Abstract
Although heparin and low-molecular-weight heparins (LMWH) have been widely used clinically as anticoagulants, their broader use has been limited by the lack of noninvasive delivery methods for this class of molecules. In this study, we demonstrate an efficient, rapid, and reproducible delivery system for heparin through the lungs that is not confined to particles of a certain geometric or aerodynamic diameter. Importantly, blood levels after intrapulmonary administration of either heparin or LMWH were comparable to that of s.c. administration but are characterized by a more rapid onset of action (t1/2 = 40 min vs. 2.5 h, respectively). Furthermore, we show in animal models, that inhaled heparin species efficiently inhibit diseases such as thrombosis and emphysema, and that the repetitive inhalation of formulated LMWH results in no observable toxicity from the delivery of reproducible systemic levels of heparin or LMWH.
The heparin-derived complex polysaccharides are a very important class of pharmaceuticals. Clinically, heparin and low-molecular-weight heparins (LMWH), such as enoxaparin and dalteparin, are the most commonly used anticoagulant and antithrombotic agents. Heparin, due to its pronounced antiactivated factor (anti-Xa) and anti-IIa activities, is the drug of choice in acute settings for the prevention and treatment of arterial thrombosis, such as in acute coronary syndrome (1). Due to its polydispersity and polyanionic nature, however, heparin is usually given via i.v. administration to avoid problems associated with interpatient variability. LMWHs, molecules with reduced size and polydispersity but with significantly reduced anti-IIa activity (2), are primarily used for the prevention and treatment of venous thromboses (3, 4). To date, parenteral injection (i.v. and s.c.) has been the only avenue for efficient delivery of these molecules. Unfortunately, in both cases, due to the inconvenience of i.v. or s.c. injection and associated side effects from injection such as local irritation and ulceration, chronic anticoagulant therapy is mediated primarily through the administration of the vitamin K antagonist warfarin. Warfarin, however, is a less than ideal anticoagulant, in that it influences an array of biologic processes beyond coagulation and thus possesses many and varied side effects (5). In short, that warfarin can be given noninvasively (orally), compared to invasive heparin injections, makes it the drug of choice for chronic anticoagulation, despite the fact that it is a less specific anticoagulant with more side effects. Thus, the development of a convenient, noninvasive, alternative route of administration for heparin and LMWHs with comparable pharmacokinetic performance to parental injection would represent a significant advance in anticoagulant therapy.
However, as polyanionic and polydisperse pharmaceuticals, the development of noninvasive delivery systems for heparin and LMWH has proved a daunting challenge. Currently, several strategies are being pursued for the oral delivery of these molecules. These strategies include formulation of heparin nanoparticles as well as covalent coupling of heparin or LMWH to amino acid analogues, cationic lipophilic detergents, or bile acids (6-8). However, these strategies result in delivery systems that are markedly inferior to s.c. delivery, including delivering <3-5% of active drug to the blood stream [compared to >98% bioavailability through s.c. injection of LMWH (3)]. In addition, due to their inability to deliver the vast majority of the drug into the bloodstream, many of these systems possess variable pharmacokinetic behavior and generally require stoichiometric or superstoichiometric levels of a carrier that itself may have unintended biologic properties or toxicological effects (9). Additionally, rapid metabolism of heparin and LMWH in the gastrointestinal tract limits the potential bioavailability through oral administration. In fact, a recent oral delivery formulation of heparin failed phase III clinical trials because of interpatient variability, poor patient compliance, and poor bioavailability. Clearly, alternative delivery systems are required for this important class of molecules. Other noninvasive routes have been suggested, including nasal (10), but these too suffer from many of the same limitations as the oral route of administration.
In the present study, we demonstrate that aerosol particle formulation of heparins and LMWHs can result in the efficient pulmonary delivery of this class of molecules, potentially making it therapeutically viable. We establish that the mechanism of delivery of the heparins is fundamentally different from existing deep-lung-mediated strategies and thus is not confined to particles of a certain geometric or aerodynamic diameter (11). Importantly, we demonstrate that pulmonary delivery of suitably formulated heparin or LMWH particles results in bioavailability that is 10-20 times that of other currently described noninvasive strategies. We verify these results in two distinct animal models and through the use of multiple dosing regimens to ensure the authenticity of our results. We also investigate the pharmacodynamic effect of inhaled LMWH by testing its efficacy in inhibiting thrombus formation and in mediating emphysema progression. Finally, through mechanistic studies, we find that delivery of formulated heparin or LMWHs results in transient, reversible opening of the tight junctions (TJs) between cells of the airway epithelium, thus facilitating delivery of therapeutic levels of heparin and LMWH.
