Abstract
Toxicity has been estimated to be responsible for the attrition of ~ 1/3 of drug candidates and is a major contributor to the high cost of drug development, particularly when not recognized until late in the clinical trials or post-marketing. The causes of drug toxicity can be organized in several ways and include mechanism-based (on-target) toxicity, immune hypersensitivity, off-target toxicity, and bioactivation/covalent modification. In addition, idiosyncratic responses are rare but one of the most problematic issues; several hypotheses for these have been advanced. Although covalent binding of drugs to proteins was described almost 40 years ago, the significance to toxicity has been difficult to establish; recent literature in this field is considered. The development of more useful biomarkers and short-term assays for rapid screening of drug toxicity early in the drug discovery/development process is a major goal, and some progress has been made using “omics” approaches.
Keywords: Drugs, toxicity, mechanisms, idiosyncratic responses, covalent binding, short-term assays
Introduction
The cost of pharmaceutical development has been increasing for many years, and the estimated average cost of developing a profitable drug has been estimated at > US $1.7 billion.1) However, a graph of the number of new approved drugs per year is relatively flat.2) Some reasons for the problem include the difficulties of target validation—in approaching increasingly complex disease areas—and the rising regulatory barriers.
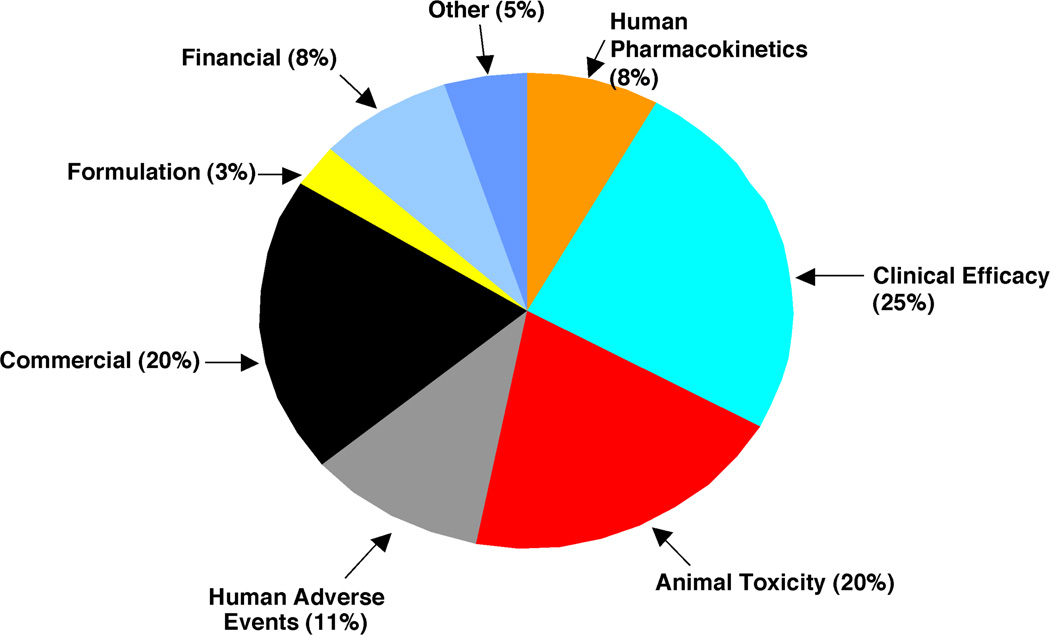
It has been estimated that an average of between 10,000 and 25,000 individual chemicals are considered in the course of development of a new drug. What are the major reasons for attrition of lead compounds? A major issue 25 years ago was poor pharmacokinetics in humans. This aspect has been addressed through advances in the understanding of human cytochrome P450 (P450) and other enzymes, knowledge about transporters, and the development of predictive in vitro assays. However, as metabolic issues have been reduced, toxicity issues have increased (Figure 1). Together, pre-clinical toxicity (animal) and adverse events (human toxicity) account for ~ 1/3 of the cases of attrition.2) If one excludes the “non-scientific” issues (e.g. commercial, financial) then the fraction is even higher.
Fig. 1.
Estimates of fractions of reasons for attrition of drug candidates in pre-clinical and clinical development (ca. 2000).2)
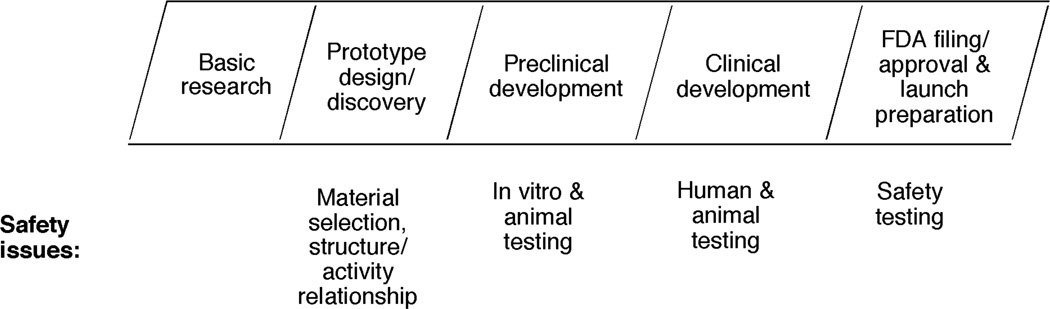
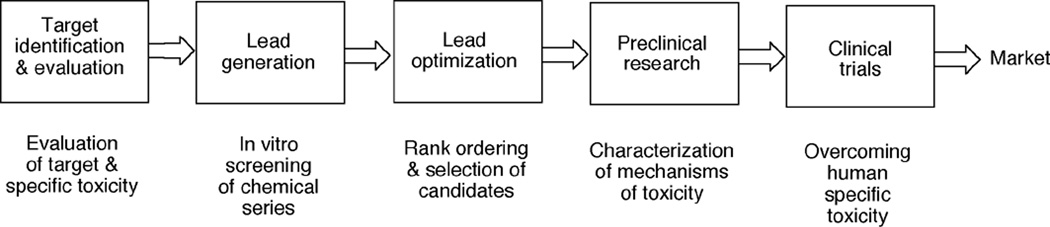
The real issue is the expenditure of resources (of time and money) on compounds that have toxicity issues and ultimately have to be dropped from development. Toxicity and safety assessment are done at many steps in the drug discovery/development pathway (Figure 2). If compounds with toxicity issues are not dropped until a very late time, then the loss may run into hundreds of millions of dollars and years of research. Thus, earlier decisions are very important in drug development, and the initial decisions must be accurate. In this review, toxicity issues mostly relevant to drugs will be covered here.
Fig. 2.
Safety issues at different stages of drug discovery and development.1)
Contexts of Drug Toxicity
All compounds are toxic at high doses and all are safe at very low doses, using the axiom of Paracelsus.3) What we are considering here are not accidental drug overdoses but toxicity and adverse events at doses that are relevant to patients using a medicine. What the context of toxicity is will affect how one approaches the matter of circumventing toxicity or developing alternate compounds that will not have this liability. The most commonly encountered problems are with cardiovascular and hepatic toxicity (Table 1).
Table 1.
Sites for toxicology attrition. Based on experience from DuPont-Merck and Bristol-Myers Squibb, 1993–2006. Information kindly provided by B. D. Car, Bristol-Myers Squibb.
| Target organ or tissue | % of all advanced moleculesa |
|---|---|
| Cardiovascular | 27.3 |
| Liver | 14.8 |
| Teratogenicity | 8.0 |
| Hematologic | 6.8 |
| Central and peripheral nervous system | 6.8 |
| Retina | 6.8 |
| Mutagenicity/clastogenicity | 4.5 |
| Reproductive toxicity | 4.5 |
| Gastrointestinal/pancreatic | 3.4 |
| Muscle | 3.4 |
| Carcinogenicity | 3.4 |
| Lung | 2.3 |
| Acute death (unspecified cause) | 2.3 |
| Renal | 2.3 |
| Irritant | 2.3 |
| Skeletal (arthritis/bone development) | 1.1 |
Total = 100%.
Several classifications are possible. What is presented here is a systematic one previously described (Table 2).4) Others have presented alternate but similar classifications.5)
Table 2.
Contexts of drug toxicity.4)
| Type | Example |
|---|---|
| On-target (mechanism-based) | Statins |
| Hypersensitivity and immunological | Penicillins |
| Off-target | Terfenadine |
| Biological activation | Acetaminophen |
| Idiosyncratic | Halothane |
The first context of toxicity is on-target (or mechanism-based) toxicity. That is, the toxicity is due to interaction of the drug with the same target that produces the desired pharmacological response. The concept is not one of competitive inhibition but rather that the biological response that the drug exhibits upon binding to its target is the same one that produces both the efficacious and the toxic effects. In principle this type of toxicity is difficult to deal with because all classes of compounds developed to treat the disease will show the toxicity. Changing the target for the disease may be necessary. However, another strategy is exemplified in the case of statins. All statins produce hypercholesterolemic properties by inhibiting 3-hydroxy-3-methylglutaryl CoA (HMG CoA) reductase in the liver, i.e. the target. The adverse effects of statins are also due to inhibition of HMGCoA reductase in muscle and possibly other tissues, i.e. geranylgeranylation of proteins6) is inhibited. Fortunately the distribution of statins between tissues can be modulated by various transport proteins, and although on-target toxicity is an issue it can be controlled by inter-tissue distribution.7)
The second context of drug toxicity is hypersensitivity and immune responses. For instance, allergic reactions to penicillins have been recognized for many years. The concept, developed largely on the basis of the pioneering work of Landsteiner,8) is that drugs (or their metabolites) react with proteins in the body (as haptens) to induce antibodies and immune responses. In this example (penicllins) the chemical is not completely stable and has the potential to bind covalently to proteins and initiate antibody production.
