Abstract
Carbon nanotubes (CNT) have been developed into new materials with a variety of industrial and commercial applications. In contrast, the physicochemical properties of CNT at the nanoscale render them the potency to generate toxic effects. Indeed, the potential health impacts of CNT have drawn a great deal of attention in recent years, owing to their identified toxicological and pathological consequences including cytotoxicity, inflammation, fibrosis, genotoxicity, tumorigenesis, and immunotoxicity. Understanding the mechanisms by which CNT induce toxicity and pathology is thus urgently needed for accurate risk assessment of CNT exposure in humans, and for safe and responsible development and commercialization of nanotechnology. Here, we summarize and discuss recent advances in this area with a focus on the molecular interactions between CNT and mammalian systems, and the signaling pathways important for the development of CNT toxicity such as the NF-κB, NLRP3 inflammasome, TGF-β1, MAPK, and p53 signaling cascades. With the current mechanistic evidence summarized in this review, we expect to provide new insights into CNT toxicology at the molecular level and offer new clues to the prevention of health effects resulting from CNT exposure. Moreover, we disclose questions and issues that remain in this rapidly advancing field of nanotoxicology, which would facilitate ascertaining future research directions.
Keywords: Carbon nanotubes, fiber toxicity, mechanism, signaling pathway, nanotoxicology
Introduction
The development of nanotechnology has led to the creation of a vast array of nanomaterials in the recent few decades. The engineered nanomaterials have at least one dimension of less than 100 nm, but vary in size, shape, chemical composition, and surface characteristics considerably, giving rise to distinct and unique physicochemical and conducting properties highly desirable for industrial and commercial applications. As such, nanotechnology and nanomaterials are poised to revolutionize numerous fields (IWGN, 1999; NSF, 2011). However, the rapid increase in the production and use of nanomaterials may lead to greater exposure of workers, consumers, and the environment, which, alongside the uncertainty of the biological effects of nanoexposure, has raised considerable concerns over their potential effects on human health (Council, 2012; NIOSH, 2013b).
The large number and many variations of nanomaterials produced and their properties at the nanoscale have made it difficult, if not all impossible, to examine the entire nanomaterials through conventional toxicological characterizations, making risk assessment of nanoexposure a formidable task in the field of nanotoxicology. Therefore, it is imperative for toxicologists not to test every variation of a new nanomaterial, but to elucidate the mode of action from representative nanomaterials, such as identification of key factors and pathways that govern the interactions between nanomaterials and biological systems and their pathological consequences. Such information could then be used to predict toxicity, guide targeted screening, and allow safety to be built into the design of nanomaterials and their applications, with a goal to ultimately foster the safe and responsible development of the nanoindustry (Maynard et al., 2011; Stone & Donaldson, 2006). From this prospect, carbon nanotubes (CNT) with sp2 carbon bonding and excellent mechanical, electrical, thermal, and transport properties have been selected as a model nanomaterial to demonstrate the road of nanomaterials towards industry (De Volder et al., 2013; Zhang et al., 2013). Collateral to the rapid development of CNT nanotechnology, a large body of toxicological data has accumulated to characterize the potential health effects of CNT and as a result, the study on CNT toxicity has had a major impact on our understanding of the potential health effects of nanoexposure on humans over the past decade (Donaldson et al., 2010; Johnston et al., 2010).
CNT are made of one-atom-thick carbon walls called graphene that roll into long and hollow nanostructures with either a single layer (single-walled CNT, SWCNT) or concentric multiple layers (multi-walled CNT, MWCNT). SWCNT and MWCNT have large surface areas that can be modified to introduce specific functions on pristine nanofibers, further increasing the complexity and diversity of CNT. As a newly developed material, the current annual production capability of CNT has reached several thousand tons, and because of their outstanding tensile and electro- and thermal-conducting properties, CNT have been developed with a variety of applications in both industrial and consumer products, ranging from electronics, such as rechargeable batteries, to biomedical uses, such as medical devices and drug delivery (De Volder et al., 2013; Zhang et al., 2013).
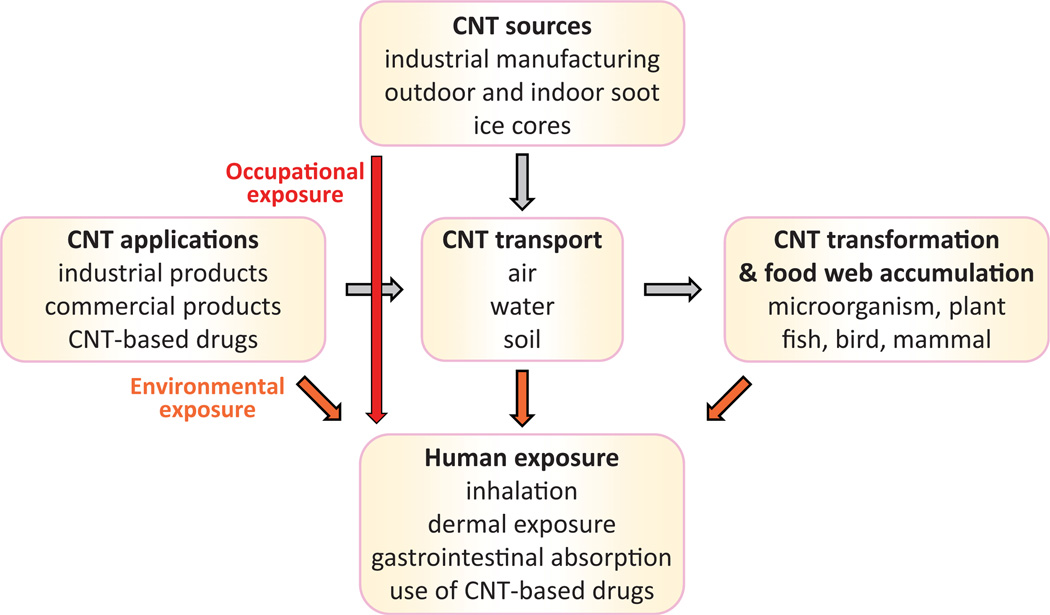
The initial concern over the health impact of CNT exposure stemmed from the notion that most CNT are respirable fibers with a high aspect (length to width) ratio, and are presumed to have substantial biodurability and insolubility in biological systems, attributes known to cause the fibrotic and tumorigenic effects of toxic fibers such as asbestos (Donaldson et al., 2006; Morgan & Gee, 1995). Animal testing has demonstrated that certain forms of CNT can cause fibrosis in the lungs of mice and rats upon pulmonary exposure, and the formation of mesotheliomas when injected into the peritoneal cavity of susceptible mice (Lam et al., 2004; Muller et al., 2005; Poland et al., 2008; Porter et al., 2010; Shvedova et al., 2005). Exposure to CNT can occur from industrial, commercial, and environmental sources, as the life cycles of CNT and their commercial products include not only their innovation and manufacturing but also their commercialization, consumer usage, disposal, recovery, and recycling (Figure 1). Owing to the above factors, the potential adverse impacts of CNT exposure in humans have drawn a great deal of attention, and some basic understanding on CNT pathology has been achieved, but detailed characterization awaits further investigation. Nonetheless, progress has been made in several aspects in the recent few years, which provides a necessary knowledge base to guide future mechanistic and translational studies on nanotoxicity and safety.
Figure 1.
Human exposure to carbon nanotubes. The major source of CNT is industrial manufacturing, owing to a diversity of CNT applications. The production activity leads to a direct occupational exposure to humans, a release of CNT to natural environment, and potentially an accumulation of CNT in food web as CNT are resistant to degradation mechanisms. Humans can be exposed to CNT through inhalation, skin absorption, ingestion, or the use of CNT-carried drugs.
First and foremost, characterization of CNT toxicity in a variety of animal and in vitro models has revealed an increasing list of toxic effects of CNT exposure, which includes, in addition to the originally suspected fibrotic and mesothelioma-causing effects, a range of cytotoxic effects, inflammation, genotoxicity, and immune modulation. These findings have created a broader basis for assessing the structure–activity relationship, mechanism of action, and health risk of CNT toxicity. Second, analysis of the structure–activity relationship has uncovered a close correlation between the toxicity and the size, shape, composition, and surface characteristics of CNT fibers, which impact the distribution, clearance, internal dose, and intrinsic pathogenicity of CNT fibers. Such knowledge is highly desirable for improving nanosafety by the way of prevention-through-design. Third, a body of information has been gathered to reveal CNT’s actions at cellular, subcellular, and molecular levels, which provided considerable new insights into the mechanisms of CNT toxicity. Fourth, a number of signaling pathways have been shown to be significantly activated by CNT and to play important roles in the development of CNT pathologic effects, such as tumorigenesis, inflammation, and fibrosis. A better understanding of the pathways in CNT toxicity would be critical for future research on the mechanisms, biomarkers, and intervention of CNT toxicity.
This review is intended to display the current understanding on CNT toxicity at the molecular level with a focus on the mechanisms and signaling pathways that play major roles in determining the dynamic behavior and pathologic effects of CNT in mammalian systems. Implications of the findings and questions that remain to be addressed in the field of nanotoxicology will be discussed to facilitate the founding of future research directions. Although CNT toxicity may be generated from a number of routes of exposure that include dermal and oral exposures in addition to inhalation (Johnston et al., 2010), the health concerns and toxicological studies on CNT exposure have mostly involved the pulmonary effects of CNT fibers at the present stage. Therefore, to facilitate mechanistic understanding of the major toxic effects of CNT, we focused the current review on CNT pulmonary toxicity from respiratory exposures.
The expanding CNT toxicity
The manifestations of the pulmonary effects of inhaled particles and fibers, which typically include inflammation and tumorigenesis, vary considerably. Those caused by inert or “nuisance dusts”, i.e. carbon black (CB) and titanium dioxide (TiO2) particles, follow a similar dose–response curve, characteristic of poorly soluble low toxicity (PSLT) particles; whereas those of more chemically active materials, i.e. crystalline silica and asbestos fibers, demonstrate a markedly different dose–response relationship, typical of high toxicity dusts (Borm et al., 2004; Maynard & Kuempel, 2005). A major accomplishment in the study of CNT toxicity over the past decade has been the recognition that CNT are capable of eliciting a wide range of biological effects in experimental systems, which exceed beyond what would be predicted from insoluble and “nuisance dust”-like materials, but are more similar to those of high toxicity dusts.
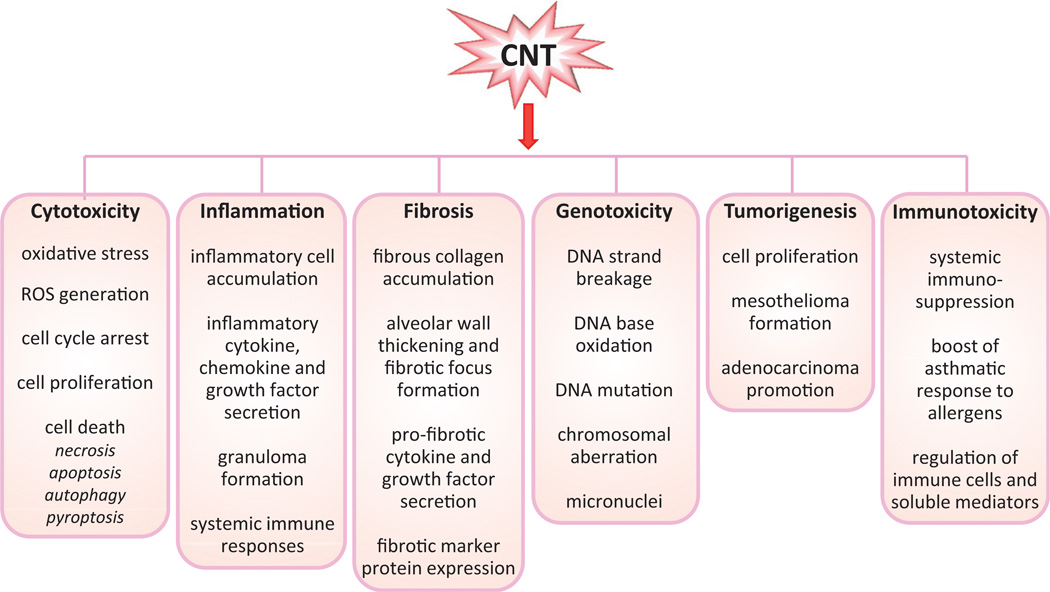
Demonstrated CNT toxicity includes various cytotoxic effects such as oxidative stress, mitochondrial damage, cell cycle arrest, and cell death; acute and chronic inflammation; interstitial fibrosis and formation of granulomas; genotoxicity with DNA and chromosomal aberrations; tumorigenesis such as formation of mesotheliomas in the mesothelial spaces and promotion of adenocarcinomas in the lungs; and modulation of immune functions such as immunosuppression and boosting asthmatic responses to allergens. To facilitate our discussion on the mechanisms of CNT action, we summarized recent findings on CNT toxicity in Figure 2. Detailed discussions on CNT toxicity can be found in several recent reviews by others (Johnston et al., 2010; Nerl et al., 2011; Zhao & Liu, 2012).
