal. The mean count and scatter from lymphocytes and monocytes remained unchanged after both meals. On the other hand, there was a postprandial increase in granulocytes' count with both meals (HFHCM-C versus baseline: p < 0.01; HFHCM-S versus baseline: p < 0.05; Figure 3(b)). However, this increase, after both meals, was accompanied neither by a reduction of SS in unstimulated samples nor by a different decrease in SS after PMA-activation (Figure 3(c)).
On the contrary, the increase in the RATIO of fluorescence of granulocytes after both PMA and AAPH treatment versus unstimulated samples appeared lower 3 hours after both meals (PMA or AAPH versus UNST: HFHCM-C at baseline: p < 0.001; HFHCM-C at 3 hours: p < 0.01; HFHCM-S at baseline: p < 0.001; HFHCM-S at 3 hours: p < 0.01; Figure 3(d)).
Similarly, nonsignificant effects were observed on monocytes with both meals.
4. Discussion
4.1. Postprandial Dysmetabolism
The functional food Snello cookie significantly improved postprandial metabolic stress. In particular, Snello cookie reduced the postprandial TG rise. Furthermore, glucose and insulin levels returned to baseline values at 3 hours after HFHCM-S, but not after HFHCM-C.
The effect on postprandial insulin could be due to the content of glucomannan in Snello cookie. In fact, McCarty [27] suggested that glucomannan reduces the postprandial insulin surge. Besides, results of a meta-analysis of randomized controlled trials pointed out that glucomannan significantly lowered TG and GLU [11].
On the contrary, a systematic review reported that studies evaluating the effects of inulin and FOS on glucose concentration in humans gave contrasting results [28]. On the other hand, inulin-enriched pasta [29] and breakfast cereal containing inulin [30] decreased TG. Inulin markedly increased bifidobacteria count and faecal concentration of lactate [30]. Lactic acid is involved in the immunomodulating effect of lactobacilli [31]. Although the prebiotic effects of inulin-type prebiotics, including FOS and inulin, occur after long term consumption [32], it has been reported that acute inulin ingestion increased postprandial serum short-chain fatty acids and reduced free fatty acids [33, 34].
On the other hand, also the chocolate contained in Snello cookie could improve postprandial dysmetabolism. Although acute cocoa supplementation showed no clear overall benefit on postprandial GLU, INS, and TG [35, 36], it has been reported that an oral supplement of (−)-epicatechin (the major flavanol contained in chocolate) significantly lowered GLU and TG 2 hours after meal [37].
Therefore, the overall improvement of postprandial dysmetabolism induced by Snello cookie could be due to the synergistic effect of its constituents.
4.2. Postprandial Leukocytes' Recruitment and Activation
We observed the previously described [38–41] postprandial increase of granulocytes' count at 3 hours. In this context, the leukocytes' excursion, after meal, was significantly reduced with acarbose in patients with T2D and subclinical inflammation (leucocytes > or = 6.2 gigaparticles/L) [42]. Rosiglitazone reduced the incremental area under the curves for leukocytes, due to a specific reduction of neutrophils (−39%, p < 0.05), in patients with T2D [43]. On the contrary, rosuvastatin did not affect baseline leukocytes' count or the postprandial neutrophils' increase in CAD patients [44]. Furthermore, results of statin withdrawal demonstrated that the expression of leukocytes' markers of activation is not affected by the use of statins [45]. However, the studies that investigated the effect of glucose on leukocytes' markers of activation have shown conflicting results in T2D [45–48]. On the other hand, postprandial studies reported that leukocytes are activated by lipids [38, 49, 50]. The expression of leukocytes' markers of activation (i.e., CD11b, CD11c) increased on monocytes or neutrophils after a high fat meal [50, 51]. The extent of upregulation of the expression of leukocytes' markers of activation correlated with TG and was accompanied by an altered scatter profile [50, 51]. In particular, CD11b expression on neutrophils was negatively correlated with the mean SS of neutrophils, reflecting granularity [50].
In our study, the increase in granulocytes' count was accompanied neither by a reduction of SS in unstimulated samples nor by a different decrease in SS after PMA-activation. In this context, the fact that, even if white blood cells' count increased and intracellular myeloperoxidase decreased within 2–4 hours after meal, waist-to-hip ratio influenced the degranulation of PMN must be taken into account [52]. Besides, recent results showed that only obese subjects had higher postprandial endotoxemia, the mechanism of postprandial leukocytes' activation, despite the lipaemia increased in both normal-weight and obese men after meal [53]. Furthermore, postprandial leukocytes' activation was highest in patients with T2D and hyperlipidaemia [45]. Therefore, our pilot study has the major limitation that subjects were healthy, of normal weight, with a moderate physical activity and none of them presented risk factors for CVD.
