Abstract
Cancer vaccines are designed to promote tumor specific immune responses, particularly cytotoxic CD8 positive T cells that are specific to tumor antigens. The earliest vaccines, which were developed in 1994-95, tested non-mutated, shared tumor associated antigens that had been shown to be immunogenic and capable of inducing clinical responses in a minority of people with late stage cancer. Technological developments in the past few years have enabled the investigation of vaccines that target mutated antigens that are patient specific. Several platforms for cancer vaccination are being tested, including peptides, proteins, antigen presenting cells, tumor cells, and viral vectors. Standard of care treatments, such as surgery and ablation, chemotherapy, and radiotherapy, can also induce antitumor immunity, thereby having cancer vaccine effects. The monitoring of patients’ immune responses at baseline and after standard of care treatment is shedding light on immune biomarkers. Combination therapies are being tested in clinical trials and are likely to be the best approach to improving patient outcomes.
Introduction
Cancer is one of the major killers in the Western world, with lung, breast, prostate, and colorectal cancers being the most common. As demographics and treatments change, thyroid, liver, and pancreatic cancers are predicted to increase significantly.1 Each histological type of cancer contains several molecularly defined subtypes, which have altered gene expression patterns and differing arrays of mutations.2 Several clinical approaches to the treatment of cancer are available, including surgery, chemotherapy, radiotherapy, and treatment with small molecule signaling pathway inhibitors (such as vemurafenib for BRAF mutant melanoma), which can each be used in specific clinical settings. Importantly, each of these “standard” approaches has been shown to modulate antitumor immunity.
Glossary
Adjuvants: Non-antigen specific triggers of the immune system that increase the immune response to an associated antigen. Adjuvants can include components of infectious agents, immune cell growth factors, and tissue damage signals
Allogeneic cell lines: Cell lines derived from another person; autologous cell lines are derived from the person being treated
Antigen presenting cells (APCs): Cells that capture proteins by various mechanisms and process and present them in association with MHC class I and II molecules to activate CD8+ and CD4+ T cells, respectively. APCs include dendritic cells, B cells, monocytes, and macrophages
Avidity: Strength of molecular interaction
Chimeric antigen receptor (CAR) therapeutics: Use of an antibody-T cell receptor fusion molecule to retarget a transfected cell to the specificity of the antibody with the signaling of the T cell receptor
CD8+ T cells: T cells that can be cytotoxic (“killer” cells) or secretors of cytokines (and other molecules). They are triggered by recognition of peptide-MHC class I complexes on the surface of cells
CD4+ T cells: T cells that generally secrete cytokines to support (or “help”) T cells and B cells
Checkpoint blockade: Antibody mediated blockade of a molecule that serves as a “checkpoint” for the immune response—for example the use of anti-CTLA-4 (anti-cytotoxic T lymphocyte associated protein 4) and anti-PD-1 (anti-programmed death 1) or anti-PD-L1 (anti-programmed death ligand-1).
CpG molecule: Section of DNA comprising cytosine then guanine, with a phosphate group in between
Epitope: Linear section of a protein (peptide) that is processed and presented by APCs in association with MHC class I and II molecules and is recognized by T cell receptors clonally expressed on the surface of CD8+ and CD4+ T cells. CD8+ T cells recognize 8-11 amino acid long peptides in association with MHC class I molecules and CD4+ T cells recognize longer (13-17 amino acids) peptides in association with MHC class II molecules
Foreign pathogen pattern recognition receptors: Family of receptors that recognize pathogen molecules shared by multiple pathogens, such as lipopolysaccharide or flagellin
Foreign helper proteins: A foreign protein designed to promote a non-specific immune response to “help” or assist in the desired immune response
G-Vax: Tumor cells transfected to express high amounts of granulocyte-macrophage colony stimulating factor
Heterologous helper peptide: A peptide that is used to activate CD4+ T cells against a non-cancer related protein (such as tetanus toxoid) with the goal that the tetanus specific CD4+ helper T cells will promote cancer specific CD8+ T cells as a bystander effect
Induced antibody neutralization: An antibody response that blocks an interaction between other proteins
MART-1/Melan-A, tyrosinase, and gp100: Antigens that are overexpressed in melanoma
MHC class I and II molecules: Major histocompatibility molecules that help to define an individual’s tissue type
MHC restriction: MHC class I molecules present intracellular peptides to CD8+ T cells; MHC class II molecules present mostly extracellular peptides to CD4+ T cells
MHC restricted epitope: A short, linear string of amino acids from a protein that is digested through a series of steps involving several intracellular proteases that fits into the peptide-binding groove of an MHC molecule expressed by the individual
Oncolytic viruses: Viruses that lyse host tumor cells during viral replication
Prime boost: To start, or “prime,” an immune response with one type of vaccine and enhance, or “boost,” the response with a different type of vaccine
PolyIC: a string of double stranded RNA of inosine-cytosine molecules; polyICLC: a string of double stranded RNA that includes inosine and cytosine molecules stabilized with polylysine, and that is meant to mimic viral infection
Small molecule signaling pathway inhibitors: Small molecules that are used as drugs to inhibit intracellular signaling pathways
Transactivator early genes: Viral genes expressed early after infection that turn on other viral genes
Transfection: Transfer of DNA or RNA into a cell
Tumor infiltrating lymphocytes (TILs): Generally CD8+ and CD4+ T cells that spontaneously infiltrate tumor deposits. TILs may also include B, natural killer, or suppressive cells
Tumor associated antigen (TAA): A protein expressed by a tumor that can be immunogenic
WT-1: Wilms’s tumor 1—a shared tumor associated antigen
Acronyms
CEA: Carcinoembryonic antigen
GM-CSF: Granulocyte-macrophage colony stimulating factor
HPV: Human papillomavirus
HMGB1: High mobility group B1
hTERT: Human telomerase reverse transcriptase
IFA: Incomplete Freund’s adjuvant
IL: Interleukin
KLH: Keyhole limpet hemocyanin
MDSC: Myeloid derived suppressor cell
NCI: National Cancer Institute
PADRE peptides: Pan-DR binding synthetic helper peptides
PSA: Prostate specific antigen
TLR: Toll-like receptor
Treg: Circulating regulatory T cell
It has been known for decades that the immune system can be activated against tumors spontaneously, despite the lack (in most cancers) of an infectious agent. In melanoma and renal cancer, the presence of tumor infiltrating lymphocytes (TILs; see Glossary) in tumor deposits is a positive prognostic factor.3 More recently, CD8+ memory T cells (see Glossary) have been identified as a significant positive prognostic factor in colorectal cancer,4 a tumor type not previously considered to be “immunogenic.” TILs are also a positive prognostic factor in breast and ovarian cancer.5 6
Cancer vaccination comprises an array of approaches that seek to generate, amplify, or skew (or a combination thereof) antitumor immunity. To accomplish this goal many approaches involve administration of tumor antigens, often with antigen presenting cells (APCs; ; see Glossary) or other immune modulators, or direct modulation of the tumor. Importantly, standard of care cancer treatments (surgery and ablation, chemotherapy, and radiotherapy) and small molecule signaling pathway inhibitors (see Glossary) can have similar effects to cancer vaccines. They do this by increasing the expression of tumor antigens within the tumor or causing the release of antigens from dying tumor cells and by promoting antitumor immunity for therapeutic benefit.7 8
This review summarizes the field of cancer vaccination, including different types of tumor antigens, approaches to inducing antitumor immunity, and the preclinical and clinical data so far. It also discusses aspects of standard of care treatments that promote antitumor immunity. Recent controversies in the field and the importance of careful immunologic monitoring of patients in clinical trials are also covered.
Prevalence of cancer
Cancer is a leading cause of death worldwide. Currently, more than 8.2 million people die from cancer each year, and the current burden of 14 million cases of cancer a year is expected to increase to 22 million cases in the next two decades. The most common types of cancer worldwide are lung, liver, stomach, colorectal, and breast cancers.9 In the United States, the most common cancers are prostate or breast, lung, and colorectal cancers, and the highest mortality is from lung, breast or prostate, colorectal, and pancreatic cancers.10 The molecular characterization of human cancers has shown the complexity of individual tumors and the diversity between tumors in different patients. Therefore, a more personalized approach to treatment may maximize benefit. Treatments are increasingly changing from being tissue based to being based on the mutation profile of the specific tumor. For example, anti-Her2 antibody (trastuzumab) was originally used just for breast cancer but it is now used for other tumors with increased cell surface Her2.
