Abstract
The Gram-negative pathogen Vibrio cholerae causes diarrheal disease through the export of enterotoxins. The V. cholerae RTX toxin was previously identified and characterized by its ability to round human laryngeal epithelial (HEp-2) cells. Further investigation determined that cell rounding is caused by the depolymerization of actin stress fibers, through the unique mechanism of covalent actin cross-linking. In this study, we identify a domain within the full-length RTX toxin that is capable of mediating the cross-linking reaction when transiently expressed within eukaryotic cells. A structure/function analysis of the actin cross-linking domain (ACD) reveals that a 412-aa, or a 47.8-kDa, region is essential for cross-linking activity. When this domain is deleted from the full-length toxin gene, actin cross-linking, but not cell rounding, is eliminated, indicating that this toxin carries multiple dissociable activities. The ACD shares 59% amino acid identity with a hypothetical protein from V. cholerae, VC1416, and transient expression of the C-terminal domain of VC1416 also results in actin cross-linking in eukaryotic cells. The presence of this second ACD linked to an Rhs-like element suggests that V. cholerae acquired the domain by horizontal gene transfer and the ACD was inserted into the RTX toxin by gene duplication through the evolution of V. cholerae.
Keywords: Vibrio vulnificus, VgrG, covalent actin cross-linking
Vibrio cholerae is a Gram-negative pathogen that causes an estimated 1 million cases of severe dehydrating diarrhea per year, resulting in as many as 120,000 deaths. The bacterium inhabits estuarine environments throughout the world and is transmitted to humans through consumption of contaminated water or food. Upon colonization of the intestinal epithelium, V. cholerae elicits disease through the export of enterotoxins, including the major virulence factor, the ADP-ribosylating cholera toxin (CT) (1). However, proposed live attenuated vaccine strains and clinical isolates that lack the ctx genes still cause mild to moderate diarrheal disease (2, 3). This milder form of cholera disease could be elicited by export of enterotoxins other than CT, including the V. cholerae RTX (VcRtxA) toxin encoded by the rtxA gene.
The rtxA gene was discovered in 1999 as a 13,635-bp-long ORF located adjacent to the ctx genes on the large chromosome of V. cholerae. The deduced VcRtxA protein is 4,545 aa in length, with a predicted molecular mass of 484 kDa, making it the largest single polypeptide toxin that had been reported at the time (4). Based on the presence of Gly-Asp (GD)-rich repeats at the C terminus of the protein and the presence of genes located adjacent to the toxin that encode a consensus type I secretion apparatus and a protein responsible for toxin activation, VcRtxA was initially identified as a member of the RTX family of cytolysins (4, 5). However, VcRtxA is not associated with the hemolytic or cytolytic activities typical of other RTX toxins (4, 6).
Rather, VcRtxA causes the depolymerization of actin stress fibers resulting in the rapid rounding of cells in culture (4, 6). This toxin-induced depolymerization of actin occurs via a unique mechanism, covalent cross-linking of actin monomers, as shown by the appearance of actin dimers, trimers, and higher-order multimers in cells exposed to bacteria expressing VcRtxA (6). In addition, VcRtxA disrupts the integrity of the polarized T84 intestinal epithelial cell monolayer, as observed through an increase in permeability and a decrease in electrical resistance after incubation with V. cholerae expressing the toxin (7). The activity of VcRtxA may also contribute to the overall pathogenesis of V. cholerae through its stimulation of the proinflammatory immune response (8).
VcRtxA activity was first observed in the O1 El Tor strain N16961. Additional analyses demonstrate that the gene encoding VcRtxA is absent in O1 classical biotype strains of V. cholerae because of a 7-kb deletion within the rtx operon (4), but is present in El Tor, O139, and non-O1 clinical and environmental isolates (9-11). The ubiquitous presence of VcRtxA suggests that this toxin is an important virulence factor of V. cholerae.
In this study, we further investigate the mechanism of actin depolymerization. We identify a domain within the VcRtxA toxin that covalently cross-links actin when transiently expressed within cells. We then demonstrate that a deletion of this domain from the full-length rtxA gene completely abolishes the ability of the toxin to cross-link actin. Lastly, VC1416, a hypothetical protein from V. cholerae that is homologous to this unique domain, can also mediate the covalent cross-linking of actin when expressed in eukaryotic cells. These data suggest that this domain was inserted into the V. cholerae RTX toxin by gene duplication. Discovery of this unique domain of VcRtxA will aid in future studies focusing on delineating the catalytic mechanism of covalently cross-linking actin.