Figure 2.
Toxicological and pathological effects of carbon nanotubes. A variety of CNT-induced effects have been identified in recent studies performed in cultured mammalian cells and experimental animals. These effects demonstrate the potential health impacts of CNT exposure on humans.
The effect of CNT on mammalian cells has been reported in multiple cell types, which provides guidance for screening, and mechanistic and in vivo investigations on CNT toxicity. CNT conferred cytotoxicity in a dose- and time-dependent manner in different cell types (Donaldson et al., 2006; Jia et al., 2005; Johnston et al., 2010; Pacurari et al., 2008). Both SWCNT and MWCNT induced secretion of inflammatory cytokines, chemokines, and growth factors such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, monocyte chemotactic protein (MCP)-1, and transforming growth factor (TGF)-β1, in mouse RAW264.7 macrophages (He et al., 2011, 2012; Shvedova et al., 2005), indicating that CNT have the potential to trigger inflammatory responses. CNT-treated cells demonstrated elevated levels of intracellular reactive oxygen species (ROS) in a variety of cell types, which leads to oxidative stress and toxicity (Alarifi et al., 2014; He et al., 2011, 2012; Pacurari et al., 2008). In addition, CNT generate genotoxic effects in cultured cells, such as DNA strand breakage, DNA base oxidation, formation of micronuclei, and chromosomal aberrations, which were recently reviewed by van Berlo et al. (2012). These genotoxic effects of CNT suggest a potential of CNT to cause cancer in animals and humans.
In vivo characterization of the responses to CNT exposure in animals has been pivotal in disclosing the hazardous effects of CNT. First, CNT were found to induce lung inflammation and fibrosis in mice and rats, as have been predicted from their size and fiber shape (Aiso et al., 2010; Dong et al., 2014; Lam et al., 2004; Mangum et al., 2006; Muller et al., 2005; Park et al., 2011; Porter et al., 2010, 2013; Reddy et al., 2012; Shvedova et al., 2005; Wang et al., 2013; Warheit et al., 2004). The inflammatory response includes the accumulation of inflammatory cells and elevated secretion of cytokines, chemokines and growth factors in the lungs and the bronchoalveolar lavage (BAL) fluid, and formation of epithelioid granulomas in the lung parenchyma. The fibrogenic response was evaluated by histopathological analysis of lung tissues, such as increased fibrous collagen level and thickened alveolar wall. In addition, inflammation and fibrosis were observed in the pleural space and the abdominal cavity upon direct injection into the spaces (Murphy et al., 2011; Poland et al., 2008; Ryman-Rasmussen et al., 2009a). Second, from the genotoxicity of CNT identified in cultured cells (discussed above) and in different strains of mice (Kato et al., 2013; Patlolla et al., 2010; Shvedova et al., 2008a), carcinogenesis has been linked to CNT-induced pathologic effects. Mesotheliomas were observed in mice and rats after exposure to MWCNT through intraperitoneal or intrascrotal injection (Nagai et al., 2011; Sakamoto et al., 2009; Takagi et al., 2012). Recently, it was reported that inhalation of MWCNT at a dose relevant to human occupational exposures, following administration of a tumor initiator methylcholanthrene (MCA), induced a high incidence of lung adenocarcinomas in mice, demonstrating MWCNT is a potent tumor promoter in the lungs (Sargent et al., 2014). Third, CNT modulate immune functions both systemically and locally. Inhalation of MWCNT caused systemic immunosup-pression in mice characterized by reduced T-cell-dependent antibody to sheep erythrocytes, reduced T-cell proliferation in the presence of Concanavalin A, and decreased NK (natural killer) cell activity (Mitchell et al., 2007). Inhalation of MWCNT also exacerbated airway remodeling in a murine allergic asthma model pre-challenged with ovalbumin (OVA), revealing a potential of CNT to increase asthmatic responses (Inoue et al., 2009; Ryman-Rasmussen et al., 2009b). In addition, CNT have been shown to modulate the expression of multiple cytokines and other factors critical in immune functions such as Th2 (T helper 2) cytokines and IgE (immunoglobulin E) (Park et al., 2009; Yamaguchi et al., 2012).
Although the spectrum of CNT toxicity has expanded substantially, many of the observed biological effects of CNT are scalable and thus, are predictable from those of non-nanoscaled materials. Nonetheless, it has been postulated that some CNT fibers, because of their similarity in size and shape to certain structures in the cell, such as the spindle microtubules, may replace or perturb the function of the cellular structures to result in harm to the cell, for instance, disrupt the mitotic spindle during mitosis to lead to clastogenic effects (Muller et al., 2008; Sargent et al., 2012). However, further evidence from molecular, cellular, and animal studies are needed to substantiate this hypothesis. Therefore, whether and how the unique properties of CNT at the nanoscale, which defines nanotechnology, can be applied to the propensity of the materials to cause harm in biological systems remain a major challenge for future study.
Another major challenge in the study of CNT toxicity derives from the fact that the manufactured nanomaterials differ considerably in their structures and physicochemical properties, making it very difficult, if not impossible, to compare among different forms of CNT, for instance, between SWCNT and MWCNT, despite all CNT use graphene as the building block and have a fiber-like shape. Even with the same CNT product, minor variations among different batches of manufacturing may alter their behaviors and toxicological endpoints in biological systems considerably, underscoring the importance of identifying CNT properties critical for their toxicity in nanosafety evaluation.
Factors affecting the intrinsic pathogenicity and internal dose of CNT
Considerable information has been gathered to disclose the physicochemical properties and in vivo kinetic behaviors of CNT that determine the intrinsic pathogenicity and internal dose of the nanomaterials, inasmuch as such knowledge would facilitate predicting the health risks of CNT and designing safer nanomaterials.
Solubility and biopersistence
On one hand, pristine CNT are insoluble in biological fluids. Moreover, CNT are generally considered highly biopersistent, i.e. having a long half-life, in biological systems compared with most other carbonaceous substances for several reasons. First, CNT are resistant to biological degradation mechanisms, because their building block, graphene, is an exceedingly strong material made of strong sp2, instead of the more common sp3, carbon–carbon bonds. Second, CNT fibers with a large aspect ratio, for instance, a length of >10 µm, are more difficult to be cleared off from the lungs and plural space by macrophages and through size-dependent mechanisms, such as the stomatal openings, as discussed in more detail below. Third, CNT also tend to form large bundles and aggregates in tissues making them more difficult to be removed from their site of deposition.
On the other hand, a recent study indicated that SWCNT may be susceptible to myeloperoxidase (MPO)-catalyzed and reactive radical-dependent degradation in vitro in neutrophils and, to a lesser extent, in macrophages. In this scenario, the basic amino acids of MPO interact with the carboxyl groups of SWCNT to position the nanotubes near the enzyme’s catalytic site. Hypochlorite and the reactive radical intermediate of MPO carry out the degradation (Kagan et al., 2010). Consistent with these findings, MPO knockout mice were shown to have impaired clearance and enhanced pulmonary inflammatory and fibrotic responses to SWCNT in the lungs (Shvedova et al., 2012a). Whether MPO catalyzes the degradation of more sophisticated CNT including MWCNT effectively remains to be examined. Nonetheless, the findings provide new insights into CNT biodegradation and biopersistence, and suggest potential new ways of reducing the pathogenicity of carbonaceous nanomaterials including CNT in the body via MPO.
As a result of the CNT’s insolubility and biopersistence, CNT toxicity, typified by pulmonary inflammation and fibrosis, resembles a response to foreign body deposition, wherein the deposition of CNT in alveoli and small airways causes local damage and triggers acute inflammation characterized by marked infiltration of inflammatory cells. The alveolar and interstitial macrophages engulf deposited CNT to facilitate their clearance via the mucociliary clearance system of the airway or to the circulation via local lymphatic vessels. As discussed above, fibrogenic CNT are resistant to these mechanisms of clearance, leading to their continued presence and accumulation in the lungs and consequently, chronic inflammation, interstitial fibrosis, and granuloma formation (Mercer et al., 2011).
Rigidity and physical state
MWCNT and SWCNT differ in their rigidity and physical state, which affect the dispersion, distribution, and pathogenicity of the fibers.
Long and straight (i.e. needle-like) MWCNT fibers with lengths of 5–20 µm, but not short, entangled MWCNT (1–5 µm), were shown to exhibit asbestos-like behavior and induced mesothelial granulomatous inflammation upon injection into mouse peritoneal cavity (Poland et al., 2008). Long, needle-like CNT and asbestos, but not CB, short CNT, or long and tangled CNT, stimulated the secretion of IL-1β from macrophages, indicating activation of the inflammasome pathway responsible for the maturation and secretion of IL-1β (Palomaki et al., 2011).
In recent studies, MWCNT and SWCNT were compared: the MWCNT tested are fiber-like with a mean diameter of 49 nm and a mean length of 3.9 µm; whereas the SWCNT are thread filament-like with a mean diameter of 1–4 nm and a length of several hundred nm. Thus, the MWCNT are more rigid and straighter than the SWCNT. The MWCNT appeared to be more easily dispersed than the SWCNT in solution; and importantly, the MWCNT showed a greater tendency to penetrate cells and membranous structures, and to reach distal spaces and organs from the port of entry in vivo, including the pleural space of the thorax and extra-pulmonary organs such as the kidneys and liver. These findings raised the possibility of extra-pulmonary effects from inhalation of CNT (Mercer et al., 2011, 2013b).
CNT may aggregate to form agglomerates in the form of ropes (fiber-like), loose bundles, or large agglomerate mass (particlelike). Aggregation of CNT fibers would change the overall surface area available for interaction with target cells and molecules and thereby, affect CNT biological effects. Compared with singlet or small bundles of CNT, large agglomerates of CNT are more difficult to be cleared off and thus, tend to stimulate the formation of granulomas in which macrophages are transformed to epithelioid cells to segregate CNT from the surrounding tissues. CNT agglomerates may also release singlet CNT fibers over time, resulting in the redistribution and alteration of the internal dose of CNT in different compartments and organ systems in the body, causing extended and potentially, unexpected effects in extrapulmonary organs (Mercer et al., 2013a).
In one study, four SWCNT preparations from the same CNT source (raw CNT, CNT agglomerates, well-dispersed CNT bundles, and pellet from centrifugation of the CNT bundles that contains non-tube shaped carbonaceous particulate matters) were compared. The CNT raw material and agglomerates, but not CNT bundles, were found to inhibit the proliferation of a mesothelioma cell line, supporting the notion that the physical states of CNT affect toxicity (Wick et al., 2007). In another study, treatment with acetone was used to reduce Van der Waals attractions among CNT fibers to result in a better “dispersed” preparation. Acetone treatment reduced the diameters of SWCNT aggregates from ~15.2 µm to ~0.69 µm. The untreated SWCNT were shown to be easily encased by macrophages to form granulomas, whereas the acetone-treated SWCNT induced an interstitial fibrotic response without apparent granuloma formation. These findings suggest that agglomeration of CNT promotes macrophage engulfment of CNT fibers to induce granulomatous inflammation (Mercer et al., 2008).
In the comparison between SWCNT and MWCNT discussed above, the SWCNT were mostly present within the interstitial space (up to 90% of the lung burden) with few being incorporated into alveolar macrophages upon inhalation into the lungs; but the MWCNT were predominantly distributed within the alveolar and interstitial macrophages (68% of the lung burden). The differential distribution patterns of MWCNT and SWCNT in the lungs appeared to correlate with their ability to induce the formation of granulomas (Mercer et al., 2011). What accounts for the differential distribution of the SWCNT and MWCNT fibers is currently unclear. Presumably, the properties of SWCNT and MWCNT, such as rigidity and physical state, as well as surface area and fiber length, affect their interactions with macrophages and the microenvironment to result in preferential distributions in different compartments in the lungs.
Fiber length
Fibrogenic and tumorigenic fibers with a high aspect ratio, such as asbestos and CNT, differ from particles in that the inhaled fibers may reach the pleural space and cause parietal pleural lesions leading to pleural fibrosis, effusion, and mesotheliomas, in addition to causing interstitial fibrosis and tumors in the lungs. In the case of asbestos, the capacity of the pathogenic fibers to induce both lung and pleural lesions is directly correlated with the length of the fibers, which was summarized in the so-called “fiber pathogenicity paradigm”, that is, to be hazardous, a fiber must be biopersistent and thinner than 3 mm, but longer than 10–20 µm (Adamson et al., 1993; Davis et al., 1986; Stanton, 1973). The regulated forms of “asbestos”, as defined by World Health Organization (WHO), Occupational Safety and Health Administration (OSHA), and National Institute for Occupational Safety and Health (NIOSH), denote asbestos fibers with a length longer than 5 µm and an aspect ratio of 3:1–5:1, which was largely based on the fiber length–pathogenicity relationship of pathogenic fibers (Case et al., 2011, Liu et al., 2013).
