Abstract
The pharmacological activation of the cholesterol-binding Translocator Protein (TSPO) leads to an increase of endogenous steroids and neurosteroids determining benefic pleiotropic effects in several pathological conditions, including anxiety disorders. The relatively poor relationship between TSPO ligand binding affinities and steroidogenic efficacies prompted us to investigate the time (Residence Time, RT) that a number of compounds with phenylindolylglyoxylamide structure (PIGAs) spends in contact with the target. Here, given the poor availability of TSPO ligand kinetic parameters, a kinetic radioligand binding assay was set up and validated for RT determination using a theoretical mathematical model successfully applied to other ligand-target systems. TSPO ligand RT was quantified and the obtained results showed a positive correlation between the period for which a drug interacts with TSPO and the compound ability to stimulate steroidogenesis. Specifically, the TSPO ligand RT significantly fitted both with steroidogenic efficacy (Emax) and with area under the dose-response curve, a parameter combining drug potency and efficacy. A positive relation between RT and anxiolytic activity of three compounds was evidenced. In conclusion, RT could be a relevant parameter to predict the steroidogenic efficacy and the in vivo anxiolytic action of new TSPO ligands.
The 18 kDa Translocator Protein (TSPO) is an outer mitochondrial membrane high affinity cholesterol- and drug-binding protein abundant in steroid-producing tissues, including gonads, adrenal, and brain1. Previous pharmacological, biochemical and genetic studies, as well as in vivo experiments, have provided several lines of evidence demonstrating that TSPO is a key member of the multiprotein complex transduceosome, as it participates to the cholesterol translocation into mitochondria, which is considered the rate-limiting step of steroidogenesis2.
Consistent with the important role of TSPO in steroidogenesis3 and the involvement of steroids in numerous fundamental processes8,9, TSPO ligands have been proposed as innovative therapeutic tools in several pathological conditions. For example, TSPO ligands have been implicated in axonal regeneration10,11,12, in anti-inflammatory13, anxiolytic14,15,16,17,18, antidepressant19 and anti-post-traumatic stress20 activities, both in animal models, and in individuals with neurological or psychiatric disorders. To date, phase II and III clinical trials have been concluded or are ongoing for the treatment of diabetic peripheral neuropathy (ClinicalTrials.gov identifier: NCT00502515), chemotherapy-induced peripheral neuropathy (NCT00868166), and generalized anxiety disorder (NCT00108836).
However, one of the most recurrently issue concerning TSPO ligands consists in the lack of correlation between the binding affinity and the in vitro efficacy, including steroidogenic efficacy21. This phenomenon has limited not only the identification of lead compounds during the traditional affinity-based drug discovery processes, but also questioned the specificity of the observed effects22,23,24. Recent studies have shown that the affinity of a ligand for its target could not directly define its biological action effectiveness, that it may instead be related to the period for which a drug interacts with its target defined as ‘Residence Time’ (RT)25,26.
In the present work, it was investigated whether the RT could be a crucial measure to estimate the steroidogenic efficacy of a TSPO ligand. To this aim, a number of our previous reported TSPO ligands, belonging to phenylindolylglyoxylamides (PIGAs) was selected based on their different abilities to stimulate in vitro steroidogenesis27,28,29, Table 1. Among such selected TSPO compounds, three presented in vivo anxiolytic effects28,30,31.
Table 1. Structures of PIGAs, PK11195, and Ro5-4864.
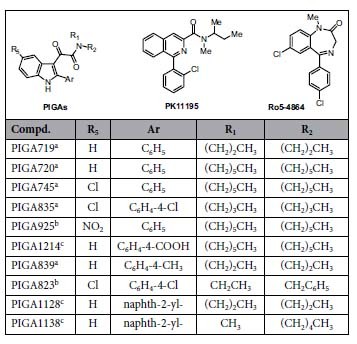
aPrimofiore et al., 2004.
bDaSettimo et al., 2008.
cBarresi et al., 2015.
As a first step, given the poor availability of kinetic parameters for TSPO ligands, a kinetic radioligand binding assay was set up and validated for TSPO ligand RT determination by the use of the theoretical mathematical model of Motulsky and Mahan32(RT = 1/koff, where koff is the dissociation rate constant). This model has been applied to several ligand-target systems and shown to be highly accurate in determining the binding kinetics of synthetic ligands33,34,35.
Results
RT determination: initial setting
The [3H]PK11195 radioligand was used as a probe for the determination of the kinetic parameters using membrane homogenates of rat kidney, a tissue highly rich in TSPO. Initial experiments were performed to fully characterize [3H]PK11195 binding kinetic parameters as few literature data were available36,37. [3H]PK11195 equilibrium binding parameters, Kd and Bmax, were 3.60 ± 0.41 nM and 6498 ± 500 fmol/mg of proteins, respectively (Table 2). The Ki value of unlabeled PK11195 determined by [3H]PK11195 displacement binding assay was 3.39 ± 0.34 nM (Table 2).
Table 2. TSPO ligand binding affinity and kinetic parameters obtained by equilibrium binding and kinetic association assays.
| TSPO ligand | Equilibrium Ki orKd (nM) | Kon (M−1. min−1) | Koff (min−1) | Kinetically-derivedKd = Koff/Kon (nM) | RT = 1/Koff(min) |
|---|---|---|---|---|---|
| PK11195 (Saturation binding)a | 3.60 ± 0.41 | NA | NA | NA | NA |
| PK11195 (Displacement)b | 3.39 ± 0.34 | NA | NA | NA | NA |
| PK11195 (Association and dissociation)c | 8.30 ± 0.64 × 106 | 0.030 ± 0.002 | 3.61 ± 0.21 | 33 ± 4 | |
| PK11195 (Standard competition association)d | 9.20 ± 0.71 × 106 | 0.034 ± 0.004 | 3.70 ± 0.43 | 29 ± 3 | |
| PK11195 (Simplified competition assay)e | 9.30 ± 0.94 × 106 | 0.029 ± 0.003 | 3.12 ± 0.37 | 34 ± 3 | |
| Ro5-4864 | 20.04 ± 2.36 | 1.29 ± 0.16 × 106 | 0.031 ± 0.002 | 24.04 ± 2.01 | 32 ± 3 |
| PIGA719 | 12.24 ± 1.1 | 7.89 ± 0.62 × 106 | 0.094 ± 0.007 | 11.95 ± 1.00 | 11 ± 2 |
| PIGA720 | 1.40 ± 0.28 | 2.18 ± 0.29 × 107 | 0.039 ± 0.004 | 1.79 ± 0.24 | 26 ± 2 |
| PIGA745 | 13.14 ± 1.16 | 3.84 ± 0.51 × 106 | 0.058 ± 0.003 | 15.12 ± 1.16 | 17 ± 1 |
| PIGA823 | 3.30 ± 0.31 | 4.30 ± 0.33 × 106 | 0.008 ± 0.001 | 1.86 ± 0.17 | 127 ± 4 |
| PIGA835 | 0.91 ± 0.11 | 5.78 ± 0.56 × 107 | 0.058 ± 0.005 | 1.00 ± 0.19 | 17 ± 1 |
| PIGA839 (M-PIGA) | 5.50 ± 0.47 | 1.69 ± 0.27 × 106 | 0.009 ± 0.001 | 5.45 ± 0.60 | 109 ± 4 |
| PIGA925 | 12.23 ± 3.14 | 6.78 ± 0.42 × 106 | 0.068 ± 0.004 | 10.01 ± 1.13 | 15 ± 2 |
| PIGA1128 | 0.31 ± 0.02 | 8.10 ± 0.36 × 107 | 0.018 ± 0.001 | 0.22 ± 0.05 | 55 ± 2 |
| PIGA1138 | 0.34 ± 0.03 | 4.30 ± 0.30 × 107 | 0.007 ± 0.001 | 0.17 ± 0.03 | 141 ± 4 |
| PIGA1214 | 343.01 ± 15.94 | 9.05 ± 0.51 × 104 | 0.030 ± 0.004 | 332.05 ± 15.04 | 39 ± 2 |
Values are means ± SEM of three experiments performed in duplicate. NA = not applicable.
a[3H]PK11195 binding performed using increasing concentration of radioligand.
bdisplacement of [3H]PK11195 binding using increasing concentration of PK11195.
cthe binding kinetics of [3H]PK11195 were determined by ‘traditional’ association and dissociation assays.
dthe binding kinetics of unlabeled PK11195 were determined by adding a concentration equivalent to one-, three- and ten-fold the Ki value of PK11195.
ethe binding kinetics of unlabeled PK11195 were determined by adding a concentration equivalent to only three-fold the Ki value of PK11195.