Sources and selection criteria
In May and June 2014, I performed PubMed searches for “cancer vaccine” and “immunotherapy and cancer” to identify recent publications (within the past five years) and accessed World Health Organization and American Cancer Society websites for current cancer statistics. The recent cancer immunotherapy presentations at the 2013 annual Society for Immunotherapy of Cancer and at the 2013 and 2014 American Society for Clinical Oncology meetings were also reviewed. Peer reviewed publications in English language journals covering the areas of investigation were included and studies that have led to clinical trials were prioritized. I also prioritized studies that included immune monitoring and biomarkers of response.
Vaccination approaches
Vaccination with target antigens: tumor specific and tumor associated antigens
The most common approach to cancer vaccination involves immunization with shared tumor antigens expressed by many different patients’ tumors. The earliest tumor associated antigens (TAAs; see Glossary) identified were proteins that were overexpressed in tumor cells but minimally expressed in untransformed normal tissues.11 12 13 14 Antigens were also identified after the cloning of genes that encoded proteins that make up epitopes (see Glossary) recognized by tumor reactive TILs.
The initial classes of antigens used in cancer vaccines included overexpressed antigens, cancer-testis antigens, oncofetal antigens, and mutated antigens (or tumor specific (private) antigens) (table).15
Strengths and weaknesses of different types of tumor antigens
| Antigens | Examples | Strengths | Weaknesses |
|---|---|---|---|
| Tumor associated antigens | MART-1, gp100, CEA, PSA | Highly upregulated in many tumors; low level expression in some normal tissues; immunogenic | Activated T cell clones may be weak; antigens are not drivers of oncogenesis |
| Mutated, tumor specific antigens | p53, RAS, unique mutations | Activate high avidity and effective T cells; may be tumor drivers | Difficult to identify and use for vaccination |
| Cancer-testis antigens | NY-ESO-1, MAGE-A3 | Expressed by many tumor types and in immunoprivileged sites, immunogenic | Activated T cell clones may be weak; antigens are not drivers of oncogenesis |
| Oncofetal antigens | α fetoprotein | Highly upregulated in many tumors; not expressed in normal adult tissues; immunogenic | Activated T cell clones may be weak; antigens are not drivers of oncogenesis |
CEA=carcinoembryonic antigen; PSA=prostate specific antigen.
Overexpressed antigens
Overexpressed antigens are proteins that are amplified at the DNA, mRNA, or protein level and are therefore expressed at a much higher level in tumor cells than in adjacent normal tissue. They include carcinoembryonic antigen (CEA; colorectal cancer and others); prostate specific antigen (PSA; prostate cancer); Her2/neu (breast cancer); melanoma lineage antigens such as MART-1/Melan-A, tyrosinase, and gp100 (see Glossary); and mucin 1 (several cancers including colorectal and pancreatic cancer). All have shown efficacy as targets in vitro and in mouse models, and have been tested in clinical trials with some objective RECIST (response evaluation criteria in solid tumors) clinical responses.16 17 18 A concern about targeting such TAAs is that the highest avidity (see Glossary) T cells specific to these normal self antigens may be deleted, leaving only less effective, lower avidity T cells to stimulate.
Cancer-testis antigens
Cancer-testis antigens are a class of proteins that are expressed in a proportion of most tumor tissue types and in germ cells, which because of their physiologic location are ignored by the immune system. Such antigens include the large MAGE-A, MAGE-B, and MAGE-C families, and NY-ESO-1. These antigens have been tested in human clinical trials (because of the lack of expression in mice, the ability to model therapeutic strategies in mouse models is limited) and implicated in some therapeutic responses.19
Oncofetal antigens
Oncofetal antigens are normal fetal antigens that are turned off during development but turned back on in some transformed cells; they include oncofetal antigen and α fetoprotein. These antigens have been tested in vitro, in murine models, and in clinical trials and have shown immunogenicity and some efficacy as targets.20 21 22 23
In 2008 the National Cancer Institute (NCI) organized a workshop to rank and prioritize the antigens identified at that time, publishing the highest priority, most experimentally confirmed tumor rejection antigens.24 Most of the antigens were non-mutated shared antigens, and they were ranked according to agreed criteria, including therapeutic function, immunogenicity, oncogenicity, tumor specificity, frequency of overexpression (in tumors and in patients), and expression in cancer stem cells. None of the classes of tumor antigen described above is truly tumor specific.
Tumor specific mutations have been known for decades and common mutations in the RAS oncogene and P53 tumor suppressor gene have been identified. However, it was thought unlikely that such mutations would result in a processed, presented, and immunogenic MHC restricted epitope (see Glossary) in sufficiently common MHC class I or II molecules (see Glossary), and that this would be difficult to predict and virtually impossible to target by vaccination in a clinical trial.25 But—as sequencing technology has improved, and software capable of predicting processed, presented, and properly binding peptide epitopes has improved—highly therapeutic T cells that target these mutated patient specific mutations have begun to be identified.26 Currently, it takes a long time to identify targetable, patient specific mutations, which results in treatment delay; however, several investigators are focusing on ways to identify such tumor specific targets more quickly.
Vaccination with autologous tumor cells
This technique involves a therapeutic agent that is produced by isolating tumor cells from a patient and processing them into a vaccine in vitro; the vaccine is then given to the same patient. The vaccine would include all known tumor specific mutations and could take the form of killed tumor cells; tumor cells engineered with a cytokine such as granulocyte-macrophage colony stimulating factor (GM-CSF) to improve immunogenicity; tumor lysates, with or without adjuvants (see Glossary); or a tumor lysate pulsed with APCs, such as dendritic cells.
Tumors also express mostly normal self proteins, which could promote autoimmunity if presented in the environment of an ongoing immune response. It is not necessary for the tumor specific mutations that could be used in tumor vaccination approaches to be identified for their role in antitumor responses to be tested. Despite remaining technical hurdles in identifying patient specific targetable mutations, many investigators are focusing on utilizing these mutated antigens, which have been shown to be capable of inducing a potent and avid immune response.27
Determinant spreading
Although the identification of an optimal antigen is important for directing antitumor immunity to the tumor, the spread of the immune response from one antigen to another antigen expressed in the same tissue (“determinant spreading”28 or “epitope spreading”) has been linked to superior clinical outcome.29 30 31 32 33 34 35 This spontaneous phenomenon has been studied in autoimmune diseases and has also been noted in cancer immunotherapy studies.
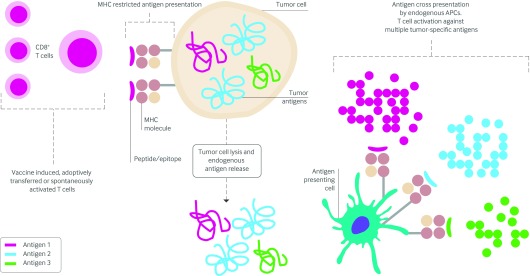
The phenomenon of in vivo cross presentation of tumor derived antigens released in one wave of T cell attack to promote subsequent waves of antitumor T cells directed against different antigens may be an important mechanism for tumor rejection (fig 1).33 A vaccine that targets shared antigens can facilitate subsequent rounds of immunity to unidentified, patient specific mutated antigens and may induce clinically significant tumor rejection. The observation from many clinical trials that the immune response to vaccine delivered antigens does not correlate with clinical outcome may be related to this mechanism.29 The most important role of vaccines containing TAAs may be to induce determinant spread to tumor specific antigens that activate higher avidity T cells, which more effectively mediate tumor rejection. The in vivo mechanism of cross priming (see Glossary) may also result in autoimmunity,36 which has been found to be a biomarker of clinical response to interferon in patients with melanoma.37
Fig 1 Determinant, or epitope, spreading. Central to this phenomenon is the activation of specific CD8+ T cells that recognize a particular MHC class I restricted peptide (derived from a tumor antigen) expressed on the surface of a tumor cell. These activated T cells kill and lyse the tumor cell, resulting in the release of a variety of tumor antigens. Endogenous antigen presenting cells (APCs) take up and present these newly released antigens to additional T cells that specifically recognize the newly released antigens, thereby broadening and spreading the response from one to multiple antigens
Many antigens have been identified as suitable for cancer vaccination, but the most important step may be the approach taken and the vehicle used for immune activation.38
Types of cancer vaccines
Most cancer vaccines aim to activate tumor specific CD8+ cytotoxic T cells because studies in mice support the key therapeutic role played by these cells.