A fiber length–toxicity relationship was demonstrated for CNT recently by comparing the pathologic effects of CNT fibers with different lengths injected into the peritoneal or pleural cavity. In these scenarios, long, but not short, CNT fibers (i.e. >15 µm in length) induced significant inflammatory and fibrotic responses and granuloma formation on the parietal mesothelium (Murphy et al., 2011; Poland et al., 2008), as well as mesotheliomas in the case of peritoneal injection in p53+/− mice (Takagi et al., 2008). By using single-photon emission computed tomographic imaging, it was shown that the long, but not short, CNT fibers were retained along the parietal mesothelium. Presumably, on one hand, short CNT fibers were cleared off quickly from the cavities through the stomatal openings (3–10 µm in diameter) on the surface of the parietal pleural wall and the diaphragm, which drain into local lymphatic vessels. On the other hand, long CNT fibers could not negotiate through the stomata leading to their accumulation on the parietal surface. Retained CNT would then be engulfed by macrophages, which are 10–15 µm in diameter; but long CNT could not be effectively phagocytized, resulting in “frustrated phagocytosis” that damages the mesothelium and stimulates inflammation, which ultimately lead to chronic inflammation, fibrosis, and malignancy (Donaldson et al., 2010). These findings imply that the parietal pleural mesothelium is the site of initial pathological alterations, thus providing a rationale for estimating the internal dose of pathogenic CNT for pleural lesions at the parietal pleural surface. Because mesothelioma has a long latency and is generally difficult to study with regard to its course of tumorigenesis and development, the pleural findings at the stomata also provide an opportunity for analyzing the early events that would lead to mesothelioma.
It remains to be examined whether and how this fiber length– pathogenicity relationship demonstrated for CNT pleural toxicity are applied to the fibrotic and tumorigenic effects of CNT in the lungs, because the lung parenchyma does not seem to have a “sieve” mechanism similar to the stomata of the pleural parietal mesothelium for selective retention of CNT fibers in the lungs. Nonetheless, such a relationship is predicted for CNT by analogy with the findings on asbestos’ effects in the lungs, i.e. asbestosis and lung cancer, and on the basis of a critical role of macrophages in the clearance of CNT fibers from the lungs, both of which are fiber length-dependent. This notion was supported by two recent studies. First, long MWCNT (NM400, 0.7–3 µm and NM402, 0.7–4 µm) as well as crocidolite asbestos, but not short MWCNT (MWCNTg2400, 0.7 µm) and crushed NM400 (NM400c, 0.14–0.5 mm), were shown to stimulate fibroblast proliferation in vitro and induce fibrosis in the lungs (Vietti et al., 2013). Second, spontaneously hypertensive (SH) rats administered with long MWCNT (20–50 µm), but not short MWCNT (0.5–2 mm), were found to exhibit increased fibroblast proliferation, collagen deposition, granuloma formation in the lungs in a TGF-β-depend-ent manner (Wang et al., 2013).
Surface area
The intrinsic pathogenic activity of CNT is closely correlated with their surface area, as is true for most other insoluble particulate matters. For this reason, surface area, as well as biopersistence and fiber length (discussed above) and surface reactivity (discussed below), are considered key elements of the “biologically effective dose” of CNT fibers.
A correlation between surface area and toxicity was clearly observed by comparing between SWCNT and MWCNT. When the target site dose was used to assess the fibrotic potential of CNT in the lungs, SWCNT were estimated to be approximately 8.5-fold more fibrogenic than MWCNT per µg dose. Presumably, on one hand, SWCNT fibers are lighter and have a larger surface area than MWCNT and thus, are more pathogenic on an equal weight basis. On the other hand, when adjusted for surface area, MWCNT is 2.5-time more toxic than SWCNT (Mercer et al., 2008, 2011; Porter et al., 2010; Shvedova et al., 2008a). Using the above data, a recent NIOSH Current Intelligence Bulletin suggests a benchmark dose of CNT exposure associated with a 10% increase in abnormal response to be 3.6 and 0.48 µg/lung for MWCNT and SWCNT, respectively, or an approximately 7.5-fold difference (NIOSH, 2013a).
It is noteworthy to point out that, for safety evaluation and risk assessment of CNT toxicity, multiple dose metrics, including surface area, mass concentration, and fiber number, should be used where possible, even though there is a growing evidence supporting a better correlation between toxicity and surface area compared with other metrics (Seaton et al., 2010).
Surface reactivity
Modification of the surface of CNT would alter their toxicity. On one hand, acid treatment would oxidize CNT to introduce hydroxyl and carboxyl groups on the surface of CNT, which alters the bioactivity and interaction of CNT with other molecules. Compared with pristine MWCNT, acid-oxidized MWCNT showed increased toxicity toward Jurkat cells (Bottini et al., 2006). On the other hand, nitrogen-doped MWCNT demonstrated significantly reduced toxicity and increased tolerance than their pure CNT counterparts in exposed mice (Carrero-Sanchez et al., 2006).
Covalent modification or surfactant addition is sometimes called functionalization of CNT. Functionalized CNT are being increasingly used in industrial and commercial applications. Functionalization of CNT potentially affects their toxicity. In one study, on one hand, pristine MWCNT were dispersed in mouse serum or were functionalized with ammonium. Functionalization of the CNT promoted rapid excretion of the CNT from the body and thus, reduced toxicity in mice; on the other hand, the serum-coated MWCNT induced respiratory distress, which was associated with accumulation of CNT in pulmonary vasculature (Lacerda et al., 2008). In another study, lung cells were exposed to pristine or carboxylated MWCNT (MWCNT or MWCNT– COOH) and a panel of toxic responses were analyzed including cell survival, DNA damage, and cytokine expression. MWCNT– COOH were found to have increased cytotoxicity in bronchial cells compared with pristine MWCNT, whereas the pristine MWCNT showed higher toxicity toward alveolar cells than MWCNT–COOH (Ursini et al., 2014). Recently, atomic layer deposition (ALD), a novel process to enhance functional properties of MWCNT, was used to coat MWCNT with a thin film of aluminum oxide (Al2O3). The “Al2O3”-coated MWCNT showed reduced fibrosis in mice compared with pristine MWCNT (Taylor et al., 2014).
These examples demonstrate that surface modification can both increase and decrease toxicity, depending on the particular modification taken, providing new ways of prevention-through-design for CNT and other nanomaterials.
Composition
The chemical composition of CNT is generally not considered as a major determinant of toxicity compared with fiber length, surface area, and biopersistence. However, in the case of surface functionalization and coating (discussed above), and when the graphene structure is altered by elements other than carbon or substantial impurity is present, the chemical composition would be important in CNT toxicity, as it potentially alters the surface reactivity and/or the distribution and half-life of CNT in the body. Additionally, it remains possible that the coated materials, functionalized groups, and contaminants would be detached from CNT fibers upon entering the body and be released into tissues over time to cause harm to cells locally or systemically. However, few studies have been conducted to address these possibilities.
Metals present in a CNT preparation may affect the biological effects of CNT. Metals, such as iron and nickel, are used as catalysts during the production of CNT. The metal content of a CNT preparation appeared to affect some, but not all, toxicological effects of CNT. For instance, SWCNT with an iron content of ~26% showed a greater effect in inducing ROS production than the iron-depleted preparation that has an iron content of ~0.23%, in both cell-free systems and cultured cells (Kagan et al., 2006). In a separate study, unpurified SWCNT (iron content, 30%) were shown to be more cytotoxic to skin cells in vitro compared with acid-treated counterparts (iron content, 0.23%), and their toxicity was decreased with the addition of a metal chelator. The unpurified CNT also caused skin pathology upon topical application, compared with acid-washed CNT. These findings support a critical role of metals in the toxic effects of CNT (Murray et al., 2009). In contrast, the iron contents of a number of different CNT samples (iron contents, 0.53–26.9%) did not appear to affect their potentials to induce granulomas in mice, which may be more related to the CNT’s tendency to aggregate and induce a foreign body response in the lungs (Lam et al., 2004).
Molecular mechanisms of CNT toxicity
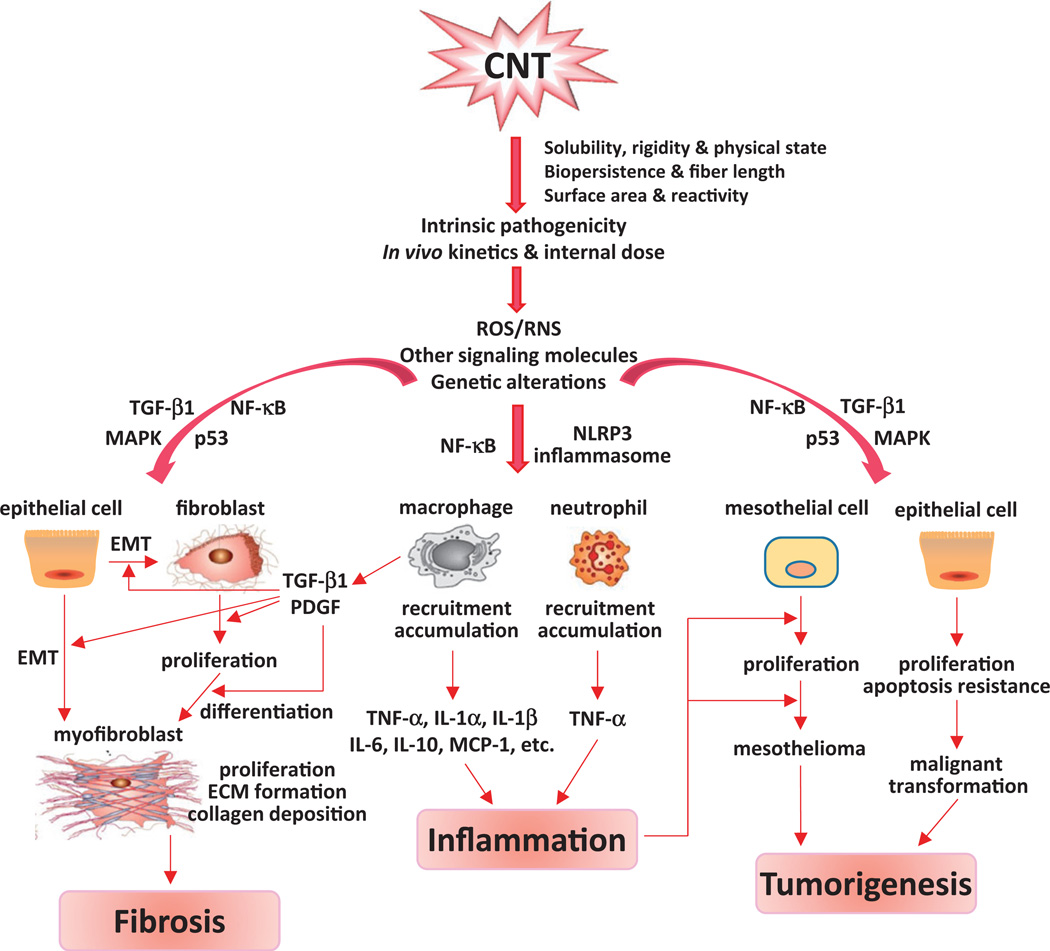
Given the considerable progress made in understanding the structure-toxicity relationship of CNT, it remains an important task to elucidate how CNT interact with cellular macromolecules to elicit specific pathologic effects, such as inflammation, fibrosis, and tumorigenesis; and how the physicochemical properties of CNT affect the CNT-biological interactions at the molecular level, both of which remain poorly understood. In this regard, several molecular processes associated with toxicities of pathogenic particles and fibers were observed in animal and in vitro models exposed to CNT, which would allow certain broad conclusions to be drawn with regard to the mechanisms underlying CNT toxicity.
Oxidative stress
One of the most consistent observations that would associate CNT exposure to toxicity mechanistically is oxidative stress, a cellular stress state caused by an imbalance between the production of ROS and antioxidant defense to result in harm to the cell. Biologically relevant ROS include oxygen radicals (, •OH, RO2•, and RO•) and oxygen species that are oxidizing agents and/ or are easily converted to radicals (H2O2, HOCl, O3, and 1O2). Eukaryotes are constantly exposed to ROS, resulting from both internal metabolism, such as the mitochondrial oxidative phosphorylation and the plasma membrane-bound NADPH (nicotinamide adenine dinucleotide phosphate) oxidase (NOX)-catalyzed reactions, as well as exogenous exposures, such as bacterial infection and exposure to particles, fibers, and transition metals. ROS may serve useful purposes under a physiological condition, such as regulation of cell proliferation and immune response, and killing of invading microbes during phagocytosis. However, overproduction of ROS would overwhelm the body’s antioxidant capacity, leading to damages to macromolecules such as DNA strand break, DNA mutation, protein peptide chain break, and lipid peroxidation, and eventually cell death. Therefore, ROS production and anti-oxidation are consequences of oxygen utilization in mammalian physiology, and oxidative stress contributes to the development of a range of diseases including aging, cancer, neurodegeneration, and chronic inflammatory pathology such as asbestosis and silicosis (Finkel, 2005; Ma, 2010).