Materials and Methods
Formulation of Heparins. Preweighed heparin (178 U.S. Pharmacopeial Convention units/mg) or LMWH, typically ardeparin (93 units/mg, anti-Xa from Celsus Laboratories, Cincinnati) was dissolved in water, ion exchanged, and lyophilized. We formed particles by two different means that possessed identical characteristics. Certain particles, especially those containing 60% dipalmitoyl phosphatidylcholine, were formed by using a spray dryer Buchi B-190 (Brinkmann). For these formulations, the dipalmitoyl phosphatidylcholine percentage was made by mixing powder in a 7:3 ethanol/water solution and then adding this to an aqueous solution of drug prior to spray drying. Alternatively, particles containing only heparin or LMWH (used in the majority of experiments) were formed by mechanical grinding followed by molecular sieving (W. S. Tyler, Mentor, OH). Sieves of size cutoffs of 20, 53, 75, and 100 μm were used to form size-defined particles. In both cases, particle dimensions were confirmed both visually by using scanning electron microscopy and through particle measurements by laser size diffraction (see supporting information, which is published on the PNAS web site), and the activity of the heparin or LMWH particles was confirmed via an in vitro activity assay. Instillation of liquid heparin was completed as described (12).
Pharmacokinetics Studies. For the rat model, male Sprague-Dawley rats weighing 350-450 g (Charles River Breeding Laboratories) were housed for 5-7 days before experiments. Rats were fed on rat chow and tap water ad libitum. After anesthetization with Ketamine (80 mg/kg) and Xylazine (10 mg/kg) i.p., the right carotid artery was isolated and intubated with a Teflon catheter. A three-way stopcock was connected to the catheter for blood sample collection. Blood collection followed published procedures (13, 14). Pulmonary inhalation was completed by using an insufflator (Penn Century, Philadelphia) specially designed for aerosol inhalation in small animals. Blood samples were collected in an aqueous solution of sodium citrate (3.8%; 1/9, vol/vol), centrifuged at 2,000 × g for 20 min, and the resulting plasma was shock frozen and stored in -80°C freezer until assayed.
For the rabbit model, 2- to 2.5-kg New Zealand male rabbits were used with four to five rabbits per group. Rabbits were allowed to adapt for 7 days before the experiment, with free access to water and food. Ketamine (40 mg/kg) and Xylazine (5 mg/kg) were injected intramuscularly to anesthetize the rabbits. A 24-gauge Teflon catheter was inserted into the center auricular artery. The catheter was connected to an injection plug filled with 3.8% sodium citrate solution. Then a 15-cm tracheal tube was inserted into the trachea of the anesthetized rabbits via mouth. The position of tracheal tube was about 5 mm above the tracheal bifurcates (confirmed by necropsy). After intubation of the animals, the front arm of the insufflator was inserted into the tracheal tube above the bifurcates. After pulmonary administration of heparin or LMWH, blood samples were processed as described above and assayed for anti-Xa levels. In selected experiments, LMWH was also given by s.c. or i.v. bolus injection via the contralateral marginal ear vein.
In each case, pharmacokinetic parameters were calculated as described (15).
Pulmonary Lavage. To determine the rate of disappearance of heparin from the lungs of rats and rabbits after inhalation, lungs were harvested en bloc 0, 5, or 30 min or 1, 2, 4, 6, or 8 h after inhalation, with one rabbit per time point. The trachea was cannulated with an 18-guage animal feeding needle and lavaged with five sequential aliquots of 6 ml (rabbits) or 3 ml (rats) of normal saline. Lavage fluid was centrifuged at 2,000 × g for 10 min. The supernatant was shock frozen immediately and transferred to -80°C shortly after. The resulting cell pellets was resuspended in saline. Twenty microliters of the cell suspension was smeared on a slide and stained with Diff-Quik (American Scientific Products, McGaw Park, IL). The rest of the cell suspension was homogenized and centrifuged, and the supernatant was tested for anti-Xa activity. The lavaged lungs were homogenized in saline (1 g in 5 ml of saline) with a polytron device. The homogenate was centrifuged at 12,000 × g for 10 min, and the supernatant was tested for anti-Xa activity, as described below.
Activity Assays. Whole-blood recalcification times were used to indirectly determine the amount of heparin present in the blood, as described (16). In most cases with LMWH, anti-Xa activity was used as a surrogate marker to monitor plasma drug levels. An anti-Xa assay was performed by modification of the amidolytic method (17) with a Coatest heparin test kit by using S-2222 as the chromogenic substrate (Diapharma Group, West Chester, OH). The detailed procedure has been described (15).
