Abstract
Objectives:
To determine characteristics and treatment outcomes of multidrugs resistant tuberculosis (MDR-TB) patients and risk factors for poor outcomes in MDR-TB patients in a tertiary care hospital in Peshawar, Pakistan.
Methods:
This retrospective study was conducted at the Programmatic Management of Drug Resistant TB Unit, Lady Reading Hospital Peshawar, Pakistan and included all MDR-TB patients registered between January 2012 and December 2012. A special proforma was used for data collection. Analysis was performed using SPSS version 16, after exporting data from the proforma. Differences in proportions were assessed using Pearson’s Chi square test whereas for predictors of poor outcomes, multivariate logistic regression analysis with Wald Statistical criteria using backward elimination method was performed.
Results:
The treatment success rate was 74.3%. In univariate analysis, poor outcomes were associated in patients with age ≥44 years (odds ratio [OR]=0.250; 95% confidence interval [CI]: 0.114-0.519, p=0.001), rural residence (OR=0.417; 95% CI: 0.18-0.937, p=0.03), lung cavitation (OR=0.22; 95% CI, 0.007-0.067, p=0.001), resistance to second line drugs (SLD) (OR=3.441; 95% CI: 1.579-7.497, p=0.001), and resistance to ofloxacin (OR=2.944; 95% CI: 1.361-6.365, p=0.005); whereas multivariate logistic regression analysis, poor outcomes were associated in patients with age ≥44 years (OR=0.249, 95% CI: 0.075-0.828, p=0.023), rural residence (OR=0.143, 95% CI: 0.052-0.774, p=0.032), and cavitatory lungs (OR=0.022, 95% CI: 0.007-0.072, p=0.000).
Conclusion:
The MDR-TB patient needs special attention for better treatment outcomes. The presence of older age, rural area residence, resistance to ofloxacin, SLD resistance, and cavitary disease are independent prognostic factors for poor outcome in patients with MDR-TB.
Tuberculosis (TB) is a communicable, chronic granulomarous disease that ranks second as the leading cause of death from a single infectious agent after the human immunodeficiency virus (HIV). Due to world-wide efforts its control, the mortality rate of TB has decreased by 45% since 1990. However, despite all the possible measures to fight against TB, its global burden remains the same. According to the World Health Organization (WHO) report in 20131 TB infected 9 million people with 1.5 million deaths. Though the disease has been controlled by programmatic directly observed treatment short courses (DOTS) strategies, still its resurgence in the form of drug-resistant TB (DR-TB) is alarming. Among various resistance patterns, multi drug-resistant TB (MDR-TB) and extensive DR-TB (XDR-TB) demands more attention of health professionals. Multi drug-resistant TB is a purely man-made problem, which originates from wrong diagnosis, improper treatment, and non-compliance; thus, demanding more profound efforts. On average, 9% of estimated MDR-TB patients fail to achieve the desired therapeutic outcome and become extensive by DR. Globally in 2013,1 an estimated 480,000 people were infected by MDR-TB with estimated deaths of 210,000. The WHO data reveals that 3.7% of new cases, and 20% of previously treated cases are estimated to have MDR-TB.1 Multi drug-resistant TB offers a great challenge to TB control programs, and is significantly more difficult to treat than drug-susceptible TB. It requires the use of less effective second line drugs associated with more intolerable adverse effects compared with drug susceptible TB. It has a prolonged treatment of a minimum of 24 months with a low treatment success rate.2 Hence, it is very important to find out and efficiently treat an MDR-TB patient. Inadequate treatment of MDR-TB may worsen patients’ outcome, consequently increasing the risk of extensive drug resistance.3 Without proper treatment, multidrug resistant strains can spread rapidly within vulnerable populations.4 Because standard short-course chemotherapy for MDR-TB have been associated with unacceptably high rates of failure and relapse, and new approaches to treatment in poor countries are needed.5 Due to a rapid increase in the incidence of MDR-TB, it is very important to identify the predictors for unsuccessful treatment outcomes. Once the predictors are identified, they may be elaborated expeditiously for more effective control of DR-TB. Pakistan is one of the top listed countries ranking fourth among the top 22 MDR-TB countries. Based on 4.2% primary resistance and 19% resistance in re-treatment cases, the WHO has an estimated annual incidence of approximately 15000 MDR-TB cases in Pakistan.6 The increasing rate of MDR and XDR-TB in Pakistan underscores the importance of effective treatment programs of DR-TB. There is an urgent need for extensive research to elaborate efficient diagnosis, treatment, and control of MDR-TB as poor implementation of treatment protocols leads to further resistance, which emerge in the form of XDR-TB. Understanding the risk factors responsible for poor treatment outcomes among MDR-TB, patients is necessary to improve the treatment outcomes.7 This study was conducted to determine the overall treatment outcome and predictors attributing towards poor treatment outcome.