The koff determination was obtained by: (i) pre-labelling of TSPO to equilibrium with one [3H]PK11195 concentration (approximately 10 × Kd) that provides high initial TSPO occupancy; (ii) inducing radioligand dissociation by addition of TSPO-saturating concentration (about 1000 × Kd) of unlabeled competing compound PK11195. Then, the dissociation time-course was analyzed using an exponential function. An example of [3H]PK11195 dissociation kinetic curve is shown in Fig. 1A; [3H]PK11195 dissociation was monophasic and gave a half-life of radioligand-TSPO complex (t1/2) of 23 min, which when applied to Equation 1 (see Materials and Methods section) gave a koff of 0.030 ± 0.002 min−1. The RT value, which is the reciprocal of koff, was 33 ± 4 min (Table 2).
Figure 1. [3H]PK11195 kon and koff by ‘traditional’ dissociation and association kinetics assays.
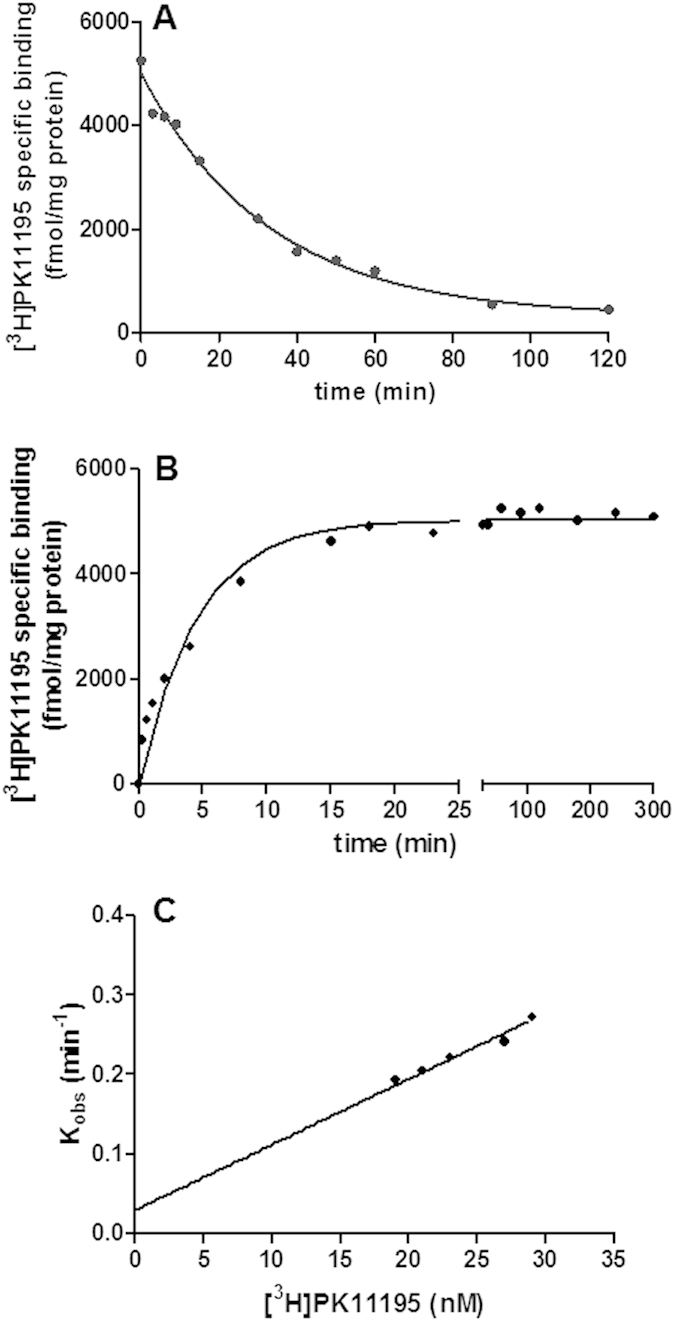
(A) [3H]PK11195 binding dissociation Kinetics: Data were best fitted using a one-phase exponential decay function to produce a t1/2 estimate. This was converted into a koff value by using Equation 1, as detailed in the Materials and Methods section. The ordinate reports the specific [3H]PK11195 binding expressed as fmol/mg of protein. The abscissa reports the incubation time expressed in min. (B,C) [3H]PK11195 binding association Kinetics: a family of association kinetics curves were constructed incubating membrane homogenates with a range of [3H]PK11195 concentrations as described in the Materials and Methods section. Data were fitted using a one phase exponential association function to yield a kob. (B) A representative association curve performed using 23 nM [3H]PK11195 up to 5 h incubation time is showed. The ordinate reports the specific [3H]PK11195 binding expressed as fmol/mg of protein. The abscissa reports the incubation time expressed in min. (C) kobs values plotted against the corresponding [3H]PK11195 concentration employed are showed.
To determine [3H]PK11195 kon, a family of association kinetic curves using a range of radioligand concentrations were constructed. Each association curve was monitored until equilibrium. In Fig. 1B, a representative [3H]PK11195 association kinetics curve is showed. If [3H]PK11195 binding follows a simple law of mass action model, the observed kinetic association constant (kob) should increase in a linear manner with radioligand concentration38. In this case, the slope of the line should equate to the association rate and extrapolation of the plot to the Y-intercept (at x = 0) should equal the dissociation rate39. When the kob values were plotted against radioligand concentration, the data were consistent with a straight line (r2 = 0.98), indicating that binding of [3H]PK11195 to TSPO was consistent with the law of mass action (Fig. 1C). The obtained values were kon = 8.30 ± 0.64 × 106 M−1 min−1 and koff = 0.030 ± 0.002 min−1 (Fig. 1C and Table 2). The kinetically derived Kd (3.61 ± 0.21 nM; kinetic Kd = koff/kon) was in good agreement with the value obtained from [3H]PK11195 saturation experiments (equilibrium Kd = 3.60 ± 0.41) (Table 2).
RT Determination: TSPO ligand koff and kon by competition kinetic association assays
With the predetermined kon (k1) and koff (k2) values of [3H]PK11195 from ‘traditional’ kinetic association and dissociation experiments, kon (k3) and koff (k4) of an unlabeled ligand could be determined by fitting the kinetic parameters into the model of ‘Kinetics of competitive binding’ described in Materials and Methods section. This method is based on a framework developed by Motulsky and Mahan32, where an unlabeled competitor is added simultaneously with a radioligand to the receptor preparation of interest. Then, the experimentally derived rate of specific radioligand binding can be modelled to provide the association and dissociation rates of the unlabeled compound.