Thrombosis Experiments. The antithrombotic effects of inhaled LMWH were evaluated by Wessler's stasis model (18) adapted for the rat by using Russell Viper Venom as the thrombogenic stimulus (19). The procedure was performed as described (15). Briefly, 1 h after pulmonary inhalation or s.c. injection of LMWH, a laparotomy was performed under anesthesia. A vena cava segment was isolated between the left renal and iliac veins. All of the side branches of vena cava were ligatured, and Russell Viper Venom (0.03 units/kg) was administered via the penile vein. Two minutes later, the vena cava was ligatured by cotton threads just below the left renal vein and above the iliac veins. Ten minutes later, the thrombus (if formed) was removed and weighed wet.
Emphysema Model. Aerosol heparin at 3 mg/kg was given to Sprague-Dawley rats 1 h before instillation of 250 μg of human sputum leukocyte elastase via the trachea (20). After administration of elastase, rats were kept head up at a 30° slope for 30 min and allowed to recover. Eight weeks later, the rats were killed. The lungs of treated and control rats were harvested and fixed in 10% formalin at 20-cm H2O pressure for 24 h and then analyzed.
Calu-3 Cell Culture. Calu-3 cells (American Type Culture Collection no. HTB-55) were cultured in MEM supplemented with 10% FBS/2 mM L-glutamine/0.5% with penicilin and streptomycin under standard condition. Passage 4-10 cells were seeded into 24-well polyester 0.4-μm pore size Transwells (Corning) at a density of 105 cells per cm-2. Confluent cells [represented by a stable trans-epithelial electrical resistance (Millicell-ERS, Millipore)] of ≈1,000 Ω·cm-2) were then doped with either aerosol or liquid heparin. For the aerosol particles, the material was delivered with an insufflator onto the calu-3 cell layer in the apical chamber depleted of media via the air interface chamber model (21). In either case, 9 mM heparin (equal to 23 mg in 250 μl of media) or 4 mg of heparin aerosol was applied into the apical chamber in triplicate. The media in the basal lateral chamber were collected at different time points: 0, 5 min, 10 min, 15 min, 30 min, 1 h, or 2 h after introduction of heparin.
Immunofluorescence and Confocal Microscopy. Calu-3 cells grown on glass coverslips were fixed with 10% buffered formalin for 30 min. Then the cells were permeabilized with 0.5% Triton X-100 (Sigma) in Tris-buffered saline with 0.1% Tween 20 (TBST) for 15 min at room temperature. Nonspecific antibody binding was blocked by administration of 0.1% BSA in TBST for 30 min at room temperature and then incubated for 4 h with a polyclonal rabbit anti-ZO-1 antibody (Santa Cruz Biotechnology) diluted at a ratio of 1:400 in 0.1% BSA. After washing with TBST (three times for 5 min), the coverslips were incubated with a rhodamine-conjugated goat anti-rabbit IgG (1:400 in 0.1% BSA; Santa Cruz Biotechnology) and FITC-phalloidin (1:50 in 0.1% BSA; Molecular Probes) for 1 h in a dark at room temperature and then washed three times with TBST. Coverslips were mounted with SlowFade light antifade kit (Molecular Probes) and viewed under confocal microscopy (Zeiss LSM510 laser confocal scanning microscopy).
For immunohistology, lung and trachea tissues from male Sprague-Dawley rats (250≈300 g; Charles River Breeding Laboratories) were harvested after administration of either aerosol ardeparin or FITC-labeled ardeparin. Harvested tissues were embedded in OCT compound (Electron Microscopy Sciences, Fort Washington, PA) and immediately put into liquid nitrogen. The tissues were then cut by cryosection and stained and viewed as outlined above.
