Abstract
The chronic lung diseases, asthma and chronic obstructive pulmonary disease (COPD), are common affecting over 500 million people worldwide and causing substantial morbidity and mortality. Asthma is typically associated with Th2-mediated eosinophilic airway inflammation, in contrast to neutrophilic inflammation observed commonly in COPD. However, there is increasing evidence that the eosinophil might play an important role in 10–40% of patients with COPD. Consistently in both asthma and COPD a sputum eosinophilia is associated with a good response to corticosteroid therapy and tailored strategies aimed to normalize sputum eosinophils reduce exacerbation frequency and severity. Advances in our understanding of the multistep paradigm of eosinophil recruitment to the airway, and the consequence of eosinophilic inflammation, has led to the development of new therapies to target these molecular pathways. In this article we discuss the mechanisms of eosinophilic trafficking, the tools to assess eosinophilic airway inflammation in asthma and COPD during stable disease and exacerbations and review current and novel anti-eosinophilic treatments.
Keywords: ACOS, anti-CRTh2, anti-IL5, anti-IL-13, anti-IL4R, asthma, biomarker, COPD, corticosteroid, eosinophil
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) cause considerable morbidity affecting over 500 million people worldwide and managing these conditions consumes substantial healthcare resources exceeding €56 billion per year in the European Union [Burrowes et al. 2014]. Both conditions are characterized by airflow obstruction, which is typically variable and reversible in asthma, but fixed in COPD. Similarly the symptoms in asthma are usually intermittent, although persistent in more severe disease, whereas in COPD symptoms occur daily with reduced exercise capacity and respiratory failure occurring as the disease progresses. Both conditions are characterized by exacerbations whereby sufferers have worsening of their symptoms often precipitated by infection and these exacerbations amplify the morbidity and contribute substantially to the mortality of these airway diseases.
Eosinophilic inflammation orchestrated by allergic sensitization and T helper 2 lymphocytes (Th2)-mediated immune response is the hallmark of airway inflammation in asthma, but importantly eosinophilic inflammation can occur independent of allergy and the presence of eosinophilic inflammation is neither necessary nor sufficient for the development of asthma [Eltboli and Brightling, 2013]. Conversely, COPD is typically associated with T helper 1 lymphocytes (Th1)-mediated immunity with a neutrophilic response [Stanescu et al. 1996] often in association with bacterial colonization. However, this probably represents only half of COPD patients and in a subgroup of 10–40% eosinophilic airway inflammation is a feature [Brightling et al. 2000, 2005; Eltboli et al. 2014; Leigh et al. 2006; Pizzichini et al. 1998; Saetta et al. 1994]. Therefore, eosinophilic inflammation cannot be assumed to be present or absent dependent on the airway disease, but needs to be measured as part of the assessment and phenotyping of airways disease. This is critical when considering the potential immunopathogenic role of eosinophilic inflammation in disease; its role as a biomarker to direct current therapies and for the future development of eosinophil-directed therapies.
Herein, we shall discuss the mechanisms of eosinophilic trafficking, the tools to assess eosinophil airway inflammation, cross-sectional and longitudinal analysis of eosinophilic airway inflammation in asthma and COPD during stable disease and exacerbations and review anti-eosinophilic treatments including therapies in development.
Eosinophil biology: mechanisms of eosinophilic airway inflammation
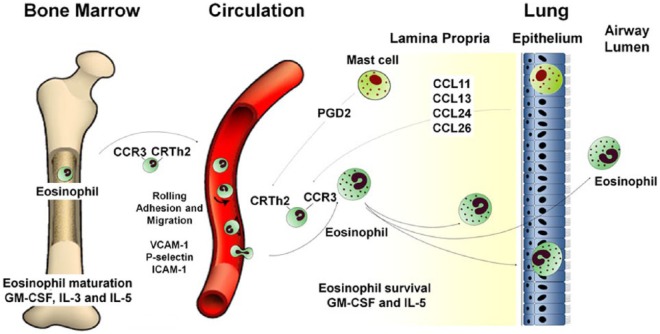
Eosinophils are granulocytic leukocytes first discovered by Heinrich Caro in 1874 and described by Paul Ehrlich in 1879 [Kay, 2014]. They constitute 1–4% of circulating white cells and are distinguished phenotypically by their bilobed nuclei and large acidophilic cytoplasmic granules. The pathological role of eosinophils primarily occurs in tissues and therefore a major focus has been to outline the molecular mechanisms involved in selective eosinophil recruitment summarized in Figure 1 [Rosenberg et al. 2007; Wardlaw, 1999].
Figure 1.
Eosinophil trafficking from the bone marrow to the airway.
Eosinophils are derived from pluripotent CD34+ progenitor stem cells found in normal bone marrow. This differentiation occurs under the influence of granulocyte-monocyte colony stimulating factor (GM-CSF) and interleukin (IL)-3 in early phases and IL-5 in the latter phases of differentiation [Denburg, 1998]. Mature eosinophils are released from bone marrow into circulation and this is primarily regulated by IL-5. Eosinophils are transformed from a quiescent state to a state of increased hyper responsiveness by priming agents such as these cytokines IL-3, IL-5, GM-CSF [Luijk et al. 2005]. The priming process does not fully activate the cell but increases its responsiveness to chemotaxis, degranulation and cytokine production [Fulkerson and Rothenberg, 2013]. As eosinophils flow in the blood stream they roll onto the bronchial vascular endothelium. Infiltration of eosinophils into the airway will not occur unless there is adhesion and transmigration across the bronchial vascular endothelium. This preferential adhesion of eosinophils from the blood stream into various tissues results from specific interactions between integrins on surface of eosinophils and adhesion receptors on the surface of vascular endothelium, which include P-selectin/P-selectin glycoprotein ligand-1 and very late activation antigen/vascular cell adhesion molecule, VCAM-1 ligand [Fukuda et al. 1996; Symon et al. 1996]. IL-4 and IL-13 have been found to increase VCAM-1 and P-selectin expression [Patel, 1998; Woltmann et al. 2000]. Recruitment to the airway is under the control of the chemokines CCL5, 7, 11, 13, 15, 24 and 26 and their cognate receptor CCR3 [Smit and Lukacs, 2006; Ying et al. 1997], which play a critical role together with chemo-attractant receptor homologous molecule expressed on Th2 cells (CRTh2) and its ligand prostaglandin D2 (PGD2) [Hirai et al. 2001; Mutalithas et al. 2010]. PGD2 acts both as an eosinophil chemoattractant in its own right, but also augments the effects of the CCR3 chemokines [Mesquita-Santos et al. 2006]. Activated mast cells are the predominant source of PGD2 with release via IgE-dependent and independent activation [Lewis et al. 1982].
Eosinophils have four specific basic proteins that include major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPO) and eosinophil derived neurotoxin (EDN), which are stored in secondary granules. ECP has been found to attach to cell membrane altering permeability as well as increasing production of reactive oxygen species [Navarro et al. 2008]. MBP was also found to cause alveolar epithelium cell lysis allowing for penetration of inhaled antigen [Ayars et al. 1985]. In combination these granules are cytotoxic and disrupt the protective pulmonary epithelial barrier allowing further inflammatory responses to occur [Frigas et al. 1991]. Eosinophils have also been characterized by the ability to store preformed mediators as well as synthesize and secrete a number of pro-inflammatory cytokines, chemokines and growth factors including IL-2, IL-3, IL-4, IL-5, IL-10, IL-12, IL-13, IL-16, IL-25, tumour necrosis factor, transforming growth factor (TGF) α/β, CCL5, CCL11, 13 facilitating their key role as immunoregulators [Davoine and Lacy, 2014; Moqbel et al. 1994].
Thus, although eosinophils have an immunomodulatory role they are recruited into the airway orchestrated by other cells and can be considered largely a final important effector cell.
Phenotyping the heterogeneity of eosinophilic airway inflammation
How can you measure eosinophilic inflammation?
Eosinophilic airway inflammation can be measured in the airway noninvasively by sputum analysis and invasively by bronchoscopic sampling. Bronchoscopy enables investigators to sample airway inflammation directly in the airway wall by bronchial biopsy, in the large airway lumen by proximal bronchial wash and in the smaller distal airways with bronchoalveolar lavage. Bronchoscopic sampling is invasive costly, time consuming and requires the need of experienced investigators. The relationship between sputum and bronchoscopic sampling has been investigated in several studies and although they generally report correlations between compartments these correlations are weak [Keatings et al. 1997; Maestrelli et al. 1995; Rutgers et al. 2000]. The use of sputum cytology to explore airway inflammation has been in existence since the first descriptions by Ernst Leyden and Jean Charcot of Charcot-Leyden crystals [Sakula, 1986] and was used clinically in the 1950s by Dr Morrow-Brown [Brown, 1958] and then optimized in the 1990s by Freddy Hargreaves and colleagues [Pavord et al. 1997]. Normal sputum eosinophil count ranges in large populations have been defined. A blood eosinophil count has been used as a surrogate for eosinophilic airway inflammation and to determine the intensity of eosinophilic inflammation in the blood compartment [Bafadhel et al. 2012; Wagener et al. 2015]. Studies have shown a moderate correlation between blood eosinophil count and sputum eosinophil count (r = 0.6; p < 0.0001) [Schleich et al. 2013] (r = 0.59; p < 0.001) [Wagener et al. 2015]. Using the (ROC) curves the diagnostic accuracy of a blood eosinophil count was 0.85 [95% confidence interval (CI) 0.78–0.93] [Bafadhel et al. 2011; Wagener et al. 2015] to detect a sputum eosinophilia ⩾3%. Blood eosinophils can be assessed in most hospitals worldwide and thus presents a biomarker that has advantages due to its simplicity and availability. The relationship between blood and sputum eosinophils is complicated in obesity. Recent evidence demonstrates that in obese asthmatics the relationship is distinct with strong associations between blood and tissue eosinophilia, but a consistently reduced sputum eosinophilia suggesting altered eosinophil trafficking in obesity [Desai et al. 2013].