Both SWCNT and MWCNT increased the production of ROS, often accompanied with elevated levels of oxidative markers, depletion of antioxidants, and induction of antioxidant enzymes in animals (Han et al., 2010; Rothen-Rutishauser et al., 2010; Shvedova et al., 2007). CNT may stimulate ROS production via a direct effect on cells, as they induced oxidative stress in a number of cell types including macrophages, bronchial and alveolar epithelial cells, and fibroblasts in vitro (Brown et al., 2007; He et al., 2011, 2012; Rothen-Rutishauser et al., 2010; Thurnherr et al., 2011). On one hand, deficiency of vitamin E in animal diet, which decreases the body’s antioxidant capacity, caused oxidative stress and increased the sensitivity to SWCNT-induced lung lesions in mice (Shvedova et al., 2007). On the other hand, addition of antioxidants or ROS scavengers alleviated oxidative stress and inflammatory cytokine expression in cells exposed to MWCNT (Brown et al., 2010; Han et al., 2010).
In addition to directly damaging macromolecules and cellular structures to result in cytotoxicity and cell death (Ma, 2010), ROS can activate signaling pathways, such as the NF-κB (nuclear factor-κB) signaling pathway, to boost the production and secretion of proinflammatory and profibrotic cytokines and growth factors that promote inflammation and fibrosis (He et al., 2011, 2012). ROS may also serve as signaling molecules to regulate growth factor-induced proliferation and differentiation of fibroblasts critical in the fibrogenic response to CNT. ROS have been implicated in transducing the signals of epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) to stimulate cell proliferation by boosting tyrosine phosphorylation signaling through their plasma membrane receptors and NADPH oxidases (Dickinson & Chang, 2011; Finkel, 2011), as well as activating cellular programs such as the inflammasome and autophagy signaling pathways (Ma, 2013; Shvedova et al., 2012b). Given the long half-life of CNT fibers in the body, it would be expected that there is continued production of ROS and prolonged oxidative stress in CNT-deposited tissues over a long period of time. Therefore, strategies to suppress ROS production by administering antioxidants would not be sufficient to prevent or halt CNT-induced lung fibrosis, if the tissue burden of CNT is not effectively reduced.
CNT may boost ROS production in several ways. First, in accordance with the fiber length paradigm, long CNT fibers (415 mm in length) can cause “frustrated phagocytosis” in which macrophages are activated but cannot effectively engulf the long fibers that exceed their body diameter. “Frustrated” macrophages release the contents of their phagosomes including digestive enzymes, anti-microbial agents, and highly toxic ROS and reactive nitrogen species (RNS), resulting in local inflammation and destruction of surrounding tissues (Brown et al., 2007). Second, many SWCNT and MWCNT fibers are shorter than 10 mm, but are capable of inducing ROS production in vitro and in vivo. In these scenarios, phagocytosis of CNT by activated macrophages are also necessary for induction of oxidative stress, and entangled or long and straight CNT generate considerably more ROS than CNT that are short and have a relatively small surface area (Rothen-Rutishauser et al., 2010). Third, in addition to being the power house and a major source of ROS production in mammalian cells under physiologic conditions, the mitochondria are prone to damage by toxic agents including pathogenic fibers to result in oxidative stress. Both SWCNT and MWCNT have been shown to induce mitochondrial damage and elevated ROS production in lung cells (He et al., 2011, 2012). Fourth, NOXs, a group of membrane enzymes, can generate superoxide anion and hydroxyl radicals near the plasma membrane. NOX2 (gp91phox) is responsible for the “respiratory burst” during phagocytosis by neutrophils and other phagocytic cells. NOX2 was shown to play a role in the transition of CNT-induced acute inflammation to chronic fibrosis in mice, as the NOX2 knockout mice exposed to SWCNT exhibited significantly more proinflammatory, but not profibrotic, phenotypes compared with wild-type (Shvedova et al., 2008b). Lastly, impurities from CNT preparations, such as iron and other transition metals, can stimulate the Fenton (Fe2+ + H2O2 →Fe3+ + •OH + OH−) or Fenton-like (Mnn+ + H2O2→Mn(n+1)+ + •OH + OH−) reactions to result in ROS production and oxidative stress in biological systems.
In addition to ROS, exposure to particles and fibers, such as silica and asbestos, has been shown to stimulate the expression of inducible nitric oxide synthase (iNOS) in the lungs that produces nitric oxide (NO•) (Kang et al., 2000). NO• serves as a gaseous signaling molecule for blood vessel relaxation or as a neurotransmitter in the brain physiologically, but it may also react with ROS to form stronger oxidants such as the potent peroxynitrite (ONOO−). Overproduction of NO•, ONOO−, and their derivatives, which are collectively called reactive nitrogen species, causes nitrosative stress that contributes to toxicity and disease pathogenesis in the body (Pacher et al., 2007). RNS have been implicated in the development of silicosis and asbestosis, but whether CNT stimulate RNS production to cause nitrosative stress and toxicity remains controversial, as both positive and negative results for iNOS induction have been observed (Lee et al., 2012; Pulskamp et al., 2007).
Inflammation
The tissue response to CNT deposition in the lungs and in the pleural and abdominal cavities resembles a foreign body-induced response in that it initiates with a marked acute inflammatory response, followed by prolonged or chronic pathologic alterations (Dong et al., 2014; Mercer et al., 2011). Acute inflammation is characterized by rapid infiltration of neutrophils and macrophages and high titers of proinflammatory mediators, which peak at around 1–7 d and subside at 14 d post-exposure (Dong et al., 2014; Porter et al., 2010). The chronic response, marked by interstitial or pleural fibrosis and granulomas, is contingent on continued presence and accumulation of CNT in the tissue and is considered to reflect the function of inflammation to promote tissue repair (i.e. fibrosis) and clearance of CNT (i.e. granuloma formation). Thus, inflammation is a major component of the acute phase and, arguably, the chronic phase of the tissue response to CNT.
In vitro studies reveal that both MWCNT and SWCNT stimulate the production and secretion of inflammatory mediators such as TNF-α, IL-1β, IL-6, and IL-8, from a variety of cell types, including macrophages, bronchial and alveolar epithelia, kera-tinocytes, and fibroblasts (He et al., 2011, 2012). In vivo induction of inflammatory mediators by CNT has also been detected (Dong et al., 2014). It is believed that elevated levels of the inflammatory mediators in the local matrix and circulation would drive the recruitment and activation of inflammatory cells to cause the acute inflammatory infiltration at the site of CNT deposition. CNT are likely to activate specific signaling pathways, such as the NF-κB and the NLRP3 (nucleotide-binding oligomerization domain-like receptor, pyrin domain-containing 3) inflammasome pathways, to up-regulate gene transcription and post-translational processing of inflammatory mediators, which will be discussed in more detail in later sections. The mediators and mechanisms that control the transition from acute to chronic inflammation and the subsequent propagation of the chronic phase response to CNT remain unclear for the most part. Together, these findings imply that anti-inflammation by targeting key mediators may be used to help in preventing or halting the pathologic processes caused by CNT. However, because CNT may persist and continuously stimulate inflammatory and pathogenic responses in the tissue, any anti-inflammation therapy would need to be administered in conjunction with measures that reduce the tissue load of CNT fibers to be effective.
Proliferation
Cell proliferation has been recognized as a prominent molecular and cellular mechanism critical in tumorigenesis, fibrosis, and inflammation, which are major pathologic outcomes of CNT exposure in animals. Recent research has provided ample evidence demonstrating that CNT modulate the proliferation of a number of types of cells in vitro and in animals. Long MWCNT induced the proliferation of primary mouse lung fibroblasts and a number of fibroblast cell lines including human fetal lung fibroblasts (HFL-1), mouse embryonic fibroblasts (BALB-3T3), and mouse lung fibroblasts (MLg), in a dose-dependent manner; moreover, the potentials of different preparations of MWCNT to induce proliferation of the cells in vitro correlated with their fibrotic effects in vivo in C57BL/6 mice (Vietti et al., 2013). The role of fibroblast proliferation in CNT-induced fibrosis is further discussed in the section below.
MWCNT exposure induced hyperplastic proliferative lesions of the visceral mesothelium in which the proliferating cell nuclear antigen levels were approximately 10-fold higher than vehicle control in F344 rats; furthermore, the pleural cavity lavage fluid from the dosed rats or conditioned culture media of macrophages treated with MWCNT increased mesothelial cell proliferation in vitro, further supporting the notion that stimulation of mesothelial proliferation by CNT potentially leads to the formation of mesotheliomas (Xu et al., 2012). Therefore, MWCNT clearly produce pathological effects by promoting cell proliferation in certain cell types.
The fibroblastic response
Fibroblasts play a major role in tissue fibrosis (Wynn, 2011). Upon exposure to CNT, fibroblasts increase in number and transform to myofibroblasts to secret a large number of matrix proteins such as collagens and fibronectin, as well as matrix modulating enzymes such as metalloproteinases, resulting in the deposition and processing of collagen proteins and, eventually, replacement of the parenchyma with collagen fibers, near CNT deposits. Pulmonary interstitial fibroblasts may derive from several sources during fibrosis, which conceivably include the following: (a) proliferation of resident fibroblasts in the lungs; (b) recruitment of bone marrow-derived fibrocytes from blood into the lung interstitial space; and (c) epithelial–mesenchymal transition (EMT) of airway and alveolar epithelial cells. MWCNT and SWCNT have been shown to stimulate fibroblast proliferation in vitro (Vietti et al., 2013; Wang et al., 2010a,b), transformation of fibroblasts into myofibroblasts (He et al., 2011, 2012), and induction of EMT (Chang et al., 2012, Chen et al., 2014). The role of fibrocyte recruitment in CNT toxicity has not been demonstrated.
Two mechanisms have been hypothesized to account for the effects of CNT on fibroblasts. First, CNT fibers may stimulate fibroblasts to proliferate and differentiate by mimicking endogenous collagen fibers or other fibrous structures to attract fibroblasts to adhere to their surface, leading to activation of the fibroblasts. Alternatively, CNT stimulate the secretion of profibrogenic mediators, such as the inflammatory cytokines TNF-α and IL-1β, and growth factors TGF-β1 and PDGF, which exert potent mitogenic effects on fibroblasts to promote the proliferation and differentiation of the cells (Dong et al., 2014). Although some in vitro and in vivo evidence has been obtained to support these posits, a definitive proof of either mechanism to account for the fibrotic effects of CNT in the lungs in vivo has not been available.
Genotoxicity and tumorigenesis
MWCNT and SWCNT have been shown to damage DNA both in vitro and in vivo, though negative results have also been observed in studies with some CNT preparations (van Berlo et al., 2012). These potentially controversial reports likely reflect the heterogeneity in the physicochemical properties and the genotoxic potentials of the CNT fibers tested, as well as differences in the assay types and testing conditions of the studies. The spectrum of genotoxicity by CNT is similar to that known to be caused by tumorigenic fibers such as asbestos, including (a) single- and double-DNA strand breaks, as demonstrated by comet assay, formation of γH2AX foci, and activation of poly(ADP-ribose) polymerase 1; (b) oxidation of DNA base such as formation of 8-hydroxydeoxyguanosine (8-OHdG); (c) micronucleus formation; (d) clastogenic and aneugenic effects; and (e) increased mutation frequency in mutation-screening model systems (Schins & Knaapen, 2007). Mutagenicity tests in bacterial strains have been mostly negative for CNT (and asbestos in this regard). In light of these findings, it was proposed that CNT do not damage DNA by directly acting on DNA. Instead, CNT cause genetic lesions indirectly via several mechanisms. First, CNT may stimulate cells to produce DNA-damaging ROS as discussed above, to cause DNA base oxidation and DNA strand breaks. Second, CNT may suppress DNA repair and thereby facilitate genotoxic processes. This notion was supported by the findings that a decrease of the tumor suppressor p53 in mice enhanced tumorigenesis by MWCNT, and p53 is known to be critical in initiating DNA repair in the presence of DNA impairment. Third, CNT may cause damage to DNA as a consequence of elevated inflammatory response to CNT. Lastly, CNT may enter the nucleus and interfere with the mitotic machinery, i.e. the centrosomes and mitotic spindle, due to their similarities to the microtubules of the spindle, to result in clastogenic and aneugenic phenotypes (Sargent et al., 2010, 2012).
Tumorigenesis is a multi-step process involving an initial insult(s) to the genome, failure of the defense and repair mechanisms, and neoplastic growth and progression. CNT tumorigenicity was studied in two types of animal cancer models, i.e. induction of abdominal mesothelioma and promotion of lung adenocarcinoma. Intraperitoneal injection of MWCNT induced mesothelioma formation in heterozygous p53+/− mice at high (3 mg/mouse) and low (30 or 300 µg/mouse) doses in two separate studies (Takagi et al., 2008, 2012). In a study on rats, thin MWCNT (diameter approximately 50 nm) with high crystallinity showed inflammogenicity and mesotheliomagenicity, but thick (diameter approximately 150 nm) or tangled (diameter approximately 2–20 nm) MWCNT were found less toxic, inflammogenic, and carcinogenic. Moreover, the mesothelioma induced by MWCNT had homozygous deletion of Cdkn2a/2b tumor suppressor genes similarly to asbestos-induced mesothelioma (Nagai et al., 2011). In a separate study, MWCNT with short length (<1 µm) were found incapable of inducing inflammation and mesothelioma in the rat abdominal cavity, which is in accordance with the notion that short CNT would be rapidly cleared from mesothelial cavities through the parietal stomatal openings and would not induce frustrated phagocytosis (Donaldson et al., 2010; Muller et al., 2009). Taken together, these findings indicate that the ability of MWCNT to induce inflammation and mesothelioma in the abdominal cavity is associated with their diameter-dependent piercing of the cell membrane to cause mesothelial injury as well as their resistance to clearance from the mesothelial surface, and is significantly enhanced by disruption of tumor suppression mechanisms.
