Abstract
Cholinergic axons and nicotinic receptors are abundant in all layers of the olfactory bulb (OB), the main region of newborn neuron integration in the adult brain. Here, we report that the OB granule cell layer in mice lacking the predominant form of brain high-affinity nicotinic acetylcholine receptors (β2-/- mice) displayed nearly 50% more newborn neurons and significantly fewer apoptotic cells than did β2+/+ mice. Conversely, in vivo chronic nicotine exposure significantly decreased the number of newborn granule cells in β2+/+ but not β2-/- adult mice, confirming that the survival of newborn neurons can be controlled by the activation of β2-containing nicotinic acetylcholine receptors. Unexpectedly, investigating the behavioral consequence of an increased number of granule cells in β2-/- mice revealed that these animals have a less robust short-term olfactory memory than their wild-type counterparts. Taken together, these results provide evidence that high-affinity nicotinic receptors are involved in the maturation of adult OB local circuits. They also indicate that an increase in the number of granule cells does not necessarily correlate with better olfactory performance and further highlight the importance of cholinergic afferents for olfactory processing.
Throughout adulthood, the olfactory bulb (OB) is furnished with a constant supply of neuroblasts originating from the subventricular zone (SVZ) and rostral migratory stream (RMS) (1, 2). These cells first migrate tangentially along the RMS before migrating radially in the OB and differentiating into functional inhibitory granule or periglomerular cells (3), a process that takes 3-4 weeks to complete (4, 5). Within the bulb, the activity of these cells can be strongly modulated, via muscarinic (mAChRs) and nicotinic (nAChRs) acetylcholine receptors (6), by the dense cholinergic innervation pervading all OB layers (7).
The family of nAChRs is composed of heteromeric and homomeric pentamers forming gated ion channels. The main nAChRs in the brain are composed of α4 and β2 subunits (high affinity for nicotine) or of α7 subunits only (high affinity for α-bungarotoxin) (8). In vitro activation of the latter by low doses of nicotine has been shown to lead to apoptotic cell death of undifferentiated primary and immortalized progenitor cells from the dentate gyrus (DG), the other main region of neurogenesis in the mature brain (9). In accordance with these results, in vivo chronic nicotine exposure in adult rats was shown to reduce proliferation in the DG (10, 11). Interestingly, nicotine exposure had no effect on proliferation in the SVZ, suggesting that nAChRs might not be involved in adult OB neurogenesis. However, given the strong expression of high-affinity nAChRs in the OB (12, 13), a study on the involvement of these receptors in events downstream to SVZ proliferation was warranted. In this context, we undertook a study of OB neurogenesis in β2-/- and β2+/+ mice subjected or not subjected to chronic nicotine exposure and report here that β2-containing nAChRs (*β2-nAChRs) are specifically involved in the survival of newborn granule cells in the adult bulbar circuitry.
Methods
Animals. Animals were used in accordance with the Centre National de la Recherche Scientifique guidelines for care and use of laboratory animals. Age-matched adult (2 to 4 months old) male β2+/+ and β2-/- mice, backcrossed for 12 generations to the C57BL/6J parental strain, were obtained from Charles River Laboratories and housed in climate-controlled facilities, with ad libitum access to food and water. Details concerning the generation of β2-/- mice are provided in ref. 14. The mice were coded, and all experiments and analyses were conducted blind to genotype.
Ligand-Binding Autoradiography. Mice were decapitated, and the brains were rapidly dissected out and frozen in dry-ice powder. Serial 20-μm-thick sections were cut on a Reichert-Jung cryostat, mounted, dried at room temperature (RT) on glass slides, and stored at -80°C until use. The day of the experiment, sections from both genotypes were thawed and incubated 30 min at RT with 200 pM 125I-epibatidine (125I-EB, 2,200 Ci/mmol; 1 Ci = 37 GBq) (PerkinElmer) diluted in 50 mM Tris (pH 7.4). Then, they were washed in Tris for 30 min at RT and dried on a hot plate before being exposed to BioMax MR-1 film (Sigma) for 72 h. Films were developed with a Kodak X-Omat 2000 Processor, and binding was quantified in relation to brain paste standards, as described in ref. 15. Binding was always abolished by adding 1 mM nicotine (Sigma) to the 125I-EB solution.