Interestingly, sputum cytology also reveals information about longer term eosinophil exposure beyond the simple sputum eosinophil differential cell count. The apoptosis and subsequent removal of dead cells by phagocytes (efferocytosis) is a critical mechanism for the noninflammatory clearance of granulocytes including eosinophils. Macrophage red hue due to eosin staining within sputum macrophages reflects eosinophil efferocytosis and can be used as a biomarker of eosinophil load and its clearance [Eltboli et al. 2014; Kulkarni et al. 2010].
Other biomarkers that are associated with eosinophilic airway inflammation include fractional exhaled nitric oxide (FeNO) or Th2-activation marker, e.g. serum periostin [Berry et al. 2005]. Breath analysis is an attractive alternative as a near-patient test. Nitric oxide is produced by various cells including airway epithelial cells and inflammatory cells under the action of inducible NO synthase enzyme which converts L-arginine to L-citrulline. FeNO levels are correlated with peripheral blood eosinophils [Zietkowski et al. 2006] and sputum eosinophils particularly in mild-to-moderate disease whereas the relationship is more complex in severe disease. FeNO in severe asthma was poorly related to blood eosinophils as highlighted in a national registry audit in refractory asthma, which showed that though blood eosinophils levels decreased, FeNO levels paradoxically increased in response to oral corticosteroids [Sweeney et al. 2012]. Novel ‘breathomic’ approaches also have promise to identify different inflammatory phenotypes.
Periostin is a matricellular protein secreted basolaterally from epithelial cells in response to various stimuli including IL-13 and its levels are highly correlated with Th2 signature [Woodruff et al. 2007]. Two major studies have highlighted apparently conflicting views on the correlation of periostin with eosinophilia. An observational study found periostin to be a superior predictor of eosinophil inflammation in the airway [Jia et al. 2012] while a more recent a cross-sectional study [Wagener et al. 2015] showed a strong correlation of sputum eosinophils with blood eosinophils, but failed to demonstrate a relationship between serum periostin and eosinophils in sputum. The most likely explanation for these apparent differences are that former study investigated the sensitivity and specificity of periostin to identify an airway eosinophilia using a composite score rather than specifically comparing the relationship between blood periostin and a sputum eosinophil count as performed in the later study. This suggests that blood periostin might provide additional value as a biomarker of Th2 inflammation, but is not directly comparable with either blood or sputum eosinophilia. Periostin may be more useful as a marker to assess specific IL-13 activity and responses to anti IL-13 therapy [Corren et al. 2011]. IL-13 induces epithelial-derived periostin directly and is associated with upregulated FENO expression, whereas the relationship between airway eosinophilic inflammation and IL-13 is more complex and indirect such as via upregulation of epithelial-derived chemoattractants. These variations in biomarker measurements in various studies further highlights the heterogeneity and complexity of airway inflammation and the possible need of measuring a number of biomarkers in conjunction with one another to accurately try and determine a phenotype.
How common is eosinophilic inflammation in asthma and COPD and is it a stable phenotype?
In asthma up to 80% of corticosteroid-naïve patients and 50% of corticosteroid treated patients have a sputum eosinophilia [Douwes et al. 2002; Eltboli and Brightling, 2013]. Short-term repeatability of a sputum eosinophilia is very good [Boorsma et al. 2007; Pizzichini et al. 1996]. However, there is emerging evidence that in some patients their inflammatory profile is stable whereas in others it is highly variable. McGrath and colleagues in a population of 995 asthma patients reported that persistent eosinophilic inflammation was less frequent than subjects with an intermittent eosinophilia, 22% versus 31% [McGrath et al. 2012]. Interestingly Newby and colleagues [Newby et al. 2014a] have demonstrated that in severe asthma those subjects with intermittent eosinophilia had increased rate of lung function decline compared with those without eosinophilic inflammation or persistent eosinophilic inflammation.
Further insights into how common eosinophilic inflammation is in asthma can be derived from corticosteroid withdrawal studies as emergence of eosinophilic inflammation following treatment withdrawal in apparently noneosinophilic disease is informative. In a 1-year follow-up study of asthmatic patients in whom treatment was stepped-down in the absence of a sputum eosinophilia, persistent noneosinophilia was observed in 12.5% of patients [Kulkarni et al. 2010]. Interestingly, the macrophage red hue predicts the emergence of eosinophilic inflammation in treatment withdrawal with continued corticosteroid step-down only possible in those subjects without evidence of a sputum eosinophilia nor elevated macrophage red hue [Kulkarni et al. 2010]. This suggests that in severe asthma the absence of an underlying eosinophilic inflammation is probably uncommon.
In COPD, although neutrophilic inflammation is commonly reported, a sputum eosinophilia is present in 10–40% of patients with COPD [Brightling et al. 2000; Leigh et al. 2006; Pizzichini et al. 1998; Saetta et al. 1994] Similarly to asthma the repeatability of eosinophilic inflammation is very good, but again there are three groups with either persistent, variable or absent eosinophilic inflammation. In the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End points) cohort repeated blood eosinophil counts were available in 1483 subjects over 3 years [Singh et al. 2014]. Using a cutoff for blood eosinophils of 2%, 37% of subjects had persistently elevated eosinophils, 49% were intermittent and the remainder were noneosinophilic. The persistently eosinophilic group were older, predominantly male with milder disease and fewer symptoms. Emphysema progression was greater in the non-eosinophilic group [Singh et al. 2014] and in contrast to severe asthma there were no differences in lung function decline between groups.
To further inform the understanding of the heterogeneity of airway disease unbiased statistical approaches such as cluster analysis have been applied to large clinical datasets [Amelink et al. 2013; Haldar et al. 2008; Newby et al. 2014b; Wardlaw et al. 2005]. Interestingly, these have underscored the importance of eosinophilic airway inflammation in asthma and COPD and perhaps most importantly in asthma-COPD overlap syndrome (ACOS). For example, Ghebre and colleagues combined data from asthma and COPD and identified three clusters [Ghebre et al. 2015]. Cluster 1 consisted of asthma subjects with increase IL-5, IL-13 and CCL26 mediators and eosinophil predominance. Cluster 2 consisted of an overlap between asthma and COPD with neutrophil predominance with 11% of patients with asthma having sputum eosinophilia. Cluster 3 consisted mainly of COPD patients with a mixed granulocytic airway inflammation. The differences seen between neutrophilic COPD in cluster 2 and eosinophilic COPD in cluster 3 included the presence of increased bacterial colonization in the former and increased CCL13 in the latter possibly explaining the observed airway inflammation differences seen between these clusters. Importantly few studies have investigated cluster stability and predictably due to the variability of eosinophilic inflammation in some patients as described above cluster membership can change overtime. This further highlights the importance of considering the dynamics of eosinophilic inflammation within an individual to appreciate the relationship of this inflammatory phenotype with natural history of disease and response to therapy.
Is eosinophilic inflammation related to disordered airway physiology and remodelling?
Eosinophil-derived granule proteins and pro-inflammatory mediators promote persistent inflammation, which has been associated with increased exacerbations and lung function decline [Green et al. 2002b; Jatakanon et al. 2000; Siva et al. 2007]. However, the relationship between eosinophilic inflammation and airflow obstruction and airway hyper-responsiveness is weak exemplified by the presence of eosinophilic inflammation in eosinophilic bronchitis without asthma [Gibson et al. 1989, 1995]. Indeed comparisons with this disease group have revealed the important role of mast cell localisation to the airway smooth muscle bundle in driving disordered airway physiology [Brightling et al. 2002]. Eosinophils also contribute to airway remodelling [Flood-Page et al. 2003; Kay et al. 2004]. Airway remodelling is a consequence of increased extracellular matrix deposition, increased subepithelial mesenchymal cells and most importantly increased airway smooth muscle mass, which is the major determinant of airflow obstruction. Eosinophil-derived TGF-β activates fibroblast release of matrix proteins and although increased airway smooth muscle mass was eosinophil dependent in a mouse model of asthma [Humbles et al. 2004] whether the eosinophil is necessary for this critical feature of remodelling in human disease is questionable as evidenced by intervention studies described later. Whereas in contrast, reticular basement membrane thickening is associated with eosinophilic inflammation in asthma, COPD and nonasthmatic eosinophilic bronchitis [Brightling et al. 2003; Eltboli et al. 2015; Siddiqui et al. 2008].
Is eosinophilic inflammation a feature of asthma and COPD exacerbations?
