Abstract
The maize abscisic acid responsive protein Rab17 is a highly phosphorylated late embryogenesis abundant protein involved in plant responses to stress. In this study, we provide evidence of the importance of Rab17 phosphorylation by protein kinase CK2 in growth-related processes under stress conditions. We show the specific interaction of Rab17 with the CK2 regulatory subunits CK2β-1 and CK2β-3, and that these interactions do not depend on the phosphorylation state of Rab17. Live-cell fluorescence imaging of both CK2 and Rab17 indicates that the intracellular dynamics of Rab17 are regulated by CK2 phosphorylation. We found both CK2β subunits and Rab17 distributed over the cytoplasm and nucleus. By contrast, catalytic CK2α subunits and a Rab17 mutant protein (mRab17) that is not a substrate for CK2 phosphorylation remain accumulated in the nucleoli. A dual-color image shows that the CK2 holoenzyme accumulates mainly in the nucleus. The importance of Rab17 phosphorylation in vivo was assessed in transgenic plants. The overexpression of Rab17, but not mRab17, arrests the process of seed germination under osmotic stress conditions. Thus, the role of Rab17 in growth processes is mediated through its phosphorylation by protein kinase CK2.
The plant hormone abscisic acid (ABA) plays a major role in adaptation to osmotic stress and induces a number of genes that encode proteins generally assumed to be involved in protecting the cell and promoting recovery from stress. Proteins responsive to ABA accumulate during seed maturation; they naturally disappear during seed germination and can be induced to reappear by ABA treatment or osmotic stress in vegetative tissues (1, 2).
Late embryogenesis abundant proteins (Lea) from group 2, responsive to ABA (Rab), or dehydrins are among the most common plant proteins involved in adaptation to water or osmotic stress. Several hypothetical roles have been proposed for Rab/dehydrin proteins based on different experimental evidence, including binding to phosphate or sulfate ions (3), nuclear localization signal (NLS) peptides (4), calcium (5), and lipid vesicles containing acidic phospholipids (6), among others. All are aimed toward a protective role as chaperones to stabilize molecules or structures under stress conditions (4, 7, 8).
Many proteins included in this Lea family contain the S domain (8), consisting of a tract of serines with several phosphorylation sites (9). Maize Rab17 protein is one of the most heavily phosphorylated proteins in mature embryos and is found both in nucleus and cytoplasm (4). The S domain of Rab17 is followed by a protein kinase CK2 phosphorylation consensus site (9). We previously established that Rab17 is phosphorylated by CK2 in serine residues of the S domain (10). Phosphorylation/dephosphorylation is an important mechanism that may regulate the function of Rab17 in the cell; however, the actual physiological function for Rab/dehydrin proteins is still unknown, and a precise understanding of the function of Rab17 and the importance of its phosphorylation by CK2 is not yet available.
Protein kinase CK2 is a multifunctional enzyme (reviewed in refs. 11 and 12) composed of two types of subunits, the catalytic CK2α subunits and the regulatory CK2β subunits, which tetramerize to adopt an αββα structure. There is increasing evidence for specific functions of the individual subunits themselves apart from those of the holoenzyme (13, 14). We have demonstrated the existence and functionality of the CK2 holoenzyme in maize: there is a constitutive expression of the three CK2α catalytic subunits, whereas the three CK2β regulatory subunits are differently expressed during the embryo developmental stages. Moreover, we have also shown the existence of preferential interactions between the CK2α/β and CK2β/β isoforms (15, 16). Biochemical data suggest a high heterogeneity in maize CK2 that may affect both interactions with substrates and the holoenzyme structure and function (17). The structure of the plant CK2 holoenzyme has not yet been determined, but it will likely be different from that found in other organisms, because plant CK2β regulatory subunits contain an N-terminal extension whose functionality is still not defined. CK2 has been implicated in the response to physiological stress. Heat treatment induces relocalization of CK2 in eukaryotic cells (18). In yeast, saline hypersensitivity is associated with the lack of CK2β regulatory subunits (19, 20), and an increase in salt tolerance as a consequence of the overexpression of plant CK2α (21) and CK2β (16) has been reported. Moreover, CK2 has been involved in vital processes such as cell cycle (22) and cell survival (12, 23). However, the biological function of CK2 in stress situations remains poorly understood.
In the present work, we address the question of how Rab17 phosphorylation is implicated in cellular responses to osmotic stress. Toward this end, we provide evidence of the mechanistic, functional, and biological role of CK2 phosphorylation of Rab17 in the cell. Imaging of cells expressing the GFP-fusion protein revealed differential dynamics and specific localization of CK2α/β subunits and Rab17 protein in cell compartments. We show that CK2 and Rab17 protein associate as a functional molecular complex. Finally, a comparison of the constitutive overexpression of Rab17 and mRab17 (a mutated version of Rab17 carrying a disruption of its CK2 phosphorylation consensus site) in transgenic Arabidopsis plants indicates a function for the phosphorylated Rab17 during seed germination. Ours findings point to a role of Rab17 modulated by CK2 phosphorylation in growth processes during stress conditions.
