Abstract
Pulmonary focal Ground-glass Opacities (fGGOs) would frequently be identified after widely implementation of low-dose computed tomography (LDCT) screening. Because of the high false-positive rate of LDCT, antibiotics should be regarded as advocates in clinical management for detected fGGOs. Retrospectively review consecutive patients with fGGOs between August 2006 and August 2012. Then, relative Glasgow prognostic score (GPS) were constructed in three different systems, traditional GPS system (tGPS), modified GPS system 1 (m1GPS), and modified GPS system 2 (m2GPS). Moreover, propensity score matching (PSM) was employed in balancing baseline covariates. After PSM, patients were matched and included in benign and malignant groups as 1:1 ratio. All reported parameters were balanced in both groups and no statistical differences could be detected. Finally, m1GPS exhibited remarkable different distribution between benign and malignant fGGOs. In detail, m1GPS 1 was more frequently observed in benign fGGOs nodules, while m1GPS 2 in malignant fGGOs nodules. Modified inflammation-based score was identified as an independent predictor of malignancies in patients with pulmonary fGGOs. Patients with m1GPS 1 were more likely to be benign fGGOs, while victims with m1GPS 2 more likely to be malignant.
Pulmonary focal Ground-glass Opacities (fGGOs), defined as “Hazy increased attenuation of lung, but with preservation of bronchial and vascular margins; caused by partial filling of air spaces, interstitial thickening, partial collapse of alveoli, normal expiration, or increased capillary blood volume”1, were commonly recognized as nonspecific lung computed tomography (CT) findings2. After success of the National Lung Screening Trial3, more widely implementation of low-dose computed tomography (LDCT) screening would identify even more nodules4, although approximately 150,000 new lung nodules has been detected annually in the United States5.
However, the false-positive rate of LDCT was extremely high6; and only 1% to 12% detected fGGOs were malignant7. Despite the fact that a wide range existed in the differential diagnosis of lung fGGOs8, evidences demonstrated the most common cause of lung fGGOs would be infection9, especially in developing countries and areas10. As a result, antibiotics should be regarded as advocates in clinical management for detected fGGOs to obviate unnecessary follow-up testing, radiation exposure, anxiety, depression, and substantial financial costs11. Nevertheless, selecting candidates for antibiotic use remained controversial12, which resulted in concerns in the likelihood of meaningful infections among detected nodules and the potential for antibiotics resistance13.
In order to better and easier evaluating inflammation status, elevated systemic C-reactive protein (CRP), as a typical index and a sensitive measure of the systemic inflammatory response14, and hypoalbuminemia, an indicator of malnutrition15, has been combined to construct an inflammation-based score system, named as Glasgow prognostic score (GPS)16. Aiming to improve the predictive effect of the GPS system, modified versions of the GPS system were developed, either adjusted cut-off values of both serum CRP and albumin levels17, or omitted hypoalbuminaemia alone as a negative prognostic indicator18.
In observational non-randomized studies, the baseline characteristics between the compared groups would be statistically different19. Specifically, potential confounding factors that might affect the outcomes of benign and malignant fGGOs would be statistically different, which would result in inaccurate assessment of inflammation-based score in patients with pulmonary fGGOs. To minimizing selection bias in non-randomized cohorts, propensity score matching (PSM) has been proposed as a statistical tool since 198320. The constructed score, describing the condition of unbalanced baseline covariates for participants in either experimental or control group21, could be used for matching in order to control the confounding between different groups22.
Given the fact of increasing detection of lung fGGOs and a paucity of evidence on clinical antibiotics utilities23, this preliminary study was designed with the aim of identifying an effective predictor of antibiotics use in treatment after lung fGGOs detection. This subset of victims should be recommended for antibiotics application because of the potential benefits.
Result
Clinical outcomes
128 patients with pulmonary fGGOs nodules were eligible for the final analysis. In this group of 128 patients, the mean age was 55.4 years. Additionally, malignant fGGOs were pathologically diagnosed as adenocarcinoma in 26patients, squamous cell carcinoma in 10patients, carcinoma in situ in 29 patients, and lymphoepithelioma in 12 patients. Accordingly, benign fGGOs were pathologically diagnosed as tuberculoma in 14 patients, pneumonia in 31 patients, and hamartomastage in 6 patients. Malignant fGGOs nodules were statistically correlated with presence of symptoms (p = 0.007), dominant nodule(s) with part-solid component (p < 0.001), and spiculation (p = 0.017), present of history of lung cancer (p = 0.015) and history of other cancers (p = 0.001), as well as larger lesions (p = 0.001). These imbalance parameters were proved to be risk factors of malignant fGGOs in previous studies and guidelines2,11,24. Other parameters included in guidelines were all reviewed and reported in these 128 patients, although no significantly differences were observed (Tables 1 and 2).