The definition of asthma exacerbations vary according to literature source and asthma severity. In summary asthma exacerbations are defined as a decrease in peak flow with worsening of symptoms requiring the use of reliever treatment and or systemic corticosteroids [Reddel et al. 2009; see also http://www.ginasthma.org]. Severe asthma exacerbations require 3 or more days of high-dose oral corticosteroids. GOLD (see http://www.goldcopd.org) have defined a COPD exacerbation as ‘an acute event characterised by worsening of the patients’ respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication’. The differences in exacerbation definitions for asthma and COPD poses limitations in comparisons between the diseases. However, both asthma and COPD exacerbations are commonly associated with identification of pathogens usually viral although also bacterial particularly in COPD. However, pathogens are not identified at exacerbations in 20–50% of cases [Bafadhel et al. 2011; Sethi and Murphy, 2008] either due to insensitivity of assays or as a consequence of a lack of a role of new infections in some exacerbations. Therefore, airway inflammation at exacerbations might represent a consequence of an acute environmental insult on an intrinsically unstable state reflecting an emergent phenomenon in a complex system [Brightling, 2013].
Eosinophilic airway inflammation is present in exacerbations of asthma and COPD, particularly in those subjects with eosinophilic inflammation in stable state. A high baseline sputum eosinophil count is a predictor of loss of control of asthma and an increased number of subsequent exacerbations [Jatakanon et al. 2000]. Treatment strategies aiming to maintain sputum eosinophil counts <2–3% is associated with a significant decrease in the number of exacerbations as compared with treatments focused on clinical history or spirometry [Brightling et al. 2000; Green et al. 2002a; Jayaram et al. 2006]. In COPD sputum eosinophilia has been seen in 10–40% of patients at baseline [Brightling et al. 2000; Pizzichini et al. 1998; Singh et al. 2014] with higher eosinophilic inflammation seen at exacerbation states compared to stable states [Saetta et al. 1994]. In a study aimed at phenotyping COPD exacerbations and identifying biomarkers a sputum eosinophilia was identified in 28% of exacerbations [Bafadhel et al. 2011]. The combination of sputum eosinophil count and macrophage red hue was used to categorize COPD subjects into four groups according to high and low sputum eosinophils (cutoff ⩾3%) and high and low percentage macrophage red hue area (cutoff >6%) [Eltboli et al. 2014]. The group with high eosinophil count, but low red hue had the greatest fall in lung function during an exacerbation and also increased exacerbation frequency. In this group macrophage efferocytosis is defective contributing to persistent eosinophilic inflammation, which in turn is likely to impact upon the severity of the exacerbation event.
Is the future risk of an adverse outcome or response of an outcome to an intervention the same for given eosinophil cutoffs?
Studies have not consistently used the same sputum or blood eosinophil cutoff. This has led to the question of what cutoff should be applied in clinical trials and clinical practice. Importantly, different clinical outcomes have different relationships with eosinophilic inflammation at a single point in time, longitudinally in stable disease, at an exacerbation and in response to therapy. For example, in the study of benralizumab in COPD described below beneficial effects were observed in terms of lung function, health status and exacerbations at different baseline blood and sputum eosinophil counts [Brightling et al. 2014]. Thus, the application of different cutoffs to direct therapies and monitor disease are likely to vary depending on the intervention of interest together with consideration of the potential health economic benefits for these interventions at different cutoffs. For this reason although normal ranges are established a single cutoff to direct all therapy is unlikely.
Therapies that modulate eosinophilic inflammation
The importance of eosinophilic inflammation in asthma and COPD and its role in different states of diseases is perhaps best informed by response to therapies. We describe here current and emerging therapies targeting eosinophilic inflammation and consider their overall clinical effusiveness; their impact upon eosinophilic inflammation and consequent new insights into the relationship between changes in eosinophilic inflammation and clinical outcomes.
Corticosteroids
Inhaled and systemic corticosteroids are the mainstay anti-inflammatory therapy for asthma and COPD in stable disease and at exacerbations as reflected in their central position in international guidelines supported by extensive meta-analyses and Cochrane reviews of multiple randomized controlled trials [Edwards et al. 2010; Loymans et al. 2014; Yang et al. 2012]. The mechanism of action of corticosteroids has been studied extensively, but remains incompletely understood. In brief, corticosteroids diffuse across the eosinophil cell membrane and enter the cell cytoplasm where they bind with glucocorticoid receptors causing exposure of nuclear localization signals [Heitzer et al. 2007]. This results in rapid transport of the glucocorticoid receptor complex into the nucleus which then binds to DNA altering the transcription of target genes which code for pro-inflammatory proteins thereby inhibiting the synthesis of cytokines such as IL-3, IL-5 and GM-CSF, promoting eosinophil apoptosis.
Inhaled and oral corticosteroids decrease eosinophilic bronchial mucosal inflammation [Djukanović et al. 1992] and induced sputum differential eosinophil count [Brightling et al. 2000; Jatakanon et al. 2000; Pizzichini et al. 1998]. Importantly, the presence of sputum eosinophilia (>3%) in asthma and COPD [Gibson et al. 1995; Green et al. 2002a,b] and the Th2 gene signature [Woodruff et al. 2009] has shown to be a good predictor of the response to corticosteroid treatment in stable disease. These observations have been extended to acute exacerbations of COPD with the blood eosinophil count predicting a good response to oral prednisolone [Bafadhel et al. 2012]. Studies further showed that treatment aimed to maintain eosinophil counts <2–3% decreased the number of exacerbations and hospital admissions in asthma and COPD. Interestingly in the study by Green and colleagues the macrophage red hue was further studied and identified that in those subjects with increased red hue but normal eosinophil count that corticosteroid withdrawal was associated with onset of poor control and increased exacerbation risk whereas only in those subjects without evidence of either sputum eosinophils nor increased macrophage red hue could corticosteroids be successfully withdrawn [Kulkarni et al. 2010].
Leukotriene receptor antagonists
Leukotrienes are potent lipid mediators derived from arachidonic acid metabolism and released predominately by mast cells and eosinophils. They are potent bronchoconstrictors, causing airway smooth muscle contraction and mucus hyper-secretion [Hallstrand and Henderson, 2010]. Leukotriene receptor antagonists decrease eosinophilic airway inflammation in blood and sputum in asthma [Pizzichini et al. 1999], albeit to a lesser extent than inhaled corticosteroids [Jayaram et al. 2005]. In moderate-to-severe disease an effect of leukotriene receptor antagonists upon eosinophilic airway inflammation is minimal or absent [Green et al. 2006; Pavord et al. 2007]. In a study where montelukast was used as an add on to a steroid treatment regime as compared to arm where steroid dose was doubled both arms showed improvement in forced expiratory volume in 1 second (FEV1) but there was no difference in sputum eosinophilic inflammation with either arms [Barnes et al. 2007]. In COPD small studies suggest limited clinical benefit and to date no studies have reported effects upon eosinophilic airway in-flammation [Chauhan and Ducharme, 2012; Ducharme et al. 2011].
Theophylline
Theophylline is indicated as third line with most studies showing an equivalent effect and few studies showing a very minimal benefit as compared to doubling steroid regimens or adding a long acting beta agonist (LABA) [Lim et al. 2000; Subramanian et al. 2015; ZuWallack et al. 2001]. Theophylline is commonly prescribed for asthma and COPD as add on treatment in poorly controlled disease [Barnes, 2006; see also http://www.ginasthma.org and http://www.goldcopd.org]. It has both bronchodilator and anti-inflammatory actions with the latter possibly due to its effects upon the activity of a key corticosteroid-associated corepressor protein, histone deacetylase (HDAC)2. Histone acetylation and deacetylation is key in regulating expression of inflammatory genes [Barnes et al. 2005]. Theophylline is an activator of HDAC2 even at low therapeutic levels and enhances the action of corticosteroids [Ito et al. 2006]. In asthma theophylline caused significant reduction in the number of eosinophils in bronchial biopsies, bronchoalveolar lavage (BAL) and induced sputum [Lim et al. 2001] as compared with placebo. Low-dose theophylline when used in combination with inhaled corticosteroids significantly decreased sputum eosinophils counts in COPD patients as compared to theophylline alone [Ford et al. 2010].
Immunomodulators
Immunomodulators such as methotrexate and cyclosporin have steroid-sparing effects, but also predictable side-effects that have limited their value in severe asthma. To date only one study has specifically investigated the effect of methotrexate upon peripheral blood eosinophil count and found no difference between the placebo or treatment arms [Erzurum et al. 1991]. These therapies have not been tested in COPD.
Anti-IgE omalizumab
Omalizumab is the only biological therapy approved for the treatment of asthma. It is a humanized monoclonal anti-IgE antibody. A recent Cochrane review of 25 omalizumab trials in moderate-to-severe asthma showed effectiveness in decreasing exacerbations and hospitalizations as compared with placebo with increased potential to withhold inhaled corticosteroids in patients on anti-IgE treatment as compared with placebo [Normansell et al. 2014]. The effects of omalizumab upon eosinophilic inflammation have been studied in a single multicentre study in which omalizumab reduced the sputum, epithelial and sub mucosal eosinophil count [Djukanović et al. 2004] and high blood eosinophils, serum periostin or FeNO prior to treatment initiation predict favourable responses to omalizumab [Hanania et al. 2013].