Methods
Study design and settings
Due to an increase in the incidence of DR-TB in Pakistan, patients are treated through Programmatic Management of Drug Resistant TB (PMDT). Different PMDT sites are functional in the country; Lady Reading Hospital Peshawar (LRH), Pakistan is one of most sophisticated health care setup in the Khyber Pakhtunkhwa Province. By utilizing its own resources, the LRH provided support for MDR patients between 2008 and 2012. In 2012 the National TB Control Programme declared the hospital as its PMDT site.
This retrospective cohort study was conducted at PMDT-LRH Peshawar, Pakistan. All confirmed pulmonary MDR-TB patients who were consecutively enrolled for treatment at the study site between January 2012 and December 2012 were included in the study. All the enrolled patients were treated on an ambulatory based care model and were properly examined by a team of clinicians on a monthly basis. All registered patients were initially started on a standardized treatment regimen, and then shifted to individualized regimens according to individual drug susceptibility test results.
Sputum smear, culture, and chest radiographs (CXR) were obtained at the time of enrollment, and then monthly during the intensive phase of treatment, whereas the continuation phase was accompanied by monthly smear and bimonthly culture and chest radiograph. All patients were tested at baseline for HIV, and blood investigations were performed at baseline, and at every month as per national DR-TB guidelines. Patients’ compliance and medication adherence was closely monitored by trained treatment supporters and directly observed therapy facilitators. All patients were psychologically assessed, and personalized counseling was provided on monthly follow up visits. Home visits were arranged for individual patient for contact screening, infection control measures at home and to create a liaison with the regional district TB officer. Adverse events if any, associated with second line drugs were monitored and managed promptly, utilizing multiple approaches of counselling, administering ancillary drugs and in rare cases, permanent removal of any culprit drug from the treatment regimen as a last resort. Patients received counseling to maximize adherence, nutritional support, and transportation reimbursement for their ambulatory visits.
Regimen design and patient management
All the registered patients were started on a standardized/empiric treatment regimen (ETR). The ETR was comprised of pyrazinamide, levofloxacin, ethionamide, cycloserine, and amikacin. The dose was individualized according to weight and clinical condition of each patient as per guidelines of the National TB Control Programme. Treatment on ETR was carried out until the results of drug susceptibility testing (DST). After confirmation of the resistance pattern determined through DST, all patients were shifted to an individualized treatment regimen (ITR) based on the DST profile for an individual patient.8 In general, regimens contained at least 5 drugs on which the infecting strain was susceptible, including a second-line injectable drug for at least 6 months after documented sputum culture conversion. Total treatment duration included a minimum of 12-18 months of post-culture conversion, which was regularly pursued afterwards by Mycobacterium tuberculosis (M. tuberculosis) smears and cultures up to the end of treatment.
Bacteriologic studies and drug susceptibility testing. Specimen processing
All samples were decontaminated with N-acetyl-L-cysteine (NALC) sodium hydroxide according to the standard protocol. All specimens were concentrated by centrifugation for 30 min and sediments were used for acid-fast bacilli microscopy and culture.
Microscopy
Smears for microscopy were screened using auramine-rhodamine staining. Positive slides were further confirmed by staining with Kinyoun modification of Ziehl-Neelsen stain.
Isolation of M. tuberculosis
Mycobacterial cultures were performed on both liquid and solid media. Sediments were cultured at 37°C using Lowenstein-Jensen (LJ) medium and the mycobacteria growth indicator tube (MGIT) (Becton Dickinson Diagnostic Instruments Systems, Sparks, MD, USA). For the LJ slant, 0.1 ml of concentrated specimen was inoculated and incubated for 8 weeks. Mycobacteria growth indicator tube vials were inoculated with 0.5 ml of specimen and incubated at 37°C after supplementation of the medium with oleic acid-albumin-dextrose-catalase and PANTA (Polymyxin B, Amphotericin B, Nalidixic Acid, Trimetho-Prim and Azlocillin). Growth from the positive LJ slant and MGIT vials were first stained with Kinyoun. Mycobacterium tuberculosis was then identified using the Bactec Nap TB differentiation test (Becton Dickinson, Sparks, MD, USA).
Drug susceptibility testing
Drug susceptibility testing was performed using the standard agar proportion method on enriched Middlebrook 7H10 medium (Becton Dickinson, Sparks, MD, USA) at the following drug concentrations: rifampicin (RMP) 1 g/ml and 5 g/ml, isoniazid (INH) 0.2 µg/ml and 1 µg/ml, streptomycin (SM) 2 µg/ml and 10 µg/ml, and ethambutol (EMB) 5 µg/ml and 10 µg/ml.8,9 To ensure the selection of high-level resistance strains for the purposes of this paper, only the higher concentrations were reported. Disc elution susceptibility plates were prepared using paper susceptibility discs (Becton Dickinson, Sparks, MD, USA). McFarland No. 1 the standard suspension of isolates was made from growth on LJ slant and diluted to 102 and 104. The inoculated plates were incubated at 35°C and examined for growth each week for 8 weeks. Mycobacterium tuberculosis was considered resistant to a given drug when growth of 1% above the drug-free control was observed in the drug-containing area. Pyrazinamide (PZA) sensitivity was carried out using Bactec 7H12 medium pH 6.0 at 100 µg/ml (Bactec PZA test medium, Becton Dickinson, Sparks, MD, USA) in accordance with the manufacturer’s instructions. Mycobacterium tuberculosis H37Rv was used as a control with each batch of DST.