The third context of drug toxicity is off-target toxicity. The issue here is that the drug is not specific in its interactions. Binding to an alternate target is the cause of toxicity. With our current knowledge of the complexity of biological regulatory pathways and multi-gene families (e.g. protein kinases), it is not surprising that a drug might not be totally specific. The example in Table 2 is terfenadine, which binds not only to the H1 receptor (eliciting the desired antihistaminic response) but also to the hERG channel and thus causing arrhythmias. In principle, this liability can be addressed by more screening and development of drug candidates with lower IC50 and Kd values, in that a lower dose might avoid the specificity issue.
The fourth context of drug toxicity is bioactivation. Many drugs are converted to reactive products (often termed (reactive) “metabolites”). These entities modify the proteins they react with and somehow cause toxicity, although mechanisms have been evasive (vide infra). One theory is that important regulatory or other proteins are modified, with loss of function. Another possibility is that the modified proteins induce immune responses, linking with the second context of toxicity. An analysis of drugs at one company, Bristol-Myers Squibb, indicated that “metabolism” was an issue in 28% of cases in which drug candidates had been dropped from development (Table 3).
Table 3.
Mechanistic causes of toxicology attrition. Based on experience from DuPont-Merck and Bristol-Myers Squibb, 1993–2006. Information kindly provided by B. D. Car, Bristol-Myers Squibb.
| % of all advanced moleculesa |
|
|---|---|
| Biotransformation-related | 27 |
| Target-based | 28 |
| Single or multiple ion channel inhibition | 18 |
| Immune-mediated | 7 |
| All other mechanisms | 36 |
n = 88. Because categories are partially overlapping, the total is > 100%.
The fifth context of toxicity is idiosyncratic reactions. Idiosyncratic means “individual,” and these are rare events (1/103 to 1/104 individuals), which are not well understood. Such responses are highly problematic in that few (if any) animal models are very predictive. The low incidence makes such adverse events difficult to find even in large clinical trials. However, with widely-used drugs for which millions of prescriptions may be written, even an incidence of 1/104 can yield hundreds of problems.
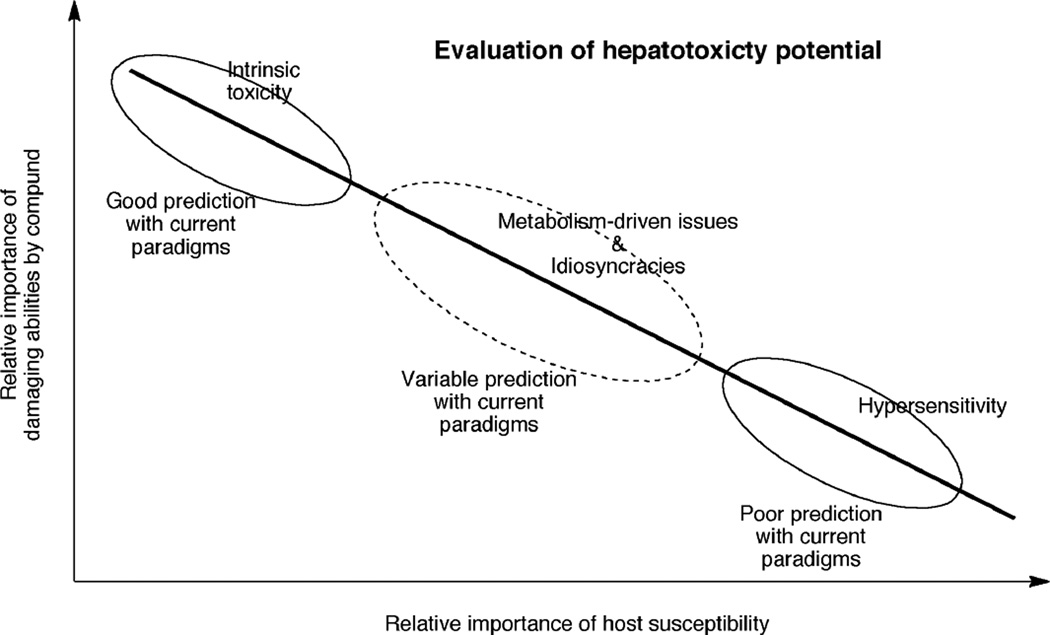
The context of toxicity has bearing on how difficult it is to predict safety problems (Figure 3).
Fig. 3.
Hypothetical relationship between the inherent toxicity of drugs and the variability of the response among hosts (e.g. test animals, humans). The dose is not a consideration in this treatment, adapted from Zimmerman.9,10) At toxic doses, the most readily understood compounds are those with high toxicity in all animal species. Variation among species introduces more uncertainty in extrapolation to humans. Predictions can be made if the issue is metabolism but idiosyncratic problems are very difficult to understand with animal models.
Theories regarding mechanisms of idiosyncratic reactions
This topic has been reviewed by others11–14) (Table 4). At least five theories have been proposed to explain idiosyncratic reactions, and these may not be exclusive of each other in considering all drugs for which idiosyncrasies have been reported (Table 5). The first theory is polymorphisms or rare alleles of metabolism enzymes. (The term polymorphism may not be applicable in that this is generally reserved for incidences of ≥ 1–2%; otherwise the term “rare alleles” applies.) The concept is that the sensitivity is due to lack of metabolism of a drug, including a lack of detoxication. For instance, an individual might be the ~1% of a (Caucasian) population with a high propensity to activate a drug (e.g., the ultra-rapid metabolizers in the P450 2D6 group17)) and also be deficient in a glutathione (GSH) transferase or other enzyme to detoxicate the product. Thus, two polymorphisms at the 1% level would be multiplied to yield an incidence of 1/104. This scenario is possible but no solid examples exist to explain any observed idiosyncratic reactions.13)
Table 4.
| Intrinsic | Idiosyncratic |
|---|---|
| Predictable (?) | Unpredictable |
| Relatively common occurrence | Rare occurrence (< 1/104) |
| Detected pre-clinically | Not detected until post-launch |
| Dose dependent | Occurs at any dose (?) |
| Acute or sub-acute onset | Delayed onset |
| No immune component | Immune/metabolic component (usually see fever, rash, eosinophilia) |
| Animal models useful | Few (any?) animal models available |
| Example: acetaminophen | Examples: isoniazid, halothane |
Table 5.
Idiosyncratic toxicity: proposed mechanisms.
|
The second is the hapten theory, which has already been introduced. The concept is that some individuals will show more activation of a drug to yield a hapten, and also the variations in the immune systems of individuals will dictate that only a few will show this response.11) There is some support in the cases of tienilic acid18) and hydralazine,19) which are activated by human P450s and bound covalently to P450s, generate anti-P450 antibodies in humans, and cause hepatitis. The deficiency is that it has not been possible to prove that these events are causal for the hepatotoxicity or simply all happening at the same time but unrelated.20)
The third theory is sometimes referred to as the inflammagen model.21) The concept is that bioactivation and other events occur in many people and that inflammation (or other predisposing episodes) render only some individuals more sensitive. It is possible to demonstrate this phenomenon in rats treated with lipopolysaccharide to cause oxidative stress.22) However, exactly how representative this is as a model for human idiosyncrasies is subject to debate.12)
The fourth (theory) is the danger hypothesis.23,24) Here the injured tissue produces danger signals (e.g. lipid oxidation products, cytokines) that evoke a toxic response, not the drug or its metabolites. As Uetrecht has pointed out,12) this hypothesis is not mutually exclusive from the hapten theory (if an immunological response is involved), although there is no hard evidence that this is a mechanism in clinical drug idiosyncracies.
The fifth theory is the pharmacological intervention model.25,26) In this model, drugs elicit immunological responses by reversible binding to proteins, i.e. without covalent binding. One of the bases for this proposal was sulfamethoxazole, an arylamine prone to forming reactive metabolites. There is some evidence that ximelegatran, a peptide-like substance, might act in this manner.27)
Where does covalent binding fit in?