Materials and Methods
Transient Onion Transformation. Rab17, mRab17, all three CK2α, and CK2β-3 cDNAs were cloned in the ppk100 vector containing a double CamV 35S promoter. The cDNAs were amplified by PCR and fused in the 3′ region with the GFP gene. The CK2β-3 cDNA was digested and also cloned in the pGJ1425 vector containing the RFP gene. CK2β-1 and CK2β-2 cDNA were cloned in pCAMBIA1302 under the control of a CamV 35S promoter and fused in the 3′ region with the GFP. Transformation of onion epidermal monolayer cells was performed as described (24). After 24 h, samples were visualized by a Leica TCS SP confocal laser-scanning microscope (Leica, Heidelberg).
Two-Hybrid Interaction Assays. For the two-hybrid assays, delCK2β-1, a truncated version of CK2β-1 lacking the first 80 amino acids in the N-terminal region, was amplified by PCR and cloned into the pGBT9 vector (Clontech). Rab17 and mRab17 cDNAs were cloned into pGAD424 vector (Clontech). All of the other CK2 constructs used have been described (16). For interaction studies, plasmids containing fusion proteins were cotransformed into Saccharomyces cerevisiae AH109 and selected on medium lacking -Leu-Trp-His-Ade. β-Galactosidase activity filter assays were done according to the manufacturer (Clontech).
Recombinant Protein Purification, Protein Extraction, and Phosphorylation Assays. Recombinant proteins Rab17 and mRab17 [a mutated version in the CK2 phosphorylation consensus site, previously described (25), where amino acids EDD in position 85-87 have been changed to AAA by direct mutagenesis] were expressed and purified as His-tag fusion proteins according to the pET system manual (Novagen). In vitro phosphorylation assays as well as purification of CK2 holoenzymes/subunits and total protein extracts were performed as described (16, 17). Phosphorylation of maize extracts was carried out by mixing 200 μg of protein extracts (60 and 15 days after pollination) with CK2 buffer (16). In some lanes, 1 μg of recombinant Rab17 or mRab17 was added. Apigenin (100 μM) was used to inhibit CK2 activity in the extracts. Rab17 protein was identified by using a specific rabbit antiserum (26); alkaline phosphatase treatments and 2D analysis were performed as described (4).
Arabidopsis Transgenic Plants and Germination Assays. Rab17 and mRab17 cDNAs were cloned into pBin19 vector including the cauliflower mosaic virus 35S promoter. Transgenic Arabidopsis plants were obtained as described (25). Seeds were incubated for 3 days at 4°C before germination in Murashige and Skoog standard medium, with or without 100 mM NaCl, at 22°C in light/dark cycle conditions of 16/8 h. Experiments were performed in triplicate with a minimum of 50 seeds in each plate, and average germination percentages with standard error were calculated.
Results
Subcellular Localization of Rab17 and CK2. In maize embryonic tissues, Rab17 is found as a mixture of phosphorylated and unphosphorylated forms in nuclear and cytoplasmic compartments (4). We have also observed that in transient expression assays using Rab17-GUS constructs, the nuclear or cytoplasmic targeting of the hybrid protein seems to depend on the integrity of the CK2 phosphorylation consensus site in the Rab17 protein (25). However, the mechanism of Rab17 phosphorylation, as well as the cellular compartment in which the protein is phosphorylated, remains unknown. On the other hand, there is no agreement on the subcellular localization of the CK2 holoenzyme and its individual subunits (27). Here, we analyze in detail by confocal microscopy the subcellular distribution of Rab17 and CK2 fused to GFP in transformed onion cells.
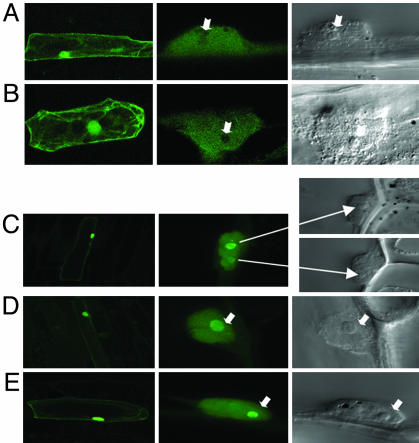
Fig. 1 A and B show cells transformed with Rab17-GFP. As expected, Rab17 is distributed in the cytoplasm, where a large proportion of the protein is localized, and in the nucleus, with absence of nucleolar staining. Surprisingly, in cells transformed with the nonphosphorylable form (mRab17-GFP), fluorescent staining is found predominantly in the nucleolus (see Fig. 1 C-E).
Fig. 1.
Subcellular localization of Rab17 and mRab17 in onion cells. Confocal microscopy images of onion cells transfected with Rab17-GFP (A and B) and mRab17-GFP (C-E). (Left) General views of transfected cells (×20). (Center) Detail of fluorescent cell nucleus (×60). (Right) View of the same nucleus under Nomarsky optics (arrows indicate the position of the nucleoli).