Table 1. Clinical characteristics of benign and malignant fGGOs before and after PSM.
| Characteristic | Before PSM |
After PSM |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 128) | Benign (n = 51) | Malignant (n = 77) | P | All (n = 82) | Benign (n = 41) | Malignant (n = 41) | P | |||
| Age, yrs | 56.6† (range: 18–79) | 53.8† (range: 18–77) | 58.7† (range: 33–79) | 0.078 | 54.4† (range: 18–79) | 55† (range: 18–77) | 54† (range: 33–79) | 0.817 | ||
| Smoking history, pack-yr | 7.8† (range: 0–60) | 9.7† (range: 0–60) | 6.6† (range: 0–60) | 0.587 | 5.9† (range: 0–60) | 9.3† (range: 0–60) | 2.5† (range: 0–60) | 0.173 | ||
| Time since smoking cessation, yr | 0.8† (range: 0–20) | 1.2† (range: 0–20) | 0.6† (range: 0–20) | 0.204 | 1.1† (range: 0–20) | 1.3† (range: 0–20) | 0.9† (range: 0–20) | 0.340 | ||
| Sex(%) | 0.149 | 0.659 | ||||||||
| Male | 62 | 48.4% | 29 | 33 | 41 | 50.0% | 22 | 19 | ||
| Female | 66 | 51.6% | 22 | 44 | 41 | 50.0% | 19 | 22 | ||
| Symptom | 0.007 | 0.372 | ||||||||
| Absent | 69 | 53.9% | 35 | 34 | 47 | 57.3% | 26 | 21 | ||
| Present | 59 | 46.1% | 16 | 43 | 35 | 42.7% | 15 | 20 | ||
| History of other lung diseases | 0.702 | 1.000 | ||||||||
| Absent | 121 | 94.5% | 49 | 72 | 80 | 97.6% | 40 | 40 | ||
| Present | 7 | 5.5% | 2 | 5 | 2 | 2.4% | 1 | 1 | ||
| History of lung cancer | 0.015 | 0.312 | ||||||||
| Absent | 112 | 87.5% | 40 | 72 | 72 | 87.8% | 34 | 38 | ||
| Present | 16 | 12.5% | 11 | 5 | 10 | 12.2% | 7 | 3 | ||
| History of other cancers | 0.001 | 0.198 | ||||||||
| Absent | 98 | 76.6% | 31 | 67 | 62 | 75.6% | 28 | 34 | ||
| Present | 30 | 23.4% | 20 | 10 | 20 | 24.4% | 13 | 7 | ||
| Family history of lung cancer | 0.682 | 0.999 | ||||||||
| Absent | 122 | 95.3% | 48 | 74 | 77 | 93.9% | 38 | 39 | ||
| Present | 6 | 4.7% | 3 | 3 | 5 | 6.1% | 3 | 2 | ||
PSM: propensity score matching.
†Values are given as the median fGGOs.
Table 2. Radiological characteristics of benign and malignant fGGOs before and after PSM.
| Characteristic | Before PSM |
After PSM |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 128) | Benign (n = 51) | Malignant (n = 77) | P | All (n = 82) | Benign (n = 41) | Malignant (n = 41) | P | |||
| Size of fGGOs, mm | 21.5† (range: 5–45) | 16.0† (range: 5–45) | 26.0† (range: 8–45) | 0.001 | 20.0† (range: 5–45) | 16.0† (range: 5–45) | 22.0† (range: 8–45) | 0.160 | ||
| GGO numbers | 0.194 | 0.547 | ||||||||
| Solitary | 110 | 85.9% | 41 | 69 | 69 | 84.1% | 33 | 36 | ||
| Multiple | 18 | 14.1% | 10 | 8 | 13 | 15.9% | 8 | 5 | ||
| GGO type | <0.001 | 1.000 | ||||||||
| Pure GGO without a dominant lesion(s) | 43 | 33.6% | 31 | 12 | 42 | 51.2% | 21 | 21 | ||
| Dominant nodule(s) with part-solid component | 85 | 66.4% | 20 | 65 | 40 | 48.8% | 20 | 20 | ||
| Cavitation | 0.062 | 0.116 | ||||||||
| Present | 17 | 13.3% | 3 | 14 | 12 | 14.6% | 3 | 9 | ||
| Absent | 111 | 86.7% | 48 | 63 | 70 | 85.4% | 38 | 32 | ||
| Spiculation | 0.017 | 0.262 | ||||||||
| Present | 52 | 40.6% | 14 | 38 | 34 | 41.5% | 14 | 20 | ||
| Absent | 76 | 59.4% | 37 | 39 | 48 | 58.5% | 27 | 21 | ||
| Calcification | 0.275 | 0.241 | ||||||||
| Present | 3 | 2.3% | 0 | 3 | 3 | 3.7% | 0 | 3 | ||
| Absent | 125 | 97.7% | 51 | 74 | 79 | 96.3% | 41 | 38 | ||
fGGOs: focal Ground-glass Opacity.
PSM: propensity score matching.
†Values are given as the median.