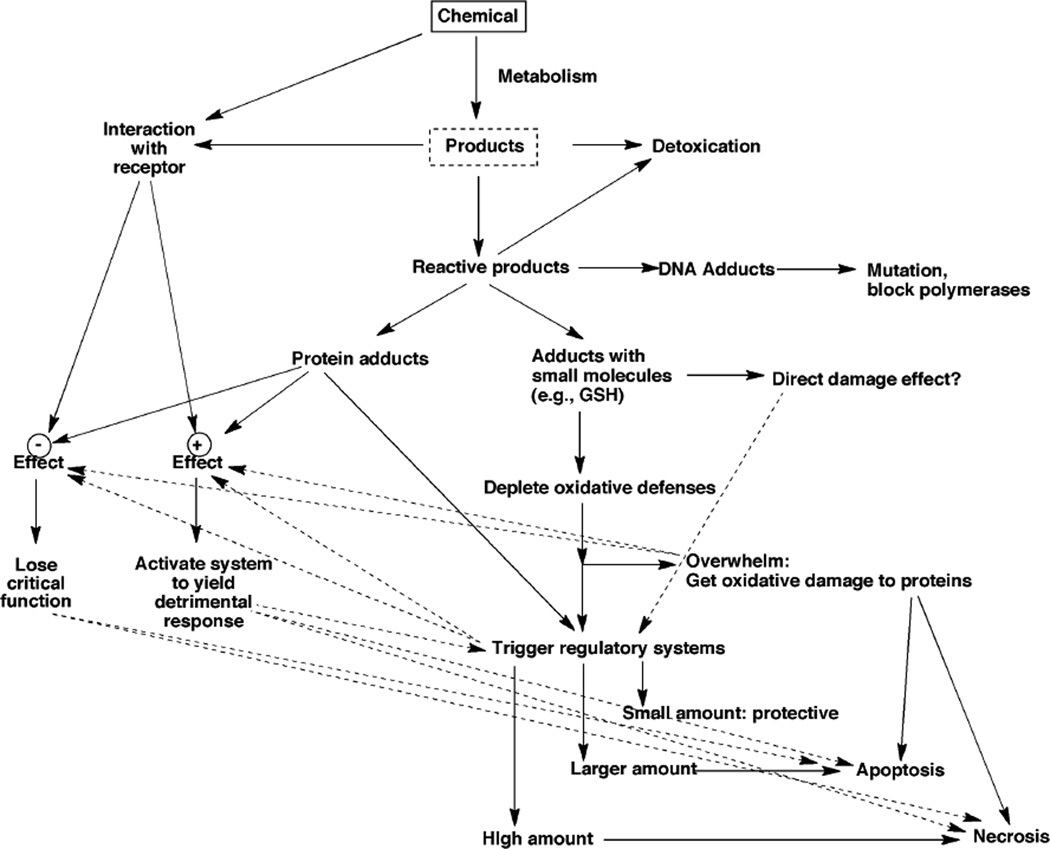
An overall scheme of drug toxicity includes many aspects, some of which are related to metabolism (Figure 4). Covalent binding of drugs to proteins has been with us at least since 1973, with the classic papers of Gillette and Brodie on acetaminophen.29,30) Even before then the covalent binding of carcinogens to proteins had been demonstrated by the Millers.31) However, two important questions remain unanswered. One is how important this process really is with drug toxicity, in that the evidence remains highly correlative. The other question is, if covalent binding to proteins causes toxicity, what exactly is the mechanism (or does a general mechanism even exist?).
Fig. 4.
A general scheme of biological events related to the toxicity of drugs and other chemicals.4,28)
One of the major areas in which covalent binding has been studied is hepatoxicity, which is both a pre-clinical and clinical issue (Tables 3, 6). In a seminal review, Walgren et al.35) listed 14 drugs which have been withdrawn from the market due to hepatoxicity (Table 7). Of these, nine (64%) have been shown to be activated to reactive products. Another list of drugs includeds those that had been withdrawn in other countries due to hepatotoxicity (and never introduced into the United States) (Table 8). Of the 11 tested for generation of reactive products, all 11 (100%) were positive. Finally, a third list of 14 marketed drugs with “Black Box” warnings for hepatoxicity had ten (71%) that showed reactive metabolites (out of all 14 examined) (Table 9).
Table 6.
| Time period | Drug | Total number |
|---|---|---|
| Pre-1960 | Cinchophen, iproniazid | 2 |
| 1960–1969 | Benziodarone, ibufenac, phenoxypropazine, pipamazine, xenazoic acid | 5 |
| 1970–1979 | Fenclozic acid, mebanazine, nialamide, oxyphenisatin | 4 |
| 1980–1989 | Benoxaprofen, clomacron, clometacin, cyclofenil, exifone, glafenine, isaxonine, nitrofazole, nomifensine, perhexiline, suloctidyl, tienilic acid, zimelidine | 13 |
| 1990–1999 | Alpidem, amineptine, bendazac, benzarone, bromfenac, chlormezanone, dilevalol, ebrotidine, fipexide, moxisylyte, niperotidine, pirprofen, tolrestat | 13 |
| 2000–2006 | Ximelagatran, pemoline, nefazodone, troglitazone | 4 |
Table 7.
Drugs withdrawn for hepatotoxicity (U. S.).35)
| Drug | Date | Dose (mg/day) |
Reactive products |
Reference |
|---|---|---|---|---|
| Cincophen | 1930 | 300 | No | |
| Iproniazid | 1959 | 25–150 | Yes | 36) |
| Pipamazine | 1969 | 15 | No | |
| Fenclozic acid | 1970 | 300 | Yes | 37) |
| Oxyphenisatin | 1973 | 50 | No | |
| Nialamide | 1974 | 200 | Yes | 38) |
| Tienilic acid | 1980 | 250–500 | Yes | 20) |
| Benoxaprofen | 1982 | 300–600 | Yes | 39) |
| Nomifensine | 1986 | 125 | Yes | 40) |
| Chlormezanone | 1996 | 600 | No | |
| Bromfenac | 1998 | 25–50 | Yes | |
| Troglitazone | 2000 | 400 | Yes | 41) |
| Nefazodone | 2004 | 200 | Yes | 42) |
| Pemoline | 2005 | 38–110 | No | |
| 9/14 = 64% | ||||
Table 8.
Drugs withdrawn for hepatotoxicity (non-U. S.).35)
| Drug | Date | Dose (mg/day) |
Reactive products |
Reference |
|---|---|---|---|---|
| Alpidem | 1993 (France) | 25–150 | Yes | 43) |
| Amineptine | 1999 (France, Thailand) | 200 | Yes | 44) |
| Bendazac | 1993 (Spain) | 500 | ||
| Benzarone | 1992 (Greece) | 300 | Yes | |
| Benziodarone | 1964 (U. K.) | 300 | ||
| Clomacron | 1982 (U. K.) | |||
| Clometacin | 1987 (France) | 450 | ||
| Cyclofenil | 1987 (France | 200 | ||
| Dilevalol | 1990 (U. K.) | 200–400 | ||
| Ebrotidine | 1998 (Spain) | 400–800 | ||
| Exifone | 1989 (France) | 1200 | Yes | |
| Fipexide | 1991 (France, Greece) | 600 | Yes | |
| Glafenine | 1984 (France, Greece) | 400 | Yes | |
| Ibufenac | 1968 (U. K.) | 2400 | Yes | 45) |
| Isaxonine | 1984 (France) | 1500 | Yes | 46) |
| Mebanazine | 1975 (U. K.) | 30 | Yes | |
| Moxisylyte | 1993 (France) | 480 | ||
| Niperotidine | 1995 (Italy) | 230–460 | ||
| Nitrofazole | 1984 (Greece) | 1200 | ||
| Perhexiline | 1985 (U. K.) | 300 | ||
| Phenoxypropazine | 1966 (U. K.) | 10–20 | Yes | |
| Pirprofen | 1990 (Europ. Union) | 800 | Yes | |
| Suloctidyl | 1985 (Spain) | 600 | ||
| Tolrestat | 1996 (Europ. Union) | 400 | ||
| Xenazoic acid | 1965 (France) | Unknown | ||
| Ximelagatran | 2006 (Europ. Union) | 48 | ||
| Zimelidine | 1985 (U. K.) | 100–300 | ||
| 11/11 = 100% | ||||
Table 9.
Drugs with Black Box warnings for hepatotoxicity.35) A “Black Box” warning is the strongest type of warning that the U. S. Food and Drug Administration can require for a drug and is generally reserved for warning prescribers about adverse drug reactions that can cause serious injury or death. At issue here is the benefit/risk ratio.
| Drug | Dose (mg/day) | Reactive products | Reference |
|---|---|---|---|
| Acitretin | 25–50 | No | |
| Bosentan | 125–250 | No | |
| Dacarbazine | 140–315 | Yes | 47) |
| Dantrolene | 300–400 | Yes | 48) |
| Felbamate | 1200 | Yes | 49) |
| Flutamide | 750 | Yes | 50) |
| Gemtuzumab | (9 mg m−3) | Yes (?) | 51) |
| Isoniazid | 300 | Yes | 52) |
| Ketoconazole | 200 | Yes | 53) |
| Naltrexone | 50 | No | |
| Nevirapine | 200 | Yes | |
| Tolcapone | 300 | Yes | 54) |
| Trovafloxacin | 100–500 | No | |
| Valporic acid | 1000–2400 | Yes | 55) |
| 10/14 = 71% | |||
Collectively, two conclusions can be reached in examining these retrospective studies.35) One is that a large fraction of problematic drugs produce reactive products, which may be an issue. However, there are caveats: one is that any relationship is not necessarily causal, and another being that the binding of non-toxic drugs was not compared here. The other point to make about the review of Walgren et al.35) is that all of the drugs producing idiosyncratic hepatotoxicity were used at high doses. Uetrecht56) has made the point that idiosyncratic problems are seldom seen with drugs used at doses of ≤ 10 mg/day. (Some drugs certainly can be hepatoxic at lower doses, e.g. cerivastatin at sub-mg per day doses, but in this case the situation is explained by the high potency, on-target toxicity, and some cytochrome P450 polymorphisms.7)) The tendency for more hepatotoxicity with higher dose drugs may be consistent with the view that a low dose drug, even if extensively bioactivated to reactive products, might produce damage that would not exceed the usual threshold of protective systems in the body.
