Abstract
Nobiletin, a citrus flavonoid has been associated with various beneficial biological activities. 4′-Demethylnobiletin (4DN) is a major metabolite of nobiletin and its tissue level was found to be much higher than that of nobiletin after oral administration of nobiletin in mice. Anti-inflammatory effects of 4DN were studied in lipopolysaccharide (LPS)-treated RAW 264.7 macrophages. The results showed 4DN not only dose-dependently inhibited LPS-induced nitric oxide production, but also significantly reduced expression of pro-inflammatory mediators, namely PGE2, IL-1β and IL-6. 4DN potently suppressed the expression of iNOS and COX-2 at both protein and mRNA levels. 4DN also inhibited nuclear translocation of NF-κB and AP-1. Furthermore, we demonstrated that 4DN activated transcription factor Nrf2 and its dependent genes including HO-1 and NQO1 whose expression may contribute to anti-inflammatory effects. The results demonstrated anti-inflammatory effects of 4DN and provided a scientific basis for using nobiletin as a nutraceutical to inhibit inflammation–driven diseases.
Keywords: 4′-demethylnobiletin, nobiletin, anti-inflammation, iNOS, COX-2, NF-κB, Nrf2, AP-1, metabolites
1. Introduction
It is well recognized that there is a strong association of inflammation with various types of chronic diseases such as cancer. Targeting inflammation has been recognized as a promising diet-based strategy for prevention of inflammation-driven chronic diseases. Polymethoxyflavones (PMFs) are unique flavonoids mainly found in citrus fruits. Nobiletin is a major PMF and has been reported to have with a wide range of biological activities, including anti-inflammation (Guo et al., 2012). Accumulating evidence supported the use of nobiletin as an anti-inflammatory functional food ingredient. In vitro studies showed that nobiletin inhibited LPS-induced production of NO and PGE2 in RAW 264.7 macrophages (Guo et al., 2012), and suppressed gene expression of pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α in J774A.1 macrophages (Lin et al., 2003). In vivo studies demonstrated that nobiletin inhibited TPA-induced skin inflammation (Murakami et al., 2000) and suppressed dextran sulfate sodium-induced colitis (Miyamoto, Yasui, Tanaka, Ohigashi, & Murakami, 2008).
Biotransformation plays critical roles in the biological activities of dietary components (Lotito, Zhang, Yang, Crozier, & Frei, 2011; Yang, Cheng, Yang, & Guo, 2012). A compound can be biotransformed to produce metabolites that may exhibit enhanced or reduced biochemical and pharmacological activities. We and others have studied biotransformation of polymethoxyflavones including nobiletin and identified their major metabolites in mice (S. Li et al., 2007; S. Li, Wang, Sang, Huang, & Ho, 2006; Wu et al., 2015; Zheng et al., 2015b; Zheng et al., 2013). After oral administration, nobiletin was transformed to its demethylated metabolites such as 3′-demethylnobiletin, 4′-demethylnobiletin (4DN), and 3′, 4′-didemethylnobiletin. Interestingly, 4DN was the most abundant metabolite, and its abundance was even much higher than that of nobiletin in certain tissues (Wu et al., 2015). Moreover, it has been reported that 4DN had stronger bioactivities than nobiletin such as anti-inflammation and anti-cancer effects (Wu et al., 2015). However, the detailed information on anti-inflammatory effects of 4DN and its molecular mechanisms remains unknown. Therefore, the aim of this study was to determine the inhibitory effects of 4DN on LPS-induced inflammation in RAW 264.7 macrophage cells, and elucidate the underlying molecular mechanisms.
Epidemiological studies and molecular studies have shown that inflammatory conditions increase the risk of malignancy. Chronic inflammation has tumor-promoting effects by various mechanisms including enhancing proliferation of malignant cells, promoting tumor angiogenesis and metastasis, and altering tumor response to chemotherapeutic drugs (Mantovani, Allavena, Sica, & Balkwill, 2008). In tumor cells and inflammatory cells, eukaryotic transcription factor nuclear factor-kappa B (NF-κB) contributes to the activation of genes coding pro-inflammatory enzymes, such as induce inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), which in turn stimulates the expression of nitric oxide (NO) and prostaglandins, respectively. Prostaglandin E2 (PGE2), the major bioactive lipid derived from COX-2 activity, is a key inflammatory mediator that promote tumor growth and metastasis. Similarly, iNOS-mediated overproduction of NO can cause modifications and structure damage of DNA, and promote tumor angiogenesis (Surh et al., 2001). NF-κB also triggers the expression of pro-inflammatory cytokines, such as interleukin-1β (IL-1β). Besides NF-κB, activator protein 1 (AP-1) is another transcription factor that plays an important role in the regulation of the expression of genes responsible for inflammatory responses including iNOS and COX-2 (Guha & Mackman, 2001). Nuclear factor erythroid 2-related factor 2 (Nrf2), a redox-sensitive transcription factor, translocates to the nucleus and triggers the expression of a set of phase II detoxification and antioxidant enzymes including heme oxygenase-1 (HO-1) and NADH quinone oxidoreductase 1 (NQO1) when stimulated by prooxidant stimulus. In contrast to the pro-inflammatory effects of NF-κB and AP-1, Nrf2 was reported to inhibit abnormal inflammatory response (Zhang, 2006), and alleviate adverse effects of LPS-induced NF-κB activation (Thimmulappa et al., 2006). Previous studies have shown that increased HO-1 and NQO1 expression contributed to the adaptive increase of cellular anti-oxidant and anti-carcinogenic capacity (Lee & Surh, 2005). Since Nrf2 and its downstream anti-oxidative enzymes play critical roles in down-regulation of inflammation, they are important targets for anti-inflammation remedy. Herein, we will determine the effects of 4DN on these important signaling molecules to establish its molecular mechanism.
