Abstract
Objectives:
To assess current practices of different healthcare providers for treating extensively drug-resistant (XDR) Acinetobacter baumannii (AB) infections in tertiary-care centers in Saudi Arabia.
Methods:
This cross-sectional study was performed in tertiary-care centers of Saudi Arabia between March and June 2014. A questionnaire consisting of 3 parts (respondent characteristics; case scenarios on ventilator-associated pneumonia [VAP] and tracheobronchitis [VAT], and antibiotic choices in each scenario) was developed and sent electronically to participants in 34 centers across Saudi Arabia.
Results:
One-hundred and eighty-three respondents completed the survey. Most of the respondents (54.6%) preferred to use colistin-based combination therapy to treat VAP caused by XDR AB, and 62.8% chose to continue treatment for 2 weeks. Most of the participants (80%) chose to treat VAT caused by XDR AB with intravenous antibiotics. A significant percentage of intensive care unit (ICU) fellows (41.3%) and clinical pharmacists (35%) opted for 2 million units (mu) of colistin every 8 hours without a loading dose, whereas 60% of infectious disease consultants, 45.8% of ICU consultants, and 44.4% of infectious disease fellows preferred a 9 mu loading dose followed by 9 mu daily in divided doses. The responses for the scenarios were different among healthcare providers (p<0.0001).
Conclusion:
Most of the respondents in our survey preferred to use colistin-based combination therapy and intravenous antibiotics to treat VAP and VAT caused by XDR AB. However, colistin dose and duration varied among the healthcare providers.
Mechanical ventilation is commonly used as a therapeutic option when caring for critically ill patients in the intensive care unit (ICU). Although mechanical ventilation may be lifesaving, it is associated with an increased risk of infections, including ventilator-associated pneumonia (VAP) and ventilator-associated tracheobronchitis (VAT). Ventilator-associated pneumonia has an estimated incidences of 10-25% and VAT has 1.4-11% with an estimated all-cause mortality of 25-50% for VAP, and 39% for VAT.1-3 Late-onset VAP (occurring after 5 days) is usually caused by multidrug-resistant (MDR) organisms and is associated with an increase in morbidity and mortality.1,2 The increased incidence of infections with these MDR pathogens is a major concern to health care providers worldwide and in particular Acinetobacter species, which were recognized as a cause of infection in critically ill patients in the past decade. With an increase in the use of broad-spectrum antibiotics, MDR and extensively drug-resistant (XDR) Acinetobacter baumannii (AB) have emerged as common pathogens causing late-onset VAP in the Middle East and Europe.4-6 The attributable mortality for ICU infected with AB was 10-43%, and for in-hospital patients (those who did not require ICU) was 8-23%.7 These high rates are likely related to the limited number of drugs available to treat XDR strains, as AB has an exceptional pathogenicity and capability to develop inherent and acquired resistance. The current knowledge on its treatment is insufficient as the quality of evidence, and the clinical practice guidelines are lacking. There are many controversies regarding different treatment options such as the superiority of combination therapy over monotherapy and the optimal dose of colistin, which is usually the only antibiotic to which XDR AB is susceptible.8,9 These have led to a variation in practice. The objective of this study was to investigate the current practices of clinicians and clinical pharmacists (CPs) caring for patients with XDR AB infections in Saudi Arabia. A survey was conducted to find answers to the following questions: Is combination therapy superior to monotherapy? Is there any role for colistin nebulization? What is the optimal dose of colistin?.
Methods
This cross-sectional study was performed in tertiary-care centers of Saudi Arabia between March and June 2014. The PubMed database was used to find prior related research.
The study subjects were physicians who were specialized in infectious disease (ID), critical care medicine, and clinical pharmacology in all major hospitals in different regions of Saudi Arabia. Informed consent was not required as the survey consisted of voluntary anonymous responses to a web-based questionnaire with no risk of breaching the participants’ confidentiality.
The sample size calculation was not performed a priori as we aimed at surveying all the specified healthcare providers in Saudi Arabia that were accessible via emails or social networks. After personal communication with acquaintances in the target hospitals, snowball sampling was used to reach the target healthcare providers. Assuming that the number of healthcare providers who were the target for this survey was 300, a reasonable estimate, the calculated sample size would be 169 at 95% confidence interval and 5% margin of error.
Item generation and development of questions
Five experts (3 ICU and 2 ID physicians) generated items through group discussion. Multiple meetings and discussions were held to shorten the list of items to reduce the burden on respondents and minimize redundancy while retaining the important items. Questions were developed based on the items of interest and were structured as multiple-choice questions that allowed a single answer for each question. The questionnaire was piloted with 10 participants before it was finalized. Feedback was obtained on the clarity and terminology of questions, and the questions were adjusted according to the feedback received and then the questionnaire was retested by the same 10 participants.