Therapies in clinical development
There is tremendous interest in targeting eosinophilic/Th2-mediated inflammation and this has led to the development of both biological and small molecule therapies. Clinical trials in this space published since 2010 are summarized in Table 1.
Table 1.
Phase II and III parallel double-blind placebo-controlled randomized controlled trials of anti-Th2 cytokine/chemokine therapies since 2010.
| Duration | Intervention | Subjects | Primary outcomes | Secondary outcomes | |
|---|---|---|---|---|---|
| Corren et al. [2011] | 6 months | Anti-IL-13 Lebrikizumab |
Severe asthma, n = 219 |
↑FEV1% predicted Greater in periostinHigh |
↔ symptoms Trend in ↓exacerbation rate |
| Hanania [2014] | 12 weeks | Anti-IL-13 Lebrikizumab |
Severe asthma n = 463 |
↓ exacerbation rate Greater in periostinHigh |
↑FEV1% predicted Greater in periostinHigh |
| Piper et al. [2013] | 12 weeks | Tralokinumab Anti-IL-13 |
Moderate-to-severe asthma, n = 194 |
↔ ACQ | ↑ FEV1
↓ β2 agonist use |
| Brightling et al. [2014] | 52 weeks | Tralokinumab Anti-IL-13 |
Severe asthma n = 452 |
Trend in ↓exacerbation rate in periostinHigh DPP4High |
↑ FEV1
Improved symptom scores in DPP4High |
| Wenzel et al. [2013] | 12 weeks | Dupilumab Anti IL4Rα |
Moderate to severe asthma, n = 104 |
↓ exacerbation rate | ↑ FEV1
Improved symptom scores |
| Wenzel et al. [2014] | 12 weeks | Dupilumab Anti IL4Rα |
Moderate to severe asthma n = 104 |
↑ ACQ | - |
| Pavord et al. [2012] | 12 months | Mepolizumab Anti-IL-5 |
Severe asthma, n = 621 |
↓ exacerbation rate ↓ blood, sputum eosinophils |
Small ↓ FEV1
Small ↑ symptom scores |
| Bel et al. [2014] | 20 weeks | Mepolizumab Anti-IL-5 |
Severe asthma, n = 135 |
↓ in glucocorticoid daily dose |
↓ exacerbation rate |
| Ortega et al. [2014] | 32 weeks | Mepolizumab Anti-IL-5 |
Severe asthma, n = 576 |
↓ exacerbation rate | ↑ FEV1
Improved symptom scores |
| Castro et al. [2014] | 12 months | Benralizumab Anti-IL-5Rα |
Moderate to severe asthma n = 609 |
↓ exacerbation rate. | ↑ FEV1
Improved ACQ |
| Brightling et al. [2014] | 48 weeks | Benralizumab Anti-IL-5Rα |
Moderate to severe COPD n = 101 |
↔ exacerbation rate | ↑ FEV1
Trend in ↓ exacerbation rate in ↑ blood and sputum eosinophil group |
| Castro et al. [2011] | 12 weeks | Reslizumab Anti-IL5 |
Moderate to severe asthma n = 106 |
Improved ACQ | ↓ blood, sputum eosinophils Trend in ↓ exacerbation rate |
| Castro et al. [2015] | 12 months | Reslizumab Anti-IL5 |
Moderate to severe asthma n = 953 |
↓ exacerbation rate | ↑ FEV1
Improved symptom scores |
| Corren et al. [2014] | 16 weeks | Reslizumab Anti-IL5 |
Moderate to severe asthma, n = 492 |
↑ FEV1 greater in patients with blood eosinophil > 400/µl | ↑ ACQ and ↓ rescue medication use in patients with blood eosinophils > 400/µl |
| Barnes et al. [2012] | 28 days |
OC000459 CRTh2 antagonist |
Moderate asthma n = 107 |
↑ FEV1
Improved symptom scores |
Improved AQLQ score |
| Gonem et al. [2014] | 12 weeks | QAW039 Dp2/CRTh2 Antagonist |
Severe asthma n = 61 |
↓ in sputum eosinophils | Improved AQLQ score ↑ post bronchodilator FEV1 |
| Molfino et al. [2013] | 6 weeks | KB002 Anti-GM-CSF |
Severe asthma n = 24 |
↑ FEV1
↓ in sputum eosinophils |
↔ symptom scores |
| Wenzel et al. [2014] | 4 weeks | ARRY 502 DP2 antagonist |
Mild Asthma n = 184 |
↑ FEV1 | ↑ ACQ ↑ symptom free days |
| Hall et al. [2012] | Six weeks | BI-671800 DP2 antagonist |
Asthma n = 243 |
↑ FEV1 | ↑ ACQ |
| Sutherland et al. [2012] | 4 weeks | BI-671800 DP2 antagonist |
Asthma n = 388 |
↔ FEV1 | ↓ ACQ |
| Krug et al. [2012] | 2 weeks | BI-671800 DP2 antagonist |
Allergic rhinitis n = 146 |
↓ nasal symptoms, ↓ nasal eosinophils, ↓ eosinophil activation | ↓ IL-4 ↓ eotaxin levels |
| Fitzgerald et al. [2013] | 14–28 days | ADC-3680 DP2 antagonist |
Asthma (n = 24) Healthy (n = 88) |
Blocked PGD2 mediated eosinophil activation by >90% | - |
| Pettipher et al. 2014 | 12 weeks |
OC000459 DP2 antagonist |
Asthma n = 482 |
↑ FEV1 and ↑ PEF in eosinophilic subgroup | ↑ AQLQ ↑ ACQ ↓ Exacerbation rate |
| Busse et al. [2013] | 12 weeks | AMG 853 D2 antagonist |
Asthma n = 79 |
↔ ACQ | ↔ FEV1 ↔ symptom scores ↔ exacerbation |
| KaloBios [2014] | 24 weeks | KB003 Anti-GM-CSF |
Severe Asthma n = 160 |
↔ FEV1
↑FEV1 in eosinophilic subgroup |
↔ ACQ ↔ exacerbations |
| Gaureauv et al. [2011] | 9 weeks | TPI ASM8 Anti-IL-3,-4,GM-CSF, CCR3 |
Mild –Moderate Asthma n = 14 |
↓ sputum eosinophil count ↓sputum ECP |
Safe at all doses tested |
| Greiff et al. [2010] | 10 weeks | AZD3778 CCR3 antagonist |
Seasonal allergic rhinitis n = 38 | ↓ eosinophil | ↓ allergic rhinitis symptoms |
| Neighbour et al. [2014] | 22 days | GW76694 CCR3 antagonist |
Asthma n = 60 |
↔ eosinophils↔ eosinophil progenitor cell count | ↔ FEV1 ↑ airway hyper responsiveness ↑ ACQ |
Abbreviations: AQLQ, asthma quality of life questionnaire; ACQ, asthma control questionnaire; COPD, chronic obstructive pulmonary disease; ECP, eosinophil cationic protein; FEV1, forced expiratory volume in 1 second; GM-CSF, granulocyte-monocyte colony stimulating factor; IL, interleukin.
Anti-IL-5/anti-IL-5R
As described above, IL-5 has a pivotal role in the differentiation and maturation of eosinophils in bone marrow and survival in tissue. Monoclonal antibody therapies are in late phase clinical development neutralising IL-5 or blocking IL-5Rα.
Mepolizumab is a humanized monoclonal antibody against free IL-5 [Desai and Brightling, 2009; Haldar et al. 2009]. Earlier trials confirmed that anti-IL-5 monoclonal antibody therapy attenuated markedly blood, sputum and, to a lesser extent, bronchial mucosal eosinophils, but failed to demonstrate significant benefits in clinical endpoints [Flood-Page et al. 2007; Leckie et al. 2000]. Although the clinical outcomes from these studies were disappointing together they suggested a possible effect upon exacerbation frequency. This gave encouragement to undertake a phase IIa study in severe asthmatics with evidence of eosinophilic inflammation and frequent exacerbations, which demonstrated a reduction in exacerbation frequency together with reductions in eosinophilic inflammation [Haldar et al. 2009; Pavord et al. 2012]. This observation was replicated in phase IIb and III studies confirming that mepolizumab reduced severe asthma exacerbations, together with benefits in health status, a small increase in lung function as well as a safe glucocorticosteroids sparing effects [Bel et al. 2014; Ortega et al. 2014]. Benefits were associated with the blood eosinophil count and previous exacerbation frequency. Following cessation of mepolizumab therapy benefits are lost within 6 months after treatment withdrawal [Haldar et al. 2014].
Reslizumab is another humanized monoclonal antibody, which neutralises circulating IL-5, showed in phase II studies improvements in lung function and a decrease in both sputum and blood eosinophil counts [Castro et al. 2011]. Improvements in asthma control were associated with high baseline blood eosinophil counts. Two duplicate phase III trials confirmed reduction in asthma exacerbations; improvements in health status and asthma control [Castro et al. 2015].