4.3. PLIR and UA in the Postprandial Phase
Snello cookie improved PLIR of lymphocytes, but not of monocytes and granulocytes. To understand this result further considerations should be made.
Although PLIR is a functional index that is independent of baseline levels of oxidation, measuring the ratio between the resistance to exogenous and resistance to endogenous ROS injury [16], this ratio calculation could mask the effect of foods that inhibit both the exogenous ROS injury and the oxidative burst. In particular, the calculation of PLIR includes the PMA-induced oxidation. Lower increases in the RATIO of fluorescence of granulocytes after both PMA and AAPH treatment versus unstimulated samples were recorded 3 hours after both HFHCM-C and HFHCM-S compared to baseline. However, the unchanged mean SS (reflecting granularity) after meal suggests that the decrease in the RATIO of fluorescence is more likely due to the antioxidant effect of UA and cocoa flavanols after HFHCM-C and HFHCM-S, respectively, rather than an effect on oxidative burst. In agreement with this hypothesis, Sodré et al. [7] reported that the intracellular ROS in PBMC, assessed by flow cytometry as the ethidium (ETH) fluorescence, decreased 2 and 4 hours after meal not only in monocytes but also in lymphocytes, which do not produce ROS by oxidative burst. On the contrary, others [6] reported that the release of superoxide radical by PMN, as measured by chemiluminescence, was significantly lower when orange juice was added to the meal than when water or glucose was added to the meal. However, extracellular free radicals' measurements, such as the chemiluminescence assay, are deeply affected by cell count and viability. Therefore, the postprandial increase of granulocytes' count [38–41] could bias these methods.
On the other hand, we observed an increase in UA after HFHCM-C. This increase could be due to the healthy status of the subjects. In agreement with this, increases in TRAP and UA have been reported following HFHCM in healthy subjects [4], despite the lower TRAP values after meal in T2D patients [23].
The increase in UA was lower after HFHCM-S. The inhibition of UA increase could be due to the cocoa flavanols contained in Snello cookie. In agreement with this hypothesis, tea flavanols could have UA lowering effect [54, 55] and fruit-based juice drinks, providing exogenous antioxidants, prevented the endogenous antioxidant response to HFHCM, by inhibiting the production of UA [56].
In this context, UA levels could affect PLIR in two different ways: acting as antioxidant [57] on all leukocytes and inducing oxidative burst in ROS-producing cells [58]. The effect of UA depends on its concentration. AAPH-induced lipid peroxidation, in vitro, was strongly inhibited by UA at concentration ranging between 50 and 400 μM (0.84–6.72 mg/dL) [57]. The increase in UA after HFHCM-C ingestion could justify why HFHCM-C did not change PLIR. In this context, it has been suggested that in healthy people the body responds to postprandial stress by inducing endogenous defenses [4]. In the presence of dietary antioxidants (i.e., the chocolate contained in Snello cookie), the resistance to AAPH-induced oxidation is increased in lymphocytes despite the reduced UA increase.
On the other hand, although the level at which UA concentration becomes abnormal is still disputed, ranging between 3.5 and 7.2 mg/dL in adult males and postmenopausal women and between 2.6 and 6.0 mg/dL in premenopausal women [59], a threshold value below the saturation concentrations (<6 mg/dL or <360 μmol/L), in order to prevent monosodium urate (MSU) crystals formation, has been suggested [59, 60]. In fact, in response to MSU, the neutrophils recruited to sites of inflammation undergo oxidative burst [58]. In our study, after HFHCM-C, UA reached the concentration of 5.46 ± 0.13 mg/dL, a value below the threshold value of 6 mg/dL [59, 60]. From that, the healthy status of the subjects could be a limitation of this study for PLIR evaluation on cells that produce ROS (i.e., monocytes and granulocytes).
5. Conclusion
In conclusion, the functional food Snello cookie significantly improved postprandial metabolic stress and reduced the postprandial increase of UA.
After HFHCM-S, PLIR was improved on lymphocytes, but not on monocytes and granulocytes.
The healthy status of the subjects could be a limitation of this pilot study for PLIR evaluation on cells that produce ROS (i.e., monocytes and granulocytes). From that, further studies on subjects who are at risk of cardiovascular diseases are needed in order to investigate the relationship between postprandial dysmetabolism and PLIR.
