Abstract
Blocking the activity of IL-1β has entered the clinical arena of treating autoimmune diseases. However, a successful outcome of this approach requires a clear definition of the mechanisms controlling IL-1β release. These are still unclear as IL-1β, lacking a secretory signal peptide, follows a nonclassical pathway of secretion. Here, we analyze the molecular mechanism(s) undergoing IL-1β processing and release in human monocytes and provide a unifying model for the regulated secretion of the cytokine. Our data show that in a first step, pro-caspase-1 and endotoxin-induced pro-IL-1β are targeted in part to specialized secretory lysosomes, where they colocalize with other lysosomal proteins. Externalization of mature IL-1β and caspase-1 together with lysosomal proteins is then facilitated by extracellular ATP. ATP triggers the efflux of K+ from the cell, followed by Ca2+ influx and activation of three phospholipases: phosphatidylcholine-specific phospholipase C and calcium-independent and -dependent phospholipase A2. Whereas calcium-independent phospholipase A2 is involved in processing, phosphatidylcholine-specific phospholipase C and calcium-dependent phospholipase A2 are required for secretion. Dissection of the events that follow ATP triggering allowed to demonstrate that K+ efflux is responsible for phosphatidylcholine-specific phospholipase C induction, which in turn allows the rise in intracellular free calcium concentration required for activation of phospholipase A2. This activation is ultimately responsible for lysosome exocytosis and IL-1β secretion.
Interleukin L-1β (IL-1β) is a powerful proinflammatory cytokine that represents a potential target of therapeutic intervention in inflammatory and autoimmune diseases (1). An entanglement to this approach is the poor definition of the mechanism of IL-1β release. Indeed, IL-1β lacks a secretory signal peptide and is secreted through a pathway that avoids the classical exocytotic route; its release undergoes unprecedented mechanisms of control only partially understood (2).
It is generally accepted that secretion of IL-1β by monocytes occurs in two steps. In a first step, an inflammatory signal, such as lipopolysaccharide (LPS), promotes the synthesis and cytoplasmic accumulation of the inactive precursor (pro-IL-1β). A second signal, exogenous ATP, triggers caspase-1-mediated processing of pro-IL-1β (1-3) and secretion of the mature cytokine (4-9). Exogenous ATP is provided autocrinally/paracrinally by endotoxin-activated monocytes; furthermore, in vivo the signal is amplified by ATP released by cells participating in inflammation, such as platelets (10). ATP engagement of P2X7 purinergic receptors results in K+ efflux and increase in the concentration of cytosolic Ca2+ ([Ca2+]i) (reviewed in ref. 10).
The ensuing events are object of controversies. Some studies suggested that IL-1β release is associated to ATP-induced cell death (11, 12), whereas others ruled out a role for cell lysis in secretion (5, 6, 13). K+ depletion seems crucial for the generation of active caspase-1 (4, 14, 15), possibly through activation of calcium-independent phospholipase A2 (iPLA2) (16): however, the link between caspase-1 activation and IL-1β secretion remains unknown. Discrepancies also exist about the way of release; MacKenzie et al. (9) proposed that ATP promotes shedding of microvesicles packaged with IL-1β; conversely, other reports exclude that ATP-induced blebbing is coupled to IL-1β secretion (17, 18). Even the role of Ca2+ is controversial: whereas we (13, 19) and others (18) have shown that an increase in [Ca2+]i induces IL-1β secretion, Walev et al. (16) suggest that Ca2+ influx inhibits both processing and release.
Thus, several questions remain to be answered. Where and how do IL-1β processing and release occur? Why are only a portion of intracellular pro-caspase-1 and pro-IL-1β processed and secreted after stimulation? Which is the contribution of [Ca2+]i? The present paper clarifies these points and merges some previous discordant data in a unifying model for IL-1β secretion. We started from our observations that IL-1β secretion is mediated by a subset of secretory lysosomes, where the cytokine colocalizes with lysosomal enzymes (6, 19). Secretory lysosomes are Ca2+-regulated organelles, abundant in hemopoietic cells, that deliver their content extracellularly in response to triggering signals. These organelles play a crucial role in most steps of inflammatory and immune responses; deficit of their function is associated to a number of severe immunodeficiency syndromes (20, 21). Here, we show that in human LPS-activated monocytes, secretory lysosomes are the site of ATP-induced IL-1β processing; ATP also triggers exocytosis of these organelles with secretion of IL-1β and caspase-1.
Experimental Procedures
Chemicals. Arachidonyl trifluoromethylketone (AACOCF3) and bromoenol lactone were from Alexis Biochemicals (Lausen, Switzerland); acetyl-Tyr-Val-Ala-Asp-chloromethyl ketone (ac-YVAD-cmk) was from Bachem. All other chemicals were from Sigma-Aldrich.
Cell Cultures. Human monocytes were isolated from buffy coats from healthy donors, enriched by adherence, and activated with 1 μg/mlLPSfor4hin RPMI medium 1640 supplemented with 10% FBS (all from Sigma-Aldrich) as described in ref. 6. Various drugs were added during the incubation, as indicated. Supernatants were then replaced with RPMI medium 1640, NaCl buffer (150 mM NaCl/10 mM Hepes/1 mM MgCl2/1 mM CaCl2/1gof LD-glucose, pH 7.4), or KCl buffer (150 mM KCl/10 mM Hepes/1 mM MgCl2/1 mM CaCl2,/1gof LD-glucose, pH 7.4) (15) supplemented with 1% Nutridoma-HU (Roche Applied Science) in the presence or absence of 1 mM ATP or 2 μM ionomycin or other drugs as indicated, and incubation was carried out for the indicated times. Supernatants were collected, and cells were lysed in 1% Triton X-100 lysis buffer (6, 19).