Results
Efficient Pulmonary Delivery of Heparin Using Aerosol Particles. In an effort to define conditions for the efficient pulmonary delivery of heparin and LMWHs, a number of experimental parameters were tested. For most experiments, blood levels of anti-Xa activity were measured as surrogate markers for systemic levels of the drug, but in selected cases, actual heparin (or other polysaccharide) levels were directly measured by using a modification of published procedures (16). Initial experiments were predicated on the concept that significant absorption of heparin into the bloodstream required efficient delivery of heparin to the deep lung, where particles or droplets of heparin could effectively cross the alveoli-blood barrier. Consistent with this concept as well as with previous observations, instillation of liquid heparin into the lungs of rabbits resulted in low bioavailability and poor pharmacokinetics (Fig. 1A). However, when pharmacologic doses of heparin were formulated as aerosol-porous particles that possessed an aerodynamic diameter of <5 μm (11, 22), efficient delivery of heparin was observed (Fig. 1A), consistent with previous observations. Surprisingly, however, when aerosol particles with measured mass densities of 0.4-0.45 g/cm3 were created that possessed an aerodynamic diameter >5 μm and thus could not travel to and be maintained in the deep lung, efficient absorption was still observed (Fig. 1A). Several additional lines of evidence suggest that these dense nonporous particles are predominantly deposited in the upper airway. Measurement of particle size before and after passage through the insufflator confirmed the large geometric size of the particles (see supporting information). That the insufflator seemingly did not alter the particle characteristics is consistent with previous observations (23). In addition, the delivery of dye-labeled heparin, followed by histological examination of animals after pulmonary administration, showed that the vast majority of material was deposited in the upper airway. Also, use of a ventilator as an alternate delivery methodology resulted in high bioavailability as well (see supporting information), indicating that the observed phenomenon is not contingent on the use of an insufflator. In every case, absolute bioavailability for the pulmonary delivery of heparin, depending on formulation, was measured as 35-60%, similar to or greater than the bioavailability after s.c. administration. Thus, taken together, these observations suggest that delivery of therapeutic levels of heparin can be accomplished via deposition of particles in the upper airway. To further explore this possibility, additional experiments were completed with aerosol particles that possessed aerodynamic diameters >5 μm.
Fig. 1.
Pharmacokinetic profile of the pulmonary delivery of heparin. (A) Heparin (8 mg/kg) was given to New Zealand rabbits via either s.c. injection (•), inhalation of porous particles with an aerodynamic diameter of <5 μm (▴), inhalation of aerosol particles with an aerodynamic diameter of >5 μm (□), or instillation of liquid heparin (○), and drug levels were monitored (n = 6). (B) Lavage studies completed in rabbits after administration of 8 mg/kg inhaled heparin indicate the rapid clearance of heparin from the lung (n = 3). Levels of heparin at various times were measured in both the lavage fluid (•) and plasma of rabbit (○) after administration of heparin aerosol particles with an aerodynamic diameter >5 μm.
In the case of LMWH, an investigation of differently sized particles, with geometric diameters of 20-80 μm indicated that absorption and bioavailability were particle size independent in this range (Table 1). In addition, as we observed above with heparin particles, imaging of fluorescently labeled aerosol particles of LMWH confirmed that these particles did not reach the deep lung but were in fact deposited in the upper airway and bronchi, where they were rapidly absorbed into the bloodstream. Lavage studies completed by using either fluorescently tagged or unlabeled LMWH confirmed these findings, e.g., LMWH from the lavage fluid disappeared sharply after inhalation and the level of LMWH in the lavage fluid reached baseline in <1 h in both rats and rabbits (Fig. 1B). From these data, the calculated elimination half-life from the lavage fluid is 5.8 min. When converted to the total amount of LMWH in the fluid, >90% of the drug disappeared from the lung air passage surface in the first half hour. This fast disappearance of heparin or LMWH coincides with the rapid appearance of heparin in the plasma. As a control, it has been reported that >50% of instilled heparin remains in the lavage fluid 1.5 h after instillation, which we also observe when unformulated heparin is instilled (data not shown) (24). Consequently, from these data, it is clear that the deposition, absorption, and elimination profiles of inhaled aerosolized heparin are distinct from those of instilled heparin.
Table 1. Particle size independence of inhaled LMWH.
| 6 mg/kg inhaled LMWH, μm
|
|||
|---|---|---|---|
| 20 | 40 | 80 | |
| AUC, units·h/ml | 3.3 ± 0.2 | 3.5 ± 0.3 | 3.2 ± 0.2 |
| Cmax, units/ml | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 |
| tmax, h | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 |
AUC, area under the curve.
Efficient delivery of a number of other LMWHs, including enoxaparin and dalteparin (currently the leading clinically available LMWHs), was achieved. As was the case with heparin, absorption was independent of particle size (Table 2). In every case, intrapulmonary delivery was characterized by rapid onset of action (tmax of 40 min compared to 2-4 h for s.c. delivery), as well as a Cmax comparable to s.c. administration (Table 2). To ensure the authenticity of these observations and to test the extent of this phenomenon, we further investigated the intrapulmonary delivery of heparin and LMWHs.