Data collection and analysis
Both paper-based as well as electronic documentation were used for efficient and proper utilization of data. Parameters included in documentation were patients’ demographics and their clinical and microbiological data. Demographics data included gender, age, weight, co-morbidities, area of residence, and close contacts. Clinical data included history and outcome of previous TB treatment, previous medication history of any second line drugs and radiological findings at baseline chest x-ray. Microbiological data was composed of baseline sputum smear grading, DST result, and monthly microbiological culture status.
All the data was analyzed using the Statistical Package for Social Sciences version 16.0 (SPSS Inc., Chicago, IL, USA). Comparisons of demographics, socioeconomic status, HIV findings and TB-related characteristics, as well as treatment outcome parameters between patient subgroups were calculated using the Chi-squared test to find out any association between categorical variables, and the Mann-Whitney U-test was used for continuous variables. To estimate the predictors of poor treatment outcome, multivariate logistic regression analysis with Wald statistical criteria using the backward elimination method was performed. All the factors considered in the univariate analysis were entered into the multivariate analysis. P<0.05 was considered statistically significant. The study was approved by the Research and Ethics Committee of the Postgraduate Medical Institute, Peshawar, Pakistan.
Treatment outcomes were defined according to the recommendations from the WHO MDR-TB working group.1 Cured was defined as “treatment completed as recommended by the national policy (minimum 18 months past culture conversion) without evidence of failure and 5 consecutive cultures taken at least 30 days apart, are negative after the intensive phase. If only one positive culture is reported during that time, and there is no concomitant clinical evidence of deterioration, the patient may still be considered cured provided that this positive culture is followed by a minimum of 3 consecutive negative cultures taken at least 30 days apart”.
Treatment failure was defined as “Treatment terminated or need for permanent regimen change of at least 2 anti-TB drugs due to: a) lack of conversion by the end of the intensive phase, or b) bacteriological reversion in the continuation phase after conversion to negative, or c) evidence of additional acquired resistance to fluoroquinolones or second-line injectable drugs, or d) adverse drug reactions (ADRs)”.
Default means an interruption of >2 consecutive months of treatment. Patients were recorded as dead if they died during treatment, regardless of the cause. The XDR-TB is “resistance to any fluoroquinolone and to at least one of 3 second-line injectable drugs (capreomycin, kanamycin and amikacin), in addition to multidrug resistance”. Serious adverse events were defined as those that resulted in any change to the anti-TB drug regimen, either changing the dose of a drug, or temporarily or permanently removing a drug from the regimen. Culture is considered to have converted to negative when 2 consecutive cultures, taken at least 30 days apart, are found to be negative. In such a case, the specimen collection date of the first negative culture is used as the date of conversion.
Results
Between January 2012 and December 2012, a total of 200 DR-TB patients were registered for treatment at the PMDT LRH site. Among the 200 registered cases, 179 (90.6%) were found to be infected with MDR-TB, out of which 7 (3.9%) had XDR-TB. The remaining 21 (9.4%) patients was infected with strains of mycobectarium resistent to other drugs, which did not fulfil the criteria for MDR-TB and XDR-TB; hence, they were excluded from the study. Treatment outcome results were reported for those 179 patients who completed their treatment duration according to the prescribed guidelines.
Baseline patient characteristics
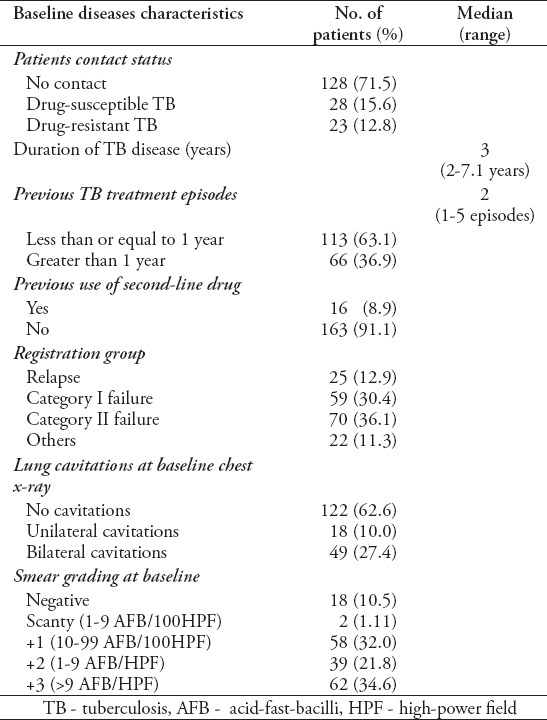
Baseline characteristics of patients treated in the 2012 cohort are summarized in Tables 1 & 2. All patients came from different districts of Khyber Pakhtunkhwa Province, Federally Administrative Tribal Areas (FATA), and Afghanistan, but most patients (34.6%) were from the District of Peshawar where LRH is located. Among the population, 121 (67%) cases were from rural areas. The median age was 26 years, ranging from 12-79 years, and 52.2% were female. Most patients (n=115 [64.2%]) placed in 40-60 kg baseline weight ranging from 22-78 kg with an average of 44 kg. Sixty-seven percent of the study cases were married at the time of treatment. Twenty-eight (15.6%) patients were in close contacts with drug-susceptible TB, and 23 (12.8%) patients were of DR-TB and 90% of patients were previously treated with first line anti-TB drugs (FLDs). Patients had been ill with TB for a median of 3 years and had received a median of 3 previous TB treatment episodes. More than half of the patients had taken retreatment regimen (category-II) treatment regimen for at least once, and out of these patients, 36.1% failed to respond to category-II regimen at least one time. Sixteen patients (8.9%) had taken at least one second-line drug in their previous treatment regimen.