The most common vaccination strategies used to activate CD8+ T cells have been based on MHC class I restricted peptide epitopes on TAAs. These have been delivered in a variety of adjuvant formulations (including cytokines and toll-like receptor (TLR) ligands) to promote in vivo presentation by endogenous APCs.
Peptide based vaccines take advantage of the ability of computer algorithms to screen protein amino acid sequences for candidate MHC class I restricted peptide epitopes derived from TAAs. Candidate epitopes are then tested experimentally for those that bind commonly expressed HLA molecules, are naturally processed and presented by tumor cells, and are immunogenic (capable of activating CD8+ cells). Many have also been tested in mouse models (to either mouse self antigen homologs, highly similar human antigens, or transfected (see Glossary) or transgenic models), and were found to have the therapeutic properties of a tumor rejection antigen.
Another common approach to activating TAA specific CD8+ T cells is to include autologous APCs, such as dendritic cells, in TAA based vaccines. This approach takes into account the observation that tumors can have negative effects on endogenous APCs.39 Antitumor T cell responses can be triggered more effectively by preparing optimized APCs ex vivo, without the presence of tumor derived factors but with specific positive growth factor and immune signals.
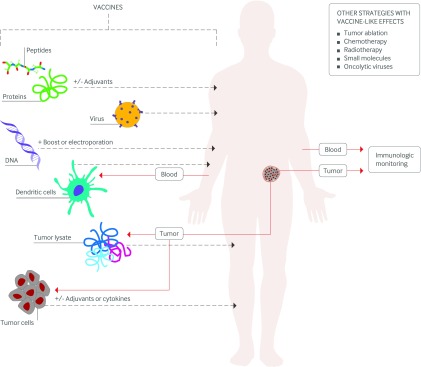
A third strategy makes use of tumor cells (and, therefore, a complex and incompletely characterized array of antigens including tumor specific mutated antigens), often engineered with cytokines such as GM-CSF or formulated with adjuvants (or both). These cells carry the full array of tumor specific mutations and all of the patient’s MHC class I and II molecules. Another strategy uses the naturally efficient infectivity of viruses to infect cells in vivo with encoded tumor antigens. Viral strategies also include the use of oncolytic viruses (see Glossary) that are engineered to replicate specifically in tumor cells (fig 2).
Fig 2 Cancer vaccine approaches. Vaccines include short peptides, full length proteins (with and without adjuvants), viruses, DNA (or virus plus DNA in sequence), dendritic cells, tumor cells (killed), and tumor lysates. These elements can be modified, added to adjuvants, or combined together. Standard of care approaches such as tumor ablation, certain chemotherapies, and radiation can also have vaccine-like effects, promoting tumor specific immunity
One therapeutic cancer vaccine—Sipuleucel-T manufactured by Dendreon—has been approved by the Food and Drug Administration. It consists of autologous antigen presenting cells loaded with the TAA prostatic acid phosphatase and GM-CSF. It is approved for metastatic prostate cancer on the basis of a 4.1 month improvement in overall survival seen in data from large scale phase III clinical trials.40
Peptide based strategies
One of the most common approaches for cancer vaccination is the delivery of MHC class I restricted peptide epitopes derived from shared TAAs with the aim of activating rare specific CD8+ T cell clones that react against self antigens. Data from animal models support the potential for such vaccines to have a substantial therapeutic effect.41 42 43
Peptides formulated in adjuvants (such as Montanide, which is analogous to incomplete Freund’s adjuvant (IFA)) with or without cytokines, such as GM-CSF and interferon γ, or TLR agonists, have shown clinical benefit (partial responses, complete responses, and durable disease stabilization) in small and large scale clinical trials.44 45 46 In smaller trials, peptides on APCs such as dendritic cells have also resulted in positive immune and clinical effects.29 47
Vaccines based on one or more peptides can be injected alone or with the simple adjuvant Montanide ISA-51 combined with a cytokine (such as GM-CSF to activate APCs), with other adjuvants (such as a specific TLR ligand to activate and mature APCs), or with the peptides pulsed on to autologous antigen presenting cells (or a combination of these tactics). Montanide ISA-51, an oil in water emulsion similar to IFA that is used in mouse models, has led to activation of TAA specific cytotoxic T cells.48 49 50 Data suggested that altering the production of Montanide from a beef source to an olive oil source changed its adjuvanticity.51 A recently identified concern is that IFA can result in the accumulation of T cells at vaccination sites instead of promoting systemic immunity.52 Hence, as peptide based vaccines are tested, optimal adjuvants and formulations of these vaccines are still being identified. Recent clinical trials of peptide based vaccines were recently reviewed.53
A benefit of peptide based approaches is that nine to 10 amino acid peptides are simple and cheap to manufacture. Large scale manufacture is possible and the peptides are stable when stored and shipped.
Unfortunately, however, because of HLA restriction, people who do not express common HLA types cannot be treated with this type of vaccine. In addition, the usual MHC class I binding short peptides do not activate CD4+ helper T cells, which can limit the functionality of CD8+ cytotoxic T cells. This problem has been overcome by the addition of non-tumor specific help (inclusion of keyhole limpet hemocyanin (KLH), tetanus, or (pan-DR binding synthetic helper (PADRE) peptides), although data are limited over the nature of the “help” provided by heterologous helper peptides (see Glossary).
Another strategy that has shown significant clinical efficacy is the use of synthetic peptides that are long enough to include multiple MHC class I and II epitopes.54 55 These 23-45 amino acid long peptides, delivered subcutaneously, have been shown to be especially effective, possibly because of a more efficient processing and presentation pathway, which leads to superior T cell activation.56
By contrast, a whole tumor antigen protein approach, which also allows for uptake, processing, and presentation of multiple MHC class II and MHC class I antigen peptides (with perhaps less efficiency), has been tested in phase III clinical trials. Despite formulation with an optimized adjuvant, thus far, this full length protein vaccine has not met its primary clinical endpoints,57 although trial analysis is ongoing.
Multiple peptides can be given at the same time, targeting several T cell clones and antigens at once.58 59 60 A trial combining pre-vaccine cyclophosphamide with multiple peptides and GM-CSF showed that improved survival was associated with antigenic breadth of response and reduced suppressive circulating regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs).60
Mouse models support combinations of multi-antigen peptide vaccines and chemotherapy.61 Peptide vaccines may also be useful for preventing the progression of premalignant lesions to cancer. A recent human clinical trial of the prevention of progression of colon adenoma to colorectal cancer tested a mucin 1 TAA peptide based approach.62 The vaccine was highly immunogenic in almost half of the 39 patients who were evaluated. Importantly, high numbers of immunosuppressive MDSCs were already present in more than half of patients with advanced colonic adenoma. This indicates that these cells might provide a biomarker of vaccine response (circulating numbers of MDSCs) and that earlier vaccination should be tested, because even premalignant lesions may induce systemic immunosuppression.
APC based strategies
Many types of APCs have been investigated, including peripheral blood mononuclear cells, activated B cells, and, more commonly, dendritic cells. Dendritic cells are a heterogeneous population of APCs that can efficiently take up antigens and sample their environment. They then process and present these antigens to CD4+ and CD8+ T cells and incorporate immune response modulating cues (including the secretion of cytokines such as interleukin 12 (IL-12) p70, which skews the immune response to a type 1 response) to determine the type of response. A type 1 response involves interferon γ, IL-2, and tumor necrosis factor and it promotes the activation of cytotoxic CD8+ T cells. Several recent reviews summarize the history, biology, and clinical application of these cells.63 64 65
Dendritic cells
Clinical trials of dendritic cell vaccines are usually unique, involving individualized patient vaccination approaches and single clinical trial arms. It is therefore difficult to compare trials and draw firm conclusions about the efficacy or different approaches. Monocytes and CD34+ progenitor cells have been tested, and antigens including complex tumor lysates that contain normal, tumor associated, and tumor specific antigens, or synthetic MHC class I restricted peptides, have all been used. Vaccines have been injected into the blood, skin (subcutaneously or intradermally), and lymph nodes. All these parameters could affect the clinical responses seen. The initial lessons learnt were that dendritic cell based vaccines are safe, feasible, and immunogenic, and that they can promote clinically significant tumor regression in some patients.66 67 68
Several important clinical trials of dendritic cell vaccines have been published more recently (2004-12). Objective clinical responses and immunologic responses were seen in a trial of dendritic cells that had been pulsed with mucin 1 derived peptide plus heterologous PADRE peptides (delivered subcutaneously) in patients with renal cell carcinoma.34 Mucin 1 was a highly ranked antigen in the NCI prioritization project.24 These dendritic cell vaccines were combined with low dose IL-2. In another study, patients with acute myeloid leukemia who were in remission after standard treatment were given vaccines in which dendritic cells were loaded with WT-1 (see Glossary). TAA mRNA and these patients showed both immune activation and improved clinical outcomes.69 WT-1 was also a highly ranked antigen in the NCI prioritization project.24 Another trial that tested dendritic cell-tumor fusions in patients before and after autologous stem cell transplantation found both antitumor immune activation and a reduction in disease.70 Interestingly, these trials all used a dendritic cell vaccine combination strategy, where the antigen loaded vaccines were added to standard of care treatments and systemic cytokine therapy. Recent dendritic cell based vaccine trials have been summarized.71
Tumor based strategies
Early cancer vaccine studies found that mice could be immunized with tumor cells that were killed and engineered to express immune stimulatory cytokines,72 73 including GM-CSF. The data supporting syngeneic, autologous, or allogeneic tumor cells transfected to express high amounts of GM-CSF (G-Vax; see Glossary) supported clinical testing, with some immune and clinical responses.74 75 76 77 Preparation of vaccines based on autologous tumor cells was feasible but complex.