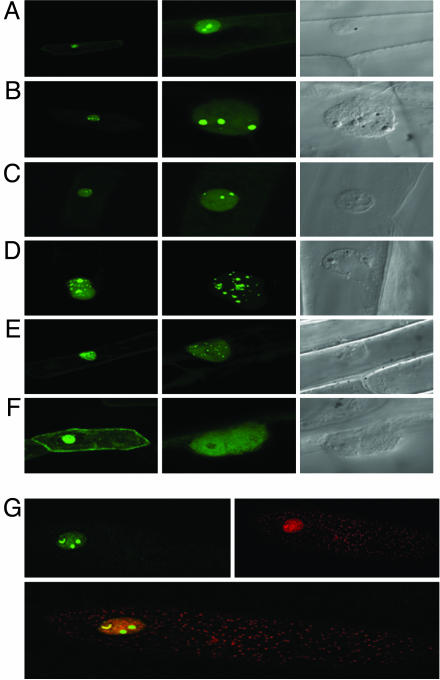
Cells transformed with CK2α and CK2β GFP fusion constructs show a different subcellular localization of the CK2 subunits (Fig. 2). The three CK2α catalytic subunits have a similar subcellular location; they are found in the nucleus and predominantly in the nucleolus as bright fluorescent speckles without significant staining in the cytoplasm (Fig. 2 A-C). By contrast, CK2β-1 and CK2β-2 are found predominantly in the nucleus where bright fluorescent speckles are clearly identified (Fig. 2 D and E) with some faint staining in the cytoplasm. CK2β-3 is mostly found in the cytoplasm where a few bright speckles can be observed, having a diffused staining pattern in the nucleus with no staining in the nucleoli (Fig. 2F). To gain insight into the specific localization of the CK2 holoenzyme complex and the free populations of the individual subunits, cells were cotransformed with differently labeled CK2 subunits (CK2α2-GFP and CK2β3-RFP), and dual-color image analysis was performed to monitor their localization in the cell. As expected, green and red fluorescence followed the pattern found for the individual subunits; however, CK2α/β partially colocalize in the cell nucleus. Several cells exhibited brighter yellow spots in the nucleus, but the physiological relevance is still unknown (Fig. 2G). Our results indicate that both subunits are distributed into the cell and targeted to the nuclei independently, and that a large portion of the stable holoenzyme accumulates mainly in the nucleus.
Fig. 2.
Subcellular localization of protein kinase CK2 subunits in onion cells. Confocal microscopy images of onion cells transfected with CK2α1-GFP (A), CK2α2-GFP (B), CK2α3-GFP (C), CK2β1-GFP (D), CK2β2-GFP (E), and CK2β3-GFP (F). (Left) General views of transfected cells (×20). (Center) Detail of fluorescent cell nucleus (×60). (Right) View of the same nucleus under Nomarsky optics. (G Upper) Confocal microscopy images of onion cells transfected with CK2α2-GFP and CK2β3-RFP, respectively. (Lower) Onion cell cotransfected with CK2α2-GFP/CK2β3-RFP.
It is noteworthy that unphosphorylated mRab17 is retained in the nucleolus in contrast to Rab17, which is efficiently removed from the nucleolus. These data, together with the nuclear/nucleolar localization of the three catalytic subunits of CK2, suggest that after translation in the cytoplasm, Rab17 may be targeted to the nucleus where it is probably efficiently phosphorylated by CK2.
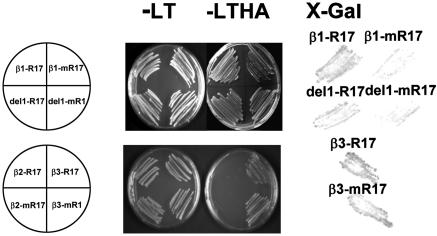
Rab17 Specifically Interacts with CK2β Regulatory Subunits. We have previously proposed that Rab17 could be transported to the nucleus through protein-protein interaction. Binding of Rab17 to NLS peptides was found to depend on phosphorylation (4). The presence of unphosphorylated Rab17 in the nucleus indicates that other partner molecules are involved in the nucleocytoplasmic trafficking of Rab17. Toward the identification of Rab17-associated partners, and because Rab17 is an in vitro substrate of protein kinase CK2 (10, 16), we examined whether a physical interaction between Rab17 and CK2 could occur. The interactions between CK2 regulatory subunits and Rab17 are shown in Fig. 3. Rab17 specifically interacts with CK2β-1 and CK2β-3 but not with CK2β-2 regulatory subunits. During late embryogenesis, there is a correlation in the pattern of expression of the CK2β-1 and Rab17 proteins, whereas CK2β-3 is predominantly expressed in the early stages of embryogenesis where Rab17 is absent (16). Our results indicate that the specific interaction of Rab17 with the two CK2 isoforms CK2β-1 and CK2β-3 is independent of their expression pattern.
Fig. 3.
Interactions between CK2β regulatory subunits and Rab17 substrate with the two-hybrid system. (Left) The indicated transformants were selected in Leu-Trp plates and replated in selective plates lacking Leu-Trp-His-Ade. (Right) Filter β-galactosidase assays after 4-h incubation with substrate.
Strong interactions were obtained by using the mutated mRab17, suggesting that the disruption of the CK2 phosphorylation consensus site does not affect its interaction with CK2β-1 and CK2β-3 and raising the possibility that both phosphorylated and unphosphorylated Rab17 can interact with CK2β in vivo.