Table 2. Pharmacokinetic parameters of three LMWHs: ardeparin, enoxaparin, and dalteparin.
| s.c. injection, 3 mg/kg
|
Inhalation, 80-μm particle, 3 mg/kg
|
|||||
|---|---|---|---|---|---|---|
| Ardeparin | Enoxaparin | Dalteparin | Ardeparin | Enoxaparin | Dalteparin | |
| Ka, h−1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 2.5 ± 0.5 | 3.6 ± 0.4 | 3.3 ± 0.6 |
| Ke, h−1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 |
| AUC, units·h/ml | 4.7 ± 1.1 | 7.8 ± 0.6 | 8.2 ± 0.5 | 2.2 ± 0.2 | 4.8 ± 0.9 | 4.4 ± 0.6 |
| Cmax, units/ml | 0.7 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.2 | 0.6 ± 0.1 | 0.9 ± 0.3 | 0.9 ± 0.2 |
| tmax, h | 2.4 ± 0.2 | 2.7 ± 0.2 | 2.5 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.2 | 0.7 ± 0.3 |
AUC, area under the curve.
Dose-Response of Intrapulmonary Delivery. To test the utility of intrapulmonary delivery of heparin and LMWHs, dose-response studies were completed in both Sprague-Dawley rats and New Zealand rabbits. In both animal models, pulmonary delivery of either heparin or LMWH resulted in therapeutic blood levels of the anticoagulant and a linear dose-response (Fig. 2A). Taken together, these pharmacokinetic data with multiple dosing schedules in two different animal models indicate that the observed phenomenon is reproducible.
Fig. 2.
Dosing studies of inhaled LMWH. (A) We delivered ardeparin aerosol particles (≈40 μm) to the lung at doses of 1 mg/kg (▴),3mg/kg (▪), or 6 mg/kg (♦) and followed the blood levels of LMWH (n = 6). (Inset) We plotted the maximum blood levels of ardeparin (Cmax) from pulmonary delivery vs. the delivered dose (•) and compared these values to those from s.c. injection (○). (B) Rabbits were treated once per day with 3 mg/kg ardeparin aerosol particles for 3 consecutive days.
Because the potential clinical application of inhaled heparin or LMWH is in the treatment or prevention of thrombosis, it is essential to know whether stable pharmacokinetic performance can be maintained upon repetitive inhalation. To address this question, we dosed rabbits once every 12 h for 3 days. Measurement of blood levels of LMWH indicated no decrease in absorption or alteration of the pharmacokinetic profile over the time of the experiment, as measured either by tmax, Cmax, or bioavailability (Fig. 2B). Histological examination of rabbits after multiple inhalations revealed complete clearance of aerosol particles and no gross toxological effects, including no detectable bleeding in the lungs. In addition, after consecutive inhalation treatments, no signs of morbidity such as weight loss or labored breathing were observed in animals, either before, during, or after dosing. Thus, we find no obvious side effects from pulmonary inhalation of heparin or LMWH, even after repeated dosings.
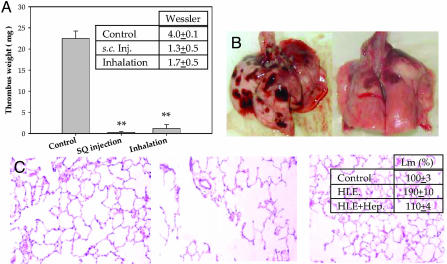
Therapeutic Efficacy of Inhaled Heparin. To date, LMWHs like enoxaparin and dalteparin are primarily used in the prevention and treatment of deep vein thrombosis. To extend our pharmacokinetic findings, we examined the ability of inhaled ardeparin to prevent the formation of venous thrombi in a rat model. Given the rapid onset of action, we surmised that pulmonary delivery of LMWH aerosol particles would represent an effective way of rapidly delivering therapeutic levels of heparin or LMWH, a hypothesis that was shown to be correct (Fig. 3A). In this case, delivery of 3 mg/kg of LMWH through a pulmonary route resulted in efficient prevention of thrombus formation, which is statistically equivalent to delivery of an identical dose via s.c. injection.
Fig. 3.
Therapeutic efficacy of the pulmonary delivery of heparins. (A) We treated Sprague-Dawley rats with PBS (control), 3 mg/kg LMWH delivered s.c., or 3mg/kg aerosol LMWH delivered via inhalation 20 min before the onset of venous thrombosis through a stasis model (n = 6). Shown is the extent of thrombus formation, as measured by weight (**, P < 0.001). Also shown is the Wessler score (Inset). (B) We also measured the effect of nonanticoagulant heparins in a human sputum elastase (HLE)-induced emphysema model. (Left) A representative lung from the positive control group that received HLE but not heparin (n = 3). (Right) A representative lung of the treated group that received pulmonary heparin after HLE instillation. (C) Histological examination of lung tissue confirmed the result obtained from gross inspection. (Left) The control normal lung tissue is from animals that received neither HLE nor heparin. (Center) Tissue from animals that were administered HLE but not heparin consistently showed histological changes. (Right) Lung tissue of animals that received both HLE and heparin showed reduced change as compared to control. (Inset) Quantification of the mean linear intercept (Lm) measured upon histological examination of the control group and the two treatment groups.