Materials and Methods
Cell Lines, Bacterial Strains, and Reagents. HEp-2 and COS-7 cells were cultured at 37°C with 5% CO2 in DMEM containing 50 units/ml penicillin, 50 μg/ml streptomycin, and 10% FBS (Invitrogen). All V. cholerae strains in this study are derived from KFV43 (12), a streptomycin-resistant isolate of the V. cholerae O1 El Tor Inaba strain N16961 with a deletion in the hapA gene. KFV43 was further modified to delete the genes rtxA (KFV92) and hlyA (KFV119) by double homologous recombination using the plasmids pCWΔrtxA and pCWΔhlyA and the sacB-lethality counterselection method as described (8). Strains were grown at 30°C in Luria broth (LB) containing streptomycin (100 μg/ml). All restriction enzymes were obtained from Invitrogen. All chemicals were purchased from Sigma.
Construction of Actin Cross-Linking Domain (ACD) Constructs. Primers VgrG1963 5′-GAAGATCTCGCCACCATGGGAAGTCAACCAACGGGTCAA-3′ and VgrGEcoRI 5′-CGGAATTCGTCTCATGGTTATCAGTATAAG-3′ were designed to amplify the DNA corresponding to amino acids 1963-2419 of VcRtxA with flanking BglII and EcoRI sites. Subsequent primers were also designed to amplify the truncated forms of the gene region. To construct the pVC1416-EGFP (eukaryotic GFP) expression plasmid, primers VC1416F 5′-GAAGATCTCGCCACCATGAACGCGCAGCCTAATCTTGGAAGA-3′ and VC1416R 5′-CGGA AT TCGCGCGT TGCCAT TCT TGAGGAT T-3′ were used amplify the DNA corresponding to amino acids 716-1161 of the VC1416 ORF. PCR products were then cloned into the pCR-BluntII-TOPO vector (Invitrogen) and then sub-cloned into the pEGFP-N3 vector (Clontech) as a BglII-EcoRI fragment to create an in-frame fusion to the egfp gene. Plasmid DNA prepared by using the Qiaprep Spin Miniprep kit (Qiagen, Valencia, CA) was sequenced at the Cancer Research Center DNA Sequencing Facility at the University of Chicago (Chicago) to confirm gene sequence and egfp fusion.
Construction of In-Frame Deletion of the ACD Within VcRtxA. An in-frame deletion within the rtxA gene on the V. cholerae chromosome was created by double homologous recombination using the sacB-lethality counterselection method (12). Briefly, an 877-bp fragment upstream and 967-bp fragment downstream of the region was amplified from N16961 genomic DNA, and the two fragments were separately cloned into the pCR-BluntII-TOPO vector (Invitrogen). An in-frame fusion of these two regions was generated by using engineered AvaI restriction sites on the respective fragments and ligation into the pCR-BluntII-TOPO vector. The fused fragments were then moved into the pWM91 vector, which contains the sacB counterselection gene (13), by using the SpeI and XhoI sites. The donor bacterium Escherichia coli SM10λpir was transformed with the resulting plasmid pTCO16 and mated with the recipient strain from KFV119. Colonies containing the cointegrated plasmid were subjected to counterselection on sucrose. The colonies obtained after this selection process were then screened by PCR to detect the 1,587-nt deletion on the V. cholerae chromosome. The plasmid pTCO16 as well as the PCR product from the mutant strain were sequenced at the Cancer Research Center DNA Sequencing Facility at the University of Chicago. To test the cell rounding and actin cross-linking activity of this strain, cultures of KFV92, KFV119, and CCO5 were grown for 16 h in LB containing streptomycin at 30°C, washed with PBS, and added to the media of HEp-2 cells. Cytotoxicity assays were also performed on these cells at 3 and 6 h after addition of bacteria by using the CytoTox96 Nonradioactive Cytotoxicity kit (Promega) and the Live/Dead Reduced Biohazard Viability/Cytotoxicity kit (Molecular Probes) according to the manufacturer's instructions.
Transient Transfection. Approximately 2 × 105 cells, COS-7 or HEp-2, were seeded in a six-well dish 24 h before transfection. The media was changed to DMEM without serum and antibiotics, and the lipofectamine reaction (2 μg of DNA, 8 μg of lipofectamine, 18 μg of PLUS reagent in DMEM alone) was incubated with the cultured cells at 37°C with 5% CO2 for 3-5 h. DMEM containing 10% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin was then added to the cells for continued incubation.
Microscopy. Transfected cells were observed after 24 h for expression of EGFP at ×400 magnification under fluorescence microscopy at 550-575 nm. Phase contrast images were acquired at ×200 magnification of HEp-2 cells that had been incubated with PBS, KFV119, KFV92, or CCO5. Live cell images were captured by using an inverted Leica DMIRE2 microscope (Leica) with a C4742-95-12ERG digital charge-coupled device (CCD) camera (Hamamatsu Photonics, Tokyo) in conjunction with the openlab software (Improvision, Coventry, U.K.) for image processing.
