Abstract
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease that progresses to end-stage liver disease and cirrhosis. Recurrent biliary inflammation is thought to lead to dysplasia, and as such PSC confers a high risk of cholangiocarcinoma. PSC accounts for 10% of all UK liver transplants, although transplantation does not guarantee a cure with 20% recurrence in the graft. At present there are no effective medical treatment options for PSC, and trials of novel therapeutic agents are limited by the time taken to reach clinically significant endpoints with no well defined early surrogate markers for disease outcome. Moreover, PSC appears to be a heterogeneous disease with regards to disease distribution, associated inflammatory bowel disease and subsequent disease outcome, further compounding the issue. Thus existing trials have taken place in heterogeneous groups, are likely to be underpowered to detect any individual subgroups effect. The current mainstay of medical treatment is still with ursodeoxycholic acid, although there is no evidence that it alters long-term outcome. Small pilot studies of immunosuppressive agents have taken place, but despite evidence that may support studies in larger groups, these have not been conducted. Recent advances in our understanding of the disease pathogenesis may therefore pave the way for trials of novel therapeutic agents in PSC, even given the limitations described. This review explores the controversial evidence underlying current treatment strategies and discounted treatments, and explores prospective agents that may bring new hope to the treatment of PSC in the 21st century.
Keywords: cholestasis, primary sclerosing cholangitis, ursodeoxycholic acid
Introduction
Primary sclerosing cholangitis (PSC) is one of the archetypal autoimmune liver diseases alongside autoimmune hepatitis and primary biliary cirrhosis (PBC). Considered a rare disease, it characteristically affects young patients with a slight male predominance. At present a truly conceptual pathogenic framework is lacking and there is no recognized therapy that has been shown to alter the outcome of patients with this condition.
PSC is a chronic, cholestatic liver disease characterized by biliary inflammation and fibrosis of both small and large bile ducts, that can potentially lead to cholestasis and cirrhosis. This can result in end-stage liver failure and as such PSC is the fifth commonest indication for liver transplantation in the UK [Adam et al. 2003]. Patients with PSC carry a high lifetime risk of gastrointestinal malignancy; 44% of PSC deaths are cancer related, with 7–13% developing cholangiocarcinomas possibly due to inflammation-associated epithelial dysplasia [Bergquist et al. 2002; Fevery et al. 2007]. Inflammatory bowel disease (IBD) coexists in 60–80% of patients, with a 10-fold increased risk of colorectal carcinoma compared with the general population [Bergquist et al. 2002; Sano et al. 2011; Ye et al. 2011]. The IBD associated with PSC is unusual in that it is usually a pancolitis with activity worse on the right, backwash ileitis and rectal sparing [Loftus et al. 2005; Sano et al. 2011]. Furthermore, 25% of patients with PSC have concurrent autoimmune diseases [Saarinen et al. 2000]. Thus, despite being a rare disease with a prevalence of 0.2–16 per 100,000, PSC represents a significant burden on hepatobiliary and oncological services [Hurlburt et al. 2002; Bambha et al. 2003; Boonstra et al. 2012].
Existing evidence suggests that PSC is a heterogeneous condition, with different subgroups conferring different prognoses. For example, patients with concomitant ulcerative colitis (UC) and PSC have an increased risk of developing colorectal cancer compared with patients with UC or PSC alone [Broome et al. 1995]. Patients with PSC with small duct disease have an improved survival and lower risk of cholangiocarcinoma compared with patients with large-duct PSC [Bjornsson et al. 2002]. Patients with PSC and a raised immunoglobulin G4 (IgG4) have a more severe liver disease, and patients who demonstrate a significant reduction in their serum alkaline phosphatase (ALP) in a median time of 2 years following diagnosis have an improved transplant-free survival and reduced risk of cholangiocarcinoma [Mendes et al. 2006; Al Mamari et al. 2013].
Disease pathogenesis
An understanding of disease pathogenesis is fundamental to the selection of potential treatments. Although the exact pathogenesis remains unclear, PSC is thought to have both environmental and genetic causes, with 16 genetic loci currently identified and further genetic loci undergoing evaluation in international genome-wide-association studies (GWAS) meta-analysis [Liu et al. 2013]. These analyses and other experimental results are starting to shape a pathogenic model of PSC.
An autoimmune aetiology is strongly supported by several factors; the presence of concurrent autoimmune disease in up to 25% of patients, strong linkage of PSC to the human histo-compatibility complex, tissue infiltration with immune cells, and GWAS/Immunochip (Illumina Infinium single-nucleotide polymorphism (SNP) microarray) genetic studies [Saarinen et al. 2000; Karlsen et al. 2010; Liu et al. 2013]. At the heart of the pathogenic model appears to be a dysregulated autoimmune network that results in the loss of peripheral tolerance to self cholangiocytes and colonocytes. There is strong evidence supporting the production of pathogenic T cells in the colon and small bowel, which subsequently attack the biliary tree and bowel. This is based upon the fact that pathogenic liver T cells are enriched in a subset that express the small intestinal homing C–C chemokine receptor type 9 (CCR9), and home to the liver endothelium by the expression of gut-enriched homing chemokines; mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1) and chemokine (C–C motif) ligand 25 (CCL25). In addition, infiltration of the liver by T-helper 17 (TH-17) cells further supports their proposed origin from the gut.
TH-17 are a subset of T-helper cells that produce interleukin 17 (IL-17), IL-22, tumour necrosis factor α (TNFα) and CCL20. They differentiate from naïve T cells in the presence of dendritic cells and cytokines IL-1β, IL-6, IL-23, transforming growth factor β (TGFβ) and IL-21. In order to maintain immunological tolerance, this production of TH-17 cells in the bowel and biliary tree in PSC should be kept in careful check. This is usually secured by the favoured production of T-regulatory cells (CD4+/CD25+/CTLA-4+/Foxp3 positive cell) by tolerogenic plasmacytoid dendritic cells. Therefore an alteration favouring the production of mucosal TH-17 axis (CD4+ and CD8+ T cells) may underpin PSC pathogenesis. This TH-17, T-regulatory imbalance would perhaps also suggest why single nucleotide polymorphisms of genome-wide significance are embedded in the IL-2, IL-2 receptor and CD28 axis, with macrophage stimulating 1 having a potential role in dendritic cell tolerance.
These autoreactive T cells are postulated to recognize cholangiocytes and colonocytes via class I and class II human leukocyte antigen, resulting in inflammation, apoptosis, necrosis and tissue fibrosis. Furthermore, increases in bile duct permeability result in leakage of bile into the surrounding peribiliary tissues, secondary vascular injury with endarteritis obliterans and biliary ischemia. The end result is activation of portal fibroblasts and stem cells that lay down elastin and collagen to form scar tissue, with associated biliary cell apoptosis/mesenchymal transition and the induction of cellular senescence. Furthermore damage and impaired drainage of the bile ducts promotes bacterial and fungal colonization, secondary invasion and tissue damage.
An additional role for involvement of the gut microbiota has been highlighted in PSC. In vitro studies of biliary epithelial cells (BECs) from patients with and without alcohol-related liver disease demonstrate inappropriate innate immune responses to intestinal endotoxins and subsequent endotoxin intolerance due to enhanced pattern recognition receptor signalling in BECs of those with chronic liver disease [Mueller et al. 2011]. This is thought to contribute to chronic cholangitis. Rats with small bowel bacterial overgrowth induced by jejunual ligation develop liver lesions similar to those seen in PSC [Lichtman et al. 1990]. Subsequent antibiotic therapy with metronidazole and tetracycline leads to an improvement in these lesions, suggesting that modification of gut microbiota may be important in PSC [Lichtman et al. 1991]. Furthermore, a unique microbiome may be present in PSC [Sabino et al. 2015].
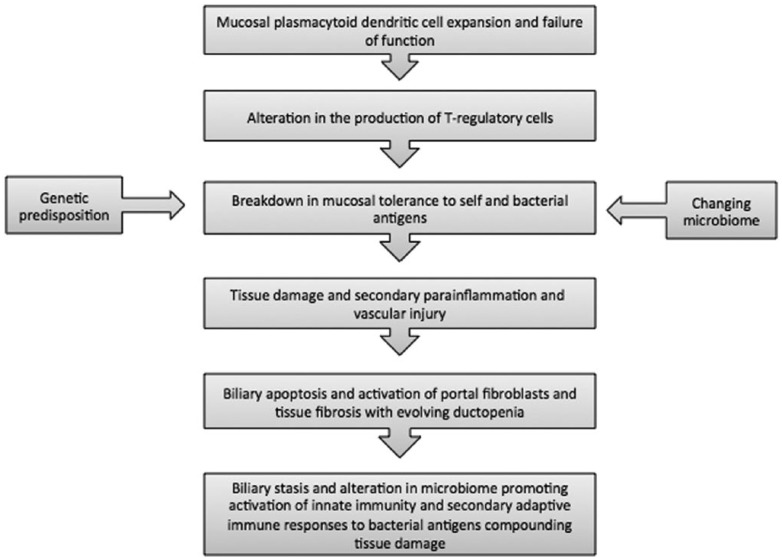
It is this growing understanding of the pathogenesis of PSC (see Figure 1) that brings new hope for emerging treatments in PSC. It is likely that by tackling the different aspects of pathogenesis, inflammation, ischaemia, fibrosis, vascular injury and alteration of the biome, a new successful treatment approach will be found either in isolation or with a combination of agents.