Notably, our findings are not confined to the delivery of anticoagulant heparin or LMWH. In fact, previous studies have demonstrated that heparins that do not possess anticoagulant activity are still effective inhibitors of diseases such as emphysema and asthma (25-27), both through inhibition of the immune system (thus limiting damage arising from chronic inflammation) and directly through inhibiting the action of elastase (in the case of emphysema). We find that pulmonary delivery of heparin aerosol particles in an animal model of emphysema results in a marked decrease of elastase-induced injury (Fig. 3B, and supporting information).
Mechanism of Rapid Absorption via Pulmonary Delivery. In an effort to understand, at a fundamental level, the pulmonary delivery of heparin and LMWH, we examined mechanisms by which rapid absorption could be explained. To this end, a number of in vitro and in vivo studies were completed.
One of the obvious factors involved in the efficient delivery of heparin or LMWH particles is that, mechanically, large aerosol particles remain in the upper lung, enabling rapid absorption and inhibiting clearance by the mucocilliary apparatus. Furthermore, in vivo, we find that large particles (10-100 μm) are not readily engulfed by macrophages. Homogenized lung tissue and macrophage cell pellets from centrifugation contained little heparin, as observed using the anti-Xa assay (data not shown). To confirm this finding and to study the impact of particle size on bioavailability, LMWH particles were formulated and delivered that possessed smaller geometric diameters (<5 μm) but that were otherwise identical to the larger particles. As expected, delivery of these small particles yielded lower blood levels of LMWH (approximately one-half to one-third of the area under the curve) compared to the large aerosol particles reported here despite similarities between the two formulations. This decrease in bioavailability is expected because small particles, unlike the others formulated here, are subject to phagocytosis by macrophages (28) and increased clearance. Importantly, however, this mechanical argument is not sufficient to explain entirely the rapid absorption profile and marked bioavailability that we observe. Therefore, additional mechanistic studies were completed as described below.
Administration of instilled heparin or LMWH results in slow transport across Calu-3 cells in vitro, consistent with our in vivo findings. Alternatively, administration of the same amount of heparin or LMWH as aerosol particles results in much more efficient transport (Fig. 4A). Monitoring the transepithelial electrical resistance in Calu-3 cells over time upon administration of heparin or LMWH aerosol particles indicates that this opening is rapid but transient and reversible (Fig. 4B). Staining and visual examination of both cells and in vivo tissue sections after heparin or LMWH delivery demonstrate that the particles modulate the opening of the TJs between cells, allowing for the efficient delivery of heparin or LMWH (Fig. 4C). Given the pronounced effect of heparin on the TJ complex, we further explored the role of heparin in mediating TJ action and thus influencing paracellular permeability (29, 30).
Fig. 4.
Effects of heparin or LMWH on the TJs. (A) In vitro delivery of aerosol particles of heparin (•) results in greater transport across the transwell than does delivery of instilled heparin (○). (B) Calu-3 cells were treated with aerosol ardeparin (•) or PBS (○) for 8 h (n = 3), and transepithelial electrical resistance was tested at each time point. Results are presented as a percentage of the original transepithelial electrical resistance measured before treatment. (Inset) We used the same procedure, except that, 1 h after treatment, ardeparin was removed from both the apical and basal-lateral chambers. (C) Fluorescence microscopy to examine the localization of labeled heparin. (Left) In vivo staining of the trachea and upper airway of rats after administration of aerosol heparin. After administration of aerosol heparin, treated animals were killed, and tissue was stained for ZO-1 or visualized for FITC-heparin. Superposition of the two images revealed that there was complete overlap between the two, indicating that aerosolized heparin localizes to the TJs. (Right) Administration of aerosol heparin to Calu-3 cells results in a reorganization of the actin-cytoskeleton, leading to reversible opening of the TJ. (Upper) Control cells that were not treated with aerosol heparin. (Lower) Cells treated with aerosolized heparin. The boxed portion (Lower) represents the area of aerosol administration.