Western Blotting. Transiently transfected cells were collected 24 h after transfection, whereas cells exposed to KFV119, KFV92, or CCO5 were collected 90 min after addition of bacteria. All cells were washed with PBS, collected by scraping, centrifuged at 4,500 × g, and resuspended in SDS/PAGE sample buffer. Boiled cell lysates were electrophoresed on SDS/8% polyacrylamide gel and transferred to Hybond-ECL nitrocellulose (Amersham Pharmacia). Detection of actin cross-linking was performed through Western blotting with a polyclonal anti-actin antibody at 1:1,000 dilution (Sigma) and a 1:5,000 dilution of anti-rabbit IgG horseradish peroxidase (HRP) (Sigma). EGFP expression was detected by using a monoclonal anti-GFP antibody at 1:1,000 dilution (Santa Cruz Biotechnology) followed by the anti-mouse IgG HRP (Sigma) secondary antibody at 1:5,000 dilution.
Concentrated supernatants were prepared from a 1-liter culture of KFV119, KFV92, or CCO5 as described (6). Protein concentration was determined by using a bicinchoninic acid protein assay kit (Pierce), and 7 μg of total protein was loaded in each lane of a 4-15% gradient SDS/polyacrylamide gel (Bio-Rad). Gels were transferred for 16 h at 200 mA at 4°C. VcRtxA toxin expression was detected by using a 1:1,000 dilution of the polyclonal RtxA antibody as described (6).
Results
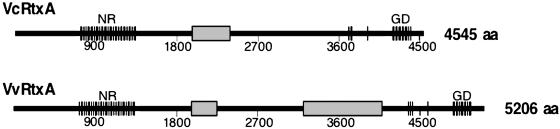
V. cholerae and Vibrio vulnificus RTX Toxins Have Unique Domains.Recently, the sequence of the V. vulnificus RTX toxin (VvRtxA) was reported (14). The deduced primary amino acid sequences of VcRtxA and VvRtxA were compared and revealed that the two toxins are ≈80-90% identical throughout most regions of the toxins. Previously, the repeats consisting of 19 amino acids with the consensus sequence GXAN(I/V)XT(K/H)VGDGXTVAVMX were reported to be present in the N-terminal portion of VcRtxA (4). Upon alignment of VcRtxA and VvRtxA, these repeats are also present in VvRtxA as depicted in Fig. 1. The GD-rich calcium binding repeats (C-terminal) involved in target cell binding and characteristic of all RTX toxins are also present within VvRtxA (4, 5). Although most regions are highly conserved, further comparison of these two toxins reveals extensive sequence divergence at two internal regions. As shown in Fig. 1, VcRtxA contains a domain at amino acids 1963-2419 [according to annotation by Lin et al. (4); GenBank accession no. AF119150], which is absent in VvRtxA. VvRtxA also contains a domain at the same relative location and a second domain located at amino acid 3204-4095 [according to annotation by Chen et al. (14); GenBank accession no. NC005140].
Fig. 1.
Comparative analysis of the primary amino acid sequences of VcRtxA and VvRtxA reveals that the two toxins are similar. Both toxins possess identical N-terminal novel repeats (NR) and C-terminal GD-rich repeats (GD) depicted by hatch marks. The domains unique to each toxin are depicted as shaded boxes.
Based on sequence comparison, we hypothesized that these distinct domains may confer different catalytic activities to these toxins on an otherwise highly conserved backbone. Indeed, the presence of the gene for VvRtxA is correlated with rapid cell lysis (P. Gulig, University of Florida, Gainesville, personal communication) and not with covalent actin cross-linking. Therefore, we hypothesize that the domain in VcRtxA confers the actin cross-linking activity specifically to the V. cholerae RTX toxin.
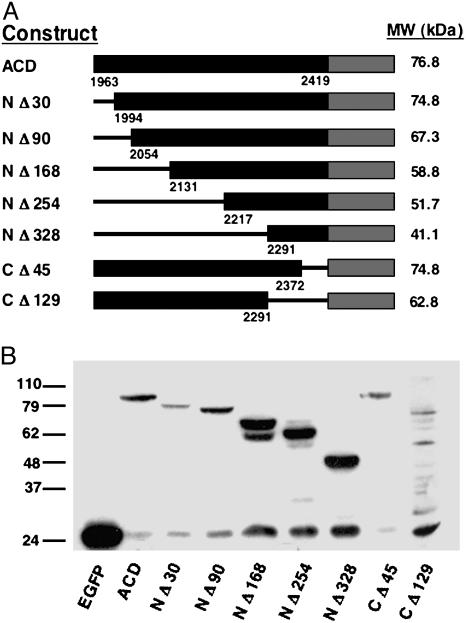
Discovery of the ACD. To investigate whether the recently discovered domain of VcRtxA is the portion of the toxin responsible for covalent cross-linking of actin, DNA from the V. cholerae chromosome corresponding to residues 1963-2419 of VcRtxA was amplified by PCR and cloned into the eukaryotic expression vector pEGFP-N3, such that the domain is expressed with EGFP fused to the C terminus. As shown in Fig. 2B, COS-7 cells transiently transfected with the resulting plasmid pACD express an EGFP fusion protein of 76.8 kDa, whereas cells transfected with vector pEGFP-N3 express the 27-kDa EGFP protein.