Figure 1.
Pathogenesis of primary sclerosing cholangitis.
Factors hindering therapeutic trials in PSC
Despite some progress in our understanding of disease pathogenesis, medical therapy for PSC has been hindered by two major factors: disease characteristics and lack of clear endpoints for clinical trials.
As a disease of low prevalence, studies of PSC have, until recently, been limited by small cohort size, relying on meta-analyses to improve statistical power [Schrumpf and Boberg, 2001]. As previously discussed, PSC displays a multitude of clinical phenotypes that differ with respect to prognosis. These phenotypes encompass disease with and without ulcerative colitis, overlap with other autoimmune conditions, raised IgG4 and the location and site of the biliary injury [Trivedi and Hirschfield, 2012]. Thus patient heterogeneity with regards to level of baseline fibrosis and rate of disease progression means true randomization in clinical trials is difficult. A number of prognostic models have been developed to help identify at an early stage, patients who are more likely to progress to a poor outcome [Wiesner et al. 1989; Farrant et al. 1991; Dickson et al. 1992; Broome et al. 1996; Kim et al. 2000]. However, with the exception of the revised Mayo Clinic model, they all include a histological staging parameter, which necessitates invasive liver biopsy. In a disease diagnosed via cholangiogram, this limits the clinical utility of these prognostic models. Furthermore, these models do not address the impact of cholangiocarcinoma in PSC, which is of paramount importance when considering prognosis and future treatments. Guidelines therefore suggest that the use of existing risk scores should be restricted to cohort studies rather than for individual patient outcomes [Wiesner et al. 1989; Farrant et al. 1991; Dickson et al. 1992; Broome et al. 1996; Kim et al. 1999; Chapman et al. 2010].
The lack of consensus over clinically relevant endpoints has further hindered interpretation of results from therapeutic trials. The most commonly used endpoints include liver biochemistry, symptoms, transplantation and disease-related death. However, the time taken for the latter to occur is not practical for most pharma-sponsored studies. Utility of histology is variable in study design, and importantly, whether histological progression directly reflects clinical progression is yet to be established given the heterogeneous distribution of fibrosis in PSC. Furthermore, use of fibroscan, magnetic resonance imaging (MRI) elastography, Enhanced Liver Fibrosis (ELF) and serum fibrosis panels is yet to be validated in PSC, although it is currently under study by the International PSC Study Group.
It is likely that future PSC trials will take two forms. The first is likely to be trials to establish if the drug of investigation has a biological signal (mediated via fibrosis, cholangiography or an immunological signal) without necessarily demonstrating that it alters clinically meaningful endpoints. The second is to stratify patients into groups that have either a low, intermediate or high risk of progression, and target the intermediate- or high-risk patients for recruitment into trials to establish if a clinically meaningful signal is detected. Whilst at present stratification according to risk of progression is not possible, large-scale phenotyping studies from the International PSC Study Group and UK PSC Consortium mean that this is likely to be possible in the near future.
This review will therefore explore the established and emerging medical therapies for PSC at a time that hopes to be a 21st century turning point in our understanding of the disease.
Therapies altering bile composition
Ursodeoxycholic acid
Ursodeoxycholic acid (UDCA) is the most commonly prescribed drug in PSC. Given its proven efficacy in the treatment of other cholestatic diseases such as PBC, UDCA has biological plausibility in the treatment of PSC. UDCA is postulated to have two mechanisms of action: reducing hydrophobicity of bile and a direct effect on adaptive immunity by inhibiting dendritic cell response. However, it is not an agonist for farnesoid X receptor (FXR) or pregnane X receptor (PXR), which is protective in cholestatic disorders [Paumgartner and Beuers, 2004; Beuers, 2006].
Low-dose UDCA
The first placebo-controlled pilot studies of low-dose UDCA (10–15 mg/kg/day) demonstrated efficacy in improving liver biochemistry, histology and symptoms [Chazouilleres et al. 1990; Beuers et al. 1992; Stiehl et al. 1994]. However, statistical power was limited by small sample sizes of 14–20 patients per trial. A larger placebo-controlled study of 105 patients confirmed improvement of liver biochemistry with low-dose UDCA (13–15 mg/kg), but did not demonstrate any effect on symptoms or time to transplantation [Lindor, 1997]. Yet unfortunately these early studies are notable for their short duration compared with the natural history of PSC (see Table 1).
Table 1.
Characteristics of trials of UDCA in PSC.
| Study | Year | UDCA dose | Design | UDCA (n) | Placebo (n) | Trial duration (months) | Liver biochemistry improved | Symptomatic improvement | Mayo risk score | Liver histology improvement | Cholangiographic improvement | Progression to end-stage liver disease | Progression to transplant/death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O’Brien et al. | 1991 | 10 mg/kg/day | OL | 12 | N/A | 30 | Y | Y | Not done | Y | N | Not done | Not done |
| Beuers et al. | 1992 | 13–15 mg/kg/day | DB, PC | 6 | 8 | 12 | Y | N | Not done | Y | N | Not done | Not done |
| Lo et al. | 1992 | 10 mg/kg/day | DB, PC | 8 | 10 | 24 | Y | Not done | Not done | Y | N | Not done | Not done |
| Stiehl et al. | 1994 | 750 mg/day | DB, PC | 10 | 10 | 12–48 | Y | N | Not done | Y | N | Not done | Not done |
| De Maria et al. | 1996 | 600 mg/day | DB, PC | 20 | 20 | 24 | N | Not done | Not done | Not done | N | Not done | Not done |
| Lindor | 1997 | 13–15 mg/kg/day | DB, PC | 51 | 51 | 34 | Y | N | Not done | N | Not done | N | N |
| Van Hoogstraten et al. | 1998 | 10 mg/kg/day | DB, PC | 24 | 24 | 24 | Y | N | N | N | N | N | N |
| Mitchell et al. | 2001 | 20 mg/kg/day | DB, PC | 13 | 13 | 24 | Y | N | Not done | Y | Y | Y | N |
| Harnois et al. | 2001 | 25–30 mg/kg/day | OL | 15 | 15 | 12 | Y | Not done | Y | Not done | N | Not done | Y |
| Okolicsanyi et al. | 2003 | 8–13 mg/kg/day | RA | 69 | 17 | N/R | Y | Y | N | N | N | Not done | Not done |
| Olsson et al. | 2005 | 17–23 mg.kg.day | DB, PC | 97 | 101 | 60 | Y | N | N | Not done | N | Not done | Not done |
| Lindor et al. | 2009 | 28–30 mg/kg/day | DB, PC | 76 | 73 | 60 | Y | Not done | Not done | Not done | N | N | N |
DB, double blind; N/A, not applicable; N/R, not reported; OL, open label; PBC, primary biliary cirrhosis; PC, placebo controlled; RA, retrospective analysis; UDCA, ursodeoxycholic acid.
The largest multicentre, randomized, double-blind, placebo-controlled study (RCT) to date recruited 219 patients, treated with UDCA (17–23 mg/kg/day) and followed up for 5 years [Olsson et al. 2005]. Despite significant efforts, researchers failed to recruit the 346 patients required to detect a statistically significant difference in primary endpoint, reflecting the difficulty of studying a rare disease. The study demonstrated only a non-significant trend towards improved survival and time to liver transplantation, which may reflect type II error resulting from an underpowered study. However, secondary outcome measures (change in symptoms, quality of life and change in liver biochemistry) were also not supportive of UDCA. Furthermore, a 15-year follow up to this trial supported no role for UDCA [Lindstrom et al. 2013].
High-dose UDCA
Pilot studies of UDCA up to 30 mg/kg/day demonstrated a significant improvement in Mayo risk score, which perhaps unadvisedly was used as a surrogate marker for improved survival [Harnois et al. 2001; Cullen et al. 2008]. However, the largest RCT of high-dose UDCA was terminated at interim analysis when, despite a statistically significant improvement in liver biochemistry, there was an unexpected, 2.3-fold increased risk of progression to liver transplantation and varices in the treatment group [Lindor et al. 2009]. Subgroup analysis revealed that the risk of adverse events, particularly oesophageal varices, were more apparent in patients with early histological stage disease and normal total bilirubin. Importantly, recent analysis has suggested that there is an increased serum concentration of lithocholic acid, a potent hydrophobic bile acid, in patients given high-dose UDCA, which may cause these adverse outcomes [Sinakos et al. 2010].
Due to conflicting evidence and the limited sample size in these major studies, we rely upon meta-analyses to improve statistical power. Recent meta-analysis of nine RCTs concluded that UDCA at any dose conferred no significant improvement in mortality, symptoms, cholangiocarcinoma and histological progression [Triantos et al. 2011]. Similarly, a Cochrane systematic review of eight RCTs found no significant reduction in the relative risk of death, treatment failure, liver transplant, varices, ascites or encephalopathy [Poropat et al. 2011]. Interestingly, a significant improvement in liver biochemistry was observed, the clinical significance of which is uncertain. Although meta-analyses are considered the highest class of evidence, the trials included were subject to high risk of publication bias. Variable dosage, treatment time course and follow up, and different primary endpoints in UDCA trials means that the role of UDCA is still uncertain; a point that is reflected in current international guidance, and the fact that many European countries no longer prescribe the drug [Chapman et al. 2010; Imam et al. 2011]. More recently, several studies have shown that patients with PSC, who normalize their serum ALP, whether this occurs spontaneously or more often with UDCA therapy, have a better prognosis. Despite this, recent guidelines from the American College of Gastroenterology recognize that many practitioners, particularly in the US and UK, are still prescribing UDCA at a dose of 15–20 mg/kg/day, but that data from well controlled trials are lacking [Lindor et al. 2015]. The only recommendation from these new guidelines is that UDCA of more than 28 mg/kg/day should not be used in the management of patients with PSC.