As a first step, the competition association assay was performed using unlabeled PK11195. Three different concentrations of PK11195 were tested to ensure that the rate parameters calculated were independent of ligand concentration (Fig. 2A). The kon (k3) and koff (k4) values determined in this assay were 9.20 ± 0.71 × 106 M−1 min−1 and 0.034 ± 0.004 min−1, respectively (Table 2; PK11195 RT = 29 ± 3 min), which corresponded rather well to the kinetics rates determined by ‘traditional’ association and dissociation experiments (Table 2). Moreover, the kinetically derived Kd obtained from the competition association assay for unlabeled PK11195 was similar to Ki obtained from displacement experiments and Kd derived from saturation experiments (Table 2). Taken together, these findings proved that the competition association assay could be applied to determine the binding kinetics of an unlabeled TSPO ligand.
Figure 2. PK11195 kon and koff by competitive association kinetics assays.
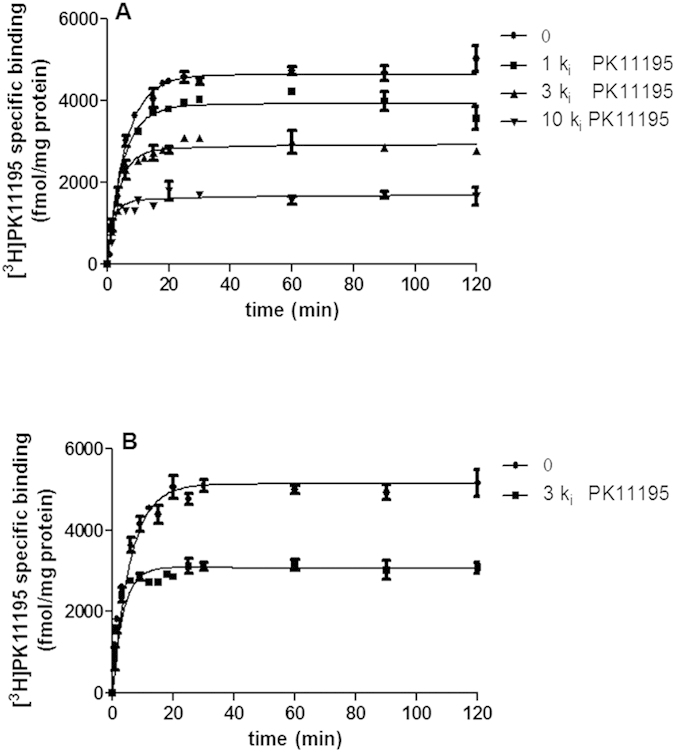
(A) The curves were obtained by incubation of membranes with either radioligand alone or radioligand and unlabeled PK11195 for the indicated time points. Data were fitted to the equation 2 to calculate the kon and koff of PK11195. Representative curves obtained using one-, three-, ten-fold Ki value of unlabeled PK11195. (B) Representative curves obtained using three-fold Ki value of unlabeled PK111195.
The competition association assay approach has been shown to be highly accurate in determining the binding kinetics at several targets33,34,35. However, when the kinetics of multiple compounds need to be determined, the standard model is laborious and time consuming because it implies the use of three concentrations of each unlabeled ligand. Recently, it has been demonstrated that the use of one concentration of unlabeled ligand is able to yield an accurate determination of kinetic rates of unlabeled ligands at their receptor, too34. These findings prompted us to modify the three-concentration-dependent assay into a one-concentration-based method. The data analyzed at three-fold Ki of unlabeled PK11195 showed a comparable result (kon = 9.30 ± 0.94 × 106 M−1 min−1 and Koff = 0.029 ± 0.003 min−1; RT = 34 ± 3 min) (Fig. 2B) to that generated in a standard (three-concentration-dependent) competition association experiment (Table 2). This result indicates that this simplified method is strong enough to quantify the binding kinetics, which eventually enables testing in a faster medium-throughput format, yet without loss of accuracy.
By using the ‘simplified’ competition kinetic association assay, the TSPO ligands, including the classical ones Ro5-4864 and PIGA compounds (Table 1) were tested at three-fold respective Ki concentration and data were fitted using Equation 2 (see Material and Methods section) to calculate kon and koff simultaneously (Table 2). Competition association assay results demonstrated two patterns of [3H]PK11195 binding in dependence of the competing ligand used. In general, if the competitor dissociates from its target faster than the radioligand, the specific binding of the radioligand will approach its equilibrium time slowly and monotonically. However, when the competitor dissociates slower, the association curve of the radioligand will consist of two phases, starting with a typical “overshoot” and then a decline until a new equilibrium is reached. The results obtained are consistent with a rapid (PIGA719, PIGA720, PIGA745, PIGA835, PIGA925, PIGA1214, Ro5-4864) and a slow (PIGA823, PIGA839 (M-PIGA), PIGA1128, PIGA1138) dissociation rate of the ligands from TSPO. Representative curves for rapid dissociating (PIGA1214) and slow dissociating (PIGA1138) TSPO ligands were shown in Fig. 3A,B, respectively.
Figure 3. Competitive association kinetics assays of TSPO ligands and correlation between Ki and ‘Kinetic Kd’ values.
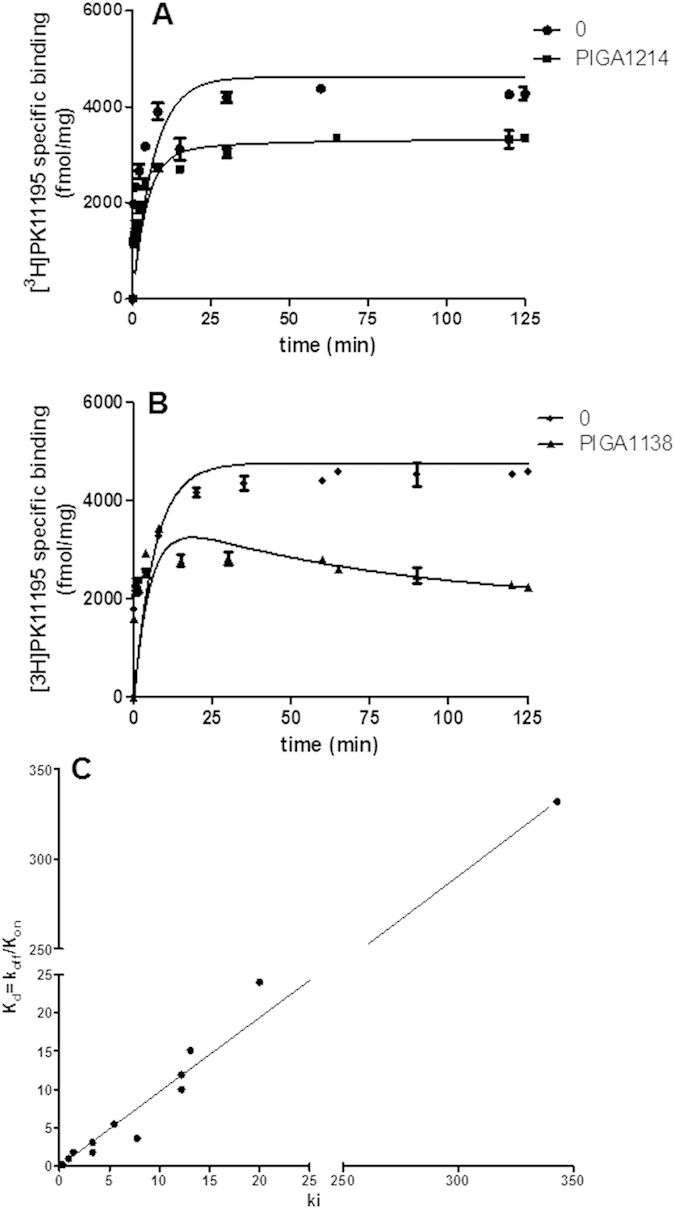
(A) Representative curves obtained using three-fold Ki of PIGA1214 (B) or PIGA1138. (C) Ki values were obtained from [3H]PK11195 competition binding experiments at equilibrium. The kinetically Kd values were derived from the competition association experiments.