BrdUrd Injections. Dividing cells were labeled with the DNA synthesis marker BrdUrd (50 mg/kg, i.p.) (Sigma) dissolved in 0.9% NaCl with 0.4 M NaOH. To evaluate proliferation, mice were killed 2 h after receiving a single BrdUrd injection. To evaluate neurogenesis (proliferation, migration, and survival combined), animals were killed 21 days after receiving four BrdUrd injections at 2-h intervals.
Nicotine Administration. Two hours after the last of four repeated BrdUrd injections (as above), mice were anesthetized with a xylazine-ketamine solution (Sigma) and implanted s.c. with miniosmotic pumps (model 2004) from Alzet (Palo Alto, CA), delivering either saline or 6 mg of nicotine per kg per day for the next 21 days, until killing.
BrdUrd Immunocytochemistry. Mice were deeply anesthetized with chloral hydrate (400 mg/kg, i.p.) and the brains were fixed by cardiac perfusion with ice-cold saline followed by 4% paraformaldehyde (RT) prepared in 0.1 M phosphate buffer (pH 7.4). Brains were postfixed overnight at 4°C in fixative, included in agar, cut serially in 40-μm-thick coronal or sagittal sections with a vibrating Microtome and processed for free-floating immunocytochemistry. In brief, sections were rinsed thoroughly with 50 mM PBS, pretreated for 2 h at RT in PBS containing 0.2% Triton X-100 and for 30 min at 37°C in 2 M HCl in PBS to denature DNA. After several rinses, sections were incubated for 48 h at 4°C with monoclonal rat anti-BrdUrd (Accurate Scientific, Harlan Sera-Lab, Loughborough, U.K.) diluted 1/200 in PBS containing 0.5% gelatin, 4% BSA, and 0.2% Triton X-100. After several rinses in PBS, sections were incubated for2hatRT with biotinylated rabbit anti-rat antibodies (1/200; Vector Laboratories), followed for 1 h by the avidin-biotin complex procedure (ABC Kit, VECTASTAIN Elite, Vector Laboratories). Labeling was revealed with 0.05% diaminobenzidine, to which 0.005% H2O2 was added. Sections then were rinsed thoroughly and mounted on glass slides with DPX.
Image Analysis and Quantification. BrdUrd-immunostained (BrdUrd+) nuclei were visualized with a Leica light microscope (×40 objective) equipped with a charge-couple device Sony digital camera. For the 21 days postinjection, immunostained profiles contained in the granule cell and glomerular layers of the OB and in the granular layer of the DG were counted in one in three sections (120 μm apart). For the 2-h postinjection period, the numbers of BrdUrd+ nuclei were counted in all sagittal sections containing SVZ, RMS, and/or DG. The respective areas of these layers/regions were determined with the simple pci software and the quantified values were converted to densities (numbers of BrdUrd+ profiles per mm2 of tissue).
BrdUrd/NeuN Double-Labeling Immunocytochemistry. OB coronal sections were incubated with monoclonal rat anti-BrdUrd (as above) and mouse anti-NeuN (1/200; Chemicon) antibodies, followed by the fluorescent Alexa 568-labeled goat anti-rat IgG antibodies and Alexa 488-labeled goat anti-mouse IgG antibodies (both diluted at 1/500, Molecular Probes). Sections were analyzed by using a Zeiss confocal microscope equipped with its complementary software package for image acquisition and data analysis.
Terminal Deoxynucleotidyltransferase-Mediated Biotinylated dUTP Nick End Labeling (TUNEL) Staining. TUNEL staining, used to detect DNA fragmentation in situ, was performed with the Apoptag kit (Serological Corp., Norcross, CA) according to the manufacturer's instructions. In brief, after deparaffinization and rehydration, 8-μm-thick serial coronal OB sections were pretreated with 0.5% Triton X-100 for 10 min followed by addition of equilibrium buffer. Sections then were incubated for 1 h in a humidified chamber at 37°C with a solution containing terminal deoxynucleotidyltransferase enzyme and digoxigenin nucleotides. After vigorous washing, peroxidase-labeled anti-digoxigenin Ab was added for 30 min at RT, and revelation of labeled profile was achieved by diaminobenzidine reaction. TUNEL-positive (TUNEL+) nuclei were counted in one-in-three series (120 μm apart) of five adjacent sections and expressed as above in densities (number of nuclei per mm2 of tissue).