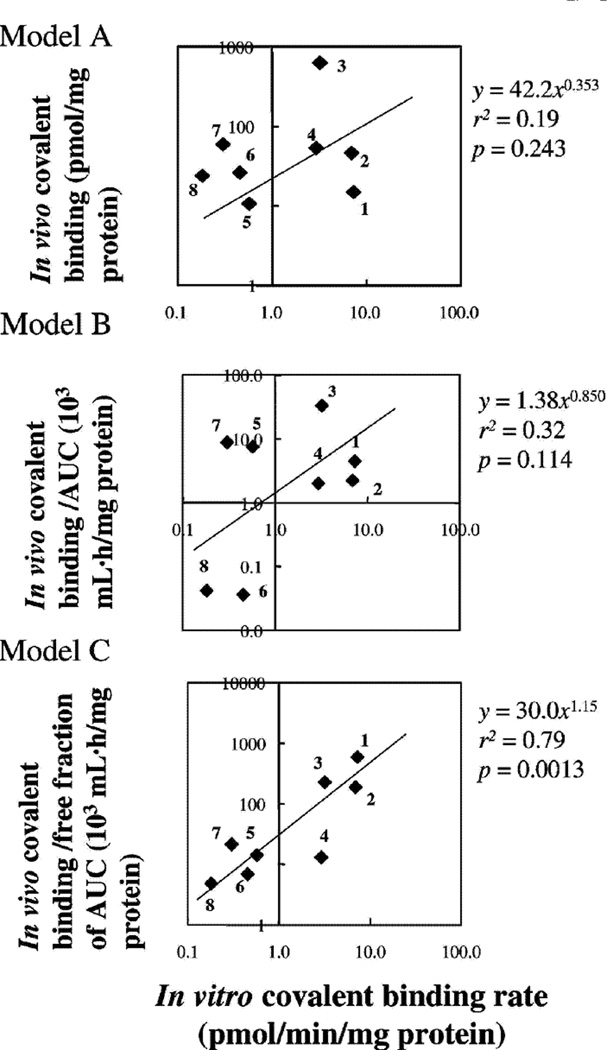
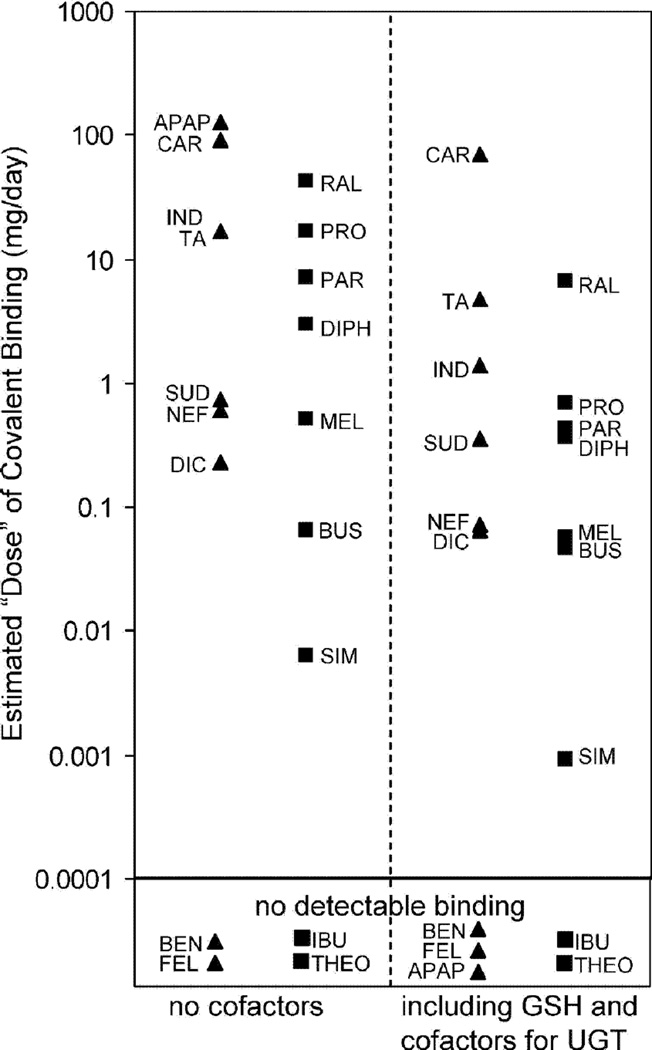
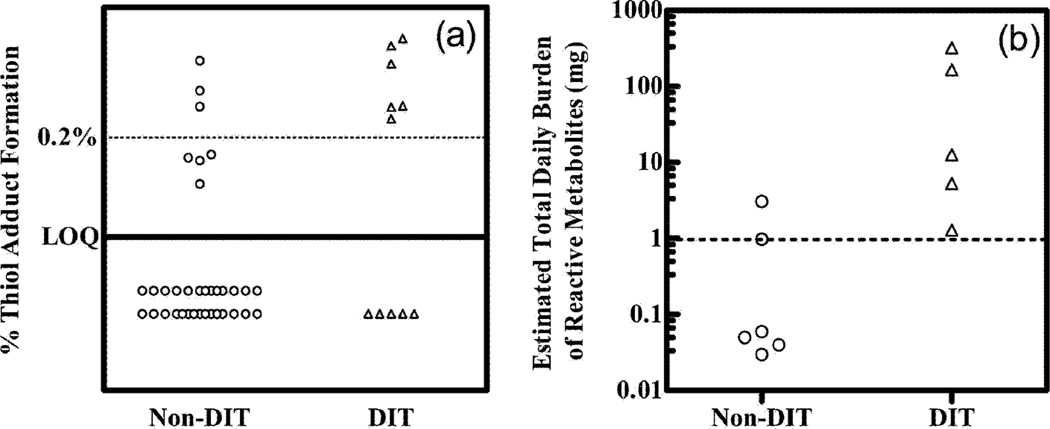
Recently several studies have made careful comparisons of the extent of covalent binding of drugs (in animals) and correlated these with hepatoxicity. Masubuchi et al.57) showed good correlation between rat and human liver microsomes with a series of drugs. Reasonably good in vitro/in vivo correlations were observed57) (Figure 5). In other studies, the degree of covalent binding was higher for hepatotoxic drugs than non-hepatotoxic drugs (Figures 6–8). Nevertheless, the variation in covalent binding was considerable for both the hepatoxic and nonhepatotoxic drugs, and the two sets showed considerable overlap.
Fig. 5.
Relationship between in vitro covalent binding (rate of reactive metabolites to microsomal proteins (10 µM substrate) and in vivo covalent binding (rate) in rat liver tissue after administration of labeled compounds (at 20 mg/kg). Three different models were used.57) 1, Furosemide; 2, tienilic acid; 3, clozapine, 4, imipramine; 6, acetaminophen; 6, indomethacin; 7, carbamazepine; 8, diclofenac.
Fig. 6.
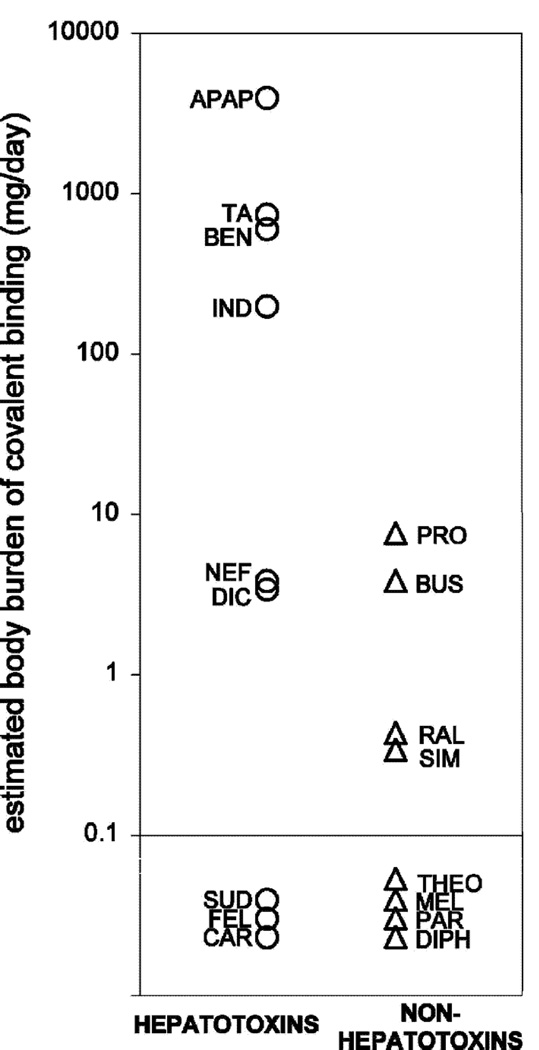
Comparisons of hepatoxins (▲) and non-hepatoxins (■) for estimates of total daily dose of covalently bound material extrapolated from in vitro liver microsomal covalent binding and doses. APAP, acetaminiophen; BEN, benoxaprofen; BUS, buspirone; CAR, carbamazepine; DIC, diclofenac; DIPH, diphenhydramine; FEC, felbamate; IBU, ibuprofen; IND, indomethacin; MEL, meloxicam; NEF, nefazodone; PAR, paroxetine; PRO, propranolol; RAL, raloxifene; SIM, simvastatin; SUD, sudoxicam; TA, tienilic acid; THEO, theophylline.58)
Fig. 8.