Subcellular Fractionation by Differential Ultracentrifugation. Subcellular fractionation was carried out as described (6), with slight modifications. Briefly, monocytes were washed, resuspended in homogenizing buffer, and broken in a Dounce homogenizer. The postnuclear supernatants (PNS) were centrifuged at 50,000 × g for 5 min, leading to a pellet enriched in endolysosomes, as confirmed by electron microscopy (data not shown). Supernatants were spun again 40 min at 100,000 × g to obtain soluble cytosol.
Western Blot Analysis. Cell lysates, subcellular fractions, trichloroacetic acid-concentrated supernatants, and cytosolic fractions were boiled in reducing Laemmli sample buffer, resolved on 12% SDS/PAGE, and electrotransferred as described (6). Filters were stained with anti-cathepsin D mAb (IgG2a, Calbiochem), anti-IL-1β mAb (3ZD, IgG1, obtained from the National Cancer Institute Biological Resources Branch), or rabbit anti-caspase-1 Ab (DBA Italia, Segrate, Milan) followed by a horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (DAKO) and developed with ECL-plus (Amersham Biosciences). Densitometric analyses were performed by analyzing at least three different exposures of the same blot (6, 19).
Determination of Cell Lysis. The putative toxic effect of ATP and other drugs on monocytes was evaluated by measuring the release of lactate dehydrogenase (LDH) by using the LDH colorimetric assay from Sigma-Aldrich.
Conventional and Immunoelectron Microscopy. Endolysosomal fractions were processed for postembedding immunocytochemistry as described in ref. 6. Thin sections were double immunolabeled with rabbit anti-caspase-1 antiserum followed by protein-A gold (18 nm) and then alternatively with anti-IL-1β mAb, anti-LAMP mAb H4A3 (Development Studies Hybridoma Bank, Iowa City, IA), or anti-cathepsin-D mAb followed by goat anti-mouse IgG gold conjugates (10 nm, BB International, Cardiff, U.K.). Control experiments were performed by using as primary Ab a rabbit preimmune serum and an unrelated isotype matched (IgG1) mAb (both kindly provided by A. Santoni, University La Sapienza, Rome). All sections were then poststained with uranyl acetate and lead citrate.
Calcium Mobilization Assay. Monocytes adherent on a glass coverslip, activated with 1 μg/ml LPS for 4 h at 37°C, were loaded with the acetoxymethyl-ester of Fura-2 (Fura-2-AM, 1 μM) for 1 h (22), placed under an AXIOVERT 10 microscope (Zeiss) maintained at 37°C, and stimulated with different substances as indicated. Fura-2-AM was excited at 340 and 380 nm, and emitted light was filtered at 510 nm. The fluorescence ratio at 340/380 was evaluated by a charged-coupled device camera (Atto Instruments, Rockville, MD). Results are the mean of fluorescence of at least 50 cells in each experiment monitored for 15 min. [Ca2+]i increase was calculated as described in ref. 22.
Determination of Phospholipase Activity. Phosphatidylcholine-specific phospholipase C (PC-PLC), phospholipase D (PLD), or PLA2 activities was investigated by using the Amplex Red PC-PLC and Amplex Red PLD assay kits by Molecular Probes or the calcium-dependent phospholipase A2 (cPLA2) assay kit by Cayman Chemical (Ann Arbor, MI) according to the manufacturer's instructions. Briefly, 50 μg of cell lysates were used for each sample. In the case of PLA2, a half sample was pretreated with 5 μM bromoenol lactone to inhibit the iPLA2 present. Phospholipase C (PLC) and PLD activity (milliunits/μg) was determined by comparison with the positive controls. PLA2 activity (nmol/μg/min) was calculated as indicated in the kit instructions.
Results
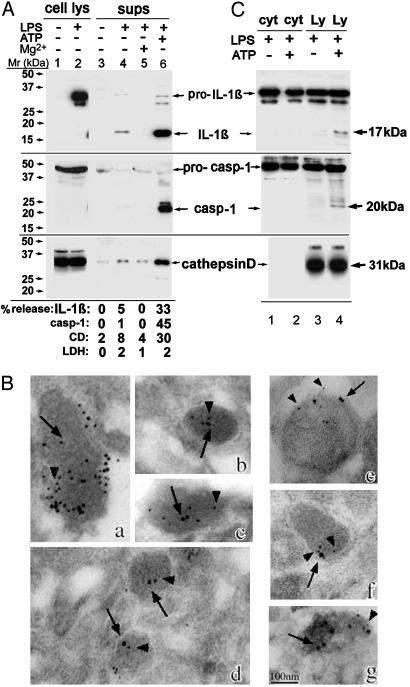
ATP Induces Secretion of IL-1β, Caspase-1, and Lysosomal Cathepsin D. Fig. 1A shows that resting monocytes do not express IL-1β (Top, lane 1), whereas they show elevated levels of intracellular pro-caspase-1 (Middle, 45 kDa, lane 1). LPS stimulation (3 h) triggers pro-IL-1β synthesis, leaving unchanged the levels of pro-caspase-1 (Top and Middle, lane 2). LPS-activated monocytes secrete a low amount of IL-1β (Top, lane 4) that is prevented by inhibitors of exogenous ATP (6, 10) such as Mg2+ (lane 5), oxidized ATP, or KN-62 (data not shown). This indicates that IL-1β secretion by LPS-activated monocytes is induced by autocrinally produced ATP. The autocrine production of ATP in vitro is low, and exposure to higher ATP concentrations (1 mM) strongly increases maturation and secretion of IL-1β (lane 6). ATP-induced IL-1β secretion is paralleled by secretion of the active form of caspase-1 (12), appearing as a 20-kDa band (Middle, lane 6) and of the lysosomal hydrolase cathepsin D (Bottom, lane 6). The percent of secretion of the three proteins ranged from 30% to 45%. In contrast, the percent of release of the cytosolic marker LDH was 2% in the experiment shown (Fig. 1 A Bottom) and never exceeded 7%, clearly indicating that ATP triggers secretion in the absence of cell lysis.