Table 1.
Baseline sociodemographic characteristics of multi drug-resistant tuberculosis patients from Pakistan (n=179).
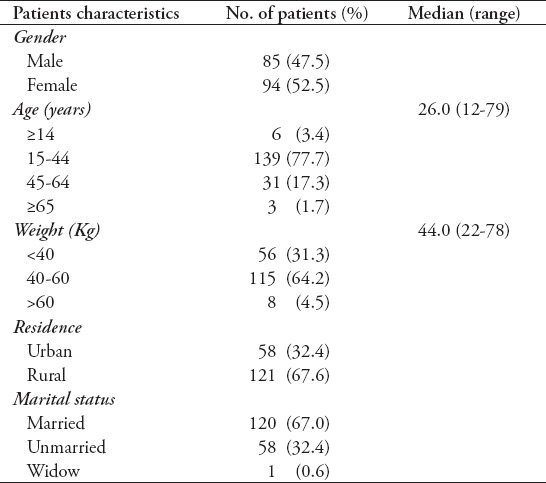
Table 2.
Baseline disease characteristics of MDR-TB patients from Pakistan (n=179).
Drug resistance pattern
High levels of first-line resistance was observed. All of the strains were resistant to INH and RMP followed by PZA, EMB, and SM (Table 3). Overall, 104 (58.1%) strains were resistant to at least one second-line drugs (SLD). The most common second-line resistance observed was ofloxacin, followed by ethionamide, amikacin, capreomycin, and kanamycin. Significant overlap was also observed in 99 strains resistant, to ofloxacin that were also resistant to PZA.
Table 3.
Pattern of resistance to first and second line anti-tuberculosis drugs in study cases (n=179).

Treatment regimens
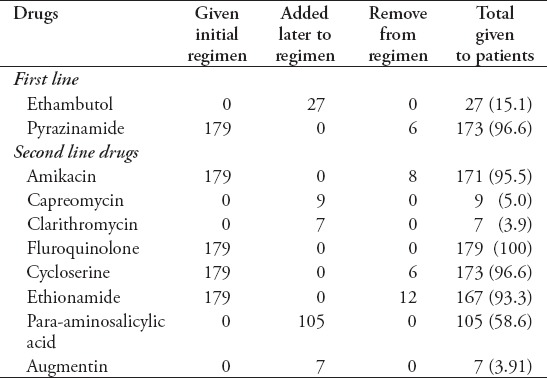
All the registered MDR-TB patients whose diagnosis was confirmed by Xpert MTB/Rif were started with standardized regimen as per National TB guidelines until confirmation of DST pattern. Drug susceptibility testing results were available at a median of 52.50 days (range 21-102 days) after treatment initiation. In 66 (36.9%) patients initiated with empirical regimen, the confirmation of DST results did not cause any change to the treatment regimen. In 113 (63.1%) patients, the most common change to the regimen upon receiving DST results was the removal of amikacin (n=8) based on resistance level. The most common drugs added to the treatment regimens after treatment initiation were PAS (n=105), followed by ethambutol (n=27), which is not included in the ETR and susceptibility was later on demonstrated by DST: augmentin (n=7), clarithromycin (n=7), and capreomycin (n=9) (added as an additional agents when more efficacious SLD were no longer imperative). The 2 most common reasons for stopping drugs in the regimen was the development of resistance and severe adverse events (Table 4). To all those cases, which showed resistance to fluoroquinolone, PAS was added as a clean drug and pyrazinamide was given to all cases irrespective of DST results as per National DR-TB guidelines.
Table 4.
Overview of drugs given to the multi drug-resistant TB patient from Pakistan during treatment (n=179).