All 128 patients (51 benign fGGOs and 77 malignant fGGOs) were eligible for PSM under one-to-one nearest neighbor matching algorithm at a caliper of 0.2. The calculated PS, constructed for the entire 128 cases, ranged from 0.03 to 1.0 and had a median of 0.67. Before matching, the mean propensity score was 0.39 for patients with benign fGGOs (n = 51) and 0.74 for patients with malignant fGGOs (n = 77) (P = 0.003). After PSM under one-to-one nearest neighbor matching algorithm at a caliper of 0.2, 82 patients (41 benign fGGOs and 41 malignant fGGOs) were matched and included in benign and malignant groups. The mean propensity score was 0.46 for patients with benign fGGOs (n = 41) and 0.70 for patients with malignant fGGOs (n = 41) (P = 0.805). The standardized difference in means and distribution of propensity scores consistently illustrated improvement of covariate balance after PSM (Figs 1 and 2). In this group of 82 patients, the mean age was 53.5 years. All reported parameters were balanced in both groups and no statistical differences could be detected, including symptoms (p = 0.372), dominant nodule(s) with part-solid component (p = 1.000), spiculation (p = 0.262), history of lung cancer (p = 0.312), history of other cancers (p = 0.198), and tumor size (p = 0.160) (Tables 1 and 2). Additionally, histopathological analyses showed no significant difference before and after PSM (p = 0.152), which illustrated consistent outcomes of benign and malignant fGGOs before and after the balancing procedure of PSM, thus confirming the reliability of PSM in balancing baseline demographic characteristics (Table S1).
Figure 1. Parallel line plot of the standardized difference in means before and after PSM in patients with benign and malignant fGGOs.
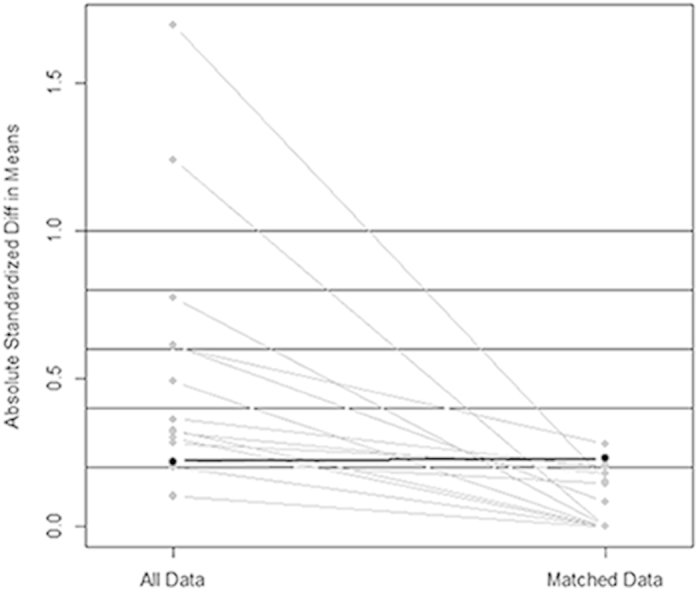
As the standardized difference in means was reduced, covariate balance was improved in the matched samples.
Figure 2. Dot plot of the propensity scores of patients with benign and malignant fGGOs showing individual units in the dataset and whether they were matched or discarded.
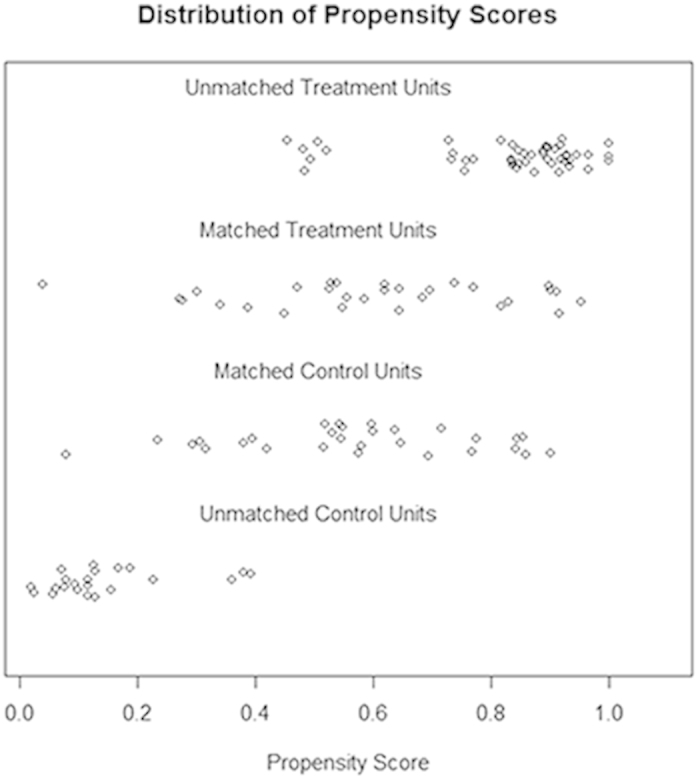
Treatment units: patients with malignant fGGOs; Control units: patients with benign fGGOs.