2. Materials and methods
2.1 Cell cultures and treatments
RAW 264.7 cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and were cultured in RPMI-1640 media supplemented with 10% heat-inactivated FBS (Medistech, Herndon, VA, USA), 100 units/mL of penicillin, and 0.1 mg/ mL of streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C with 5% CO2 and 95% air. 4DN was chemically synthesized as previously described (Zheng et al., 2015a; Zheng et al., 2013). Chemical structure of 4DN was illustrated in Figure.1. Dimethylsulfoxide (DMSO) was used as the vehicle to deliver 4DN to the cells. The final concentration of DMSO in all experiments was 0.1% (v/v) in cell culture media.
Figure 1.
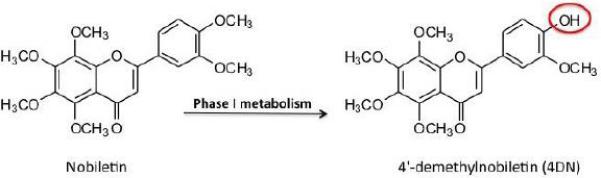
Chemical structures of 4′-demethylnobiletin (4DN), a major metabolite of nobiletin.
2.2 Cell viability and nitric oxide assay
The cell viability was determined as previously described (Charoensinphon et al., 2013; Guo et al., 2012; Qiu et al., 2010; Xiao, Zhang, Lin, Reddy, & Yang, 2008). RAW 264.7 cells were seeded in 96-well plates. After 24 hours, cells were treated with serial concentrations of 4DN in 200 μL of serum complete media. After another 24 hours of treatment, cells were subject to MTT assay. Media were replaced by 100 μL of fresh media containing 0.1 mg/mL of MTT (Sigma-Aldrich, St. Louis, MO, USA). After 2 hours of incubation, MTT-containing media were removed, and the reduced formazan dye was solubilized by adding 100 μL of DMSO to each well. After gentle mixing, the absorbance was monitored at 570 nm using a plate reader (Elx800TM absorbance microplate reader, BioTek Instrument, Winooski, VT, USA). The nitrite concentration in the culture media was measured as an indicator of NO production by the Griess reaction (Guo et al., 2012). The culture media were mixed with an equal volume of Griess reagent A and B (A: 1% sulphanilamide in 5% phosphoric acid, and B: 0.1% naphthylethylenediamine dihydrochloride in water). Absorbance at 540nm was measured by a plate reader, and concentrations of nitrite were calculated according to a standard curve constructed with sodium nitrite as a standard as we previously reported (Guo et al., 2012).
2.3 ELISA for PGE2 and cytokines
RAW 264.7 cells (5 × 106 cells/well) were seeded in 6-well plates. After 24 hours, cells were treated with 1 μg/mL LPS alone or with serial concentrations of 4DN in 2 mL of serum complete media. After another 24 hours of incubation, the culture media were collected and analyzed for PGE2, IL-1β and IL-6 levels by ELISA kits, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).
2.4 Immunoblotting analysis
Whole Cell lysate were prepared as previously described (Charoensinphon et al., 2013; Guo et al., 2012; Qiu et al., 2010; Xiao et al., 2008). RAW 264.7 cells were seeded in 10 mm culture dishes. After 24 h of incubation, cells were treated with 1 μg/mL LPS alone or with serial concentrations of 4DN. After 24 h of incubation, the cells were harvested and extracted with RIPA lysis buffer (Tris-HCl pH 7.2, 25 mM; SDS 0.1%; Triton X-100 1%; sodium deoxycholate 1%; NaCl 0.15 M; ethylenediaminetetraacetic acid (EDTA) 1 mM) (Boston Bioproducts, Ashland, MA, USA) containing 1% protease inhibitor cocktail. The nuclear and cytosolic fractions were prepared using NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Rockford, IL, USA). Protein concentrations were determined using the BCA method (Pierce, Rockford, IL, USA). The cell lysates were further subjected to Western blotting analysis as we previously described(Charoensinphon et al., 2013; Guo et al., 2012; Qiu et al., 2010; Xiao et al., 2008). Antibodies for iNOS, COX-2, p-IκBα, IκBα, PI3K, p-Akt, Akt, p-JNK, JNK, HO-1 and PARP were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies for NF-κB, p50, p65, c-Fos, c-Jun, p-PI3K, NQO1 and Nrf2 were obtained from Santa Cruz (Santa Cruz, CA, USA). Anti β-actin antibody was from Sigma-Aldrich.
2.5 qRT-PCR analysis
Real-Time qRT-PCR analysis was conducted as previously described (Guo et al., 2012). The primer pairs were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA) with the following primers: iNOS F: 5′- TCC TAC ACC ACA CCA AAC-3′, R: 5′- CTC CAA TCT CTG CCT ATC C-3′; COX-2 F: 5′- CCT CTG CGA TGC TCT TCC-3′, R: 5′- TCA CAC TTA TAC TGG TCA AAT CC-3′; HO-1 F: 5′-AAG AGG CTA AGA CCG CCT TC-3′, R: 5′-GTC GTC GTC AGT CAA CAT GG-3′; NQO1 F: 5′-TCG GAG AAC TTT CAG TAC CC-3′, R: 5′-TGC AGA GAG TAC ATG GAG CC-3′; GADPH F: 5′- TCA ACG GCA CAG TCA AGG-3′, R: 5′- ACT CCA CGA CAT ACT CAG C-3′. At least three independent experiments were carried out, and triplicate samples were used for each treatment. The copy number of each transcript was calculated with respect to the GADPH copy number, using the 2−ΔΔCt method.
2.6 Statistical analysis
Data were presented as means ± SD for the indicated number of independently performed experiments. Student's t-test was used to assess the mean difference between treatment groups and the control group. A p-value <0.05 was considered statistically significant.
