Abstract
Reproductive isolation (RI) plays an important role for speciation, but assessing reproductive barriers at all life-cycle stages remains challenging. In plants, most studies addressing the topic have been focusing on herbs with short generation times. The present study attempted to quantify several reproductive barriers between a hybridizing species pair of long-lived woody rhododendrons. Consistent with findings of previous studies, pre-zygotic reproductive barriers contributed more to total RI than post-zygotic reproductive barriers. Especially in the more widespread species geographic isolation was an important barrier, and pollinator constancy contributed exceptionally to RI in both species. Additionally to strong pre-zygotic reproductive barriers, post-zygotic reproductive barriers were considerable, and had asymmetric tendencies favoring one of the species as maternal parent. Overall, despite occasional hybridization, the present study provides evidence for strong RI between R. cyanocarpum and R. delavayi.
A still unresolved question in speciation is to trace the mode and speed of the evolution of barriers to gene flow1,2. Therefore, comprehensive analyses of mechanisms preventing gene flow between populations of sexually reproducing organisms are crucial for understanding how biological species arise and how they are maintained.
Speciation can be considered as a continuous process3, and if a species pair has proceeded from the initial stages of divergence, multiple reproductive barriers would be expected to have emerged4. Thus, studying a limited number of barriers for a species pair may skew our view of the relative importance of these barriers for certain reproductive stages5. Different isolating barriers act in a hierarchical order6, and therefore, early barriers can contribute more to total isolation than late barriers. For example a geographical barrier has to be overcome before any barriers related to pollination start playing a role. With regards to their timing in life history, barriers are usually classified using the zygote as a reference point. Hence barriers contributing to reproductive isolation (RI) can be classified as pre- and post-zygotic5. In recent years more attempts have been made to identify all barriers that contribute to RI, however, the prevalence of herbs in these studies is still considerable6,7. This might be due to this type of assessment being much more challenging for woody plants as opposed to annual plants because of issues arising from the long generation times involved, and hence problems identifying barriers related to later stages in life-history (e.g. between seedling establishment and reaching reproductive age). This is one of the main reasons why we still have very little knowledge about RI in trees and tropical taxa, when compared to temperate herbs7.
The genus Rhododendron is the largest genus in the family Ericaceae, containing approx. 1000 species, all of which are woody shrubs or trees. Subgenus Hymenanthes, one of its eight recognized subgenera, comprises 225 species in 24 subsections8,9, with all of the investigated subgeneric species being diploids (2n = 26)9. Generally reproductive barriers in the genus are weak, especially between species belonging to the same subsection, which is evidenced by several reports of natural hybrids in the wild10,11,12,13,14. Furthermore, even hybrids between less closely related species are often obtainable with ease, which resulted in a large amount of artificial hybrids for ornamental purposes, undoubtedly contributing to the popularity of the genus in horticultural circles. It has been hypothesized that most members of the genus in the Sino-Himalayan region originated from a relatively recent radiation event triggered by the uplift of the Himalayas15. This could be one possible factor for the overall high reproductive compatibility between members of the genus. However, how much other factors, as for example long generation time or demography contributed is completely unknown. Furthermore, most studies in the genus have focused on a small subset of reproductive barriers, such as hybrid swarm composition11,13,14,16, and genetic incompatibilities14. Hence, to date no comprehensive study considering all possible stages potentially conveying reproductive barriers between any two members of the genus has been published.
A previous study of hybrids between the two species Rhododendron delavayi and R. cyanocarpum, found an exceptionally high number of F1 and F2 hybrids in a contact zone, and provided evidence for asymmetric backcrossing towards R. delavayi11. Relatively recent habitat disturbance of the area in the 1950 s was put forward as one possibility for the emergence of this hybrid zone12. However, as ecological data for the species was lacking, it remained unclear how factors such as seed viability and seedling establishment contributed to the unusual pattern, and how strong reproductive isolation between the species remained despite the occurrence of hybrids.
Therefore, the specific goal of the present study was to conduct a comprehensive assessment of diverse barriers that can contribute to RI (see table 1). The questions we wanted to answer were (1) How strong are the different reproductive barriers, and what is their relative contribution to overall RI? (2) Do some of these barriers act asymmetrically, providing an explanation for the observed asymmetric hybridization patterns? (3) Is there evidence for ecological selection during seed germination with regards to soil type, hence are hybrid seeds affected differently depending on location?
Table 1. Equations used to quantify components of reproductive isolation.
| Barrier | Equation for calculating RI |
|---|---|
| Prezygotic | |
| Geographic | 1 – (no. of counties in which the species co-occur/no. of counties in which the species occurs) |
| Phenology | 1 – (no. of days in which both species are in flower at the same time/no. total flowering days for the species) |
| Pollinator assemblage | 1 – (flower visits by shared pollinators/total number of flower visits) |
| Pollinator behavior | 1 – (no. of flower visits by pollinators coming from the respective other species/total no. of flower visits) |
| Postzygotic | |
| Pollen precedence | 1 – (the ration of pollen tube length to the total length of the style from interspecific/the ration from intraspecific pollination) |
| Seed production | 1 – (no. of seeds per interspecific/no. seeds per intraspecific pollination) |
| Seed germination | (1 – no. of germinated seeds resulting from interspecific pollination/no. germinated seeds resulting from intraspecific pollination) |
Each RI value was generally varying from 0 to 1, with 0 representing no barrier to inter-specific gene flow and 1 representing a complete barrier.
Results
Geographic isolation
In total, 553 specimens of R. delavayi were listed on the website of the Chinese virtual herbarium, and 83 specimens of R. cyanocarpum. The R. delavayi specimens represented records from 56 counties, of which 43 were counties of Yunnan province, eight counties of Guizhou province, three counties of Tibet, and two counties of Guangxi province. All the specimens of R. cyanocarpum were from Cangshan mountain, confirming its restricted distribution. Therefore, for R. delavayi RIgeograph = 1 − (1/56) = 0.98 whereas RIgeograph = 0 for R. cyanocarpum as the only location where it was recorded was sympatric with R. delavayi.
Phenological Isolation
In 2008, R. cyanocarpum flowered from 18th of February to 19th of May, whereas the flowering period of R. delavayi lasted from 25th of February to 2nd of June. Therefore, R. cyanocarpum flowered for 92 days (February in 2008 had 29 days) and R. delavayi flowered for 99 days; for 85 days R. cyanocarpum and R. delavayi were flowering at the same time. Hence RIphenology = 1 − (85/92) = 0.043 for R. cyanocarpum, and RIphenology = 1 − (85/99) = 0.141 for R. delavayi.