The final questionnaire was administered in English and investigated 3 components: respondent characteristics, antibiotic choice for 3 different clinical scenarios (VAP, VAT, and septic shock) and the duration of therapy for each of the clinical scenarios. Respondent characteristics included the medical specialty, job title (consultant, or fellow/registrar), and number of years practicing in that specialty. Questions regarding antibiotics, including the antibiotic preferred to treat VAP caused by XDR AB, monotherapy versus combination therapy, duration, dose (in patients with normal kidney function and in those with acute kidney injury [AKI]), treatment of VAT caused by XDR AB, and the route (intravenous versus nebulization) used to treat VAT. The questionnaire also contained questions to assess physician’s specialty title, years of experience, level of care, and perceptions of current clinical practice.
Questionnaire administration
The questionnaire was distributed to all centers (N=41) that provided ICU care in all major geographic regions of Saudi Arabia. Survey Monkey (www.surveymonkey.com) was used to design and distribute the questionnaire and to collect the responses. Emails and smart phone applications (Facebook messenger and Whatsapp) were also used to distribute the questionnaire link and to send reminders. Three reminders were sent to all recipients one week apart, and the data were collected between March and June 2014. The research coordinators were responsible for reminding non-respondents by phone or email. No financial or other incentives were provided to respondents.
Statistical analysis
Data were analyzed using MedCalc Statistical Software version 13.2.2 (MedCalc Software bvba, Ostend, Belgium). The categorical study variables were presented as frequencies with percentages. Responses to the survey items were compared according to the respondents’ speciality, job title, and length of clinical experience using Pearson’s Chi-square test. Using SPSS for Windows, Version 16.0 (SPSS Inc., Chicago, IL, USA), we performed multivariate binary logistic regression analysis to determine the predictors of the most prevalent management options, defined as those options chosen by the highest proportion of respondents. The independent variables in the model were the following: specialty (clinical pharmacists and intensivists with ID physicians being the reference group), position (consultants versus other healthcare practitioners), length of clinical experience (>10 years versus <10 years) and type of hospital (tertiary-care versus other hospitals). The results were presented as odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). A p-value of <0.05 was used to indicate statistical significance.
Results
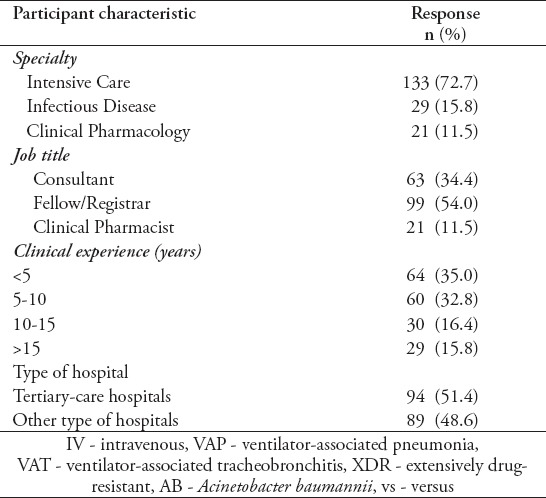
Out of the 204 healthcare practitioners (174 physicians and 30 CPs) from 29 government hospitals and 12 private hospitals responded (68% response rate), 183 completed the survey. The main characteristics of respondents are summarized in Tables 1 & 2. Most of the respondents were specialized in intensive care medicine (70.6%) followed by ID and clinical pharmacology (14.7% each).
Table 1.
Survey participant’s specialty and title according to the years of practice (N=204).
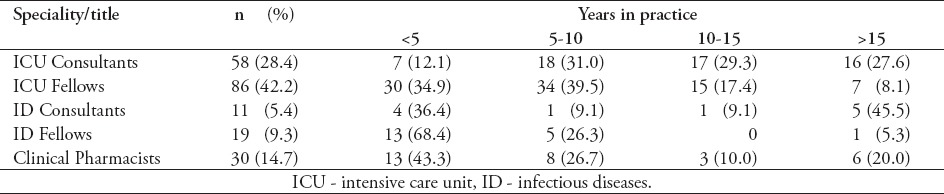
Table 2.
Characteristics of survey participants based on the completed survey (N=183).
Responses of all study subjects
Most of the physicians (54.6%) preferred colistin-based combination therapy as the first-choice treatment for VAP caused by XDR AB. The remaining 45.4% believed that IV colistin alone was sufficient. Carbapenems were the most frequently preferred antibiotics in combination with colistin (26.8%) followed by tigecycline (16.4%). Sixty-three percent of respondents believed that 2 weeks of therapy were sufficient for treating VAP caused by XDR AB (Table 3). For VAT caused by XDR AB, 80% of respondents agreed that patients should be treated with antibiotics (p<0.0001).
Table 3.
Distribution of survey responses towards the treatment of VAP and VAT among the ICU clinical staff (N=83).