The tumorigenicity of MWCNT in the lungs was investigated in a two-stage initiation/promotion model (Sargent et al., 2014). B6C3F1 mice, with an intermediate susceptibility for spontaneous lung tumor formation, were exposed to a single intraperitoneal injection of vehicle or MCA (10 µg/g body weight, i.p.); followed, 1 week later, by inhalation of either filtered air (control) or MWCNT at 5 mg/m3, 5 h/d, 5 d/week for a total of 15d. This dose gave a lung burden of 31.2 mg/mouse, which is relevant to feasible human occupational exposures. Lung tumors were examined 17 months post-exposure. Exposure to MCA followed by MWCNT caused lung adenocarcinoma and adenoma formation in 90% of the mice with a mean of 2.9/mouse, compared with 23% in the filtered air controls (mean of 0.25/mouse), 26.5% in the MWCNT-exposed (mean of 0.38/mouse), and 51.9% in the MCA followed by the filtered air-exposure (mean of 0.81/mouse) group. In addition, MCA plus MWCNT increased the incidence of serosal tumor, which is consistent with sarcomatous mesothelioma, by 4.5-fold, compared with MCA alone (9% versus 2%). These findings would not support MWCNT as an initiator or a complete carcinogen, but demonstrate that MWCNT inhaled at a dose relevant to human exposures is a strong tumor promoter in mouse lungs. The mechanism(s) by which MWCNT promote tumor progression remains to be elucidated. By analogy with the findings from asbestos, tumor promotion by MWCNT is likely to relate to MWCNT’s capacity to induce cytotoxicity, inflammation, fibrosis, cell proliferation, and cellular atypia in the lungs (Poland et al., 2008; Porter et al., 2010).
Modulation of immune functions
Inhalation of MWCNT caused systemic immunosuppression in mice, suggesting that the signals originated in the lungs were transduced to directly affect the functions of T cells in distal organs such as the spleen (Mitchell et al., 2007). Suppression of T cell functions was partially rescued by administering ibuprofen, a common anti-inflammatory drug that blocks the cyclooxygenase-2 (COX-2) pathway; moreover, mice deficient in COX-2 did not develop overt lung inflammation after inhaling MWCNT; in addition, proteins from the lungs of exposed mice suppressed the immune functions of spleen cells from normal mice, but not those from COX-2 knockout mice (Mitchell et al., 2009). From this study, it was posited that signals from the CNT-exposed lungs activate signals in the spleen to suppress the immune functions of exposed mice, which, in part, involves the COX-2 pathway. The role of COX-2 was also investigated in a murine asthmatic model using COX-2 knockout mice. MWCNT were shown to exacerbate the OVA-induced airway remodeling, which was associated with activation of a mixed Th1/Th2/Th17 immune response, and COX-2 appeared to protect against the inflammation and mucous cell metaplasia, but not the fibrosis induced by OVA and MWCNT. The results demonstrate a role for COX-2 in the exacerbation of allergen-induced airway remodeling by MWCNT and suggest different pathways in the development of fibrotic and allergic responses, respectively (Sayers et al., 2013).
CNT alter the expression of multiple immune-related cytokines and factors, which contribute to their effects on the immune system in a tissue and organ-dependent manner. Intratracheal instillation of MWCNT resulted in elevated levels of proin-flammatory cytokines in a dose-dependent manner in the blood and BAL fluid, elevated Th2 and Th1 cytokine levels, increased numbers of B cells in the spleen and blood, and enhanced production of IgE in mice; the findings suggest that MWCNT induce allergic responses in mice through B cell activation and production of IgE (Park et al., 2009). Intraperitoneal administration of MWCNT in mice led to increased mRNA expression of proinflammatory cytokines and chemokines (IL-1β, IL-33, TNF-α, and MCP-1), Th2 cytokines (IL-4, IL-5, and IL-13), and Th17 cytokine (IL-17) in peritoneal cells at early stage and increased mRNA expression of Th1 cytokines (IL-2 and interferon (IFN)-g) at a later stage, elevated numbers of inflammatory cells in the peripheral blood, and enhanced production of ovalbumin-specific IgM and IgG1 (Yamaguchi et al., 2012).
Signaling pathways of CNT toxicity
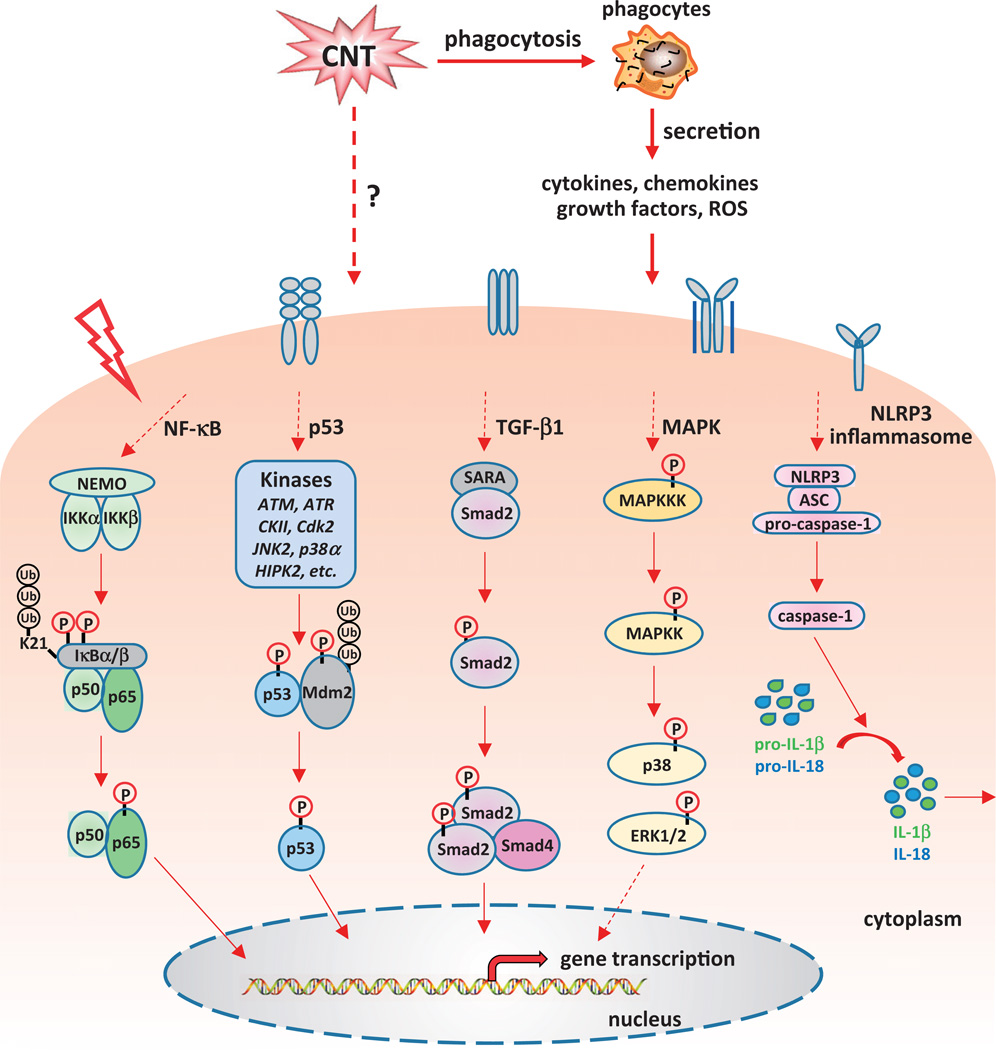
Many of the mechanisms for CNT toxicity discussed above would reflect, to a large extent, common strategies that cells use to perform physiologic functions and/or to cope with adverse insults. At the molecular level, these functions are carried out through specific cellular signaling pathways and programs. An increasing body of evidence indicates that a number of signaling pathways are activated by CNT and mediate the biological response to CNT, providing new molecular insights into the mechanism by which CNT induce toxic effects (Figure 3).
Figure 3.
Signaling molecules and pathways activated by carbon nanotubes. It has been demonstrated that CNT can positively or negatively influence several signaling cascades that play critical roles in physiological and pathological conditions, including the NF-κB, NLRP3 inflammasome, p53, TGF-β1, and MAPK pathways. Dysregulation of these pathways leads to abnormal gene expression and protein function, and eventually results in disease occurrence and progression.
NF-κB
The transcription factor NF-κB plays an important role in immune and inflammatory responses by regulating the expression of genes that serve as inducers or effectors at multiple levels in the inflammatory networks in response to stimuli. NF-κB is activated by CNT in a variety of experimental conditions both in vivo and in vitro. As a result, NF-κB has become one of the most studied pathways involved in CNT biological effects.
CNT induced NF-κB activation in a number of cell lines. In human HaCaT keratinocytes, SWCNT stimulated NF-κB signaling in a dose-dependent manner, revealed by sequential events of the signaling pathway, including activation of IκB kinase a (IKKα), enhanced phosphorylation and degradation of the NF-κB inhibitor IκBα, accumulation of NF-κB subunit p65 in the nucleus, elevated binding of NF-κB p50/p65 complex to DNA, and increased NF-κB-dependent reporter gene expression (Manna et al., 2005). SWCNT induced NF-κB activation in a dose-dependent manner in human normal mesothelial and malignant mesothelial cells detected by enzyme-linked immunosorbent assay (ELISA) (Pacurari et al., 2008). Both unpurified (30% iron) and partially purified (0.23% iron) SWCNT activated NF-κB in mouse epidermal JB6 P+ cells (Murray et al., 2009). NF-κB was activated by SWCNT in rat aortic endothelial cells (RAEC) (Zhiqing et al., 2010). In a recent study, SWCNT were shown to activate the NF-κB signaling cascade and increase the secretion of a panel of NF-κB-regulated proinflammatory cytokines and chemokines, including TNF-α, IL-1β, IL-6, IL-10, and MCP-1, in mouse RAW264.7 macrophages (He et al., 2012). Treating human alveolar epithelial A549 cells with MWCNT led to NF-κB activation and increased IL-8 mRNA expression; moreover, induction of IL-8 expression was suppressed by NF-κB inhibitors N-tosyl-l-phenylalanine chloromethyl ketone (TPCK) and parthenolide (Ye et al., 2009). In a separate study, MWCNT were shown to activate the NF-κB signaling pathway and increase the secretion of a number of NF-κB-regulated proinflammatory cytokines and chemokines, including TNF-α, IL-1β, IL-6, IL-10, and MCP-1, in mouse RAW264.7 macrophages; moreover, activation of NF-κB involved degradation of IκBα, nuclear translocation of NF-κB subunit p65, binding of NF-κB to specific κB-binding sites, and elevated NF-κB-controlled reporter gene expression (He et al., 2011). In addition, MWCNT induced time-dependent phosphorylation of the NF-κB inhibitor IκBα, an essential step leading to IκBα degradation, NF-κB nuclear translocation, and nuclear accumulation of NF-κB in rat lung epithelial cells (Ravichandran et al., 2010).
Intratracheal instillation of SWCNT caused airway hyper-reactivity, airflow obstruction, and granuloma formation, as well as alveolar macrophage activation and chronic inflammatory responses in mouse lungs. Pathway analysis of Affymetrix microarray data from the mouse lungs indicated that NF-κB-related inflammatory responses and downstream signals affecting tissue remodeling played an important role to account for the SWCNT-induced effects (Chou et al., 2008; Hsieh et al., 2012). Treatment of mice with NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) attenuated the pathologic phenotypes and induction of inflammatory genes by SWCNT significantly, further supporting an important role of NF-κB in the toxicity of SWCNT in vivo (Hsieh et al., 2012).
Given the complexity of the NF-κB pathway and the marked activation and involvement of NF-κB in CNT-induced cytotoxicity and pathology, it remains critical to elucidate the complicated layers of molecular mechanisms through which CNT affect NF-κB activity. In particular, how CNT activate the signaling pathway of NF-κB remains largely elusive. In this respect, several upstream signaling molecules leading to activation of NF-κB in response to physiologic and microbial cues have been identified, including tumor necrosis factor receptor (TNFR), interleukin-1 receptor (IL1R), toll-like receptors (TLRs), and growth factor receptors (GFRs), which may guide future studies of the interactions between CNT and the NF-κB pathway in the development of inflammation, fibrosis, and tumorigenesis by CNT.