Floral Isolation
Nectar composition
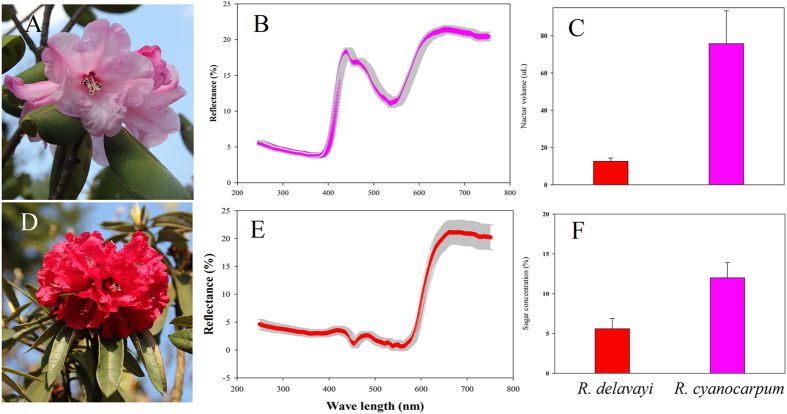
R. cyanocarpum flowers contained on average 75.71 ± 17.64 ul of nectar (n = 20), while R. delavayi flowers contained 12.6 ± 1.75 ul (n = 20), which was significantly different (t = 19.15, p < 0.001; Fig. 1C). This large difference was mostly due to R. cyanocarpum having five nectar pouches, all of which produce nectar, while R. delavayi has one dominant nectar pouch, which is the only one producing nectar. Looking at nectar production per nectar pouch, R. cyanocarpum nectar pouches produced 15.14 ± 0.75 ul of nectar, which is still significantly different (t = 2.918, p = 0.006). The sugar concentration in R. cyanocarpum nectar was 12 ± 2% and 6 ± 1% for R. delavayi nectar (both n = 20), which was also significant (t = 10.51; p < 0.001; Fig. 1F).
Figure 1. Flowers with its petal color reflectance, nectar volume and sugar concentration of R. cyanocarpum and R. delavayi.
(A) R. cyanocarpum flower. (B) Petal color reflectance of R. cyanocarpum. (C) Nectar volume. (D) R. delavayi inflorescence. (E) Petal color reflectance of R. delavayi. (F) Nectar sugar concentration.
Petal color reflectance
While R. cyanocarpum flowers showed a marked peak in the reflectance spectrum at 430 nm, this peak was completely absent in R. delavayi flowers (Fig. 1B,E). In addition, a peak in the reflectance spectrum above 600 nm was detected in both species.
Volatile compounds in flowers
In the floral extracts of R. cyanocarpum 21 volatile compounds were identified, accounting for 96.73% of total extracted mass, and in extracts of R. delavayi 18 volatile compounds were identified, accounting for 70.38% of total extracted mass (Supplementary Table 1).
Most of the detected compounds belonged to either aliphatics (accounting for 64.37% in R. cyanocarpum, and 56.2% in R. delavayi) or terpenoids (accounting for 28.88% in R. cyanocarpum, and 11.4% in R. delavayi) (Table 2). Additional quantification of produced volatile compounds showed that production of all compound groups in R. cyanocarpum exceeded production in R. delavayi by about one order of magnitude (Aliphatics: 595.98 ng/h/flower vs. 38.26 ng/h/flower; Terpenoids: 276.65 ng/h/flower vs. 7.52 ng/h/flower; Benzenoids: 22.96 ng/h/flower vs. 2.14 ng/h/flower; Table 2). If we taking the one third of flower numbers per inflorescence of RC to RD (on average 6 flowers of RC vs. 19 flowers of RD) into account, the much larger amount of compounds were still examined in R. cyanocarpum than in R. delavayi per inflorescence (Aliphatics: 3575.88 ng/h/inflorescence vs. 726.94 ng/h/inflorescence; Terpenoids: 1659.9 ng/h/inflorescence vs. 142.88 ng/h/inflorescence; Benzenoids: 137.76 ng/h/inflorescence vs. 40.66 ng/h/inflorescence; Table 2).
Table 2. Volatile compounds extracted from flowers of R. cyanocarpum and R. delavayi.
| Species | R. delavayi |
R. cyanocarpum |
||||
|---|---|---|---|---|---|---|
| Relative content (%) | Absolute production (ng/h/flower) | Absolute production (ng/h/inflorescence) | Relative content (%) | Absolute production (ng/h/flower) | Absolute production (ng/h/inflorescence) | |
| Aliphatics | 56.2 | 38.26 | 726.94 | 64.37 | 595.98 | 3575.88 |
| Terpenoids | 11.04 | 7.52 | 142.88 | 29.88 | 276.65 | 1659.9 |
| Benzenoids | 3.14 | 2.14 | 40.66 | 2.48 | 22.96 | 137.76 |
| Total | 70.38 | 47.91 | 910.29 | 96.71 | 895.41 | 5372.46 |
Shown are the relative contribution of compound groups to the total mass, and the absolute production per hour per flower and average production per hour per inflorescence.
Pollinator mediated isolation
In 2008, 32 bumblebees and 1 butterfly were recorded visiting flowers of R. cyanocarpum; 39 bumblebees and 8 wasps were recorded visiting R. delavayi flowers. As experimental plots for pollinator constancy had not been set up in 2008, RI was calculated for pollinator assemblage only. RI due to shared pollinators (bumblebees) for R. cyanocarpum = 1 − (31/32) = 0.031; and RI = 1 − (39/47) = 0.17 for R. delavayi.
In 2011, observations were carried out in three plots: plot-1 (R. cyanocarpum dominated population); plot-2 (R. delavayi dominated population); plot-3 (hybrid zone). Over the course of the observation time, 30 bumblebees were recorded visiting flowers of R. cyanocarpum (plot-1: 28, plot-2: 2, plot-3: 0), and 11 bumblebees visiting flowers of R. delavayi (plot-1: 6, plot-2: 5, plot-3: 0). Furthermore, 14 visits of birds (Yuhina occipitalis) to flowers of R. delavayi were recorded (plot-1: 0, plot-2: 14, plot-3: 0). During the whole of the observation time there were no pollinator visits to plot-3. Although bumblebees visited both species, we never witnessed a transfer visitation between flowers of the two species in any of the plots. Therefore, reproductive isolation for pollinator assemblage was relatively weak (RI for R. cyanocarpum = 1 − (30/30) = 0; and RI = 1 − (11/25) = 0.56 for R. delavayi) in comparison to RI resulting from pollinator constancy: RI = 1 for both species.