Treatment outcomes
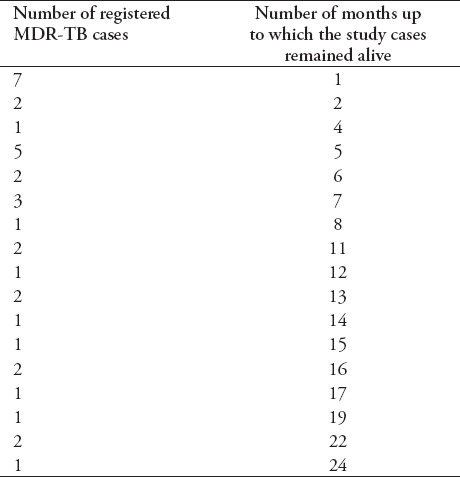
Overall, 133 (74.3%) of total cases were successfully treated, while 46 (25.7%) patients were recorded as being ineffectively treated. One hundred and thirty-three cases (74.3%) were declared cured, 34 (19%) patients died during treatment, 10 (5.6%) were classified as treatment failures, and 2 (1.1%) patients defaulted during treatment. Among the 2 patients recorded as treatment defaults, the median time for default was 9.5 months (range 2-17 months). The sputum conversion of one patient defaulted after the second month, whereas other patient was defaulted after 17 months, and conversion of the culture was occurred at the third month and was negative until the time of default. Ten patients were classified as treatment failure and were removed from treatment due to failure of therapy after a median of 24.5 months on treatment (range 19-30 months). The survival time of 34 (19%) cases is shown in Table 5.
Table 5.
Survival time of registered multi drug-resistant TB (MDR-TB) patients who died during the study period (n=34).
Treatment duration
Among successfully treated patients, the median total treatment duration was 24 months (range 14-34 months), which was a median of 21 months after culture conversion (range 17-27 months). Injectable drugs were given for a median of 9 months after culture conversion (range 6-12 months), giving a total median duration for the injectable drugs among successfully treated patients. All patients classified as failure in treatment were continued on the injectables for the entire duration of treatment.
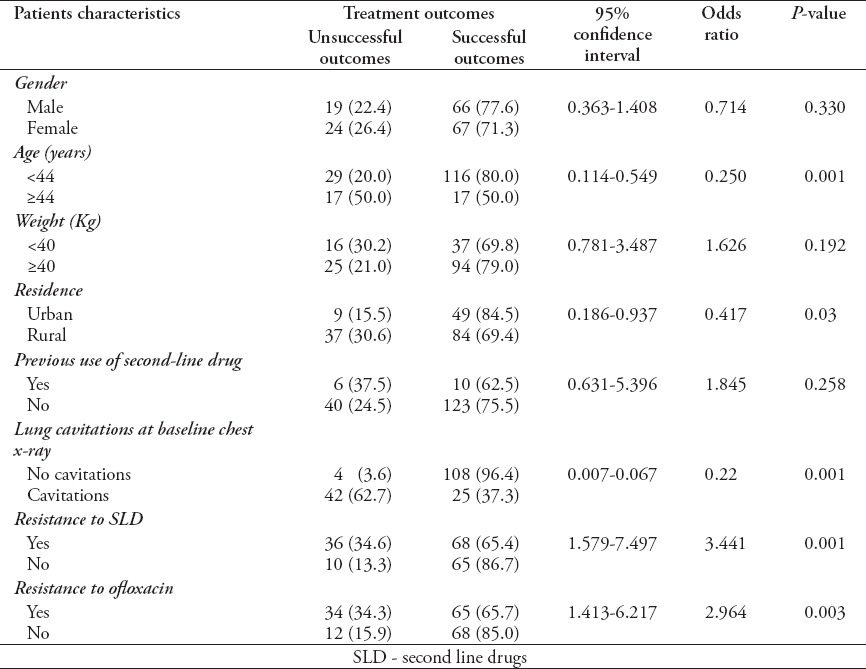
Factors associated with poor outcomes
Univariate analysis (Table 6) showed that certain demographic and clinical characteristics, such as patient with, or greater than 44 years of age (p=0.001), rural residence (p=0.03), cavitatory lungs at baseline (p=0.001), resistance to SLD at treatment initiation (p=0.001), and resistance to ofloxacin (p=0.003) were associated with poor treatment outcomes.
Table 6.
Univariate analysis of factors potentially contributing to unsuccessful treatment outcomes (n=179).
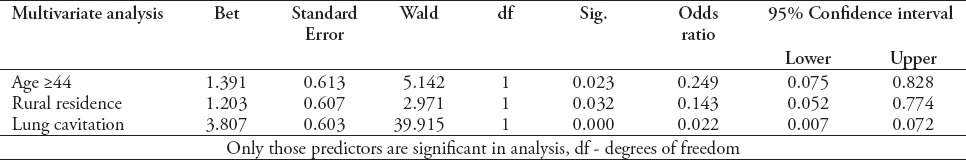
In a multivariate regression model, only patients with age ≥44, rural residence, and cavitation of lungs at baseline, chest x-ray had a positive significant association with unsuccessful treatment outcomes (Table 7). This model was based on non-significant Hosmer and Lemeshow test (p=0.182) and overall percentage of 84.6% from classification table.
Table 7.
Multivariate analysis showing predictors for unsuccessful treatment outcomes of multidrug-resistant TB patients (n=172).