3. Results
3.1 Inhibition of LPS-induced production of NO, PGE2, IL-1β and IL-6 by 4DN
To investigate the anti-inflammatory effect of 4DN in LPS-stimulated macrophage cells, we firstly established nontoxic dose range of 4DN in RAW 264.7 cells. As shown in Figure 2A, 4DN at up to 50 μM showed no effects on the viability of RAW 264.7 cells. Using the nontoxic dose range established, we further examined the effects of 4DN on LPS-induced production of inflammation mediators, namely NO, PGE2, IL-1β and IL-6. As shown in Figure 2B, the production of NO was significantly increased by LPS (1 μg/mL) treatment by 12.1-fold in macrophages. Treatment with 4DN dose-dependently inhibited LPS-induced NO production by 90-100%, at a dose range of 10 - 50 μM. Furthermore, we determined the effects of 4DN on LPS-induced production of PGE2, IL-1β and IL-6 by using ELISA assay. The LPS treatment resulted in significant increases in the concentration of PGE2, IL-1β and IL-6 in the cell culture media (Figure 2C). Treatments with 4DN at 30 μM suppressed the levels of PGE2 by 98%, as compared to the LPS-treated positive control cells. Similarly, treatments with 4DN at 30 μM decreased the production of pro-inflammatory cytokines by 92% (for IL-1β), and 99% (for IL-6).
Figure 2.
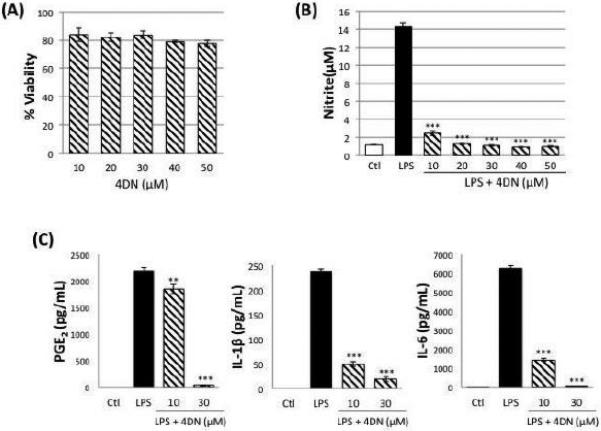
(A) Cytotoxicity profile of 4DN in RAW 264.7 macrophages. Macrophages were seeded in 96-well plates. After 24 hours, cells were treated with serial concentrations of 4DN. After another 24 hours of incubation, cells were subject to MTT assay for viability. The viability of control cells was set as the reference with a value of 100%. Results were presented as mean ± SD from six replicates (n = 6). No significant difference was found between any treatment and the control group.
(B) Effects of 4DN on LPS-induced NO production in RAW 264.7 macrophages. The cells were treated with LPS (positive control) or LPS plus serial concentrations of 4DN as indicated in the bar graph. After 24 hours of incubation, the culture media were collected to determine the levels of NO as described in the Method section. Results were presented as mean ± SD from six replicates (n = 6). All treatments showed statistical significance in comparison with the LPS-treated positive control. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate statistically significant differences from the LPS-treated positive control group.
(C) Effects of 4DN on LPS-induced production of PGE2, IL-1β and IL-6 in RAW 264.7 macrophages. The cells were treated with LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 hours of incubation, the culture media were collected and analyzed for protein levels of PGE2, IL-1β and IL-6 by ELISA. Results were presented as mean ± SD from six replicates (n = 6). *p < 0.05, **p < 0.01, and ***p < 0.001 indicate statistically significant differences from the LPS-treated positive control group.
3.2 Inhibition of LPS-induced iNOS and COX-2 gene expression by 4DN
To investigate the molecular mechanism by which 4DN inhibits the production of aforementioned inflammation mediators, we next determined the effects of 4DN on the protein levels of iNOS and COX-2 by immunoblotting analysis. We found that 4DN at 10 and 30 μM significantly decreased protein levels of iNOS by 69 and 88%, respectively, when compared with LPS-treated positive control (Figure 3A). Similarly, 4DN at 10 and 30 μM suppressed the protein levels of COX-2 by 54% and 91%, respectively. We further examined the mRNA levels of iNOS and COX-2 using qRT-PCR technique. As shown in Figure 3B, RAW 264.7 macrophages expressed very low levels of iNOS and COX-2 mRNA without LPS stimulation. After 24 hours of LPS treatment, the mRNA levels of iNOS and COX-2 significantly elevated by 408- and 153-fold, respectively, which is in accordance with the protein levels of iNOS and COX-2 shown in Figure 3A. The results further showed that 4DN significantly reduced the mRNA levels of both iNOS and COX-2 in a dose-dependent manner. Treatments with 4DN at 30 μM resulted in a decrease on mRNA levels of iNOS by 83%. Similarly, 4DN at 30 μM suppressed mRNA level of COX-2 by 81%. The effects of 4DN on the gene expression levels of iNOS and COX-2 were consistent with their effects on the protein levels of iNOS and COX-2.
Figure 3.
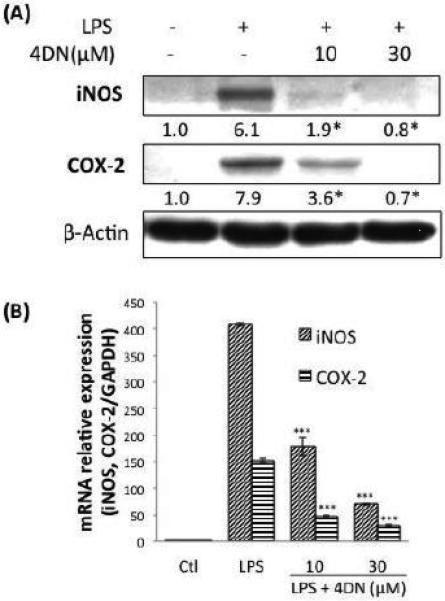
Inhibitory effects of 4DN on LPS-induced protein and gene expression of iNOS and COX-2 in RAW 264.7 macrophages. (A) The cells were seeded in 10-cm dishes for 24 h, and then treated with LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 hours of incubation, cells were harvested for immunblotting as described in Methods. The numbers underneath of the blots represent band intensity (normalized to β-actin, means of 3 independent experiments) measured by Image J software. The standard deviations (all within ±15% of the means) were not shown. β-Actin was served as an equal loading control. *indicates statistical significant difference compared to the LPS-treated positive control group (p < 0.05, n = 3). (B) The cells were treated with LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 hours of incubation, total RNA was subjected to quantitative real-time RT-PCR as described in Methods section. The RT products were labeled with SYBR Green dye. Relative iNOS and COX-2 mRNA expression (2−ΔΔCt) was determined by real-time PCR and calculated by the Ct value for iNOS and COX-2 from GAPDH mRNA. ΔΔCt=(Ct target gene-Ct GAPDH) –(Ct control-Ct GAPDH). Each value represents the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate statistically significant differences compared to the LPS-treated positive control group.