Pollen tube growth
For all four pollination treatments the pollen tubes had grown a very short distance 24 hours after application of the pollen to the stigmas. With R. cyanocarpum as maternal species, pollen tubes from intra-specific crosses had on average grown 12.32 ± 1.3% of the total length of the style, whereas pollen tubes from inter-specific pollination reached only 2.2 ± 0.3% of total style-length. This difference is highly significant (t = 7.66, p < 0.001). When R. delavayi was the maternal species, pollen tubes from intra-specific pollinations had reached 6.1 ± 2.0% of the total style-length, compared to 2.7 ± 2.0% of total style-length for inter-specific pollinations. This difference was also highly significant (t = 11.41, p < 0.001). As no pollen tubes had reached the ovules, only a tentative RI can be estimated. Making the very simplified assumption that the difference in pollen tube growth at the measured stage reflects the likelihood for later fertilization, RI due to conspecific pollen precedence for R. cyanocarpum = 1 − (2.2/12.32) = 0.821, and RI = 1 − (2.7/6.1) = 0.557 for R. delavayi.
Seed production
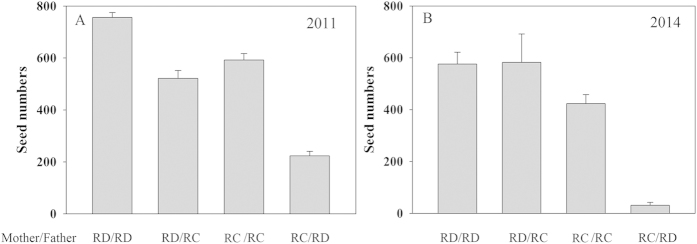
All pollination treatments resulted in seed-set (Fig. 2A,B). Although the number of seeds produced was significantly affected by years (F = 5.03; p = 0.027), there were no significant interactions between year and the other factors (Table 3). Furthermore, pollen source (F = 21.33, p < 0.001) and maternal species (F = 29.58, p < 0.001) had a significant effect on seed number, with a significant interaction between these two factors (F = 6.2, p = 0.014). RIseed production for R. cyanocarpum = 1 − (223.2/592.6) = 0.623 and RIseed production for R. delavayi = 1 − (521.8/756) = 0.310 in 2011, respectively. In 2014, RIseed production for R. cyanocarpum = 1 − (31.06/423.59) = 0.927 and RIseed production for R. delavayi = 1 − (582.33/576.12) = −0.011 (Fig. 2A,B).
Figure 2. Seed production from inter- and intra-specific pollinations in 2011 (A) and 2014 (B).
RD: R. delavayi; RC: R. cyanocarpum. The error bars indicate S. E.
Table 3. ANOVA results for the effect of the factors (year, pollen source, and mother species) on the number of seeds produced.
| Factors | Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Year | 425756.64 | 1 | 425756.64 | 5.03 | 0.03 |
| Pollen source | 1806197.96 | 1 | 1806198 | 21.33 | <0.001 |
| mother | 2504887.08 | 1 | 2504887.1 | 29.58 | <0.001 |
| Year * Pollen source | 86980.96 | 1 | 86980.96 | 1.03 | 0.31 |
| Year * mother | 107904.48 | 1 | 107904.48 | 1.27 | 0.26 |
| Pollen source * mother | 525313.38 | 1 | 525313.38 | 6.20 | 0.01 |
| Year * Pollen source * mother | 128169.48 | 1 | 128169.48 | 1.51 | 0.22 |
Soil physico-chemical analysis
With the exception of available potassium, all physico-chemical soil properties showed significant differences between the three sites (Table 4). In measures of pH, soil organic matter, and total potassium the hybrid site is intermediate between the two parental species dominated sites (Table 4). However, measures of total nitrogen and total phosphorus are significantly higher for the hybrid site than for either parental site. Available phosphorous was comparable between the hybrid site and the R. cyanocarpum dominated site, but both were significantly higher than the R. delavayi dominated site (Table 4).
Table 4. Soil physicochemical properties of samples taken from three different localities (one R. delavayi dominated population, one R. cyanocarpum dominated population, and the hybrid zone).
| Site | R. delavayi dominated | R. cyanocarpum dominated | Hybrid zone |
|---|---|---|---|
| Altitude (m) | 2800 ~ 3000 | 3200 ~ 4000 | 3100 |
| pH | 4.97 ± 0.05a | 4.02 ± 0.04b | 4.48 ± 0.1c |
| Organic matter (g/kg) | 210.42 ± 3.78a | 257.35 ± 3.50b | 234.56 ± 1.17c |
| Total nitrogen (g/kg) | 7.73 ± 0.13a | 8.97 ± 0.09b | 9.92 ± 0.56b |
| Total phosphorus (g/kg) | 1.16 ± 0.03a | 1.24 ± 0.04a | 1.42 ± 0.06b |
| Total potassium (g/kg) | 2.92 ± 0.02a | 1.85 ± 0.08b | 3.64 ± 0.10c |
| Available phosphorus (g/kg) | 18.14 ± 0.41a | 33.88 ± 2.07b | 34.86 ± 7.32b |
| Available potassium (g/kg) | 276.18 ± 2.97a | 303.65 ± 5.25a | 243.10 ± 42.76a |
Values with different letter superscripts are significantly different on the p < 0.05 level.
Seed germination
Medium: distilled water
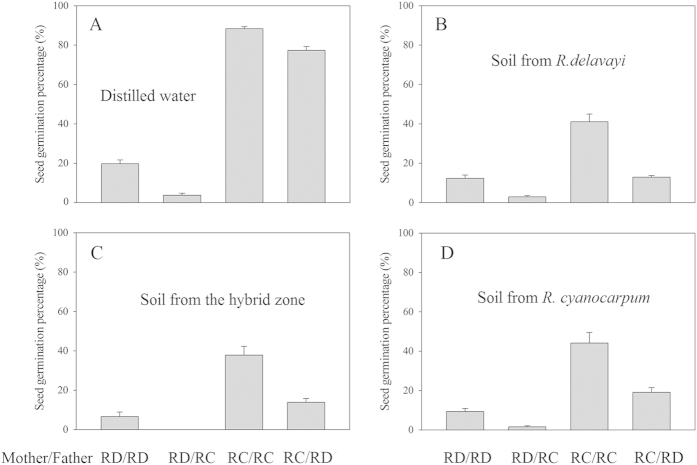
When seeds were germinated on filter paper soaked with distilled water, seed germination rate was higher for seeds obtained from R. cyanocarpum plants when compared to seeds from R. delavayi plants (F = 1060, p < 0.001; Table 5, Fig. 3A). In addition, pollen source also had a significant effect on seed germination (F = 38.15, p < 0.001), with the interaction term between the two factors being not significant (Table 5). For seed obtained from R. cyanocarpum, the germination rate was 0.883 ± 0.01 for seeds from intra-specific pollinations compared to 0.773 ± 0.019 for seeds from inter-specific pollinations (Fig. 3A). For seed from R. delavayi, the germination rate was 0.197 ± 0.019 for seeds from intra-specific pollination compared to 0.037 ± 0.01 for seeds from inter-specific pollination (Fig. 3A). Thus RIseed germination of R. cyanocarpum = 1 − (0.773/0.883) = 0.125 while RIseed germination of R. delavayi = 1 − (0.037/0.197) = 0.812 (Fig. 3A).