Benralizumab is a human afucosylated monoclonal antibody that targets IL-5Rα expressed by eosinophils and basophils and following receptor blockade initiates antibody-dependent cell-mediated cytotoxicity [Ghazi et al. 2012]. A small phase I double-blind placebo-controlled trial showed that benralizumab decreased airway mucosal and sputum eosinophils in addition to a completely suppressing blood and bone marrow eosinophils and their precursors when compared with placebo [Laviolette et al. 2013]. Benralizumab showed a significant reduction in exacerbation rates, lung function and asthma control in severe asthmatics with high baseline blood eosinophil count [Castro et al. 2014]. Although targeting the IL-5Rα is likely to have more profound effects upon eosinophilic inflammation than IL-5 neutralization whether this translates into differential clinical effects remains unclear.
In the first study of a biologic therapy in COPD, benralizumab improved lung function, but did not decrease exacerbation rates, although there was a trend to reduction in exacerbations and improvement in health status in those COPD subjects with higher baseline blood and sputum eosinophil counts [Brightling et al. 2014]. These encouraging findings have supported progression to phase III studies.
Anti-IL-13 and IL-4Rα
The Th2-derived cytokines IL-4 and IL-13 have multiple effects upon inflammatory and structural cells. IL-4 and IL-13 activate epithelial cells to release important eosinophil chemoattractants, promote IgE class switching and IL-13 has direct effects upon airway smooth muscle augmenting hyper-contractility. IL-13 binds with IL-13Rα1 with a low affinity and then with IL-4Rα to form a high affinity cytokine binding heterodimer. [Brightling et al. 2010]. Therefore monoclonal antibody therapies directed towards IL-4Rα inhibit the function of IL-4 in concert with IL-13 via IL-13RI. However, IL-13 neutralization has no effect upon IL-4, but inhibits the activation of IL-13RI and exerts effects upon IL-13RII which are antibody dependent. Inhibition of IL-13RII activation occurs with tralokinumab but not lebrikizumab [Ultsch et al. 2013]. Increased IL-13 expression is a consistent feature of asthma in peripheral blood, sputum, BAL and bronchial biopsies. IL-13 concentrations in sputum have been seen to be related to asthma control [Saha et al. 2008].
Phase II trials have shown consistent positive results for anti-IL-13 therapy [Corren et al. 2011; Hanania, 2014; Piper et al. 2013] with improvement in lung function. This effect was more pronounced in patients who had higher levels of pretreatment periostin, dipepityl-pepitidase-4 or evidence of increased sputum IL-13 [She et al. 2014]. Similarly in those with evidence of activation of the IL-13 pathway had lower exacerbation rates in response to anti-IL13 therapy.
Anti-IL4Rα, dupilumab, also improves lung function and asthma control and in an inhaled corticosteroid withdrawal study there was significant reduction in the exacerbation rates in those subjects that received dupilumab versus placebo [Teper et al. 2014; Wenzel et al. 2013].
In the anti-IL-13 and anti-IL-4Rα trials the blood eosinophil count increased possibly due to its indirect effects upon generation of eosinophil chemokines or directly due to the effect of IL-13 upon eosinophil endothelial adhesion as described above. Interestingly whether anti-IL-4/13 approaches reduce sputum or bronchial mucosal eosinophilia is unknown.
Anti-CRTh2
Prostaglandin D2 (PGD2) is a product of arachidonic acid metabolism and is a major prostanoid found within the airways of asthmatics immediately following an airway challenge. PGD2 is known to have chemokinetic and chemotactic effects on eosinophils and Th2 lymphocytes acting via DP2/CRTh2 receptor [Hirai et al. 2001; Nagata et al. 1999]. This interaction plays a key role in airway hyper-responsiveness, IgE and cytokine production [Pettipher and Whittaker, 2012]. An in vitro study using a potent CRTH2 antagonist was the first to show decrease in PGD2-mediated human eosinophil migration [Sugimoto et al. 2003]. Since this discovery a variety of DP2/CRTH2 antagonists including ARRY 502, BI-671800, QAW-039, OC000459 have been studied in phase II trials. BI-671800 showed better improvement in FEV1 when compared with montelukast [Hall et al. 2012], but did not show much difference in effects on FEV1 when compared with an inhaled steroid treatment. Phase II trials investigating the DP2 antagonists OC000459 and QAW 039 DP2, respectively, showed a reduction in sputum eosinophil counts and improved symptoms in mild [Barnes et al. 2012] and severe asthmatics [Gonem et al. 2014]. These effects were also demonstrated with BI-671800 in seasonal allergic rhinitis [Krug et al. 2012]. A more recent phase II trial continued to show benefits of oral CRTH2 treatment on FEV1 and symptom scores [Wenzel et al. 2014].
Anti-GM-CSF
GM-CSF causes the differentiation proliferation and survival of monocytes, macrophages and granulocytes including eosinophils and neutrophils. The development of the anti-GM-CSF IgG1 antibody MT203 showed decrease in eosinophil activation and survival [Krinner et al. 2007]. The safety and efficacy of other anti-GMCSF therapies are in early clinical development and to date whether it impacts upon eosinophilic inflammation is uncertain. Phase I and II trials using anti-GM-CSF treatments KB002 [Bardin et al. 2013] and KB003 [KaloBios, 2014] have been done in the hope of finding a treatment to target atopic and non-atopic asthma. These anti-GM-CSF therapies have shown no improvements compared to placebo in overall study populations, but a statistically significant improvement in FEV1 and decrease in sputum eosinophil count was demonstrated in the eosinophilic subgroups (blood eosinophil ⩾300 cells/µl).There was no effect on symptom scores or exacerbations. Whether there will be further development and phase III trials of these agents to establish their effects is uncertain at present.
Anti-CCR3
The CCR3 chemokine receptor is expressed on the surface of eosinophils and is activated by chemokines Eotaxin 1 (CCL11)/Eotaxin 2 (CCL 24) and Eotaxin 3(CCL26). Eotaxin 1(CCL11) has been shown to be actively involved in eosinophil recruitment and in survival along with IL-5. Airway epithelial cells and airway smooth muscle cells express CCR3 and also produce cytokines and fibroblast growth factor. Recent studies using CCR3 antagonists including GSK766994, GSK 766904,GW824575, DPC 168 and QAP 642 [Pease and Horuk, 2014] in asthma or in allergic rhinitis have not demonstrated significant effects upon eosinophilic airway inflammation and have questioned the importance of CCR3 in eosinophil recruitment versus other mechanisms such as CRTh2 [Neighbour et al. 2014].
Conclusion
Current treatments and the emergence of new therapies targeting eosinophilic inflammation have informed our understanding of the relationship between eosinophilic inflammation and different characteristics of asthma and COPD. Identification of eosinophilic and Th2-meduiated inflammation predicts a good response to corticosteroid, anti-IgE, anti-IL5, anti-IL5Rα, and anti-13 therapies. Eosinophilic inflammation has a clear role in asthma exacerbations and its inhibition by anti-IL5 improves lung function, symptoms and health status related to the intensity of eosinophilic inflammation and possibly with greater effects upon lung function following IL-5Rα blockade rather than IL-5 neutralization. In COPD, anti-IL-5 improved lung function in eosinophilic COPD, but the role of eosinophils in COPD exacerbations remains uncertain. Anti-IL-13 has consistent effects upon lung function in asthma, but the benefits in exacerbations have to date been less marked than with anti-IL-5 and current late phase studies in selected populations will be critical in determining the role of IL-13 in asthma exacerbations. CCR3 antagonism has been disappointing whereas anti-CRTh2 is emerging as a potentially important target that impacts inflammation, lung function and symptoms with effects upon exacerbations to be determined. The potential role of therapies targeting IL-4/13 and CRTh2 in eosinophilic COPD is unknown.
In the forthcoming 5 years the mechanistic role of the eosinophil in asthma and COPD, the proportion of the asthma and COPD patients in whom the eosinophil is critical and the magnitude of clinical efficacy of anti-eosinophil therapies will become clear. During this time attention will need to focus on the aspects of disease that are independent of eosinophilic inflammation and in those where the eosinophil plays a key role then there is a need to understand the relative merits of each therapy in an individual and how can we apply biomarkers to predict long term outcomes. Addressing this challenge will both further inform our understanding of the role of the eosinophil in asthma and COPD and sharpen our focus on the need for precision medicine.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interest: CEB has received grant and consultancy funds via his Institution from AstraZeneca/MedImmune, GSK, Roche/Genentech, Novartis, Chiesi and Boehringer-Ingelheim.
Contributor Information
Leena George, Institute for Lung Health, NIHR Respiratory Biomedical Research Unit, Department of Infection, Immunity and Inflammation, University of Leicester and University Hospitals of Leicester NHS Trust, Leicester, UK.
Christopher E. Brightling, Institute for Lung Health, Clinical Science Wing, University Hospital of Leicester, Leicester LE3 9QP, UK.