Short-Term Olfactory Memory. Olfactory memory was evaluated with a slightly modified version of a test described in ref. 16. One week before the first day of testing, mice were adapted to an inverted light-dark cycle (light on 2000 to 0800), and the experiment was conducted under dim red-light illumination during subjective night. Ten simple odors (heptanol, limonene, amyl acetate, propanol, propionaldehyde, octanol, hexanol, xylene, terpinene, and carvone) were diluted in mineral oil to 10-3 M from pure liquids (Fisher) and applied (20 μl) on small circular pieces of filter paper, which were hung 8 cm above the cage bottom. The test consisted of scoring the total time mice spent investigating (sniffing or rearing) the filter paper during 5-min periods. The time spent by mice investigating odors upon first and second presentation was measured, with interpresentation intervals ranging from 30 to 360 min. Animals, odors, and time intervals were randomly tested over the 4-week experimentation period. A significant decrease in time investigating an odor on the second presentation showed retention of that odor.
Olfactory Threshold. To compare the olfactory threshold of β2-/- and β2+/+ mice, we presented mineral oil (control) or increasing concentrations (10-10 to 10-4 M) of freshly prepared solutions of heptanol to mice as above. Mineral oil and heptanol always were tested on two consecutive 5-min periods, and a single heptanol concentration was used each day, from the least to the most concentrated. A significant increase in investigation time between the consecutive mineral oil and odorant trials was taken to mean that mice detected the odor.
Results
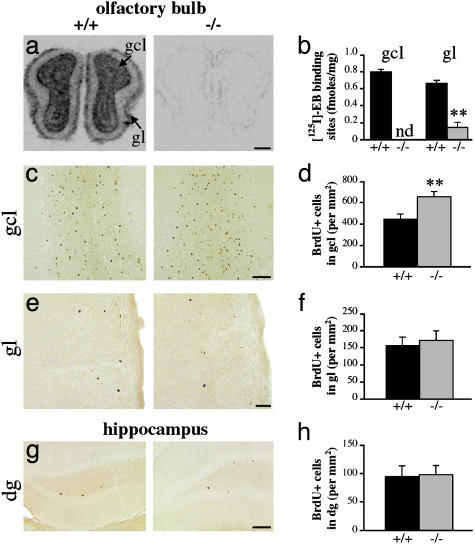
Preponderance of *β2-nAChRs in the OB. As illustrated in Fig. 1 a and b, ligand-binding autoradiography with 125I-EB revealed a strong expression of neuronal heteromeric nAChRs in the adult WT mouse OB and particularly in both recipient layers of newborn neurons, i.e., the granule cell and glomerular layers (0.79 ± 0.03 and 0.66 ± 0.03 fmol/mg ± SEM, respectively). In β2-/- mice, this binding disappeared completely in the granule cell layer and was reduced by 88% in the glomerular layer (0.14 ± 0.06 fmol/mg ± SEM), indicating that *β2-nAChRs constitute the great majority of high-affinity nAChRs in the OB. No 125I-EB binding was ever detected in the olfactory nerve layer.
Fig. 1.
Increased survival of newborn neurons in olfactory bulb of adult β2-/- mice. (a) 125I-EB binding to high-affinity nAChRs in the OB of adult β2+/+ (Left) and β2-/- mice (Right). Note the high levels of binding in the granule cell layer (gcl) and glomerular layer (gl) of WT mice. Such binding could not be detected (nd) in the gcl and was reduced by 88% in the gl of β2-/- mice (b). (c, e, and g) Representative micrographs illustrating the layers in which densities of BrdUrd+ cells were quantified 21 days after repeated BrdUrd injections, i.e., the granule cell layer and glomerular layer of the OB and the granular layer of the dentate gyrus (dg), in both β2+/+ and β2-/- mice (Left and Right, respectively). (d, f, and h) Densities of BrdUrd+ cells determined from the gcl, gl, and dg, respectively. The only statistically significant difference between genotypes was found for the gcl, in which β2-/- mice had on average 46% more granule cells than their WT counterparts. **, P < 0.01, by Student's t test; n = 6 per genotype, from two independent experiments. (Scale bars: a, 500 μm; c and g, 100 μm; e, 25 μm.)