PSM analysis
Before PSM, the distribution of tGPS score was tGPS 0 in 22 (43.1%) patients, tGPS 1 in 21 (41.2%) patients, and tGPS 2 in 8 (15.7%) of benign fGGOs; and tGPS 0 in 27 (35.1%) patients, tGPS 1 in 33 (42.8%) patients, and tGPS 2 in 17 (22.1%) of malignant fGGOs, accordingly (Table 3). Additionally, the distribution of m1 GPS score was m1 GPS 0 in 26 (51.0%) patients, m1 GPS 1 in 17 (33.3%) patients, and m1 GPS 2 in 8 (15.7%) of benign fGGOs; and m1 GPS 0 in 30 (39.0%) patients, m1 GPS 1 in 30 (39.0%) patients, and m1 GPS 2 in 17 (22.0%) of malignant fGGOs, accordingly (Table 4). Furthermore, the distribution of m2 GPS score was m2 GPS 0 in 17 (33.3%) patients, m2 GPS 1 in 18 (35.3%) patients, and m2 GPS 2 in 16 (31.4%) of benign fGGOs; and m2 GPS 0 in 20 (26.0%) patients, m2 GPS 1 in 17 (22.1%) patients, and m2 GPS 2 in 40 (51.9%) of malignant fGGOs, accordingly (Table 5). Consequently, no statistical differences could be observed between benign and malignant fGGOs nodules among these 128 patients in aspects of tGPS (p = 0.553), m1GPS (p = 0.383) and m2GPS (p = 0.064) (Tables 3, 4, 5).
Table 3. Distribution of traditional GPS in patients with benign and malignant fGGOs before and after PSM.
| Before PSM (n = 128) | After PSM (n = 82) | |||||||
|---|---|---|---|---|---|---|---|---|
| tGPS 0, n (%) | tGPS 1, n (%) | tGPS 2, n (%) | Total, n | tGPS 0, n (%) | tGPS 1, n (%) | tGPS 2, n (%) | Total, n | |
| Benign, n (%) | 22 (43.1%) | 21 (41.2%) | 8 (15.7%) | 51 | 17 (41.5%) | 17 (41.5%) | 7 (17.0%) | 41 |
| Malignant, n (%) | 27 (35.1%) | 33 (42.8%) | 17 (22.1%) | 77 | 15 (36.6%) | 17 (41.5%) | 9 (21.9%) | 41 |
fGGOs: focal Ground-glass Opacity.
PSM: propensity score matching.
tGPS: traditional Glasgow prognostic score system.
Table 4. Distribution of modified GPS 1 in patients with benign and malignant fGGOs before and after PSM.
| Before PSM (n = 128) | After PSM (n = 82) | |||||||
|---|---|---|---|---|---|---|---|---|
| m1GPS 0, n (%) | m1GPS 1, n (%) | m1GPS 2, n (%) | Total, n | m1GPS 0, n (%) | m1GPS 1, n (%) | m1GPS 2, n (%) | Total, n | |
| Benign, n (%) | 26 (51.0%) | 17 (33.3%) | 8 (15.7%) | 51 | 13 (31.7%) | 22 (53.7%) | 6 (14.6%) | 41 |
| Malignant, n (%) | 30 (39.0%) | 30 (39.0%) | 17 (22.0%) | 77 | 10 (24.4%) | 3 (7.3%) | 28 (68.3%) | 41 |
fGGOs: focal Ground-glass Opacity.
PSM: propensity score matching.
m1GPS: modified Glasgow prognostic score system 1.
Table 5. Distribution of modified GPS 2 in patients with benign and malignant fGGOs before and after PSM.
| Before PSM (n = 128) | After PSM (n = 82) | |||||||
|---|---|---|---|---|---|---|---|---|
| m2GPS 0, n (%) | m2GPS 1, n (%) | m2GPS 2, n (%) | Total, n | m2GPS 0, n (%) | m2GPS 1, n (%) | m2GPS 2, n (%) | Total, n | |
| Benign, n (%) | 17 (33.3%) | 18 (35.3%) | 16 (31.4%) | 51 | 13 (31.7%) | 15 (36.6%) | 13 (31.7%) | 41 |
| Malignant, n (%) | 20 (26.0%) | 17 (22.1%) | 40 (51.9%) | 77 | 10 (24.4%) | 10 (24.4%) | 21 (51.2%) | 41 |
fGGOs: focal Ground-glass Opacity.
PSM: propensity score matching.
m2GPS: modified Glasgow prognostic score system 2.