Discussion
Currently, DR-TB is a serious public health concern. Accurate diagnosis and effective treatment of DR-TB is essential not only to cure the effected population, but most importantly to prevent further transmission of resistant strains. Compared to drug susceptible TB, successful outcome rate of DR-TB is low especially MDR-TB. It is important to evaluate effective management, treatment outcomes, and predictors responsible for poor treatment outcomes for better understanding and highlighting opportunities for improvement in the treatment outcomes of MDR-TB. This present study was designed to identify the characteristics and treatment outcomes of MDR-TB patients, and to identify the critical predictors of poor treatment outcomes. The demographic profile of patients in the present study was similar to other series,10,11 with a majority of female patients in the economically productive age group (25-54 years). Out of these 179 patients, 133 (74.3%) patients achieved successful outcome, which is close to the target set by “The Global Plan to stop TB 2011-2015” namely, >75%.12 The result is comparatively better compared with other studies such as 49% in a study conducted in south Africa,13 54.9% from a study conducted in Shanghai,14 64% in New York,15 and 48.2% in South Korean report.16 The target rate of the present study was lower than Germany (80%).17 Similar result were also found in previous studies18-23 namely 51-77%. Treatment failure among this cohort approached 5.6%, which is approximately comparable with other study where failure rates range between 0% and 4% among MDR-TB patients.24,25 In the current study, low default rate (1.1%) contributed to better treatment success rate. Default rate is lower than other studies conducted in Uzbekistan (14%),25 South Africa (29%),26 and South Korea (32%) for MDR-TB).27 The low default rate could be attributed to free treatment cost, tracing patients by phone in case of delay in scheduled monthly visits, provision of monthly food rations, and conveyance allowance to both patients and their treatment supporters, and home visits carried out by the hospital DOTs linkages coordinator (treatment coordinator HDL) create and establish linkages between patients, district TB control officer, and nearest TB treatment centers (DOT center), and PMDT LRH. A 19% mortality rate is similarto a study conducted in South India28 and lower than a study conducted in South Africa (36%).26 Differences in mortality rates were found when compared with other studies (5-19%).29-33 The mortality rate is high in the present study compared with other studies, and the possible reason for this might be the high default rates in other studies (11%, 12%).29,33 For predictors of poor outcomes, different variables were tested in univariate and multivariate analysis. Different variables were tested in univariate and multivariate analysis. Some of the factors that did not influence the treatment outcome include gender, weight, previous TB treatment, duration of sickness and resistance to first line drugs, while some factors such as age, residence, lung cavitation, resistance to SLD’s, and resistance to ofloxacin were found to be associated with poor outcomes, as also shown in other studies.17,25,30 The present study suggested that age is positively associated with poor outcomes. Similar to our findings, older age has been previously reported as a predictor of poor treatment outcomes in MDR-TB patients DOTS-plus projects in 5 resource limited countries.34 This is because older people have comorbidities, respond poorly to anti-TB drugs and their recovery is slow compared with to the younger people. The study shows that poor outcome has been observed with increased age. We also found that poor outcome of MDR-TB treatment is strongly associated with living in rural areas. One speculative explanation for this phenomenon is that patients belong to rural zones were from far-flung areas with limited health facilities, difficult implementation of DOTS, low education level, and poor socioeconomic conditions with malnutrition leading to an ineffective pharmacological response to drugs. Rationally, the most important tool for improving the treatment outcome of MDR-TB in rural areas is patient education. A strong emphasis should be placed on effective patient counselling and education in rural areas for better results.
Cavitation of the lungs was also a predictor of poor outcomes in the present study. Patients with lung cavitation documented on their first visit have poorer outcomes as compared with no cavitation. Patients with bilateral cavities were more vulnerable to poor outcomes as compared with unilateral or no cavitatory lungs. Similar findings were also observed in previous studies.34,35 The possible reason was the presence of cavities in lungs associated with poor penetration of drugs resulting in decreased efficacy.7 Several studies emphasize the important role of resistance to ofloxacin in poor MDR-TB treatment outcome.16,20,21,30,32,36 In the present study, resistance to ofloxacin was 55.3%. Two reasons could contribute to the high drug resistant proportions: First, fluoroquinolones have been widely used in the treatment of respiratory tract bacterial infections with better effects and minimal adverse effects. Second, fluoroquinolones are also prescribed for drug resistant TB patients as well as in some drug susceptible TB patients who cannot tolerate the first line anti-TB drugs. This finding further emphasizes the importance of ofloxacin in MDR-TB treatment regimens and highlights the need for preserving susceptibility to ofloxacin, as well as pointing out the clinical value of ofloxacin resistance in the definition of XDR-TB. One alarming point is that patients resistant to ofloxacin were also resistant to pyrazinamide and both the drugs were used for treatment of MDR and XDR-TB. This issue is of great importance and needs efficient clinical attention. This study also suggests the use of SLD’s as the strongest risk factor for poor MDR-TB outcomes. Rational use of SLD’s with proper monitoring should be highly encouraged.