Scatter plots of (A) percentage GSH adduct formation and (B) estimated total daily covalent adduct burden in “drug-induced toxicity” (DIT, Δ) and non-drug-induced toxicity (Non-DIT, O) groups of chemicals.60) Horizontal lines are drawn at a (A) 0.2% adduct level and (B) 1 mg body level.
Thus, the proposals of Evans et al.61) to utilize in vitro and in vivo in making decisions about advancing drugs have support from these studies. Another question that one can ask is what fraction of the attrition of drug candidates is related to the five individual contexts of drug toxicity described earlier (Table 2). A definitive answer is not easy, in that this would require information about proprietary compounds (and many compounds are probably not followed up after attrition). However, one estimate has been that 27% of (preclinical) candidates were dropped due to biotransformation-related issues and 28% due to on-target problems (from experience at DuPont-Merck and Bristol-Myers Squibb) (Table 3).28)
Biological Mechanisms: Mitochondrial Toxicity
One of the classic cases of utilizing structure-function relationships in understanding the toxicity of drugs involves the comparison of acetaminophen with its meta-isomer, 3-hydroxyacaetanilide. Both compounds yield similar levels of total covalent binding, both in vitro and in vivo.62,63) However, different reactive intermediates are produced from acetaminophen and the meta-isomer, an iminoquinone and an ortho-quinone respectively. More careful analysis established that acetaminophen generated more mitochondrial binding and the meta-congener more cytosolic binding, apparently related to the stability of the reactive Michael acceptors produced in the two cases.64)
Mitochondrial stress has since developed in terms of a major aspect of drug toxicity.65) Some of the evidence suggest a combination of drug (or drug metabolite) promoting oxidative stress (a “direct” effect) and alteration of signal transduction systems result in further loss of mitochondrial function (an “indirect” effect).65) Oxidative stress can be defined as an imbalance of pro-oxidants and anti-oxidants in a cell, or a cellular compartment. An example has been offered with acetaminophen, i.e. the reactive iminoquinone product reacts with mitochondrial proteins64) and produces mitochondrial injury and reactive oxygen species, the latter which in turn activate cytoplasmic signal transduction pathways. Thioredoxin is one of the proteins oxidized by the reactive oxygen species, which then dissociates from ASK-1 and leads to ASK-1 activation and its phosphorylation and activation of KMM 4/7, which in turn phosphorylates and activates JNK. GSK-3β activity is also enhanced, and JNK and GSK-3β translocate to mitochondria and promote cell death, in part by binding to voltage-dependent anion channels and thus altering the mitochondrial permeability transition.65) Thus, acetaminophen hepatotoxicty can be considered an active process, involving specific signaling molecules and net up-regulation of activity, as opposed to the older concepts of massive inactivation of cellular proteins by reactive metabolites. In addition, recent work has demonstrated that the fraction of a cytochrome P450 (2E1) localized in the mitochondria is much more uncoupled (than the fraction in the endoplasmic reticulum) and generates more reaction oxygen species, as judged by both dye and isoprostane measurements.66)
In part because of the above-mentioned role of oxidative stress, animal models with compromised anti-oxidant capacity have been utilized in efforts to gain insight into drug-induced liver injury and, by extension, to idiosyncratic hepatotoxicity.65,67) Heterozygous sod2 mice (missing one superoxide dismutase 2 allele) have been used and shown to be more sensitive to a number of drugs, with an initial adaptive response followed by a toxic response.68) This model is being utilized not so much for directly evaluating the role of human superoxide dismutase but as a probe for a role of impaired anti-oxidant capacity as a factor in idiosyncratic hepatotoxicity, in that regard resembling the inflammagen model (Table 5). The model also relates to the hypothesis that underlying genetic or acquired mitochondrial abnormalities are a major determinant of susceptibility for a number of drugs that target mitochondria and cause drug-induced liver injury.67) In the future, the availability of extensive genetic analysis methods with patients (i.e., DNA sequencing) may be used to critically test this hypothesis.
High throughput approaches
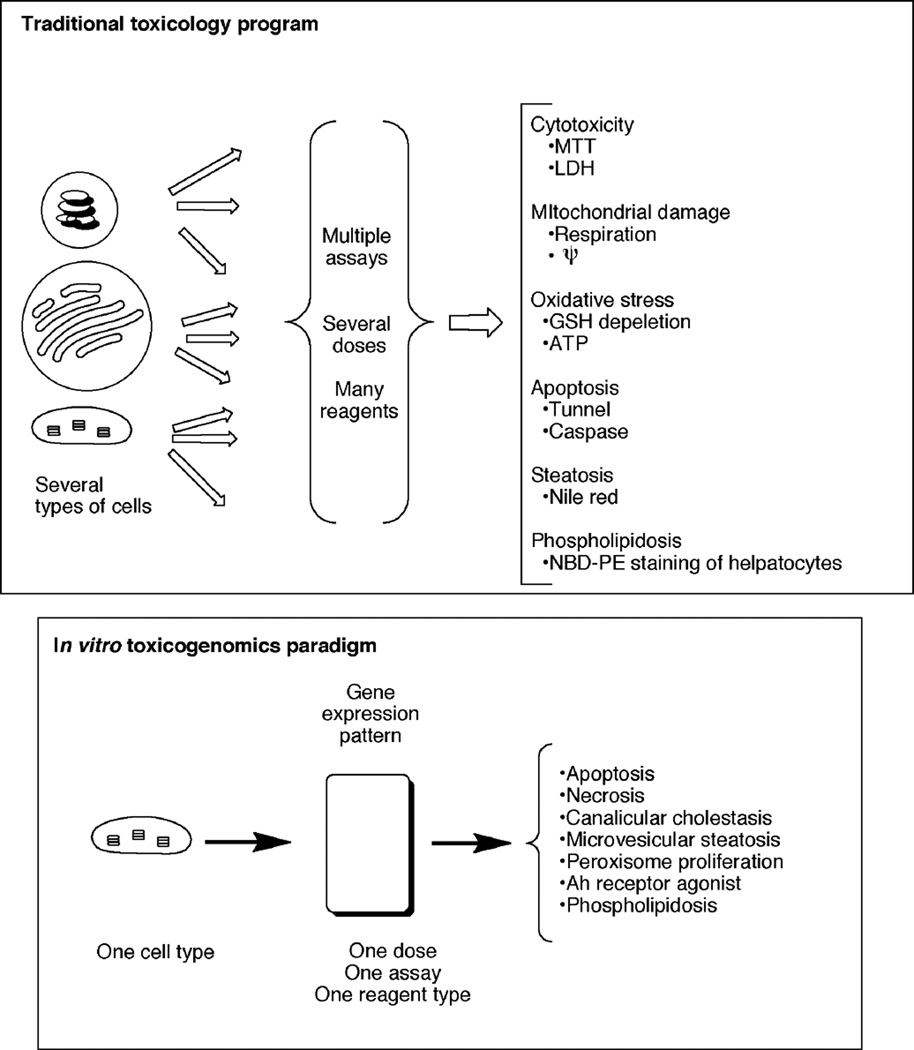
Traditional toxicology approaches are relatively slow, directed toward individual elements of toxicity, and not necessarily relevant to human issues if done with experimental animals. A goal of many researchers in the field is to develop a very simple in vitro assay that will accurately predict multiple toxicities in vivo (Figure 9). Ideally, this all could be done in a human cell line and be predictable for humans (Figure 10). To some extent, the anticipation was that (mRNA) microarrays might be able to achieve such results, to the same extent that an Ames Salmonella typhimurium assay is used as a primary screen for genotoxicity.
Fig. 9.
(A) Traditional in vitro or in vivo toxicity program. (B) Idealized in vitro toxicogenomics system.
Fig. 10.