Table 5. ANOVA results testing for the effect of the factors medium (water vs soil), pollen source, and mother species on the seed germination rate of seed obtained from four different pollination treatments.
| Source of variation | Sum of Squares | d.f. | F | p |
|---|---|---|---|---|
| Different soil treatments | ||||
| Soil type | 0.014 | 2 | 2.311 | 0.115 |
| Pollen source | 0.258 | 1 | 83.814 | 0 |
| Mother | 0.652 | 1 | 211.702 | 0 |
| Pollen source * Mother | 0.121 | 1 | 39.376 | 0 |
| Soil type * Pollen source | 0.001 | 2 | 0.158 | 0.854 |
| Soil type * Mother | 0.003 | 2 | 0.409 | 0.667 |
| Soil type * Pollen source * Mother | 0.007 | 2 | 1.181 | 0.319 |
| Distilled water treatment | ||||
| Pollen source | 0.055 | 1 | 38.145 | 0 |
| Mother | 1.519 | 1 | 1060.300 | 0 |
| Pollen source * Mother | 0.002 | 1 | 1.308 | 0.286 |
| Combined analysis of soil and water germination | ||||
| Medium | 0.874 | 1 | 307.989 | 0 |
| Pollen source | 0.217 | 1 | 76.542 | 0 |
| Mother | 2.079 | 1 | 732.960 | 0 |
| Medium * Pollen source | 0.003 | 1 | 0.922 | 0.342 |
| Medium * Mother | 0.555 | 1 | 195.572 | 0 |
| Pollen source * Mother | 0.010 | 1 | 3.400 | 0.071 |
| Medium * Pollen source * Mother | 0.031 | 1 | 10.800 | 0.002 |
Figure 3. Seed germination percentage under distilled water and three soil treatments.
(A) Distilled water. (B) Soil samples collected from R. delavayi population. (C) Soil samples collected from the hybrid zone. (D) Soil samples collected from R. cyanocarpum population. RD: R. delavayi; RC: R. cyanocarpum. The error bars indicate S. E.
Medium: different soil types
Compared to germination using distilled water, seed germination in soil overall was significantly reduced (F = 307.99, p < 0.001; Table 5). However, between the three different soil types no significant differences in germination rate were detected (F = 2.31, p = 0.115). On the other hand, the pollen source and mother species had both a significant effect on germination rate (pollen source: F = 83.81, p < 0.001; mother species: F = 211.7, p < 0.001), with their interaction term being significant as well (F = 39.38, p < 0.001).
Because the soil type had no significant effect on seed germination, we averaged the three different soil treatments. Taking the mean of all three soil treatments, the germination rate for seed from R. cyanocarpum was 0.41 ± 0.018 for seeds from intra-specific pollinations compared to 0.153 ± 0.019 for seeds from inter-specific pollinations. For seed from R. delavayi, the germination rate was 0.095 ± 0.017 for seeds from intra-specific pollination compared to 0.015 ± 0.009 for seeds from inter-specific pollination. Thus RIseed germination of R. cyanocarpum = 1 − (0.153/0.41) = 0.627 while RIseed germination of R. delavayi = 1 – (0.015/0.095) = 0.833.
Discussion
Our assessment of multiple reproductive barriers indicated that R. cyanocarpum and R. delavayi are nearly completely reproductively isolated (overall RI = 1 for pre-pollination stages, and overall RI = 0.94 for R. cyanocarpum and 0.937 for R. delavayi in post-pollination stage (Table 6).
Table 6. Contribution of assessed barriers to RI between R. cyanocarpum and R. delavayi.
| Isolating barrier | Components of RI |
Absolute contributions to total RI |
In sympatry (pre-pollination) |
|||
|---|---|---|---|---|---|---|
| R. cyanocarpum | R. delavayi | R. cyanocarpum | R. delavayi | R. cyanocarpum | R. delavayi | |
| Geographic | 0 | 0.98 | 0 | 0.98 | – | – |
| Phenology | 0.043 | 0.141 | 0.043 | 0.0028 | 0.043 | 0.141 |
| Pollinator assemblage1 | 0.016 | 0.365 | 0.015 | 0.006 | 0.015 | 0.316 |
| Pollinator behavior2 | 1 | 1 | 0.942 | 0.0112 | 0.942 | 0.543 |
| In total | 1 | 1 | 1 | 1 | ||
| In sympatry (post-pollination) | ||||||
| Pollen precedance3 | 0.281 | 0.557 | 0 | 0 | 0.281 | 0.557 |
| Seed production4 | 0.775 | 0.15 | 0 | 0 | 0.557 | 0.066 |
| Seed germination5 | 0.627 | 0.833 | 0 | 0 | 0.102 | 0.314 |
| In total | 0 | 0 | 0.94 | 0.937 | ||
1Average RI for data obtained in 2008 and in 2011.
2RI = 1 due to no observed transfer visits. As hybrids do occur in the wild this is obviously an overestimate, but might be correct for certain years.
3RI obtained by extrapolating data, likely to be a very rough estimate.
4Averaged from data obtained in 2011 and 2014.
5RI as calculated for seed germination in soil, which better reflects natural conditions.
Prezygotic barriers
Geographic isolation
Geographic isolation protects most individuals of R. delavayi completely from geneflow originating from R. cyanocarpum, while on the other hand all known plants of R. cyanocarpum grow at least in parapatry with R. delavayi, and hence may hybridize with it. This results in a diametrically opposite contribution of geographic isolation to RI in the two species, and while it is the most important barrier for R. delavayi (RI = 0.98) it is non-existent in R. cyanocarpum (RI = 0). This should apply to any case for which one species of a hybridizing species-pair is a narrow endemic, which should then have by default a lower barrier to geneflow than the wide-spread species. However, this only applies to the species as a whole, and the situation in sympatry is likely to be different in each case.