References
- Amelink M., Nijs S., Groot J., Tilburg P., Spiegel P., Krouwels F., et al. (2013) Three phenotypes of adult-onset asthma. Allergy 68: 674–680. [DOI] [PubMed] [Google Scholar]
- Ayars G., Altman L., Gleich G., Loegering D., Baker C. (1985) Eosinophil-and eosinophil granule-mediated pneumocyte injury. J Allergy Clin Immunol 76: 595–604. [DOI] [PubMed] [Google Scholar]
- Bafadhel M., McKenna S., Terry S., Mistry V., Pancholi M., Venge P., et al. (2012) Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 186: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafadhel M., McKenna S., Terry S., Mistry V., Reid C., Haldar P., et al. (2011) Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 184: 662–671. [DOI] [PubMed] [Google Scholar]
- Bardin P., Thompson P., Luckey A., Yarranton G., Molfino N. (2013) A randomized placebo-controlled safety and pharmacodynamic study of KB002, a chimeric antiGMCSF monoclonal antibody, in patients with asthma. Am J Respir Crit Care Med 187: A3867. [Google Scholar]
- Barnes P. (2006) Theophylline for COPD. Thorax 61: 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P., Adcock I., Ito K. (2005) Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J 25: 552–563. [DOI] [PubMed] [Google Scholar]
- Barnes N., Laviolette M., Allen D., Flood-Page P., Hargreave F., Corris P., et al. (2007) Effects of montelukast compared to double dose budesonide on airway inflammation and asthma control. Respir Med 101: 1652–1658. [DOI] [PubMed] [Google Scholar]
- Barnes N., Pavord I., Chuchalin A., Bell J., Hunter M., Lewis T., et al. (2012) A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy 42: 38–48. [DOI] [PubMed] [Google Scholar]
- Bel E., Ortega H., Pavord I. (2014) Glucocorticoids and mepolizumab in eosinophilic asthma. N Engl J Med 371: 2434. [DOI] [PubMed] [Google Scholar]
- Berry M., Shaw D., Green R., Brightling C., Wardlaw A., Pavord I. (2005) The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy 35: 1175–1179. [DOI] [PubMed] [Google Scholar]
- Boorsma M., Lutter R., van de Pol M., Out T., Jansen H., Jonkers R. (2007) Repeatability of inflammatory parameters in induced sputum of COPD patients. J Chron Obstruct Pulmon Dis 4: 321–329. [DOI] [PubMed] [Google Scholar]
- Brightling C. (2013) Biomarkers that predict and guide therapy for exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 10: S214–S219. [DOI] [PubMed] [Google Scholar]
- Brightling C., Bleecker E., Panettieri R., Bafadhel M., She D., Ward C., et al. (2014) Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med 2: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightling C., Bradding P., Symon F., Holgate S., Wardlaw A., Pavord I. (2002) Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 346: 1699–1705. [DOI] [PubMed] [Google Scholar]
- Brightling C., McKenna S., Hargadon B., Birring S., Green R., Siva R., et al. (2005) Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 60: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightling C., Monteiro W., Ward R., Parker D., Morgan M., Wardlaw A., et al. (2000) Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 356: 1480–1485. [DOI] [PubMed] [Google Scholar]
- Brightling C., Saha S., Hollins F. (2010) Interleukin-13: prospects for new treatments. Clin Exp Allergy 40: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightling C., Symon F., Birring S., Bradding P., Wardlaw A., Pavord I. (2003) Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax 58: 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. (1958) Treatment of chronic asthma with prednisolone significance of eosinophils in the sputum. Lancet 272: 1245–1247. [DOI] [PubMed] [Google Scholar]
- Burrowes K., Doel T., Brightling C. (2014) Computational modeling of the obstructive lung diseases asthma and COPD. J Transl Med 12(Suppl. 2): S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse W., Wenzel S., Meltzer E., Kerwin E., Liu M., Zhang N., et al. (2013) Safety and efficacy of prostaglandin D2 receptor antagonist AMG 853 in asthmatic patients. J Allergy Clin Immunol 131: 339–345. [DOI] [PubMed] [Google Scholar]
- Castro M., Mathur S., Hargreave F., Boulet L., Xie F., Young J., et al. (2011) Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 184: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Castro M., Wenzel S., Bleecker E., Pizzichini E., Kuna P., Busse W., et al. (2014) Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2: 879–890. [DOI] [PubMed] [Google Scholar]
- Castro M., Zangrilli J., Wechsler M., Bateman E., Brusselle G., Bardin P., et al. (2015) Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 3: 355–366. [DOI] [PubMed] [Google Scholar]
- Chauhan B., Ducharme F. (2012) Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 5: CD002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corren J., Lemanske R., Jr., Hanania N., Korenblat P., Parsey M., Arron J., et al. (2011) Lebrikizumab treatment in adults with asthma. N Engl J Med 365: 1088–1098. [DOI] [PubMed] [Google Scholar]
- Corren J., Weinstein J., Janka L., O’Brien C., Zangrilli J. (2014) A randomised phase 3 study of reslizumab efficacy in relation to blood eosinophil levels in patients with moderate to severe asthma. Eur Respir J 44(Suppl. 58): 4673. [Google Scholar]
- Davoine F., Lacy P. (2014) Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol 5: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg J. (1998) The origins of basophils and eosinophils in allergic inflammation. J Allergy Clin Immunol 102: S74–S76. [DOI] [PubMed] [Google Scholar]
- Desai D., Brightling C. (2009) Cytokine and anti-cytokine therapy in asthma: ready for the clinic? Clin Exp Immunol 158: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai D., Newby C., Symon F., Haldar P., Shah S., Gupta S., et al. (2013) Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med 188: 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukanović R., Wilson J., Britten K., Wilson S., Walls A., Roche W., et al. (1992) Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis 145: 669–674. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Wilson S., Kraft M., Jarjour N., Steel M., Chung K., et al. (2004) Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 170: 583–593. [DOI] [PubMed] [Google Scholar]
- Douwes J., Gibson P., Pekkanen J., Pearce N. (2002) Non-eosinophilic asthma: importance and possible mechanisms. Thorax 57: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme F., Lasserson T., Cates C. (2011) Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev 5: CD003137. [DOI] [PubMed] [Google Scholar]
- Edwards S., Von Maltzahn R., Naya I., Harrison T. (2010) Budesonide/formoterol for maintenance and reliever therapy of asthma: a meta analysis of randomised controlled trials. Int J Clin Pract 64: 619–627. [DOI] [PubMed] [Google Scholar]
- Eltboli O., Bafadhel M., Hollins F., Wright A., Hargadon B., Kulkarni N., et al. (2014) COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm Med 14: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltboli O., Brightling C. (2013) Eosinophils as diagnostic tools in chronic lung disease. Expert Rev Respir Med 7: 33–42. [DOI] [PubMed] [Google Scholar]
- Eltboli O., Mistry V., Barker B., Brightling C. (2015) Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease. Respirology 20: 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurum S., Leff J., Cochran J., Ackerson L., Szefler S., Martin R., et al. (1991) Lack of benefit of methotrexate in severe, steroid-dependent asthma: a double-blind, placebo-controlled study. Ann Intern Med 114: 353–360. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Snape S., Febbraro S., Banyard A., Patnell C., Ruparelia B., et al. (2013) The safety, PK, PD profile of AD3680, a potent and selective CRTH2 antagonist in healthy volunteers and partly controlled atopic asthmatic subjects. C-23 Novel therapeutic in asthma. ATS Conference Proceedings, Pennsylvania, 21 May 2013. Abstract A3874. [Google Scholar]
- Flood-Page P., Menzies-Gow A., Phipps S., Ying S., Wangoo A., Ludwig M., et al. (2003) Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest 112: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood-Page P., Swenson C., Faiferman I., Matthews J., Williams M., Brannick L., et al. (2007) A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 176: 1062–1071. [DOI] [PubMed] [Google Scholar]
- Ford P., Durham A., Russell R., Gordon F., Adcock I., Barnes P. (2010) Treatment effects of low-dose theophylline combined with an inhaled corticosteroid in COPD. Chest 137: 1338–1344. [DOI] [PubMed] [Google Scholar]
- Frigas E., Motojima S., Gleich G. (1991) The eosinophilic injury to the mucosa of the airways in the pathogenesis of bronchial asthma. Eur Respir J Suppl 13: 123s–135s. [PubMed] [Google Scholar]
- Fukuda T., Fukushima Y., Numao T., Ando N., Arima M., Nakajima H., et al. (1996) Role of interleukin-4 and vascular cell adhesion molecule-1 in selective eosinophil migration into the airways in allergic asthma. Am J Respir Cell Mol Biol 14: 84–94. [DOI] [PubMed] [Google Scholar]
- Fulkerson P., Rothenberg M. (2013) Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov 12: 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau G., Pageau R., Séguin R., Carballo D., Gauthier J., D’Anjou H., et al. (2011)Dose response effects of TPI ASM8 in asthmatic after allergen. Allergy 66: 1242–1248. [DOI] [PubMed] [Google Scholar]
- Ghazi A., Trikha A., Calhoun W. (2012) Benralizumab–a humanized mAb to IL-5α with enhanced antibody-dependent cell-mediated cytotoxicity–a novel approach for the treatment of asthma. Expert Opin Biol Ther 12: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebre M., Bafadhel M., Desai D., Cohen S., Newbold P., Rapley L., et al. (2015) Biological clustering supports both “Dutch” and “British” hypotheses of asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 135: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P., Denburg J., Dolovich J., Ramsdale E., Hargreave F. (1989) Chronic cough: eosinophilic bronchitis without asthma. Lancet 333: 1346–1348. [DOI] [PubMed] [Google Scholar]