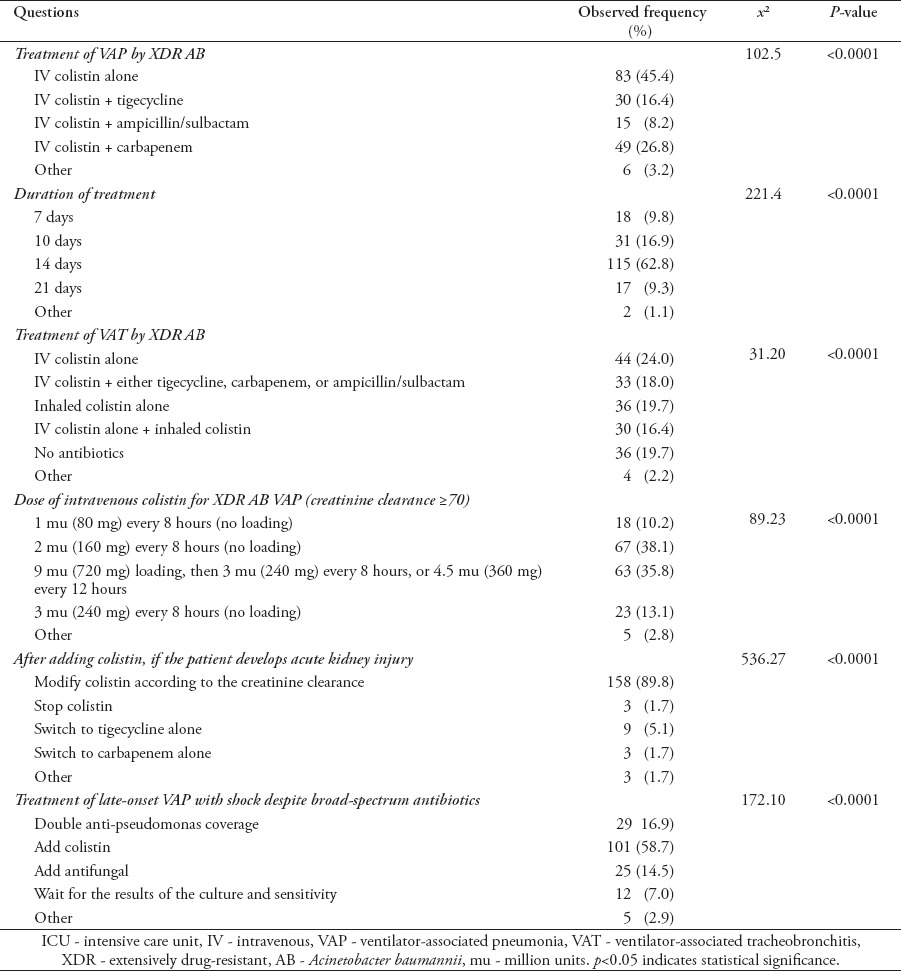
Approximately 38% of respondents preferred to use 2 million units (mu) (160 mg) of colistin every 8 hours without a loading dose, whereas 35.8% chose a 9 mu (720 mg) loading dose of colistin, followed by 3 mu (240 mg) every 8 hours, or 4.5 mu (360 mg) every 12 hours to treat VAP caused by XDR AB (p<0.0001). In case of AKI, 89.8 of respondents preferred to modify colistin dosing according to the creatinine clearance. When septic shock was present in patients with late-onset VAP on broad-spectrum antibiotics, most of the respondents (58.7%) preferred to add IV colistin empirically (Table 3).
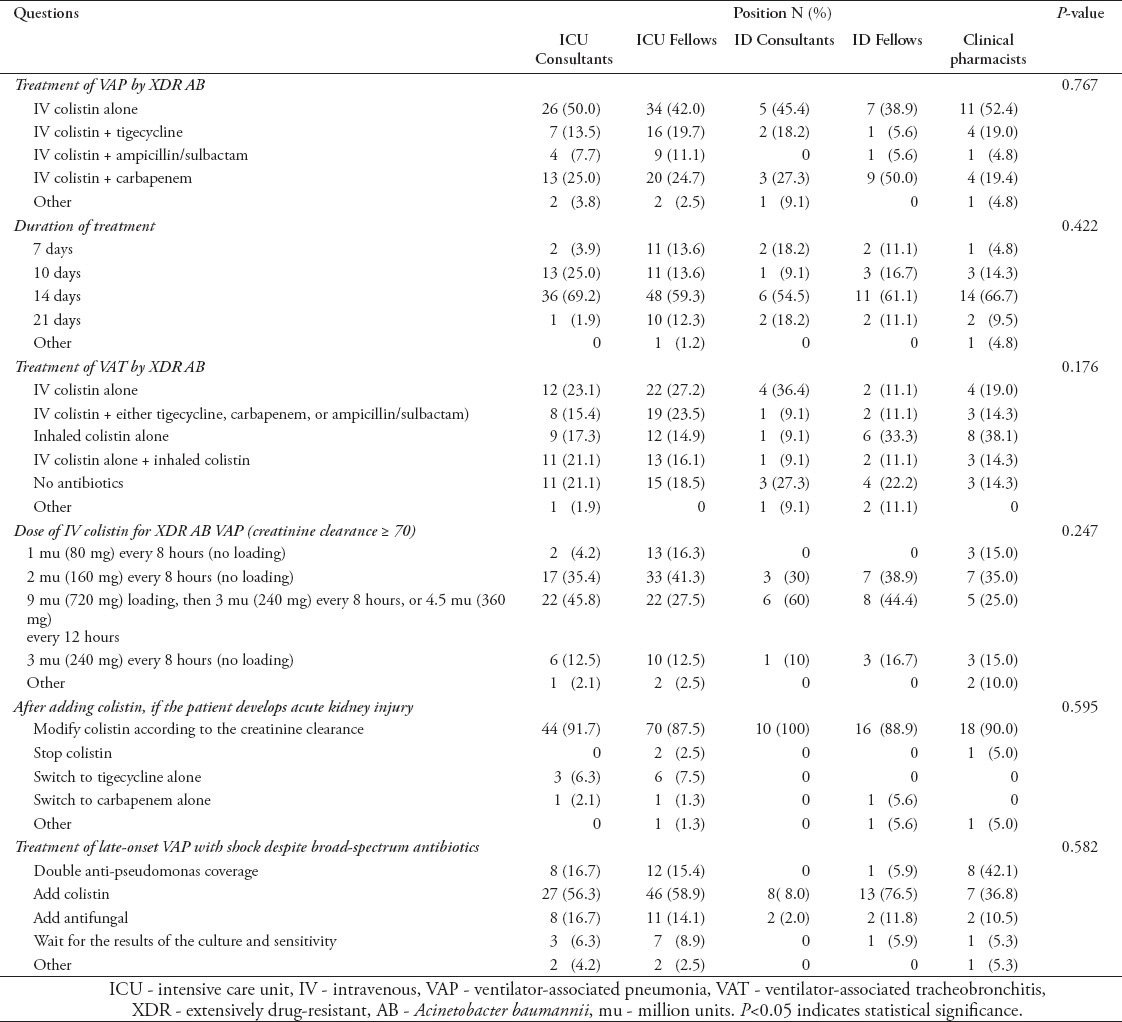
Responses according to position
Table 4 describes responses according to position. Most fellows (61% of ID and 58% of ICU) and ID consultants (55%) preferred combination therapy to treat VAP caused by XDR AB. Half of the ICU consultants and 52.4% of the CPs opted for IV colistin alone. Almost 41% of ICU fellows and 35% of CPs preferred to treat XDR AB VAP patients with normal renal function using 2 mu of colistin every 8 hours without a bolus dose. Most consultants (60% of ID and 45.8% of ICU) and ID fellows (44.4%) preferred to use a colistin loading dose of 9 mu followed by 3 mu every 8 hours, or 4.5 mu every 12 hours. Most CPs (66.7%), ICU physicians (64.3%), and ID physicians (57.8%) believed that a 2-week regimen was appropriate. When treating patients with late-onset VAP and shock despite broad-spectrum antibiotics, 80% of ID consultants recommended adding colistin empirically compared with 36.8% of CPs.
Table 4.
Distribution of survey responses regarding treatment of VAP and VAT among ICU clinical staff according to position (N=183).
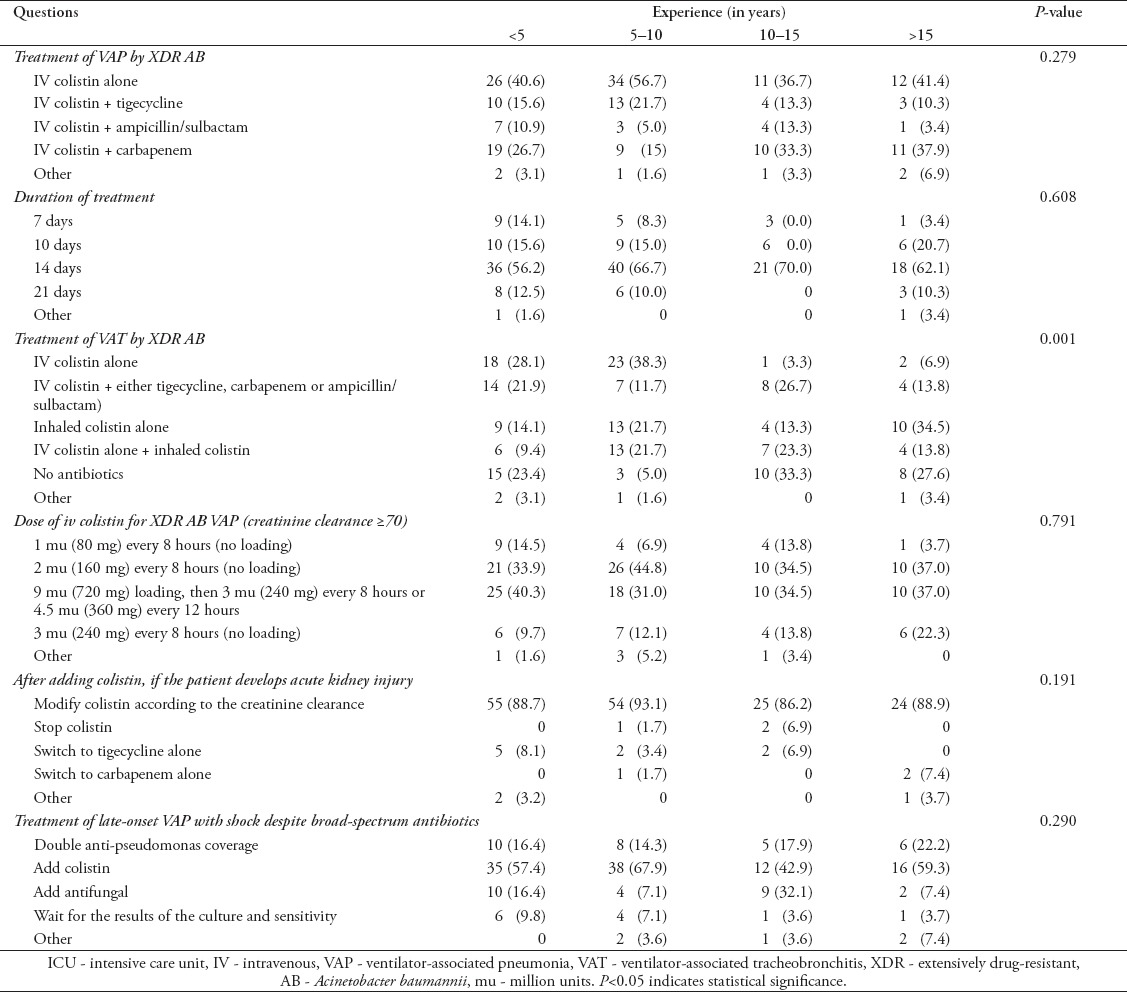
Responses according to clinical experience