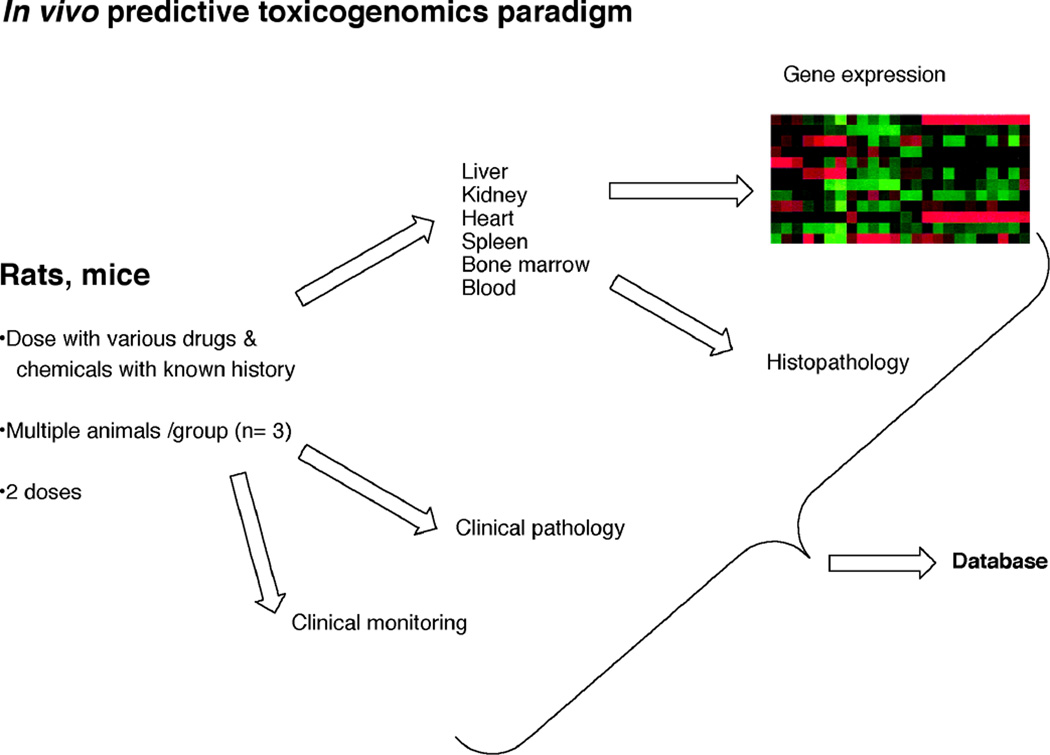
An in vivo predictive toxicogenomics paradigm for database development. The model shown here is the DrugMatrix® system developed by Iconix (now part of Entelos).28)
Microarrays have not been that successful in this regard (at least in providing a single readout diagnostic of all potential toxicities), nor have any proteomics or metabolomics approaches. Such a goal may be unrealistic, in that even if hepatocyte cell mRNA analysis was successful it would probably have limited use in extrapolation to endocrine tissues, kidneys, etc. Moreover, in vitro and in vivo microarray results correspond but the correlation is not perfect.69)
“Omics” applications have been useful, but on a more limited basis and in addressing specific mechanistic questions, rather than as a broad sweep screens. For instance, microarrays allow for detailed pathway analysis of select sets of drug candidates. Considerable effort has been made to develop databases of mRNA responses to known toxic chemicals for which many aspects of toxicity are understood, with the goal of then being able to rapidly assess the potential toxicities of new drug candidates. This approach has been used by the Iconix company (now part of Entelos) (Figure 10), in collaboration with several pharmaceutical companies.70–72)
Recently proteomics efforts have identified a number of candidates that have potential as biomarkers of toxicity.72) In particular, a panel of biomarkers has been evaluated in preclinical studies on nephrotoxicity, and urinary cystatin C, β2-microgloulin, trefoil factor 3, albumin, and kidney injury molecule-1 (Kim-1) have emerged as potentially useful biomarkers.74–77) An advantage to this (proteomics) approach is that it should be transposable to clinical studies.
In silico approaches are also under consideration and, in principle, may be the ultimate goal. Some insight has been obtained with such methods.78–81) To date most of the success has come from correlative relationships as opposed to mechanistic ones. The difficulty with structure-based relationships is that these are not well-established for toxicity, i.e. the targets are often not established, and the results are developed in the absence of basic biological knowledge. For example, structure-activity relationships are relatively well established for gross dioxin toxicity even though there is no structural information available about the Ah receptor.
Conclusions
Although the field of drug toxicity is a difficult one it can also be viewed as one of great opportunities, both in terms of basic science and practical application. To conclude, there are three major issues: (i) identifying useful biomarkers of toxicity; (ii) establishing in vitro/in vivo relationships; and (iii) linking animal models with human toxicity. There are still many known discrepancies in the effects of chemicals on experimental animals and humans (and between species of experimental animals) (Tables 10, 11). The challenges and opportunities can be summarized largely in these three items.
Table 10.
Applying mechanisms of toxicity to human safety.
| What is toxic? (parent drug or metabolite(s)) |
| How is it toxic? |
| What is the dose-response relationship? |
| Does toxicity occur in humans? |
| Can a screen be developed to assess the liability? |
| Can the liability be eliminated? |
Table 11.
Rodent carcinogenic responses not likely to apply to humans.
| Tumor site | Illustrative chemical agents |
|---|---|
| Male rat kidney | d-Limonene, unleaded gasoline |
| Male bladder | Saccharin, nitrilotriacetic acid |
| Rat thyroid | Goitrogens, some alkylcarbamates, fungicides |
| Forestomach | Butylated hydroxyanisole, propionic acid, ethyl acrylate |
| Mouse liver | Barbiturates, peroxisome proliferators |
Fig. 7.
Categorization of hepatoxins and nonhepatotoxins based on estimated total daily body burden covalent binding from human hepatocyte data.59) See Figure 6 for drug abbreviations.
Fig. 11.
Uses of toxicity data at various stages of drug discovery and development. Major steps in the process are shown in boxes, with relevant screens listed below.28)
Acknowledgments
Thanks are extended to K. Trisler for assistance in preparation of the manuscript. Research in this area in the author’s laboratory is supported in part by United States Public Health Service grants R37 CA090426, R01 ES010546, and P30 ES000267.
Reference
- 1.U. S. Food and Drug Administration. Challenge and opportunity on the critical path to new medical products. US Department of Health and Human Services; 2004. Mar, [Google Scholar]
- 2.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 3.Borzelleca JF. Profiles in Toxicology - Paracelsus: herald of modern toxicology. Toxicol. Sci. 2000;53:2–4. doi: 10.1093/toxsci/53.1.2. [DOI] [PubMed] [Google Scholar]
- 4.Liebler DC, Guengerich FP. Elucidating mechanisms of drug-induced toxicity. Nat. Rev. Drug Discov. 2005;4:410–420. doi: 10.1038/nrd1720. [DOI] [PubMed] [Google Scholar]
- 5.Park BK, Kitteringham NR, Maggs JL, Pirmohamed M, Williams DP. The role of metabolic activation in drug-induced hepatotoxicity. Annu. Rev. Pharmacol. Toxicol. 2005;45:177–202. doi: 10.1146/annurev.pharmtox.45.120403.100058. [DOI] [PubMed] [Google Scholar]
- 6.Johnson TE, Zhang X, Bleicher KB, Dysart G, Loughlin AF, Schaefer WH, Umbenhauer DR. Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol. Appl. Pharmacol. 2004;200:237–250. doi: 10.1016/j.taap.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Zipes DP, Zvaifler NJ, Glassock RJ, Gilman S, Munoz A, Gogolak V, Gordis L, Dedon PC, Guengerich FP, Wasserman SI, Witztum JL, Wogan GN. Rosuvastatin: an independent analysis of risks and benefits. MedGenMed. 2006;8:73. [PMC free article] [PubMed] [Google Scholar]
- 8.Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J. Exp. Med. 1935;61:643–656. doi: 10.1084/jem.61.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman HJ. Hepatotoxicity. The Adverse Effects of Drugs and Other Chemicals in the Liver. 2nd. Philadelphia: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 10.Bjornsson E. Drug-induced liver injury: Hy's rule revisited. Clin. Pharmacol. Ther. 2006;79:521–528. doi: 10.1016/j.clpt.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu. Rev. Pharmacol Toxicol. 2007;47:513–539. doi: 10.1146/annurev.pharmtox.47.120505.105150. [DOI] [PubMed] [Google Scholar]
- 12.Uetrecht J. Idiosyncratic drug reactions: past, present, and future. Chem. Res. Toxicol. 2008;21:84–92. doi: 10.1021/tx700186p. [DOI] [PubMed] [Google Scholar]
- 13.Uetrecht J. Immune-mediated adverse drug reactions. Chem. Res. Toxicol. 2009;22:24–34. doi: 10.1021/tx800389u. [DOI] [PubMed] [Google Scholar]
- 14.Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol. Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uetrecht JP. New concepts in immunology relevant to idiosyncratic drug reactions: the "danger hypothesis" and innate immune system. Chem. Res. Toxicol. 1999;12:387–395. doi: 10.1021/tx980249i. [DOI] [PubMed] [Google Scholar]
- 16.Shenton JM, Chen J, Uetrecht JP. Animal models of idiosyncratic drug reactions. Chem.-Biol Interact. 2004;150:53–70. doi: 10.1016/j.cbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Johansson I, Lundqvist E, Bertilsson L, Dahl ML, Sjoqvist F, Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11825–11829. doi: 10.1073/pnas.90.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaune P, Pessayre D, Dansette P, Mansuy D, Manns M. Autoantibodies against cytochromes P450: role in human diseases. Adv. Pharmacol. 1994;30:199–245. doi: 10.1016/s1054-3589(08)60175-1. [DOI] [PubMed] [Google Scholar]