Cell lines
Allogeneic cell lines (see Glossary) were also tested, with or without autologous tumor cells. The G-Vax platform continues to be tested and is part of a “prime boost” (see Glossary) combination vaccine study in which patients with pancreatic cancer receive recombinant listeria bacteria expressing the TAA mesothelin, with or without a G-Vax composed of two allogeneic pancreatic cancer cell lines.78 79 Importantly, multiple injections are possible without inhibition from induced antibody neutralization (see Glossary), and the inclusion of bacteria serves to mimic many aspects of a natural infection by triggering TLRs and foreign pathogen pattern recognition receptors (see Glossary).
Other strategies that use the personalized approach of harnessing autologous tumor antigens include using tumor lysates to load APCs ex vivo and fusion of tumor cells and autologous APCs. These have been tested in early phase clinical trials and several have shown promising results. Immunity to undefined tumor lysates and foreign helper proteins (see Glossary) has been demonstrated in some cases.80 81 82
Autologous tumor cells
Autologous tumor cells can also be used to transfect APCs (autologous or derived from allogeneic cell lines) with tumor genomic DNA.83 This allows uncharacterized mutated gene products specific to the tumor to be processed and presented for immune activation.
Virus based strategies
As mentioned above, the inclusion of pathogens in cancer vaccines can greatly increase immune stimulation in the context of presenting tumor antigens. Although peptide based vaccines have sometimes included TLR ligands such as CpG molecules (see Glossary) or polyIC/polyICLC (see Glossary), pathogens have complex arrays of molecules that can trigger multiple immune activation pathways.
The human papillomavirus vaccines Cervarix and Gardasil are licensed to prevent human papillomavirus (HPV) mediated cervical cancers in uninfected adolescents. They work by activating humoral immunity against viral capsid proteins assembled into non-infectious viral-like particles. They are not, however, effective against cancers that develop in infected patients.
Viruses, including adenovirus, have been used as vectors to directly immunize with tumor antigens by injection into muscle tissue, which is relatively easily transfected,84 85 taking advantage of their natural infectivity. Viruses have also been used ex vivo to transduce antigens into APCs,86 87 88 89 90 91 and each virus has unique effects on these transduced cells, from activation to suppression. Direct administration of viral vectors can induce neutralizing antibody responses that block repeated use, whereas the induction of neutralizing antibodies is minimal after vaccination with ex vivo virally transduced APCs. However, clinical use of viral vectors can involve complex logistics, including the requirements of clinical grade virus production.
A strategy that resolves some of these limitations involves virus based prime boost (see Glossary) with heterologous viral backbones expressing tumor antigens. This approach is exemplified by the vaccinia virus and fowl pox virus prime boost for use in patients with prostate cancer, which expresses the TAA, PSA, as well as the costimulatory molecules B7-1 (CD80), intercellular adhesion Molecule 1 (CD54), and lymphocyte function-associated antigen 3 (CD58).92 This promising approach has improved survival in patients with prostate cancer,93 94 and it continues to be tested in late phase clinical trials. The same group is also developing viruses encoding CEA and mucin 1 instead of PSA to use against other carcinomas.
Oncolytic viruses have been used for decades. They include adenoviruses that drive the transactivator early genes (see Glossary) E1a and E1b and viral replication from a tumor specific promoter (including promoters that drive TAAs such as α fetoprotein, PSA, and human telomerase reverse transcriptase (hTERT).95 Vaccinia viruses have also been used. One strategy has created mutations in viral serpin (serine peptidase inhibitor) genes to improve tumor selectivity for viral replication and killing,96 and additional engineering with chemokine genes or combinations with costimulation are being pursued.97 98
Herpesviruses have also been used as oncolytic viruses for cancer vaccination. A promising strategy has tested GM-CSF as an adjuvant or APC growth factor engineered into replicating herpesvirus vectors. One such vector, T-VEC, was recently tested in patients with melanoma in a randomized phase III trial.99 The trial found an objective response rate of 26% and a complete clinical response in 11% of patients with stage IIIB-IV melanoma.100 The immune mechanisms of response will be important to investigate.
What else can be considered a “cancer vaccine”?
Tumor ablation
Several tumor ablative techniques can remove large and small tumors, and this process can reduce both systemic and intratumoral suppressive environments. Elimination or reduction of the tumor mass can eliminate a broad array of tumor derived suppressive factors while simultaneously releasing tumor antigens from dying tumor cells.
Radiofrequency ablation
This localized heating technique causes necrosis and inflammation and transiently allows the re-emergence of circulating antitumor effector cells that recognize the TAA α fetoprotein.7 Although these memory cells are functional they are not potent enough to be therapeutic alone. In a larger scale study in patients with hepatocellular carcinoma, this technique enhanced both a memory cell response and the development of new effector cells against multiple TAAs. The immune responses correlated positively with the prevention of disease recurrence and negatively with the numbers of MDSCs.101 Radiofrequency ablation has also been shown to promote the activation of natural killer cells.102
High intensity focused ultrasound
This technique is being investigated for its immune stimulatory effects alone and combined with other therapeutic strategies. It has been shown to affect STAT5 (signal transducer and activator of transcription 5) signaling,103 increase cytotoxic T cell responses,104 and alter tissue architecture.105 Embolization (therapeutic blood vessel blockade) of tumors has also been shown to allow re-emergence of circulating TAA specific CD4+ T cells.106 The frequency and function of these cells correlated with improved clinical outcome.