UDCA and chemoprotection
Current evidence demonstrates an increased incidence of right-sided colonic cancers in patients with PSC, perhaps caused by colonic exposure to secondary bile acids [Shetty et al. 1999]. In vitro and animal studies suggest that UDCA might act as a chemoprotective agent by modifying bile acid composition and reducing faecal levels of secondary bile acids [Rodrigues et al. 1995; Wali et al. 1995; Batta et al. 1998; Im and Martinez, 2004; Khare et al. 2008].
A phase III study of 1285 patients (without PSC) who had undergone removal of colorectal adenomas within the previous 6 months reported that low-dose UDCA (8–10 mg/kg/day) prevented adenoma recurrence [Alberts et al. 2005]. A cross-sectional study of 59 patients, and a retrospective analysis of 52 previously randomized patients with PSC IBD, reported a significantly decreased prevalence of colorectal dysplasia with UDCA: adjusted odds ratio (OR) 0.14 [95% confidence interval (CI) 0.03–0.64] (p = 0.01) [Tung et al. 2001]. However, a short follow-up period of 2 years, short 6-month exposure period, and low patient numbers limited the reliability of these conclusions.
Contrasting results were reported in a retrospective cohort study of patients with PSC IBD, comparing 28 cases treated with UDCA against 92 controls [Wolf et al. 2005]. Whilst UDCA appeared to confer a beneficial effect in decreasing mortality (adjusted relative risk for death 0.44; 95% CI 0.22–0.90), cumulative incidence of dysplasia or cancer was not significantly different between cases and controls (p = 0.17 by log-rank test). This was also suggested in a second long-term follow-up study of a previously randomized cohort of 98 patients with PSC IBD, treated with high-dose UDCA (17–23 mg/kg/day) or placebo. Frequency of colorectal dysplasia was similar in treatment and control groups, with no difference in cancer-free survival [Lindstrom et al. 2012]. Moreover, in a retrospective analysis of data from an RCT including 56 patients with PSC IBD treated with high-dose UDCA (28–30 mg/kg/day) and followed up for 235 patient-years, long-term intake of UDCA was surprisingly associated with an increased risk of colorectal neoplasia (hazard ratio 4.44; 95% CI 1.30–20.10; p = 0.02) [Eaton et al. 2011]. Conversely a recent meta-analysis of 763 patients with PSC IBD concluded that UDCA may reduce the risk of advanced colorectal neoplasia (OR 0.35; 95% CI 0.17–0.73), or all colorectal neoplasia at doses of 8–15 mg/kg/day (OR 0.19; 95% CI 0.08–0.49) [Singh et al. 2013]. However, the results of this meta-analysis should be treated with caution, as several follow-up studies were included and no adjustment made for patients who switched between treatment groups. Furthermore, the observations with low-dose UDCA were based upon only two early studies of 70 cases and 41 controls.
There is even further limiting evidence for UDCA as a chemoprotectant for cholangiocarcinoma. A Scandinavian study of 255 patients with PSC awaiting liver transplantation over an 11-year period reported that lack of treatment with UDCA was an independent risk factor for development of hepatobiliary malignancy [Brandsaeter et al. 2004]. However, the two largest placebo-controlled RCTs observed no effect on rates of cholangiocarcinoma in patients with PSC, though they were not powered to detect a difference in rates of hepatobiliary malignancy [Olsson et al. 2005; Lindor et al. 2009].
Immunosuppressive-based therapies
Glucocorticoids
Glucocorticoids are the most commonly used drug in the treatment of immune-mediated conditions. Patients with PSC and autoimmune hepatitis overlap or high IgG4 levels benefit from corticosteroids [Gregorio et al. 2001; Boberg et al. 2003; Floreani et al. 2005; Mendes et al. 2006; Webster et al. 2009]. Surprisingly, there have been no published RCTs comparing oral corticosteroids with placebo in patients with PSC alone (See Table 2).
Table 2.
Characteristics of trials of immunosuppressant agents in PSC.
| Agent | Study | Year | Design | Treatment (Tx) | Tx n | Control (C) | C n | Trial duration (months) | Liver biochemistry improvement | Symptomatic improvement | Liver histology improvement | Cholangiographic improvement | Progression to end-stage liver disease | Transplant/survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azathioprine | Schramm et al. | 1999 | Case series | Azathioprine | 15 | N/A | N/A | 41 | N | Not done | Y | N | Not done | Not done |
| 1–1.5 mg/kg/day | ||||||||||||||
| Prednisolone 1 mg/kg/day | ||||||||||||||
| UDCA | ||||||||||||||
| 500–750 mg/day | ||||||||||||||
| Cyclosporin | Wiesner et al. | 1991 | DB, PC | Cyclosporin | 20 | Placebo | 10 | N\R | N | N | Y | Not done | N | N |
| Sandborn et al. | 1993 | DB, RCT | Cyclosporin 4.1 mg/kg/day | 19 | Placebo | 11 | 12 | N | N | N | N | N | N | |
| Methotrexate | Knox and Kaplan | 1991 | OL | Methotrexate 0.2 mg/kg/week | 10 | N/A | N/A | 12 | Y | Not done | Y | Y | Not done | Not done |
| Knox and Kaplan | 1994 | PC | Methotrexate | 12 | Placebo | 12 | 24 | N | Not done | N | N | Not done | Not done | |
| Tacrolimus | Van Thiel et al. | 1995 | OL | Tacrolimus 3 mg twice daily | 10 | N/A | N/A | 3 | Y | Not done | Not done | Not done | Not done | Not done |
| Talwalkar et al. | 2007 | OL | Tacrolimus 0.05 mg/kg twice daily | 16 | N/A | N/A | 12 | Y | Y | Not done | Not done | Not done | Not done |
N/A, not applicable; DB, double blind; PC, placebo controlled; N/R, not reported; RCT, randomized controlled trial; OL, open label; C, control; Tx, treatment; n, number.
A cohort study of 21 patients treated with 9 mg of budesonide for 1 year showed an improvement in portal inflammation but no change in Mayo risk score [Angulo et al. 2000]. A significant decrease in serum ALP and Aspartate Transaminase (AST) compared with baseline was observed (p = 0.001), however this effect was lost 3 months post treatment cessation. Furthermore, significant loss of bone density prompted the authors to conclude that overall there was minimal benefit. A nonrandomized, placebo-controlled trial in 12 patients with PSC compared combined therapy with prednisolone 10 mg/day and colchicine 0.6 mg/twice daily with placebo, administered for 2 years [Lindor et al. 1991]. After 24 months, no significant difference in liver biochemistry or histology was detected, and only a nonsignificant trend towards less clinical deterioration. Furthermore bone density in the prednisolone group was significantly lower compared with the placebo group.
The only published meta-analysis of corticosteroids in PSC included just two trials [Giljaca et al. 2010]. The first, an unblinded trial of hydrocortisone administered via biliary lavage versus placebo in 11 randomized patients with PSC [Allison et al. 1986], and the second, an RCT comparing oral budesonide (3 and 9 mg/day) with oral prednisolone (10 mg/day) in 19 patients with PSC [Van Hoogstraten et al. 2000]. No effect on liver biochemistry, symptoms or mortality was observed. However, small sample sizes and the absence of power calculations or intention-to-treat analysis may have resulted in systematic error overestimating beneficial effects and underestimating harmful effects, such as increased rates of cholangitis and sepsis [Allison et al. 1986].
Azathioprine
Azathioprine is a steroid-sparing immunosuppressant and purine antimetabolite widely used for the maintenance of remission in IBD [Mowat et al. 2011]. However, studies of its efficacy in PSC have been limited. Azathioprine inhibits ribonucleotide synthesis and induces T-cell apoptosis by modulating Rac-1 cell signalling [Tiede et al. 2003]. Several cases of azathioprine use in PSC have been reported; two patients improved with treatment and one died of a liver abscess [Javett, 1971; Wagner, 1971]. A case series of 15 patients with PSC treated with combination azathioprine (1–1.5 mg/kg/day), prednisolone (1 mg/kg/day) and UDCA (500–750 mg/day) observed significant improvement in liver histology and biochemistry [Schramm et al. 1999]. Unfortunately the commonality of concomitant PSC IBD means that many patients with PSC are taking azathioprine at the time of PSC diagnosis and progression, which may explain the lack of enthusiasm for further evaluation of azathioprine. However, trials employing use of thiopurine metabolite measurements in PSC are also lacking.