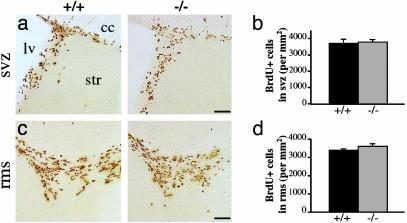
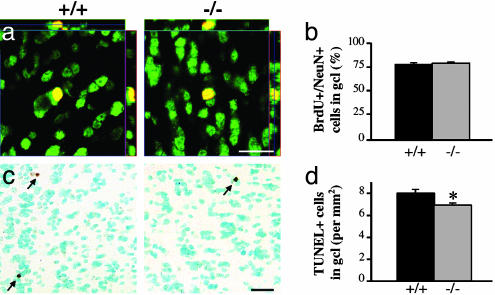
Increased Survival of Granule Cells in OB of β2-/- Mice. Twenty-one days after BrdUrd injections, a significantly greater number of BrdUrd+ profiles were found in the granule cell layer (654.8 ± 48.4 vs. 447.3 ± 45.9 cells per mm2 ± SEM), with no difference in the glomerular layer (172.2 ± 27.2 vs. 155.8 ± 25.4 cells per mm2 ± SEM) or DG (98.0 ± 15.9 vs. 94.5 ± 19.1 cells per mm2 ± SEM) of β2-/- compared with β2+/+ mice, respectively (Fig. 1 c-h). Because the increase in density of newborn cells in the OB was layer-specific, it is unlikely that this effect resulted from changes in proliferation and/or tangential or radial migrations. In agreement with this finding, the densities of neuroblasts measured 2 h after a single BrdUrd injection did not vary significantly between genotypes (SVZ, 3,806.5 ± 142.1 vs. 3,728.9 ± 234.9; RMS, 3,603.2 ± 129.1 vs. 3,392.4 ± 59.5; DG, 140.6 ± 9.6 vs. 138,5 ± 4.6; all values expressed in mean numbers of BrdUrd+ cells per mm2 ± SEM, for β2-/- and β2+/+ mice, respectively) (Fig. 2). Furthermore, increase in the density of newborn granule cells was not accompanied by the altered fate of newborn cells because both genotypes had the same proportion of BrdUrd+ profiles, which were double-labeled for NeuN, a neuron-specific marker (80.9% ± 1.4 vs. 79.6% ± 1.6 for β2-/- and β2+/+ mice, respectively) (Fig. 3 a and b). Taken together, these results indicate that *β2-nAChRs are not involved in the proliferation, migration, or fate determination but, rather, specifically in the survival of newborn granule cells in the adult OB. This finding was confirmed by TUNEL staining, as the number of apoptotic profiles in the granule cell layer was found to be significantly lower in β2-/- compared with β2+/+ animals, with respective values of 6.9 ± 0.2 and 8.0 ± 0.4 cells per mm2 ± SEM (Fig. 3 c and d).
Fig. 2.
Proliferation is not affected in β2-/- mice. Representative micrographs of BrdUrd-immunostained sections of SVZ (a) and RMS (c) in β2+/+ and β2-/- mice (Left and Right, respectively), and for which densities of BrdUrd+ profiles were determined 2 h after a single injection of BrdUrd (b and d). lv, lateral ventricle; cc, corpus callosum; str, striatum. (Scale bars: 100 μm.)
Fig. 3.
The fate of newborn cells is not affected in β2-/- mice. (a and b) Confocal microscopy images of BrdUrd (red) and NeuN (green) double-immunofluorescent labeling in the OB granule cell layer, 21 days after BrdUrd injections (n = 3 per genotype). Reconstructed orthogonal projections are presented as viewed in the x-z (Upper) and y-z (Right) planes. The proportion of double-labeled (yellow) cells did not differ between β2+/+ and β2-/- mice (b), as determined from the analysis of almost 1,000 cells per group. (c and d) Decreased apoptosis in the granule cell layer of β2-/- mice. (c) TUNEL staining in granule cell layer sections from adult β2+/+ (Left) and β2-/- mice (Right). Arrows point to apoptotic cells. (d) Significantly fewer apoptotic profiles were found in β2-/- mice. β2+/+, n = 3, and β2-/-, n = 4; *, P < 0.05 by Student's t test. (Scale bars: a, 10 μm; c, 25 μm.)