Cryoablation
Cryoablation of tumors can also result in the release of TAAs, thereby activating antitumor immunity.107
Chemotherapy
Data from mouse models from the past decade show that different classes of chemotherapeutic agents have very different effects on the tumor in terms of the type of death induced (for example, necrosis or apoptosis). The specific death signals induced also have an impact on immunity. “Immunogenic” death can be induced by the upregulation of tumor cell surface calreticulin and release of ATP, HMGB1 (high mobility group B1), and tumor antigens.8 It had previously been assumed that chemotherapy in general is immunosuppressive because it results in the depletion of white blood cells. However, the loss of these cells also removes suppressive Tregs and MDSCs,108 109 110 and reduces tumor secretion of immune inhibiting cytokines and growth factors.111 With the additional benefit of the release of tumor antigens for uptake by endogenous APCs and reduction in tumor burden, specific chemotherapy drugs are thought to work with and promote antitumor immunity in certain settings. Examples of the most recent clinical trials in this area have recently been summarized.112
Radiotherapy
Radiotherapy induces cell death in the irradiated tissues by causing genomic DNA damage. In addition, irradiation of a tumor can sometimes cause shrinkage not only of the treated tumor but also of unirradiated lesions, a phenomenon known as the “abscopal effect.” This effect has been shown to be mediated by the immune system.113 114 115 In one recent clinical case report with extensive immunologic monitoring, the radiation induced abscopal effect was shown to induce both a strong antibody response to TAAs and potent T cell activation.116 Tumor reactive T cell activation has also been shown to be induced by radiotherapy in mouse models.117 Immune effects in radiotherapy studies were recently reviewed.118
Targeted therapy
The ability to design small molecules that can bind to the active sites of signal transduction molecules that drive the proliferation of tumor cells and block proliferation has been a major advance in the treatment of cancer. However, many of the molecules are not specific and have off target effects on normal tissues and other signaling pathways, and they also lead to compensatory mutations that limit the duration of their clinical effects.119
Such targeted therapy can also have immunologic effects. The BRAF inhibitor vemurafenib increased the tumor cell expression of TAAs in melanomas and, therefore, improved the immune recognition of tumor cells.120 In melanoma biopsies from patients treated with a BRAF inhibitor, plus or minus a MEK (mitogen activated protein kinase) inhibitor, increased TAA expression was accompanied by increased CD8+ T cell infiltration and reduced cell surface and soluble markers of immunosuppression.119 Recently, an increase in tumor infiltration by adoptively transferred tumor specific T cells was also shown in mice treated with a BRAF inhibitor.121
Methodological optimization of cancer vaccination strategies
Cancer vaccines still show only modest success in clinical trials. Many trials have shown potent therapeutic responses in a proportion of patients with late stage cancer, but it has been rare for trials to obtain more than a 5-10% partial or complete response. One reason is the broad range of platforms that are being developed. Some are “off the shelf” allogeneic, synthetic peptide, protein, or pathogen based vaccines. Others are autologous tumor or APC based vaccines that have a high level of inherent variability over and above the requirements for platform development.122
The development of chimeric antigen receptor (CAR) therapeutics (see Glossary) is a good example of the optimization of a cancer vaccination strategy. The genetically engineered CAR has undergone three generations of development, from CD3 zeta chains, through the addition of CD28 or 4-1BB regions, to multiple signaling domain constructs using CD27, CD28, ICOS, 4-1BB, and OX40.123 124 125 Electroporation of constructs and the use of coding cassettes in recombinant retroviruses and lentiviruses have also been used to introduce the receptor into cells. Since the initial description of the CAR approach, immune depleting regimens and cytokine support procedures have been developed to allow the persistence of CAR bearing cells.126 The success of CAR therapy is based on decades of painstaking work by many groups of dedicated investigators to identify constructs, domains, mechanisms, targets, and combination treatments that enable the basic CAR bearing cell to be effective. Similarly, the field of “cancer vaccines” continues to refine technical aspects of multiple vaccine platforms to improve efficacy.
Animal models do not always predict outcomes in humans
Several recent commentaries have discussed the role of animal models and the need for genetically engineered mouse models with mutations that recapitulate human tumors and which spontaneously develop tumors with homologous histology and metastatic spread.127 128 Results of simple and short timeframe studies of in vitro cultured tumors that are implanted subcutaneously have predicted the success of some immune therapies but many have proved to have no significant clinical impact in human clinical trials.
For example, DNA vaccines have had very different effects in mouse models and human studies. This simple, safe, and cost effective approach showed potent immunogenicity and intriguing therapeutic effects in several mouse models.20 129 130 131 Part of the efficacy in mice was related to CpG dinucleotides in bacterially derived plasmid backbones and recognition by TLR9 expressing myeloid and plasmacytoid dendritic cells.132 When this approach was tested in human trials the naked DNA injections were mostly ineffective and minimally immunogenic.133 134 Subsequent studies that have tested the addition of more potent adjuvants; improved delivery approaches, including in vivo electroporation135; and priming with DNA, followed by boosting with a viral vector based vaccine, have shown promising results.136 137
Controversies
In 2004 a high profile review that summarized many clinical trials of cancer vaccines concluded that cancer vaccine trials had shown minimal efficacy in patients with cancer (3.8% objective response rate, with the highest (7.1%) response rate for dendritic cell based trials).138 Subsequent correspondence pointed out that many variables in cancer vaccination (certainly for dendritic cell based cancer vaccines) remain to be tested, and that biomarkers that might identify optimal vaccine characteristics and patient variables have yet to be validated.139
Another controversial review covered antigenic targets for immunotherapy.122 As discussed above, most of the antigens targeted to date are non-mutated shared antigens that are overexpressed in tumor tissues. The review discusses several instances of unexpected adverse events, severe toxicities, and death from adoptive transfer of very high avidity lymphocytes bearing genetically engineered T cell receptors or CARs specific for non-mutated self antigens. The review’s authors support a focus on viral antigens such as those found on HPV (implicated in cervical cancer, as well as head and neck cancer) and Epstein-Barr virus (important for nasopharyngeal cancers and lymphoproliferative diseases), mutated antigens (KRAS mutations in HLA-A2+ patients), and antigens like CD19, where complete elimination of all normal cells and cancer expressing cells is safe or has an acceptable level of toxicity.
Current technologies and mouse models cannot completely mimic the biology of each of these target antigens or predict the level of antigen expression in normal tissue, the expression of T cell receptors or CARs, or crossreactivity with homologous antigens, which can lead to toxicity. Shared antigens may be “safe” targets for cancer vaccines that harness the naturally positively and negatively selected endogenous T cell repertoire. However, they are not necessarily safe targets in situations where large numbers of T cells, engineered to be of high affinity, are adoptively transferred, and where prolonged or permanent deletion of any cell that expresses the target antigen may occur. Elimination of the CD19+ B cell population by CAR T cells is considered to be an acceptable toxicity.140 141
Lessons learnt from immune monitoring of cancer vaccine clinical trials
Many cancer therapeutics with an incompletely characterized mechanism of action have been used successfully for decades. Some of these mechanisms of action have subsequently been identified and are important for patient enrollment and stratification, outcome prediction, and identification of suitable combination treatments to improve patient outcomes.
Regardless of platform, the primary mechanism of action of all cancer vaccines is the same—activation of tumor specific T cells. Because cancer vaccines have passed the hurdles of feasibility, safety, immunogenicity, biologic activity, and therapeutic efficacy (albeit in a small minority of patients), the crucial questions are:
What qualities in vaccine activated T cells lead to therapeutic tumor destruction?
How can vaccines be formulated and delivered to activate antitumor T cells effectively?
Which patients are the most likely to respond to cancer vaccines?
Is prevention the best clinical outcome for cancer vaccines?
Immunologic monitoring and detailed vaccine analysis are needed to answer these questions (fig 3). The Society for Immunotherapy of Cancer, with the NCI and FDA, formed a task force that considered several questions and made recommendations to the field.142 This report updated previous work from the society,143 and incorporated important recommendations from other groups,144 as well as results of worldwide immunologic monitoring proficiency panels and harmonization efforts.145 146 147 148
Fig 3 Immunologic monitoring approaches. Summary of the processing of patient specimens and the immune monitoring assays. Tumors can be frozen or formalin fixed and paraffin embedded and slices tested by immunohistochemistry (IHC). They can also be enzymatically digested into viable single cell suspensions. Blood can be separated using density gradient centrifugation (Ficoll-Paque) and a variety of tests performed; TIL=tumor infiltrating lymphocyte
Specifically, to determine key aspects of the biology of antitumor T cells, functional assessments are needed to determine not only the prevalence and phenotype of tumor reactive T cells, but also the cytokines, effector molecules, and chemokines produced. Many trials have determined the prevalence of circulating peptide specific CD8+ T cells to an immunizing TAA epitope, but such measures have rarely correlated significantly with clinical outcome. This type of measure is more likely to be a reflection of successful vaccination.
An exception to this is when vaccines include multiple peptides that are found in several antigens. The breadth of response to multiple peptides has correlated with clinical outcome in several trials. In the past 10 years an increasing number of trials have included well designed and carefully performed immunologic monitoring, including multiple functional assessments of CD8+ and CD4+ T cells, natural killer cells, and antibody responses.
Examples of immunologic measures correlating with clinical response
A study of patients with vulvar intraepithelial neoplasia who were vaccinated with long peptides from HPV-16 found a correlation between immunologic measures and clinical outcome.54 Investigators tested lymphocyte proliferation and cytokine production to immunizing antigens as well as circulating Tregs. Patients with a clinical response to treatment showed the highest levels of lymphocyte proliferation, interferon γ, and IL-5 as well as the lowest numbers of Tregs.