To elucidate the downstream signals for the opening of the TJs after heparin particle administration, we studied the localization of ZO-1, a critical component in the assembly of TJs (29), and the associated actin cytoskeleton by labeling the former with antibodies and the latter with FITC-phalloidin. Confocal imaging indicates that administration of heparin particles results in the elimination of ZO-1 localization within the TJs, leading to a transient disruption of actin cytoskeletal orientation (Fig. 4C). Thus the above data taken together suggest that heparin influences the TJ complex of epithelial cells and is therefore readily transported into the bloodstream. This is in direct contrast to other mechanisms for heparin delivery that appear to be transcellular and result in less efficient, slower absorption of heparin or LMWH (31).
Discussion
From the above studies, we discovered three unexpected findings: (i) pulmonary delivery of heparin and LMWH is primarily mediated in the upper lung and thus is not confined to particles of a certain geometric or aerodynamic diameter; (ii) delivery depends on optimal formulation of aerosol particles; and (iii) delivery resulted in rapid absorption of heparin or LMWH such that Cmax is achieved at an earlier time post-administration compared to s.c. delivery.
In this study, we have also shown that this strategy permits the delivery of therapeutic levels of drug to the blood to treat both thrombotic and nonthrombotic disorders. For example, the formulations studied herein show a rapid onset of action, very similar to i.v. administration and markedly faster than s.c. injection. As such, this delivery system may be suited for the treatment of existing thrombi (for example, during stroke or other emergency situations) via the rapid introduction of active drug into the bloodstream. In addition, this delivery strategy shows potential promise with regard to safety. As noted in Results, reproducible systemic levels of drug could be readily achieved with low interanimal variability. Notably, a number of studies, primarily involving instillation of heparin, have delivered heparin doses to patients that are >100 times those used here, and these studies reported no adverse events, further supporting the possibility of pulmonary delivery of heparin as a viable clinical strategy (24, 32, 33).
Unlike other delivery strategies, we find that the pulmonary delivery of heparin appears to occur via a fundamentally different mechanism that is not primarily deep-lung mediated. Given the transport characteristics of heparin and LMWH, as well as the underlying biology of the system, this raises the possibility that the findings described herein could provide a basis for the delivery of additional molecules, such as peptides, proteins, and/or small molecule therapeutics. In conclusion, the present study shows the efficient noninvasive delivery of heparin and LMWH. The inhaled aerosolized heparin is comparable in terms of bioavailability to that of s.c. injection and thus represents a promising alternative in chronic antithrombotic treatment and perhaps in other disease areas as well.
Acknowledgments
We acknowledge financial assistance from the National Institutes of Health (GM 57073 to R.S.).
Abbreviations: LMWH, low-molecular-weight heparins; TJ, tight junction.
References
- 1.Hirsh, J., Dalen, J. E., Deykin, D. & Poller, L. (1992) Chest 10, 337S-351S. [DOI] [PubMed] [Google Scholar]
- 2.Linhardt, R. J. & Gunay, N. S. (1999) Semin. Thromb. Hemostasis 25, Suppl 3, 5-16. [PubMed] [Google Scholar]
- 3.Hirsh, J., Warkentin, T. E., Raschke, R., Granger, C., Ohman, E. M. & Dalen, J. E. (1998) Chest 114, 489S-510S. [DOI] [PubMed] [Google Scholar]
- 4.Merli, G. J. (2003) Expert Opin. Pharmacother. 4, 55-65. [DOI] [PubMed] [Google Scholar]