In some experiments, we used a truncated version of the N-terminal region of CK2β-1 (delCK2β-1) lacking the first 80 amino acid residues that have been described only in plant CK2β regulatory subunits. This deletion does not affect interactions between CK2β and other CK2α/β subunits (M.R., unpublished data). We show that both Rab17 and mRab17 are able to efficiently interact with delCK2β-1, indicating that this interaction is also independent of the presence of the N-terminal domain. The absence of interaction between Rab17 and CK2α catalytic subunits (not shown) indicates that the interaction between Rab17 and CK2 holoenzyme occurs through CK2β subunits.
Phosphorylation of Rab17 by Protein Kinase CK2. The existence of several forms of CK2α/β in maize raises the possibility that Rab17 might be phosphorylated in the cell by a CK2 enzyme containing specific CK2α/β subunits.
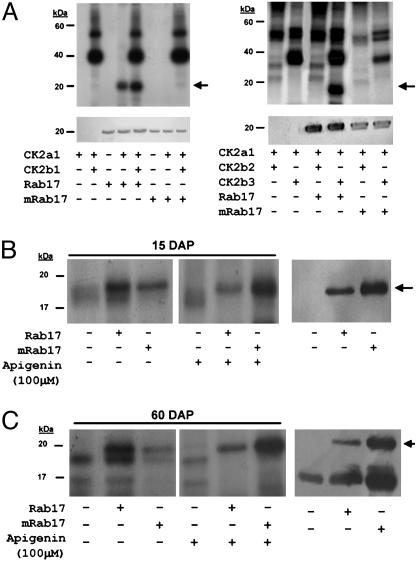
To determine whether Rab17 might be phosphorylated by the different CK2 holoenzymes, recombinant protein kinase CK2α/β subunits were assembled to fully active heterotetrameric complexes in vitro. Reconstituted CK2 were used for in vitro phosphorylation assays with recombinant proteins Rab17 and with mRab17 expressed in E. coli. Fig. 4A shows that Rab17 is not autophosphorylated but can be phosphorylated by the CK2α subunit alone and to a greater extent by the CK2 holoenzyme when reconstituted with CK2β-1 or CK2β-3. By contrast, no significant Rab17 phosphorylation occurs, assembling the holoenzyme with CK2β-2. These results correlate with those using two-hybrid assays, where only CK2β-1 and CK2β-3 subunits interact with Rab17 (Fig. 3). When using mRab17 as a substrate, and despite the interaction of mRab17 with CK2β-1 and CK2β-3, neither the CK2α subunit nor the holoenzyme reconstituted with the different CK2β subunits is able to phosphorylate the protein. This demonstrates that the mutation in the CK2 phosphorylation site completely prevents the phosphorylation of mRab17 by CK2.
Fig. 4.
Phosphorylation of Rab17 by protein kinase CK2. (A) In vitro phosphorylation of Rab17 by CK2. (Upper) Autoradiography of the different in vitro CK2 assays. (Lower) Western blot of the above samples using anti-Rab17 antibody, confirming the position and amounts of recombinant Rab17 or mRab17 added to the in vitro CK2 phosphorylation reaction. The combinations of different CK2α/β subunits, addition of recombinant Rab17 or mRab17 as substrate, and presence or absence of phosphorylation are indicated underneath each lane. (B and C Left) Autoradiography of in vitro CK2 assays with young embryo extracts 15 days after pollination (B) or dry embryo extracts 60 days after pollination (C), plus recombinant Rab17 or mRab17 as substrate, with or without the CK2 inhibitor apigenin. (Right) Western blot of the first three lanes on the left using the antiRab17 antibody indicating the presence of the endogenous Rab17 present in the mature maize extracts and in those samples where exogenous recombinant Rab17 and mRab17 proteins have been added. Addition of recombinant Rab17, mRab17, or apigenin is indicated underneath for each lane.
During maize embryogenesis, CK2 activity is mainly constitutive (15). On the contrary, Rab17 is not synthesized in young embryos but is very abundant and highly phosphorylated in the mature maize embryo (26). Accordingly, we performed phosphorylation assays by using embryo extracts from different developmental stages to assess their potential capacity of in vitro phosphorylation of the Rab17 protein (Fig. 4 B and C). Recombinant Rab17 added to protein extracts from young immature or mature maize embryo extracts, using either GTP or ATP as a phosphate donor, was effectively phosphorylated in vitro, indicating that CK2 present in mature or immature embryonic maize tissues is capable of phosphorylating Rab17. Interestingly, using recombinant mRab17 as substrate and ATP, but not GTP, as phosphate donor, the mutated Rab17 protein is phosphorylated, suggesting the possibility that another protein kinase might also be able to phosphorylate Rab17 in both young and mature embryos. To confirm these results, we added apigenin to the tissue extracts, a compound known to inhibit CK2 in cells (28). Phosphorylation of endogenous Rab17 in mature embryos is strongly reduced (Fig. 4C), and the number of phosphorylated proteins in extracts treated with apigenin is dramatically reduced (not shown); however, both recombinant Rab17 and mRab17 are still phosphorylated, confirming the hypothesis that not only CK2 but also another protein kinase could be involved in Rab17 phosphorylation in vivo.