Fig. 1.
ATP induces lysosome exocytosis. (A) Monocytes untreated (lanes 1 and 3) or treated for 3 h with LPS (lanes 2, 4, and 6) or LPS plus 10 mM MgCl2 (lane 5) were incubated for 20 min without (lanes 1-5) or with (lane 6) 1 mM ATP. Cell lysates (lanes 1 and 2) and supernatants (lanes 3-6) were analyzed by Western blotting for the presence of IL-1β (Top), caspase-1 (Middle), and cathepsin D (Bottom). Mr markers are shown on the left. The percent of release of the three proteins and of LDH is stated at the bottom. Values are expressed as percentage of total protein released after Triton X-100 lysis of untreated cells. (B) Immunoelectron microscopy analysis of caspase-1, IL-1β, lamp-1, and cathepsin D in lysosomal-enriched fractions (see Experimental Procedures). Double immunolabeling with anti-caspase-1 Ab (18-nm gold particles, arrows) and anti-IL-1β mAb (a-d) or anti-Lamp-1 (e and f) or anti-cathepsin D (g) (all 10-nm gold particles, arrowheads). (Bar, 100 nm.) (C) Western blotting as in A of an aliquot (1/10) of cytosolic fraction (cyt, lanes 1 and 2) and the whole endolysosomal-enriched fractions (Ly, lanes 3 and 4) from LPS-activated monocytes untreated (lanes 1 and 3) or treated for 20 min with 1 mM ATP (lanes 2 and 4). Arrows point to the mature forms of IL-1β and caspase-1 detected in lysosomes from ATP-treated monocytes only. A representative experiment of the three performed is shown.
We then investigated whether, like pro-IL-1β (6, 19), procaspase-1 also is contained in secretory lysosomes. Immunoelectron microscopy analysis (Fig. 1B) of an endolysosome-enriched fraction from LPS-activated monocytes (6) revealed the presence of caspase-1 (large gold, arrows, panels a-g) and its colocalization with IL-1β (small gold, arrowheads, panels a-d) and lysosomal markers (lamp-1, panels e and f; and cathepsin D, panel g, small gold, arrowheads). Western blot analysis of this fraction and of soluble cytosol showed that caspase-1 and IL-1β are present both in the cytosol and in endolysosomes in their precursor forms (45 kDa and 35 kDa, respectively, Fig. 1C). Interestingly, after 20 min of exposure to ATP, a processed band of both proteins (20-kDa caspase-1 and 17-kDa IL-1β) appears in the endolysosomal fraction (Fig. 1C, lane 4) but not in the cytosol (Fig. 1C, lane 2), suggesting that endolysosomes are the site of processing. As a control, cathepsin D (Lower) is entirely contained in lysosomes.
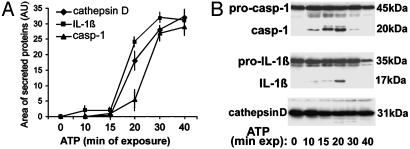
Kinetics studies show that ATP-induced secretion is similar for IL-1β, caspase-1, and cathepsin D (Fig. 2A), which appear in the medium at 20 min from ATP addition and reach the plateau at 40 min. Intracellular conversion of pro-caspase-1 to caspase-1 starts to be detected after 10 min from ATP stimulation and increases until 20 min (Fig. 2B Top). In keeping with the caspase-1 dependency of pro-IL-1β processing, the appearance of intracellular 17-kDa IL-1β is slightly delayed (Fig. 2B Middle). Both intracellular mature caspase-1 and IL-1β decrease at longer times, when secretion is higher. As a control, intracellular cathepsin D (Fig. 2B Bottom) is also present and decreases slightly with ATP stimulation.
Fig. 2.
Kinetics of IL-1β, caspase-1, and cathepsin D secretion. LPS-activated monocytes were exposed to 1 mM ATP for various times. Supernatants (A) and cell lysates (B) were analyzed by Western blotting for the presence of the three proteins. (A) Densitometric analyses of mature IL-1β, caspase-1, and cathepsin D secreted at the different times. Results are expressed as average ± SE of a single experiment performed in triplicate. One experiment of five performed is shown.
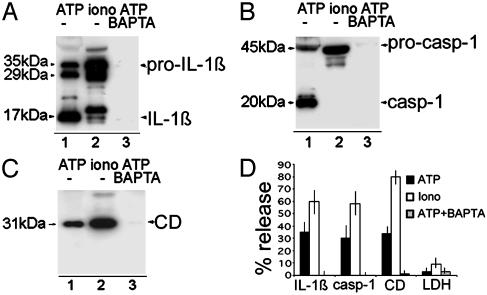
IL-1β Processing and Secretion Are Independent Events. Processing has been proposed to depend on the K+ loss resulting from P2X7 receptor stimulation (4, 10, 15, 16); lysosome exocytosis is a Ca2+-dependent process (20, 21). To investigate the role of K+ and Ca2+ ions in IL-1β processing and secretion, we compared the effects of ATP (which decreases K+ and increases Ca2+ (10) with that of the calcium ionophore ionomycin (which induces a Ca2+ influx without affecting intracellular K+). Fig. 3 shows that whereas ATP stimulation (lane 1) triggers secretion of mature IL-1β (A) and caspase-1 (B), the Ca2+ influx induced by ionomycin (Fig. 3 A and B, lane 2) results in secretion of pro-caspase-1 and of a number of molecular forms of IL-1β, not one of which derives from caspase-1 processing, because they are not prevented by caspase-1 inhibitors (data not shown and ref. 23). This finding suggests that IL-1β processing and secretion, even if associated, are autonomous events. As expected, both ATP and ionomycin induce the release of cathepsin D (Fig. 3C), although at different extents. Intracellular calcium chelation by 1,2-bis(2-aminophenoxy)ethane-tetra-acetic acid (Fig. 3 A-C, lane 3) results in block of ATP-induced secretion, supporting the hypothesis that ATP-triggered exocytosis is calcium-dependent. Ionomycin is a stronger inducer of secretion than ATP (Fig. 3D); the percent of release of LDH following both treatments is very low (<10%), ruling out a role for cell lysis in ATP or ionomycin-induced secretion.