Fig. 2.
Construction and expression of ACD-EGFP fusions. (A) Schematic representation of the ACD-EGFP full-length and truncated constructs used in this study. The predicted molecular weights of each fusion protein are stated to the right of the construct. Numbers at bottom correspond to the amino acids of the translated product according to the annotation of Lin et al. (4) (GenBank accession no. AF119150). (B) Expression of the full-length and truncation ACD proteins was detected in transiently transfected COS-7 cells. Twenty-four hours after transfection, cell lysates were subjected to SDS/PAGE and Western blotting with anti-GFP antibody. The last lane has been overexposed to detect the expression of CΔ129-EGFP.
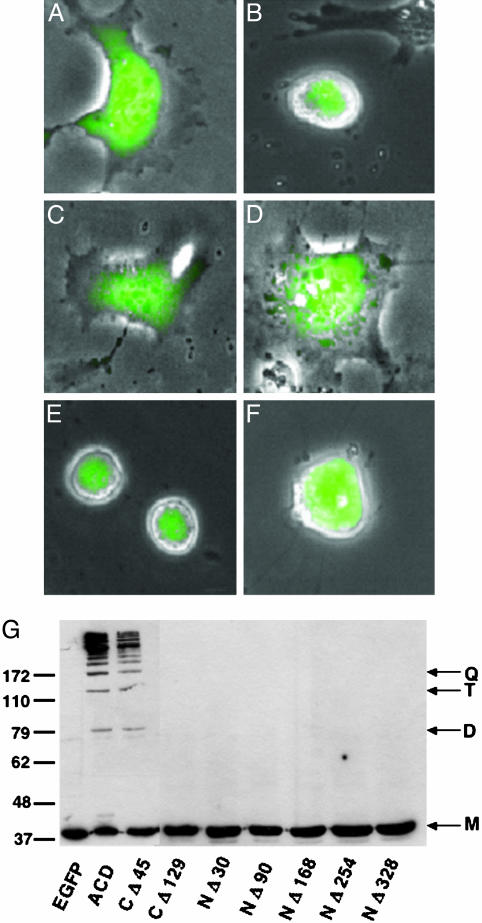
Twenty-four hours after transfection, COS-7 cells expressing the EGFP fusion protein appear rounded by phase microscopy, whereas untransfected neighboring cells appear similar to cells expressing EGFP alone (Fig. 3 A and B). These data demonstrate that this domain from VcRtxA is associated with cell rounding.
Fig. 3.
Functional analysis of ACD-EGFP fusions. COS-7 cells transiently expressing EGFP (A), ACD-EGFP (B), ACD NΔ30-EGFP (C), ACD CΔ129-EGFP (D), ACD CΔ45-EGFP (E), and VC1416-EGFP (F) were observed by using fluorescence microscopy. Images are an overlay of the phase contrast image with the fluorescence micrograph measured at 550-575 nm of a single field viewed at ×400 magnification. (G) COS-7 cells transiently expressing EGFP (lane 1), ACD-EGFP (lane 2), or truncated forms of ACD fused to EGFP (lanes 3-9) were harvested 24 h after transfection. Collected cells were resuspended in SDS buffer, boiled, and subjected to SDS/PAGE and Western blotting with an anti-actin antibody. Arrows mark monomer (M), dimer (D), trimer (T), and quatramer (Q) forms of actin.
To determine whether cell rounding occurs because of covalent actin cross-linking, the COS-7 cells were collected and lysed in SDS sample buffer. When subjected to Western blotting using anti-actin antibody, the actin from cells expressing the recently discovered domain from VcRtxA appear as a ladder of cross-linked forms of actin (Fig. 3G, lane 2), whereas cells expressing only EGFP possess monomeric actin (Fig. 3G, lane 1). These data demonstrate that this domain carries the actin cross-linking activity of VcRtxA, and this region of the protein was named the ACD.
Structure/Function Analysis of ACD. A Chou-Fasman secondary structure prediction performed by using the macvector software (Oxford Molecular Group) showed that the 457-aa ACD contains both α-helices and β strands. Based on the predicted structure, a series of primers were designed to create truncated forms of the ACD. Five 5′ deletion constructs were created, resulting in proteins with truncations of 30 (NΔ30), 90 (NΔ90), 168 (NΔ168), 254 (NΔ254), and 328 (NΔ328) amino acids. Similarly, two 3′ deletions were constructed resulting in 129 (CΔ129) and 45 (CΔ45) amino acid truncations from the C terminus of the ACD representing the last two predicted α-helices. The truncated DNA sequences were amplified from N16961 chromosomal DNA and cloned into the pEGFP-N3 vector to create C-terminal fusions with EGFP. Fig. 2 A depicts a schematic representation of these constructs.