To validate the rate constants, the kinetically derived Kd were compared with Ki obtained from equilibrium competition binding experiments. Notably, an excellent correlation (r2 = 0.999, p < 0.0001; Fig. 3C) was observed between Ki determined in equilibrium-binding studies and Kd values derived from the competition association assays (Table 2). This further proved that the simplified model is able to quantify the association and dissociation rates of unlabeled TSPO ligands accurately.
TSPO ligand steroidogenic efficacy
The steroidogenic efficacy of TSPO ligands was measured in terms of pregnenolone production in C6 glioma cells following exposure with increasing ligand concentrations for a fixed incubation time. For each TSPO ligand, potency (EC50 value) was derived by sigmoidal concentration-dependent curve and efficacy (Emax value, relative to the highest tested concentration of TSPO ligand) was calculated with respect to control (DMSO-treated sample), corresponding to basal pregnenolone production. In Fig. 4, the curves of TSPO ligand-stimulated pregnenolone production are shown. The EC50 and Emax values are detailed in Table 3. Specifically, among all tested TSPO ligands, M-PIGA, PIGA823 and PIGA1138 had the highest efficacy. The majority of the tested TSPO ligands showed efficacy to stimulate pregnenolone production ranging from 140% up to 179% (basal value was set to 100%).
Figure 4. Effects of the TSPO ligands (PK11195, Ro4-54864, PIGAs) on glioma C6 cell steroid synthesis.
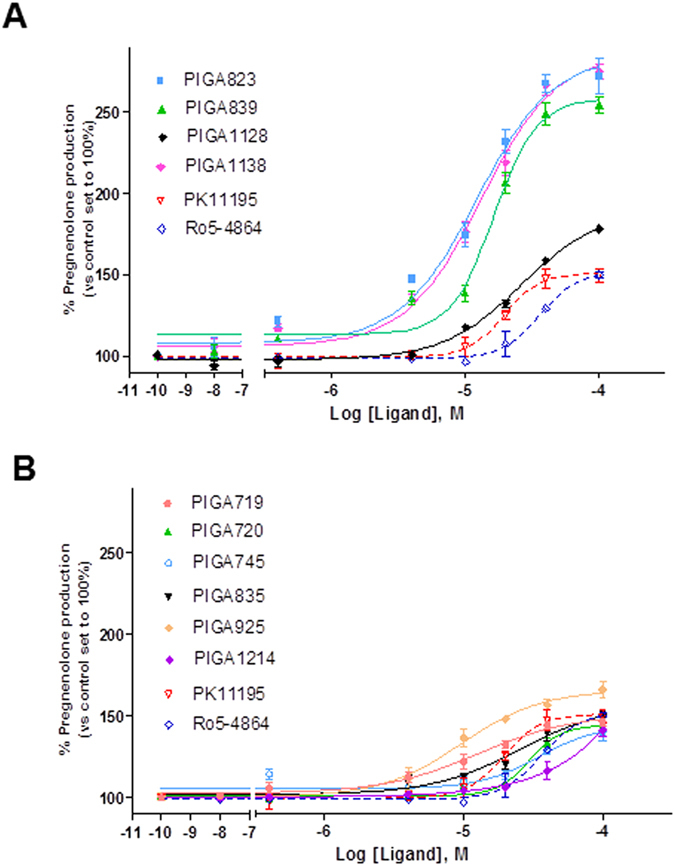
(A) Dose-dependent effects of the compounds with longest RTs (>40 min) on C6 steroidogenesis (B) Dose-dependent effects of the compounds with shortest RTs (<40 min) on C6 steroidogenesis. C6 cells were cultured with increasing concentrations of ligands (0–100 μM) for 2 h in serum-free media and pregnenolone released into the media was assessed by ELISA. The results are expressed as the mean of three separate experiments, and data were fitted with a dose–response curve with Hill slope of 1.0.
Table 3. Experimental kinetic/thermodynamic data and steroidogenic parameters for TSPO ligands.
| TSPO ligand | EC50(μM) | Emax (at 100 μM)(vehicle set to 100%) | Equilibrium Ki or Kd(nM) | RT (min) |
|---|---|---|---|---|
| PK11195 | 19.5 ± 1.8 | 153 ± 4 | 3.30 ± 0.34 | 34 ± 3 |
| Ro5-4864 | 36.2 ± 2.5 | 150 ± 4 | 20.04 ± 2.36 | 32 ± 3 |
| PIGA719 | 12.9 ± 1.5 | 146 ± 2 | 12.24 ± 1.1 | 11 ± 2 |
| PIGA720 | 29.7 ± 3.1 | 144 ± 4 | 1.40 ± 0.28 | 26 ± 2 |
| PIGA745 | 34.6 ± 3.6 | 140 ± 5 | 13.14 ± 1.16 | 17 ± 1 |
| PIGA823 | 12.2 ± 1.3 | 272 ± 11 | 3.30 ± 0.31 | 127 ± 4 |
| PIGA835 | 25.6 ± 2.3 | 149 ± 4 | 0.91 ± 0.11 | 17 ± 1 |
| PIGA839 (M-PIGA) | 16.3 ± 1.4 | 254 ± 5 | 5.50 ± 0.47 | 109 ± 4 |
| PIGA925 | 9.71 ± 1.0 | 166 ± 5 | 12.23 ± 3.14 | 15 ± 2 |
| PIGA1128 | 26.1 ± 1.5 | 179 ± 7 | 0.31 ± 0.02 | 55 ± 2 |
| PIGA1138 | 13.1 ± 1.4 | 275 ± 5 | 0.34 ± 0.03 | 141 ± 4 |
| PIGA1214 | 92.0 ± 5.6 | 141 ± 4 | 343.01 ± 15.94 | 39 ± 2 |
Values are means ± SEM of three experiments performed in duplicate. Potency (EC50 value) of TSPO ligands was derived by sigmoidal concentration-dependent curves. Efficacy (Emax) corresponds to amount of pregnenolone production by 100 μM TSPO ligand concentration.