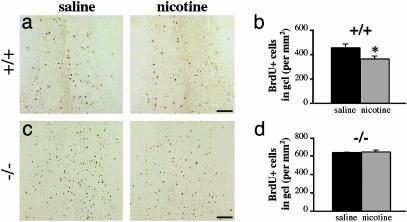
Chronic Nicotine Exposure Decreases the Number of BrdUrd+ Cells in the OB Granule Cell Layer via *β2-nAChRs. Because the examination of β2-/- mice revealed that nAChRs take part in the process of apoptosis, we then investigated the consequence of chronic in vivo nicotine exposure on the survival of newborn OB neurons. Twenty-one days after BrdUrd injections, a significantly lower number of BrdUrd+ profiles were found in the granule cell layer of β2+/+ mice chronically exposed to nicotine vs. saline (364.6 ± 19.6 vs. 453.5 ± 32.4 cells per mm2 ± SEM, respectively) (Fig. 4 a and b). In contrast, no difference was found between groups for the glomerular layer (77.4 ± 2.8 vs. 90.4 ± 8.2 cells per mm2 ± SEM) or DG (57.9 ± 1.6 vs. 66.9 ± 5.9 cells per mm2 ± SEM). These results are in agreement with the layer-specific increase in cell density found in the OB of β2-/- mice. The same experiment performed in β2-/- mice yielded strikingly similar numbers of BrdUrd+ cells in nicotine- and saline-exposed mice, with values of 644.5 ± 21.3 and 636.6 ± 5.6 cells per mm2 ± SEM, respectively (Fig. 4 c and d). This result confirmed that the survival of WT granule cells was decreased specifically by *β2-nAChR activation.
Fig. 4.
Chronic nicotine exposure decreases survival of newborn OB neurons via *β2-nAChR activation. (a and c) Representative micrographs of BrdUrd+ cells in the granule cell layer of chronic saline- and nicotine-exposed β2+/+ and β2-/- mice, and corresponding densities of BrdUrd+ cells (b and d). β2+/+, n = 6 per group; β2-/-, n = 3 per group. *, P < 0.05 by Student's t test. (Scale bars: 100 μm.)
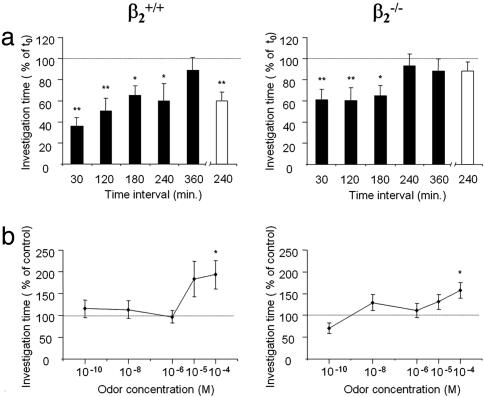
Short-Term Olfactory Memory Is Less Robust in β2-/- Mice. As presented in Fig. 5a, both β2-/- and β2+/+ mice spent significantly less time investigating simple odors 30, 120, and 180 min after a first exposure, indicating the ability to recognize the odors after these intertrial intervals. The time β2+/+ mice spent investigating odors on second exposure increased in parallel with these intervals (Fig. 5a Left), whereas it remained fairly constant for β2-/- mice (Fig. 5a Right). The most striking difference between genotypes, however, was that β2+/+, but not β2-/-, animals continued to remember simple odors after a 240-min interval. This difference was confirmed with an independent series of tests (white columns in Fig. 5a) and was not caused by a difference in odor perception because both genotypes had the same threshold of odor detection (Fig. 5b).
Fig. 5.