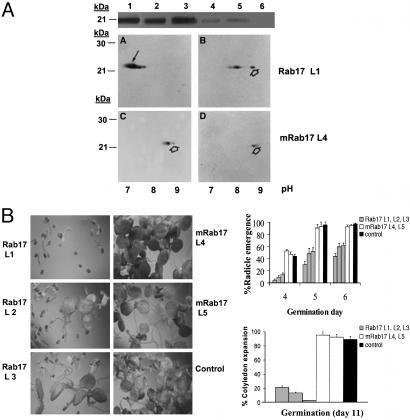
Rab17 Inhibits Germination in Stress Situation. The overexpression of maize Rab17 protein in Arabidopsis vegetative tissues confers a protective effect on plants under osmotic stress conditions; moreover, several physiological and biochemical parameters, such as relative water content, transpiration rates, and carbohydrate and proline content, are altered in these transgenic plants (29). To assess the effect of Rab17 phosphorylation, we produced transgenic Arabidopsis plants overexpressing Rab17 or mRab17 under a constitutive promoter. Transgenic plants accumulate in the vegetative tissues, Rab17 (Fig. 5A, transgenic lines 1-3) or, in lower amounts, mRab17 (Fig. 5A, transgenic lines 4 and 5). The degree of Rab17 or mRab17 phosphorylation attained in vivo was assessed by 2D electrophoresis. The mobility shift of Rab17, from the acid to a more alkaline pH after alkaline phosphatase treatment of protein extracts (Fig. 5A A and C), indicates extensive Rab17 phosphorylation in Arabidopsis tissues, with a phosphorylation pattern similar to that described for maize Rab17 (26). On the contrary, extracts from transgenic plants expressing mRab17 protein show a poor level of phosphorylation (Fig. 5A B and D). This reduced phosphorylation may compromise protein stabilization and thus could account for its lower accumulation in transgenic plants.
Fig. 5.
Overexpression of Rab17 and mRab17 in Arabidopsis thaliana. (A Top) Western blot of protein extracts from leaves from Arabidopsis transgenic plants using the anti-Rab17 antibody. Samples correspond to lines overexpressing Rab17 protein (L1 to L3), mRab17 protein (L4 and L5), and the nontransgenic control (L6). (Bottom) 2D electrophoresis and immunodetection of Rab17 in protein extracts from line L1 (A) and line L4 (C). (A, B, and D) Analysis of the same samples after dephosphorylation with alkaline phosphatase. The black arrow indicates the position of phosphorylated Rab17, and the open arrow indicates the nonphosphorylated or dephosphorylated Rab17. (B Left) Germination assays of seeds from lines L1 to L5 and nontransgenic control seeds after 11 days of germination in plates containing 100 mM NaCl. (Right) Differences in radicle emergence (days 4-6 of germination) and cotyledon expansion (day 11 of germination) in Rab17 and mRab17 transgenic lines.
A physiological response of Rab17 transgenic Arabidopsis plants, as opposed to mRab17, was observed in seed germination under saline conditions. In the presence of salt, expression of Rab17 strongly delays seed germination (Fig. 5B). The germination capacity was quantified first at the time of radicle emergence and later when cotyledons were fully expanded (Fig. 5B). There are no differences under standard in vitro conditions; however, in the presence of 100 mM NaCl, the three transgenic lines overexpressing Rab17 showed a marked reduction in germination capacity, in comparison to germination of two transgenic lines overexpressing mRab17, which behaved as the nontransgenic control. By day 11 of germination, most seeds of Rab17 expressing lines remained in the first stages of radicle emergence with few cotyledons developed, whereas all seeds of mRab17 expressing lines and control lines had their cotyledons completely expanded. At higher NaCl concentrations (up to 200 mM) or in the presence of KCl (100-200 mM), the reduced germinability of Rab17-expressing lines was maintained (not shown). Germination delay is reversed by salt removal from the medium. Moreover, plants resulting from crosses of different lines overexpressing Rab17 are equally delayed in their germination, suggesting that this effect is phosphorylation more than gene-dose-dependent (A.G., unpublished work).
Discussion
In maize, Rab17 is strongly induced during late embryogenesis and also in vegetative tissues subjected to water stress. The high degree of phosphorylation of the protein found in the mature embryo before desiccation led us to investigate the importance of phosphorylation for its physiological role.
To gain insight into the mechanism of Rab17 phosphorylation by CK2, we performed a detailed analysis of the spatial distribution in the cell of both CK2 and Rab17 by using confocal microscopy. Bombardment of onion cells using GFP fusions showed that Rab17 was found over the cytoplasm and the nucleus but mainly excluded from nucleoli, whereas mRab17 was unexpectedly found highly accumulated in nucleoli. Fluorescent nucleoli become visible only when the unphosphorylable form of Rab17 was used, suggesting that the phosphorylated protein does not accumulate in the nucleolus. These results point to a regulation of the Rab17 distribution in the cell mediated by CK2 phosphorylation. The unphosphorylated form of Rab17 would be retained in the nucleolus probably as a means of inhibiting Rab17 protein function. Alternatively, Rab17 may also play a role in preserving nucleolar structure/function during late embryogenesis and desiccation. The lower CK2 activity detected during embryo desiccation (15) may account for the accumulation of unphosphorylated Rab17 in the nucleolus. Rab17 would be released from the nucleolus by a process mediated by CK2 phosphorylation, and consequently CK2 phosphorylation would regulate the Rab17 function. However, Rab17 is a highly basic protein of a pI 9.4, and thus we cannot exclude the possibility of a direct entry of the mRab17 protein into the nucleolus. It has been proposed that nucleolar accumulation of proteins containing basic domains proceeds by diffusion and retention rather than by an active transport process. Using Rab17-β-glucosidase (GUS) fusion proteins, no GUS activity was detected in the nucleoli of onion cells or transgenic Arabidopsis plants transformed with mRab17-GUS (25). The absence of nucleolar staining may be explained by diffusion of GUS staining or by the inability of the recombinant Rab17-GUS protein to localize to the nucleolus. Several reports are in line with this observation, showing the exclusion from the nucleoli of otherwise nucleolar proteins, such as the CK2 interacting mouse protein FAF1, when transfected with a FLAG epitope-tagged quail (30).