A trial testing vaccination with the TAA WT-1 in patients with acute myeloid leukemia identified significant changes in numbers of WT-1 specific CD8+ T cells, and more dramatic activation of circulating natural killer cells in patients with a clinical response.69
In another example, from a large multicenter clinical vaccine trial in patients with melanoma, immunity to multiple vaccine peptide epitopes correlated with clinical outcome. The trial used two assays to measure specific immunity: MHC tetramer flow cytometry to measure the numbers of vaccine antigen specific T cells and ELISPOT (enzyme linked immunospot) assays of interferon γ release by peripheral blood mononuclear cells. The functional ELISPOT assay correlated with overall survival,44 but although the phenotypic MHC tetramer assay detected increased numbers of vaccine specific T cells, this did not correlate with clinical outcome.149
A study that tested a multi-peptide vaccine in patients with renal cancer found that inhibition of Tregs strengthened antitumor immunity. The number of specific subsets of MDSCs at baseline was crucial, and the improvement in clinical outcome correlated with the number of peptides responded to.60
Prognostic markers
Other potential prognostic markers include “polyfunctionality,” which includes simultaneous secretion of cytokines, lytic granules, and chemokines in peripheral blood cells.150
Another important parameter is tumor infiltration. A strong or “brisk” lymphocytic infiltrate strongly correlates with a positive outcome in melanoma,151 and recently, the infiltrate in pretreatment colorectal cancers has been shown to be an important biomarker of T cells. Although the antigen specificity is not known, the cells are CD3+/CD8+/CD45RO+ and thoroughly infiltrate tumor tissue.4 152
Predictive and prognostic tissue biomarkers in melanoma, including type 1 interferon signatures, have recently been identified by tissue analysis,153 and by the use of molecular approaches instead of immunohistochemistry in formalin fixed tissues.154
Patient specific factors
Patient specific parameters that affect the antitumor response can be identified at several levels. Analysis of baseline tumor specific T cells can identify patients in whom vaccination will boost an existing response. Investigation of baseline circulating suppressor cells could identify those who are unlikely to respond owing to high numbers of Tregs and MDSCs,155 156 157 and those who would benefit from a combination treatment that reduces immunosuppression.
Tests for a wide range of immunomodulating factors in plasma or serum can also indicate immune skewing (to a regulatory or suppressive (type 2) response involving IL-4, IL-5, IL-13, and other molecules that do not support cytotoxic CD8+ T cells) and could indicate cytokines that need to be blocked or augmented. Genetic analysis of patient specific small nucleotide polymorphisms can identify subpopulations with altered gene expression patterns.
Lastly, as described above, identification of patients with infiltrated or “inflamed” tumors could pinpoint those with an ongoing immune response that needs to be boosted or to have checkpoint blockade (anti-CTLA-4, anti-PD-1; see Glossary) used to amplify or de-repress the immune response. By contrast, patients with non-infiltrated tumors would be good candidates for a vaccination strategy to initiate an antitumor immune response.
Approaches to test these different immunologic parameters have recently been reviewed.158
Emerging treatments: cancer vaccine combinations
As noted above, some recent and relatively successful vaccination approaches have included combinations of vaccine elements and vaccines combined with standard of care treatments. On the basis of preclinical mouse models, combinations of adjuvant cocktails that target different TLRs are being investigated and are showing promise.159
Prime boost vaccines are also inherently combination vaccines. Cancer vaccines aimed at supporting the survival and function of adoptively transferred T cells (by the optimal presentation of tumor antigens) have developed from mouse models and are being tested clinically.
The use of multiple antigens has also become more common because so many antigens have been shown to be immunogenic and safe to use. Strategies to reduce tumor induced immunosuppressive Tregs and MDSCs after vaccination are also being tested.
Lastly, the combination of cancer vaccines with checkpoint blockade (anti-CTLA-4, anti-PD-1) is an obvious area for investigation (fig 4). Because the immune system self regulates, activated T cells upregulate CTLA-4, PD-1, and other cell surface molecules that can be triggered to inhibit T cell activity. Blockade of CTLA-4 and PD-1 binding through antibodies alone has led to highly significant clinical responses, and combining these antibodies with a vaccine might strengthen the antitumor effects of either alone.
Fig 4 Cancer vaccines can be combined with various other treatments such as standard of care approaches, checkpoint blockade, immunotherapy, and strategies to reduce suppression. CTLA-4=cytotoxic T lymphocyte associated protein 4; GM-CSF=granulocyte-macrophage colony stimulating factor; IFN=interferon; IL-2=interleukin 2; MDSC=myeloid derived suppressor cell; PD-1/L1=programmed death 1/L1; Tregs=circulating regulatory T cells
Conclusion
The field of cancer vaccination encompasses a broad array of strategies that can promote tumor specific immune responses. TAAs have been shown to be safe and immunogenic, and several trials have produced objective clinical responses. To improve responses, multiple antigens, patient specific mutated antigens, viral vectors, prime boost strategies, and combinations with standards of care are being tested in new clinical trials.
Research questions
Recent studies have investigated cancer vaccination to prevent progression from premalignant lesions to a fully malignant state. Should they be further investigated with multiple cancer vaccine platforms?
Investigation of immunologic biomarkers seems to be crucial to providing standardized benchmarks of immune response, levels of immune suppression, and to identify predictive and prognostic biomarkers of clinical outcome. Should this be emphasized in clinical studies, particularly rigorous banking of blood and tumor samples?
Autologous, personalized therapies based on patient specific cellular vaccines, tumors, and peptide epitopes are potentially potent immunogens capable of activating tumor specific and high avidity T cell responses. Should such approaches be prioritized?
Thanks to Robert Vonderheide (University of Pennsylvania) for helpful discussions.
Contributors: As sole author I accept responsibility for all aspects of this review and act as guarantor.
Funding: LHB is supported by NIH/NCI RO1 CA138635, NIH P50 CA121973, and NIH P30 CA047904.
Competing interests: I have read and understood BMJ policy on declaration of interests and have none to declare.
Provenance and peer review: Commissioned; externally peer reviewed.
Cite this as: BMJ 2015;350:h988
References
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol 1983;9:689-96. [DOI] [PubMed] [Google Scholar]
- 4.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [DOI] [PubMed] [Google Scholar]
- 5.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend KN, Spowart JE, Huwait H, et al. Markers of T cell infiltration and function associate with favorable outcome in vascularized high-grade serous ovarian carcinoma. PLoS One 2013;8:e82406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zerbini A, Pilli M, Penna A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res 2006;66:1139-46. [DOI] [PubMed] [Google Scholar]
- 8.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011;8:151-60. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Cancer fact sheet. 2015. www.who.int/mediacentre/factsheets/fs297/en/.
- 10.American Cancer Society. Cancer facts and figures 2014. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/.
- 11.Kawakami Y, Eliyahu S, Delgado CH, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A 1994;91:6458-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A 1994;91:3515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulie PG, Brichard V, Van Pel A, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med 1994;180:35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traversari C, van der Bruggen P, Luescher IF, et al. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med 1992;176:1453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn, OJ, Binder, RJ, Brickner, AG, et al. Human tumor antigens as targets of immunosurveillance and candidates for cancer vaccines. In: Gires O, Seliger B, eds. Tumor-associated antigens: identification, characterization and clinical applications. Wiley-VCH Verlag, GmbH & Co, 2009:23-43.