Same detection threshold but decreased olfactory memory in β2-/- mice. (a) Time spent investigating (sniffing or rearing) 10-3 M dilutions of 10 different simple odors. Data represent the ratio of time spent investigating odors during second compared with first presentation. Animals, odors, and time intervals were randomly tested over the 4-week period of experimentation. Whereas β2+/+ mice spent significantly less time investigating odors after up to 240 min (Left), β2-/- mice did not (Right). Animals were retested for this time interval (white columns), and the difference between groups was confirmed. (b) Time spent by mice investigating a simple odor of increasing concentration. Mineral oil (control) or diluted solutions of heptanol were presented to mice on consecutive 5-min periods. A single heptanol concentration was used each day, from the least to the most concentrated. Both genotypes detected heptanol at 10-4 M. β2+/+, n = 15, and β2-/-, n = 16; **, P < 0.01 and *, P < 0.05, with Wilcoxon's test.
Discussion
The experimental data presented in this study reveal that, in addition to its classical neuromodulatory properties, the cholinergic input to the OB also participates in the ongoing process of granule cell regeneration. This phenomenon is mediated by *β2-nAChRs, which were found here to constitute the sole population of high-affinity nAChRs in the mouse granule cell layer. In contrast, non-*β2-nAChRs were found to constitute a significant portion of high-affinity nAChRs in the glomerular layer. With a protocol designed to evaluate proliferation, migration, and survival of newborn cells, knockout mice lacking the β2-nAChR subunit had on average 46% more new granule cells than their WT counterparts, with no significant difference in densities of periglomerular neurons. Because proliferation in the SVZ and RMS was similar between genotypes, such an increase likely reflected a difference in the rate of granule cell survival. This prediction was confirmed by TUNEL, which stained fewer apoptotic profiles in the granule cell layer of β2-/- than β2+/+ mice. The complementary experiments aimed at examining the effects of in vivo chronic nicotine exposure further confirmed the involvement of *β2-nAChRs in survival, as this drug significantly reduced the number of granule cells in β2+/+ but not in β2-/- mice.
Despite the important increase in new granule cells found in adult β2-/- mice, the size of the granule cell layer (and overall bulb) did not vary between genotypes. Interestingly, olfactory deprivation in the adult decreases importantly the number of newborn OB neurons but has little (17) or no effect (18) on OB size. In contrast, a similar deprivation early in postnatal life shrinks the adult OB (19). These studies suggest that after a certain postnatal critical period, bulb size is quite stable and is not affected by increasing or decreasing granule cell neurogenesis. In all likelihood, this stability stems from yet-to-be-investigated mechanisms that control granule cell packing and/or shape. In this context, if *β2-nAChRs are expressed in the mouse OB during the first postnatal weeks, as they are in the rat (20), these receptors are unlikely to be involved in the survival of newborn granule cells during early development.
It has been shown previously that in vivo nicotine exposure reduces proliferation in the DG, but not in the SVZ (10, 11). In light of these reports, the data obtained here with chronic nicotine administration starting after the proliferation phase suggest that once differentiation has started, the survival of DG cells is no longer affected by nAChR activity. This finding is consistent with the in vitro demonstration by Berger et al. (9) that nicotine can induce apoptosis in both primary and immortalized hippocampal cells only before the onset of differentiation. These authors further demonstrated that nicotine's cytotoxic properties stem from the activation of α7-containing nAChRs (*α7-nAChRs) and the induction of the tumor suppressor p53 and the cdk inhibitor p21. The fact that DG proliferation is not perturbed in β2 knockouts strongly suggests that *α7-nAChRs are central for this phenomenon in the hippocampus. Thus, it is tempting to speculate that the regional specificities in nicotinic modulation of neurogenesis simply reflect the involvement of different nAChR subtypes, where *α7-nAChRs regulate proliferation in the hippocampus and *β2-nAChRs control granule cell survival in the OB.