Fig. 3.
Lysosome exocytosis is calcium-dependent. LPS-activated monocytes were exposed to 1 mM ATP (lane 1) or 2 μM ionomycin (lane 2) for 20 min or preexposed to 50 μM 1,2-bis(2-aminophenoxy)ethane-tetra-acetic acid (BAPTA) for 1 h and then to ATP for 20 min (lane 3). Supernatants were analyzed for the presence of IL-1β (A), caspase-1 (B), and cathepsin D (CD) (C) by Western blot. One experiment of five performed is shown. (D) Percent of release of pro- and mature IL-1β and caspase-1, cathepsin D, and LDH ± SD.
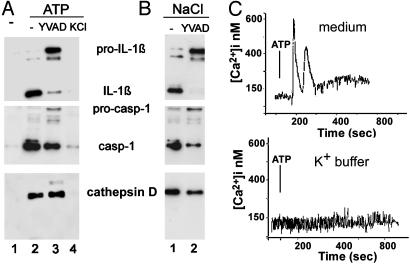
Blocking Caspase-1 Activity with Caspase-1 Inhibitors or by Preventing K+ Efflux Has Different Effects on Exocytosis. To further analyze the relationship between IL-1β maturation and release, we tested whether inhibition of caspase-1-mediated processing has any effects on secretion. We exploited two treatments to block IL-1β processing in monocyte cultures: first, the specific caspase-1 inhibitor ac-YVAD-cmk (24), and second, a high extracellular concentration of K+ that blocks caspase-1 activation by preventing K+ efflux (15). Fig. 4A shows that ac-YVAD-cmk prevents the ATP-induced IL-1β maturation, but not lysosome exocytosis, as shown by the detection of secreted pro-IL-1β, caspase-1, and cathepsin D (lane 3). In contrast, hampering K+ efflux hinders both processing and secretion; under conditions of high extra-cellular K+, the ATP-induced externalization of pro-IL-1β, pro-caspase-1, and cathepsin D is completely blocked (Fig. 4A, lane 4). K+ efflux is also responsible for the ATP-induced [Ca2+]i increase, as high extracellular K+ (Fig. 4C Lower) prevents the Ca2+ rise triggered by ATP (Upper). Together, these data suggest that intracellular K+ depletion not only activates pro-IL-1β processing but also plays a role in exocytosis. In keeping with this, monocytes incubated in K+-free Na+ buffer, which promotes K+ exit from the cell (15, 16), undergo lysosome exocytosis even without ATP stimulation (Fig. 4B) with release of precursor or mature forms of IL-1β and caspase-1, and of cathepsin D either in the absence (lane 1) or presence (lane 2) of ac-YVAD-cmk.
Fig. 4.
K+ regulates both IL-1β processing and lysosome exocytosis. (A) LPS-activated monocytes were exposed for 20 min to medium (lane 1) or to 1 mM ATP (lanes 2-4) in the absence (lanes 2) or in the presence of ac-YVAD-cmk (lanes 3) or of K+ buffer (KCl, lane 4). At the end of incubation, supernatants were collected and analyzed for the presence of IL-1β (Top), caspase-1 (Middle), and cathepsin D (Bottom) by Western blot. (B) Monocytes activated with LPS as in A were incubated for 30 min in Na+ buffer without ATP in the absence (lane 1) or presence (lane 2) of ac-YVAD-cmk. Supernatants were processed as in A. One representative experiment of three is shown. (C) LPS-activated monocytes were incubated in RPMI medium (Upper) or K+ buffer (Lower) supplemented with 1% FBS and challenged with 1 mM ATP (arrow), and Ca2+ waves were monitored. Results are expressed as [Ca2+]i and represent the mean of calcium response of 15 different cells.
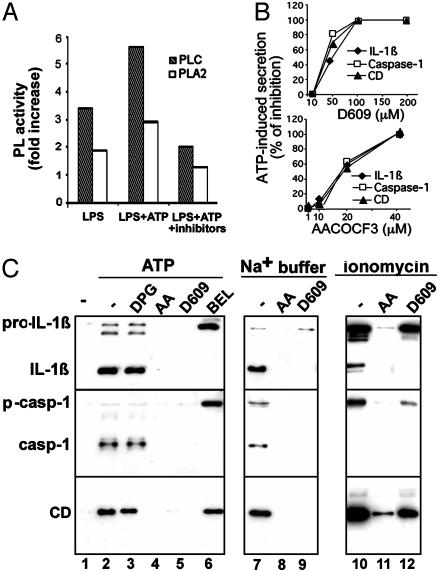
Lysosome Exocytosis Requires PC-PLC and cPLA2 Activity. We then investigated whether phospholipases, which play a crucial role in membrane fusion events (25-27), are involved in lysosome exocytosis. Only a low PLD activity was detected in resting monocytes and did not increase significantly after activation (data not shown). Differently, LPS stimulation increases PC-PLC and, at a lesser extent, PLA2 activity (Fig. 5A). Two minutes of ATP treatment further enhance LPS-induced PC-PLC and PLA2 activity; this increase, however, is prevented if cells are incubated with D609 or AACOCF3, which inhibit PC-PLC and PLA2, respectively (28-30).
Fig. 5.