3.3 Inhibition on the activation of NF-κB by 4DN
Activation and nuclear translocation of NF-κB plays central roles in mediating inflammation, and it is involved in regulation of iNOS and COX-2 gene expression. Since 4DN can inhibit iNOS and COX-2 on transcriptional levels, we next investigated the inhibitory effect of 4DN on the activation and nuclear/cytosolic levels of p65 and p50, the functional subunits of NF-κB. As shown in Figure 4A, we found that LPS treatment caused nuclear translocation of both p65 and p50, as evidenced by the increased nuclear accumulation of p65 and p50, and their decreased cytosolic levels after LPS stimulation. Treatments with 4DN significantly and dose-dependently inhibited LPS-induced nuclear translocation of both p65 and p50, which was evidenced by lower nuclear levels of p65 and p50, and higher cytosolic levels of p65 and p50 (Figure 3A) in comparison with those found in LPS-treated positive control cells. In the unstimulated state, NF-κB binds to IκBα, which sequesters NF-κB in an inactive cytoplasmic complex (Mantovani et al., 2008). Upon stimulation, phosphorylation of IκBα can occur, which results in dissociation of IκBα from NF-κB, and activated NF-κB can then be translocated into the nucleus where it acts as a transcription factor to initiate pro-inflammatory responses. We examined the expression and phosphorylation levels of IκBα protein and found that LPS treatment significantly increased phosphorylation of IκBα. Treatments with 4DN dose-dependently suppressed the phosphorylation levels of IκBα. Treatments with LPS or 4DN did not change the expression levels of IκBα.
Figure 4.
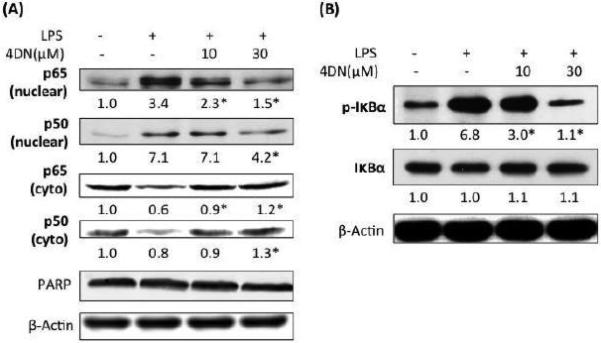
Inhibitory effects of 4DN on LPS-induced NF-κB activation in RAW 264.7 macrophages. The cells were seeded in 10-cm dishes for 24 hours, and then treated with LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 hours of incubation, cytoplasmic and nuclear fractions were prepared for immunblotting as described in Methods. The numbers underneath of the blots represent band intensity that was normalized to PARP (nuclear fraction) or β-actin (cytosolic fraction) and measured by Image J software (means of three independent experiments). The standard deviations (all within ±15% of the means) were not shown. PARP and β-Actin were served as an equal loading control for nuclear fraction and cytosolic fraction, respectively. *indicates statistical significance from the LPS-treated group (p < 0.05, n = 3).
3.4 Inhibition on the activation of AP-1, JNK, PI3K and Akt by 4DN
Besides NF-κB, AP-1 is another critical transcription factor whose activation is involved in the pro-inflammatory responses (Guha & Mackman, 2001). We examined the effects of 4DN on the levels of c-Jun and c-Fos, the key components of AP-1 protein complex. As shown in Figure 5, LPS treatment increased nuclear levels of c-Jun and c-Fos, indicating nuclear accumulation of AP-1 and hence its activation. LPS treatment also increased the cytosolic level of c-Jun, but not that of c-Fos. Treatments with 4DN dose-dependently decreased nuclear levels of c-Jun and c-Fos, as well as cytosolic levels of c-Jun and c-Fos.
Figure 5.
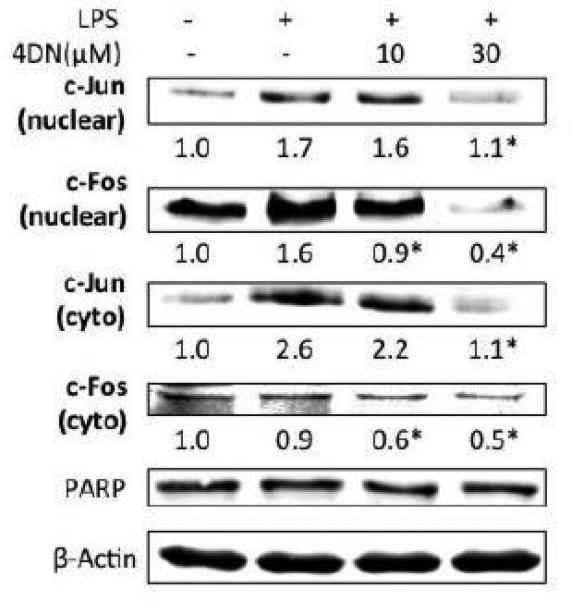
Inhibitory effects of 4DN on LPS-induced AP-1 activation in RAW 264.7 macrophages. The cells were seeded in 10-cm dishes for 24 h, and then treated with 1 μg/mL of LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 hours of incubation, cytoplasmic and nuclear fractions were prepared for immunoblotting as described in Methods. The numbers underneath of the blots represent band intensity that was normalized to PARP (nuclear fraction) or β-actin (cytosolic fraction) and measured by Image J software (means of three independent experiments). The standard deviations (all within ±15% of the means) were not shown. PARP and β-Actin were served as an equal loading control for nuclear fraction and cytosolic fraction, respectively. *indicates statistical significance from the LPS-treated group (p < 0.05, n = 3).