Ciclosporin
Ciclosporin binds to cytosolic cyclophilin of T cells and inhibits calcineurin, subsequently inhibiting the transcription of IL-2, which whilst inhibiting T-cell response may also limit T-regulatory cell production. Following 24 months of treatment, ciclosporin prevented progression of liver histological change: 9 of 10 patients on placebo demonstrated histological progression compared with 11 of 20 patients on ciclosporin (p < 0.05) [Wiesner et al. 1991]. However, lack of effect on symptoms, liver biochemistry or disease progression led to conclusions that cyclosporine was ineffective in the treatment of PSC. cyclosporine has also been further evaluated in a double-blind RCT of 35 patients with precirrhotic PSC with concomitant UC. Whilst patients with PSC UC experienced improvement in symptomatic bowel disease, the trial was primarily powered to establish an effect on UC and no difference in PSC-related endpoints were observed with this therapy [Sandborn et al. 1993].
Tacrolimus
A preliminary open-label trial of tacrolimus in 10 patients with PSC demonstrated a significant improvement of liver biochemistry [Van Thiel et al. 1995]. This effect was confirmed in an open-label, phase II study of 16 patients with PSC treated with tacrolimus (0.05 mg/kg/day). However, only 50% of patients completed 1 year of therapy and 31% withdrew from the trial due to drug-related adverse events. Moreover, inclusion of large numbers of patients with proctocolectomy may explain the greater frequency of gastrointestinal side effects. The study concluded that clinical benefit was limited and tacrolimus poorly tolerated in this patient group [Talwalkar et al. 2007]. Sirolimus and everolimus are inhibitors of mechanistic target of rapamycin (mTOR) and recent evidence shows these mTOR inhibitors improve liver fibrosis and reduce inflammation in bile duct ligated rats [Patsenker et al. 2011]. These could be potential therapeutic targets in cholangiocarcinoma and PSC [Herberger et al. 2007; Pignochino et al. 2010]. Further studies are therefore needed to evaluate these drugs.
Methotrexate
Methotrexate is a dihydrofolate reductase inhibitor that targets enzymes involved in purine metabolism, suppressing T-cell activation and adhesion molecule expression, thus conveying anti-inflammatory properties [Johnston et al. 2005]. A preliminary trial of 0.2 mg/kg/week oral methotrexate demonstrated a statistically significant improvement in liver biochemistry [Knox and Kaplan, 1991]. Six of none (66%) showed histological improvement at 1 year and three of six (50%) who underwent repeat cholangiograms showed improvement. In contrast, a prospective, placebo-controlled RCT of oral methotrexate in 24 patients with PSC demonstrated no change in liver histology, cholangiographic findings or liver biochemistry following 2 years of treatment [Knox and Kaplan, 1994]. However, it should be noted that 58% of patients in the treatment group had established cirrhosis compared with only 42% on placebo, which could explain the lack of observed efficacy with methotrexate in this trial [Lindor et al. 1996].
Mycophenolate
Mycophenolate mofetil (MMF) is a potent immunosuppressant that has largely replaced azathioprine as a second-line agent in solid-organ transplantation. MMF attenuates B- and T-lymphocyte proliferation by inhibiting de novo purine synthesis [Allison and Eugui, 1993; Eugui and Allison, 1993; Fulton and Markham, 1996]. A pilot study of 1–3 g MMF in 30 patients with PSC aimed to determine safety and efficacy; 77% completed 1 year of treatment, with 33% experiencing adverse reactions that resolved with dose reduction [Talwalkar et al. 2005]. A significant, but clinically marginal reduction in serum ALP was observed, and the pilot study did not support the sole use of MMF in PSC. These results were corroborated by a 2-year RCT of combined MMF (1 g/twice daily) and UDCA (13–15 mg/kg/day) (n = 12) versus UDCA alone (n = 13). Small sample size, open label and high dropout rate could have led to type II error, however results from this trial were not supportive of combination therapy with MMF in PSC [Sterling et al. 2004].
Antibiotics
The potential role of antibiotics in PSC therapy was initially derived from experimental evidence in rat models of small intestinal bacterial overgrowth, leading to biliary strictures and portal inflammation [Lichtman et al. 1990]. Importantly, patients with advanced PSC suffer repeated episodes of bacterial cholangitis, which may fuel disease progression [Broome et al. 1996]. Studies of bile fluid obtained at Endoscopic Retrograde Cholangiopancreatography (ERCP) and from explanted livers show a wide range of bacteria and fungi in both patients with multiple biliary interventions and 25% of patients who are ERCP naïve [Olsson et al. 1998; Bjornsson et al. 2000; Negm et al. 2010]. Current guidelines thus advocate the use of prophylactic antibiotics in patients with recurrent bacterial cholangitis and those undergoing biliary intervention [Chapman et al. 2010].
There have been a few small trials of antibiotics in PSC. A 1-year pilot study of minocycline, with antiapoptotic and anti-inflammatory properties, in 16 patients with PSC observed a significant improvement in ALP (p < 0.05), however improvements in Mayo risk score were not statistically significant [Silveira et al. 2009]. The largest antibiotic trial including 80 patients with PSC evaluated the efficacy of metronidazole and UDCA versus UDCA alone in a 3-year RCT. A significant decrease in serum ALP and Mayo risk score was observed, however improvements in liver histology and cholangiographic findings were statistically insignificant [Farkkila et al. 2004]. Long-term treatment with oral vancomycin in 14 children with PSC IBD significantly improved liver biochemistry, inflammatory markers and symptoms, especially in the absence of cirrhosis [Cox and Cox, 1998; Davies et al. 2008]. More recently, a small RCT of 35 patients with PSC randomized to receive vancomycin 125 or 250 mg four times per day or metronidazole 250 or 500 mg three times per day for 12 weeks demonstrated some efficacy [Tabibian et al. 2013a]. The primary endpoint of ALP normalization was achieved in both the low- and high-dose vancomycin groups. Mayo risk score significantly decreased in both the low-dose vancomycin and metronidazole groups and pruritus significantly decreased in the high-dose metronidazole groups. This promising data have prompted a larger clinical trial to determine the efficacy of vancomycin in improving liver biochemistry in PSC, which is currently ongoing [ClinicalTrials.gov identifier: NCT01802073]. Therefore, antibiotic therapy appears promising, especially given mounting evidence for the role of the intestinal microbiome in PSC [Tabibian et al. 2013b], yet the concern of evolving resistance remains a real concern for clinicians. Future therapies involving faecal and bile transplantation which can alter the microbiome may be an important consideration in PSC.
Other treatments
Periductal fibrosis is a characteristic histopathological hallmark of PSC, however trials of antifibrotic agents including colchicine and pirfenidone have failed to demonstrate efficacy [Olsson et al. 1995; Angulo et al. 2002]. Fibrates are agonists at the nuclear peroxisome proliferator activated receptor α, thus decreasing IL-1 induced C-reactive protein expression. However, a case series of seven patients with PSC treated with bezafibrate for 6 months observed no effect on symptoms, progression or survival [Mizuno et al. 2010]. Other potential antifibrogenic agents include candesartan, an angiotensin II receptor blocker that attenuates liver fibrosis in rats [Ueki et al. 2006] and propranolol, a β-adrenoceptor antagonist [Strack et al. 2011]. No data are yet available for these agents.
Rats with small bowel overgrowth develop hepatobiliary injury from peptidoglycan polysaccharide mediated activation of Kupffer cells and release of proinflammatory cytokines such as TNFα, which is inhibited by xanthine-derived phosphodiesterase inhibitor, pentoxyfylline [Kucuktulu et al. 2007]. A pilot study of pentoxyfylline in 20 patients with variable stages of PSC showed no improvement in liver biochemistry, serum TNFα or TNF-receptor subtype following 1 year of treatment [Bharucha et al. 2000]. Cellular proliferation and liver-derived lymphocyte function are impaired in patients with PSC, perhaps a result of exposure to high levels of TNFα in vivo [Spengler et al. 1992; Bo et al. 2001]. A double-blind, placebo-controlled RCT of anti-TNFα antibody, infliximab, in 24 patients with PSC was halted following interim analysis demonstrating no difference in liver biochemistry or histology after 4–6 months [Hommes et al. 2008]. Furthermore, a pilot study of etanercept in 10 patients demonstrated no change in liver biochemistry or stricture formation, however two of five patients with symptomatic pruritus experienced resolution of pruritus during treatment, which returned on cessation of therapy, and resolved on reintroduction [Epstein and Kaplan, 2004].
Future treatments
A current priority in PSC research is the identification of short-term biomarkers for disease outcome. It is hoped that the identification of disease biomarkers will pave the way for the development and trial of new treatments in PSC (See Table 3).
Table 3.