Correlation between RT and steroidogenesis efficacy
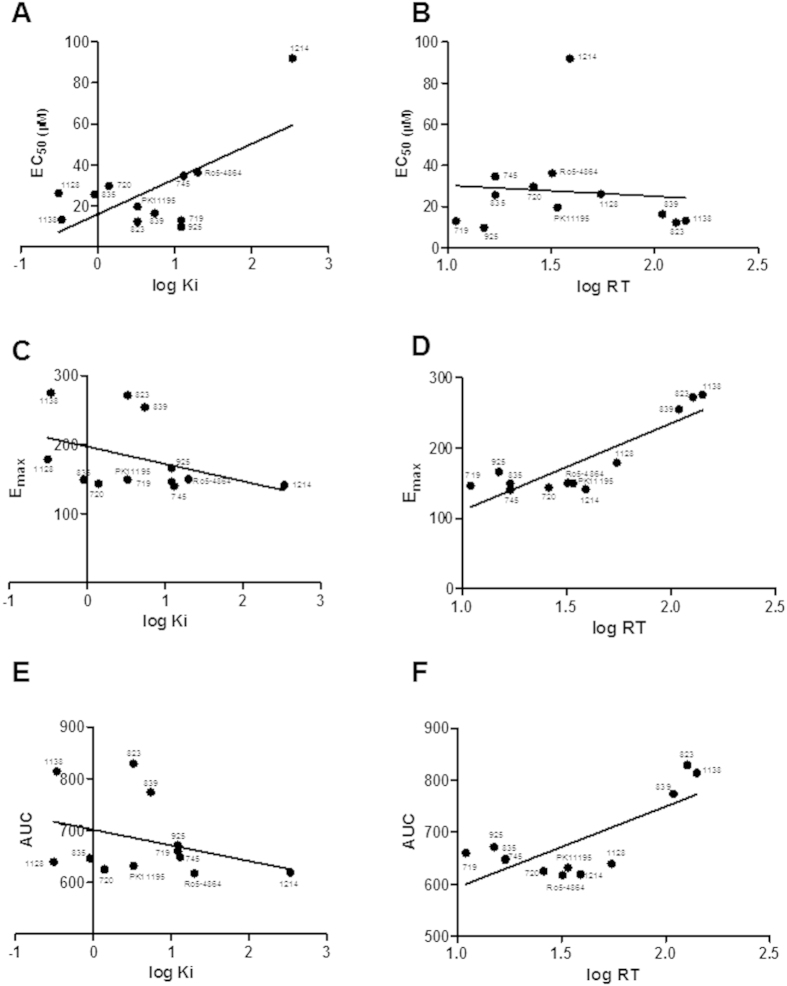
In Fig. 5, correlation analyses are reported between the TSPO ligand-mediated steroidogenic potency or efficacy and either binding affinity (Ki) or Residence Time (RT). The steroidogenic potency of TSPO ligands correlated with the logarithm of Ki (Pearson r = 0.6553; P = 0.0207; r2 = 0.4295) (Fig. 5A), in agreement with previous reported data40,41,42, but not with RT (Pearson r = 0.08962; P = 0.7818; r2 = 0.0803) (Fig. 5B). The steroidogenic efficacy of TSPO ligands did not significantly correlate with Ki (Pearson r = −0.3489; P = 0.2663; r2 = 0.1217) (Fig. 5C). Conversely, a highly significant correlation was observed between steroidogenic efficacy and RT (Pearson r = 0.8526; P = 0.0004; r2 = 0.7270) (Fig. 5D). Correlation analyses were also performed between the logarithm of Ki or RT and the area under the dose-response curve (AUC), a value that combines potency and efficacy of a drug into a single parameter43. When the relationship between AUC and the logarithm of Ki was analyzed, no correlation was found (Pearson r = −0.3308; P = 0.2937; r2 = 0.1094) (Fig. 5E). On the contrary, the AUC values significantly correlates with logarithm of RT (Pearson r = 0.7563; P = 0.0044; r2 = 0.5720) (Fig. 5F).
Figure 5. Correlation analyses between kinetic/thermodynamic and steroidogenic parameters.
TSPO compound names were included next to their respective data points (for PIGA compounds only the ID numbers were shown). (A) Scatter plot of the EC50 values against thermodynamic parameters (logKi) of test TSPO ligands; (B) Scatter plot of the EC50 values against kinetic parameters (logRT) of test TSPO ligands; (C) Scatter plot of the Emax values against thermodynamic parameters (logKi) of test TSPO ligands; (D) Scatter plot of the Emax values against kinetic parameters (logRT) of test TSPO ligands; (E) Scatter plot of the AUC values against thermodynamic parameters (logKi) of test TSPO ligands; (F) Scatter plot of the AUC values against kinetic parameters (logRT) of test TSPO ligands.
Discussion
The TSPO drug discovery has followed the traditional thermodynamic equilibrium constant (Kd or Ki) paradigm to identify lead compounds. However, binding kinetics parameters, especially the Residence Time of a drug on its target, are becoming critical predictors for in vivo drug efficacy25,26. In the present manuscript, accurate kinetic parameters for TSPO ligands were obtained by the use of the mathematical model of Motulsky and Mahan32 that has been shown to be highly accurate in determining the binding kinetics of unlabeled ligands at several targets33,34,35. This mathematical model has overcame the high cost of compound radiolabeling in order to measure directly the association and dissociation rates of a drug to the target44. For binding kinetics determination, classical TSPO ligands (the isoquinoline PK11195 and the benzodiazepine Ro5-4864) and the TSPO ligand PIGAs27 (Table 1) were selected. Specifically, the majority of the tested TSPO compounds resulted rapid dissociating competitors of [3H]PK11195 binding sites (PIGA719, PIGA720, PIGA745, PIGA835, PIGA925, PIGA1124 and Ro5-4864) (Table 2). Conversely, the compound PIGA823, PIGA839 (M-PIGA), PIGA1128, and PIGA1138 resulted slow dissociating competitors (Table 2). Concerning the molecular determinants underling the TSPO-ligand kinetic interactions, no literature data are available. The estimation of the RT for a large number of TSPO ligands could support in determining new Structure Activity Relationship (SAR) based on the ligand kinetic parameters. Indeed up to date, SAR has been rationalized only on binding affinity parameters using pharmacophore hypotheses27,28,45,46 and a 3D model29 based on the newly published NMR structure of mouse TSPO (PDB code 2MGY)47. Notably, the steroidogenic efficacy of TSPO ligands did not correlated with the compound binding affinity21, suggesting the limitation of a SAR affinity-based strategy.
Herein, a highly significant positive correlation between the efficacy to stimulate in vitro steroidogenesis of TSPO ligands and their kinetic parameter RT was found. Remarkably, the efficacy did not correlate with the thermodynamic equilibrium constant Ki of the TSPO ligands. Taken together, these results indicate that the key factor for robust steroidogenic TSPO ligand efficacy is not the binding affinity per se, but rather the time the compound spends on the target. Such this novel concept for TSPO ligands suggests the importance of using the RT parameter rather than the constant Ki during the in vitro characterization of TSPO compounds in relation to their steroidogenic activity.
The results discussed thus far allow us to outline a preliminary efficacy-based SAR for the interaction of PIGAs with TSPO. Data in Table 2 suggest a cooperative effect between the size of the substituents on the amide nitrogen and the lipophilicity of the aryl group at the 2-indole position. In particular, high retention times and high efficacy seem to derive from the presence of an highly lipophilic moiety at the 2-position (C6H4-4-CH3, C6H4-4-Cl, naphth-2-yl), combined with at least one of the two N-alkyl groups with a number of carbon atoms in the 1–3 range (PIGA839, PIGA823, PIGA1138).