- 16.Topalian SL, Rivoltini L, Mancini M, et al. Human CD4+ T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc Natl Acad Sci U S A 1994;91:9461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn OJ, Jerome KR, Henderson RA, et al. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol Rev 1995;145:61-89. [DOI] [PubMed] [Google Scholar]
- 18.Bakker AB, Marland G, de Boer AJ, et al. Generation of antimelanoma cytotoxic T lymphocytes from healthy donors after presentation of melanoma-associated antigen-derived epitopes by dendritic cells in vitro. Cancer Res 1995;55:5330-4. [PubMed] [Google Scholar]
- 19.Boon T, Cerottini JC, Van den Eynde B, et al. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 1994;12:337-65. [DOI] [PubMed] [Google Scholar]
- 20.Vollmer CM Jr, Eilber FC, Butterfield LH, et al. Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res 1999;59:3064-7. [PubMed] [Google Scholar]
- 21.Butterfield LH, Koh A, Meng W, et al. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res 1999;59:3134-42. [PubMed] [Google Scholar]
- 22.Irie RF, Giuliano AE, Morton DL. Oncofetal antigen: a tumor-associated fetal antigen immunogenic in man. J Natl Cancer Inst 1979;63:367-73. [PubMed] [Google Scholar]
- 23.Coggin JH Jr, Barsoum AL, Rohrer JW. 37 kiloDalton oncofetal antigen protein and immature laminin receptor protein are identical, universal T-cell inducing immunogens on primary rodent and human cancers. Anticancer Res 1999;19:5535-42. [PubMed] [Google Scholar]
- 24.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009;15:5323-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vonderheide RH, Nathanson KL. Immunotherapy at large: the road to personalized cancer vaccines. Nat Med 2013;19:1098-100. [DOI] [PubMed] [Google Scholar]
- 26.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overwijk WW, Wang E, Marincola FM, et al. Mining the mutanome: developing highly personalized immunotherapies based on mutational analysis of tumors. J Immunother Cancer 2013; published online 29 Jul. [DOI] [PMC free article] [PubMed]
- 28.Lehmann PV, Forsthuber T, Miller A, et al. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 1992;358:155-7. [DOI] [PubMed] [Google Scholar]
- 29.Butterfield LH, Ribas A, Dissette VB, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res 2003;9:998-1008. [PubMed] [Google Scholar]
- 30.Ribas A, Glaspy JA, Lee Y, et al. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother 2004;27:354-67. [DOI] [PubMed] [Google Scholar]
- 31.Ribas A, Timmerman JM, Butterfield LH, et al. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol 2003;24:58-61. [DOI] [PubMed] [Google Scholar]
- 32.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 2002;20:2624-32. [DOI] [PubMed] [Google Scholar]
- 33.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother 2011;60:433-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wierecky J, Müller MR, Wirths S, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res 2006;66:5910-8. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y, Petroni GR, Olson WC, et al. Immunologic hierarchy, class II MHC promiscuity, and epitope spreading of a melanoma helper peptide vaccine. Cancer Immunol Immunother 2014;63:779-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krauze MT, Tarhini A, Gogas H, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. Semin Immunopathol 2011;33:385-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gogas H, Ioannovich J, Dafni U. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 2006;354:709-18. [DOI] [PubMed] [Google Scholar]
- 38.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [DOI] [PubMed] [Google Scholar]
- 39.Kiertscher SM, Luo J, Dubinett SM, et al. Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells. J Immunol 2000;164:1269-76. [DOI] [PubMed] [Google Scholar]
- 40.Kantoff PW, Higano CS, Shore ND, et al; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X, Bose A, Komita H, et al. Vaccines targeting tumor blood vessel antigens promote CD8(+) T cell-dependent tumor eradication or dormancy in HLA-A2 transgenic mice. J Immunol 2012;188:1782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komita H, Zhao X, Taylor JL, et al. CD8+ T-cell responses against hemoglobin-beta prevent solid tumor growth. Cancer Res 2008;68:8076-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Disis ML, Gralow JR, Bernhard H, et al. Peptide-based, but not whole protein, vaccines elicit immunity to HER-2/neu, oncogenic self-protein. J Immunol 1996;156:3151-8. [PubMed] [Google Scholar]
- 44.Kirkwood JM, Lee S, Moschos SJ, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine ±granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res 2009;15:1443-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slingluff CL Jr, Lee S, Zhao F, et al. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602). Clin Cancer Res 2013;19:4228-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol 2014;32:2050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 2011;29:330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fourcade J, Kudela P, Andrade Filho PA, et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother 2008;31:781-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenter GG, Welters MJ, Valentijn AR, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008;14:169-77. [DOI] [PubMed] [Google Scholar]
- 50.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011;364:2119-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg S, Yang J, Kammula U, et al. Different adjuvanticity of incomplete Freund’s adjuvant derived from beef or vegetable components in melanoma patients immunized with a peptide vaccine. J Immunother 2010;33:557-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med 2013;19:465-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aranda F, Vacchelli E, Eggermont A, et al. Trial watch: peptide vaccines in cancer therapy. Oncoimmunology 2013;2:e26621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welters MJ, Kenter GG, de Vos van Steenwijk PJ, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A 2010;107:11895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009;361:1838-47. [DOI] [PubMed] [Google Scholar]
- 56.Rosalia RA, Quakkelaar ED, Redeker A, et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol 2013;43:2554-65. [DOI] [PubMed] [Google Scholar]
- 57.Hirschler B. 2-GSK cancer vaccine fails again but testing continues. Reuters 2014. www.reuters.com/article/2014/03/20/gsk-vaccine-idUSL6N0MH1IR20140320.
- 58.Slingluff CL Jr. The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J 2011;17:343-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slingluff CL Jr, Petroni GR, Chianese-Bullock KA, et al. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol 2011;29:2924-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012;18:1254-6. [DOI] [PubMed] [Google Scholar]
- 61.Disis ML, Gad E, Herendeen DR, et al. A multiantigen vaccine targeting neu, IGFBP-2, and IGF-IR prevents tumor progression in mice with preinvasive breast disease. Cancer Prev Res (Phila) 2013;6:1273-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura T, McKolanis JR, Dzubinski LA, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palucka K, Ueno H, Fay J, et al. Dendritic cells and immunity against cancer. J Intern Med 2011;269:64-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013;39:38-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996;2:52-8. [DOI] [PubMed] [Google Scholar]
- 67.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998;4:328-32. [DOI] [PubMed] [Google Scholar]
- 68.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 2001;61:6451-8. [PubMed] [Google Scholar]
- 69.Van Tendeloo VF, Van de Velde A, Van Driessche A, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A 2010;107:13824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenblatt J, Avivi I, Vasir B, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res 2013;19:3640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vacchelli E, Vitale I, Eggermont A, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology 2013;2:e25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 1993;90:3539-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McBride WH, Thacker JD, Comora S, et al. Genetic modification of a murine fibrosarcoma to produce interleukin 7 stimulates host cell infiltration and tumor immunity. Cancer Res 1992;52:3931-7. [PubMed] [Google Scholar]
- 74.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A 1998;95:13141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nemunaitis J, Sterman D, Jablons D, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst 2004;96:326-31. [DOI] [PubMed] [Google Scholar]
- 76.Tani K, Azuma M, Nakazaki Y, et al. Phase I study of autologous tumor vaccines transduced with the GM-CSF gene in four patients with stage IV renal cell cancer in Japan: clinical and immunological findings. Mol Ther 2004;10:799-816. [DOI] [PubMed] [Google Scholar]
- 77.Luiten RM, Kueter EW, Mooi W, et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients. J Clin Oncol 2005;23:8978-91. [DOI] [PubMed] [Google Scholar]
- 78.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 2012;18:858-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le DT, Dubenksy TW Jr, Brockstedt DG. Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol 2012;39:311-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chakraborty NG, Sporn JR, Tortora AF, et al. Immunization with a tumor-cell-lysate-loaded autologous-antigen-presenting-cell-based vaccine in melanoma. Cancer Immunol Immunother 1998;47:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geiger J, Hutchinson R, Hohenkirk L, et al. Treatment of solid tumours in children with tumour-lysate-pulsed dendritic cells. Lancet 2000;356:1163-5. [DOI] [PubMed] [Google Scholar]
- 82.Barth RJ Jr, Fisher DA, Wallace PK, et al. A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res 2010;16:5548-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim TS, Chopra A, O-Sullivan IS, et al. Enhanced immunity to breast cancer in mice immunized with fibroblasts transfected with a complementary DNA expression library from breast cancer cells: enrichment of the vaccine for immunotherapeutic cells. J Immunother 2006;29:261-73. [DOI] [PubMed] [Google Scholar]
- 84.Meng WS, Butterfield LH, Ribas A, et al. α-Fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res 2001;61:8782-6. [PubMed] [Google Scholar]
- 85.Butterfield LH, Economou JS, Gamblin TC, et al. Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients. J Transl Med 2014;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arthur JF, Butterfield LH, Roth MD, et al. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther 1997;4:17-25. [PubMed] [Google Scholar]