As described in Table 5, more than 50% of the subjects at all experience levels preferred to treat patients with colistin for 14 days. From the subjects who had 5-10 years of experience, 38.3% favored using IV colistin alone to treat VAT while the respondents with more experience chose not to treat (33.3%), or to treat with inhaled colistin alone (34.5%). More than two-thirds of the subjects preferred using either 2 mu (160mg) every 8 hours without a loading dose, or a loading dose with a higher maintenance dose of intravenous colistin to treat patients with XDR AB VAP irrespective of the length of experience. For late-onset, VAP patients in shock despite broad-spectrum antibiotics, the empirical coverage recommendations were similar regardless of the length of experience.
Table 5.
Distribution of survey responses regarding treatment of VAP and VAT among ICU clinical staff according to clinical experience (N=83).
Predictors of the prevalent management choices
As represented in Table 6, IV colistin alone was the prevalent choice for VAP management. Healthcare practitioners at tertiary-care hospitals were more likely to choose this response compared with those working at other hospitals (OR=1.96; 95% CI: 1.07-5.58). Specialty, job title, and the length of experience did not predict this response.
Table 6.
Predictors of the prevalent survey responses using multivariate analysis (N=83).
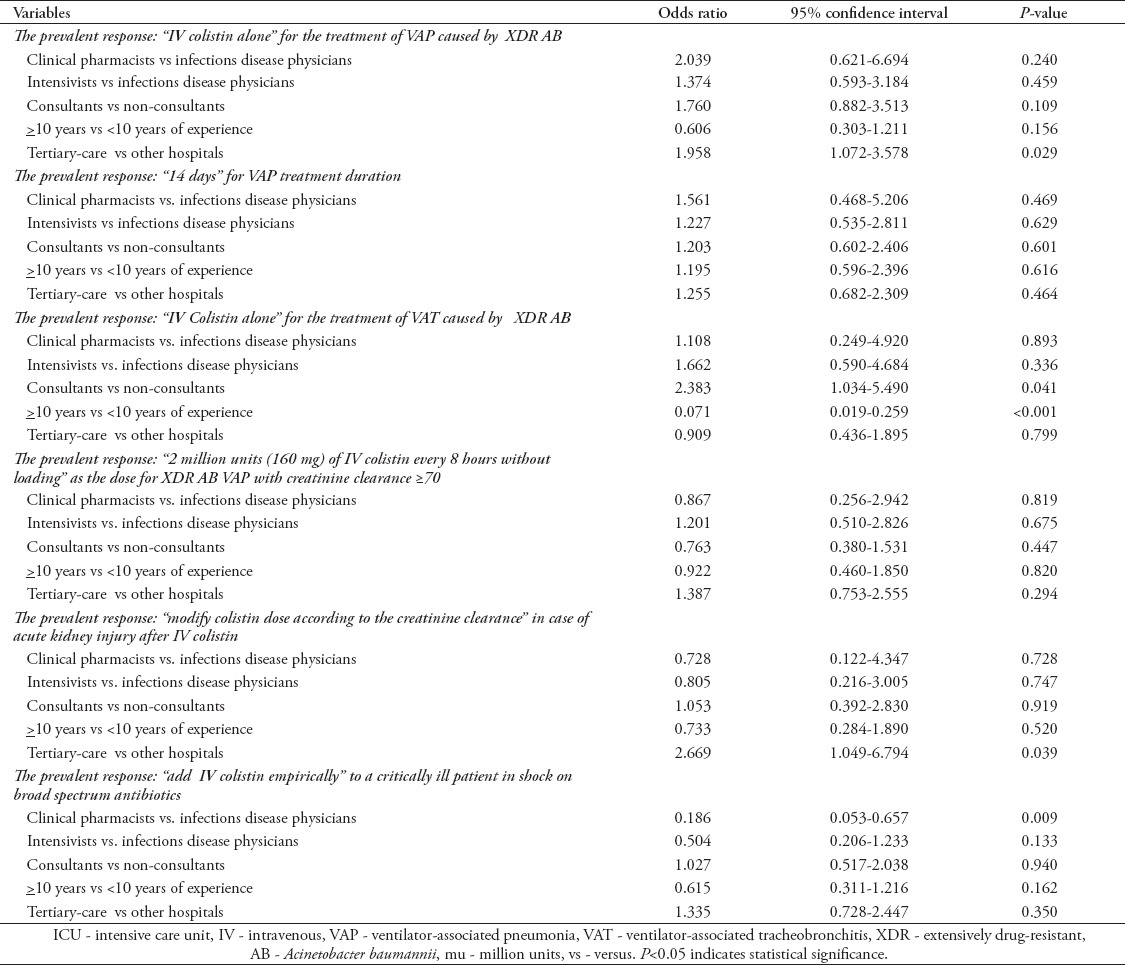
For VAP treatment duration, 14 days was the prevalent choice. None of the independent variables entered in the multivariate model predicted this response. Intravenous colistin alone was the prevalent choice for treatment of VAT. Being a consultant (OR=2.38; 95% CI: 1.03-5.49) and the length of experience more than or equal 10 years (OR=0.07; 95% CI: 0.02-0.26) were predictors of this response compared with healthcare practitioners of a different professional status and less experience.