Currently registered therapeutic trials in PSC.
| Agent | ClinicalTrials.gov identifier | Principal investigators | Start date | Estimated completion date | Trial phase | Design | Treatment | Primary endpoint | Secondary endpoints |
|---|---|---|---|---|---|---|---|---|---|
| Human monoclonal anti-VAP-1 antibody (BTT1023) | NCT02239211 | Hirschfield, GM, University of Birmingham, UK | March 2015 | January 2017 | II | Single arm, two stage, multicentre | BTT1023 8 mg/kg intravenous infusion, every 14 days (total of 7 infusions) | ALP | Liver fibrosis |
| Oral vancomycin | NCT01802073 | Cox, KL, Stanford University | January 2012 | December 2015 | III | Single arm, open label | Adults and children >30 kg, vancomycin 500 mg 3 times per day. Dose increased to 750 mg 3 times per day for the second month and 1000 mg 3 times per day for the third month if bloods not normalized | ALT, ALP | |
| MRCI/MRCP | |||||||||
| Liver histology | |||||||||
| Obeticholic acid (OCA) | NCT02177136 | Shapiro, D, Intercept Pharmaceuticals | November 2014 | November 2018 | II | Randomized, double blind, placebo controlled, dose finding | 1.5 mg OCA, 5 mg OCA, or placebo for 12 weeks, followed by titration for further 12 weeks; 1.5 mg OCA treatment group then titrate to 3 mg, the 5 mg OCA treatment group then titrate to 10 mg OCA, placebo group remain on placebo | ALP | |
| Nor-ursodeoxycholic acid | NCT01755507 | Trauna M, Med. Uni Wien | December 2012 | March 2014 | II | Double blind, randomized, placebo controlled | 500, 1000 or 1500 mg/day norursodeoxycholic acid capsules versus placebo | Change in serum ALP in 12 weeks | Proportion of patients with at least 50% reduction in serum ALP |
| Manns MP, Med. Hochschule Hannover, Boberg K, Dr Falk Pharma | |||||||||
| LOXL-2 inhibitor simtuzumab (GS-6624) | NCT01672853 | Myers R, Gilead Sciences | February 2013 | July 2016 | II | Dose ranging, randomized, double blind, placebo controlled | Subcutaneous injection weekly for 96 weeks | Change from baseline in morphometric quantitative collagen on liver biopsy | Safety of GS-6624 in subjects with PSC |
| All-trans retinoic acid (ATRA) and ursodeoxycholic acid (UDCA) | NCT01456468 | Boyer JL, Yale University | October 2011 | June 2014 | I | Single-arm | ATRA 45 mg/m2 divided into 2 doses, with UDCA 15 mg/kg/day | 30% improvement in serum alkaline phosphatase | |
| LUM001 (apical sodium-dependent bile acid transporter inhibitor (ASBTi) | NCT02061540 | Lumina Pharma | March 2014 | December 2015 | II | Open label, single arm | LUM001 administered orally once each day | Adverse events, changes in vital signs, laboratory and other safety parameters from baseline to week 14 | Changes in serum bile acids, pruritus, and other biochemical markers of cholestasis and liver disease from baseline to week 14 |
| Safety and tolerability |
ALT, ALP, alkaline phosphatase; MRCI; MRCP, Magnetic Resonance Cholangiopancreatography.
CCR9 is a chemokine receptor, expressed on most lamina propria and intraepithelial T cells of the small intestine, with up to 25% of T cells on the large bowel positive for CCR9 [Zaballos et al. 1999; Norment et al. 2000]. CCR9 binds CCL25, causing activation of α4β7 T cells, thereby binding to MadCAM-1 in the bowel endothelium resulting in homing of T cells to the bowel in the healthy state. In IBD this process is enhanced and blocking of MAdCAM-1 and binding to α4β7 T cells by the VAP-1 blocker BTT1023 or vedolizumab alters the recruitment of pathogenic T cells. Furthermore vedolizumab is now licensed for the treatment of IBD, particularly UC [Jin et al. 2015]. Aberrant expression of CCR9 and its ligand CCL25 in the liver of patients with PSC, but not healthy liver or liver disease controls, has been demonstrated [Eksteen et al. 2004]. Therefore, targeted blockade of this pathway using vedolizumab is a natural therapeutic development in PSC, a trial of which is currently ongoing [ClinicalTrials.gov identifier: NCT02239211]. CCX282-B is also a selective antagonist of CCR9 with some therapeutic efficacy in CD, which may inhibit B- and T-cell entry to the liver, and may also be therapeutically important [Keshav et al. 2013].
Matrix enzyme, lysyl-oxidase 2 (LOXL2), is implicated in nonorgan-specific pathological fibrogenesis by promoting cross-linking of type I collagen [Barry-Hamilton et al. 2010]. GS-6624 is a humanized monoclonal antibody with an immunoglobulin IgG4 isotype directed against human LOXL2. Pilot studies of its safety and tolerability in 10 patients with liver fibrosis of variable aetiology experienced no serious adverse effects, with a decline in serum Alanine Transaminase (ALT) [Talal et al. 2012]. Such agents may prove efficacious in attenuating the fibrosis of PSC and is the basis for a current phase II trial [ClinicalTrials.gov identifier: NCT01672853].
All-trans retinoic acid (ATRA) is a ligand for nuclear receptors involved in modulation of bile salt homeostasis [Cai et al. 2010]. ATRA possesses immunomodulatory effects through inhibition of proinflammatory cytokines [Montrone et al. 2009], and is currently used in the treatment of acute promyelocytic leukaemia, rheumatoid arthritis and psoriasis [Reichrath et al. 2007]. In some environments, ATRA can also induce T cells to become CCR9 positive [Eksteen et al. 2009]. In bile-duct ligated rats, treatment with UDCA and ATRA significantly reduces liver fibrosis, bile duct proliferation, liver necrosis and bile salt pool size compared with ATRA or UDCA alone [He et al. 2011]. However, this observation has not yet been verified in humans and phase II trials of ATRA and UDCA are now underway in PSC [ClinicalTrials.gov identifier: NCT01456468].
Manipulation of the UDCA molecule by shortening a side chain by one methylene group produces 24-norUDCA (norUDCA), a C23 homologue of UDCA. High-dose UDCA in patients with PSC leads to increased rates of adverse outcomes, especially in patients with normal serum bilirubin or early histological-stage disease [Imam et al. 2011]. Post hoc analysis of serum bile acid composition in 56 patients included in the aforementioned trial observed markedly increased levels of hepatotoxic bile acid: lithocholic acid (LCA) in patients on high-dose UDCA [Sinakos et al. 2010]. In rabbits, accumulation of toxic LCA following ingestion of UDCA causes inflammation of hepatic portal tracts and bile duct proliferation [Cohen et al. 1986]. In contrast to UDCA, norUDCA is secreted into bile in an unconjugated, glucoronidated form [Hofmann et al. 2005]. Its metabolite, nor-lithocholate, does not accumulate in hepatocytes or cause hepatotoxicity in animal models [Cohen et al. 1986]. Moreover, norUDCA administered to Mdr2–/– mice increases hydrophilicity of bile and stimulates canalicular flow [Fickert et al. 2006]. It remains unclear whether these promising results will translate to human studies, however phase II trials in humans are now also underway [ClinicalTrials.gov identifier: NCT01755507].
Activating protective pathways in hepatocyte cholestasis may limit hepatic damage in a cholestatic disorder. One of the master regulators of this is FXR. The natural ligand for FXR are bile salts and one of the key roles of FXR is downregulating cytochrome P450 7A1, a rate-limiting enzyme in bile salt production. Obeticholic acid, an FXR agonist with encouraging results in PBC phase II studies, may also prove efficacious in PSC with a clinical trial ongoing [ClinicalTrials.gov identifier: NCT02177136]. Furthermore inhibition of the Apical Sodium Dependent Bile Acid Transporter (ASBT) in the terminal ileum may also reduce the enterohepatic circulation of bile salts, which could bring therapeutic benefit in PSC. Once again, a phase II study of an ASBT inhibitor, LUM001, is ongoing [ClinicalTrials.gov identifier: NCT02061540].
Conclusion
Over the past two decades many clinical trials of medical therapy in PSC have been conducted; however, none have demonstrated real improvements in hard clinical endpoints. This reflects our lack of understanding of disease heterogeneity, basic mechanisms of disease pathogenesis and perhaps a lack of robust biomarkers to act as early disease endpoints in clinical trial design. Several potential therapeutic agents have been widely accepted as ineffective despite inadequate trial data to support these conclusions. A renewed approach to evaluation of such agents is thus justified. Future trials in PSC are speculated to focus on two areas: the search for a biologically plausible signal of drug efficacy (e.g. fibrosis markers, MRI changes, histological changes) and trials of therapeutic agents in high-risk individuals who have yet to reach end-stage disease, but whose disease is advanced enough to provide a true signal of drug efficacy. With GWAS highlighting new potential pathogenic mechanisms, the development of national collaborative disease consortia and new antifibrotic agents, the 21st century should hold new excitement for the treatment of PSC.
Acknowledgments
SMR conceptualized the review and ECG and SMR were involved in preparation of the manuscript. ECG will act as the guarantor for the paper.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Elizabeth C. Goode, Department of Hepatology, Norfolk and Norwich University Hospital, Norwich, UK
Simon M. Rushbrook, Department of Hepatology, Norfolk and Norwich University Hospital, Colney Lane, Norwich NR4 7UY, UK.