Isolation due to phenology and pollinators
Phenology and pollinator assemblage conveyed a higher RI for R. delavayi than for R. cyanocarpum. Both barriers were negligible in R. cyanocarpum (RIphenology = 0.043, RIpollinator assemblage = 0), while considerable in R. delavayi (RIphenology = 0.141, RIpollinator assemblage = 0.56). However, pollinator behavior contributed the strongest barrier in sympatry for both species (RIpollinator behavior = 1). It is obvious that the estimate of RI = 1 for pollinator behavior, meaning a complete barrier, is flawed, as hybrids do occur in the wild, but nonetheless, pollinator constancy is probably a crucial barrier for this species pair. A just-published work of quantifying RI in sympatric woody species of Cyrtandra (Gesneriaceae) revealed strong RI in post-zygotic stages, but reproductive barriers mediated from pollinators that were proved to be important by our current study, were neglected17.
The relatively high RI for the pollinator assemblage in R. delavayi (0.56) was due to birds visiting the flowers, while no birds were observed visiting R. cyanocarpum flowers. That R. delavayi flowers have floral characteristics indicative of ornithophily is also supported by the floral analyses, as their nectar had a low sugar concentration18, overall lower emission of floral volatile compounds, and were lacking the characteristic peak at 430 nm in the reflectance spectrum, which has been shown to be attractive for insects19. However, despite the evidence for ornithophily, R. delavayi flowers were frequently visited by bumblebees (a total of 58 visitations by bees in 2008 and 2013).
Flowers of R. cyanocarpum on the other hand had many features which are generally observed in insect pollinated plants. They possessed a characteristic peak at 430 nm in the reflectance spectrum and showed overall high emissions of volatile compounds rich in aliphatics and terpenoids which are often attributed to insect attraction20. Previous pollinator observations showed that R. cyanocarpum flowers do indeed attract several different groups of insects, but only bumblebees were ever observed to effect pollination21. The reason that we only recorded bumblebees in the present study is probably due to the circumstance that observations were carried out in spring, early in the flowering season. In the study area both species grow at relatively high altitudes (>2800 m) where temperatures are quite low in spring, and pollinators that can cope with colder conditions, such as bumblebees, are therefore likely to be overrepresented in the current data. However, as they are the only reported effective pollinators, this bias should have a minor effect on pollinator assemblage.
The largest barrier in sympatry was pollinator constancy, as no transfer visits were observed, which indicates that cross-pollination is a relatively rare occurrence. It would have been interesting to ascertain this behavior also in the hybrid zone, as only pollinator visitation data for the two parent-dominated plots (plot-1, plot-2) could be obtained. Although a plot was established in the hybrid zone (plot-3), not a single pollinator was attracted to the flowers. Other studies have, however, reported that the occurrence of hybrids can facilitate pollen transfer between parental species22. It also remains unclear if the experimental setup could have lead to increased constancy, for example the preferred flower was always available in close proximity, which would not be the case if a bumblebee crossed the border from a site dominated by one of the species into a larger patch dominated by the other species. Hence, in certain settings accidents might occur more often than the data suggest.
Pollen precedence
The RI conveyed by pollen precedence was estimated to be high for both species (0.821 and 0.557), but these values are only tentative, as only data from the first 24 hours was available, and at that stage the furthest some pollen tubes had grown was about 12% of the distance to the ovules. Furthermore, it is unclear how often pollen from both species is transferred to the same stigma under natural conditions, as frequently several flowers in one inflorescence remain un-pollinated, indicating that several flowers may receive pollen scarcely, thus making it less probable for the pollen from the different species getting to the same stigma at the same time.
Post-zygotic barriers
The amount of hybrid seed produced and its viability were affected differently in the two species. While R. cyanocarpum produced significantly fewer seeds when the pollen source was heterospecific (492 vs. 125), the picture was less clear for R. delavayi. In 2011 there was a noticeable effect on hybrid seed number (756 vs. 522), but in 2014 there was no significant difference between numbers of seed obtained from the two pollination treatments (576 vs. 582). Thus in a scenario most favorable for hybridization there is no evidence for RI resulting from a reduction in seed in R. delavayi.
However, RI resulting from a difference in seed germination was overall high in R. delavayi, with no significant difference between germination in water or soil (RI > 0.8, Table 6). On the other hand, for hybrid seed from R. cyanocarpum mothers the germination rates were significantly different between these two media (0.125 vs. 0.627, Table 6). Thus, while most hybrid seed from R. delavayi mothers was inviable, hybrid seed from R. cyanocarpum mothers seemed to be mostly viable, but apparently germination was somehow negatively affected in soil. In the natural environment (soil) the RI is therefore likely to be high for both species, which would favor R. delavayi as mother species for hybrids, as the higher number of hybrid seeds produced counteracts the lower germination success. Genetic data suggests that this is the case, as most hybrids seem to have a R. delavayi chloroplast type11.
That a large portion of R. delavayi hybrid seed was inviable could perhaps partly be explained if genetic incompatibilities affecting seed-set were acting at earlier stages in R. cyanocarpum. If potentially inviable seeds were aborted in R. cyanocarpum before they are formed, but seed development proceeded in R. delavayi to a stage when it is difficult to distinguish viable seeds from inviable ones, the germination rate would necessarily be lower for R. delavayi hybrid seed. The observed lower hybrid seed production for R. cyanocarpum would also fit this pattern. However, even if this would be the case, it would not explain the significantly different germination rates of R. cyanocarpum hybrid seed in soil as opposed to water. If some form of ecological selection plays a role for RI in R. cyanocarpum seed germination would be an interesting question for further research, as the current data does not provide clues as to which factors might have resulted in the difference.
Summary
For the widespread species R. delavayi geographic isolation is the most important barrier at the species level. In sympatry the highest contribution to RI for both species comes from pollinator constancy, with other pre-zygotic barriers being very weak in R. cyanocarpum but considerable in R. delavayi. Additionally, post-zygotic barriers are strong, and seem to be slightly asymmetric, indicating higher likelihood for occurrence of hybrid seedlings originating from R. delavayi mothers. With pre-zygotic barriers contributing considerably more to overall RI than post-zygotic barriers, the data fits the pattern already observed by Lowry et al.23.