Much in vivo and in vitro evidence has been published showing that nicotine can be neuroprotective for some neuron populations submitted to toxic or ischemic insults (see review in ref. 21). The neuroprotective properties of nicotine have been described to involve mostly *α7-nAChRs but also *β2-nAChRs. However, as mentioned above, some studies also have highlighted the fact that nicotine can be cytotoxic. In particular, recent work with primary cortical cultures from β2-/- and α7-/- mice has led to the finding that *α7-nAChR activation can potentiate toxic insults in the developing brain (22). In line with this finding, the present and previous studies clearly show that nAChR stimulation also can be proapoptotic for neuron precursors born in the adult brain. At present, we can only speculate on the molecular mechanisms underlying *β2-nAChRs' effects in OB granule cell regeneration. As previously proposed, it could be related to immature cells having an increased, but less efficiently buffered, calcium load (21, 23). It is noteworthy that *β2-nAChRs have high-affinity acetylcholine-binding sites and are therefore more suitable to activation through volume transmission (24). Therefore, diffusion of acetylcholine from cholinergic terminals could represent an activity-dependent mechanism by which the number of surviving newborn neurons could be regulated.
What are the behavioral consequences of increased neurogenesis in the OB granule cell layer? An earlier report by some of us had revealed that olfactory enrichment in adult mice (daily presentations of novel odors during 40 days) leads to an increase in the number of newborn bulbar interneurons (16). This increase, comparable to that observed in β2-/- mice, was accompanied by an enhanced short-term olfactory memory. Thus, we performed the same behavioral test with β2-/- mice and unexpectedly found that these animals have a less robust performance than WT, without displaying any difference in odor detection sensitivity. These results imply that an increase in GABAergic granule cell number is not sufficient to improve olfactory memory capacities. Previous behavioral studies have described that performance in different olfactory tasks was modulated by mAChRs (25, 26). In particular, local injections of the mAChR antagonist scopolamine in the OB were found to impair short-term olfactory memory (26). By revealing that deletion of *β2-nAChRs results in a similar behavioral impairment, this study further highlights how an adequate balance between the cholinergic and other OB neural systems is required for an efficient processing of olfactory information (27).
In conclusion, this study provides experimental evidence indicating that *β2-nAChRs modulate the survival of newborn cells in the adult brain. It also demonstrates that nAChRs are involved in the integration (i.e., “selection and stabilization,” ref. 28) of new inputs to the adult OB circuitry. Furthermore, the behavioral data obtained with β2-/- mice underline the importance of the cholinergic system in maintaining efficient olfactory processing. Finally, in vivo chronic nicotine exposure confirmed that nicotine can be cytotoxic for regenerating neuron populations in the adult brain. This finding will have to be taken into consideration for the development of therapeutic strategies involving nicotinic agonists.
Acknowledgments
This work was supported by grants from the Collège de France, the Pasteur Institute, the Annette Gruner-Schlumberger Foundation, the French Ministry of Research and Education (ACI Biologie du Développement et Physiologie Intégrative 2003), the Centre National de la Recherche Scientifique, the Association pour la Recherche sur le Cancer, and the Association Française contre les Myopathies, and by European Economic Community contracts. N.M. was a recipient of Natural Sciences and Engineering Research Councils of Canada and European Molecular Biology Organization fellowships.
Abbreviations: DG, dentate gyrus; mAChRs, muscarinic acetylcholine receptors; nAChRs, nicotinic acetylcholine receptors; *β2-nAChR, β2-containing nAChR; *α7-nAChR, α7-containing nAChR; OB, olfactory bulb; RMS, rostral migratory stream; RT, room temperature; SVZ, subventricular zone; TUNEL, terminal deoxynucleotidyltransferase-mediated biotinylated UTP nick end labeling.