References
- Adam R., McMaster P., O’Grady J., Castaing D., Klempnauer J., Jamieson N., et al. (2003) Evolution of liver transplantation in Europe: report of the European liver transplant registry. Liver Transpl 9: 1231–1243. [DOI] [PubMed] [Google Scholar]
- Alberts D., Martinez M., Hess L., Einspahr J., Green S., Bhattacharyya A., et al. (2005) Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst 97:846–853. [DOI] [PubMed] [Google Scholar]
- Allison A., Eugui E. (1993) Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev 136: 5–28. [DOI] [PubMed] [Google Scholar]
- Allison M., Burroughs A., Noone P., Summerfield J. (1986) Biliary lavage with corticosteroids in primary sclerosing cholangitis. A clinical, cholangiographic and bacteriological study. J Hepatol 3: 118–122. [DOI] [PubMed] [Google Scholar]
- Al Mamari S., Djordjevic J., Halliday J., Chapman R. (2013) Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol 58: 329–334. [DOI] [PubMed] [Google Scholar]
- Angulo P., Jorgensen R., Keach J., Dickson E., Smith C., Lindor K. (2000) Oral budesonide in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology 31: 318–323. [DOI] [PubMed] [Google Scholar]
- Angulo P., Maccarty R., Sylvestre P., Jorgensen R., Wiesner R., Larusso N., et al. (2002) Pirfenidone in the treatment of primary sclerosing cholangitis. Dig Dis Sci 47: 157–161. [DOI] [PubMed] [Google Scholar]
- Bambha K., Kim W., Talwalkar J., Torgerson H., Benson J., Therneau T., et al. (2003) Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 125: 1364–1369. [DOI] [PubMed] [Google Scholar]
- Barry-Hamilton V., Spangler R., Marshall D., McCauley S., Rodriguez H., Oyasu M., et al. (2010) Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 16: 1009–1017. [DOI] [PubMed] [Google Scholar]
- Batta A., Salen G., Holubec H., Brasitus T., Alberts D., Earnest D. (1998) Enrichment of the more hydrophilic bile acid ursodeoxycholic acid in the fecal water-soluble fraction after feeding to rats with colon polyps. Cancer Res 58: 1684–1687. [PubMed] [Google Scholar]
- Bergquist A., Ekbom A., Olsson R., Kornfeldt D., Loof L., Danielsson A., et al. (2002) Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 36: 321–327. [DOI] [PubMed] [Google Scholar]
- Beuers U. (2006) Drug insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol 3: 318–328. [DOI] [PubMed] [Google Scholar]
- Beuers U., Spengler U., Kruis W., Aydemir U., Wiebecke B., Heldwein W., et al. (1992) Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo-controlled trial. Hepatology 16: 707–714. [DOI] [PubMed] [Google Scholar]
- Bharucha A., Jorgensen R., Lichtman S., Larusso N., Lindor K. (2000) A pilot study of pentoxifylline for the treatment of primary sclerosing cholangitis. Am J Gastroenterol 95: 2338–2342. [DOI] [PubMed] [Google Scholar]
- Bjornsson E., Boberg K., Cullen S., Fleming K., Clausen O., Fausa O., et al. (2002) Patients with small duct primary sclerosing cholangitis have a favourable long term prognosis. Gut 51: 731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson E., Kilander A., Olsson R. (2000) Bile duct bacterial isolates in primary sclerosing cholangitis and certain other forms of cholestasis—a study of bile cultures from ERCP. Hepatogastroenterology 47: 1504–1508. [PubMed] [Google Scholar]
- Bo X., Broome U., Remberger M., Sumitran-Holgersson S. (2001) Tumour necrosis factor alpha impairs function of liver derived T lymphocytes and natural killer cells in patients with primary sclerosing cholangitis. Gut 49: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg K., Egeland T., Schrumpf E. (2003) Long-term effect of corticosteroid treatment in primary sclerosing cholangitis patients. Scand J Gastroenterol 38: 991–995. [DOI] [PubMed] [Google Scholar]
- Boonstra K., Van Erpecum K., Van Nieuwkerk K., Drenth J., Poen A., Witteman B., et al. (2012) Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis 18: 2270–2276. [DOI] [PubMed] [Google Scholar]
- Brandsaeter B., Isoniemi H., Broome U., Olausson M., Backman L., Hansen B., et al. (2004) Liver transplantation for primary sclerosing cholangitis; predictors and consequences of hepatobiliary malignancy. J Hepatol 40: 815–822. [DOI] [PubMed] [Google Scholar]
- Broome U., Lofberg R., Veress B., Eriksson L. (1995) Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology 22: 1404–1408. [DOI] [PubMed] [Google Scholar]
- Broome U., Olsson R., Loof L., Bodemar G., Hultcrantz R., Danielsson A., et al. (1996) Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 38: 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., He H., Nguyen T., Mennone A., Boyer J. (2010) Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J Lipid Res 51: 2265–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R., Fevery J., Kalloo A., Nagorney D., Boberg K., Shneider B., et al. (2010) Diagnosis and management of primary sclerosing cholangitis. Hepatology 51: 660–678. [DOI] [PubMed] [Google Scholar]
- Chazouilleres O., Poupon R., Capron J., Metman E., Dhumeaux D., Amouretti M., et al. (1990) Ursodeoxycholic acid for primary sclerosing cholangitis. J Hepatol 11: 120–123. [DOI] [PubMed] [Google Scholar]
- Cohen B., Hofmann A., Mosbach E., Stenger R., Rothschild M., Hagey L., et al. (1986) Differing effects of nor-ursodeoxycholic or ursodeoxycholic acid on hepatic histology and bile acid metabolism in the rabbit. Gastroenterology 91: 189–197. [DOI] [PubMed] [Google Scholar]
- Cox K., Cox K. (1998) Oral vancomycin: treatment of primary sclerosing cholangitis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 27: 580–583. [DOI] [PubMed] [Google Scholar]
- Cullen S., Rust C., Fleming K., Edwards C., Beuers U., Chapman R. (2008) High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol 48: 792–800. [DOI] [PubMed] [Google Scholar]
- Davies Y., Cox K., Abdullah B., Safta A., Terry A., Cox K. (2008) Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic.J Pediatr Gastroenterol Nutr 47: 61–67. [DOI] [PubMed] [Google Scholar]
- De Maria N., Colantoni A., Rosenbloom E., Van Thiel D. (1996) Ursodeoxycholic acid does not improve the clinical course of primary sclerosing cholangitis over a 2-year period. Hepatogastroenterology 43: 1472–1479. [PubMed] [Google Scholar]
- Dickson E., Murtaugh P., Wiesner R., Grambsch P., Fleming T., Ludwig J., et al. (1992) Primary sclerosing cholangitis: refinement and validation of survival models. Gastroenterology 103: 1893–1901. [DOI] [PubMed] [Google Scholar]
- Eaton J., Silveira M., Pardi D., Sinakos E., Kowdley K., Luketic V., et al. (2011) High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol 106: 1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksteen B., Grant A., Miles A., Curbishley S., Lalor P., Hubscher S., et al. (2004) Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med 200: 1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksteen B., Mora J., Haughton E., Henderson N., Lee-Turner L., Villablanca E., et al. (2009) Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology 137: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M., Kaplan M. (2004) A pilot study of etanercept in the treatment of primary sclerosing cholangitis. Dig Dis Sci 49: 1–4. [DOI] [PubMed] [Google Scholar]
- Eugui E., Allison A. (1993) Immunosuppressive activity of mycophenolate mofetil. Ann N Y Acad Sci 685: 309–329. [DOI] [PubMed] [Google Scholar]
- Farkkila M., Karvonen A., Nurmi H., Nuutinen H., Taavitsainen M., Pikkarainen P., et al. (2004) Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology 40: 1379–1386. [DOI] [PubMed] [Google Scholar]
- Farrant J., Hayllar K., Wilkinson M., Karani J., Portmann B., Westaby D., et al. (1991) Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology 100: 1710–1717. [DOI] [PubMed] [Google Scholar]
- Fevery J., Verslype C., Lai G., Aerts R., Van Steenbergen W. (2007) Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig Dis Sci 52: 3123–3135. [DOI] [PubMed] [Google Scholar]
- Fickert P., Wagner M., Marschall H., Fuchsbichler A., Zollner G., Tsybrovskyy O., et al. (2006) 24-Norursodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 130: 465–481. [DOI] [PubMed] [Google Scholar]
- Floreani A., Rizzotto E., Ferrara F., Carderi I., Caroli D., Blasone L., et al. (2005) Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome.Am J Gastroenterol 100: 1516–1522. [DOI] [PubMed] [Google Scholar]
- Fulton B., Markham A. (1996) Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs 51: 278–298. [DOI] [PubMed] [Google Scholar]
- Giljaca V., Poropat G., Stimac D., Gluud C. (2010) Glucocorticosteroids for primary sclerosing cholangitis. Cochrane Database Syst Rev: CD004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio G., Portmann B., Karani J., Harrison P., Donaldson P., Vergani D., et al. (2001) Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology 33: 544–553. [DOI] [PubMed] [Google Scholar]
- Harnois D., Angulo P., Jorgensen R., Larusso N., Lindor K. (2001) High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol 96: 1558–1562. [DOI] [PubMed] [Google Scholar]