The activation of JNK MAP kinase was found to be involved in the upregulation of iNOS and COX-2 gene expression(Chiang et al., 2005). Since phosphorylation is essential in the activation of JNK, we examined the effect of 4DN on the phosphorylation of JNK. As shown in Figure 6, LPS treatment showed marginal effects on the phosphorylation of JNK, and 4DN at 30 μM significantly decreased phosphorylation of JNK. PI3K/Akt pathway is another signaling pathway whose activation has been found to be associated with pro-inflammatory effects such as up-regulation of the transcriptional activity of NF-κB (Lai et al., 2008). We examined the phosphorylation levels of PI3K and Akt. The results showed that 4DN showed moderate inhibitory effects on the phosphorylation of PI3K, but had potent inhibitory effects on the phosphorylation of Akt. For example, 5HN at 30 μM decreased the phosphorylation level of Akt by 90%. 4DN treatments had no effects on the protein levels of JNK, PI3K, or Akt.
Figure 6.
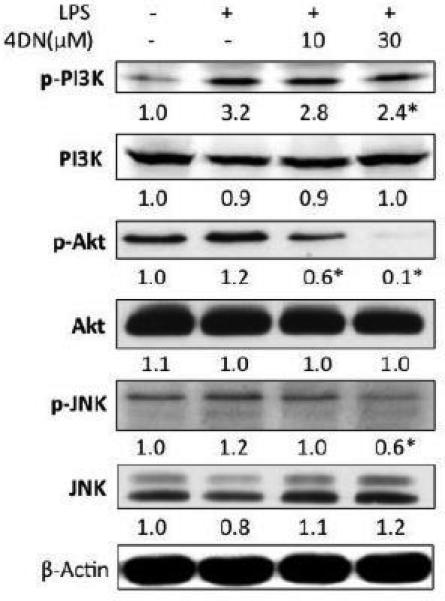
Inhibitory effects of 4DN on phosphorylation of PI3K, Akt and JNK in RAW 264.7 macrophages. The cells were seeded in 10-cm dishes for 24 hours, and then treated with LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 hours of incubation, cells were prepared for immunoblotting as described in Methods. The numbers underneath of the blots represent band intensity that was normalized β-actin and measured by Image J software (means of three independent experiments). The standard deviations (all within ±15% of the means) were not shown. β-Actin were served as an equal loading control. *indicates statistical significance from the LPS-treated group (p < 0.05, n = 3).
3.6 Induction of anti-oxidative enzymes by 4DN
HO-1 and NQO1 are anti-oxidative enzymes that are associated with anti-inflammatory effects (Guo et al., 2012; Lyu et al., 2012). We determined the effect of 4DN on the protein and gene expression of HO-1 and NQO1. As shown in Figure 7A, treatments with 4DN at 10 and 30 μM significantly increased the protein levels of HO-1 by 2.0 and 2.12-folds, respectively, when compared with LPS-treated positive control cells. Similarly, the protein levels of NQO1 were also increased by 4DN treatment as compared to the LPS-treated positive control group. Next, we analyzed the mRNA levels of HO-1 and NQO1 using qRT-PCR technique (Figure 7B). It was found that 4DN treatments does-dependently and significantly elevated the mRNA expressions of HO-1 and NQO1, which is in accordance with the effects of 4DN on the protein levels of HO-1 and NQO1. Treatment with 4DN at 30 μM resulted in an increase on mRNA level of HO-1 by 72% in comparison with the LPS-treated positive control cells. Treatments with 4DN at 30 μM enhanced mRNA level of NQO1 by 76%, compared to the LPS-treated positive control cells. The results demonstrated that 4DN treatment induced gene and protein expression of HO-1 and NQO1.
Figure 7.
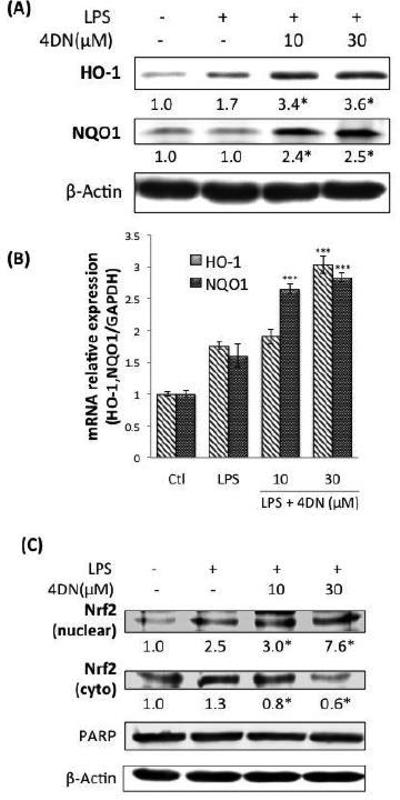
Effects of 4DN on protein and gene expression of anti-oxidative enzymes HO-1 and NQO1 in LPS-stimulated RAW 264.7 macrophages. The cells were seeded in 10-cm dishes for 24 hours, and then treated with LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 h of incubation, cells were harvested for western immunoblotting as described in Methods. The numbers underneath of the blots represent band intensity (normalized to β-actin, means of 3 independent experiments) measured by Image J software. The standard deviations (all within ±15% of the means) were not shown. β-Actin was served as an equal loading control. *indicates statistical significance from the LPS-treated positive control group (p < 0.05, n = 3). (B) The cells were treated with LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 h of incubation, total RNA was subjected to quantitative real-time RTPCR. Relative HO-1 and NQO1 mRNA expression (2−ΔΔCt) was determined by real-time PCR and calculated by the Ct value for HO-1 and NQO1 from GAPDH mRNA. Each value represents the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate statistically significant differences from the LPS-treated positive control group. (C) Effects of 4DN on nucler translocation of Nrf2 in RAW 264.7 macrophages. The cells were seeded in 10-cm dishes for 24 h, and then treated with LPS or LPS plus 4DN at concentrations indicated in the figure. After 24 hours of incubation, cytoplasmic and nuclear fractions were prepared for western immunoblotting as described in Methods. The numbers underneath of the blots represent band intensity (normalized to PARP or β-actin, means of three independent experiments) measured by Image J software. The standard deviations (all within ±15% of the means) were not shown. PARP and β-Actin were served as an equal loading control. *indicates statistical significance from the LPS-treated group (p < 0.05, n = 3).