Two million units (160 mg) of IV colistin every 8 hours without loading was the prevalent dose with creatinine clearance being more than or equal 70 ml/min. None of the independent variables entered in the multivariate model predicted this response. For AKI after IV colistin, modifying the colistin dose according to creatinine clearance was the prevalent choice. None of the independent variables entered in the multivariate model predicted this response. For septic shock not improving after 3 days of meropenem and vancomycin, “adding IV colistin” was the prevalent choice. The CP’s were less likely to choose this response compared with ID physicians (OR=0.19; 95% CI: 0.05-0.66). The other factors did not predict this response.
Discussion
The purpose of this survey was to assess current knowledge and practice among physicians and CPs in tertiary centers of Saudi Arabia. The main finding was that most respondents recommended for treating VAP caused by XDR AB with a combination of IV colistin and carbapenem for 2 weeks. Most respondents recommended using 2 mu of colistin every 8 hours, preceded by a loading dose, and 50% of participants opted for colistin therapy empirically when treating patients that had VAP and persistent septic shock while on broad-spectrum antibiotics. Most consultants preferred to treat VAT with IV colistin. In contrast, 38.1% of CPs and 33.3% of ID fellows preferred to use inhaled colistin alone. Most respondents (54.6%) recommended colistin-based combination therapy for the treatment of VAP due to XDR AB. Although we did not address the downsides of using monotherapy in our study, heteroresistance, rapid selection for