- 19.Bourdi M, Larrey D, Nataf J, Berunau J, Pessayre D, Iwasaki M, Guengerich FP, Beaune PH. A new anti-liver endoplasmic reticulum antibody directed against human cytochrome P-450 IA2: a specific marker of dihydralazine-induced hepatitis. J. Clin. Invest. 1990;85:1967–1973. doi: 10.1172/JCI114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dansette PM, Bonierbale E, Minoletti C, Beaune PH, Pessayre D, Mansuy D. Drug-induced immunotoxicity. Eur. J. Drug Metab. Pharmacokinet. 1998;23:443–451. doi: 10.1007/BF03189993. [DOI] [PubMed] [Google Scholar]
- 21.Ganey PE, Luyendyk JP, Maddox JF, Roth RA. Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem.-Biol. Interact. 2004;150:35–51. doi: 10.1016/j.cbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Luyendyk JP, Shores KC, Ganey PE, Roth RA. Bacterial lipopolysaccharide exposure alters aflatoxin B1 hepatotoxicity: benchmark dose analysis for markers of liver injury. Toxicol. Sci. 2002;68:220–225. doi: 10.1093/toxsci/68.1.220. [DOI] [PubMed] [Google Scholar]
- 23.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 24.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 25.Pichler WJ. Delayed drug hypersensitivity reactions. Ann. Intern. Med. 2003;139:683–693. doi: 10.7326/0003-4819-139-8-200310210-00012. [DOI] [PubMed] [Google Scholar]
- 26.Gerber BO, Pichler WJ. Cellular mechanisms of T cell mediated drug hypersensitivity. Curr. Opin. Immunol. 2004;16:732–737. doi: 10.1016/j.coi.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Kindmark A, Jawaid A, Harbron CG, Barratt BJ, Bengtsson OF, Andersson TB, Carlsson S, Cederbrant KE, Gibson NJ, Armstrong M, Lagerström-Fermér ME, Dellsén A, Brown EM, Thornton M, Dukes C, Jenkins SC, Firth MA, Harrod GO, Pinel TH, Billing-Clason SME, Cardon LR, March RE. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests an underlying immune athogenesis. Pharmacogenetics J. 2008;8:186–195. doi: 10.1038/sj.tpj.6500458. [DOI] [PubMed] [Google Scholar]
- 28.Guengerich FP, MacDonald JS. Applying mechanisms of chemical toxicity to predict drug safety. Chem. Res. Toxicol. 2007;20:344–369. doi: 10.1021/tx600260a. [DOI] [PubMed] [Google Scholar]
- 29.Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Expt. Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- 30.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Expt. Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- 31.Miller EC, Miller JA. The presence and significance of bound amino azodyes in the livers of rats fed p-dimethylaminoazobenzene. Cancer Res. 1947;7:468–480. [Google Scholar]
- 32.Bakke OM, Wardell WM, Lasagna L. Drug discontinuations in the United Kingdom and the United States, 1964 to 1983: issues of safety. Clin. Pharmacol. Ther. 1984;35:559–567. doi: 10.1038/clpt.1984.78. [DOI] [PubMed] [Google Scholar]
- 33.Bakke OM, Manocchia M, de Abajo F, Kaitin KI, Lasagna L. Drug safety discontinuations in the United Kingdom, the United States, and Spain from 1974 through 1993: a regulatory perspective. Clin. Pharmacol. Ther. 1995;58:108–117. doi: 10.1016/0009-9236(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 34.Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch. Intern. Med. 2005;165:1363–1369. doi: 10.1001/archinte.165.12.1363. [DOI] [PubMed] [Google Scholar]
- 35.Walgren JL, Mitchell MD, Thompson DC. Role of metabolism in druginduced idiosyncratic hepatotoxicity. Crit. Rev. Toxicol. 2005;35:325–361. doi: 10.1080/10408440590935620. [DOI] [PubMed] [Google Scholar]
- 36.Nelson SD, Mitchell JR, Timbrell JA, Snodgrass WR, Corcoran GB., 3rd Isoniazid and iproniazid: activation of metabolites to toxic intermediates in man and rat. Science. 1976;193:901–903. doi: 10.1126/science.7838. [DOI] [PubMed] [Google Scholar]
- 37.Bradbury A, Powell GM, Curtis CG, Rhodes C. The enhanced biliary secretion of a taurine conjugate in the rat after intraduodenal administration of high doses of fenclozic acid. Xenobiotica. 1981;11:665–674. doi: 10.3109/00498258109049086. [DOI] [PubMed] [Google Scholar]
- 38.Mathison BH, Murphy SE, Shank RC. Hydralazine and other hydrazine derivatives and the formation of DNA adducts. Toxicol. Appl. Pharmacol. 1994;127:91–98. doi: 10.1006/taap.1994.1143. [DOI] [PubMed] [Google Scholar]
- 39.Qiu Y, Burlingame AL, Benet LZ. Mechanisms for covalent binding of benoxaprofen glucuronide to human serum albumin. Studies by tandem mass spectrometry. Drug Metab. Dispos. 1998;26:246–256. [PubMed] [Google Scholar]
- 40.Obach RS, Dalvie DK. Metabolism of nomifensine to a dihydroisoquinolinium ion metabolite by human myeloperoxidase, hemoglobin, monoamine oxidase-A, and cytochrome P450 enzymes. Drug Metab. Dispos. 2006;34:1310–1316. doi: 10.1124/dmd.106.010173. [DOI] [PubMed] [Google Scholar]
- 41.Kassahun K, Pearson PG, Tang W, McIntosh I, Leung K, Elmore C, Dean D, Wang R, Doss G, Baillie TA. Studies on the metabolism of troglitazone to reactive intermediates in vitro and in vivo. Evidence for novel biotransformation pathways involving quinone methide formation and thiazolidinedione ring scission. Chem. Res. Toxicol. 2001;14:62–70. doi: 10.1021/tx000180q. [DOI] [PubMed] [Google Scholar]
- 42.Kalgutkar AS, Gardner I, Obach RS, Shaffer CL, Callegari E, Henne KR, Mutlib AE, Dalvie DK, Lee JS, Nakai Y, O'Donnell JP, Boer J, Harriman SP. A comprehensive listing of bioactivation pathways of organic functional groups. Curr. Drug Metab. 2005;6:161–225. doi: 10.2174/1389200054021799. [DOI] [PubMed] [Google Scholar]
- 43.Durand A, Thenot JP, Bianchetti G, Morselli PL. Comparative pharmacokinetic profile of two imidazopyridine drugs: zolpidem and alpidem. Drug Metab. Rev. 1992;24:239–266. doi: 10.3109/03602539208996294. [DOI] [PubMed] [Google Scholar]
- 44.Geneve J, Larrey D, Letteron P, Descatoire V, Tinel M, Amouyal G, Pessayre D. Metabolic activation of the tricyclic antidepressant amineptine. I. Cytochrome P-450-mediated in vitro covalent binding. Biochem. Pharmacol. 1987;36:323–329. doi: 10.1016/0006-2952(87)90289-9. [DOI] [PubMed] [Google Scholar]
- 45.Castillo M, Smith PC. Disposition and reactivity of ibuprofen and ibufenac acyl glucuronides in vivo in the rhesus monkey and in vitro with human serum albumin. Drug Metab. Dispos. 1995;23:566–572. [PubMed] [Google Scholar]
- 46.Martinat C, Amar C, Dansette PM, Leclaire J, Lopez-Garcia P, Cao TD, N'Guyen HN, Mansuy D. In vitro metabolism of isaxonine phosphate: formation of two metabolites, 5-hydroxyisaxonine and 2-aminopyrimidine, and covalent binding to microsomal proteins. Eur. J. Pharmacol. 1992;228:63–71. doi: 10.1016/0926-6917(92)90013-3. [DOI] [PubMed] [Google Scholar]
- 47.Reid JM, Kuffel MJ, Miller JK, Rios R, Ames MM. Metabolic activation of dacarbazine by human cytochromes P450: the role of CYP1A1, CYP1A2, and CYP2E1. Clin. Cancer Res. 1999;5:2192–2197. [PubMed] [Google Scholar]
- 48.Arnold TH, Jr, Epps JM, 3rd, Cook HR, Hamrick ME. Dantrolene sodium: urinary metabolites and hepatotoxicity. Res. Commun. Chem. Pathol. Pharmacol. 1983;39:381–398. [PubMed] [Google Scholar]
- 49.Dieckhaus CM, Santos WL, Sofia RD, Macdonald TL. The chemistry, toxicology, and identification in rat and human urine of 4-hydroxy-5-phenyl-1,3-oxazaperhydroin-2-one: a reactive metabolite in felbamate bioactivation. Chem. Res. Toxicol. 2001;14:958–964. doi: 10.1021/tx000139n. [DOI] [PubMed] [Google Scholar]
- 50.Berson A, Wolf C, Chachaty C, Fisch C, Fau D, Eugene D, Loeper J, Gauthier JC, Beaune P, Pompon D, Maurel P, Pessayre D. Metabolic activation of the nitroaromatic antiandrogen flutamide by rat and human cytochrome P-450, including forms belonging to the 3A and 1A subfamilies. J. Pharmacol. Expt. Ther. 1993;265:366–372. [PubMed] [Google Scholar]
- 51.Cohen AD, Luger SM, Sickles C, Mangan PA, Porter DL, Schuster SJ, Tsai DE, Nasta S, Gewirtz AM, Stadtmauer EA. Gemtuzumab ozogamicin (Mylotarg) monotherapy for relapsed AML after hematopoietic stem cell transplant: efficacy and incidence of hepatic veno-occlusive disease. Bone Marrow Transplant. 2002;30:23–28. doi: 10.1038/sj.bmt.1703602. [DOI] [PubMed] [Google Scholar]