Our data show that all three CK2α subunits are highly abundant in the nucleolus and accumulate significantly in the nucleus. No specific labeling was observed in the cytoplasm. Several studies have revealed that, although also found in the cytoplasm, CK2 is a major nuclear protein, and its presence in the nucleolus has also been reported (27). We have shown (15) that maize CK2α-2 contains one single functional NLS, consisting of a basic stretch of 20 amino acids located at amino acid position 61-81, sufficient to target the protein to the nucleus of plant cells. It is also present in the same position in the other two CK2α-1 and CK2α-3 catalytic subunits (16). In animals, it has recently been shown that the catalytic CK2α subunits shuttle between nucleus and cytoplasm (14); thus, we cannot discard the possibility that small pools of CK2α, undetectable by microscopy, may coexist in the cytoplasm. The identification of the components responsible for the accumulation of CK2α in the nucleolus and therefore its nucleolar activity, as well as the presence of the holoenzyme in nuclear structures, will constitute the next step toward understanding the mechanism of action of CK2 in the cell.
By contrast, the three CK2β regulatory subunits were localized in the nucleus and cytoplasm. Dual-color image analysis of cells transformed simultaneously with α and β CK2 subunits clearly shows that free populations of both CK2 subunits exist in the cell, in agreement with the current opinion that the individual subunits may exert different functions in the cell (30). Subunits CK2β-1 and CK2β-2 are mostly nuclear, whereas CK2β-3 is more cytoplasmic than nuclear. None of three CK2β maize regulatory subunits contain any recognizable NLS, but they are imported to the nucleus, suggesting that nuclear import of CK2β is mediated by a mechanism different from the classical NLS used by CK2α. An attractive possibility is that nuclear targeting of CK2β is achieved by interaction with various cellular proteins. In mammalian cells, it has recently been reported that dimerization of CK2β regulatory subunits is a prerequisite for nuclear localization (14). Moreover, it has been suggested that CK2β subunits contain in their zinc finger domain a nuclear signal unrelated to the classical NLSs, which has not yet been identified (14). In maize, we have shown (16) that CK2β-2 is the only isoform unable to interact with itself; this subunit holds a single amino acid change (Val-212 for Ala-212) in its zinc finger domain, and this change could affect homodimerization as well as interaction with other partners (such as with Rab17, see below) plus CK2 activity.
We have previously proposed (4) that Rab17 may play a role in nuclear protein transport by interacting with specific proteins based on its expression pattern, its phosphorylation-dependent NLS-binding activity, and its nuclear/cytosolic localization. The presence of mRab17 in the nucleolus prompted us to investigate whether CK2 could be a partner of Rab17, and whether they are associated as a molecular complex. The interaction of CK2 with a number of proteins seems to be mediated in some cases by the individual subunits but in others by the assembled holoenzyme (31). Here, we demonstrate the physical interaction between specific CK2β regulatory subunits (CK2β-1 and CK2β-3) with Rab17 by the two-hybrid system. As expected (see above), Rab17 and CK2β-2 do not interact with each other. Using in vitro phosphorylation assays, we demonstrate the functionality of these interactions because the CK2 regulatory subunits CK2β-1 and CK2β-3 not only interact specifically with Rab17 but also enhance CK2α activity toward Rab17. The unphosphorylable mRab17 is also able to interact with specific CK2β subunits (CK2β-1 and CK2β-3), showing that these interactions are independent of the integrity of the CK2 consensus sequence. Similar results have been reported for other CK2 substrates, such as the mammalian FAF1 mutated in its CK2 consensus site (30). Furthermore, our results show that the N-terminal extension so far described only in plant CK2β subunits is not essential for CK2/Rab17 interactions. Additional experiments will be needed to understand the role of this domain in the structure of the maize holoenzyme. Using the two-hybrid method, we did not find interactions between Rab17 and CK2α catalytic subunits, although, using pull-down assays, we could detect a weak interaction between Rab17 and CK2α (not shown). Additional evidence obtained in the laboratory, such as the copurification of Rab17 with CK2 (15) and coimmunoprecipitation of CK2 and in vivo phosphorylated Rab17 from maize embryos, indicates that, although the preferential interactions between Rab17 and CK2 are mediated by CK2β-1 or CK2β-3 subunits, we cannot exclude the possibility of an alternative interaction between Rab17 substrate and the whole holoenzyme.