resistance, toxicity, and lower efficacy were often common barriers for this treatment.10 Although synergism with combination therapy has been showing in vitro laboratory studies, clinical data has revealed conflicting results. A retrospective study of 27 tertiary-care centers in Turkey that included 250 patients with XDR AB infection showed that microbiological eradication and in-hospital survival rates were significantly higher with colistin-based combination therapy.11 In another retrospective study, Kalin et al12 showed a non-significant improvement in cure and bacteriological clearance rates in patients treated with a combination of colistin and sulbactam compared with those treated with IV colistin monotherapy. Although carbapenems were the preferred add-on agents in our survey, a previous study11 showed these agents did not improve 14-day mortality or clinical and microbiological clearance when used in combination therapy. Furthermore, in a recent retrospective study by Khawcharoenporn et al,13 a 28-day mortality and hospital length of stay were not significantly different among colistin-based regimens in a cohort of 236 patients with XDR AB pneumonia. The optimal duration for VAP treatment caused by AB is controversial. In our study, more than 60% of respondents preferred to administer a 14-day course. However, a systematic review favored a shorter antibiotic course (7-days) for treating VAP compared with a longer course (10-days).14 However, the recurrence rate was higher in the group of patients who were infected with non-fermenting Gram-negative bacilli and received the shorter course.14 This data demonstrates variation in practice, and reflects the need for studies examining the effects of treatment duration on outcomes in patients with VAP due to MDR gram-negative bacteria.