The truncated ACD constructs were transiently transfected into COS-7 cells, and protein expression was detected through Western blotting and fluorescence microscopy. As shown in Fig. 2B, all of the truncated ACD proteins are expressed at their respective predicted molecular masses. Cells expressing the ACD truncations NΔ30, NΔ90, NΔ168, NΔ254, NΔ328, and CΔ129 (Fig. 3 and data not shown) do not produce the rounded phenotype, whereas cells expressing the CΔ45 truncated ACD protein are rounded after 24 h (Fig. 3E). Consistent with cell rounding, the ACD CΔ45 is the only truncated protein that retains the ability to cross-link actin comparable to cells expressing the full-length construct (Fig. 3G, lane 3).
To ensure that the actin cross-linking measured in COS-7 cells is not caused by the overexpression of the ACD constructs or is cell type specific, HEp-2 cells, which do not express the small T antigen necessary for plasmid replication, were also transiently transfected with the ACD-encoding plasmids. Similar to COS-7 cells, transiently transfected HEp-2 cells only appear rounded under phase microscopy and contain cross-linked actin when cells are transfected with the full-length ACD and ACD CΔ45 expressing plasmids (data not shown and Fig. 6A, which is published as supporting information on the PNAS web site). Interestingly, expression of the ACD CΔ45, as well as some of the other constructs, is practically undetectable in HEp-2 cells by Western blotting (see Fig. 6B), suggesting that very small amounts of the ACD protein are sufficient to mediate the actin cross-linking reaction.
Deletion of the ACD of VcRtxA. Next, the activity of VcRtxA in the absence of the ACD was investigated. An in-frame deletion within the rtxA gene was constructed by double homologous recombination using the sacB-lethality counterselection method (12). Amino acids 1913-2441 of VcRtxA were deleted to remove the ACD. The resulting strain, CCO5, appears to produce normal levels of VcRtxA toxin in concentrated supernatant fluids; however, detection of toxin secretion is limited to the detection of low molecular weight breakdown products by Western blotting, as previously observed for the full-length toxin (data not shown).
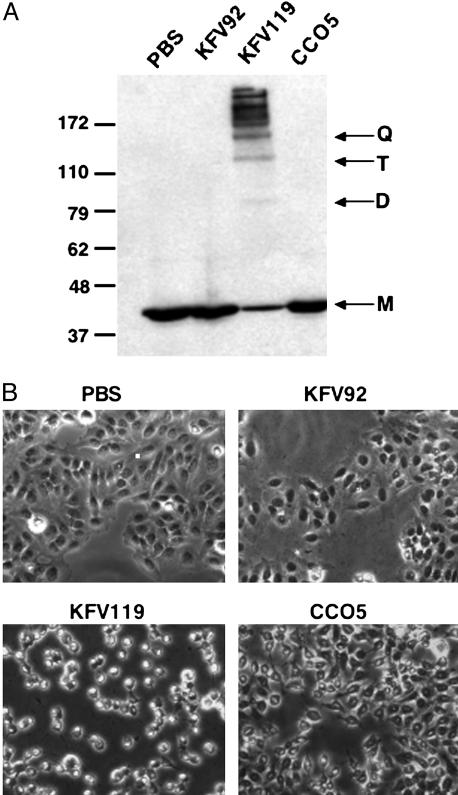
To test for actin cross-linking activity, V. cholerae strains KFV119, KFV92, or CCO5 were added to the culture media of HEp-2 cells at a multiplicity of infection of 200. Ninety minutes after addition, cells were collected and cell lysates were prepared for Western blotting with an anti-actin antibody. The actin within cells exposed to V. cholerae strain KFV119 expressing full-length VcRtxA is cross-linked (Fig. 4A, lane 3), whereas only monomeric actin is detected in cells exposed to the isogenic rtxA null mutant, KFV92 (Fig. 4A, lane 2). Cells exposed to V. cholerae strain CCO5 expressing VcRtxA lacking the ACD also have no detectable cross-linked forms of actin (Fig. 4A, lane 4). Therefore, the deletion of the ACD from VcRtxA abolishes the catalytic activity of the toxin.
Fig. 4.
HEp-2 cells were incubated with PBS or V. cholerae strains with an intact rtxA gene (KFV119), a null mutation in rtxA (KFV92), or an rtxA gene with an in-frame deletion of the ACD (CCO5). (A) Cells were harvested after 90 min of incubation, and actin cross-linking was measured by Western blotting with an anti-actin antibody. Lines at right mark monomer (M), dimer (D), trimer (T), and quatramer (Q) forms of actin. (B) Phase contrast images were acquired after 3 h of incubation at ×200 magnification.