Previous reports showed that the transcription factor Nrf2 mediates the transcriptional activation of many anti-oxidative genes. For example, activation and nuclear translocation of Nrf2 plays a key role in the induction of HO-1 and NQO1 (Lee & Surh, 2005; Zhang, 2006). To elucidate the mechanisms underlying the induction of HO-1 and NQO1 elicited by 4DN, we determined the effects of 4DN on the protein levels Nrf2. The protein levels of Nrf2 in nuclear and cytosolic fractions were determined after treatment with 4DN by immunoblotting analysis. As shown in Figure 7C, 4DN significantly enhanced the protein level of Nrf2 in nuclear fraction in comparison with LPS-treated positive control cells, and these effects were in a dose-dependent fashion. Meanwhile, the protein level of Nrf2 in cytosolic fraction was decreased by 4DN treatment. The results suggested that 4DN treatment resulted in nuclear translocation of Nrf2, which in turn induced expression of Nrf2 dependent genes such as HO-1 and NQO1.
4. Discussion
Biotransformation is critical factor that may profoundly influence the biological effects of orally ingested bioactive compounds such as nutraceuticals (Yang et al., 2012). Many nutraceuticals undergo extensive metabolism in the gastrointestinal tract and the liver to be transformed to various metabolites. These metabolites may elicit significant biological effects depending on their chemical structures and abundance in the specific tissues. As a major polymethoxyflavone found in citrus fruits, nobiletin has been shown to have various beneficial health effects. Several metabolites of nobiletin have been identified in in vivo studies (S. Li et al., 2006; Wu et al., 2015; Zheng et al., 2015b; Zheng et al., 2013). Our recent study revealed that 4DN was the most abundant colonic metabolite of nobiletin after long-term dietary administration of nobiletin in mice (Wu et al., 2015). Furthermore, it is noteworthy that the level of 4DN in the colonic mucosa was about 12-fold higher that that of nobiletin (Wu et al., 2015). These findings suggested that due to its high abundance, 4DN might play important roles in the biological effects elicited by dietary treatment with nobiletin. Inflammation is associated with multiple diseases, and anti-inflammation is considered as a promising strategy to the prevention of inflammation-driven diseases. Therefore, it is meaningful to determine the anti-inflammatory effects of 4DN. The main objective of this study is to establish the inhibitory effects of 4DN on LPS-induced inflammation and elucidate the underlying molecular mechanisms.
Our results demonstrated that 4DN (at 10 and 30 μM) effectively suppressed LPS-induced inflammation by down-regulating iNOS, COX-2, PGE2, IL-1β and IL-6, inhibiting the activation of NF-κB, AP-1, JNK MAP kinase, and PI3K/Akt pathways, and up-regulating Nrf2-regulated anti-oxidative genes such as NQO-1 and HO-1. These findings are physiologically relevant to the in vivo situation because 10 and 30 μM of 4DN are achievable in animal tissues after oral administration of nobiletin. This was based on our previous finding that long term (20 weeks) feeding of mice with nobiletin (500 ppm in diet) resulted in 20-30 μM of 4DN in the colonic mucosa (Wu et al., 2015). Inflammation plays important roles in promoting colon carcinogenesis, and previous studies have showed that dietary administration of nobiletin suppressed colon carcinogenesis in rodent (Miyamoto et al., 2008; Suzuki et al., 2004; Tang et al., 2011). Therefore, based on our results, it is reasonable to argue that 4DN as a major colonic metabolite of nobiletin may significantly contributed to the anti-colon carcinogenesis effects of nobiletin by eliciting anti-inflammatory effects in the colon.
As NF-κB and AP-1 are critical transcription factors that regulate inflammatory pathways (Guha & Mackman, 2001; Mantovani et al., 2008), we determined the effects of 4DN on those transcription factors in LPS-stimulated macrophage cells. Nuclear translocation of NF-κB is known to play a crucial role in the regulation of genes coding many pro-inflammatory enzymes and cytokines, including iNOS and COX-2 (Surh et al., 2001). Our results showed that LPS caused increased level of phosphorylation of IκBα, which led to dissociation of IκBα from NF-κB in the cytosol (Figure 4). Freed NF-κB can then be translocated to nucleus and elicit its transcriptional activity. Treatment of 4DN effectively inhibited LPS-induced phosphorylation of IκBα, which increased anchorage of NF-κB in the cytosol (Figure 4). This in turn deceased nuclear translocation of NF-κB. Nuclear translocation of AP-1, induced by a number of stimuli, including cytokines, growth factors and infections, is also crucial in regulating the induction of iNOS and COX-2 (Guha & Mackman, 2001). Our results indicated that 4DN dose-dependently inhibited LPS-induced nuclear translocation of key subunits of AP-1, namely c-Jun and c-Fos (Figure 5). Interestingly, the cytosolic levels of c-Jun and c-Fos were also significantly decreased by 4DN treatments. This result suggested that in addition to the inhibition on nuclear translocation of AP-1, 4DN might also promote degradation of AP-1 subunits in the cytosol. The inhibitory effects of 4DN on NF-κB and AP-1 were consistent with the inhibitory effect of 4DN on the gene transcription of iNOS and COX-2 (Figure 3). Furthermore, the activation of NF-κB and AP-1 has been proposed to be mediated by many signaling pathways, including PI3K/Akt and JNK pathways (Chiang et al., 2005; Johnson & Lapadat, 2002; Pan & Ho, 2008). Our results demonstrated that 4DN suppressed phosphorylation levels of JNK, PI3K and Akt. Taken together, our results suggest that 4DN exerts its anti-inflammatory effects by suppressing NF-κB and AP-1 dependent pro-inflammatory gene expression.