- 87.Schumacher L, Ribas A, Dissette VB, et al. Human dendritic cell maturation by adenovirus transduction enhances tumor antigen-specific T-cell responses. J Immunother 2004;27:191-200. [DOI] [PubMed] [Google Scholar]
- 88.Butterfield LH, Comin-Anduix B, Vujanovic L, et al. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother 2008;31:294-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kirk CJ, Mulé JJ. Gene-modified dendritic cells for use in tumor vaccines. Hum. Gene Ther 2000;11:797-806. [DOI] [PubMed] [Google Scholar]
- 90.Engelmayer J, Larsson M, Subklewe M, et al. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol 1999;163:6762-8. [PubMed] [Google Scholar]
- 91.Salio M, Cella M, Suter M, et al. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol 1999;29:3245-53. [DOI] [PubMed] [Google Scholar]
- 92.Madan RA, Bilusic M, Heery C, et al. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol 2012;39:296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith MR, Kantoff PW. Changes in PSA kinetics after DNA vaccine therapy-not so fast! J Clin Oncol 2010;28:e58. [DOI] [PubMed] [Google Scholar]
- 94.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010;59:663-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanerva A, Nokisalmi P, Diaconu I, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res 2013;19:2734-44. [DOI] [PubMed] [Google Scholar]
- 96.Guo ZS, Naik A, O’Malley ME, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res 2005;65:9991-8. [DOI] [PubMed] [Google Scholar]
- 97.John LB, Howland LJ, Flynn JK, et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res 2012;72:1651-60. [DOI] [PubMed] [Google Scholar]
- 98.Li J, O’Malley M, Sampath P, et al. Expression of CCL19 from oncolytic vaccinia enhances immunotherapeutic potential while maintaining oncolytic activity. Neoplasia 2012;14:1115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaufman HL, Bines SD. OPTIM trial: a phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol 2010;6:941-9. [DOI] [PubMed] [Google Scholar]
- 100.Ross M, Ingemar R, Puzanov A, et al. Patterns of durable response with intralesional talimogene laherparepvec (T-VEC): Results from a phase III trial in patients with stage IIIb-IV melanoma. J Clin Oncol 2014;32(5 suppl):abstr 9026. [Google Scholar]
- 101.Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013;57:1448-57. [DOI] [PubMed] [Google Scholar]
- 102.Zerbini A, Pilli M, Laccabue D, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology 2010;138:1931-42. [DOI] [PubMed] [Google Scholar]
- 103.Huang X, Yuan F, Liang M, et al. M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PLoS One 2012;7:e41632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xia JZ, Xie FL, Ran LF, et al. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol 2012;38:1363-71. [DOI] [PubMed] [Google Scholar]
- 105.Finley DS, Pouliot F, Shuch B, et al. Ultrasound-based combination therapy: potential in urologic cancer. Expert Rev Anticancer Ther 2011;11:107-13. [DOI] [PubMed] [Google Scholar]
- 106.Ayaru L, Pereira SP, Alisa A, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol 2007;178:1914-22. [DOI] [PubMed] [Google Scholar]
- 107.Gravante G, Sconocchia G, Ong SL, et al. Immunoregulatory effects of liver ablation therapies for the treatment of primary and metastatic liver malignancies. Liver Int 2009;29:18-24. [DOI] [PubMed] [Google Scholar]
- 108.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004;34:336-44. [DOI] [PubMed] [Google Scholar]
- 109.Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005;11:6713-21. [DOI] [PubMed] [Google Scholar]
- 110.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010;70:3052-61. [DOI] [PubMed] [Google Scholar]
- 111.Tsavaris N, Kosmas C, Vadiaka M, et al. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer 2002;87:21-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology 2014;3:e27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Demaria S, Pilones KA, Vanpouille-Box C, et al. The optimal partnership of radiation and immunotherapy: from preclinical studies to clinical translation. Radiat Res 2014;182:170-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [DOI] [PubMed] [Google Scholar]
- 115.Cotter SE, Dunn GP, Collins KM, et al. Abscopal effect in a patient with metastatic Merkel cell carcinoma following radiation therapy: potential role of induced antitumor immunity. Arch Dermatol 2011;147:870-2. [DOI] [PubMed] [Google Scholar]
- 116.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vacchelli E, Vitale I, Tartour E, et al. Trial watch: anticancer radioimmunotherapy. Oncoimmunology 2013;2:e25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013;19:1225-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010;70:5213-9. [DOI] [PubMed] [Google Scholar]
- 121.Liu C, Peng W, Xu C, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res 2013;19:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Butterfield LH. Dendritic cells in cancer immunotherapy clinical trials: are we making progress? Front Immunol 2013;4:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maus MV, Grupp SA, Porter DL, et al. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014;123:2625-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eshhar Z, Waks T, Bendavid A, et al. Functional expression of chimeric receptor genes in human T cells. J Immunol Methods 2001;248:67-76. [DOI] [PubMed] [Google Scholar]
- 125.Brentjens RJ, Rivière I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993;90:720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drake AC. Of mice and men: what rodent models don’t tell us. Cell Mol Immunol 2013;10:284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Di Nicolantonio F, Bardelli A. Mouse models of Kras-mutant colorectal cancer: valuable GEMMs for drug testing? Clin Cancer Res 2013;19:2794-6. [DOI] [PubMed] [Google Scholar]
- 129.Meng WS, Butterfield LH, Ribas A, et al. α-Fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res 2001;61:8782-6. [PubMed] [Google Scholar]
- 130.Meng WS, Butterfield LH. Activation of antigen-presenting cells by DNA delivery vectors. Expert Opin Biol Ther 2005;5:1019-28. [DOI] [PubMed] [Google Scholar]
- 131.Conry RM, LoBuglio AF, Loechel F, et al. A carcinoembryonic antigen polynucleotide vaccine for human clinical use. Cancer Gene Ther 1995;2:33-8. [PubMed] [Google Scholar]
- 132.Kumar V, Sercarz E. Genetic vaccination: the advantages of going naked. Nat Med 1996;2:857-9. [DOI] [PubMed] [Google Scholar]
- 133.Conry RM, Curiel DT, Strong TV, et al. Safety and immunogenicity of a DNA vaccine encoding carcinoembryonic antigen and hepatitis B surface antigen in colorectal carcinoma patients. Clin Cancer Res 2002;8:2782-7. [PubMed] [Google Scholar]
- 134.Rosenberg SA, Yang JC, Sherry RM, et al. Inability to immunize patients with metastatic melanoma using plasmid DNA encoding the gp100 melanoma-melanocyte antigen. Hum Gene Ther 2003;14:709-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Radkevich-Brown O, Piechocki MP, Back JB, et al. Intratumoral DNA electroporation induces anti-tumor immunity and tumor regression. Cancer Immunol Immunother 2010;59:409-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Diaz CM, Chiappori A, Aurisicchio, et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors. J Transl Med 2013;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mincheff M, Tchakarov S, Zoubak S, et al. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur Urol 2000;38:208-17. [DOI] [PubMed] [Google Scholar]
- 138.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004;10:909-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Timmerman JM, Levy R. Cancer vaccines: pessimism in check. Nat Med 2004;10:1279; author reply 1279-80. [DOI] [PubMed] [Google Scholar]
- 140.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013;39:49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013;13:525-41. [DOI] [PubMed] [Google Scholar]
- 142.Butterfield LH, Palucka AK, Britten CM, et al. Recommendations from the iSBTc-SITC/FDA/NCI workshop on immunotherapy biomarkers. Clin Cancer Res 2011;17:3064-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Keilholz U, Weber J, Finke JH, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother 2002;25:97-138. [DOI] [PubMed] [Google Scholar]
- 144.Dancey JE, Dobbin KK, Groshen S, et al. Biomarkers task force of the NCI investigational drug steering committee. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res 2010;16:1745-55. [DOI] [PubMed] [Google Scholar]
- 145.Janetzki S, Panageas KS, Ben-Porat L, et al. Elispot proficiency panel of the CVC immune assay working group. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol Immunother 2008;57:303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Britten CM, Janetzki S, Ben-Porat L, et al. Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Cancer Immunol Immunother 2009;58:1701-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Janetzki S, Britten CM, Kalos M, et al. “MIATA”-minimal information about T cell assays. Immunity 2009;31:527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Janetzki S, Britten CM. The impact of harmonization on ELISPOT assay performance. Methods Mol Biol 2012;792:25-36. [DOI] [PubMed] [Google Scholar]
- 149.Schaefer C, Butterfield LH, Lee S, et al. Function but not phenotype of melanoma peptide-specific CD8(+) T cells correlate with survival in a multiepitope peptide vaccine trial (ECOG 1696). Int J Cancer 2012;131:874-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med 2006;203:2865-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sondergaard K, Schou G. Prognostic factors in primary cutaneous malignant melanoma. Am J Dermatopathol 1985;7(suppl):1-4. [DOI] [PubMed] [Google Scholar]
- 152.Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. [DOI] [PubMed] [Google Scholar]
- 153.Erdag G, Schaefer JT, Slingluff CL Jr, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012;72:1070-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harlin H, Meng Y, Gajewski TF, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 2009;69:3077-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weide B, Martens A, Zelba H, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res 2014;20:1601-9. [DOI] [PubMed] [Google Scholar]
- 156.Tarhini AA, Butterfield LH, Shuai Y, et al. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-α or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother 2012;35:702-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014;9:e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Butterfield L, Vujanovic L, Pardee A. Approaches to immunologic monitoring of clinical trials. In: The tumor immunoenvironment. Springer, 2013:663-94.
- 159.Nagato T, Lee YR, Harabuchi Y, Celis E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res 2014;20:1223-34. [DOI] [PMC free article] [PubMed] [Google Scholar]