Study limitation
The study being carried out at a single center was the major limitation of the present study, and further studies should be conducted in different centers on a larger scale to identify predictors. In described limitation, it is encouraging that in such difficult and high patient-burden areas with limited resources. This center achieved such a comparable cure rate and are able to conduct such a study, which is helpful for other newly organized centers, and will encourage the PMDT staff to do more.
In conclusion, the findings of the current study have several clear implications for TB control efforts. In light of these findings, it could be concluded that to reduce DR-TB transmission in the community, improvement of treatment outcomes, via ensuring adherence, paying special attention to elderly patients, rural residents, the SLD resistant patients and those who are resistant to ofloxacin, is required, in addition to extensive use of rapid diagnostic methods and highly effective aggressive TB treatment. We know that interruption of the drug-resistant TB transmission cycle is possible if the cure rate is >60%. A cure rate of ≥80% is needed to achieve a 10-fold reduction in MDR-TB incidence within 20 years.37 Achievement of such rates, required properly implemented public information, communication and advocacy, judicious use of anti-tubercular drugs, and regular clinical, radiological, and bacteriological follow up in specialized centers with access to the standardized TB laboratory for accurate DST.
Acknowledgment
We acknowledge the outstanding contributions of Mr. Hidayat Ullah, Hasnain Ali, Muhammad Ishfaq, Shah Zaman, Umar Farooq, Salman Ahmad, and Zeeshan Ahmad from the Programmatic Management of Drug Resistant TB staff, and the National TB Control Programme, Provincial TB Control Programme, Khyber Pakhtunkhwa, Association for Community Development, and Lady Reading Hospital Peshawar, Pakistan.
Footnotes
References
- 1.World Health Organization. Global tuberculosis report. Geneva (CH): WHO; 2014. [Google Scholar]
- 2.Gupta R, Cegielski JP, Espinal MA, Henkens M, Kim JY, Lambregts-Van Weezenbeek CS, et al. Increasing transparency in partnerships for health-introductiong the Green Light Committee. Trop Med Int Health. 2002;7:970–976. doi: 10.1046/j.1365-3156.2002.00960.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan E, Iseman M. Multidrug-resistant and extensively drug-resistant tuberculosis. A review. Curr Opin Infect Dis. 2008;21:587–595. doi: 10.1097/QCO.0b013e328319bce6. [DOI] [PubMed] [Google Scholar]
- 4.Kimerling ME, Kluge H, Vezhnina N, Iacovazzi T, Demeulenaere T, Portaels F, et al. Inadequacy of the current WHO re-treatment regimen in a central Siberian prison: treatment failure and MDR-TB. Int J Tuberc Lung Dis. 1999;3:451–453. [PubMed] [Google Scholar]
- 5.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 6.Javaid A, Hasan R, Zafar A, Ghafoor A, Pattan AJ, Rab A, et al. Prevalence of primary multidrug resistance to anti-tuberculosis drug in Pakistan. Int J Tuberc Lung Dis. 2008;12:326–331. [PubMed] [Google Scholar]
- 7.Yew WW, Chan CK, Chau CH, Tam CM, Leung CC, Wong PC, et al. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest. 2000;117:744–751. doi: 10.1378/chest.117.3.744. [DOI] [PubMed] [Google Scholar]
- 8.Tabarsi P, Chitsaz E, Tabatabaei V. Revised category II regimen as an alternative strategy for retreatment of category I regimen failure and irregular treatment cases. Am J Ther. 2011;18:343–349. doi: 10.1097/MJT.0b013e3181dd60ec. [DOI] [PubMed] [Google Scholar]
- 9.Espinal MA. Time to abandon the standard retreatment regimen with first-line drugs for failures of standard treatment. Int J Tuberc Lung Dis. 2003;7:607–608. [PubMed] [Google Scholar]
- 10.Turett GS, Telzak EE, Torian LV, Alland D, Weisfuse I, Fazal BA. Improved outcomes for patients with multi-drug-resistant tuberculosis. Clin Infect Dis. 1995;21:1238–1244. doi: 10.1093/clinids/21.5.1238. [DOI] [PubMed] [Google Scholar]
- 11.Park SK, Kim CT, Song SD. Outcome of chemotherapy in 107 patients with pulmonary tuberculosis resistant to Isoniazid and Rifampicin. Int J Tuberc Lung Dis. 1998;2:877–884. [PubMed] [Google Scholar]
- 12.World Health Organization. The global plan to stop TB2011-2015: transforming the fight towards elimination of tuberculosis. Geneva (CH): World Health Organization; 2010. [Google Scholar]
- 13.Shean KP, Willcox PA, Siwendu SN, Laserson KF, Gross L, Kammerer S, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa 1992-2002. Int J Tuberc Lung Dis. 2008;12:1182–1189. [PubMed] [Google Scholar]