- 5.Wieland, H. A., Laux, V., Kozian, D. & Lorenz, M. (2003) Curr. Opin. Invest. Drugs 4, 264-271. [PubMed] [Google Scholar]
- 6.Jiao, Y., Ubrich, N., Hoffart, V., Marchand-Arvier, M., Vigneron, C., Hoffman, M. & Maincent, P. (2002) J. Pharmacol. Sci. 91, 760-768. [DOI] [PubMed] [Google Scholar]
- 7.Leone-Bay, A., Paton, D. R., Variano, B., Leipold, H., Rivera, T., Miura-Fraboni, J., Baughman, R. A. & Santiago, N. (1998) J. Controlled Release 50, 41-49. [DOI] [PubMed] [Google Scholar]
- 8.Lee, Y., Kim, S. H. & Byun, Y. (2000) Pharmacol. Res. 17, 1259-1264. [DOI] [PubMed] [Google Scholar]
- 9.Gonze, M. D., Salartash, K., Sternbergh, W. C., 3rd, Baughman, R. A., Leone-Bay, A. & Money, S. R. (2000) Circulation 101, 2658-2661. [DOI] [PubMed] [Google Scholar]
- 10.Arnold, J., Ahsan, F., Meezan, E. & Pillion, D. J. (2002) J. Pharmacol. Sci. 91, 1707-1714. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, D. A., Hanes, J., Caponetti, G., Hrkach, J., Ben-Jebria, A., Eskew, M. L., Mintzes, J., Deaver, D., Lotan, N. & Langer, R. (1997) Science 276, 1868-1871. [DOI] [PubMed] [Google Scholar]
- 12.Oberdorster, G., Oldiges, H. & Zimmermann, B. (1980) Zentralbl. Bakteriol. B 170, 35-43. [PubMed] [Google Scholar]
- 13.Bjornsson, T. D. & Levy, G. (1979) J. Pharmacol. Exp. Ther. 210, 243-246. [PubMed] [Google Scholar]
- 14.Bjornsson, T. D. & Levy, G. (1979) J. Pharmacol. Exp. Ther. 210, 237-242. [PubMed] [Google Scholar]
- 15.Sundaram, M., Qi, Y., Shriver, Z., Liu, D., Zhao, G., Venkataraman, G., Langer, R. & Sasisekharan, R. (2003) Proc. Natl. Acad. Sci. USA 100, 651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ameer, G. A., Barabino, G., Sasisekharan, R., Harmon, W., Cooney, C. L. & Langer, R. (1999) Proc. Natl. Acad. Sci. USA 96, 2350-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teien, A. N., Lie, M. & Abildgaard, U. (1976) Thromb. Res. 8, 413-416. [DOI] [PubMed] [Google Scholar]
- 18.Wessler, S. (1971) Cardiovasc. Clin. 3, 1-16. [PubMed] [Google Scholar]
- 19.Aronson, D. L. & Menache, D. (1987) Dev. Biol. Stand. 67, 149-155. [PubMed] [Google Scholar]
- 20.Herbert, J. M., Frehel, D., Rosso, M. P., Seban, E., Castet, C., Pepin, O., Maffrand, J. P. & Le Fur, G. (1992) J. Pharmacol. Exp. Ther. 260, 809-816. [PubMed] [Google Scholar]
- 21.Florea, B. I., Cassara, M. L., Junginger, H. E. & Borchard, G. (2003) J. Controlled Release 87, 131-138. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Jebria, A., Chen, D., Eskew, M. L., Vanbever, R., Langer, R. & Edwards, D. A. (1999) Pharmacol. Res. 16, 555-561. [DOI] [PubMed] [Google Scholar]
- 23.Bot, A. I., Tarara, T. E., Smith, D. J., Bot, S. R., Woods, C. M. & Weers, J. G. (2000) Pharmacol. Res. 17, 275-283. [DOI] [PubMed] [Google Scholar]
- 24.Bendstrup, K. E., Gram, J. & Jensen, J. I. (2002) Eur. Respir. J. 19, 606-610. [DOI] [PubMed] [Google Scholar]
- 25.Kanabrocki, E. L., Bremner, W. F., Sothern, R. B., Gruber, S. A., Third, J. L., Bushnell, D. L. & Olwin, J. H. (1992) Q. J. Med. 83, 259-282. [PubMed] [Google Scholar]
- 26.Tyrrell, D. J., Horne, A. P., Holme, K. R., Preuss, J. M. & Page, C. P. (1999) Adv. Pharmacol. 46, 151-208. [DOI] [PubMed] [Google Scholar]
- 27.Diamant, Z. & Page, C. P. (2000) Pulm. Pharmacol. Ther. 13, 1-4. [DOI] [PubMed] [Google Scholar]
- 28.Edwards, D. A., Ben-Jebria, A. & Langer, R. (1998) J. Appl. Physiol. 85, 379-385. [DOI] [PubMed] [Google Scholar]
- 29.Matter, K. & Balda, M. S. (2003) Nat. Rev. Mol. Cell. Biol. 4, 225-236. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Moreno, M., Jamora, C. & Fuchs, E. (2003) Cell 112, 535-548. [DOI] [PubMed] [Google Scholar]
- 31.Malkov, D., Wang, H. Z., Dinh, S. & Gomez-Orellana, I. (2002) Pharmacol. Res. 19, 1180-1184. [DOI] [PubMed] [Google Scholar]
- 32.Kohler, D. (1994) J. Aerosol. Med. 7, 307-314. [DOI] [PubMed] [Google Scholar]
- 33.Bendstrup, K. E. & Jensen, J. I. (2000) Respir. Med. 94, 174-175. [DOI] [PubMed] [Google Scholar]