Surprisingly, deletion of the ACD does not eliminate cell rounding activity. Cell rounding observed in conjunction with actin cross-linking usually occurs between 60 and 90 min after addition of bacteria to cells. However, after 3 h of incubation, cells incubated with CCO5 also round, although the cells are less refractile than cells treated with KFV119 (Fig. 4B). A Western blot for actin laddering at this time point does not suggest that this rounding is caused by a delay of activity resulting from the deletion (data not shown). This rounding is also not associated with a slow cytotoxic pore-forming activity characteristic of other RTX family members because there was no elevated release of lactate dehydrogenase into the culture medium as compared to controls at 3 and6hafter addition of bacteria (data not shown). In addition, cells coincubated with KFV1119 and CCO5, even though rounded, excluded the membrane impermeant dye DEAD-RED (see Fig. 7, which is published as supporting information on the PNAS web site). These data demonstrate that HEp-2 cells rounded by incubation with either KFV119 or CCO5 still retain their membrane integrity.
Therefore, we can conclude that, because this rounding occurs more slowly, is phenotypically distinct, and is rtxA dependent, this large toxin must contain additional cell rounding activities unveiled by the deletion of the ACD. These data also confirm that the absence of cross-linking in Fig. 4A is not caused by a disruption of toxin secretion or cell membrane interaction, because activities other than actin cross-linking are maintained. Thus, these data further demonstrate that the ACD is responsible for the cross-linking activity of VcRtxA.
Duplication of the Domain on V. cholerae Chromosome. A psi-blast search of the ACD revealed no sequence similarity to any other proteins of known function within the database (15). However, this search did reveal that the ACD is 59% identical to the C-terminal portion of the V. cholerae hypothetical protein VC1416, also known as VgrG (E value of 1 × 10-153). The N-terminal portion of VC1416 belongs to the ubiquitous family of proteins known as VgrG or Cog 3501 (www.ncbi.nlm.nih.gov/COG/new/release/cow.cgi?cog=COG3501).
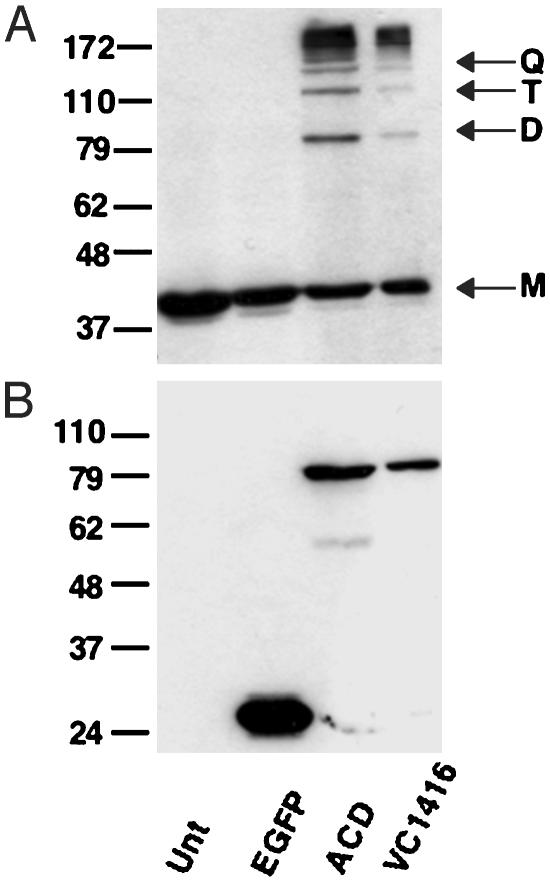
Because of the overwhelming homology of the C-terminal domain of VC1416 to the ACD, it was hypothesized that this domain may also possess actin cross-linking activity and could have been duplicated on the chromosome and inserted into VcRtxA during evolution. To test this hypothesis, pVC1416-EGFP was created to produce an EGFP fusion protein with amino acids 716-1161 of VC1416. Transient expression of this construct in COS-7 cells also produces cell rounding as determined by the visualization of VC1416-EGFP expressing cells as shown in Fig. 2F. Actin cross-linking comparable to that generated with expression of ACD-EGFP is observed when pVC1416-EGFP is expressed in COS-7 cells (Fig. 5A, lane 3). Expression of VC1416-EGFP is detectable in COS-7 cells (Fig. 5B, lane 3), but not in HEp-2 cells (see Fig. 8A, lane 3, which is published as supporting information on the PNAS web site) by Western blotting with an anti-GFP antibody. This undetectable amount of VC1416-EGFP is still capable of covalently cross-linking actin in HEp-2 cells as shown in Fig. 8B. This result further confirms that very small amounts of this protein are necessary to generate the covalently modified actin. These data also demonstrate that V. cholerae has two copies of this unique actin cross-linking domain, which is apparently not present in any other prokaryotic or eukaryotic organism.
Fig. 5.