A growing number of studies have demonstrated that increased levels of anti-oxidative enzymes, such as HO-1 and NQO1, play important roles in inhibiting LPS-induced inflammation in RAW 264.7 cells (Guo et al., 2012; Lyu et al., 2012). HO-1 can promote the production of antioxidants such as bilirubin and carbon monoxide (CO); further leads to decreased iNOS and COX-2 enzymatic activities (Suh, Jin, Yi, Wang, & Choi, 2006; Thorup, Jones, Gross, Moore, & Goligorsky, 1999). Overexpression of NQO1 has been shown to inhibit LPS-induced TNF and IL-1β expression through an NF-κB-independent pathway (Rushworth, MacEwan, & O'Connell, 2008). Nrf2 is a key transcription factor that mediates the gene expression of a number of enzymes, including HO-1, NQO1, Glutamate-cysteine ligase, catalytic (Gclc), glutamate-cysteine ligase, modifier (GCLM) and glutathione S-transferase (GST) family. It has been reported that activation and nuclear translocation of Nrf2 can protect abnormal inflammatory response by inducing cellular rescue pathways and relieve adverse effects of LPS-induced NF-κB activity (Thimmulappa et al., 2006; Zhang, 2006). Furthermore, Nrf2 has been shown to be protective against various inflammatory diseases, such as acute lung inflammation (Lyu et al., 2012), colorectal cancer (Pandurangan, Saadatdoust, Mohd Esa, Hamzah, & Ismail, 2015), cardiovascular disease (J. Li, Ichikawa, Janicki, & Cui, 2009) and diabetic retinopathy (Xu et al., 2014). Therefore, modification of Nrf2 activity has been considered as a rational approach for chemoprevention of inflammation-driven diseases. Our results demonstrated that 4DN dose-dependently increased nuclear translocation of Nrf2, which in turn led to increased expression of HO-1 and NQO1 at both mRNA and protein levels (Figure 7). These findings suggested that induction of HO-1 and NQO1 via the activation of Nrf2 contributed to the inhibitory effect of 4DN on LPS-induced inflammation in RAW 264.7 macrophages.
5. Conclusion
The 4DN, a major metabolite of nobiletin, at physiologically achievable concentrations, effectively inhibited LPS-induced inflammatory responses in RAW 264.7 macrophages, such as production of NO and gene expression of iNOS and COX-2. These effects were associated with suppression of nuclear translocation of NF-κB and AP-1 transcription factors. Furthermore, for the first time, we demonstrated that the anti-inflammatory effects of 4DN were associated with its ability to up-regulate the gene expression of anti-oxidative enzymes HO-1 and NQO1 by promoting the nuclear translocation of Nrf2. This study provided a scientific basis for using nobiletin as a nutraceutical to prevent inflammation-driven diseases in human.
Highlights.
4′-demethylnobiletin (4DN) dose-dependently inhibited lipopolysaccharide (LPS)-induced nitric oxide production in macrophages.
4DN inhibited reduced LPS-induced expression of pro-inflammatory proteins, namely iNOS, COX-2, PGE2, IL-1β and IL-6.
4DN inhibited LPS-induced nuclear translocation of NF-κB and AP-1.
4DN activated transcription factor Nrf2 and its dependent genes including HO-1 and NQO1.
Acknowledgments
This work was partially supported by a NIH grant (CA139174), an AICR grant, and a USDA Special Grant on bioactive food components.
Abbreviations
- 4DN
4′-demethylnobiletin
- LPS
lipopolysaccharide
- iNOS
inducible nitric oxide synthase
- COX-2
cyclooxygenase-2
- NF-κB
nuclear factor-κB
- AP-1
activator protein 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- HO-1
heme oxygenase-1
- NQO1
NADH quinone oxidoreductase 1
- PMF
polymethoxyflavone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared no conflict of interest.