- He H., Mennone A., Boyer J., Cai S. (2011) Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology 53: 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberger B., Puhalla H., Lehnert M., Wrba F., Novak S., Brandstetter A., et al. (2007) Activated mammalian target of rapamycin is an adverse prognostic factor in patients with biliary tract adenocarcinoma. Clin Cancer Res 13: 4795–4799. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Zakko S., Lira M., Clerici C., Hagey L., Lambert K., et al. (2005) Novel biotransformation and physiological properties of norursodeoxycholic acid in humans. Hepatology 42: 1391–1398. [DOI] [PubMed] [Google Scholar]
- Hommes D., Erkelens W., Ponsioen C., Stokkers P., Rauws E., Van Der Spek M., et al. (2008) A double-blind, placebo-controlled, randomized study of infliximab in primary sclerosing cholangitis. J Clin Gastroenterol 42: 522–526. [DOI] [PubMed] [Google Scholar]
- Hurlburt K., Mcmahon B., Deubner H., Hsu-Trawinski B., Williams J., Kowdley K. (2002) Prevalence of autoimmune liver disease in Alaska natives. Am J Gastroenterol 97: 2402–2407. [DOI] [PubMed] [Google Scholar]
- Im E., Martinez J. (2004) Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/RAF–1/ERK signaling in human colon cancer cells. J Nutr 134: 483–486. [DOI] [PubMed] [Google Scholar]
- Imam M., Sinakos E., Gossard A., Kowdley K., Luketic V., Edwyn Harrison M., et al. (2011) High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis. Aliment Pharmacol Ther 34: 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javett S. (1971) Azathioprine in primary sclerosing cholangitif. Lancet 1: 810. [DOI] [PubMed] [Google Scholar]
- Jin Y., Lin Y., Lin L., Zheng C. (2015) Meta-analysis of the effectiveness and safety of vedolizumab for ulcerative colitis. World J Gastroenterol 21: 6352–6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A., Gudjonsson J., Sigmundsdottir H., Ludviksson B., Valdimarsson H. (2005) The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol 114: 154–163. [DOI] [PubMed] [Google Scholar]
- Karlsen T., Franke A., Melum E., Kaser A., Hov J., Balschun T., et al. (2010) Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology 138: 1102–1111. [DOI] [PubMed] [Google Scholar]
- Keshav S., Vanasek T., Niv Y., Petryka R., Howaldt S., Bafutto M., et al. (2013) A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS One 8: e60094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S., Mustafi R., Cerda S., Yuan W., Jagadeeswaran S., Dougherty U., et al. (2008) Ursodeoxycholic acid suppresses COX-2 expression in colon cancer: roles of Ras, P38, and Ccaat/enhancer-binding protein. Nutr Cancer 60: 389–400. [DOI] [PubMed] [Google Scholar]
- Kim W., Poterucha J., Wiesner R., Larusso N., Lindor K., Petz J., et al. (1999) The relative role of the Child-Pugh classification and the Mayo natural history model in the assessment of survival in patients with primary sclerosing cholangitis. Hepatology 29: 1643–1648. [DOI] [PubMed] [Google Scholar]
- Kim W., Therneau T., Wiesner R., Poterucha J., Benson J., Malinchoc M., et al. (2000) A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc 75: 688–694. [DOI] [PubMed] [Google Scholar]
- Knox T., Kaplan M. (1991) Treatment of primary sclerosing cholangitis with oral methotrexate. Am J Gastroenterol 86: 546–552. [PubMed] [Google Scholar]
- Knox T., Kaplan M. (1994) A double-blind controlled trial of oral-pulse methotrexate therapy in the treatment of primary sclerosing cholangitis. Gastroenterology 106: 494–499. [DOI] [PubMed] [Google Scholar]
- Kucuktulu U., Alhan E., Tekelioglu Y., Ozekin A. (2007) The effects of pentoxifylline on liver regeneration after portal vein ligation in rats. Liver Int 27: 274–279. [DOI] [PubMed] [Google Scholar]
- Lichtman S., Keku J., Schwab J., Sartor R. (1991) Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology 100: 513–519. [DOI] [PubMed] [Google Scholar]
- Lichtman S., Sartor R., Keku J., Schwab J. (1990) Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology 98: 414–423. [DOI] [PubMed] [Google Scholar]
- Lindor K. (1997) Ursodiol for primary sclerosing cholangitis. Mayo primary sclerosing cholangitis-ursodeoxycholic acid study group. N Engl J Med 336: 691–695. [DOI] [PubMed] [Google Scholar]
- Lindor K., Jorgensen R., Anderson M., Gores G., Hofmann A., Larusso N. (1996) Ursodeoxycholic acid and methotrexate for primary sclerosing cholangitis: a pilot study. Am J Gastroenterol 91: 511–515. [PubMed] [Google Scholar]
- Lindor K., Kowdley K., Harrison M. (2015) ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol 110: 646–659; quiz 660. [DOI] [PubMed] [Google Scholar]
- Lindor K., Kowdley K., Luketic V., Harrison M., McCashland T., Befeler A., et al. (2009) High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 50: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor K., Wiesner R., Colwell L., Steiner B., Beaver S., Larusso N. (1991) The combination of prednisone and colchicine in patients with primary sclerosing cholangitis. Am J Gastroenterol 86: 57–61. [PubMed] [Google Scholar]
- Lindstrom L., Boberg K., Wikman O., Friis-Liby I., Hultcrantz R., Prytz H., et al. (2012) High dose ursodeoxycholic acid in primary sclerosing cholangitis does not prevent colorectal neoplasia. Aliment Pharmacol Ther 35: 451–457. [DOI] [PubMed] [Google Scholar]
- Lindstrom L., Hultcrantz R., Boberg K., Friis-Liby I., Bergquist A. (2013) Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 11: 841–846. [DOI] [PubMed] [Google Scholar]
- Liu J., Hov J., Folseraas T., Ellinghaus E., Rushbrook S., Doncheva N., et al. (2013) Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet 45: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S., Fleming K., Chapman R. (1994) A 2-year follow-up study of anti-neutrophil antibody in primary sclerosing cholangitis: relationship to clinical activity, liver biochemistry and ursodeoxycholic acid treatment. J Hepatol 21: 974–978. [DOI] [PubMed] [Google Scholar]
- Loftus E., Jr, Harewood G., Loftus C., Tremaine W., Harmsen W., Zinsmeister A., et al. (2005) PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 54: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes F., Jorgensen R., Keach J., Katzmann J., Smyrk T., Donlinger J., et al. (2006) Elevated serum IGG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol 101: 2070–2075. [DOI] [PubMed] [Google Scholar]
- Mitchell S., Bansi D., Hunt N., Von Bergmann K., Fleming K., Chapman R. (2001) A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology 121: 900–907. [DOI] [PubMed] [Google Scholar]
- Mizuno S., Hirano K., Tada M., Yamamoto K., Yashima Y., Yagioka H., et al. (2010) Bezafibrate for the treatment of primary sclerosing cholangitis. J Gastroenterol 45: 758–762. [DOI] [PubMed] [Google Scholar]
- Montrone M., Martorelli D., Rosato A., Dolcetti R. (2009) Retinoids as critical modulators of immune functions: new therapeutic perspectives for old compounds. Endocr Metab Immune Disord Drug Targets 9: 113–131. [DOI] [PubMed] [Google Scholar]
- Mowat C., Cole A., Windsor A., Ahmad T., Arnott I., Driscoll R., et al. (2011) Guidelines for the management of inflammatory bowel disease in adults. Gut 60: 571–607. [DOI] [PubMed] [Google Scholar]
- Mueller T., Beutler C., Pico A., Shibolet O., Pratt D., Pascher A., et al. (2011) Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver Int 31: 1574–1588. [DOI] [PubMed] [Google Scholar]
- Negm A., Schott A., Vonberg R., Weismueller T., Schneider A., Kubicka S., et al. (2010) Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest Endosc 72: 284–291. [DOI] [PubMed] [Google Scholar]
- Norment A., Bogatzki L., Gantner B., Bevan M. (2000) Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is up-regulated following pre-TCR signaling. J Immunol 164: 639–648. [DOI] [PubMed] [Google Scholar]
- O’brien C., Senior J., Arora-Mirchandani R., Batta A., Salen G. (1991) Ursodeoxycholic acid for the treatment of primary sclerosing cholangitis: a 30-month pilot study. Hepatology 14: 838–847. [DOI] [PubMed] [Google Scholar]
- Okolicsanyi L., Groppo M., Floreani A., Morselli-Labate A., Rusticali A., Battocchia A., et al. (2003) Treatment of primary sclerosing cholangitis with low-dose ursodeoxycholic acid: results of a retrospective Italian multicentre survey. Dig Liver Dis 35: 325–331. [DOI] [PubMed] [Google Scholar]
- Olsson R., Bjornsson E., Backman L., Friman S., Hockerstedt K., Kaijser B., et al. (1998) Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. J Hepatol 28: 426–432. [DOI] [PubMed] [Google Scholar]
- Olsson R., Boberg K., De Muckadell O., Lindgren S., Hultcrantz R., Folvik G., et al. (2005) High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology 129: 1464–1472. [DOI] [PubMed] [Google Scholar]
- Olsson R., Broome U., Danielsson A., Hagerstrand I., Jarnerot G., Loof L., et al. (1995) Colchicine treatment of primary sclerosing cholangitis. Gastroenterology 108: 1199–1203. [DOI] [PubMed] [Google Scholar]