Acknowledgments
This study was supported by Nutripharma S.r.l., Rome, Italy (http://www.nutripharma.it/dettprodotti.php?idx=8&pd=2#.VboVB8sw_IU). The authors thank Fondazione “Enrico ed Enrica Sovena” for the scholarship contribution to Husseen Manafikhi. The authors also thank Claudio Andrew Gobbi for English review of the paper.
Ethical Approval
Approval for the study was obtained from the Ethics Committee for Human Nonclinical Research of the Department of Physiology and Pharmacology “V. Erspamer”, “Sapienza” University of Rome, and all procedures involving human subjects complied with the Declaration of Helsinki as revised in 2000.
Consent
Written informed consent was obtained from all the participants in accordance with the Italian law (law number 196/2003, Ministry of Health Circular Letter GU number 76/2008).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Ilaria Peluso designed the research, analyzed the data, and drafted the paper. Husseen Manafikhi and Raffaella Reggi performed the analyses. Yaroslava Longhitano assessed the healthy status of the subjects, the MEDScore, and the physical activity. Christian Zanza performed the blood sampling. Maura Palmery critically reviewed the paper and supervised the whole project.
References
- 1.Sies H., Stahl W., Sevanian A. Nutritional, dietary and postprandial oxidative stress. Journal of Nutrition. 2005;135(5):96–972. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- 2.Peluso I., Manafikhi H., Reggi R., Palmery M. Effects of red wine on postprandial stress: potential implication in non-alcoholic fatty liver disease development. European Journal of Nutrition. 2015;54(4):497–507. doi: 10.1007/s00394-015-0877-2. [DOI] [PubMed] [Google Scholar]
- 3.Peluso I., Palmery M. Risks of misinterpretation in the evaluation of the effect of fruit-based drinks in postprandial studies. Gastroenterology Research and Practice. 2014;2014:9. doi: 10.1155/2014/870547.870547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miglio C., Peluso I., Raguzzini A., et al. Antioxidant and inflammatory response following high-fat meal consumption in overweight subjects. European Journal of Nutrition. 2013;52(3):1107–1114. doi: 10.1007/s00394-012-0420-7. [DOI] [PubMed] [Google Scholar]
- 5.Cao G., Prior R. L. Postprandial increases in serum antioxidant capacity in older women. Journal of Applied Physiology. 2000;89(3):877–883. doi: 10.1152/jappl.2000.89.3.877. [DOI] [PubMed] [Google Scholar]
- 6.Ghanim H., Sia C. L., Upadhyay M., et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and toll-like receptor expression. American Journal of Clinical Nutrition. 2010;91(4):940–949. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sodré F. L., Paim B. A., Urban A., Vercesi A. E., Faria E. C. Reduction in generation of reactive oxygen species and endothelial dysfunction during postprandial state. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(10):800–807. doi: 10.1016/j.numecd.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Peluso I., Morabito G., Riondino S., La Farina F., Serafini M. Lymphocytes as internal standard in oxidative burst analysis by cytometry: a new data analysis approach. Journal of Immunological Methods. 2012;379(1-2):61–65. doi: 10.1016/j.jim.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Noritake M., Katsura Y., Shinomiya N., et al. Intracellular hydrogen peroxide production by peripheral phagocytes from diabetic patients. Dissociation between polymorphonuclear leucocytes and monocytes. Clinical and Experimental Immunology. 1992;88(2):269–274. doi: 10.1111/j.1365-2249.1992.tb03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peluso I., Romanelli L., Palmery M. Interactions between prebiotics, probiotics, polyunsaturated fatty acids and polyphenols: diet or supplementation for metabolic syndrome prevention? International Journal of Food Sciences and Nutrition. 2014;65(3):259–267. doi: 10.3109/09637486.2014.880670. [DOI] [PubMed] [Google Scholar]
- 11.Sood N., Baker W. L., Coleman C. I. Effect of glucomannan on plasma lipid and glucose concentrations, body weight, and blood pressure: systematic review and meta-analysis. American Journal of Clinical Nutrition. 2008;88(4):1167–1175. doi: 10.1093/ajcn/88.4.1167. [DOI] [PubMed] [Google Scholar]