Phosphorylation assays using maize embryo extracts confirmed that Rab17 is phosphorylated by a protein kinase present in young and mature embryos that is able to use either GTP or ATP as phosphate donor, most probably being the protein kinase CK2. However, it should also be noted that in maize embryo extracts, mRab17 is phosphorylated, but only when using ATP as a phosphate donor, and that this phosphorylation is not suppressed by apigenin, a known CK2 inhibitor (28). This opens the possibility that another protein kinase, not CK2, would possibly be involved in the in vivo phosphorylation of Rab17 in maize tissues. The tomato Rab17 homolog protein TAS14 was in vitro phosphorylated by both CK2 and a cAMP-dependent protein kinase (32); however, no further data have been reported. Identification of the maize embryo protein kinase, now under way, should give us new insight into the involvement and functional role of Rab17 in different processes during stress conditions.
Here we found that CK2 modulates developmental functions of Rab17 in seed germination. In transgenic Arabidopsis, the overexpression of CK2 phosphorylated Rab17 strongly delays seed germination in a stress situation, whereas seeds accumulating the mRab17 behave identically to the nontransgenic controls and are able to germinate. Because the Rab17 protein accumulates during embryo desiccation, it is tempting to speculate that Rab17 plays a specific role in growth inhibition in embryonic tissues, probably in germination and in the induction or maintenance of dormancy of the embryos during desiccation. Both expression of phosphorylated Rab17 and osmotic stress would be required for protein functionality. Reinforcing the function of Rab17 in germination under stress conditions is the accumulation of Rab17 protein in low amounts in the viviparous mutants of maize along the embryogenesis (33). These mutant embryos do not go through a desiccation process and germinate precociously, and simultaneously the absence of desiccation in these embryonic tissues would preclude the proposed function of Rab17 in the arrest of germination.
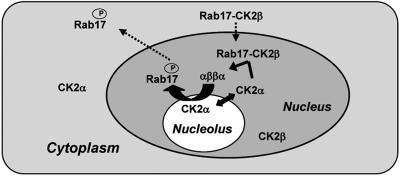
The interaction found between CK2β subunits and Rab17 should have significant physiological relevance for protein targeting and/or regulation of protein function. Based on these results, we postulate that CK2β-Rab17 binding targets both proteins to the nucleus/nucleolus where the catalytic CK2α subunit is accumulated (Fig. 6). After holoenzyme formation, Rab17 would be released under its phosphorylated form. In a stress situation, a rapid disruption of Rab17-CK2β interaction would be essential to allow Rab17 to go into the nucleoplasm and there function as a chaperone by accompanying partner molecules or forming oligomers through the nucleoplasm and/or cytoplasm.
Fig. 6.
Model for nucleo/cytoplasmic trafficking of Rab17. Because mRab17 is retained in the nucleolus, whereas Rab17 is efficiently removed from this organelle, phosphorylation of Rab17 could occur in the nucleus. In this context, the interaction between CK2β and Rab17 can be of significant physiological relevance for nuclear targeting. We propose that after translation in the cytoplasm, Rab17 may interact with CK2β, and, in this way, the Rab17/CK2β complex formed would travel to the nucleus where the three catalytic subunits of CK2 are predominantly located. In the nucleus, CK2α would disrupt Rab17/CK2β to associate with CK2β, generating the holoenzyme, and Rab17 would probably be efficiently phosphorylated by CK2. Once phosphorylated, Rab17 would go to the nucleoplasm and/or cytoplasm to exert its still-unknown function.
Our results indicate a relevant function of the Rab17 protein in growth inhibition, which is mediated by its phosphorylation status in a water-deficit situation. Further work is needed to understand the global role played by CK2 in regulating these processes.
Acknowledgments
We thank Olaf G. Issinger for advice during this work, Judit Pujal for maintenance of transgenic plants, Mónica Pons for confocal microscopy support, and Pierre Goddet for technical support. This work was funded by Grants BIO2003-01133 from McyT and QLK5-CT-2002-00841 from the European Economic Community. M.R. and C.L. were supported by Grants TDOC00012 and 2003-FI0036, respectively, from Generalitat de Catatunya Comisio Interdepartamental de Recerca i Innovacio Tecnològica.
This report was presented at the international Congress, “In the Wake of the Double Helix: From the Green Revolution to the Gene Revolution,” held May 27-31, 2003, at the University of Bologna, Bologna, Italy. The scientific organizers were Roberto Tuberosa, University of Bologna, Bologna, Italy; Ronald L. Phillips, University of Minnesota, St. Paul, MN; and Mike Gale, John Innes Center, Norwich, United Kingdom. The Congress web site (www.doublehelix.too.it) reports the list of sponsors and the abstracts.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: NLS, nuclear localization signal.