- 52.Timbrell JA, Mitchell JR, Snodgrass WR, Nelson SD. Isoniazid hepatoxicity: the relationship between covalent binding and metabolism in vivo. J. Pharmacol. Expt. Ther. 1980;213:364–369. [PubMed] [Google Scholar]
- 53.Rodriguez RJ, Acosta D., Jr N-Deacetyl ketoconazole-induced hepatotoxicity in a primary culture system of rat hepatocytes. Toxicology. 1997;117:123–131. doi: 10.1016/s0300-483x(96)03560-3. [DOI] [PubMed] [Google Scholar]
- 54.Smith KS, Smith PL, Heady TN, Trugman JM, Harman WD, Macdonald TL. In vitro metabolism of tolcapone to reactive intermediates: relevance to tolcapone liver toxicity. Chem. Res. Toxicol. 2003;16:123–128. doi: 10.1021/tx025569n. [DOI] [PubMed] [Google Scholar]
- 55.Baillie TA. Metabolic activation of valproic acid and drug-mediated hepatotoxicity. Role of the terminal olefin, 2-n-propyl-4-pentenoic acid. Chem. Res. Toxicol. 1988;1:195–199. doi: 10.1021/tx00004a001. [DOI] [PubMed] [Google Scholar]
- 56.Uetrecht JP. Is it possible to more accurately predict which drug candidates will cause idiosyncratic drug reactions? Curr. Drug Metab. 2000;1:133–141. doi: 10.2174/1389200003339081. [DOI] [PubMed] [Google Scholar]
- 57.Masubuchi N, Makino C, Murayama N. Prediction of in vivo potential for metabolic activation of drugs into chemically reactive intermediate: correlation of in vitro and in vivo generation of reactive intermediates and in vitro glutathione conjugate formation in rats and humans. Chem. Res. Toxicol. 2007;20:455–464. doi: 10.1021/tx060234h. [DOI] [PubMed] [Google Scholar]
- 58.Obach RS, Kalgutkar AS, Soglia JR, Zhao SX. Can in vitro metabolism-dependent covalent binding data in liver microsomes distinguish hepatotoxic from nonhepatotoxic drugs? An analysis of 18 drugs with consideration of intrinsic clearance and daily dose. Chem. Res. Toxicol. 2008;21:1814–1822. doi: 10.1021/tx800161s. [DOI] [PubMed] [Google Scholar]
- 59.Bauman JN, Kelly JM, Tripathy S, Zhao SX, Lam WW, Kalgutkar AS, Obach RS. Can in vitro metabolism-dependent covalent binding data distinguish hepatotoxic from nonhepatotoxic drugs? An analysis using human hepatocytes and liver S-9 fraction. Chem. Res. Toxicol. 2009;22:332–340. doi: 10.1021/tx800407w. [DOI] [PubMed] [Google Scholar]
- 60.Gan J, Ruan Q, He B, Zhu M, Shyu WC, Humphreys WG. In vitro screening of 50 highly prescribed drugs for thiol adduct formation--comparison of potential for drug-induced toxicity and extent of adduct formation. Chem. Res. Toxicol. 2009;22:690–698. doi: 10.1021/tx800368n. [DOI] [PubMed] [Google Scholar]
- 61.Evans DC, Watt AP, Nicoll-Griffith DA, Baillie TA. Drug-protein adducts: an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem. Res. Toxicol. 2004;17:3–16. doi: 10.1021/tx034170b. [DOI] [PubMed] [Google Scholar]
- 62.Streeter AJ, Bjorge SM, Axworthy DB, Nelson SD, Baillie TA. The microsomal metabolism and site of covalent binding to protein of 3'-hydroxyacetanilide, a non-hepatotoxic positional isomer of acetaminophen. Drug Metab. Dispos. 1984;12:565–576. [PubMed] [Google Scholar]
- 63.Roberts SA, Price VF, Jollow DJ. Acetaminophen structure-toxicity studies: in vivo covalent binding of a non-hepatotoxic analog, 3-hydroxyacetanilide. Toxicol. Appl. Pharmacol. 1990;105:195–208. doi: 10.1016/0041-008x(90)90181-s. [DOI] [PubMed] [Google Scholar]
- 64.Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a non-hepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- 65.Jones DP, Lemasters JJ, Han D, Boelsterli UA, Kaplowitz N. Mechanisms of pathogenesis in drug hepatotoxicity putting the stress on mitochondria. Mol. Interv. 2010;10:98–111. doi: 10.1124/mi.10.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bansal S, Liu C-P, Sepuri NBV, Anandatheerthavarada HK, Milne GL, Guengerich FP, Avadhani NG. Mitochondria-targeted cytochrome P450 2E1 preferentially induces oxidative damage and augments alcohol mediated mitochodrial dysfunction in cultured cells. J. Biol. Chem. 2010;285:24609–24619. doi: 10.1074/jbc.M110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boelsterli UA, Lim PL. Mitochondrial abnormalities—a link to idiosyncratic drug hepatotoxicity? Toxicol. Appl. Pharmacol. 2007;220:92–107. doi: 10.1016/j.taap.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 68.Lee YH, Chung MCM, Sin Q, Boelsterli UA. Troglitazone-induced hepatic mitochondrial proteome expression dynamics in heterozygous Sod2+/− mice: Two-stage oxidative injury. Toxicol. Appl. Pharmacol. 2008;231:43–51. doi: 10.1016/j.taap.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 69.Ryan TP, Stevens JL, Thomas CE. Strategic applications of toxicogenomics in early drug discovery. Curr. Opin. Pharmacol. 2008;8:654–660. doi: 10.1016/j.coph.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Davis JW, Kramer JA. Genomic-based biomarkers of drug-induced nephrotoxicity. Expert Opin. Drug Metab. Toxciol. 2006;2:95–101. doi: 10.1517/17425255.2.1.95. [DOI] [PubMed] [Google Scholar]
- 71.Ganter B, Snyder RD, Halbert DN, Lee MD. Toxicogenomics in drug discovery and develoment: mechanistic analysis of compound/class-dependent effects using the DrugMatrix® database. Future Med. 2006;7:1025–1044. doi: 10.2217/14622416.7.7.1025. [DOI] [PubMed] [Google Scholar]
- 72.Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR. Induction of Cyp1A1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol. Pharmacol. 2007;71:1475–1486. doi: 10.1124/mol.106.032748. [DOI] [PubMed] [Google Scholar]
- 73.Amacher DE. The discovery and development of proteomic safety biomarkers for the detection of drug-induced liver toxicity. Toxicol. Appl. Pharmacol. 2010;245:134–142. doi: 10.1016/j.taap.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 74.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, End P, Staedtler F, Legay F, Carl K, Laurie D, Chibout SD, Vonderscher J, Maurer G. Urinary clusterin, cystatin C, 㬲2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat. Biotechnol. 2010;28:463–469. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 75.Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, Shi S, Figueroa DJ, Clouse H, Su M, Muniappa N, Troth SP, Bailey W, Seng J, Aslamkhan AG, Thudium D, Sistare FD, Gerhold DL. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat. Biotechnol. 2010;28:470–477. doi: 10.1038/nbt.1624. [DOI] [PubMed] [Google Scholar]
- 76.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, Staedtler F, Mahl A, Grenet O, Roth DR, Wahl D, Legay F, Holder D, Erdos Z, Vlasakova K, Jin H, Yu Y, Muniappa N, Forest T, Clouse HK, Reynolds S, Bailey WJ, Thudium DT, Topper MJ, Skopek TR, Sina JF, Glaab WE, Vonderscher J, Maurer G, Chibout SD, Sistare FD, Gerhold DL. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat. Biotechnol. 2010;28:486–494. doi: 10.1038/nbt.1627. [DOI] [PubMed] [Google Scholar]
- 78.Sanderson DM, Earnshaw CG. Computer prediciton of posible toxic action from chemical structure; the DEREK system. Hum. Exp. Toxicol. 1991;10:261–273. doi: 10.1177/096032719101000405. [DOI] [PubMed] [Google Scholar]
- 79.Greene N, Judson PN, Langowski JJ, Marchant CA. Knowledge-based expert systems for toxicity and metabolism prediction: DEREK, StAR and METEOR. SAR QSAR Environ. Res. 1999;10:299–314. doi: 10.1080/10629369908039182. [DOI] [PubMed] [Google Scholar]
- 80.Deardon JC. In silico prediciton of ADMET properties: how far have we come? Expert Opin. Drug Metab. Toxciol. 2007;3:635–639. doi: 10.1517/17425255.3.5.635. [DOI] [PubMed] [Google Scholar]
- 81.Greene N, Fisk L, Naven RT, Note RR, Patel ML, Pelletier DJ. Developing structure-activity relationships for the prediction of hepatotoxicity. Chem. Res. Toxicol. 2010;23:1215–1222. doi: 10.1021/tx1000865. [DOI] [PubMed] [Google Scholar]