From a therapeutic perspective, this new concept paves the way to identify TSPO drugs with promising pharmacological activities, including anxiolytic effects. Scientific community has directed particular interest to TSPO ligands for the treatment of anxiety-related disorders, as they have shown fast-acting anxiolytic properties without the typical side-effects of benzodiazepine-based regimes15,48,49. Differently to benzodiazepines, which act as direct modulators of the GABAA receptor, TSPO ligands generally enhance GABAergic neurotransmission via the promotion of neurosteroidogenesis without direct effects at the GABAA receptor17. Neurosteroids, especially the 3-alpha-reduced steroids, are potent positive allosteric modulators of GABAA receptor17. Consistent with such evidences, previous our data30 have shown that the medium from PIGA839 (M-PIGA)-treated human glial cell model contained high levels of allopregnanolone, one of the major positive GABAA receptor allosteric modulator. The conditioned medium potentiated the 36Cl− uptake into cerebral cortical synaptoneurosomes, suggesting a positive modulation of GABAA receptor activity30. The PIGA ligands have been already tested for their potential in vivo anxiolytic effects by means of the Elevated Plus-Maze (EPM) paradigm in the rat (PIGA823: compound number 32 in28, and PIGA839 (M-PIGA) in30). In the EPM test, either PIGA823 and PIGA839 (M-PIGA), characterized by long RT and high steroidogenic efficacy, have elicited a significant anxiolytic activity (PIGA823: RT = 127 min, Emax pregnenolone = 272%; PIGA839 (M-PIGA): RT = 109 min; Emax pregnenolone = 254%). Conversely, it has been documented that the classical TSPO ligand PK11195 and Ro5-4864, which are characterized by shorter RT and lower pregnenolone Emax (PK11195: RT = 34 min, Emax pregnenolone = 153%; Ro5-4864: RT = 32 min; Emax pregnenolone = 150%) than PIGA823 and PIGA839 (M-PIGA), does not determine anxiolytic effect in EPM test50. In addition, PK11195 has been used to antagonize anxiolytic effects exerted by other TSPO ligands (high steroidogenic efficacy, such as FGIN-1-27, FGIN-1-44 and YL-IPA08)4,6,19. These retrospective assessments suggested that a long RT predicts the anxiolytic activity of a TSPO ligand. Moreover, RT is a better efficacy predictive measure than the thermodynamic equilibrium constant Kd or Ki. Indeed, PK11195, PIGA823 and PIGA839 (M-PIGA) showed comparable Ki values (PK11195: Ki = 3.60 nM; PIGA823 Ki = 3.30 nM; PIGA839 (M-PIGA) Ki = 5.50 nM), irrespective to their in vivo activity. Consistent with our data, it has been recently demonstrated discrepancy between the Ki of known anxiolytic TSPO ligands (Etifoxine and XBD173) and the enhancement of neurosteroid synthesis21. Specifically, Etifoxine, which is already clinically approved for the treatment of anxiety-related disorders, is more potent to stimulate neurosteroidogenesis than XBD173, although its binding affinity to TSPO was approximately 140 fold lower than XBD173. These findings have suggested that the efficacy of such TSPO ligands to stimulate neurosteroid synthesis, thereby leading to anxiolytic effects, cannot be concluded from their binding affinity to TSPO.
In conclusion, the present results indicate that the Residence Time is a better parameter to estimate the steroidogenic effectiveness of a TSPO ligand compared to the equilibrium thermodynamic parameters, corroborating the importance of the drug-target interaction dynamics in predicting the drug efficacy. These findings, in combination with the future availability of a RT database, open the way to optimize TSPO ligands as promising therapeutic tools.
Materials and Methods
Reagents
[3H]PK11195 (Specific Activity, 85.7 μCi/nmol) and Ultima Gold scintillation mixture were obtained from Perkin-Elmer Life Sciences. PK11195, protease inhibitors and GF/C glass fiber filters were purchased from Sigma-Aldrich. 2-arylindol-3-ylglyoxyl derivatives were synthesized as previously described27,28,29. Dulbecco’s modified Eagle’s medium, fetal bovine serum, L-glutamine, penicillin, and streptomycin were from Lonza (Milano, Italy). Enzyme immunoassay (ELISA) for pregnenolone measurement was obtained from IBL (Hamburg, Germany). SU10603 and trilostane were gifts from Novartis Farma (Varese, Italy) and Dr. Zister (University of Dublin, Dublin, Ireland), respectively. Protein assay reagent was obtained by Bio-Rad Laboratories Inc. All other chemical reagents were obtained by commercial sources.
[3H]PK11195 binding saturation assays
Membranes from rat kidneys were prepared as described previously51. All the experimental procedures were carried out following the guidelines of the European Community Council Directive 86–609 and have been approved by the Committee for animal experimentation of the University of Pisa. The resulting membrane pellets were aliquoted and frozen at –20 °C. For all radioligand binding assays, an aliquot of membranes was thawed, suspended in assay buffer (AB, Tris-HCl 50 mM, pH 7.4) and homogenized using Ultraturrax. In cell membrane homogenate, protein content was measured by the Bradford method52 using the Bio-Rad Protein Assay reagent.
Membrane homogenates (30 μg of proteins) were incubated with increasing [3H]PK11195 concentrations (0.1–20 nM; Specific Activity, 85.7 μCi/nmol) in the final volume of 500 μl of AB for 90 min at 0 °C. Non-specific [3H]PK11195 binding was obtained in the presence of 1 μM PK11195 (solubilized with ethanol); the solvent concentration was less than 1% and did not interfere with specific [3H]PK11195 binding. After incubation time, samples were filtered rapidly under vacuum through GF/C glass fiber filters. After being washed three times with 3 ml of AB, radioactivity trapped on the filter was measured by liquid scintillation counter (TopCount; PerkinElmer Life and Analytical Sciences; 65% counting efficiency).
PIGA TSPO ligands
The compounds PIGA719, PIGA720, PIGA745, PIGA835, PIGA839 (M-PIGA)27, PIGA925, PIGA823, PIGA92228, PIGA1214, PIGA1128, PIGA1138 were synthesized following experimental procedure previously described by us29.
[3H]PK11195 binding displacement assays
Membrane homogenates (20 μg of proteins) were incubated with increasing concentrations of unlabeled TSPO ligand and 1 nM [3H]PK11195 (Specific Activity, 85.7 μCi/nmol) in the same above described conditions. The inhibitory constant (Ki) determination was performed for PK11195 and additional compounds, including Ro5-4864 (Sigma-Aldrich Milano, Italy) and PIGA ligands27,28,29.
[3H]PK11195 binding ‘traditional’ kinetics assays
The [3H]PK11195 koff was determined by incubating membrane homogenates (30 μg of protein) with [3H]PK11195 at one fixed concentration, corresponding to approximately ten-fold its Kd value, in a final volume of 500 μl AB at 0 °C. To obtain [3H]PK11195 concentration, corresponding to 10 × Kd, approximately 1.5 μCi of [3H] had to be added per assay. Since 1 μCi of [3H] per assay represents the practical upper limit of radiotracer usage, the radioligand was diluted with unlabeled PK11195 to reduce its specific activity. In brief, the specific activity of [3H]PK11195 was reduced to ¼ of its initial original specific activity (Specific Activity, 21.4 μCi/nmol). This allowed us to use a maximum amount of approximately 0.38 μCi of [3H] per sample. After a pre-incubation of 2 h, the dissociation was initiated by addition of 5 μM PK11195. The amount of radioligand bound to TSPO was measured at various time intervals for a total duration of 2 h.
To determine [3H]PK11195 kon, the observed association rate constant (kob) was calculated at different concentrations of [3H]PK11195 (Specific Activity, 21.4 μCi/nmol). The experiment was initiated (t = 0) by addition of [3H]PK11195 to membrane homogenates (30 μg of proteins) in a final volume of 500 μl of AB and incubated up to 2 h. To establish whether [3H]PK11195 binding was stable for longer times than 2 h, incubation times were prolonged up to 5 h in some kinetic association binding assays. Free [3H]PK11195 was separated at multiple time points to construct association kinetic curves. Incubations for both the [3H]PK11195 dissociation and association assays were terminated and samples were obtained as above described.