A recent study15 examining the optimal dosing of colistin showed that giving a 9 mu loading dose of colistin methanesulfonate followed by 4.5 mu every 12 hours resulted in early achievement of therapeutic concentrations of colistin in the blood of patients with normal kidney function.15 In our study, there were significant differences in the preferred doses. Most of the respondents opted for 2 mu every 8 hours; however, most of the consultants believed that a loading dose was essential. The rationale for this approach is that a steady state therapeutic concentration of colistin is reached after several days if a loading dose is not given, which will cause a delay in cure rate, and a recent study16 suggested that a loading dose with a high dose extended interval colistin methanesulfonate regime had a high clinical cure rate.
More than 50% of respondents preferred to add IV colistin empirically for the treatment of late-onset VAP with septic shock despite broad-spectrum antibiotics. The study by Kumar et al17 revealed that delayed antibiotic therapy was associated with increased mortality in patients with septic shock. The choice of antibiotics in patients with VAP and septic shock depends on local epidemiology, risk factors, recent hospitalization, antibiotics use, previous colonisation, and infection with resistant strains.18 Rello et al19 demonstrated that the empirical use of colistin and meropenem decreased length of stay in the ICU in pneumonia patients who had a baseline AB prevalence more than 10%. Given the high prevalence of XDR AB infection, or colonization in most ICUs in Saudi Arabia, adding IV colistin is appropriate in situations with late-onset VAP with septic shock.20 Most of clinicians (80%) in our survey recommended treating ICU patients with VAT, but the administration route, and the agents to be used varied among respondents. Treatment of VAT remains a topic of debate in the current literature,21 and 2 randomized controlled trials (RCTs) have addressed this issue. The first RCT22 demonstrated that treated patients had lower mortality and fewer days on mechanical ventilation compared with untreated patients, and the second RCT also showed a shorter duration of mechanical ventilation in the treatment group.23
Interestingly, 36 participants (19.7%) in our study supported the use of nebulized colistin alone, which has been shown to reduce the progression rate of VAP, development of resistance, and the need for systemic antibiotic use in a small study.23 However, the effect of nebulized colistin, either alone or in combination with other antibiotics, for the treatment of VAP or VAT is controversial. Korbila et al24 described that a combination of nebulized and IV colistin resulted in a better cure rate compared with IV colistin alone in 121 patients with VAP without a significant change in mortality, and similar findings have been shown in a recent case-control study of 208 patients with VAP.25 A recent RCT26 revealed that patients with VAP caused by Gram-negative bacteria showed no clinical improvement in response to adjunctive nebulized colistin therapy compared with patients who were only given systemic antibiotics. In addition to local side effects of colistin nebulization, such as pneumonitis, bronchospasm, and respiratory failure, development of drug resistance is another concern among clinicians. A recent study27 demonstrated that 4 out of 12 patients receiving colistin nebulization developed resistance to the antibiotic. However, more research in a wider range of patients is essential to elucidate this phenomenon of drug resistance. Since there is no clear data on treatment of VAP and VAT caused by XDR AB, we hypothesized that the workplace, specialty, job title, and clinical experience of the healthcare practitioner would influence the dose, duration, and type of antibiotics. However, we found that these factors had little effect on most responses, likely due to the absence of clear recommendations and guidelines.
Study limitations
First, most of the participants were from Riyadh, thus limiting the generalizability of our results. Second, most of respondents were in the ICU field and few ID specialists responded, indicating we were unable to survey all physician stakeholders. Third, physicians were not asked on which guidelines they based their choices. Fourth, the self-reported practices in our survey may not reflect the actual practice of respondents (inherent in all surveys). We adopted a systemic approach in the development of this survey and had a good response rate of 68%.
In conclusion, this study revealed that clinicians of different specialties and length of experience varied widely in their treatment of VAP and VAT caused by XDR AB. These differences are likely related to the lack of high-quality evidence and clinical practice guidelines, as well as the conflicting results of the available studies. Given the increasing incidence of VAP and VAT caused by XDR gram-negative bacteria and the high mortality rate associated with these infections, large multicenter RCTs on the benefits of colistin-based combination therapy versus colistin monotherapy, the value of colistin loading dose versus non loading dose, the value of lower dose versus higher maintenance colistin dose and so on, are warranted to help guide the clinical practice and effectively treat these infections.
Footnotes
Related Articles.
Bukhari SZ, Hussain WM, Banjar AA, Fatani MI, Karima TM, Ashshi AM. Application of ventilator care bundle and its impact on ventilator associated pneumonia incidence rate in the adult intensive care unit. Saudi Med J 2012; 33: 278-283.
Saeed NK, Kambal AM, El-Khizzi NA. Antimicrobial-resistant bacteria in a general intensive care unit in Saudi Arabia. Saudi Med J 2010; 31: 1341-1349.
Dinc U, Bayramoglu G, Buruk K, Ulusoy H, Tosun I, Kaklikkaya N. Molecular epidemiology of Acinetobacter baumannii-Acinetobacter calcoaceticus complex isolated from clinical specimens at an intensive care unit. Saudi Med J 2010; 31: 453-455.