However, it should be pointed out that this pattern mostly results from geographic isolation and pollinator behavior, and especially the estimate for the latter is flawed in the current study. Furthermore, as the investigated species are woody perennials with long generation times, only performance of hybrid seed in early life stages could be assessed. The fitness of hybrids that are being produced despite strong RI remains, however, unclear.
Materials and Methods
Study species
Rhododendron delavayi is a widely distributed species in southwest China, generally growing at altitudes below 3000 m. Members of the genus have been reported in the provinces of Yunnan and Guizhou, and further west reaching into some areas of Tibet. R. cyanocarpum, on the other hand, is a narrow endemic which is only found in one location in China, Cangshan mountain, growing at altitudes above 3100 m. Due to its limited range R. cyanocarpum was listed in the Red List of Rhododendrons24. The two species can easily be distinguished by their bark, R. delavayi has very deeply-ridged and rough bark, whereas it is smooth in R. cyanocarpum; the leaves of R. delavayi are lanceolate and their abaxial leaf surface is covered with cream-brown hairs, which are lacking in R. cyanocarpum leaves that additionally are rounded. Furthermore, R. cyanocarpum has a big calyx in both flower and fruit, whereas it is always small in R. delavayi, and R. delavayi has deep-red flowers, while they are pink in R. cyanocarpum.
Calculation of RI
For each stage-specific mechanism that we examined, the effect of that mechanism was quantified using indices of reproductive isolation (RI). With reference to Kay5, each RI value was generally varying from zero to one, with zero representing no barrier to inter-specific gene flow and one representing a complete barrier (Table 1). In the rare case of negative values, they would be indicative of stages at which hybridization is favored.
Geographic isolation
To assess and quantify geographical overlap of species is generally problematic, as for a wider range, units will have to be chosen more or less arbitrarily7. Due to the type of records we used (herbarium specimens), we chose to use counties as units to check for co-occurrence of the two species. This is a very coarse-grained approach, but specimen information in the area was expected to be too scarce to merit an approach using a finer, custom, grid.
Specimen information for R. delavayi and R. cyanocarpum was obtained for all relevant specimens deposited in Chinese herbaria that are accessible via the Chinese virtual herbarium (http://www.cvh.org.cn, accessed: 16 June 2015). According to locality information on the specimens counties were then identified in which the species occurs.
Phenological Isolation
In the contact zone of the two species approximately 500 mature individuals of R. cyanocarpum, and 50 mature individuals of R. delavayi could be found. As co-flowering in the contact zone was deemed most important, and flowering generally occurred at the same time in the whole area, only this population was used to assess flowering time. Local people were hired for the relevant months (February to July) in 2008 to record weekly if individuals were flowering.
Floral Isolation
It is common that a pollinator assemblage can vary significantly over the course of a prolonged flowering period, and also differences between years can be expected25,26. Both species can have flowering times lasting more than three months, which could therefore lead to biased pollinator assemblage assessments, if observations would only be carried out for a selected period in the flowering season. To avoid this bias, in addition to pollinator observations, we aimed at predicting general pollinator preference by distinguishing key flower characteristics. To this purpose we measured nectar volume and sugar concentration, light reflectance spectra of petals, and extracted floral volatile compounds.
Nectar volume and nectar sugar concentration
For measurements of nectar volume and corresponding sugar concentration, ten plants from each species were selected that were at least 10 m apart, and which had flower buds about to open the next day. From each of the 20 selected plants two flower buds about to open the next day were enclosed in plastic bags to minimize evaporation, and prevent disturbance by visitors. The following day, after the buds had opened, at around 10:00, the nectar volume was directly measured by extraction from the nectar pouches with a calibrated 100 μL capillary (Sigma Chemical Co., St. Louis, MI, USA). Sugar concentration was then obtained using a hand-held refractometer (Eclipse; Bellingham & Stanley Ltd., Basingstoke, UK), measured as grams of sugar per gram of nectar, and expressed as percentage.
Flower petal reflectance
Two mature flowers each of ten different individuals per species were collected, and one petal per flower was used for measurements. Hence 40 petals were measured, 20 R. delavayi and 20 R. cyanocarpum. To assess light reflection patterns of petals at different wavelengths, we obtained spectral data from petals using an S2000 miniature fiber optic spectrometer with a PX-2 pulsed xenon lamp (Ocean Optics, Dunedin, FL, USA). All measurements were carried out in the range from 250 to 750 nm, using 0.38 nm increments. As changes in the reflectance pattern along the same petal were known to show negligible variation26, only one measurement per petal was taken.
Floral volatile compounds
For the collection of volatile compounds, three young inflorescences per species, each from a different individual were selected. The inflorescences were taken to a close by hotel, and wilted or non-opened flowers were removed until 25 flowers remained per species, though such treatment might change the scent composition of an infloresence, since those would normally be present. The three inflorescences per species were then enclosed in Tedlar bags (Dupont, USA). Extraction was started from 12:00, which corresponds to the time of highest pollinator activity. Using a pump (inlet flow rate 300 mL/min), volatiles were then extracted for 6 h from the enclosures into cartridges containing the adsorbent Porapak Q (150 mg, mesh 60/80; Waters Associates, Inc., USA). Prior to use, the adsorbent cartridges had been cleaned with 2 ml diethyl ether and dried with nitrogen gas. For analysis the trapped volatiles were eluted with 400 μL dichloromethane and the concentration was subsequently increased by reducing the original volume 1:5 through evaporation at room temperature, aided by a gentle stream of nitrogen. Then 720 ng of n-nonane was added to each sample as standard for later quantification, and the samples were stored at −20 °C.
The samples were then run on an Agilent Technologies HP 6890 gas chromatograph, equipped with an HP-5MS column (30 m × 0.25 mm inner diam, 0.25 μm film thickness), and linked to an HP 5973 mass spectrometer. Helium was used as a carrier gas at a flow rate of 1 ml/min. Split inlet and FID were held at 250 °C. Column temperature was programmed to rise from 40 °C (5-min hold) to 250 °C (20-min hold) at 3 °C/min. The mass spectra were taken at 70 eV (in EI mode) with a scanning speed of 1 scan/s from m/z 30 to 350. Compounds were tentatively identified by comparing mass spectra and relative retention times with those of standard compounds and with the Wiley NIST 05 mass spectral database. Their relative proportions (%) were calculated by dividing the absolute amounts of individual compounds by the sum of all compounds and multiplying by 100. The absolute amounts of all samples were calculated by the internal standard method. Because some flowers were removed especially from inflorescences of R. delavayi, emission quantity of compounds was tentatively calculated as the absolute emission per flower multiplied by average flower numbers per inflorescence for both species.