PC-PLC and PLA2 are involved in ATP-induced lysosome exocytosis. (A) Monocytes activated 3 h with LPS alone (LPS), or followed by 2 min with 2 mM ATP, without (LPS+ATP) or with 100 μM D609 or 40 μM AACOCF3 (LPS+ATP+inhibitors), were lysed, and the presence of active PC-PLC or PLA2 was assayed. Data are expressed as fold of increase over the basal activity of PC-PLC or PLA2 in nonstimulated monocytes. Inhibition of PC-PLC activity was 55-65% with 200 and 100 μM D609 and 35-40% with 50 μM in the different experiments; inhibition of PLA2 activity was 45% with 40 μM AACOCF3 and <10% with 20 μM (data not shown). (B) LPS-activated monocytes, preincubated for 1 h in the absence or presence of different amounts of D609 (Upper) or AACOCF3 (Lower), were exposed for 20 min to 1 mM ATP. Supernatants were analyzed for the presence of IL-1β, caspase-1, or cathepsin D by Western blotting and densitometric analyses. Data are expressed as the percentage of inhibition of secretion obtained in the absence of drugs. (C) LPS-activated monocytes, preincubated for 1 h in the absence (-, lanes 1, 2, 7, and 10) or presence of 2,3-diphosphoglycerate (DPG, 10 mM, lane 3), AACOCF3 (AA, 40 μM, lanes 4, 8, and 11), D609 (200 μM, lanes 5, 9, and 12), or bromoenol lactone (BEL, 100 μM, lane 6) were exposed for 30 min to medium (lane 1) or to 1 mM ATP (lanes 2-6) or to Na+ buffer (lanes 7-9) or for 15 min to 2 μM ionomycin (lanes 10-12) in the absence or presence of the same inhibitors. Supernatants were analyzed for the presence of IL-1β (Top), caspase-1 (Middle), and cathepsin D (CD, Bottom) by Western blot. One representative experiment of five is shown.
PLC activity (milliunits/μg) and PLA2 activity (nmol/μg/min) in a representative experiment were, respectively, 0.21 ± 0.01 and 0.16 ± 0.01 in nonactivated monocytes; 0.70 ± 0.03 and 0.30 ± 0.2 after LPS treatment; 1.15 ± 0.002 and 0.44 ± 0.01 after LPS-ATP treatment; and 0.21 ± 1.5 and 0.22 ± 0.018 after LPS-ATP treatment in the presence of D609 or AACOCF3, respectively.
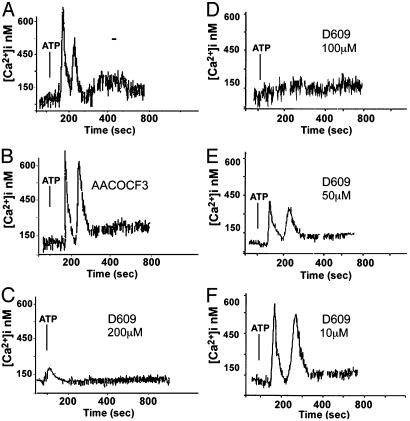
AACOCF3 inhibits different forms of PLA2 (16, 29, 30); to discriminate between iPLA2 and cPLA2, cell lysates were treated with bromoenol lactone, which specifically inhibits iPLA2 (16, 30) before measuring PLA2 activity. Results indicated that iPLA2 is about 6% of total PLA2 in unstimulated monocytes, 2% after LPS, and 13% after LPS-ATP. Specific inhibitors were also exploited to investigate further the relevance of phospholipases in IL-1β secretion. PLD inhibition by 2,3-diphosphoglycerate (31) neither affects ATP-induced IL-1β and caspase-1 processing and secretion nor the release of cathepsin D (Fig. 5C, lane 3). At variance, when monocytes are exposed to D609 or AACOCF3, a dose-dependent inhibition of lysosome exocytosis is observed (Fig. 5B), with no IL-1β, caspase-1, or cathepsin D detectable in supernatants following ATP stimulation (Fig. 5 B and C, lanes 4 and 5). Similar results are obtained when secretion is induced in K+-free Na+ buffer, without ATP (Fig. 5C, lanes 7-9). Treatment with the iPLA2 inhibitor bromoenol lactone (16, 29, 30) results in block of IL-1β processing and of caspase-1 activation in agreement with Walev et al. (16). However, secretion of pro-IL-1β, pro-caspase-1, and cathepsin D is not affected (Fig. 5C, lane 6), indicating that cPLA2 rather than iPLA2 is involved in lysosome exocytosis. Secretion driven by ionomycin is blocked by AACOCF3, but only partially by D609, suggesting that the PC-PLC requirement can be overcome by increasing [Ca2+]i (Fig. 5C, lanes 10-12). To investigate this point, we studied the effects of PLA2 or PC-PLC inhibitors on the [Ca2+]i rise induced by ATP. As shown in Fig. 6, ATP-induced calcium waves (A) are unaffected by AACOCF3 (B) but prevented by D609 (C-F) at the same concentrations that inhibited secretion (see Fig. 5B). Together, these data suggest that PC-PLC activation is required for the [Ca2+]i rise that in turn concurs to cPLA2 activation. This implies that cPLA2 is located downstream PC-PLC in the pathway leading from ATP stimulation to lysosome exocytosis.
Fig. 6.
The PC-PLC inhibitor D609, but not the PLA2 inhibitor AACOCF3, prevents the ATP-induced [Ca2+]i rise. LPS-activated monocytes untreated (A) or pretreated for 30 min with 40 μM AACOCF3 (B)or200 μM(C), 100 μM(D), 50 μM(E), or 10 μM(F) D609 were challenged with 1 mM ATP (arrow). [Ca2+]i was monitored, and results are expressed as in the legend to Fig. 4. One representative experiment of three is shown.