- Gibson P., Hargreave F., Girgis-Gabardo A., Morris M., Denburg J., Dolovich J. (1995) Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy 25: 127–132. [DOI] [PubMed] [Google Scholar]
- Gonem S., Berair R., Singapuri A., Hartley R., Laurencin M., Bacher G., et al. (2014) Late-breaking abstract: phase 2a randomized placebo-controlled trial of the oral prostaglandin D2 receptor (DP2/CRTh2) antagonist QAW039 in eosinophilic asthma. Eur Respir J 44(Suppl. 58): 2908. [Google Scholar]
- Green R., Brightling C., McKenna S., Hargadon B., Neale N., Parker D., et al. (2006) Comparison of asthma treatment given in addition to inhaled corticosteroids on airway inflammation and responsiveness. Eur Respir J 27: 1144–1151. [DOI] [PubMed] [Google Scholar]
- Green R., Brightling C., McKenna S., Hargadon B., Parker D., Bradding P., et al. (2002a) Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 360: 1715–1721. [DOI] [PubMed] [Google Scholar]
- Green R., Brightling C., Woltmann G., Parker D., Wardlaw A., Pavord I. (2002b) Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 57: 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiff L., Ahlström-Emanuelsson C., Bahl A., Bengtsson T., Dahlström K., Erjefält J., et al. (2010) Effects of dual CCR3 and H-1 antagnonist on symptoms and eosinophilic inflammation in allergic rhinitis. Respir Res 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar P., Brightling C., Hargadon B., Gupta S., Monteiro W., Sousa A., et al. (2009) Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 360: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar P., Brightling C., Singapuri A., Hargadon B., Gupta S., Monteiro W., et al. (2014) Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol 133: 921. [DOI] [PubMed] [Google Scholar]
- Haldar P., Pavord I., Shaw D., Berry M., Thomas M., Brightling C., et al. (2008) Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 178: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I., Sarno M., Disse B., Atis S., Bateman E., Finnigan H. (2012) Efficacy and safety of BI 671800, an oral CRTH2 antagonist, as add on therapy in poorly controlled asthma patients prescribed an inhaled corticosteroid. Eur Respir J 40(Suppl. 56): abstract 2551. [Google Scholar]
- Hallstrand T., Henderson W., Jr. (2010) An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol 10: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania N. (2014) Efficacy and safety of lebrikizumab in severe uncontrolled asthma: results from the lute and verse phase II randomized, double-blind, placebo-controlled trials. J Allergy Clin Immunol 133: AB402. [Google Scholar]
- Hanania N., Wenzel S., Rosén K., Hsieh H., Mosesova S., Choy D., et al. (2013) Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 187: 804–811. [DOI] [PubMed] [Google Scholar]
- Heitzer M., Wolf I., Sanchez E., Witchel S., DeFranco D. (2007) Glucocorticoid receptor physiology. Rev Endocr Metab Disord 8: 321–330. [DOI] [PubMed] [Google Scholar]
- Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., et al. (2001) Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med 193: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbles A., Lloyd C., McMillan S., Friend D., Xanthou G., McKenna E., et al. (2004) A critical role for eosinophils in allergic airways remodeling. Science 305: 1776–1779. [DOI] [PubMed] [Google Scholar]
- Ito K., Yamamura S., Essilfie-Quaye S., Cosio B., Ito M., Barnes P., et al. (2006) Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 203: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatakanon A., Lim S., Barnes P. (2000) Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med 161: 64–72. [DOI] [PubMed] [Google Scholar]
- Jayaram L., Pizzichini M., Cook R., Boulet L., Lemiere C., Pizzichini E., et al. (2006) Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J 27: 483–494. [DOI] [PubMed] [Google Scholar]
- Jayaram L., Pizzichini E., Lemiere C., Man S., Cartier A., Hargreave F., et al. (2005) Steroid naive eosinophilic asthma: anti-inflammatory effects of fluticasone and montelukast. Thorax 60: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Erickson R., Choy D., Mosesova S., Wu L., Solberg O., et al. (2012) Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 130: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KaloBios (2014) KaloBios Reports Top-Line Data for Phase 2 Study of KB003 in Severe Asthma. Available at: http://ir.kalobios.com/releasedetail.cfm?ReleaseID=821931 (accessed July 2015).
- Kay A. (2014) The early history of the eosinophil. Clin Exp Allergy 45: 575–582. [DOI] [PubMed] [Google Scholar]
- Kay A., Phipps S., Robinson D. (2004) A role for eosinophils in airway remodelling in asthma. Trends Immunol 25: 477–482. [DOI] [PubMed] [Google Scholar]
- Keatings V., Evans D., O’Connor B., Barnes P. (1997) Cellular profiles in asthmatic airways: a comparison of induced sputum, bronchial washings, and bronchoalveolar lavage fluid. Thorax 52: 372–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinner E., Raum T., Petsch S., Bruckmaier S., Schuster I., Petersen L., et al. (2007) A human monoclonal IgG1 potently neutralizing the pro-inflammatory cytokine GM-CSF. Mol Immunol 44: 916–925. [DOI] [PubMed] [Google Scholar]
- Krug N., Gupta A., Badorrek D., Mueller M., Casper A., Pivovarova A., et al. (2012) CRTH2 antagonist, BI 671800 (BI), reduces nasal symptoms and inhibits nasal cytokines and eosinophils in SAR patients exposed to grass pollen in an environmental challenge chamber (ECC). Am J Resp Crit Care Med 185: A4185. [Google Scholar]
- Kulkarni N., Hollins F., Sutcliffe A., Saunders R., Shah S., Siddiqui S., et al. (2010) Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol 126: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette M., Gossage D., Gauvreau G., Leigh R., Olivenstein R., Katial R., et al. (2013) Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 132: 1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie M., Brinke A., Khan J., Diamant Z., O’Connor B., Walls C., et al. (2000) Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356: 2144–2148. [DOI] [PubMed] [Google Scholar]
- Leigh R., Pizzichini M., Morris M., Maltais F., Hargreave F., Pizzichini E. (2006) Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J 27: 964–971. [DOI] [PubMed] [Google Scholar]
- Lewis R., Soter N., Diamond P., Austen K., Oates J., Roberts L., 2nd (1982) Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol 129: 1627–1631. [PubMed] [Google Scholar]
- Lim S., Jatakanon A., Gordon D., Macdonald C., Chung K., Barnes P. (2000) Comparison of high dose inhaled steroids, low dose inhaled steroids plus low dose theophylline, and low dose inhaled steroids alone in chronic asthma in general practice. Thorax 55: 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Tomita K., Carramori G., Jatakanon A., Oliver B., Keller A., et al. (2001) Low-dose theophylline reduces eosinophilic inflammation but not exhaled nitric oxide in mild asthma. Am J Respir Crit Care Med 164: 273–276. [DOI] [PubMed] [Google Scholar]
- Loymans R., Gemperli A., Cohen J., Rubinstein S., Sterk P., Reddel H., et al. (2014) Comparative effectiveness of long term drug treatment strategies to prevent asthma exacerbations: network meta-analysis. BMJ 348: g3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijk B., Lindemans C., Kanters D., van der Heijde R., Bertics P., Lammers J., et al. (2005) Gradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challenge. J Allergy Clin Immunol 115: 997–1003. [DOI] [PubMed] [Google Scholar]
- Maestrelli P., Saetta M., Di Stefano A., Calcagni P., Turato G., Ruggieri M., et al. (1995) Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am J Respir Crit Care Med 152: 1926–1931. [DOI] [PubMed] [Google Scholar]
- McGrath K., Icitovic N., Boushey H., Lazarus S., Sutherland E., Chinchilli V., et al. (2012) A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med 185: 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita-Santos F., Vieira-de-Abreu A., Calheiros A., Figueiredo I., Castro-Faria-Neto H., Weller P., et al. (2006) Cutting edge: prostaglandin D2 enhances leukotriene C4 synthesis by eosinophils during allergic inflammation: synergistic in vivo role of endogenous eotaxin. J Immunol 176: 1326–1330. [DOI] [PubMed] [Google Scholar]
- Molfino N., Bardin P., Thompson P., Lucky A., Yarranton G. (2013) A randomised placebo controlled safety and pharmacodynamic study of KB002, a Chimeric anti GM-CSF monoclonal antibody, in patients with asthma. Am J Respir Crit Care Med 187: A3867. [Google Scholar]
- Moqbel R., Levi-Schaffer F., Kay A. (1994) Cytokine generation by eosinophils. J Allergy Clin Immunol 94: 1183–1188. [DOI] [PubMed] [Google Scholar]
- Mutalithas K., Guillen C., Day C., Brightling C., Pavord I., Wardlaw A. (2010) CRTH2 expression on T cells in asthma. Clin Exp Immunol 161: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Hirai H., Tanaka K., Ogawa K., Aso T., Sugamura K., et al. (1999) CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor (s). FEBS Lett 459: 195–199. [DOI] [PubMed] [Google Scholar]
- Navarro S., Aleu J., Jimenez M., Boix E., Cuchillo C., Nogues M. (2008) The cytotoxicity of eosinophil cationic protein/ribonuclease 3 on eukaryotic cell lines takes place through its aggregation on the cell membrane. Cell Mol Life Sci 65: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbour H., Boulet L., Lemiere C., Sehmi R., Leigh R., Sousa A., et al. (2014) Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin Exp Allergy 44: 508–516. [DOI] [PubMed] [Google Scholar]