Unlabeled TSPO ligand competition kinetic association assays
The unlabeled TSPO ligand kinetic parameters were assessed using the theoretical model of Motulsky and Mahan32. Unlike methods in which one compound is pre-equilibrated with the receptor, this approach involves the simultaneous addition of both radioligand and competitor to receptor preparation, so that at t = 0 all receptors are unoccupied. [3H]PK11195 (approximately 30 nM; SA, 21.4 μCi/nmol) was added simultaneously with unlabeled compound to membrane homogenates (30 μg of proteins) in a final volume of 500 μl AB. The degree of bound to TSPO was assessed at multiple time points by filtration harvesting and liquid scintillation counting, as above described. The assay was performed using concentration of PK11195 corresponding to one-, three- and ten-fold its Ki. For ‘simplified’ competition kinetic association assays, the experiments were performed using concentration of unlabeled TSPO ligands corresponding to three-fold their Ki.
Pregnenolone measurement
Pregnenolone assessment was performed using rat C6 glioma cells as an in vitro steroidogenic model, as previously described29. C6 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 M L-glutamine, penicillin at 100 U/mL, and streptomycin at 100 μg/mL. Cell cultures were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Before the measurement of pregnenolone production, the cells (seeded in 96-well plates at a density of ∼105 cells/well) were washed 2 times with a salt medium, consisting of 140 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgSO4, 10 mM glucose, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid(HEPES)–NaOH (pH 7.4) plus 0.1% bovine serum albumin. For the measurement of pregnenolone secreted into the medium, the further metabolism of pregnenolone was blocked by the addition of trilostane (25 μM) and SU10603 (10 μM) (inhibitors of 3β-hydroxysteroid dehydrogenase and 17α-hydroxylase, respectively) to the salt medium. The addition of PK11195, Ro5-4864 or PIGAs to the C6 cells was accomplished by complete change of the salt medium to a medium containing increasing concentrations of the compounds (ranging from 0 to 100 μM). The final concentration of vehicle (DMSO or ethanol) was constant for all of the samples and did not exceed 0.5% (v/v), a concentration that did not affect steroid production on its own. At the end of the incubation periods (2 hours), the cell medium was collected and the amount of pregnenolone secreted into the medium was quantified by an enzyme immunoassay, under the conditions recommended by the supplier. Cross-reactivity with other steroids was typically less than 1% and cross-reactivity with progesterone is 6%. The sensitivity of the assay was 0.05 ng/ml. Unknown samples were compared with concurrently run standards of pregnenolone using a one-site competition model (calibrator curve).
Data analysis
All experiments were analyzed by either linear or non linear regression using Prism 5.0 (GraphPad Software Inc., San Diego, CA). Equilibrium Kd and maximum binding sites (Bmax) values of [3H]PK11195 at TSPO were obtained by computational analysis of saturation curves. Competition displacement binding were fitted to sigmoidal (variable slope) curves. The concentration of test compounds that inhibited [3H]PK11195 binding to kidney membranes by 50% (IC50 values) obtained from the inhibition curves were converted to Ki values using the method of Cheng and Prusoff53. [3H]PK11195 dissociation data were fitted to a one-phase exponential decay function and the t1/2 value obtained was transformed into a koff rate using the Equation:
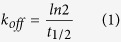 |
[3H]PK11195 association data were fitted to a single phase exponential association function to calculate an observed rate constant kob.
Association and dissociation rates for unlabeled TSPO ligands were calculated by fitting the data in the competition association model using the ‘kinetics of competitive binding’ assay, defining the amount of radioligand bound to receptor ([RL]) as a function of time32:
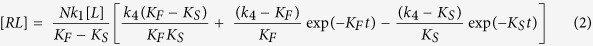 |
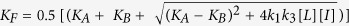 |
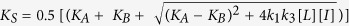 |
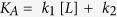 |
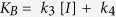 |
The abbreviations used are: [RL] is the receptor-radioligand complex, as the specific [3H]PK11195 binding (fmol/mg of protein); [L] is the concentration of [3H]PK11195 used (nM), [I] is the concentration of unlabeled ligand (nM); t is the time in min; N is the total concentration of TSPO (fmol/mg of protein); k1 and k2 are the kon (M−1 min−1) and koff (min−1) of [3H]PK11195, respectively, determined from the radioligand association assay; k3 is the kon value (M−1 min−1) of the unlabeled ligand; k4 is the koff value (min−1) of the unlabeled ligand.
Correlation analyses were performed by Pearson correlation.
Additional Information
How to cite this article: Costa, B. et al. TSPO ligand residence time: a new parameter to predict compound neurosteroidogenic efficacy. Sci. Rep. 6, 18164; doi: 10.1038/srep18164 (2016).
Acknowledgments
Funding for this study was provided by the Italian Ministry of University and Scientific Research (PRIN-prot. 20098SJX4F; PRIN-prot. 2010W7YRLZ_005 and FIRB-prot. RBFR10ZJQT_002).
Footnotes
Author Contributions B.C. conceived and conducted the experiments, analyzed the results, wrote the manuscript. E.D.P. conceived and conducted the experiments, analyzed the results. C.G. conducted the experiments. E.B. synthetized TSPO ligands, performed the 1H NMR and elemental analyses. T.S. and F.D.S. revised the manuscript. C.M. conceived the idea of the manuscript and revised the manuscript.
References
- Batarseh A. & Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol. Cell. Endocrinol. 327, 1–12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V. et al. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol. Cell. Endocrinol. 408, 90–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneyev A. et al. Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J. Neurochem. 61, 1515–1524 (1993). [DOI] [PubMed] [Google Scholar]
- Romeo E. et al. Stimulation of brain steroidogenesis by 2-aryl-indole-3-acetamide derivatives acting at the mitochondrial diazepam-binding inhibitor receptor complex. J. Pharmacol. Exp. Ther. 267, 462–471 (1993). [PubMed] [Google Scholar]
- Serra M. et al. 2-Phenyl-imidazo[1,2-a]pyridine derivatives as ligands for peripheral benzodiazepine receptors: stimulation of neurosteroid synthesis and anticonflict action in rats. Br. J. Pharmacol. 127, 177–187 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D., Foley M., Audette D., Leslie N. & Frye C. A. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 151, 64–71 (2000). [DOI] [PubMed] [Google Scholar]
- Verleye M. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol. Biochem. Behav. 82, 712–720 (2005). [DOI] [PubMed] [Google Scholar]
- Yuki A. et al. Relationship between low free testosterone levels and loss of muscle mass. Sci. Rep. 3, 1818 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrum J. A., Dastidar R. G., Ong J. F., Levy O. & Karp J. M. Performance-enhanced mesenchymal stem cells via intracellular delivery of steroids. Sci. Rep. 4, 4645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferzaz B. et al. SSR180575 (7-chloro-N,N,5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide), a peripheral benzodiazepine receptor ligand, promotes neuronal survival and repair. J. Pharmacol. Exp. Ther. 301, 1067–1078 (2002). [DOI] [PubMed] [Google Scholar]
- Girard C. et al. Etifoxine improves peripheral nerve regeneration and functional recovery. Proc. Natl. Acad. Sci. USA 105, 20505–20510 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C. Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa. Neuroendocrinol. 24, 71–81 (2012). [DOI] [PubMed] [Google Scholar]