Discussion
In this paper, we have dissected the sequence of events that, starting with ATP stimulation of LPS-activated monocytes, through ion perturbation and phospholipase activation, lead to lysosome exocytosis with release of IL-1β and caspase-1. Moreover, we describe some members of the molecular machinery involved; this will hopefully allow the selection of targets whereby control of IL-1β production may be achieved.
Our data show that a fraction of cytoplasmic pro-IL-1β and pro-caspase-1 colocalize in secretory lysosomes and are secreted together with lysosomal enzymes following exocytosis of these organelles. Processing and exocytosis are triggered by ATP and require [Ca2+]I rise and activation of phospholipases C and A2.
The colocalization of pro-IL-1β and pro-caspase-1 and the appearance of a processed band after ATP stimulation in lysosomes suggest that secretory lysosomes represent the microenvironment where pro-caspase-1 molecules encounter conditions favoring activation, such as a threshold of concentration or adaptor molecules (3, 32), and where active caspase-1 meets its substrate pro-IL-1β, allowing processing to occur. Secretory lysosomes may also represent a storage site for pro-IL-1β and pro-caspase-1, awaiting for a stimulus inducing exocytosis; in the absence of such a stimulus, they may undergo lysosomal degradation. Indeed, we have previously shown that treatment with lysosomal protease inhibitors increases the rate of IL-1β secretion (6). The hypothesis that lysosomes are a privileged site for processing is supported by the observation that the lysosomal enzyme cathepsin B may play a role in pro-caspase-1 activation (33). Along this line, febrile temperatures down-modulate IL-1β activity by inhibiting processing but not secretion (13, 34); it has been proposed that this could be accomplished by influencing cellular compartmentalization so that pro-IL-1β does not encounter caspase-1 (34). It is tempting to speculate that this trafficking implies other molecules involved in the regulation of IL-1β processing, such as inflammasome components (3, 32). Together, the above observations define a function for secretory lysosomes that is secretion of an active proinflammatory cytokine, consistent with the crucial role played by these organelles in immunoinflammatory processes (20, 21). The mechanisms underlying the import of leaderless cytosolic proteins to lysosomes, including recognition, binding, translocation, as well as the putative transporters or chaperones involved, remain to be clarified.
The analysis of ATP-triggered pathway allows to assign a crucial role to ATP-induced K+ efflux, which seems to be necessary and sufficient for triggering exocytosis of caspase-1- and IL-1β-containing secretory lysosomes. Indeed, exocytosis is induced by K+-free medium in the absence of ATP; conversely, ATP-triggered exocytosis is completely prevented if K+ efflux is blocked. The relevance of [K+]i in IL-1β secretion is in agreement with previous data, indicating that the K+ ionophore nigericin drives IL-1β processing and secretion (4, 14). However, studies with nigericin may be misleading, as this drug increases cell permeability, with release of cytoplasmic proteins (33, 35, 36), making it difficult to discriminate between active secretion and release by cell lysis. In contrast, stimulation with 1 mM ATP or incubation in K+-free medium is as effective as nigericin in inducing secretion, without release of the cytoplasmic marker LDH (Figs. 1 and 3 and data not shown).
Our data also show that ATP, through K+ depletion, induces [Ca2+]i rise and activation of three phospholipases: iPLA2, cPLA2, and PC-PLC.
Activation of iPLA2 by K+ efflux was already shown by Walev et al. (16). They proposed that iPLA2 is required to facilitate passage of caspase-1 to pro-IL-1β and, consequently, pro-IL-1β processing. Again, this could occur in secretory lysosomes, where enzyme and substrate meet and undergo activation and processing. We have confirmed here that iPLA2 is necessary for IL-1β processing; however, it has no effects on secretion. Differently, our data clearly show that both PC-PLC and cPLA2 are required for lysosome exocytosis. Activation of PLA2 by P2X7 agonists is reported in a different system in ref. 29. In contrast, the involvement of K+ homeostasis in mammal PLC activity is an unforeseen observation. However, it is worth recalling that low cellular [K+]i is implicated in phospholipase activation in toxo-plasma-infected cells, where drop in cytoplasmic K+ activates a parasite inositol phosphate-PLC that leads to increased [Ca2+]i, in turn responsible for triggering the egress of the parasite (37).
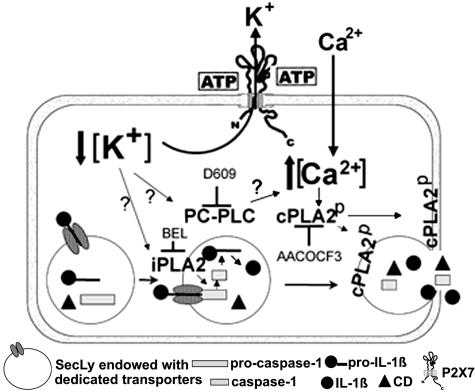
Using specific inhibitors and comparing ATP- and ionomycin-induced exocytosis, we were able to clarify that activation of PC-PLC occurs first, followed by [Ca2+]i rise and by cPLA2 activation. Thus, cPLA2 seems to be ultimately responsible for lysosome exocytosis and externalization of caspase-1 and IL-1β, whereas PC-PLC seems to contribute to IL-1β secretion by allowing the [Ca2+]i elevation needed for cPLA2 activation (38). These steps, leading from ATP stimulation to lysosome exocytosis, are outlined in Fig. 7.
Fig. 7.