Inflammasomes
CNT induced high levels of secreted IL-1β in culture media of macrophages treated with CNT (He et al. 2011, 2012; Shvedova et al., 2005) and in the BAL lavage fluid from CNT-exposed mouse lungs (Han et al., 2010; Sager et al., 2014; Shvedova et al., 2005). Secretion of IL-1β requires the proteolytic processing of the protein by the NLRP3 inflammasome (Lamkanfi & Dixit, 2014). Since the first report on the topic by Palomaki et al. (2011), activation of the NLRP3 inflammasome under CNT stimulation has drawn a large attention.
Inflammasomes are cytoplasmic sensors that detect extracellular and intracellular signals and initiate innate immune responses in response to microbe infection and tissue injury. Assembled as a large complex from multiple proteins, a number of inflammasomes have been identified, including the NLRP1b, NLRP3, NLRC4 (nucleotide-binding oligomerization domain-like receptor, caspase activation and recruitment domain-containing 4), and AIM2 (absent in melanoma 2) inflammasomes, which have distinct protein compositions and are activated by distinct and specific danger signals, such as microbial pathogens and stress cues (Lamkanfi & Dixit, 2014; Schroder & Tschopp, 2010). The NLRP3 inflammasome is the most extensively studied complex, as it is implicated in responses to a wide range of signals derived from pathogens, endogenous danger signals, and environmental stimuli. This inflammasome contains the NLR (nucleotide-binding oligomerization domain-like receptor) protein NLRP3, the adaptor protein ASC (apoptosis-associated specklike protein containing a caspase activation and recruitment domain), and the effector proteolytic enzyme caspase-1, i.e. procaspase-1. In resting cells, NLRP3 is auto-repressed in the cytoplasm. Upon stimulation, NLRP3 is activated and oligomerized, followed by recruitment of ASC and procaspase-1 to form the NLRP3 inflammasome. The inflammasome cleaves pro-caspase-1 to become active caspase-1, which in turn converts proinflammatory cytokines IL-1β and IL-18 from inert to active and secreted forms by cleavage. The matured cytokines in the extracellular space propagate inflammatory responses (Sutterwala et al., 2014; Tschopp & Schroder, 2010).
MWCNT have been reported to induce NLRP3 inflammasome activation in a number of studies. In the first report, long needlelike, but not tangled, MWCNT induced secretion of IL-1β from LPS (lipopolysaccharides)-primed human primary macrophages, which involved NLRP3 inflammasome activation because knocking down of NLRP3 diminished MWCNT-induced IL-1β secretion (Palomaki et al., 2011). Double-walled CNT (DWCNT) enhanced IL-1β release in human monocytes, which was exclusively linked to caspase-1 and NLRP3 inflammasome activation (Meunier et al., 2012). MWCNT induced IL-1β and IL-18 secretion in cultured alveolar macrophages isolated from C57BL/6 mice; and increased IL-1β secretion was repressed by a caspase-1 inhibitor (Hamilton et al., 2013). MWCNT also increased IL-1β secretion in PMA (phorbol myristate acetate)-primed THP-1 human monocytes, which was suppressed by caspase-1 inhibitor, and MWCNT treatment led to cleavage of pro-caspase-1 to mature caspase-1, indicating the activation of inflammasome by MWCNT (Kanno et al., 2014). These studies suggested that NLRP3 inflammasome activation contributes to MWCNT-induced inflammation by controlling IL-1β and IL-18 maturation and secretion.
In addition to the enhanced secretion of IL-1β and IL-18, pyroptosis, another outcome of inflammasome activation, was shown to play a role in MWCNT-induced lung injury (Hussain et al., 2014). Pyroptosis is a highly inflammatory form of cell death controlled by inflammasome-dependent caspase-1 activity (Bergsbaken et al., 2009). MWCNT induced pyroptosis in primary human bronchial epithelial (HBE) cells in a time- and dose-dependent manner; and induction of pyroptosis was mediated by NLRP3 inflammasome activation, as it was significantly reduced by treatment with NLRP3 siRNA (small interfering RNA) or caspase-1 inhibitor (Hussain et al., 2014).
It is noteworthy to point out that current studies on NLRP3 inflammasome activation by CNT were mostly conducted with MWCNT in cultured cells and the molecular understanding of the mechanism by which CNT activate the inflammasome is limited at present. Nonetheless, Palomaki et al. (2011) have shown that CNT-induced NLRP3 inflammasome activation involved ROS production, cathepsin B activity, P2X(7) receptor, and Src and Syk tyrosine kinases, providing molecular targets for further research. In addition to CNT, other particles and fibers, including silica, asbestos, alum, and nanoparticles, such as nano-TiO2 and nano-SiO2, have been shown to activate the NLRP3 inflammasome, but their underlying mechanisms and pathologic implications remain unaddressed to a large extent (Cassel et al., 2008; Dostert et al., 2008; Hornung et al., 2008; Kool et al., 2008; Peeters et al., 2013; Winter et al., 2011; Yazdi et al., 2010). Thus, although the research on the CNT-NLRP3 inflammasome interaction is at an early stage, detailed analyses on the NLRP3 inflammasome pathway under CNT exposure would provide significant new insights into the inflammatory responses triggered by CNT and other toxic particles and fibers for future studies.
TGF-β1
The molecular mechanisms underlying lung fibrosis in most animal models and human diseases have remained uncertain for the most part, but a number of cytokines, known as fibrogenic cytokines because of their capacity to induce or promote fibrosis and/or whose expression and function are altered during the development of fibrotic pathology, have been identified (Wynn, 2011). Among them, TGF-β1 is considered as a key regulator in fibrosis. TGF-β1 induces the recruitment of macrophages and fibroblasts, promotes fibroblast proliferation, and stimulates the transformation of fibroblasts to myofibroblasts, which are key cellular and molecular events in the development of lung fibrosis (Desmouliere et al., 1993; Fernandez & Eickelberg, 2012; Sime et al., 1997). TGF-β1 also drives EMT to convert epithelial cells to fibroblasts at the site of injury to boost fibrosis (Iwano et al., 2002; Kalluri & Neilson, 2003). TGF-β1 binds to its receptors on the surface of target cells to initiate the signaling pathway. In the canonical pathway, activated Type 1 receptor phosphorylates R-Smads (Smad2 and Smad3), which subsequently form a complex with the Co-Smad, Smad4. The resulting active Smad complex enters the nucleus and interacts with distinct transcription factors to activate or inhibit the transcription of many TGF-β responsive genes involved in apoptosis, cell growth and differentiation, extracellular matrix neogenesis, and immunosuppression (Schmierer & Hill, 2007). Additionally, TGF-β1 activates a number of non-Smad pathways collectively known as the non-canonical pathways including PI3K, RAS, PAR6, and JNK/p38/ MAPK pathways, which cumulatively regulate TGF-β functions (Chaudhury & Howe, 2009; Zhang, 2009).
Recent studies demonstrated that both SWCNT and MWCNT induced the production of TGF-β1 in cultured cells including mouse RAW264.7 macrophages, normal human bronchial epithelial cells BEAS-2B, and normal human lung fibroblasts WI38-VA13 (He et al., 2011, 2012). In a separate study, long SWCNT increased the expression and secretion of TGF-β1 in cultured normal human lung fibroblasts NHLF (Manke et al., 2014). Therefore, CNT possess the ability to enhance TGF-β1 expression and function, as shown by in vitro studies.
A number of in vivo studies provided evidence to support that TGF-β plays a critical role in CNT-induced lung fibrosis. SWCNT-exposed C57BL/6 mice showed EMT and injury in the lungs, with an increasing occurrence of epithelium-derived fibroblasts that produced collagen, indicating that EMT occurred and contributed to CNT-induced fibroblast expansion; moreover, an elevated number of hyperplastic epithelial cells with positive staining for TGF-β/p-Smad2 were observed, suggesting that activation of TGF-β/p-Smad2 signaling is involved in SWCNT-induced EMT and fibrosis in the lungs (Chang et al., 2012). Long MWCNT (20–50 µm) increased fibroblast proliferation, collagen deposition, and granuloma formation in the lungs of spontaneously hypertensive (SH) rats (Wang et al., 2013). Furthermore, the long MWCNT induced macrophage activation, TGF-β1 secretion, Smad2 phosphorylation, and the expression of collagen III and extracellular matrix (ECM) protease inhibitors in vivo; and in vitro studies revealed that the long MWCNT enhanced TGF-β1-induced phosphorylation of Smad2 as well as up-regulated the expression of collagen III in a TGF-β-dependent manner in mouse embryonic fibroblast NIH3T3 cells. In C57BL/6J mice, exposure to long MWCNT (5–15 µm) led to increased collagen deposition, pulmonary fibrosis, TGF-β1 secretion and Smad2 phosphoryl-ation (Chen et al., 2014). About 20% of the pro-surfactant protein-C positive epithelial cells transformed to fibroblasts at 56 d post-exposure, indicating occurrence of EMT. In vitro studies showed that the long MWCNT induced TGF-β1 production and Smad2 phosphorylation, down-regulated epithelial marker protein E-cadherin, and up-regulated mesenchymal marker protein α-smooth muscle actin (α-SMA) protein expression in human pulmonary epithelial A549 cells, which were dependent on TGF-β1 signaling. These studies demonstrated that TGF-β/Smad signaling is activated by CNT and plays an important role in CNT-induced lung fibrosis.
Despite the above findings and the common belief of TGF-β as a promoter of fibrosis, some studies demonstrated an opposite role of the TGF-β/Smad pathway in pulmonary pathologic responses. For example, blockade of TGF-β/Smad signaling was shown to enhance airway inflammation and reactivity (Hansen et al., 2000; Nakao et al., 2000), whereas over-expression of TGF-β1 in regulatory T cells inhibited bleomycin-induced lung fibrosis (Kitani et al., 2003). Thus, TGF-β may have a suppressive effect on tissue remodeling under certain conditions. This double-edged nature of TGF-b1 function demands that caution is to be taken when analyzing and interpreting studies relating TGF-β to lung fibrosis.
MAPKs
The mitogen-activated protein kinase (MAPK) pathways control a wide range of fundamental processes including cell proliferation, differentiation, apoptosis, inflammation, and organismal development (Arthur & Ley, 2013; Jeffrey et al., 2007; Munshi & Ramesh, 2013; Rose et al., 2010; Wagner & Nebreda, 2009). The MAPK pathways are activated in response to endogenous signals, such as growth factors, inflammatory cytokines, and mitogens, as well as environmental stressors, such as ultraviolet irradiation, oxidants, genotoxic agents, and microbial toxins. The MAPK pathways are activated through a well-conserved three-tiered kinase cascade, in which a MAPK kinase kinase (MAPKKK, MAP3K, MEKK, or MKKK) activates a MAPK kinase (MAPKK, MAP2K, MEK, or MKK), which in turn activates the MAPK through serial phosphorylation. This kinase cascade transduces signals from the cell membrane to the nucleus to regulate a variety of intracellular signaling pathways. Well-studied MAPKs include the extracellular signal-regulated kinase 1 and 2 (ERK1/2), the c-Jun N-terminal kinases 1, 2, 3 (JNK1, 2, 3), the p38 MAPKs (p38α, β, γ, δ), and the big MAPK (ERK5). The ERK pathway (ERK1/2) is activated by mitogens and growth factors, and plays a major role in regulating cell growth, survival, and differentiation. In contrast, JNK and p38 MAPKs respond most robustly to inflammatory cytokines and cellular stresses, and are strongly associated with stress responses such as apoptosis and inflammation, although they can also be weakly activated by growth factors. ERK5 is activated in response to both growth factors and stresses, and has various biological effects on cell growth, survival, and differentiation.
A few studies reported that SWCNT activate MAPK signaling in cultured cells. Significant phosphorylation of p38 and ERK1/2 in malignant human mesothelial cells was detected upon exposure to SWCNT (Pacurari et al., 2008). In human lung fibroblasts CRL-1490, SWCNT induced p38 phosphorylation in a dose-dependent manner, which in turn contributed to SWCNT-induced fibrogenesis and angiogenesis through the induction of TGF-b1 and vascular endothelial growth factor (VEGF) (Azad et al., 2013). Prolonged treatment of human mesothelial cells with SWCNT induced neoplastic-like transformation, which was associated with phosphorylation of ERK1/2 (Lohcharoenkal et al., 2014).
MWCNT were shown to induce MAPK activation in several in vitro systems. In a gene expression profiling study on human skin fibroblasts, a whole genome expression array analysis was performed to identify the genes whose expression was changed at transcriptional level upon exposure to MWCNT (Ding et al., 2005). Promoter analysis of the microarray results indicated that p38/ERK-MAPK cascades are critical pathways in the induced signal transduction by MWCNT. A phosphokinase array study using lysates from human bronchial epithelial BEAS-2B cells exposed to MWCNT demonstrated that phosphorylation of p38 and ERK1 was significantly increased (Hirano et al., 2010). MWCNT increased phosphorylation of ERK1/2 in mouse RAW264.7 macrophages, which was critical to the elevated COX-2 expression induced by MWCNT (Lee et al., 2012). In a co-culture system in which human small airway epithelial cells (SAEC) and human microvascular endothelial cells (HMVEC) were cultured separately by a Transwell membrane to mimic an alveolar-capillary interaction, treatment of SAEC with MWCNT induced phosphorylation of p38 in HMVEC (Snyder-Talkington et al., 2013).