- Torres S. R. Anti-inflammatory effects of peripheral benzodiazepine receptor ligands in two mouse models of inflammation. Eur. J. Pharmacol. 408, 199–211 (2000). [DOI] [PubMed] [Google Scholar]
- Bitran D., Foley M., Audette D., Leslie N. & Frye C. A. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 151, 64–71 (2000). [DOI] [PubMed] [Google Scholar]
- Kita A. et al. Antianxiety and antidepressant-like effects of AC-5216, a novel mitochondrial benzodiazepine receptor ligand. Br. J. Pharmacol. 142, 1059–1072 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R. et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug. Discov. 9, 971–988 (2010). [DOI] [PubMed] [Google Scholar]
- Da Pozzo E., Costa B. & Martini C. Translocator protein (TSPO) and neurosteroids: implications in psychiatric disorders. Curr. Mol. Med. 12, 426–442 (2012). [DOI] [PubMed] [Google Scholar]
- Costa B., Da Pozzo E. & Martini C. Translocator protein as a promising target for novel anxiolytics. Curr. Top. Med. Chem. 12, 270–285 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang L. M. et al. Antidepressant-like and anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa). Neuropharmacology. 81, 116–125 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang L. M. et al. Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. Int. J. Neuropsychopharmacol. 17, 1659–69 (2014). [DOI] [PubMed] [Google Scholar]
- Wolf L. et al. Enhancing neurosteroid synthesis–relationship to the pharmacology of translocator protein (18 kDa) (TSPO) ligands and benzodiazepines. Pharmacopsychiatry. 48, 72–77 (2015). [DOI] [PubMed] [Google Scholar]
- Scarf A. M., Auman K. M. & Kassiou M. Is there any correlation between binding and functional effects at the translocator protein (TSPO) (18 kDa)? Curr. Mol. Med. 12, 387–397 (2012). [DOI] [PubMed] [Google Scholar]
- Morohaku K. et al. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology. 155, 89–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L. N. et al. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 289, 27444–27454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland R. A. Conformational adaptation in drug-target interactions and residence time. Future. Med. Chem. 3, 1491–1501 (2011). [DOI] [PubMed] [Google Scholar]
- Mollica L. et al. Kinetics of protein-ligand unbinding via smoothed potential molecular dynamics simulations. Sci. Rep. 5, 11539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primofiore G. et al. N,N-dialkyl-2-phenylindol-3-ylglyoxylamides. A new class of potent and selective ligands at the peripheral benzodiazepine receptor. J. Med. Chem. 47, 1852–1855 (2004). [DOI] [PubMed] [Google Scholar]
- Da Settimo F. et al. Anxiolytic-like Effects of N,N-Dialkyl-2-phenylindol-3-ylglyoxylamides by Modulation of Translocator Protein Promoting Neurosteroid Biosynthesis. J. Med. Chem. 51, 5798–5806 (2008). [DOI] [PubMed] [Google Scholar]
- Barresi E. et al. Deepening the Topology of the Translocator Protein Binding Site by Novel N,N-Dialkyl-2-arylindol-3-ylglyoxylamides. J. Med. Chem. 58, 6081–6092 (2015). [DOI] [PubMed] [Google Scholar]
- Costa B. et al. Anxiolytic properties of a 2-phenylindolglyoxylamide TSPO ligand: Stimulation of in vitro neurosteroid production affecting GABAA receptor activity. Psychoneuroendocrinology. 36, 463–472 (2011). [DOI] [PubMed] [Google Scholar]
- Simorini F. et al. Medicinal chemistry of indolylglyoxylamide TSPO high affinity ligands with anxiolytic-like effects. Curr. Top. Med. Chem. 12, 333–351 (2012). [DOI] [PubMed] [Google Scholar]
- Motulsky H. J. & Mahan L. C. The kinetics of competitive radioligand binding predicted by the law of mass action. Mol. Pharmacol. 25, 1–9 (1984). [PubMed] [Google Scholar]
- Sykes D. A., Dowling M. R. & Charlton S. J. Exploring the mechanism of agonist efficacy: a relationship between efficacy and agonist dissociation rate at the muscarinic M3 receptor. Mol. Pharmacol. 76, 543–551 (2009). [DOI] [PubMed] [Google Scholar]
- Guo D., Mulder-Krieger T., IJzerman A. P. & Heitman L. H. Functional efficacy of adenosine A2A receptor agonists is positively correlated to their receptor residence time. Br. J. Pharmacol. 166, 1846–1859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvel J. et al. Agonists for the Adenosine A1 Receptor with Tunable Residence Time. A Case for Nonribose 4-Amino-6-aryl-5-cyano-2-thiopyrimidines. J. Med. Chem. 57, 3213–3222 (2014). [DOI] [PubMed] [Google Scholar]
- Butlen D. Benzodiazepine receptors along the nephron: [3H]PK 11195 binding in rat tubules. FEBS Lett. 169, 138–142 (1984). [DOI] [PubMed] [Google Scholar]
- Olson J. M. M., Ciliax B. J., Mandril W. R. & Young A. B. Presence of peripheral-type benzodiazepine binding sites on human erythrocyte membranes. Eur. J. Pharmacol. 152, 47–53 (1988). [DOI] [PubMed] [Google Scholar]
- Hill A. V. The mode of action of nicotine and curari, determined by the form of the contraction curve and the method of temperature coefficients. J. Physiol. 39, 361–373 (1909). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. & Christopoulos A. Analyzing kinetic binding data. In: Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. New-York: Oxford University Press. 245–251 (2003. Eds). [Google Scholar]
- Mukhin A. G., Papadopoulos V., Costa E. & Krueger K. E. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc. Natl. Acad. Sci. USA 86, 9813–9816 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V., Mukhin A. G., Costa E. & Krueger K. E. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J. Biol. Chem. 265, 3772–3779 (1990). [PubMed] [Google Scholar]
- Kozikowski A. P. et al. Chemistry, binding affinities, and behavioral properties of a new class of “antineophobic” mitochondrial DBI receptor complex (mDRC) ligands. J. Med. Chem. 36, 2908–2920 (1993). [DOI] [PubMed] [Google Scholar]
- Fallahi-Sichani M., Honarnejad S., Heiser L. M., Gray J. W. & Sorger P. K. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat. Chem. Biol. 9, 708–714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa P. et al. Functional and biochemical rationale for 24 h-long duration of action of olodaterol. J. Pharmacol. Exp. Ther. 337, 600–609 (2011). [DOI] [PubMed] [Google Scholar]
- Bernassau J. M., Reversat J. L., Ferrara P., Caput D. & Lefur G. A 3D model of the peripheral benzodiazepine receptor and its implication in intra mitochondrial cholesterol transport. J. Mol. Graph., 11, 236–244 (1993). [DOI] [PubMed] [Google Scholar]
- Anzini M. et al. Mapping and fitting the peripheral benzodiazepine receptor binding site by carboxamide derivatives. Comparison of different approaches to quantitative ligand-receptor interaction modeling. J. Med. Chem. 44, 1134–1150 (2001). [DOI] [PubMed] [Google Scholar]
- Jaremko L., Jaremko M., Giller K., Becker S. & Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 343, 1363–1366 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N. et al. Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: a double-blind controlled study in general practice. Human. Psychopharmacology. 21, 139–149 (2006). [DOI] [PubMed] [Google Scholar]
- Rupprecht R. et al. Translocator protein (18 kDa) as target for anxiolytics without benzodiazepine-like side effects. Science. 325, 490–493 (2009). [DOI] [PubMed] [Google Scholar]
- Rägo L. et al. The effect of chronic treatment with peripheral benzodiazepine receptor ligands on behavior and GABAA/benzodiazepine receptors in rat. Naunyn. Schmiedebergs. Arch. Pharmacol. 346, 432–436 (1992). [DOI] [PubMed] [Google Scholar]
- Chelli B. et al. PK 11195 differentially affects cell survival in human wild-type and 18 kDa Translocator protein-silenced ADF astrocytoma cells. J. Cell. Biochem. 105, 712–723 (2008). [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 7, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Cheng Y. & Prusoff W. H. Relationship between constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 (1973). [DOI] [PubMed] [Google Scholar]