- 14.Zhao M, Li X, Xu P, Shen X, Gui X, Wang L, et al. Transmission of MDR and XDR tuberculosis in Shanghai, China. PLoS ONE. 2009;4:e4370. doi: 10.1371/journal.pone.0004370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telzak EE, Sepkowitz K, Alpert P, Mannheimer S, Medard F, El-Sadr W, et al. Multidrug-resistant tuberculosis in patient without HIV infection. N Engl J Med. 1995;333:907–911. doi: 10.1056/NEJM199510053331404. [DOI] [PubMed] [Google Scholar]
- 16.Park SK, Lee WC, Lee DH, Mitnick CD, Han L, Seung KJ. Self-administered, standardized regimens for multidrug-resistant tuberculosis in South Korea. Int J Tuberc Lung Dis. 2004;8:361–368. [PubMed] [Google Scholar]
- 17.Diel R, Nieman S. Outcome of tuberculosis treatment in Hamburg: a survey, 1997-2001. Int J Tuberc Lung Dis. 2003;7:124–131. [PubMed] [Google Scholar]
- 18.Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 19.Chan ED, Laurel V, Strand MJ, Chan JF, Huynh ML, Goble M, et al. Treatment and outcome analysis of 205 patients with multi drug resistant tuberculosis. Am J Respir Crit Care Med. 2004;169:1103–1109. doi: 10.1164/rccm.200308-1159OC. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CY, Enarson DA, Yu MC, Bai KJ, Huang RM, Hsu CJ, et al. Outcome of pulmonary multidrug-resistant tuberculosis: a 6-yr follow-up study. Eur Respir J. 2006;28:980–985. doi: 10.1183/09031936.06.00125705. [DOI] [PubMed] [Google Scholar]
- 21.Migliori GB, Besozzi G, Girardi E, Kliiman K, Lange C, Toungoussova OS, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–626. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 22.Nathanson E, Lambregts-van Weezenbeek C, Rich ML, Gupta R, Bayona J, Blondal K, et al. Multidrug-resistant tuberculosis management in resource-limited settings. Emerg Infect Dis. 2006;12:1389–1397. doi: 10.3201/eid1209.051618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tahaoglu K, Torun T, Sevim T, Atac G, Kir A, Karasulu L, et al. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med. 2001;345:170–174. doi: 10.1056/NEJM200107193450303. [DOI] [PubMed] [Google Scholar]
- 24.Nathanson E, Gupta R, Huamani P, Leimane V, Pasechnikov AD, Tupasi TE, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8:1382–1384. [PubMed] [Google Scholar]
- 25.Cox HS, Kalon S, Allamuratova S, Sizaire V, Tigay ZN, Rusch-Gerdes S, et al. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: Treatment complexity and XDR-TB among treatment failures. PLoS ONE. 2007;2:e1126. doi: 10.1371/journal.pone.0001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375:1798–1807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 27.Jeon CY, Hwang SH, Min JH, Prevots DR, Goldfeder LC, Lee H, et al. Extensively drug-resistant tuberculosis in South Korea: Risk factors and treatment outcomes among patients at a tertiary referral hospital. Clin Infect Dis. 2008;46:42–49. doi: 10.1086/524017. [DOI] [PubMed] [Google Scholar]
- 28.National Tuberculosis Institute, Bangalore. Tuberculosis in a rural population of South India: a five-year epidemiological study. Bull World Health Organ. 1974;51:473–488. [PMC free article] [PubMed] [Google Scholar]
- 29.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 30.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: A systematic review and meta-analysis. PLoS ONE. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitnick C, Bayona J, Palacios E, Shin S, Furin J, Alcantara F, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 32.Leimane V, Riekstina V, Holtz TH, Zarovska E, Skripconoka V, Thorpe LE, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 33.Shin SS, Pasechnikov AD, Gelmanova IY, Peremitin GG, Strelis AK, Andreev YG, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis. 2006;10:402–408. [PubMed] [Google Scholar]
- 34.Kurbatova EV, Taylor A, Gammino VM, Bayona J, Becerra M, Danilovitz M, et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis. 2012;92:397–403. doi: 10.1016/j.tube.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez M, Monedero I, Caminero J, Encarnación M, Dominguez Y, Acosta I, et al. Successful management of multidrug-resistant tuberculosis under programme conditions in the Dominican Republic. Int J Tuberc Lung Dis. 2013;17:520–525. doi: 10.5588/ijtld.12.0481. [DOI] [PubMed] [Google Scholar]
- 36.Yew WW, Chan CK, Leung CC, Chau CH, Tam CM, Wong PC, et al. Comparative roles of levofloxacin and ofloxacin in the treatment of multidrug- resistant tuberculosis: preliminary results of a retrospective study from Honk Kong. Chest. 2003;124:1476–1481. doi: 10.1378/chest.124.4.1476. [DOI] [PubMed] [Google Scholar]
- 37.Dye C, Williams BG. Criteria for the control of drug resistant tuberculosis. Proc Natl Acad Sci USA. 2000;97:8180–8185. doi: 10.1073/pnas.140102797. [DOI] [PMC free article] [PubMed] [Google Scholar]