VC1416 contains a second copy of ACD. (A) COS-7 cells transiently expressing EGFP, ACD-EGFP, and VC1416-EGFP were measured for actin cross-linking by Western blotting with an anti-actin antibody. Lines at right mark monomer (M), dimer (D), trimer (T), and quatramer (Q) forms of actin. Unt represents untransfected cells. (B) Expression of EGFP, ACD-EGFP, and VC1416-EGFP in COS-7 cells was detected by Western blotting with an anti-GFP antibody.
Discussion
In this report, we identify a unique domain of 47.8 kDa located within the much larger full-length VcRtxA. This domain accounts for approximately one-tenth of the total toxin mass and is sufficient for the cross-linking of actin when expressed as a transgene. A structure/function analysis of the ACD reveals that a deletion of 45 aa from the C terminus did not affect the cross-linking activity of the domain. The precise catalytic mechanism of the ACD, as well as the required catalytic residues, will be elucidated in future studies using a defined mutagenesis screen.
The previously proposed model of the mechanism of VcRtxA activity speculated whether the toxin was indirectly using signaling pathways to activate endogenous cross-linking proteins or entering into host cells to catalyze the reaction directly (6). Because expression of the ACD within cells was found to generate the covalently modified actin species, VcRtxA must gain entry into the cell when added exogenously. Preliminary studies using pharmacological inhibitors of endocytosis suggest that the toxin does not enter by receptor-mediated endocytosis (K.L.S., unpublished data). Therefore, we hypothesize that VcRtxA inserts into the host cell plasma membrane similar to other members of the RTX toxin family and then, rather than forming a pore, translocates the ACD into the cell to either directly or indirectly catalyze the covalent cross-linking of actin. The RTX toxin Bordetella pertussis adenylate cyclase toxin (AC) uses this mechanism for insertion into the host cell membrane. AC then translocates the catalytic adenylate cyclase domain into the cell where it is held at the membrane to catalyze the transformation of ATP into cAMP (5). VcRtxA could employ this same mechanism to anchor the ACD at the plasma membrane. Alternatively, a cellular protease or proteolytic activity within VcRtxA could cleave and release the catalytic domain into the cytosol of the host cell.
The comparison of VcRtxA and VvRtxA reveals insight into the evolution of these two toxins. Although the two toxins possess 80-90% identity over most of their sequence, they are associated with different cellular responses. The absence of the ACD from VvRtxA indicates that this toxin does not cross-link actin and must be causing cell lysis by an as yet unidentified mechanism. Recently, the entomopathogen Photorhabdus luminescens was reported to have four complete genes that are similar to VcRtxA and VvRtxA (16). The function of these genes in pathogenesis was not discussed, but upon examination of the predicted protein sequence of these genes, we did not observe the ACD. Therefore, these members of the RTX family of toxins have a highly conserved backbone, but through the acquisition of different genetic material, have likely evolved to generate unique cellular responses that could contribute to the pathogenesis of their respective organisms. As demonstrated by our finding that cells still round in the absence of actin cross-linking, these toxins appear to have a capacity to carry multiple distinct toxic activities.
The toxins may independently acquire these different catalytic activities by horizontal gene transfer. An analysis of the entire genomic region surrounding VC1416 suggests that this gene has been recently acquired, possibly as a mobile element similar to E. coli Rhs (rearrangement hot spot) elements (17). A comparative genome alignment reveals that, although homologues of thermostable carboxypeptidase I gene VC1414 and conserved hypothetical gene VC1421 are adjacent in the same relative genomic structure within Vibrio parahemolyticus and V. vulnifi-cus, these genes are separated in V. cholerae by a >10,000-bp insertion flanked by AT-rich sequences. This insertion contains six ORFs including the hemolysin-coregulated protein (hcp) gene VC1415 followed by VC1416, a fusion of genes that encode VgrG and the second copy of the ACD (see Fig. 9, which is published as supporting information on the PNAS web site). Both hcp and vgrG are genes of unknown function that are components of Rhs elements (18). However, instead of a high GC% Rhs core gene, the remainder of the insertion encodes four hypothetical proteins and has a low GC% content (<45% G+C) compared to the flanking genomic DNA that includes other horizontally acquired elements including the CTX phage, pTLC1, and pTLC2 (see Fig. 9).
Although Rhs elements were originally characterized by the presence of the Rhs core, recent evidence suggests that the more ubiquitous vgrG is a mobile genetic element that can lead to the acquisition of novel genes, including virulence factors such as the Pseudomonas aeruginosa phospholipase D gene (19). The VgrG family, also known as Cog3501, currently consists of 45 genes identified in 10 Gram-negative bacteria, and putative Vgr-like genes have been identified in five more bacterial species (www.ncbi.nlm.nih.gov/COG/new/release/cow.cgi?cog=COG3501). The sequenced V. cholerae strain N16961 has three copies of vgrG. Previously, Wilderman et al. (19) speculated on the functional significance of the genes adjacent to these vgrG genes and their potential involvement in horizontal transfer. Indeed, the ORF VC1416 contains a vgrG gene fused to the second copy of ACD. VCA0018 is 86% identical to the VgrG domain of VC1416 and is linked to the hcp gene VCA0017, suggesting a possible gene duplication event that did not include the ACD domain of VC1416. The third copy of VgrG, VCA0123, is only 45% identical to the other VgrG proteins and is not linked to an hcp gene. Taken together, these observations suggest that the RTX toxin of V. cholerae may have acquired the ACD by horizontal gene transfer via acquisition of VC1416 as part of an Rhs-like element. It would be interesting to determine whether all strains of V. cholerae that express the RTX toxin also have the ability to cross-link actin, or whether this activity was acquired by only a subset of V. cholerae serotypes.