Pollinator mediated isolation
In April 2008, to examine the main pollinators, we recorded visitations of potential pollinators in one individual of R. cyanocarpum and one individual of R. delavayi. For each species a total of 24 h for 4 successive days in R. cyanocarpum dominant and R. delavayi dominant population were observed respectively.
In April 2011, during seven consecutive sunny days pollinator preference for the two species was observed. To assess whether insects discriminated between the species or transferred pollen between flowers of both species, three experimental plots were established, one in a population comprising predominantly R. cyanocarpum (plot-1), one in a R. delavayi dominated population (plot-2) and another one in the hybrid zone (plot-3).
In each of the plots eight flowers of each species (8 R. cyanocarpum, 8 R. delavayi) were arranged in a 4 × 4 grid structure, with flowers 0.5 m apart and alternating flowers of different species by row and column, so that row one and three started with a different species to row two and four. For the grid freshly opened flowers were collected and placed into a bottle with water. Visiting pollinators to the plots were then recorded on four sunny days in plot-1 for a total of 24 hours, and on three sunny days in plot-2 and plot-3 for a total of 18 hours of each. Only insects that were observed to visit flowers were recorded.
Hand pollination treatments
To investigate cross-fertility of the two species, four types of hand pollination treatment were carried out employing a full orthogonal design. The same pollination treatments were carried out in two years, 2011 and 2014. Per treatment anthers of more than 30 flowers of five maternal individuals were removed before anthesis; these emasculated flowers were then bagged with netbags. After opening of the corolla, the flowers were pollinated using anthers from freshly collected flowers, and consecutively re-bagged. For cross-pollination with con-specific pollen, flowers of the same species were collected from individuals that were growing at least 10 m from the experimental plants. Fruits were harvested at the end of October, and the number of seeds was counted for each fruit.
Pollen tube growth
To assess if differential pollen tube growth, depending on the maternal parent contributes to RI between the species, a subset of the flowers from the hand pollination treatments carried out in April, 2014 was used. For each of the four treatments, 10 flowers each from 5 flowering plants for each species were harvested 24 hours after pollen had been applied to the stigma. The length of each harvested pistil was recorded, after which it was dissected and softened by incubation in 0.1 M NaOH at 60 C for 1 h. Afterwards, the pistils were incubated in 0.1% aniline blue (pH 8.3) for 48 h. Then slides were examined under a fluorescence microscope with blue excitation (410 nm) and for each pistil the growth of pollen tubes. Prior to the observation, the length of style per treated flower was measured. Then the relative pollen tube growth rate was calculated as the ration of pollen tube length to the total length of the style.
Soil sampling and physico-chemical analysis
In 2013, soil was obtained from three sites, one site dominated by R. cyanocarpum, one site where mostly R. delavayi plants were found and the hybrid zone. Four plots measuring about 4 × 4 m were randomly selected in each of the three sites, and prior to soil sampling, litter and organic material was removed from the soil surface. Surface soil was then collected close to several (>4) Rhododendron individuals growing in the plot limits and subsequently mixed, giving a total of 12 soil samples (four samples each from each of the three sites). The samples were taken to the laboratory, and after removing root material and stones, were sieved using a 2 mm mesh sieve.
Aliquots of 15–20 g were oven dried at 105 °C for 24 h, and afterwards homogenized to a saturated colloid with 0.01 M NaNO3. From this colloid measurements were obtained as follows: pH was measured using a pH-meter (PHS-3C; Shanghai Precision & Scientific Instrument, Shanghai, China); total phosphorus was determined colorimetrically after nitric/perchloric acid digestion; total nitrogen was determined by UV spectrophotometric measurement after nitric/perchloric alkaline digestion; total potassium was determined by atomic absorption (spectrophotometric); available phosphorus was determined by the Olsen method; soil organic matter was determined by oil bath K2CrO7 titration; available potassium was extracted with 1 N ammonium acetate and analyzed by atomic absorption (spectrophotometric); all methods were carried out as described by Hou et al.27.
Seed germination
Seed germination using distilled water
Seeds obtained from each of the four cross-pollination treatments in 2014 were placed on filter paper maintained moist with distilled water and placed in Petri dishes at room temperature (~25 °C). From each of the four pollination treatments 200 seeds were used, separated into four replicates of 50 seeds, hence 800 seeds in total were used. The petri dishes were left for 30 days while insuring that they stayed moist, and after this period the number of germinated seeds was counted in each petri dish. The fraction of germinated seeds for each replicate was then calculated by dividing the number of germinated seeds by 50.
Seed germination in different soil types
The seeds obtained from the same treatments as mentioned above were used to also assess germination in the different soil types collected for physico-chemical analysis. For each of the three soil types, four replicates for seed obtained from each of the four pollination treatments were carried out (3 types of soil × 4 pollination treatments × 4 replicates each = 48). Petri dishes were filled to a depth of 2 cm with the respective soil, and 50 seeds were spread on the surface. The soil was then moistened with distilled water, and the petri dishes were incubated for 30 days at room temperature (~25 °C). Therefore, in total of 2400 seeds were used to do the experiment. After 30 days the number of germinated seeds was counted in each of the petri dishes, and germination rate was calculated by dividing by 50.
Data analysis
Significance of the difference of the means for nectar volume and sugar concentration between the two species was assessed using independent two-sample t-tests. Physico-chemical soil data were tested for deviation from normal distribution using a Kolmogorov-Smirnov test, and means for the three sampled sites were then compared employing a one-way ANOVA; significance of paired-comparisons was afterwards assessed using a Tukey post-hoc test. Univariate ANOVA was performed for testing the effects of multiple factors on the seed production and seed germination data. We used a three-factor ANOVA to test the effects of three factors on seed production: 1) Year (2011/2014); 2) pollen source (same species/different species); 3) mother species (R. cyanocarpum/R. delavayi). For seed germination data, we first analyzed the data from the germination in distilled water using a two-factor ANOVA to test effects of pollen source and mother species on seed germination; then a three-factor ANOVA was employed to examine the effects of three factors (soil type, pollen source and mother species) on seed germination. After the confirmation of no effect on seed germination by soil type, a combined analysis of water and soil treatments for seed germination was done using a three-factor ANOVA (medium, pollen source and mother species). All statistical analyses were performed using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA).
Additional Information
How to cite this article: Ma, Y.-P. et al. Strong reproductive isolation despite occasional hybridization between a widely distributed and a narrow endemic Rhododendron species. Sci. Rep. 6, 19146; doi: 10.1038/srep19146 (2016).