- Newby C., Agbetile J., Hargadon B., Monteiro W., Green R., Pavord I., et al. (2014a) Lung function decline and variable airway inflammatory pattern: longitudinal analysis of severe asthma. J Allergy Clin Immunol 134: 287–294. [DOI] [PubMed] [Google Scholar]
- Newby C., Heaney L., Menzies-Gow A., Niven R., Mansur A., Bucknall C., et al. (2014b) Statistical cluster analysis of the British Thoracic Society Severe refractory Asthma Registry: clinical outcomes and phenotype stability. PLoS One 9: e102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normansell R., Walker S., Milan S., Walters E., Nair P. (2014) Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 1: CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega H., Liu M., Pavord I., Brusselle G., FitzGerald J., Chetta A., et al. (2014) Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 371: 1198–1207. [DOI] [PubMed] [Google Scholar]
- Patel K. (1998) Eosinophil tethering to interleukin-4-activated endothelial cells requires both P-selectin and vascular cell adhesion molecule-1. Blood 92: 3904–3911. [PubMed] [Google Scholar]
- Pavord I., Korn S., Howarth P., Bleecker E., Buhl R., Keene O., et al. (2012) Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 380: 651–659. [DOI] [PubMed] [Google Scholar]
- Pavord I., Pizzichini M., Pizzichini E., Hargreave F. (1997) The use of induced sputum to investigate airway inflammation. Thorax 52: 498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord I., Woodcock A., Parker D., Rice L. and SOLTA Study Group. (2007) Salmeterol plus fluticasone propionate versus fluticasone propionate plus montelukast: a randomised controlled trial investigating the effects on airway inflammation in asthma. Respir Res 8: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease J., Horuk R. (2014) Recent progress in the development of antagonists to the chemokine receptors CCR3 and CCR4. Expert Opin Drug Discov 9: 467–483. [DOI] [PubMed] [Google Scholar]
- Pettipher R., Perkins M., Collins L., Baillet M., Lewis T., Steiner J., et al. (2014) The potent & selective CRTH2 antagonist OC000459 is effective in the treatment of eosinophilic asthma when given once daily. J Allergy Clin Immunol 133: AB3. [Google Scholar]
- Pettipher R., Whittaker M. (2012) Update on the development of antagonists of chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2). From lead optimization to clinical proof-of-concept in asthma and allergic rhinitis. J Med Chem 55: 2915–2931. [DOI] [PubMed] [Google Scholar]
- Piper E., Brightling C., Niven R., Oh C., Faggioni R., Poon K., et al. (2013) A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J 41: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzichini E., Leff J., Reiss T., Hendeles L., Boulet L., Wei L., et al. (1999) Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 14: 12–18. [DOI] [PubMed] [Google Scholar]
- Pizzichini E., Pizzichini M., Efthimiadis A., Evans S., Morris M., Squillace D., et al. (1996) Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med 154: 308–317. [DOI] [PubMed] [Google Scholar]
- Pizzichini E., Pizzichini M., Gibson P., Parameswaran K., Gleich G., Berman L., et al. (1998) Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med 158: 1511–1517. [DOI] [PubMed] [Google Scholar]
- Reddel H., Taylor D., Bateman E., Boulet L., Boushey H., Busse W., et al. (2009) An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 180: 59–99. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Phipps S., Foster P. (2007) Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol 119: 1303–1310. [DOI] [PubMed] [Google Scholar]
- Rutgers S., Timens W., Kaufmann H., van der Mark T., Koeter G., Postma D. (2000) Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J 15: 109–115. [DOI] [PubMed] [Google Scholar]
- Saetta M., Di Stefano A., Maestrelli P., Turato G., Ruggieri M., Roggeri A., et al. (1994) Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med 150: 1646–1652. [DOI] [PubMed] [Google Scholar]
- Saha S., Berry M., Parker D., Siddiqui S., Morgan A., May R., et al. (2008) Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol 121: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakula A. (1986) Charcot–Leyden crystals and Curschmann spirals in asthmatic sputum. Thorax 41: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleich F., Manise M., Sele J., Henket M., Seidel L., Louis R. (2013) Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med 13: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Murphy T. (2008) Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359: 2355–2365. [DOI] [PubMed] [Google Scholar]
- She D., Ranade K., Piper E., Brightling C. (2014) Efficacy and safety Of tralokinumab, an anti-Il-13 monoclonal antibody, in a phase 2b study of uncontrolled severe Asthma. Am J Respir Crit Care Med 189: A6670. [Google Scholar]
- Siddiqui S., Mistry V., Doe C., Roach K., Morgan A., Wardlaw A., et al. (2008) Airway hyperresponsiveness is dissociated from airway wall structural remodeling. J Allergy Clin Immunol 122: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Kolsum U., Brightling C., Locantore N., Agusti A., Tal-Singer R., et al. (2014) Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 44: 1697–1700. [DOI] [PubMed] [Google Scholar]
- Siva R., Green R., Brightling C., Shelley M., Hargadon B., McKenna S., et al. (2007) Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 29: 906–913. [DOI] [PubMed] [Google Scholar]
- Smit J., Lukacs N. (2006) A closer look at chemokines and their role in asthmatic responses. Eur J Pharmacol 533: 277–288. [DOI] [PubMed] [Google Scholar]
- Stanescu D., Sanna A., Veriter C., Kostianev S., Calcagni P., Fabbri L., et al. (1996) Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax 51: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian R., Jindal A., Viswambhar V., Babu A. (2015) The study of efficacy, tolerability and safety of theophylline given along with formoterol plus budesonide in COPD. J Clin Diagn Res 9: OC10–OC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H., Shichijo M., Iino T., Manabe Y., Watanabe A., Shimazaki M., et al. (2003) An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J Pharmacol Exp Ther 305: 347–352. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Tetzlaff K., Nivens C., Tsukayama M., Guerreros A., Manuel R., et al. (2012) Efficacy and safety of BI671800, an oral CRTH2 antagnoist in controller naïve patients with poorly controlled asthma. Eur Respir J 40(Suppl. 56): 3084. [Google Scholar]
- Sweeney J., Brightling C., Menzies-Gow A., Niven R., Patterson C., Heaney L., et al. (2012) Clinical management and outcome of refractory asthma in the UK from the British Thoracic Society Difficult Asthma Registry. Thorax 67: 754–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symon F., Lawrence M., Williamson M., Walsh G., Watson S., Wardlaw A. (1996) Functional and structural characterization of the eosinophil P-selectin ligand. J Immunol 157: 1711–1719. [PubMed] [Google Scholar]
- Teper A., Wang L., Pirozzi G., Radin A., Graham N., Castro M., et al. (2014) ACQ5 improvement with dupilumab in patients with persistent asthma and elevated eosinophil levels: responder analysis from a 12-week proof-of-concept placebo-controlled trial. Am J Respir Crit Care Med 189: A1323. [Google Scholar]
- Ultsch M., Bevers J., Nakamura G., Vandlen R., Kelley R., Wu L., et al. (2013) Structural basis of signaling blockade by anti-IL-13 antibody lebrikizumab. J Mol Biol 425: 1330–1339. [DOI] [PubMed] [Google Scholar]
- Wagener A., de Nijs S., Lutter R., Sousa A., Weersink E., Bel E., et al. (2015) External validation of blood eosinophils, FENO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 70: 115–120. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. (1999) Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J Allergy Clin Immunol 104: 917–926. [DOI] [PubMed] [Google Scholar]
- Wardlaw A., Silverman M., Siva R., Pavord I., Green R. (2005) Multi-dimensional phenotyping: towards a new taxonomy for airway disease. Clin Exp Allergy 35: 1254–1262. [DOI] [PubMed] [Google Scholar]
- Wenzel S., Ford L., Pearlman D., Spector S., Sher L., Skobieranda F., et al. (2013) Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 368: 2455–2466. [DOI] [PubMed] [Google Scholar]
- Wenzel S., Hopkins R., Saunders M., Chantry D., Anderson L., Aitchison R., et al. (2014) Safety and efficacy of ARRY-502, a potent, selective, oral CRTh2 antagonist, in patients with mild to moderate Th2-driven asthma. J Allergy Clin Immunol 133: AB4. [Google Scholar]
- Woltmann G., McNulty C., Dewson G., Symon F., Wardlaw A. (2000) Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood 95: 3146–3152. [PubMed] [Google Scholar]
- Woodruff P., Boushey H., Dolganov G., Barker C., Yang Y., Donnelly S., et al. (2007) Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 104: 15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff P., Modrek B., Choy D., Jia G., Abbas A., Ellwanger A., et al. (2009) T-helper type 2–driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 180: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I., Clarke M., Sim E., Fong K. (2012) Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 7: CD002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S., Robinson D., Meng Q., Rottman J., Kennedy R., Ringler D., et al. (1997) Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol 27: 3507–3516. [DOI] [PubMed] [Google Scholar]
- Zietkowski Z., Bodzenta-Lukaszyk A., Tomasiak M., Skiepko R., Szmitkowski M. (2006) Comparison of exhaled nitric oxide measurement with conventional tests in steroid-naive asthma patients. J Investig Allergol Clin Immunol 16: 239–246. [PubMed] [Google Scholar]
- ZuWallack R., Mahler D., Reilly D., Church N., Emmett A., Rickard K., et al. (2001) Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest 119: 1661–1670. [DOI] [PubMed] [Google Scholar]