- Patsenker E., Schneider V., Ledermann M., Saegesser H., Dorn C., Hellerbrand C., et al. (2011) Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine a and tacrolimus in experimental liver fibrosis. J Hepatol 55: 388–398. [DOI] [PubMed] [Google Scholar]
- Paumgartner G., Beuers U. (2004) Mechanisms of action and therapeutic efficacy of ursodeoxycholic acid in cholestatic liver disease. Clin Liver Dis 8: 67–81, vi. [DOI] [PubMed] [Google Scholar]
- Pignochino Y., Sarotto I., Peraldo-Neia C., Penachioni J., Cavalloni G., Migliardi G., et al. (2010) Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer 10: 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poropat G., Giljaca V., Stimac D., Gluud C. (2011) Bile acids for primary sclerosing cholangitis. Cochrane Database Syst Rev: CD003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichrath J., Lehmann B., Carlberg C., Varani J., Zouboulis C. (2007) Vitamins as hormones. Horm Metab Res 39: 71–84. [DOI] [PubMed] [Google Scholar]
- Rodrigues C., Kren B., Steer C., Setchell K. (1995) The site-specific delivery of ursodeoxycholic acid to the rat colon by sulfate conjugation. Gastroenterology 109: 1835–1844. [DOI] [PubMed] [Google Scholar]
- Saarinen S., Olerup O., Broome U. (2000) Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am J Gastroenterol 95: 3195–3199. [DOI] [PubMed] [Google Scholar]
- Sabino J., Vleira-Silva S., Machiels K., Joossens M., Ballet V., Ferrante M., et al. (2015) DOP087 Intestinal microbial signature in patients with primary sclerosing cholangitis. ECCO Conference Abstract. DOP Session 10 – The genome and microbiome. [Google Scholar]
- Sandborn W., Wiesner R., Tremaine W., Larusso N. (1993) Ulcerative colitis disease activity following treatment of associated primary sclerosing cholangitis with cyclosporin. Gut 34: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Nakazawa T., Ando T., Hayashi K., Naitoh I., Okumura F., et al. (2011) Clinical characteristics of inflammatory bowel disease associated with primary sclerosing cholangitis. J Hepatobiliary Pancreat Sci 18: 154–161. [DOI] [PubMed] [Google Scholar]
- Schramm C., Schirmacher P., Helmreich-Becker I., Gerken G., Zum Buschenfelde K., Lohse A. (1999) Combined therapy with azathioprine, prednisolone, and ursodiol in patients with primary sclerosing cholangitis. a case series. Ann Intern Med 131: 943–946. [DOI] [PubMed] [Google Scholar]
- Schrumpf E., Boberg K. (2001) Epidemiology of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol 15: 553–562. [DOI] [PubMed] [Google Scholar]
- Shetty K., Rybicki L., Brzezinski A., Carey W., Lashner B. (1999) The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol 94: 1643–1649. [DOI] [PubMed] [Google Scholar]
- Silveira M., Torok N., Gossard A., Keach J., Jorgensen R., Petz J., et al. (2009) Minocycline in the treatment of patients with primary sclerosing cholangitis: results of a pilot study. Am J Gastroenterol 104: 83–88. [DOI] [PubMed] [Google Scholar]
- Sinakos E., Marschall H., Kowdley K., Befeler A., Keach J., Lindor K. (2010) Bile acid changes after high-dose ursodeoxycholic acid treatment in primary sclerosing cholangitis: relation to disease progression. Hepatology 52: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Khanna S., Pardi D., Loftus E., Jr., Talwalkar J. (2013) Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 19: 1631–1638. [DOI] [PubMed] [Google Scholar]
- Spengler U., Moller A., Jung M., Messer G., Zachoval R., Hoffmann R., et al. (1992) T lymphocytes from patients with primary biliary cirrhosis produce reduced amounts of lymphotoxin, tumor necrosis factor and interferon-gamma upon mitogen stimulation. J Hepatol 15: 129–135. [DOI] [PubMed] [Google Scholar]
- Sterling R., Salvatori J., Luketic V., Sanyal A., Fulcher A., Stravitz R., et al. (2004) A prospective, randomized-controlled pilot study of ursodeoxycholic acid combined with mycophenolate mofetil in the treatment of primary sclerosing cholangitis. Aliment Pharmacol Ther 20: 943–949. [DOI] [PubMed] [Google Scholar]
- Stiehl A., Walker S., Stiehl L., Rudolph G., Hofmann W., Theilmann L. (1994) Effect of ursodeoxycholic acid on liver and bile duct disease in primary sclerosing cholangitis. A 3-year pilot study with a placebo-controlled study period. J Hepatol 20: 57–64. [DOI] [PubMed] [Google Scholar]
- Strack I., Schulte S., Varnholt H., Schievenbusch S., Tox U., Wendland K., et al. (2011) Beta-adrenoceptor blockade in sclerosing cholangitis of Mdr2 knockout mice: antifibrotic effects in a model of nonsinusoidal fibrosis. Lab Invest 91: 252–261. [DOI] [PubMed] [Google Scholar]
- Tabibian J., Talwalkar J., Lindor K. (2013a) Role of the microbiota and antibiotics in primary sclerosing cholangitis. Biomed Res Int 2013: 389537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibian J., Weeding E., Jorgensen R., Petz J., Keach J., Talwalkar J., et al. (2013b) Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis – a pilot study. Aliment Pharmacol Ther 37: 604–612. [DOI] [PubMed] [Google Scholar]
- Talal, et al. (2012) ClinicalTrials.gov Identifier NCT01452308. Available at: https://clinicaltrials.gov/ct2/show/NCT01452308
- Talwalkar J., Angulo P., Keach J., Petz J., Jorgensen R., Lindor K. (2005) Mycophenolate mofetil for the treatment of primary sclerosing cholangitis. Am J Gastroenterol 100: 308–312. [DOI] [PubMed] [Google Scholar]
- Talwalkar J., Gossard A., Keach J., Jorgensen R., Petz J., Lindor R. (2007) Tacrolimus for the treatment of primary sclerosing cholangitis. Liver Int 27: 451–453. [DOI] [PubMed] [Google Scholar]
- Tiede I., Fritz G., Strand S., Poppe D., Dvorsky R., Strand D., et al. (2003) CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 111: 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantos C., Koukias N., Nikolopoulou V., Burroughs A. (2011) Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther 34: 901–910. [DOI] [PubMed] [Google Scholar]
- Trivedi P., Hirschfield G. (2012) Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther 36: 517–533. [DOI] [PubMed] [Google Scholar]
- Tung B., Emond M., Haggitt R., Bronner M., Kimmey M., Kowdley K., et al. (2001) Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med 134: 89–95. [DOI] [PubMed] [Google Scholar]
- Ueki M., Koda M., Yamamoto S., Matsunaga Y., Murawaki Y. (2006) Preventive and therapeutic effects of angiotensin II Type 1 receptor blocker on hepatic fibrosis induced by bile duct ligation in rats. J Gastroenterol 41: 996–1004. [DOI] [PubMed] [Google Scholar]
- Van Hoogstraten H., Vleggaar F., Boland G., Van Steenbergen W., Griffioen P., Hop W., et al. (2000) Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: a randomized double-blind pilot study. Belgian-Dutch PSC Study Group. Am J Gastroenterol 95: 2015–2022. [DOI] [PubMed] [Google Scholar]
- Van Thiel D., Wright H., Carroll P., Abu-Elmagd K., Rodriguez-Rilo H., McMichael J., et al. (1995) Tacrolimus: a potential new treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial. Am J Gastroenterol 90: 771–776. [PMC free article] [PubMed] [Google Scholar]
- Wagner A. (1971) Azathioprine treatment in primary sclerosing cholangitis. Lancet 2: 663–664. [DOI] [PubMed] [Google Scholar]
- Wali R., Frawley B., Jr, Hartmann S., Roy H., Khare S., Scaglione-Sewell B., et al. (1995) Mechanism of action of chemoprotective ursodeoxycholate in the azoxymethane model of rat colonic carcinogenesis: potential roles of protein kinase C-alpha, -beta II, and -zeta. Cancer Res 55: 5257–5264. [PubMed] [Google Scholar]
- Webster G., Pereira S., Chapman R. (2009) Autoimmune pancreatitis/Igg4-associated cholangitis and primary sclerosing cholangitis–overlapping or separate diseases? J Hepatol 51: 398–402. [DOI] [PubMed] [Google Scholar]
- Wiesner R., Grambsch P., Dickson E., Ludwig J., Maccarty R., Hunter E., et al. (1989) Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology 10: 430–436. [DOI] [PubMed] [Google Scholar]
- Wiesner R., Ludwig J., Lindor K., Jorgensen R., Baldus W., Homburger H., et al. (1990) A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. N Engl J Med 322: 1419–1424. [DOI] [PubMed] [Google Scholar]
- Wolf J., Rybicki L., Lashner B. (2005) The impact of ursodeoxycholic acid on cancer, dysplasia and mortality in ulcerative colitis patients with primary sclerosing cholangitis. Aliment Pharmacol Ther 22: 783–788. [DOI] [PubMed] [Google Scholar]
- Ye B., Yang S., Boo S., Cho Y., Yang D., Yoon S., et al. (2011) Clinical characteristics of ulcerative colitis associated with primary sclerosing cholangitis in Korea. Inflamm Bowel Dis 17: 1901–1906. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Gutierrez J., Varona R., Ardavin C., Marquez G. (1999) Cutting edge: identification of the orphan chemokine receptor GPR-9–6 as CCR9, the receptor for the chemokine teck. J Immunol 162: 5671–5675. [PubMed] [Google Scholar]