After PSM, the distribution of tGPS score was tGPS 0 in 17 (41.5%) patients, tGPS 1 in 17 (41.5%) patients, and tGPS 2 in 7 (17.0%) of benign fGGOs; and tGPS 0 in 15 (36.6%) patients, tGPS 1 in 17 (41.5%) patients, and tGPS 2 in 9 (21.9%) of malignant fGGOs, accordingly (Table 3). Additionally, the distribution of m1 GPS score was m1 GPS 0 in 13 (31.7%) patients, m1 GPS 1 in 22 (53.7%) patients, and m1 GPS 2 in 6 (14.6%) of benign fGGOs; and m1 GPS 0 in 10 (24.4%) patients, m1 GPS 1 in 3 (7.3%) patients, and m1 GPS 2 in 28 (68.3%) of malignant fGGOs, accordingly (Table 4). Furthermore, the distribution of m2 GPS score was m2 GPS 0 in 13 (31.7%) patients, m2 GPS 1 in 15 (36.6%) patients, and m2 GPS 2 in 13 (31.7%) of benign fGGOs; and m2 GPS 0 in 10 (24.4%) patients, m2 GPS 1 in 10 (24.4%) patients, and m2 GPS 2 in 21 (51.2%) of malignant fGGOs, accordingly (Table 5). Interestingly, although significant differences still could not be observed between benign and malignant fGGOs nodules in aspects of tGPS (p = 0.829) and m2GPS (p = 0.195) (Tables 3 and 5), m1GPS exhibited remarkable different distribution between benign and malignant fGGOs (p < 0.001) (Table 4). In detail, m1GPS 1 was more frequently observed in benign fGGOs nodules, while m1GPS 2 in malignant fGGOs nodules. This interesting result was caused by the different definition of different GPS systems. Elevated CRP level, representing an inflammation cause of the host, would be more likely represent an inflammation cause for fGGOs, instead of hypoalbuminemia, representing malnutrition of the host. Furthermore, the suitable cut-off values should be 10 mg/L for elevated CRP level and 35 g/L for hypoalbuminemia.
Discussion
The current preliminary study, after evaluating and comparing different inflammation-based score systems, identified m1GPS as an effective predictor of antibiotics use in treatment after lung fGGOs detection. A subset of victims should be chosen for antibiotics in application because of the potential benefits.
After widely application of LDCT in lung screening, pulmonary fGGOs would be frequently identified24. fGGOs were considered to be a great challenge for biopsy due to their small size or unnecessary for immediate aggressive diagnostic procedures but only referred for follow-up with series of CT scans because of low risk for malignancy25. Nonetheless, such a strategy would be expected to result in significant anxiety, radiation exposure, and additional cost26. By contrast, a safe, simple and inexpensive option, such as antibiotics prescription, should be reckoned in fGGOs management23. Although antibiotics prescription was supported by their effectiveness against plenty of inflammatory disorders causing fGGOs, indications and exact utilities of antibiotics prescription in fGGOs remained unclear23,27. Even if clinicians suggested some clinical and radiographic characteristics and an improving trend with antibiotic use, no statistical associations between patients’ characteristics and antibiotics use could be discovered in previous studies23,28. In the present study, potential risk factors and inflammation-based score systems were analyzed though PSM method to identify a subset of candidates of antibiotics prescription with probable benefits.
In real world, treatment selection was usually influenced by a series of baseline characteristics29. For this reason, baseline characteristics should be taken into consideration when accessing therapy regimens30. PSM, designed for reducing or eliminating differences among baseline characteristics, was attracting increasing interests in medical research31,32. Before PSM, some demographic characteristics were imbalance, which might affect the outcomes of benign and malignant fGGOs , thus confounding the real role of inflammation-based score in patients with pulmonary fGGOs. After PSM, both groups illustrated similar demographic characteristics with no significant differences, which suggested that PSM effectively minimized imbalance among covariates.
Existed investigations have proved the inflammation-based prognostic score, GPS, as predictor for coexistence of systemic inflammation and malnutrition of the host33. GPS could be considered routinely applied globally depended on its plain, minimally invasive, and cost-effect measurement34. Furthermore, considerable attention was poured into improving the predict effect of GPS18,35. Some investigators modified the cut lines of abnormal serum albumin and CRP level at 38 g/L and 5 mg/L, respectively35. Additionally, other studies recommended another GPS modification as assigning normal CRP but hypoalbuminemia to GPS 0 group18. All three GPS systems were evaluated in identifying the antibiotics beneficial. Finally, only m1GPS, allocated hypoalbuminemia alone to GPS 0, was proved as an effective predictor of malignancies in patients with pulmonary fGGOs. This could be explained as that systemic inflammation would be more likely represent an inflammation cause for fGGOs, instead of malnutrition of the host. Moreover, patients with m1GPS 1 were more likely to be benign fGGOs and with m1GPS 2 malignant, while no significant different between benign and malignant fGGOs in m1GPS 0 group. A possible interpretation might be inflammation caused fGGOs would be resulting in systemic malnutrition. Thus, if both systemic inflammation and malnutrition coexisted, the fGGOs would be a higher probability of malignancies.
Although other covariates involving dynamic change during follow-up were also included in the guidelines2,36, the present study focus on the clinical management of first detected fGGOs. Due to this reason, only covariates associated with the first detection were included. Besides, as a retrospective study, clinical and survival comparison might be dependent on selection bias due to its retrospective nature. Even if PSM could significantly overcome this limitation, future prospective multi-institutional large-scale studies were still a need in validating the findings.
In conclusion, modified inflammation-based score was identified as an independent predictor of malignancies in patients with pulmonary fGGOs. Patients with m1GPS 1 were more likely to be benign fGGOs, while victims with m1GPS 2 more likely to be malignant. This pilot conclusion should be evaluated in future prospective studies involving antibiotics prescription to further clarify the clinical role of the GPS system in patients with fGGOs.