The above discussed studies on the relation between CNT and MAPK signaling were performed in cultured cells and using phosphorylation of MAPKs as the readout of MAPK activation, which limit the interpretation of the results. Therefore, detailed in vivo analyses are much needed to demonstrate the activation and functional consequences of MAPK pathways in CNT-induced toxicity. Analyses of both upstream and downstream components in the MAPK cascade under CNT exposure would facilitate to address the mechanisms by which CNT modulate the MAPK pathways in future studies.
p53
In addition to stimulating the proliferation of lung cells such as fibroblasts and mesothelial cells for their fibrotic and mesothelioma-causing effects, CNT induce cell-cycle arrest, apoptosis, and autophagy. For instance, cell-cycle analyses revealed that SWCNT induced a G2 block in cell cycle (Sargent et al., 2012; Wang et al., 2011a), whereas exposure to MWCNT caused a G1/S block in cultured cells (Han et al., 2012; Siegrist et al., 2014). Recent studies showed that SWCNT induced apoptosis in rat pheochromocytoma PC12 cells and rat aorta endothelial cells (Cheng et al., 2011; Wang et al., 2011a), and MWCNT stimulated apoptosis in rat glioma cells and RAW 264.7 cell-derived osteoclasts (Han et al., 2012; Ye et al., 2012). In addition, SWCNT were shown to stimulate autophagy in BEAS-2B cells, with up-regulated autophagy-related genes and autophagosome formation-related proteins (Park et al., 2014), and certain types of MWCNT affect autophagy, as examined in a fluorescent autophagy-reporting cell line (Wu et al., 2014).
The tumor suppressor protein p53 plays a critical role in the control of cell proliferation and cell death, two fundamental biological functions critical in many physiological, developmental, and disease processes during organismal life, by directly regulating cell cycle, apoptosis, and the response to genomic damage. Given the dominant role of p53 in the regulation of cell proliferation and cell death, it is rational to posit that the tumor suppressor protein is involved in CNT-induced toxicity, which has been supported by some experimental evidence. SWCNT were shown to affect cell proliferation and apoptosis by interrupting p53 signaling in cultured cells. Prolonged (6 months) exposure to SWCNT caused malignant transformation of human lung epithelial BEAS-2B cells showing characteristics of cancer stem cells, such as excessive cell growth and colony formation. The transformed cells were resistant to apoptosis and induced tumors in nude mice. These cells had decreased phosphorylation of p53, a major determinant of p53 function. This study indicated that prolonged exposure to SWCNT perturbs p53 signaling, leading to loss of function of p53, promotion of cell proliferation, and inhibition of apoptosis (Wang et al., 2011b). A follow-up study confirmed that, in cells transformed by prolonged exposure to SWCNT, the total p53 protein level was dramatically decreased, which involved the plasma membrane-associated protein caveolin-1 (Cav-1), supporting a role of p53 and Cav-1 in CNT tumorigenesis (Luanpitpong et al., 2014).
In the initial study with heterozygous p53+/− mice, decreased function of p53 was shown to promote induction of mesotheliomas in the peritoneal cavity injected with MWCNT at a dose of 3 mg per mouse (Takagi et al., 2008). In a follow-up study, a dose-response curve at much lower doses was pursued in p53+/− mice with MWCNT (3, 30, or 300µg/mouse, intraperitoneal injection, 1 year); mesotheliomas were observed at all doses with a dose-dependent cumulative incidence, further supporting that MWCNT induce mesotheliomas, in a p53 compromised genetic background (Takagi et al., 2012). In a recent study, intravenous injection of large-sized MWCNT into pregnant p53 heterozygous mice induced p53-dependent responses during fetal development, manifesting restriction of fetal development and deformity of the brain; moreover, molecular analyses revealed that the MWCNT triggered p53-dependent apoptosis and cell-cycle arrest in response to MWCNT-induced DNA damage; but SWCNT and small-sized MWCNT failed to induce fetotoxicity. This study demonstrates that p53 modulates MWCNT fetotoxicity by controlling cell proliferation and cell death (Huang et al., 2014).
A working model for CNT pathogenic effects
The study on CNT has served as a model for analyzing nanotoxicity. The major findings conclude that CNT can induce a variety of adverse effects in experimental systems including cytotoxicity, inflammation, fibrosis, genotoxicity, tumorigenesis, and immune effects, which extend well beyond the predictions from “nuisance dust”-like fibers (Figure 2). As a result, the health impacts of CNT on humans have evolved into an urgent issue to evaluate, discuss, and prevent. Under such a situation, a comprehensive understanding of the characteristics of pathogenic CNT and the cellular and molecular mechanisms underlying CNT-triggered and disease-related effects is imminently required. In this review, we provide a summary and a discussion of the current advances in these areas, which allow us to propose a working model to integrate determinants of CNT toxic activities, molecular mechanisms, and signaling pathways relating to the development of inflammation, fibrosis, and tumorigenesis, three major pathogenic effects observed in animals exposed to CNT (Figure 4).
Figure 4.
A working model for molecular mechanisms underlying CNT-induced diseases. The activation of NF-κB and NLRP3 inflammasome plays important functions in the inflammatory responses induced by CNT. TGF-β1 signaling triggers EMT and fibroblast to myofibroblast transformation, and results in fibrosis under CNT exposure. Abnormal p53 function plays a critical role in CNT-induced tumorigenesis, through dysregulating cell proliferation and apoptosis. Although in vivo studies have not been performed, NF-κB, p53, and/or MAPK pathways may contribute to the onset of fibrosis, and NF-κB, TGF-β1, and/or MAPK activation is expected to be involved in tumorigenesis following CNT exposure, according to the functions of these pathways. Meanwhile, as a critical and rapid-onset immune response, inflammation may initiate and facilitate both fibrosis and tumorigenesis following exposure to CNT.
Respirable CNT fibers exhibit apparent pathogenic potentials to induce toxicity and diseases similar to those by asbestos fibers. CNT also show dynamic and specific behaviors in their deposition, distribution, and clearance in tissue compartments in the body. Moreover, many toxic effects of CNT depend on their physicochemical properties, in particular, their solubility, bioper-sistence, rigidity, physical state, fiber length, surface area, and surface reactivity, which determine CNT’s intrinsic pathogenicity, in vivo kinetics, and internal dose. The molecular mechanisms by which pathogenic fibers and particles, including CNT, asbestos, and silica, cause toxicity have remained poorly understood for the most part; but a few molecular events have been observed in animal and cell models exposed to CNT, among which, oxidative stress, inflammation, proliferative response, fibroblast proliferation and differentiation, oxidative DNA base damage and clastogenic/aneugenic abnormalities, as well as immuno-modulatory effects, have been demonstrated to provide a mechanistic explanation to CNT toxicity at molecular level. Mechanistic studies have also uncovered a number of cellular signaling pathways; in particular, the NF-κB, NLRP3 inflammasome, TGF-β1, MAPK, and p53 pathways have been recognized to play important roles in CNT-induced pathologic phenotypes. More specifically, the NF-κB, NLRP3 inflammasome and MAPK signaling may be involved in inflammatory responses, whereas the NF-κB, TGF-β1, MAPK, and p53 signaling may contribute to fibrosis and tumorigenesis, following CNT exposure.
The findings on CNT toxicity have created a body of knowledge critical for understanding the health effects of nanoexposure, as well as for providing guidance to risk assessment, screening of new nanomaterials, policy making, and prevention-through-design for nanoapplications. These efforts have significantly impacted on and will continue to promote the safe and responsible development of the nanoindustry; and, at the same time, protect humans and the environment from potentially deleterious effects of nanomaterials and products.
Perspectives
Issues relating to nanotoxicity
It is evident that the research on CNT toxicity is still at its early but rapidly growing stage, and a number of major knowledge gaps relating to CNT’s structure–toxicity relationship and molecular mechanisms of action remain to be filled.
One challenging issue derives from the fact that, despite of large efforts, the properties of CNT at the nanoscale, which define their relevance to nanotechnology, have not been convincingly linked to the biological activities of CNT. For instance, CNT have been hypothesized to perturb the structure and functions of the mitotic spindle in the nucleus, which may explain their clastogenic effects. Similarly, CNT in the extracellular matrix are thought to attract and activate fibroblasts in the interstitial space of the lungs by mimicking collagen fibers, because of their nanoscale size and fiber-like shape. Conceivably, detailed molecular analyses of the interactions between CNT and the subcellular and molecular targets would be required to identify “nanotube-specific” toxic effects and the mechanisms of action underlying the effects.
From a mechanistic point of view, only a small number of cellular mechanisms and signaling pathways critical to disease occurrence and development have been detected and reported under CNT exposure. Even for the relatively well-studied mechanisms and signaling pathways as summarized above, the molecular details and biological consequences are lacking in these cases. For instance, most of the mechanistic studies have been performed in cultured cells, thus lacking the in vivo confirmatory information; many of the findings are based on the observation of positive readout of a specific signaling molecule or pathway, missing the necessary characterization of critical upstream and downstream components involved in the signal cascade, such as the initial events responsible for the activation of a specific pathway by CNT; and the detailed pathological functions of a certain pathway in the onset of disease-related phenotypes induced by CNT have not been addressed to a large extent. In contrast, these issues are largely anticipated to encounter, given that the mechanistic study of nanotoxicity is at its early stage and the molecular mechanisms of toxicity for fibers and particles including asbestosis and silicosis have remained poorly understood for the most part. Future research into these issues would promise to provide significant new insights into the toxicity and safety of pathogenic fibers and particles, including CNT and their products.
The observations that toxicity can be reduced by changing CNT’s surface reactivity, i.e. functionalization and coating, pointed out new ways to build safety into the design of nanoproducts, but the research in this area remains elementary. Further analyses of how modification of CNT surface alters CNT behavior and toxicity in vivo at cellular and molecular levels would yield valuable new insights into CNT biology and nanotoxicology.
Because there appears to be a lack of CNT-specific toxicity, and most of the identified toxic effects, mechanisms of action, and signaling pathways of toxic CNT can be found from exposure to non-nanomaterials, such as asbestos and silica, it was proposed that CNT may be grouped either as high toxicity fibers, which often represent rigid, biopersistent, and respirable fibers with a specific geometry and high aspect ratio, or low toxicity fibers, which typically reflect insoluble, granular, and biodurable particles and fibers. This categorization assumes that CNT can be distinguished by their dose response curves of toxicity, although they may share similar mode of action and signaling pathways, if a sufficiently high dose is given. Additionally, a third category was proposed for nanomaterials whose toxicity is mediated by specific chemical properties of their components and thus, should be evaluated individually (Gebel et al., 2014). It is conceivable that further mechanistic understanding of the differences in the toxicity and mechanisms of action between high and low toxicity CNT would be needed before this classification of CNT becomes meaningful for practical use.
Issues relating to new developments in nanotechnology
Nanotechnology has been advancing rapidly and will keep this rapid pace of innovation in a foreseeable future, continuously introducing novel and “smart” nanomaterials of newer generations, whose health effects would be uncertain and require toxicological evaluations. In recent years, a number of new nanomaterials or nanoscale materials have been created and used for industrial and commercial applications, raising considerable new challenges for toxicological evaluation of the materials, which would require new methodology and mechanistic insights with regard to their potential health effects.
In the first example, CNT have been exploited to produce nanomedicine for therapeutic and diagnostic purposes, owing to their unique physicochemical properties. For instance, drugs can be linked to CNT through covalent or noncovalent attachment on the surface of CNT, or can be filled within the tubular structure of CNT, for delivery. As CNT have the ability to cross the various biological barriers in the body and penetrate into the cell, they have the potential to efficiently and directly deliver drugs to target cells and tissues. Therefore, CNT have become good candidates as drug carriers recently. As examples, the breast cancer drug Paclitaxel linked to SWCNT and the human gastric carcinoma drug HCPT linked to MWCNT have been tested in vitro and in mice (Liu et al., 2008; Wu et al., 2009).
The potential applications of CNT to therapeutic platforms demand imperative toxicological considerations and evaluations of CNT-based drugs, due to the identified cytotoxic and pathologic effects induced by CNT. After the drug is released from CNT, the CNT vehicles may continue to deposit and accumulate in targeted cells and tissues, and thus may result in local toxic effects. CNT released at the targeted site may also enter the circulation and translocate to distant organs, such as the cardiovascular system and lymph nodes, leading to systemic effects. Therefore, CNT-based medicines will need to be evaluated for the in vivo distribution, pharmaco- and toxicokinetics, and internal dose of the CNT vehicles, for the purpose of developing physiologically safe and therapeutically effective nanomedicine in humans.