A schematic model outlining how ATP-induced PLC, iPLA2, and cPLA2 participate in IL-1β processing and secretion by LPS-activated monocytes. ATP binding causes the opening of P2X7 channels with K+ efflux and intracellular K+ depletion. This leads to activation of iPLA2, in turn responsible for generation of active caspase-1 (16), and of PC-PLC. PC-PLC activation results in [Ca2+]i rise, prevented by the PC-PLC-specific inhibitor D609. Although the underlying mechanism is unclear, PC-PLC involvement in [Ca2+]i increases has been described in other systems (42, 43). Ca2+ rise contributes with mitogen-activated protein kinase-mediated phosphorylation to activation and membrane trans-location of cPLA2 (38). Interestingly, the PC-PLC inhibitor D609 was also shown to inhibit in part mitogen-activated protein kinase activation by the 5-hydroxytryptamine receptor (44). Thus, PC-PLC may have a role also in P2X7 receptor-dependent PLA2 phosphorylation. In any case, PLA2 activation is ultimately responsible for lysosome exocytosis and release of IL-1β, caspase-1, and lysosomal enzymes.
Phospholipases act on membrane phospholipids, generating lipid metabolites that have an essential role in the final stages of membrane fusion (25-27). These enzymes are required in various systems of regulated secretion, including exocytosis in mast cells (39), secretion by rat submandibular gland acini (29), catecholamine release (40), and metalloproteinase externalization (41). Thus, the involvement of phospholipases in IL-1β secretion is consistent with the hypothesis of a vesicular mechanism for the release of this cytokine.
To conclude, the major intracellular ion K+ seems to play unforeseen roles in modulation of different important cell functions, in normal and pathological conditions; in particular, we have shown that all steps leading to IL-1β and caspase-1 externalization by activated monocytes are blocked if [K+]i drop is prevented. This points to a crucial role of K+ homeostasis in the control of active IL-1β secretion.
Acknowledgments
We thank Drs. G. Angelini, C. Semino, and M. R. Zocchi for helpful discussion; Dr. A. Santoni and the National Cancer Institute Biological Resources Branch for the generous gift of Ab; and the Blood Center of Galliera Hospital for providing buffy coats. The mAb H4A3 developed by J. T. August was obtained from the Development Studies Hybridoma Bank. This work was supported in part by grants from Associazione Italiana per la Ricerca sul Cancro, Ministero Salute, and Ministero dell'Istruzione, dell'Università e della Ricerca (Fondo per gli Investimenti della Ricerca di Base). C.A. was supported by a fellowship from the Fondazione Italiana per la Ricerca sul Cancro.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: [Ca2+]i, intracellular free calcium concentration; PLC, phospholipase C; PC-PLC, phosphatidylcholine-specific PLC; PLD, phospholipase D; iPLA2, calcium-independent phospholipase A2; cPLA2, calcium-dependent phospholipase A2; LPS, lipopolysaccharide; AACOCF3, arachidonyl trifluoromethylketone; ac-YVAD-cmk, acetyl-Tyr-Val-Ala-Aspchloromethyl ketone; LDH, lactate dehydrogenase.
References
- 1.Dinarello, C. A. (1998) Ann. N.Y. Acad. Sci. 856, 1-11. [DOI] [PubMed] [Google Scholar]
- 2.Rubartelli, A. & Sitia, R. (1997) in Unusual Secretory Pathways: From Bacteria to Man, eds. Kuchler, K., Rubartelli, A. & Holland, B. I. (RG Landes, Austin, TX), pp. 87-104.
- 3.Burns, K., Martinon, F. & Tschopp, J. (2003) Curr. Opin. Immunol. 15, 26-30. [DOI] [PubMed] [Google Scholar]
- 4.Perregaux, D. & Gabel. C. A. (1994) J. Biol. Chem. 269, 15195-15203. [PubMed] [Google Scholar]
- 5.Ferrari, D., Chiozzi, P., Falzoni, S., Dal Susino, M., Melchiorri, L., Baricordi, O. R. & Di Virgilio, F. (1997) J. Immunol. 159, 1451-1458. [PubMed] [Google Scholar]
- 6.Andrei, C., Dazzi, C., Lotti, L., Torrisi, M. R., Chimini, G. & Rubartelli, A. (1999) Mol. Biol. Cell 10, 1463-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perregaux, D. G., McNiff, P., Laliberte, R., Conklyn, M. & Gabel, C. A. (2000) J. Immunol. 165, 4615-4623. [DOI] [PubMed] [Google Scholar]
- 8.Mehta, V. B., Hart, J. & Wewers, M. D. (2001) J. Biol. Chem. 276, 3820-3826. [DOI] [PubMed] [Google Scholar]
- 9.MacKenzie, A., Wilson, H. L., Kiss-Toth, E., Dower, S. K., North, R. A. & Surprenant, A. (2001) Immunity 15, 825-835. [DOI] [PubMed] [Google Scholar]
- 10.Di Virgilio, F., Chiozzi, P., Ferrari, D., Falzoni, S., Sanz, J. M., Morelli, A., Torboli, M., Bolognesi, G. & Baricordi, O. R. (2001) Blood 97, 587-600. [DOI] [PubMed] [Google Scholar]