Method
Study protocol was approved by the institutional review boards of Sun Yat-Sen University Cancer Center (SYSUCC). Written informed consent was obtained from each patient: including signed consent for tissue analysis as well as consent to be recorded for potential medical research at the time of patients’ admission. All experiments were performed in accordance with relevant guidelines and regulations.
Patients
Chart review was performed on consecutive patients who had undergone CT scans at SYSUCC between August 2006 and August 2012. Both clinical and pathological data were collected and reviewed. Only patients with pulmonary fGGO lesions were recruited if definitive diagnosis of malignant or benign were recorded concurrently at SYSUCC. Moreover, patients with pulmonary fGGO lesions were excluded from analysis if: 1) lacking qualifying CT scans, 2) with history of primary lung cancer or any other malignancies and systemic treatment (i.e. chemotherapy) were not finished at the time of detecting fGGO lung nodules, 3) diagnosis of any other malignancies during follow-up period. Under these criteria, patients underwent primary lung cancer or any other malignancies, but without ongoing systemic treatment when fGGOs detection, were included in final analysis.
The fGGOs was judged as classic definition. 50% GGO area was set as the cut-off value in identifying solid lesions or dominant nodule(s) with part-solid component37. Furthermore, multiple fGGOs were also included, because of occasional reports of multicentric lung adenocarcinoma38. In addition, since relatively large pure fGGOs were pathologically diagnosed as adenocarcinomas, fGGOs size was not considered as an exclusion criterion39.
Malignant fGGOs were defined as malignant diagnosis by pathologic examination of tissue obtained via surgery or biopsy. Accordingly, benign fGGOs were defined as either pathologic examination of tissue obtained via surgery or biopsy or fGGOs resolving during follow-up. However, in the latter situation, the exact classification was recommended as non-malignant lesion because no pathological diagnoses available. All pathological data were reviewed and confirmed by two independent pathologists based on WHO classification of Lung Cancer40.
CT scans were performed by a Toshiba Aquilion 64 CT scanner (Toshiba American Medical Systems Inc, Tustin, CA) during one breath-hold with 5-mm reconstruction and 2-mm slice collimation. Both lung (width, 1,500 HU; level, –700 HU) and mediastinal (width, 400 HU; level, 20 HU) window images were obtained and reviewed. All fGGO nodules characteristics were examined by thin-section chest CT scans (section thickness < 2.5 mm). The size of fGGO was measured as maximal diameter at lung window41. The fGGO lesions were classified as pure GGO and dominant nodule(s) with part-solid component based on the tumor shadow disappearance rate (TDR): dominant nodule(s) with part-solid component (0 < TDR < 1), and pure GGO (TDR = 0)42. All radiographic images were reviewed and confirmed by the same thoracic surgeon and consultant radiologist. The final decision for each radiology finding was made by consensus between them.
GPS system
In GPS evaluation, laboratory examinations including CRP and albumin were performed within 24 hours before or after CT scans as routine clinical practice in SYSUCC. Serum CRP and albumin levels were examined by the Hitachi Auto Analyzer (Hitachi 7600, Hitachi, Tokyo, Japan). The inter- and intra-assay variability of CRP and albumin concentrations were less than 5% as established by routine quality control procedures.
Relative GPS systems were constructed as previous reports35. In traditional GPS system, victims with both hypoalbuminemia (<35 g/L) and elevated CRP level (>10 mg/L) were allocated into tGPS 2 group. And, patients with neither of these two abnormalities were allocated into tGPS 0 group. Then, remaining patients with only one biochemical abnormalities were allocated into tGPS 1 group. Differently, in modified GPS system 1, patients with hypoalbuminaemia (<35 g/L) alone were classified into m1GPS 0 group, while other criteria for m1GPS score is the same with tGPS system. In detail, m1GPS 1 was defined as patients with elevated CRP level (>10 mg/L) alone, while m1GPS 2 as patients with both hypoalbuminemia (<35 g/L) and elevated CRP level (>10 mg/L). Additionally, in modified GPS system 2, the cut-off values were changed as 5 mg/L for elevated CRP level and 38 g/L for hypoalbuminemia. Other score assigning criteria for m2GPS system is the same with tGPS system (Table 6).
Table 6. Allocation of different inflammation-based score systems.
| Biochemical abnormalities | Score |
|---|---|
| Neither hypoalbuminemia (<35 g/L) nor elevated CRP level (>10 mg/L) | tGPS 0 |
| Either hypoalbuminemia (<35 g/L) or elevated CRP level (>10 mg/L) | tGPS 1 |
| Both hypoalbuminemia (<35 g/L) and elevated CRP level (>10 mg/L) | tGPS 2 |
| Without elevated CRP level (>10 mg/L) | m1 GPS 0 |
| Elevated CRP level (>10 mg/L) but without hypoalbuminemia (<35 g/L) | m1 GPS 1 |
| Both hypoalbuminemia (<35 g/L) and elevated CRP level (>10 mg/L) | m1 GPS 2 |
| Neither hypoalbuminemia (<38 g/L) nor elevated CRP level (>5 mg/L) | m2 GPS 0 |
| Either hypoalbuminemia (<38 g/L) or elevated CRP level (>5 mg/L) | m2 GPS 1 |
| Both hypoalbuminemia (<38 g/L) and elevated CRP level (>5 mg/L) | m2 GPS 2 |
tGPS: traditional Glasgow prognostic score system.
m1GPS: modified Glasgow prognostic score system 1.
m2GPS: modified Glasgow prognostic score system 2.