References
- 1.Kalanuria AA, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18:208. doi: 10.1186/cc13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis. 2010;51(Suppl 1):S48–S53. doi: 10.1086/653049. [DOI] [PubMed] [Google Scholar]
- 3.Dallas J, Skrupky L, Abebe N, Boyle WA, 3rd, Kollef MH. Ventilator-associated tracheobronchitis in a mixed surgical and medical ICU population. Chest. 2011;139:513–518. doi: 10.1378/chest.10-1336. [DOI] [PubMed] [Google Scholar]
- 4.Alp E, Kiran B, Altun D, Kalin G, Coskun R, Sungur M, et al. Changing pattern of antibiotic susceptibility in intensive care units: ten years experience of a university hospital. Anaerobe. 2011;17:422–425. doi: 10.1016/j.anaerobe.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE!An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 6.El-Saed A, Balkhy HH, Al-Dorzi HM, Khan R, Rishu AH, Arabi YM. Acinetobacter is the most common pathogen associated with late-onset and recurrent ventilator-associated pneumonia in an adult intensive care unit in Saudi Arabia. Int J Infect Dis. 2013;17:e696–e701. doi: 10.1016/j.ijid.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care. 2007;11:134. doi: 10.1186/cc5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulikakos P, Tansarli GS, Falagas ME. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur J Clin Microbiol Infect Dis. 2014;33:1675–1685. doi: 10.1007/s10096-014-2124-9. [DOI] [PubMed] [Google Scholar]
- 9.Decker BK. Colistin dosing without pharmacokinetic data: the relic of a bygone era. Crit Care Med. 2015;43:1331–132. doi: 10.1097/CCM.0000000000000990. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batirel A, Balkan II, Karabay O, Agalar C, Akalin S, Alici O, et al. Comparison of colistin-carbapenem, colistin-sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol Infect Dis. 2014;33:1311–1322. doi: 10.1007/s10096-014-2070-6. [DOI] [PubMed] [Google Scholar]
- 12.Kalin G, Alp E, Akin A, Coskun R, Doganay M. Comparison of colistin and colistin/sulbactam for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Infection. 2014;42:37–42. doi: 10.1007/s15010-013-0495-y. [DOI] [PubMed] [Google Scholar]
- 13.Khawcharoenporn T, Pruetpongpun N, Tiamsak P, Rutchanawech S, Mundy LM, Apisarnthanarak A. Colistin-based treatment for extensively drug-resistant Acinetobacter baumannii pneumonia. Int J Antimicrob Agents. 2014;43:378–382. doi: 10.1016/j.ijantimicag.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Pugh R, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2015;8:CD007577. doi: 10.1002/14651858.CD007577.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother. 2009;53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis. 2012;54:1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 18.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rello J, Ulldemolins M, Lisboa T, Koulenti D, Manez R, Martin-Loeches I, et al. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur Respir J. 2011;37:1332–1339. doi: 10.1183/09031936.00093010. [DOI] [PubMed] [Google Scholar]
- 20.Balkhy HH, El-Saed A, Maghraby R, Al-Dorzi HM, Khan R, Rishu AH, et al. Drug-resistant ventilator associated pneumonia in a tertiary care hospital in Saudi Arabia. Ann Thorac Med. 2014;9:104–111. doi: 10.4103/1817-1737.128858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craven DE, Lei Y, Ruthazer R, Sarwar A, Hudcova J. Incidence and outcomes of ventilator-associated tracheobronchitis and pneumonia. Am J Med. 2013;126:542–549. doi: 10.1016/j.amjmed.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Nseir S, Favory R, Jozefowicz E, Decamps F, Dewavrin F, Brunin G, et al. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care. 2008;12:R62. doi: 10.1186/cc6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer LB, Smaldone GC, Chen JJ, Baram D, Duan T, Monteforte M, et al. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit Care Med. 2008;36:2008–2013. doi: 10.1097/CCM.0b013e31817c0f9e. [DOI] [PubMed] [Google Scholar]
- 24.Korbila IP, Michalopoulos A, Rafailidis PI, Nikita D, Samonis G, Falagas ME. Inhaled colistin as adjunctive therapy to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin Microbiol Infect. 2010;16:1230–1236. doi: 10.1111/j.1469-0691.2009.03040.x. [DOI] [PubMed] [Google Scholar]
- 25.Tumbarello M, De Pascale G, Trecarichi EM, De Martino S, Bello G, Maviglia R, et al. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest. 2013;144:1768–1775. doi: 10.1378/chest.13-1018. [DOI] [PubMed] [Google Scholar]
- 26.Rattanaumpawan P, Lorsutthitham J, Ungprasert P, Angkasekwinai N, Thamlikitkul V. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother. 2010;65:2645–2649. doi: 10.1093/jac/dkq360. [DOI] [PubMed] [Google Scholar]
- 27.Choi HK, Kim YK, Kim HY, Uh Y. Inhaled colistin for treatment of pneumonia due to colistin-only-susceptible Acinetobacter baumannii. Yonsei Med J. 2014;55:118–125. doi: 10.3349/ymj.2014.55.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]