- 11.Hogquist, K. A., Unanue, E. R. & Chaplin, D. D. (1991) J. Immunol. 147, 2181-2186. [PubMed] [Google Scholar]
- 12.Laliberte, R. E., Eggler, J. & Gabel, C. A. (1999) J. Biol. Chem. 274, 36944-36951. [DOI] [PubMed] [Google Scholar]
- 13.Rubartelli, A., Cozzolino, F., Talio, M. & Sitia, R. (1990) EMBO J. 9, 1503-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheneval, D., Ramage, P., Kastelic, T., Szelestenyi, T., Niggli, H., Hemmig, R., Bachmann, M. & MacKenzie, A. (1998) J. Biol. Chem. 273, 17846-17851. [DOI] [PubMed] [Google Scholar]
- 15.Walev, I., Reske, K., Palmer, M., Valeva, A. & Bhakdi, S. (1995) EMBO J. 14, 1607-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walev, I., Klein, J., Husmann, M., Valeva, A., Strauch, S., Wirtz, H., Weichel, O. & Bhakdi, S. (2000) J. Immunol. 164, 5120-5124. [DOI] [PubMed] [Google Scholar]
- 17.Verhoef, P.A., Estacion, M., Schilling, W. & Dubyak, G. R. (2003) J. Immunol. 170, 5728-5738. [DOI] [PubMed] [Google Scholar]
- 18.Brough, D., Le Feuvre, R. A., Wheeler, R. D., Solovyova, N., Hilfiker, S., Rothwell, N. J. & Verkhratsky, A. (2003) J. Immunol. 170, 3029-3036. [DOI] [PubMed] [Google Scholar]
- 19.Gardella, S., Andrei, C., Lotti, L. V., Poggi, A., Torrisi, M. R., Zocchi, M. R. & Rubartelli, A. (2001) Blood 98, 2152-2159. [DOI] [PubMed] [Google Scholar]
- 20.Blott, E. J. & Griffiths, G. M. (2002) Nat. Rev. Mol. Cell Biol. 3, 122-131. [DOI] [PubMed] [Google Scholar]
- 21.Andrews, N. W. (2000) Trends Cell Biol. 10, 316-321. [DOI] [PubMed] [Google Scholar]
- 22.Poggi, A., Rubartelli, A. & Zocchi, M. R. (1998) J. Biol. Chem. 273, 7205-7209. [DOI] [PubMed] [Google Scholar]
- 23.Gardella, S., Andrei, C., Costigliolo, S., Olcese, L., Zocchi, M. R. & Rubartelli, A. (2000) Blood 95, 3809-3815. [PubMed] [Google Scholar]
- 24.Thornberry, N. A., Bull, H. G., Calaycay, J. R., Chapman, K. T., Howard, A. D., Kostura, M. J., Miller, D. K., Molineaux, S. M., Weidner, J. R., Aunins, J., et al. (1992) Nature 356, 768-774. [DOI] [PubMed] [Google Scholar]
- 25.Goni, F. M. & Alonso, A. (2000) Biosci. Rep. 20, 443-463. [DOI] [PubMed] [Google Scholar]
- 26.Ramoni, C., Spadaro, F., Menegon, M. & Podo, F. (2001) J. Immunol. 167, 2642-2650. [DOI] [PubMed] [Google Scholar]
- 27.Brown, W. J., Chambers, K. & Doody, A. (2003) Traffic 4, 214-221. [DOI] [PubMed] [Google Scholar]
- 28.Schutze, S., Potthoff, K., Machleidt, T., Berkovic, D., Wiegmann, K. & Kronke, M. (1992) Cell 71, 765-776. [DOI] [PubMed] [Google Scholar]
- 29.Alzola, E., Perez-Etxebarria, A., Kabre, E., Fogarty, D. J., Metioui, M., Chaib, N., Macarulla, J. M., Matute, C., Dehaye, J. P. & Marino, A. (1998) J. Biol. Chem. 273, 30208-30217. [DOI] [PubMed] [Google Scholar]
- 30.Balsinde, J., Balboa, M. A., Insel, P. A. & Dennis, E. A. (1999) Annu. Rev. Pharmacol. Toxicol. 39, 175-189. [DOI] [PubMed] [Google Scholar]
- 31.Kanaho, Y., Nakai, Y., Katoh, M. & Nozawa, Y. (1993) J. Biol. Chem. 268, 12492-12497. [PubMed] [Google Scholar]
- 32.Agostini, L., Martinon, F., Burns, K., McDermott, M. F., Hawkins, P. N. & Tschopp, J. (2004) Immunity 20, 319-325. [DOI] [PubMed] [Google Scholar]
- 33.Hentze, H., Lin, X. Y., Choi, M. S. & Porter, A. G. (2003) Cell Death Differ. 10, 956-968. [DOI] [PubMed] [Google Scholar]
- 34.Boneberg, E. M. & Hartung, T. (2003) J. Immunol. 171, 664-668. [DOI] [PubMed] [Google Scholar]
- 35.Solle, M., Labasi, J., Perregaux, D. G., Stam, E., Petrushova, N., Koller, B. H., Griffiths, R. J. & Gabel, C. A. (2001) J. Biol. Chem. 276, 125-132. [DOI] [PubMed] [Google Scholar]
- 36.Warny, M. & Kelly, C. P. (1999) Am. J. Physiol. 276, C717-C724. [DOI] [PubMed] [Google Scholar]
- 37.Moudy, R., Manning, T. J. & Beckers, C. J. (2001) J. Biol. Chem. 276, 41492-41501. [DOI] [PubMed] [Google Scholar]
- 38.Gijon, M. A. & Leslie, C. C. (1999) J. Leukocyte Biol. 65, 330-336. [DOI] [PubMed] [Google Scholar]
- 39.Choi, W. S., Kim, Y. M., Combs, C., Frohman, M. A. & Beaven, M. A. (2002) J. Immunol. 168, 5682-5689. [DOI] [PubMed] [Google Scholar]
- 40.O'Connell, G. C., Douglas, S. A. & Bunn, S. J. (2003) Neurosci. Lett. 342, 1-4. [DOI] [PubMed] [Google Scholar]
- 41.Williger, B. T., Ho, W. T. & Exton, J. H. (1999) J. Biol. Chem. 274, 735-738. [DOI] [PubMed] [Google Scholar]
- 42.Tao, F. C., Shah, S., Pradhan, A., Tolloczko, B. & Martin, J. G. (2003) Am. J. Physiol. 284, L90-L99. [DOI] [PubMed] [Google Scholar]
- 43.Snetkov, V. A., Hapgood, K. J., McVicker, C. G., Lee, T. H. & Ward, J. P. (2001) Br. J. Pharmacol. 133, 243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowen, D. S., Sowers, R. S. & Manning, D. R. (1996) J. Biol. Chem. 271, 22297-22300. [DOI] [PubMed] [Google Scholar]