References
- Charoensinphon N, Qiu P, Dong P, Zheng J, Ngauv P, Cao Y, Xiao H. 5-Demethyltangeretin inhibits human nonsmall cell lung cancer cell growth by inducing G2/M cell cycle arrest and apoptosis. Mol Nutr Food Res. 2013 doi: 10.1002/mnfr.201300136. doi: 10.1002/mnfr.201300136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YM, Lo CP, Chen YP, Wang SY, Yang NS, Kuo YH, Shyur LF. Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br J Pharmacol. 2005;146(3):352–363. doi: 10.1038/sj.bjp.0706343. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Guo S, Qiu P, Xu G, Wu X, Dong P, Yang G, Xiao H. Synergistic anti-inflammatory effects of nobiletin and sulforaphane in lipopolysaccharide-stimulated RAW 264.7 cells. J Agric Food Chem. 2012;60(9):2157–2164. doi: 10.1021/jf300129t. doi: 10.1021/jf300129t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Lai CS, Li S, Chai CY, Lo CY, Dushenkov S, Ho CT, Wang YJ. Anti-inflammatory and antitumor promotional effects of a novel urinary metabolite, 3′,4′-didemethylnobiletin, derived from nobiletin. Carcinogenesis. 2008;29(12):2415–2424. doi: 10.1093/carcin/bgn222. doi: 10.1093/carcin/bgn222. [DOI] [PubMed] [Google Scholar]
- Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224(2):171–184. doi: 10.1016/j.canlet.2004.09.042. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009;13(7):785–794. doi: 10.1517/14728220903025762. doi: 10.1517/14728220903025762. [DOI] [PubMed] [Google Scholar]
- Li S, Sang S, Pan MH, Lai CS, Lo CY, Yang CS, Ho CT. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg Med Chem Lett. 2007;17(18):5177–5181. doi: 10.1016/j.bmcl.2007.06.096. doi: 10.1016/j.bmcl.2007.06.096. [DOI] [PubMed] [Google Scholar]
- Li S, Wang Z, Sang S, Huang MT, Ho CT. Identification of nobiletin metabolites in mouse urine. Mol Nutr Food Res. 2006;50(3):291–299. doi: 10.1002/mnfr.200500214. doi: 10.1002/mnfr.200500214. [DOI] [PubMed] [Google Scholar]
- Lin N, Sato T, Takayama Y, Mimaki Y, Sashida Y, Yano M, Ito A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol. 2003;65(12):2065–2071. doi: 10.1016/s0006-2952(03)00203-x. [DOI] [PubMed] [Google Scholar]
- Lotito SB, Zhang WJ, Yang CS, Crozier A, Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51(2):454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu JH, Kim KH, Kim HW, Cho SI, Ha KT, Choi JY, Joo M. Dangkwisoo-san, an herbal medicinal formula, ameliorates acute lung inflammation via activation of Nrf2 and suppression of NF-kappaB. J Ethnopharmacol. 2012;140(1):107–116. doi: 10.1016/j.jep.2011.12.043. doi: 10.1016/j.jep.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Yasui Y, Tanaka T, Ohigashi H, Murakami A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis. 2008;29(5):1057–1063. doi: 10.1093/carcin/bgn080. doi: 10.1093/carcin/bgn080. [DOI] [PubMed] [Google Scholar]
- Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, Ohigashi H. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000;60(18):5059–5066. [PubMed] [Google Scholar]
- Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008;37(11):2558–2574. doi: 10.1039/b801558a. doi: 10.1039/b801558a. [DOI] [PubMed] [Google Scholar]
- Pandurangan AK, Saadatdoust Z, Mohd Esa N, Hamzah H, Ismail A. Dietary cocoa protects against colitis-associated cancer by activating the Nrf2/Keap1 pathway. Biofactors. 2015;41(1):1–14. doi: 10.1002/biof.1195. doi: 10.1002/biof.1195. [DOI] [PubMed] [Google Scholar]
- Qiu P, Dong P, Guan H, Li S, Ho CT, Pan MH, Xiao H. Inhibitory effects of 5-hydroxy polymethoxyflavones on colon cancer cells. Mol Nutr Food Res. 2010;54(Suppl 2):S244–252. doi: 10.1002/mnfr.200900605. doi: 10.1002/mnfr.200900605. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, MacEwan DJ, O'Connell MA. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J Immunol. 2008;181(10):6730–6737. doi: 10.4049/jimmunol.181.10.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GY, Jin Y, Yi AK, Wang XM, Choi AM. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol. 2006;35(2):220–226. doi: 10.1165/rcmb.2005-0154OC. doi: 10.1165/rcmb.2005-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480-481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Kohno H, Murakami A, Koshimizu K, Ohigashi H, Yano M, Tanaka T. Citrus nobiletin inhibits azoxymethane-induced large bowel carcinogenesis in rats. Biofactors. 2004;22(1-4):111–114. doi: 10.1002/biof.552210121. [DOI] [PubMed] [Google Scholar]
- Tang MX, Ogawa K, Asamoto M, Chewonarin T, Suzuki S, Tanaka T, Shirai T. Effects of nobiletin on PhIP-induced prostate and colon carcinogenesis in F344 rats. Nutr Cancer. 2011;63(2):227–233. doi: 10.1080/01635581.2011.523506. doi: 10.1080/01635581.2011.523506. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351(4):883–889. doi: 10.1016/j.bbrc.2006.10.102. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277(6 Pt 2):F882–889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- Wu X, Song M, Wang M, Zheng J, Gao Z, Xu F, Xiao H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol Nutr Food Res. 2015 doi: 10.1002/mnfr.201500378. doi: 10.1002/mnfr.201500378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Zhang Q, Lin Y, Reddy BS, Yang CS. Combination of atorvastatin and celecoxib synergistically induces cell cycle arrest and apoptosis in colon cancer cells. Int J Cancer. 2008;122(9):2115–2124. doi: 10.1002/ijc.23315. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung ER, Duh EJ. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014;57(1):204–213. doi: 10.1007/s00125-013-3093-8. doi: 10.1007/s00125-013-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Cheng C, Yang J, Guo DA. Metabolite profiling and characterization for medicinal herbal remedies. Curr Drug Metab. 2012;13(5):535–557. doi: 10.2174/1389200211209050535. [DOI] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38(4):769–789. doi: 10.1080/03602530600971974. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zheng J, Bi J, Johnson D, Sun Y, Song M, Qiu P, Xiao H. Analysis of 10 Metabolites of Polymethoxyflavones with High Sensitivity by Electrochemical Detection in High-Performance Liquid Chromatography. J Agric Food Chem. 2015a doi: 10.1021/jf505545x. doi: 10.1021/jf505545x. [DOI] [PubMed] [Google Scholar]
- Zheng J, Bi J, Johnson D, Sun Y, Song M, Qiu P, Xiao H. Analysis of 10 metabolites of polymethoxyflavones with high sensitivity by electrochemical detection in high-performance liquid chromatography. J Agric Food Chem. 2015b;63(2):509–516. doi: 10.1021/jf505545x. doi: 10.1021/jf505545x. [DOI] [PubMed] [Google Scholar]
- Zheng J, Song M, Dong P, Qiu P, Guo S, Zhong Z, Xiao H. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Mol Nutr Food Res. 2013;57(11):1999–2007. doi: 10.1002/mnfr.201300211. doi: 10.1002/mnfr.201300211. [DOI] [PubMed] [Google Scholar]