- 12.Bauerova K., Ponist S., Navarova J., et al. Glucomannan in prevention of oxidative stress and inflammation occurring in adjuvant arthritis. Neuro Endocrinology Letters. 2008;29(5):691–696. [PubMed] [Google Scholar]
- 13.de Luis D. A., de la Fuente B., Izaola O., et al. Double blind randomized clinical trial controlled by placebo with an alpha linoleic acid and prebiotic enriched cookie on risk cardiovascular factor in obese patients. Nutricion Hospitalaria. 2011;26(4):827–833. doi: 10.3305/nh.2011.26.4.5143. [DOI] [PubMed] [Google Scholar]
- 14.Vuksan V., Sievenpiper J. L., Owen R., et al. Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome: results of a controlled metabolic trial. Diabetes Care. 2000;23(1):9–14. doi: 10.2337/diacare.23.1.9. [DOI] [PubMed] [Google Scholar]
- 15.Jensen G. S., Benson K. F., Carter S. G., Endres J. R. GanedenBC30 cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunology. 2010;11, article 15 doi: 10.1186/1471-2172-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peluso I., Adorno G., Raguzzini A., Urban L., Ghiselli A., Serafini M. A new flow cytometry method to measure oxidative status: the Peroxidation of Leukocytes Index Ratio (PLIR) Journal of Immunological Methods. 2013;390(1-2):113–120. doi: 10.1016/j.jim.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Panagiotakos D. B., Pitsavos C., Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutrition, Metabolism and Cardiovascular Diseases. 2006;16(8):559–568. doi: 10.1016/j.numecd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Panagiotakos D. B., Pitsavos C., Arvaniti F., Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Preventive Medicine. 2007;44(4):335–340. doi: 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Panagiotakos D. B., Milias G. A., Pitsavos C., Stefanadis C. MedDietScore: a computer program that evaluates the adherence to the Mediterranean dietary pattern and its relation to cardiovascular disease risk. Computer Methods and Programs in Biomedicine. 2006;83(1):73–77. doi: 10.1016/j.cmpb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Hagströmer M., Oja P., Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutrition. 2006;9(6):755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- 21. http://www.academia.edu/5346814/Guidelines_for_Data_Processing_and_Analysis_of_the_International_Physical_Activity_Questionnaire_IPAQ_Short_and_Long_Forms_Contents.
- 22.Bae J.-H., Bassengel E., Lee H.-J., et al. Impact of postprandial hypertriglyceridemia on vascular responses in patients with coronary artery disease: effects of ACE inhibitors and fibrates. Atherosclerosis. 2001;158(1):165–171. doi: 10.1016/s0021-9150(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 23.Assaloni R., Da Ros R., Quagliaro L., et al. Effects of S21403 (mitiglinide) on postprandial generation of oxidative stress and inflammation in type 2 diabetic patients. Diabetologia. 2005;48(9):1919–1924. doi: 10.1007/s00125-005-1849-5. [DOI] [PubMed] [Google Scholar]
- 24.Peluso I., Manafikhi H., Altieri F., Zanza C., Palmery M. The effect of sample storage on the Peroxidation of Leukocytes Index Ratio (PLIR) measure. Scientific Reports. 2014;4, article 6539 doi: 10.1038/srep06539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drummen G. P. C., van Liebergen L. C. M., Op den Kamp J. A. F., Post J. A. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radical Biology and Medicine. 2002;33(4):473–490. doi: 10.1016/s0891-5849(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 26.Mariani M., Colombo F., Assennato S. M., et al. Evaluation of an easy and affordable flow cytometer for volumetric haematopoietic stem cell counting. Blood Transfusion. 2014;12(3):416–420. doi: 10.2450/2014.0198-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarty M. F. Glucomannan minimizes the postprandial insulin surge: a potential adjuvant for hepatothermic therapy. Medical Hypotheses. 2002;58(6):487–490. doi: 10.1054/mehy.2001.1457. [DOI] [PubMed] [Google Scholar]
- 28.Bonsu N. K. A., Johnson C. S., Mcleod K. M. Can dietary fructans lower serum glucose? Journal of Diabetes. 2011;3(1):58–66. doi: 10.1111/j.1753-0407.2010.00099.x. [DOI] [PubMed] [Google Scholar]