References
- 1.Alvarez-Buylla, A. & Garcia-Verdugo, J. M. (2002) J. Neurosci. 22, 629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gritti, A., Bonfanti, L., Doetsch, F., Caille, I., Alvarez-Buylla, A., Lim, D. A., Galli, R., Garcia-Verdugo, J. M., Herrera, D. G. & Vescovi, A. L. (2002) J. Neurosci. 22, 437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carleton, A., Petreanu, L. T., Lansford, R., Alvarez-Buylla, A. & Lledo, P.-M. (2003) Nat. Neurosci. 6, 507-518. [DOI] [PubMed] [Google Scholar]
- 4.Petreanu, L. & Alvarez-Buylla, A. (2002) J. Neurosci. 22, 6106-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winner, B., Cooper-Kuhn, C. M., Aigner, R., Winkler, J. & Kuhn, G. (2002) Eur. J. Neurosci. 16, 1681-1689. [DOI] [PubMed] [Google Scholar]
- 6.Castillo, P. E., Carleton, A., Vincent, J.-D. & Lledo, P.-M. (1999) J. Neurosci. 19, 9180-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasa, P., Hlavati, I., Dobo, E., Wolff, A., Joo, F. & Wolff, J. R. (1995) Neuroscience 67, 667-677. [DOI] [PubMed] [Google Scholar]
- 8.Changeux, J.-P., Bertrand, D., Corringer, P.-J., Dehaene, S., Edelstein, S., Léna, C., Le Novère, N., Marubio, L., Picciotto, M. & Zoli, M. (1998) Brain Res. Rev. 26, 198-216. [DOI] [PubMed] [Google Scholar]
- 9.Berger, F., Gage, F. H. & Vijayaraghavan, S. (1998) J. Neurosci. 18, 6871-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrous, D. N., Adriani, W., Montaron, M.-F., Aurousseau, C., Rougon, G., Le Moal, M. & Piazza, P. V. (2002) J. Neurosci. 22, 3656-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang, M. H., Shin, M. C., Jung, S. B., Lee, T. H., Bahn, G. H., Kwon, Y. K., Kim, E. H. & Kim, C. J. (2002) NeuroReport 13, 1509-1513. [DOI] [PubMed] [Google Scholar]
- 12.Le Jeune, H., Aubert, I., Jourdan, F. & Quirion, R. (1995) J. Chem. Neuroanat. 9, 99-112. [DOI] [PubMed] [Google Scholar]
- 13.Tribollet, E., Bertrand, D., Marguerat, A. & Raggenbass, M. (2004) Neuroscience 124, 405-420. [DOI] [PubMed] [Google Scholar]
- 14.Picciotto, M. R., Zoli, M., Léna, C., Bessis, A., Lallemand, Y., Le Novère, N., Vincent, P., Pich, E. M., Brulet, P. & Changeux, J.-P. (1995) Nature 374, 65-67. [DOI] [PubMed] [Google Scholar]
- 15.Davenport, A. P. & Hall, M. D. (1988) J. Neurosci. Methods 25, 75-82. [DOI] [PubMed] [Google Scholar]
- 16.Rochefort, C., Gheusi, G., Vincent, J.-D. & Lledo, P.-M. (2002) J. Neurosci. 22, 2679-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maruniak, J. A., Taylor, J. A., Henegar, J. R. & Williams, M. B. (1989) Brain Res. Dev. Brain Res. 47, 27-33. [DOI] [PubMed] [Google Scholar]
- 18.Brunjes, P. C. (1985) Brain Res. Bull. 14, 233-237. [DOI] [PubMed] [Google Scholar]
- 19.Brunjes, P. C. & Frazier, L. L. (1986) Brain Res. 396, 1-45. [DOI] [PubMed] [Google Scholar]
- 20.Le Jeune, H., Aubert, I., Jourdan, F. & Quirion, R. (1996) J. Comp. Neurol. 373, 433-450. [DOI] [PubMed] [Google Scholar]
- 21.Picciotto, M. R. & Zoli, M. (2002) J. Neurobiol. 53, 641-655. [DOI] [PubMed] [Google Scholar]
- 22.Laudenbach, V., Medja, F., Zoli, M., Rossi, F. M., Evrard, P., Changeux, J.-P. & Gressens, P. (2002) FASEB J. 16, 423-425. [DOI] [PubMed] [Google Scholar]
- 23.Stevens, T. R., Krueger, S. R., Fitzsimonds, R. M. & Picciotto, M. R. (2003) J. Neurosci. 23, 10093-10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Descarries, L. & Mechawar, N. (2000) Prog. Brain Res. 125, 27-47. [DOI] [PubMed] [Google Scholar]
- 25.Ravel, N., Vigouroux, M., Elaagouby, A. & Gervais, R. (1992) Psychopharmacology 109, 439-443. [DOI] [PubMed] [Google Scholar]
- 26.Ravel, N., Elaagouby, A. & Gervais, R. (1994) Behav. Neurosci. 108, 317-324. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, D. A., Fletcher, M. L. & Sullivan, R. M. (2004) Learn. Mem. 11, 28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Changeux, J.-P. & Danchin, A. (1976) Nature 264, 705-712. [DOI] [PubMed] [Google Scholar]