Statistical analysis
Categorical data were presented as numbers and percentages and continuous data as median and range unless otherwise stated. The Pearson χ2 test and McNemar’s test were used for categorical data, and an independent sample t-test or the Mann–Whitney U test were used for numerical data. P < 0.05 was considered to be significant in all statistical analyses.
Variables with statistically significant differences between groups might have impact on the postoperative outcomes. The PSM, aiming to minimize the influence of selection bias and potential confounding variables between benign and malignant fGGOs, was generated using all reported covariates with one-to-one nearest neighbor matching algorithm at a caliper of 0.2. The included characteristics as covariates were age, smoking history (measured by pack-yr), time since smoking cessation, sex, symptoms (including cough, dyspnea, sputum production, wheezing, night sweats, fever and weight loss), history of other lung diseases (including chronic obstructive pulmonary disease and pulmonary fibrosis), history of lung cancer, history of other cancers, family history of lung cancer, fGGOs size, GGO numbers, GGO type, cavitation, spiculation, and calcification. The standardized difference in means and distribution of propensity scores were used in assessing the improvement of covariate balance after PSM. The propensity score was calculated by multiplying the coefficient for each variable in the model. The initial unmatched and matched samples were assessed by calculating standardized differences. A standardized difference of less than the absolute value of 0.2 was taken to indicate negligible difference in the mean or prevalence of a covariate between the compared groups43. All the above procedures, inclusion calculation and matching, could be conducted by IBM SPSS Statistics for Windows and SPSS PS Matching plug-in.
Data management and statistical analyses were performed using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 22.0. IBM Corp., Armonk, NY) for Windows (SPSS Inc, Chicago, IL) and SPSS PS Matching plug-in (Propensity score matching in SPSS, psmatching3.03, Felix Thoemmes, Cornell University/University of Tübingen).
Additional Information
How to cite this article: Jiang, L. et al. Modified inflammation-based score as an independent malignant predictor in patients with pulmonary focal ground-glass opacity: a propensity score matching analysis. Sci. Rep. 6, 19105; doi: 10.1038/srep19105 (2016).
Supplementary Material
Acknowledgments
We wish to thank Prof. Qing Liu, Chairman of Department of Tumor Epidemiology and Professor of Department of Medical Statistics and Epidemiology, for statistical assistant, and Prof. Pamela Derish, Scientific Publications Manager of UCSF Department of Surgery (University of California, San Francisco), for language editing.
Footnotes
Author Contributions L.J. and S.S.J. designed and performed most study. L.J. and H.Y. wrote the main manuscript text. L.J. and Y.B.L. collected the clinical data. L.J., Z.R.Z. and Z.H.X. performed data analysis. L.J., Y.B.L. and H.L. designed and directed the overall project. All authors wrote and proofed the article.
References
- Austin J. H. et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 200, 327 (1996). [DOI] [PubMed] [Google Scholar]
- Naidich D. P. et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 266, 304 (2013). [DOI] [PubMed] [Google Scholar]
- Aberle D. R. et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365, 395 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G. A. Screening for lung cancer: it works, but does it really work? Ann Intern Med 155, 537 (2011). [DOI] [PubMed] [Google Scholar]
- Ost D., Fein A. M. & Feinsilver S. H. Clinical practice. The solitary pulmonary nodule. N Engl J Med 348, 2535 (2003). [DOI] [PubMed] [Google Scholar]
- Wahidi M. M., Govert J. A., Goudar R. K., Gould M. K. & McCrory D. C. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132, 94S (2007). [DOI] [PubMed] [Google Scholar]
- Henschke C. I. et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 231, 164 (2004). [DOI] [PubMed] [Google Scholar]
- Bolukbas S., Eberlein M. & Schirren J. Management of lung nodules detected by volume CT scanning. N Engl J Med 362, 757, 758 (2010). [PubMed] [Google Scholar]
- Wiener R. S., Gould M. K., Woloshin S., Schwartz L. M. & Clark J. A. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest 143, 672 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrmann G. B., van Vollenhoven F. H. & Moodley L. Approach to a solid solitary pulmonary nodule in two different settings-“Common is common, rare is rare”. J Thorac Dis 6, 237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiselle P. M. Computed tomography screening for lung cancer. JAMA 309, 1163 (2013). [DOI] [PubMed] [Google Scholar]
- Bach P. B. et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 307, 2418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschke C. I. et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 354, 99 (1999). [DOI] [PubMed] [Google Scholar]