- 29.Russo F., Chimienti G., Riezzo G., et al. Inulin-enriched pasta affects lipid profile and Lp(a) concentrations in Italian young healthy male volunteers. European Journal of Nutrition. 2008;47(8):453–459. doi: 10.1007/s00394-008-0748-1. [DOI] [PubMed] [Google Scholar]
- 30.Brighenti F., Casiraghi M. C., Canzi E., Ferrari A. Effect of consumption of a ready-to-eat breakfast cereal containing inulin on the intestinal milieu and blood lipids in healthy male volunteers. European Journal of Clinical Nutrition. 1999;53(9):726–733. doi: 10.1038/sj.ejcn.1600841. [DOI] [PubMed] [Google Scholar]
- 31.Peluso I., Fina D., Caruso R., et al. Lactobacillus paracasei subsp. paracasei B21060 suppresses human T-cell proliferation. Infection and Immunity. 2007;75(4):1730–1737. doi: 10.1128/iai.01172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly G. Inulin-type prebiotics—a review: part 1. Alternative Medicine Review. 2008;13(4):315–329. [PubMed] [Google Scholar]
- 33.Fernandes J., Vogt J., Wolever T. M. S. Inulin increases short-term markers for colonic fermentation similarly in healthy and hyperinsulinaemic humans. European Journal of Clinical Nutrition. 2011;65(12):1279–1286. doi: 10.1038/ejcn.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarini J., Wolever T. M. S. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Applied Physiology, Nutrition and Metabolism. 2010;35(1):9–16. doi: 10.1139/h09-119. [DOI] [PubMed] [Google Scholar]
- 35.Westphal S., Luley C. Flavanol-rich cocoa ameliorates lipemia-induced endothelial dysfunction. Heart and Vessels. 2010;26(5):511–515. doi: 10.1007/s00380-010-0085-1. [DOI] [PubMed] [Google Scholar]
- 36.Basu A., Betts N. M., Leyva M. J., Fu D., Aston C. E., Lyons T. J. Acute cocoa supplementation increases postprandial HDL cholesterol and insulin in obese adults with type 2 diabetes after consumption of a high-fat breakfast. Journal of Nutrition. 2015;145(10):2325–2332. doi: 10.3945/jn.115.215772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutiérrez-Salmeán G., Ortiz-Vilchis P., Vacaseydel C. M., et al. Acute effects of an oral supplement of (-)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food and Function. 2014;5(3):521–527. doi: 10.1039/c3fo60416k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Oostrom A. J., Rabelink T. J., Verseyden C., et al. Activation of leukocytes by postprandial lipemia in healthy volunteers. Atherosclerosis. 2004;177(1):175–182. doi: 10.1016/j.atherosclerosis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Van Oostrom A. J. H. H. M., Sijmonsma T. P., Rabelink T. J., Van Asbeck B. S., Cabezas M. C. Postprandial leukocyte increase in healthy subjects. Metabolism: Clinical and Experimental. 2003;52(2):199–202. doi: 10.1053/meta.2003.50037. [DOI] [PubMed] [Google Scholar]
- 40.van Oostrom A. J., Sijmonsma T. P., Verseyden C., et al. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. Journal of Lipid Research. 2003;44(3):576–583. doi: 10.1194/jlr.m200419-jlr200. [DOI] [PubMed] [Google Scholar]
- 41.Coutinho E. R., Macedo G. M., Campos F. S., Bandeira F. A. Changes in HDL cholesterol and in the inflammatory markers of atherogenesis after an oral fat load in type-2 diabetic patients and normal individuals. Metabolic Syndrome and Related Disorders. 2008;6(2):153–157. doi: 10.1089/met.2007.0032. [DOI] [PubMed] [Google Scholar]
- 42.Hanefeld M., Schaper F., Koehler C., et al. Effect of acarbose on postmeal mononuclear blood cell response in patients with early type 2 diabetes: the AI(I)DA study. Hormone and Metabolic Research. 2009;41(2):132–136. doi: 10.1055/s-0028-1119407. [DOI] [PubMed] [Google Scholar]