Supplementary Material
Acknowledgments
We sincerely thank Mr. Ti-Wei Yang from Huadianba Medical Factory for field assistance and Dr. Gao Chen from Kunming Institute of Botany, CAS for his valuable suggestions about collection and identification of floral scents. This study was supported by the National Natural Science Foundation of China (Grant Nos. 31200247 and U1302262) and the Yunnan Natural Science Foundation (Grant No. 2012FB180).
Footnotes
Author Contributions Y.P.M. designed the research and wrote the manuscript; Y.P.M. and W.J.X. performed experiments; T.M. and W.B.S. revised the manuscript.
References
- Jennings J. H., Snook R. R. & Hoikkala A. Reproductive isolation among allopatric Drosophila Montana populations. Evolution 68, 3095–3108 (2014). [DOI] [PubMed] [Google Scholar]
- Schemske D. W. Adaptation and the origin of species. Am. Nat. 176(S1), S4–S25 (2010). [DOI] [PubMed] [Google Scholar]
- Nosil P. & Feder J. L. Genomic divergence during speciation: causes and consequences. Philos. Trans. R. Soc. B Biol. Sci. 367, 332–342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J., Bradshaw H. D. & Schemske D. W. Components of reproductive isolation between the monkey flowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57, 1520–1534 (2003). [DOI] [PubMed] [Google Scholar]
- Kay K. M. Reproductive isolation between two closely related hummingbird pollinated neotropical gingers. Evolution 60, 538–552 (2006). [PubMed] [Google Scholar]
- Dell’Olivo A., Hoballah M. E., Gübitz T. & Kuhlemeier C. Isolation barriers between Petunia axillaris and Petunia integrifolia (Solanaceae). Evolution 65, 1979–1991 (2011). [DOI] [PubMed] [Google Scholar]
- Baack E., Melo M. C., Rieseberg L. H. & Ortiz-Barrientos D. The origins of reproductive isolation in plants. New Phytol. 207, 968–984 (2015). [DOI] [PubMed] [Google Scholar]
- Chamberlain D. F. A revision of Rhododendron II. Subgenus Hymenanthes. Not. R. Bot. Garden Edinburgh 39, 209–486 (1982). [Google Scholar]
- Fang R. Z. & Min T. L. The floristic study on the genus Rhododendron. Acta Botanica Yunnanica 17, 359–379 (1995). [Google Scholar]
- Zhang J. L., Zhang C. Q., Gao L. M., Yang J. B. & Li H. T. Natural hybridization origin of Rhododendron agastum (Ericaceae) in Yunnan, China: inferred from morphological and molecular evidence. J. Plant Res. 120, 457–463 (2007). [DOI] [PubMed] [Google Scholar]
- Ma Y. P., Milne R. I., Zhang C. Q. & Yang J. B. Unusual patterns of hybridization involving a narrow endemic Rhododendron species in Yunnan (Ericaceae), China. Am. J. Bot. 97, 1749–1757 (2010a). [DOI] [PubMed] [Google Scholar]
- Ma Y. P., Zhang C. Q., Zhang J. L. & Yang J. B. Natural hybridization between Rhododendron delavayi and R. cyanocarpum (Ericaceae), from morphological, molecular and reproductive evidence. J. Integr. Plant Biol. 52, 844–851 (2010b). [DOI] [PubMed] [Google Scholar]
- Zha H. G., Milne R. I. & Sun H. Asymmetric hybridization in Rhododendron agastum: a hybrid taxon comprising mainly F1s in Yunnan, China. Ann. Bot. 105, 89–100 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczewski T., Chamberlain D. F. & Milne R. I. Hybridization in closely related Rhododendron species: half of all species-differentiating markers experience serious transmission ratio distortion. Ecol. Evol. doi: 10.1002/ece3.1570 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne R. I., Davies C., Prickett R., Inns L. H. & Chamberlain D. F. Phylogeny of Rhododendron subgenus Hymenanthes based on chloroplast DNA markers: Between lineage hybridisation during adaptive radiation? Plant Syst. Evol. 285, 233–244 (2010). [Google Scholar]
- Milne R. I., Terzioglu S. & Abbott R. J. A hybrid zone dominated by fertile F1s: maintenance of species barriers in Rhododendron. Mol. Ecol. 12, 2719–2729 (2003). [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Price D. K. & Stacy E. A. Postzygotic barriers isolate sympatric species of Cyrtandra (Gesneriaceae) in Hawaiian montane forest understories. Am. J. Bot. 102, 1870–1882 (2015). [DOI] [PubMed] [Google Scholar]
- Fang Q., Chen Y. Z. & Huang S. Q. Generalist passerine pollination of a winter-flowering fruit tree in central China. Ann. Bot. 109, 379–384 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe A. D. & Chittka L. The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471–510 (2001). [DOI] [PubMed] [Google Scholar]
- Knudsen J. T., Eriksson R., Gershenzon J. & Stahl B. Diversity and distribution of floral scent. Bot. Rev. 72, 1–120 (2006). [Google Scholar]
- Ma Y. P., Wu Z. K., Dong K., Zhang C. Q., Sun W. B. & Marczewski T. Pollination biology of Rhododendron cyanocarpum (Ericaceae), an alpine species endemic to NW Yunnan, China. J. Sys. Evo. 53, 63–71 (2015). [Google Scholar]
- Wesselingh R. A. & Arnold M. L. Pollinator behavior and the evolution of Louisiana iris hybrid zones. J. Evo. Biol. 13, 171–180 (2000). [Google Scholar]
- Lowry D. B., Modliszewski J. L., Wright K. M., Wu C. A. & Willis J. H. The strength and genetic basis of reproductive isolating barriers in flowering plants. Philos. Trans. R. Soc. B Biol. Sci. 363, 3009–3021 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D., Chamberlain D. F. & Argent G. The Red List of Rhododendrons. Botanic Gardens Conservation International, Richmond, p 128 (2011). [Google Scholar]
- Fang Q. & Huang S. Q. Relative stability of core groups in pollination networks in a biodiversity hotspot over four years. Plos one 7, e32663 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. Q. & Huang S. Q. Floral divergence, pollinator partitioning and the spatiotemporal pattern of plant–pollinator interactions in three sympatric Adenophora species. Oecologia 173, 1411–1423 (2013). [DOI] [PubMed] [Google Scholar]
- Hou X., Duan C., Tang C. Q. & Fu D. Nutrient relocation, hydrological functions, and soil chemistry in plantations as compared to natural forests in central Yunnan, China. Eco. Res. 25, 139–148 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.