- Karakiewicz P. I. et al. C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 110, 1241 (2007). [DOI] [PubMed] [Google Scholar]
- Onate-Ocana L. F. et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol 14, 381 (2007). [DOI] [PubMed] [Google Scholar]
- Shedda S., Kosmider S., Faragher I., Jones I. & Gibbs P. A critical review of the Glasgow Prognostic Score for colorectal cancer. Ann Surg 247, 1087, 1088 (2008). [DOI] [PubMed] [Google Scholar]
- Hirashima K. et al. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol 49, 1040 (2014). [DOI] [PubMed] [Google Scholar]
- Shafique K. et al. The modified Glasgow prognostic score in prostate cancer: results from a retrospective clinical series of 744 patients. Bmc Cancer 13, 292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty E. J. et al. Surgical candidacy and selection biases in nonemergent left main stenting: implications for observational studies. JACC Cardiovasc Interv 4, 1020 (2011). [DOI] [PubMed] [Google Scholar]
- ROSENBAUM P. R. & RUBIN D. B. The central role of the propensity score in observational studies for causal effects. Biometrika 70, 41 (1983). [Google Scholar]
- Austin P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28, 3083 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikwe J., Chiang Y. P., Egorova N. N., Itagaki S. & Adams D. H. Survival and outcomes following bioprosthetic vs mechanical mitral valve replacement in patients aged 50 to 69 years. JAMA 313, 1435 (2015). [DOI] [PubMed] [Google Scholar]
- Khokhar S. et al. Antibiotic use in the management of pulmonary nodules. Chest 137, 369 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. H., Schiller J. H. & Bunn P. J. Recent clinical advances in lung cancer management. J Clin Oncol 32, 973 (2014). [DOI] [PubMed] [Google Scholar]
- van Klaveren R. J. et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 361, 2221 (2009). [DOI] [PubMed] [Google Scholar]
- Libby D. M. et al. CT screening for lung cancer: the value of short-term CT follow-up. Chest 129, 1039 (2006). [DOI] [PubMed] [Google Scholar]
- Aberle D. R. et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 369, 920 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey K. W., Alwani A., Danziger L. H. & Rubinstein I. Tissue reparative effects of macrolide antibiotics in chronic inflammatory sinopulmonary diseases. Chest 123, 261 (2003). [DOI] [PubMed] [Google Scholar]
- Greenland S., Pearl J. & Robins J. M. Causal diagrams for epidemiologic research. Epidemiology 10, 37 (1999). [PubMed] [Google Scholar]
- Cheng Y. J. & Wang M. C. Estimating propensity scores and causal survival functions using prevalent survival data. Biometrics 68, 707 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik J. G. et al. Association of early imaging for back pain with clinical outcomes in older adults. JAMA 313, 1143 (2015). [DOI] [PubMed] [Google Scholar]
- Farzadfar F. et al. Effectiveness of diabetes and hypertension management by rural primary health-care workers (Behvarz workers) in Iran: a nationally representative observational study. Lancet 379, 47 (2012). [DOI] [PubMed] [Google Scholar]
- Ishizuka M., Nagata H., Takagi K., Horie T. & Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg 246, 1047 (2007). [DOI] [PubMed] [Google Scholar]
- Deans D. A. et al. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer 100, 63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest L. M., McMillan D. C., McArdle C. S., Angerson W. J. & Dunlop D. J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 89, 1028 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken M. M. et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 306, 1865 (2011). [DOI] [PubMed] [Google Scholar]
- Inoue M. et al. Clinical outcome of resected solid-type small-sized c-stage IA non-small cell lung cancer. Eur J Cardiothorac Surg 37, 1445 (2010). [DOI] [PubMed] [Google Scholar]
- Zwirewich C. V., Miller R. R. & Muller N. L. Multicentric adenocarcinoma of the lung: CT-pathologic correlation. Radiology 176, 185 (1990). [DOI] [PubMed] [Google Scholar]
- Nambu A. et al. Focal area of ground-glass opacity and ground-glass opacity predominance on thin-section CT: discrimination between neoplastic and non-neoplastic lesions. Clin Radiol 60, 1006 (2005). [DOI] [PubMed] [Google Scholar]
- Petersen I. & Warth A. [Lung cancer. Developments, concepts and preview of the new WHO classification]. Pathologe 35, 547 (2014). [DOI] [PubMed] [Google Scholar]
- Chang B. et al. Natural history of pure ground-glass opacity lung nodules detected by low-dose CT scan. Chest 143, 172 (2013). [DOI] [PubMed] [Google Scholar]
- Takamochi K. et al. Pathologic N0 status in pulmonary adenocarcinoma is predictable by combining serum carcinoembryonic antigen level and computed tomographic findings. J Thorac Cardiovasc Surg 122, 325 (2001). [DOI] [PubMed] [Google Scholar]
- Mnatzaganian G., Davidson D. C., Hiller J. E. & Ryan P. Propensity score matching and randomization. J Clin Epidemiol 68, 760 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