Overall, we have further advanced the study of the VcRtxA toxin. Discovery of the domain will greatly aid future investigations into the precise mechanism of the covalent modification of actin and the observed activity responsible for cell rounding in the absence of actin cross-linking. Further elucidation of the mechanism of action of these activities of VcRtxA will provide a greater understanding of the effect on host cell biology contributing to cholera pathogenesis.
Supplementary Material
Acknowledgments
This work was supported by a Biomedical Research Support Program Award from the Howard Hughes Medical Institute and Public Health Services Grant AI051490 from the National Institute for Allergy and Infectious Diseases (to K.J.F.S.). K.-L.S. and C.L.C. are supported by predoctoral National Research Service Award Fellowships T32-AI07476 and F31-AI52490, respectively.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACD, actin cross-linking domain; EGFP, eukaryotic GFP.
References
- 1.Kaper, J., Morris, J. G. & Levine, M. M. (1995) Clin. Microbiol. Rev. 8, 48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine, M. M., Kaper, J. B., Herrington, D., Losonsky, G., Morris, J. G., Clements, M. L., Black, R. E., Tall, B. & Hall, R. (1988) Infect. Immun. 56, 161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris, J. G., Jr. (2003) Clin. Infect. Dis. 37, 272-280. [DOI] [PubMed] [Google Scholar]
- 4.Lin, W., Fullner, K. J., Clayton, R., Sexton, J. A., Rogers, M. B., Calia, K. E., Calderwood, S. B., Fraser, C. & Mekalanos, J. J. (1999) Proc. Natl. Acad. Sci. USA 96, 1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch, R. (2001) Curr. Top. Microbiol. Immunol. 88, 137-162. [DOI] [PubMed] [Google Scholar]
- 6.Fullner, K. J. & Mekalanos, J. J. (2000) EMBO J. 19, 5315-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fullner, K. J., Lencer, W. I. & Mekalanos, J. J. (2001) Infect. Immun. 69, 6310-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fullner, K. J., Boucher, J. C., Hanes, M. A., Haines, G. K., III, Meehan, B. M., Walchle, C., Sansonetti, P. J. & Mekalanos, J. J. (2002) J. Exp. Med. 195, 1455-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow, K. H., Ng, T. K., Yuen, K. Y. & Yam, W. C. (2001) J. Clin. Microbiol. 39, 2594-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalsgaard, A., Serichantalergs, O., Forslund, A., Lin, W., Mekalanos, J., Mintz, E., Shimada, T. & Wells, J. G. (2001) J. Clin. Microbiol. 39, 4086-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dziejman, M., Balon, E., Boyd, D., Fraser, C. M., Heidelberg, J. F. & Mekalanos, J. J. (2002) Proc. Natl. Acad. Sci. USA 99, 1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fullner, K. J. & Mekalanos, J. J. (1999) Infect. Immun. 67, 1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalf, W. W., Jiang, W., Daniels, L. L., Kim, S. K., Haldimann, A. & Wanner, B. L. (1996) Plasmid 35, 1-13. [DOI] [PubMed] [Google Scholar]
- 14.Chen, C. Y., Wu, K. M., Chang, Y. C., Chang, C. H., Tsai, H. C., Liao, T. L., Liu, Y. M., Chen, H. J., Shen, A. B., Li, J. C., et al. (2003) Genome Res. 13, 2577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D. J. (1997) Nucleic Acids Res. 25, 3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duchaud, E., Rusniok, C., Frangeul, L., Buchrieser, C., Givaudan, A., Taourit, S., Bocs, S., Boursaux-Eude, C., Chandler, M., Charles, J. F., et al. (2003) Nat. Biotechnol. 21, 1307-1313. [DOI] [PubMed] [Google Scholar]
- 17.Lin, R. J., Capage, M. & Hill, C. W. (1984) J. Mol. Biol. 177, 1-18. [DOI] [PubMed] [Google Scholar]
- 18.Wang, Y., Zhao, S. & Hill, C. W. (1998) J. Bacteriol. 180, 4102-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilderman, P. J., Vasil, A. I., Johnson, Z. & Vasil, M. L. (2001) Mol. Microbiol. 39, 291-303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.