References
- 1.Gómez, J., Sánchez-Martínez, D., Steifel, V., Rigau, J., Puigdomenech, P. & Pagès, M. (1988) Nature 334, 262-264. [DOI] [PubMed] [Google Scholar]
- 2.Mundy, J. & Chua, N. H. (1987) EMBO J. 7, 2279-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dure, L., III (1993) Plant J. 3, 363-369. [DOI] [PubMed] [Google Scholar]
- 4.Goday, A., Jensen, A. B., Culiañez-Macià, F. B., Albà, M. M., Figueras, M., Serratosa, J., Torrent, M. & Pagès, M. (1994) Plant Cell 6, 351-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyen, B. J., Alsheikh, M. K., Smith, E. A., Torvik, C. F., Seals, D. F. & Randall, S. K. (2002) Plant Physiol. 130, 675-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koag, M. C., Fenton, R. D., Wilkens, S. & Close, T. J. (2003) Plant Physiol. 131, 309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaye, C. & Guy, C. L. (1995) Sci. Prog. 78, 271-299. [PubMed] [Google Scholar]
- 8.Close, T. J. (1997) Physiol. Plant 100, 291-296. [Google Scholar]
- 9.Vilardell, J., Goday, A., Freire, M. A., Torrent, M., Martínez, M. C., Torné, J. M. & Pagès, M. (1990) Plant Mol. Biol. 14, 423-432. [DOI] [PubMed] [Google Scholar]
- 10.Plana, M., Itarte, E., Eritja, R., Goday, A., Pagès, M. & Martinez, M. C. (1991) J. Biol. Chem. 266, 22510-22514. [PubMed] [Google Scholar]
- 11.Litchfield, D. W. (2003) Biochem. J. 369, 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed, K., Gerber, D. A. & Cochet, C. (2002) Trends Cell Biol. 12, 226-230. [DOI] [PubMed] [Google Scholar]
- 13.Pinna, L. A. & Meggio, F. (1997) Prog. Cell Cycle Res. 3, 77-97. [DOI] [PubMed] [Google Scholar]
- 14.Filhol, O., Nueda, A., Martel, V., Gerber-Scokaert, D., Benitez, M. J., Souchier, C., Saoudi, Y. & Cochet, C. (2003) Mol. Cell. Biol. 23, 975-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peracchia, G., Jensen, A. B., Culiáñez-Macià, F. A., Grosset, J., Goday, A., Issinger, O. G. & Pagès, M. (1999) Plant Mol. Biol. 40, 199-211. [DOI] [PubMed] [Google Scholar]
- 16.Riera, M., Peracchia, G., de Nadal, E., Ariño, J. & Pagès, M. (2001) Plant J. 25, 365-374. [DOI] [PubMed] [Google Scholar]
- 17.Riera, M., Pagès, M., Issinger, O. G. & Guerra, B. (2003) Protein Expr. Purif. 29, 24-32. [DOI] [PubMed] [Google Scholar]
- 18.Gerber, D. A., Souquere-Besse, S., Puvion, F., Dubois, M. F., Bensaude, O. & Cochet, C. (2000) J. Biol. Chem. 275, 23919-23926. [DOI] [PubMed] [Google Scholar]
- 19.Bidwai, A. P., Reed, J. C. & Glover, C. V. C. (1995) J. Biol. Chem. 270, 10395-10404. [DOI] [PubMed] [Google Scholar]
- 20.de Nadal, E., Calero, F., Ramos, J. & Ariño, J. (1999) J. Bacteriol. 181, 6456-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanhonou, R., Serrano, R. & Palau, R. R. (2001) Plant Mol. Biol. 47, 571-579. [DOI] [PubMed] [Google Scholar]
- 22.Hanna, D. E., Rethinaswamy, A. & Glover, C. V. (1995) J. Biol. Chem. 270, 25905-25914. [DOI] [PubMed] [Google Scholar]
- 23.Buchou, T., Vernet, M., Blond, O., Jensen, H. H., Pointu, H., Olsen, B. B., Cochet, C., Issinger, O. G. & Boldyreff, B. (2003) Mol. Cell. Biol. 23, 908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varagona, M. J., Schmidt, R. J. & Raikhel, N. V. (1992) Plant Cell 4, 1213-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen, A. B., Goday, A., Figueras, M., Jessop, A. C. & Pagès, M. (1998) Plant J. 13, 691-697. [DOI] [PubMed] [Google Scholar]
- 26.Goday, A., Sánchez-Martínez, D., Gómez, J., Puigdomènech, P. & Pagès, M. (1988) Plant Physiol. 88, 564-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faust, M. & Montenarh, M. (2000) Cell Tissue Res. 301, 329-340. [DOI] [PubMed] [Google Scholar]
- 28.Ford, H. L., Landesman-Bollag, E., Dagwag, C. S., Stukenberg, P. T., Pardee, A. B. & Seldin, D. C. (2000) J. Biol. Chem. 275, 22245-22254. [DOI] [PubMed] [Google Scholar]
- 29.Figueras, M., Pujal, J., Saleh, A., Pagès, M. & Goday, A. (2004) Ann. Appl. Biol., in press.
- 30.Guerra, B., Boldyreff, B. & Issinger, O. G. (2001) Int. J. Oncol. 19, 1117-1126. [DOI] [PubMed] [Google Scholar]
- 31.Guerra, B. & Issinger, O. G. (1999) Electrophoresis 20, 391-406. [DOI] [PubMed] [Google Scholar]
- 32.Godoy, J. A., Lunar, R., Torres-Schumann, S., Moreno, J., Rodrigo, R. M. & Pintor-Toro, J. A. (1994) Plant Mol Biol. 26, 1921-1934. [DOI] [PubMed] [Google Scholar]
- 33.Pla, M., Goday, A., Vilardell, J., Gómez, J. & Pagès, M. (1989) Plant Mol. Biol. 13, 385-389. [DOI] [PubMed] [Google Scholar]