- 43.van Wijk J. P. H., Cabezas M. C., Coll B., Joven J., Rabelink T. J., de Koning E. J. P. Effects of rosiglitazone on postprandial leukocytes and cytokines in type 2 diabetes. Atherosclerosis. 2006;186(1):152–159. doi: 10.1016/j.atherosclerosis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 44.van Oostrom A. J. H. H. M., Plokker H. W. M., van Asbeck B. S., et al. Effects of rosuvastatin on postprandial leukocytes in mildly hyperlipidemic patients with premature coronary sclerosis. Atherosclerosis. 2006;185(2):331–339. doi: 10.1016/j.atherosclerosis.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 45.de Vries M. A., Alipour A., Klop B., et al. Glucose-dependent leukocyte activation in patients with type 2 diabetes mellitus, familial combined hyperlipidemia and healthy controls. Metabolism. 2015;64(2):213–217. doi: 10.1016/j.metabol.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Van Oostrom A. J., Van Wijk J. P., Sijmonsma T. P., Rabelink T. J., Castro Cabezas M. Increased expression of activation markers on monocytes and neutrophils in type 2 diabetes. Netherlands Journal of Medicine. 2004;62(9):320–325. [PubMed] [Google Scholar]
- 47.Fogelstrand L., Hulthe J., Hultén L. M., Wiklund O., Fagerberg B. Monocytic expression of CD14 and CD18, circulating adhesion molecules and inflammatory markers in women with diabetes mellitus and impaired glucose tolerance. Diabetologia. 2004;47(11):1948–1952. doi: 10.1007/s00125-004-1553-x. [DOI] [PubMed] [Google Scholar]
- 48.Sampson M. J., Davies I. R., Brown J. C., Ivory K., Hughes D. A. Monocyte and neutrophil adhesion molecule expression during acute hyperglycemia and after antioxidant treatment in type 2 diabetes and control patients. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(7):1187–1193. doi: 10.1161/01.atv.0000021759.08060.63. [DOI] [PubMed] [Google Scholar]
- 49.Alipour A., van Oostrom A. J. H. H. M., Izraeljan A., et al. Leukocyte activation by triglyceride-rich lipoproteins. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(4):792–797. doi: 10.1161/atvbaha.107.159749. [DOI] [PubMed] [Google Scholar]
- 50.Klop B., van de Geijn G.-J. M., Njo T. L., et al. Leukocyte cell population data (volume conductivity scatter) in postprandial leukocyte activation. International Journal of Laboratory Hematology. 2013;35(6):644–651. doi: 10.1111/ijlh.12103. [DOI] [PubMed] [Google Scholar]
- 51.Gower R. M., Wu H., Foster G. A., et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(1):160–166. doi: 10.1161/atvbaha.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamburrelli C., Gianfagna F., D'Imperio M., et al. Postprandial cell inflammatory response to a standardised fatty meal in subjects at different degree of cardiovascular risk. Thrombosis and Haemostasis. 2012;107(3):530–537. doi: 10.1160/TH11-09-0674. [DOI] [PubMed] [Google Scholar]
- 53.Vors C., Pineau G., Drai J., et al. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose-effect trial. The Journal of Clinical Endocrinology & Metabolism. 2015;100(9) doi: 10.1210/jc.2015-2518. [DOI] [PubMed] [Google Scholar]
- 54.Peluso I., Palmery M., Vitalone A. Green tea and bone marrow transplantation: from antioxidant activity to enzymatic and multidrug-resistance modulation. Critical Reviews in Food Science and Nutrition. 2015 doi: 10.1080/10408398.2013.826175. [DOI] [PubMed] [Google Scholar]
- 55.Peluso I., Teichner A., Manafikhi H., Palmery M. Camellia sinensis in asymptomatic hyperuricaemia: a meta-analysis of tea or tea extract effects on uric acid levels. Critical Reviews in Food Science and Nutrition. 2015 doi: 10.1080/10408398.2014.889653. [DOI] [PubMed] [Google Scholar]
- 56.Miglio C., Peluso I., Raguzzini A., et al. Fruit juice drinks prevent endogenous antioxidant response to high-fat meal ingestion. British Journal of Nutrition. 2014;111(2):294–300. doi: 10.1017/S0007114513002407. [DOI] [PubMed] [Google Scholar]
- 57.Muraoka S., Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacology and Toxicology. 2003;93(6):284–289. doi: 10.1111/j.1600-0773.2003.pto930606.x. [DOI] [PubMed] [Google Scholar]
- 58.Schauer C., Janko C., Munoz L. E., et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nature Medicine. 2014;20(5):511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 59.Desideri G., Castaldo G., Lombardi A., et al. Is it time to revise the normal range of serum uric acid levels? European Review for Medical and Pharmacological Sciences. 2014;18(9):1295–1306. [PubMed] [Google Scholar]
- 60.Grassi D., Pontremoli R., Bocale R., Ferri C., Desideri G. Therapeutic approaches to chronic hyperuricemia and gout. High Blood Pressure and Cardiovascular Prevention. 2014;21(4):243–250. doi: 10.1007/s40292-014-0051-6. [DOI] [PubMed] [Google Scholar]