“Active nanomaterials” and “nanoscale sophisticated materials” are two examples of novel nanomaterials. An “active” nanomaterial is a nanostructure that changes or evolves its state during its operation, as defined according to the National Science Foundation in the Active Nanostructures and Nanosystems grant solicitation in 2006. Five types of active nanomaterials have emerged, including (a) remote actuated active nanostructure; (b) environmentally responsive active nanostructure; (c) miniaturized active nanostructure; (d) hybrid active nanostructure; and (e) transforming active nanostructure (Subramanian et al., 2010). A few examples that involve active nanostructures are nanoelec-tromechanical systems, targeted drugs and chemicals, sensors, self-healing materials, and energy storage devices. Unlike traditional or passive nanomaterials, active nanomaterials can shift from a passive stage to an active stage, which may render them extra activity and functions including potential and previously uncharacterized toxic effects.
The “nanoscale sophisticated” materials possess novel, dynamic, multifaceted, and even time- and context-specific functionality. Based on the definition, the materials that (a) demonstrate abrupt scale-specific changes in biological or environmental behavior, (b) are capable of penetrating to normally inaccessible places, (c) are active, (d) are self-assembling, or (e) exhibit scalable hazard that is not captured by conventional hazard assessments, have been classified as sophisticated materials, which were introduced and reviewed elaborately by Maynard et al., (2011). Accordingly, the nanoscale sophisticated materials may have more complicated physicochemical properties, in vivo kinetics, and bioactivities, compared with traditional nanomaterials.
The production and applications of these active nanomaterials and nanoscale sophisticated materials demand careful consideration and evaluation with regard to the health risks that these new materials might pose, bringing about new challenges in toxicology studies. Conceivably, the research methods that have been developed and the findings that have been achieved on the toxicity of CNT and other traditional nanomaterials are expected to be applicable to the health effect studies on these new materials, which, together with new tools, experimental designs, and methodologies to be established and commensurate with the novel and complex features and functions of the new materials, would allow the study into critical toxicological issues, such as how the physicochemical properties and chemical composition of active nanomaterials and sophisticated materials interact with cells and bio-molecules to determine their dynamic behaviors, molecular interactions, and ultimately, toxicity within a biological system.
Conclusions
The expanding production and applications of CNT-related materials could result in an unavoidable and increasing human exposure to CNT, which has developed into an environmental and occupational issue in the toxicology field, due to the identified effects induced by CNT. Understanding the molecular mechanisms related to CNT-induced toxicity and pathology is a necessary approach to elucidate and prevent the adverse effects of using CNT as new materials in human life. In addition, results from the research on CNT toxicity will provide new clues for developing, producing and applying novel nanomaterials that will be hazardless to humans and to the environment. We anticipate an intensive research effort and a bloom of new findings in the field of nanotoxicology during the coming few years.
Acknowledgments
The study was funded to QM by National Institute for Occupational Safety and Health, Health Effects Laboratory Division.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Abbreviations
- 8-OHdG
8-hydroxydeoxyquanosine
- α-SMA
α-smooth muscle actin
- ALD
atonic layer deposition
- ASC
apoptosis-associated speck-like protein containing a caspase activation and recruitment domain
- AIM2
absent in melanoma 2
- BAL
bronchoalveolar lavage
- Cav-1
caveolin-1
- CB
carbon black
- CNT
carbon nanotubes
- COX-2
cyclooxygenase-2
- DWCNT
double-walled CNT
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ELISA
enzyme-linked immunosorbent assay
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- GFR
growth factor receptor
- IFN
interferon
- IgE
immunoglobulin E
- IKKα
IκB kinase α
- IL
interleukin
- IL1R
interleukin-1 receptor
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharides
- MAPK
mitogen-activated protein kinase
- MAPKK
MAPK kinase
- MAPKKK
MAPK kinase kinase
- MCA
methylcholanthrene
- MCP
monocyte chemotactic protein
- MPO
myeloperoxidase
- MWCNT
multi-walled CNT
- MWCNT-COOH
carboxylated MWCNT
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-κB
- NK
natural killer
- NLR
nucleotide-binding oligomerization domain-like receptor
- NLRC4
nucleotide-binding oligomerization domain-like receptor: caspase activation and recruitment domain-containing 4
- NLRP3
nucleotide-binding oligomerization domain-like receptor: pyrin domain-containing 3
- NOX
NADPH oxidase
- OPN
osteopontin
- OVA
ovalbumin
- PAR6
partitioning defective protein 6
- PDGF
platelet-derived growth factor
- PDTC
pyrrolidine dithiocarbamate
- PI3K
phosphoinositide 3-kinase
- PMA
phorbol myristate acetate
- RAS
retrovirus-associated DNA sequences
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAEC
small airway epithelial cells
- siRNA
small interfering RNA
- Smad
contraction of Sma and Mad (mothers against decapentaplegic)
- SWCNT
single-walled CNT
- TGF
transforming growth factor
- Th2
T helper 2
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- TPCK
N-tosyl-l-phenylalanine chloromethyl ketone
- VEGF
vascular endothelial growth factor
Footnotes
Declaration of interest
The authors declare that they have no competing interests.
References
- Adamson IY, Bakowska J, Bowden DH. Mesothelial cell proliferation after instillation of long or short asbestos fibers into mouse lung. Am J Pathol. 1993;142:1209–1216. [PMC free article] [PubMed] [Google Scholar]
- Aiso S, Yamazaki K, Umeda Y, Asakura M, Kasai T, Takaya M, et al. Pulmonary toxicity of intratracheally instilled multiwall carbon nanotubes in male Fischer 344 rats. Ind Health. 2010;48:783–795. doi: 10.2486/indhealth.ms1129. [DOI] [PubMed] [Google Scholar]
- Alarifi S, Ali D, Verma A, Almajhdi FN, Al-Qahtani AA. Single-walled carbon nanotubes induce cytotoxicity and DNA damage via reactive oxygen species in human hepatocarcinoma cells. In Vitro Cell Dev Biol Anim. 2014;50:714–722. doi: 10.1007/s11626-014-9760-3. [DOI] [PubMed] [Google Scholar]
- Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- Azad N, Iyer AK, Wang L, Liu Y, Lu Y, Rojanasakul Y. Reactive oxygen species-mediated p38 MAPK regulates carbon nanotube-induced fibrogenic and angiogenic responses. Nanotoxicology. 2013;7:157–168. doi: 10.3109/17435390.2011.647929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borm PJ, Schins RP, Albrecht C. Inhaled particles and lung cancer, part B: paradigms and risk assessment. Int J Cancer. 2004;110:3–14. doi: 10.1002/ijc.20064. [DOI] [PubMed] [Google Scholar]
- Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Brown DM, Dickson C, Duncan P, Al-Attili F, Stone V. Interaction between nanoparticles and cytokine proteins: impact on protein and particle functionality. Nanotechnology. 2010;21:215104. doi: 10.1088/0957-4484/21/21/215104. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kinloch IA, Bangert U, Windle AH, Walters DM, Walker GS, et al. An in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammation mediators and frustrated phagocytosis. Carbon. 2007;45:1743–1756. [Google Scholar]
- Carrero-Sanchez JC, Elias AL, Mancilla R, Arrellin G, Terrones H, Laclette JP, et al. Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Lett. 2006;6:1609–1616. doi: 10.1021/nl060548p. [DOI] [PubMed] [Google Scholar]
- Case BW, Abraham JL, Meeker G, Pooley FD, Pinkerton KE. Applying definitions of “asbestos” to environmental and “low-dose” exposure levels and health effects, particularly malignant mesotheli-oma. J Toxicol Environ Health B Crit Rev. 2011;14:3–39. doi: 10.1080/10937404.2011.556045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Tsai ML, Huang HC, Chen CY, Dai SX. Epithelial-mesenchymal transition contributes to SWCNT-induced pulmonary fibrosis. Nanotoxicology. 2012;6:600–610. doi: 10.3109/17435390.2011.594913. [DOI] [PubMed] [Google Scholar]
- Chaudhury A, Howe PH. The tale of transforming growth factor-beta (TGFbeta) signaling: a soigne enigma. IUBMB Life. 2009;61:929–939. doi: 10.1002/iub.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Nie H, Gao X, Yang J, Pu J, Chen Z, et al. Epithelial-mesenchymal transition involved in pulmonary fibrosis induced by multi-walled carbon nanotubes via TGF-beta/Smad signaling pathway. Toxicol Lett. 2014;226:150–162. doi: 10.1016/j.toxlet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Cheng WW, Lin ZQ, Wei BF, Zeng Q, Han B, Wei CX, et al. Single-walled carbon nanotube induction of rat aortic endothelial cell apoptosis: reactive oxygen species are involved in the mitochondrial pathway. Int J Biochem Cell Biol. 2011;43:564–572. doi: 10.1016/j.biocel.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, Chen HW, et al. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8:437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- Council NR. A Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials. Washington, DC: The National Academic Press; 2012. [PubMed] [Google Scholar]
- Davis JM, Addison J, Bolton RE, Donaldson K, Jones AD, Smith T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 1986;67:415–430. [PMC free article] [PubMed] [Google Scholar]
- De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Stilwell J, Zhang T, Elboudwarej O, Jiang H, Selegue JP, et al. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005;5:2448–2464. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Porter DW, Battelli LA, Wolfarth MG, Richardson DL, Ma Q. Pathologic and molecular profiling of rapid-onset fibrosis and inflammation induced by multi-walled carbon nanotubes. Arch Toxicol [Epub ahead of print] 2014 doi: 10.1007/s00204-014-1428-y. [DOI] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–976. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebel T, Foth H, Damm G, Freyberger A, Kramer PJ, Lilienblum W, et al. Manufactured nanomaterials: categorization and approaches to hazard assessment. Arch Toxicol. 2014;88:2191–2211. doi: 10.1007/s00204-014-1383-7. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Jr, Xiang C, Li M, Ka I, Yang F, Ma D, et al. Purification and sidewall functionalization of multiwalled carbon nanotubes and resulting bioactivity in two macrophage models. Inhal Toxicol. 2013;25:199–210. doi: 10.3109/08958378.2013.775197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Andrews R, Gairola CG. Acute pulmonary response of mice to multi-wall carbon nanotubes. Inhal Toxicol. 2010;22:340–347. doi: 10.3109/08958370903359984. [DOI] [PubMed] [Google Scholar]
- Han YG, Xu J, Li ZG, Ren GG, Yang Z. In vitro toxicity of multi-walled carbon nanotubes in C6 rat glioma cells. Neurotoxicology. 2012;33:1128–1134. doi: 10.1016/j.neuro.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Hansen G, Mcintire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, et al. CD4(+) T helper cells engineered to produce latent TGF-beta1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest. 2000;105:61–70. doi: 10.1172/JCI7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Young SH, Ferback JE, Ma Q. Single-walled carbon nanotubes induce fibrogenic effect by disturbing mitochondrial oxida-tive stress and activating NF-κB signaling. J Clin Toxicol. 2012;S5:005. doi: 10.4172/2161-0495.S5-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Young SH, Schwegler-Berry D, Chisholm WP, Fernback JE, Ma Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-kappaB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem Res Toxicol. 2011;24:2237–2248. doi: 10.1021/tx200351d. [DOI] [PubMed] [Google Scholar]
- Hirano S, Fujitani Y, Furuyama A, Kanno S. Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2010;249:8–15. doi: 10.1016/j.taap.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh WY, Chou CC, Ho CC, Yu SL, Chen HY, Chou HY, et al. Single-walled carbon nanotubes induce airway hyperreactivity and parenchymal injury in mice. Am J Respir Cell Mol Biol. 2012;46:257–267. doi: 10.1165/rcmb.2011-0010OC. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang F, Sun X, Choi KY, Niu G, Zhang G, et al. The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials. 2014;35:856–865. doi: 10.1016/j.biomaterials.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Sangtian S, Anderson SM, Snyder RJ, Marshburn JD, Rice AB, et al. Inflammasome activation in airway epithelial cells after multi-walled carbon nanotube exposure mediates a profibrotic response in lung fibroblasts. Part Fibre Toxicol. 2014;11:28. doi: 10.1186/1743-8977-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Koike E, Yanagisawa R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol. 2009;237:306–316. doi: 10.1016/j.taap.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWGN. Nanotechnology Research Directions: IWGN Workshopreport. Vision for Nanotechnology R&D in the Next Decade. Washington, DC: National Science and Technology Council Committee on Technology Interagency Working Group on Nanoscience, Engineering and Technology (IWGN); 1999. [Google Scholar]
- Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Aschberger K, et al. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico-chemical characteristics. Nanotoxicology. 2010;4:207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett. 2006;165:88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JL, Lee K, Castranova V. Nitric oxide up-regulates DNA-binding activity of nuclear factor-kappaB in macrophages stimulated with silica and inflammatory stimulants. Mol Cell Biochem. 2000;215:1–9. doi: 10.1023/a:1026581301366. [DOI] [PubMed] [Google Scholar]
- Kanno S, Hirano S, Chiba S, Takeshita H, Nagai T, Takada M, et al. The role of Rho-kinases in IL-1beta release through phagocytosis of fibrous particles in human monocytes. Arch Toxicol. 2014;89:73–85. doi: 10.1007/s00204-014-1238-2. [DOI] [PubMed] [Google Scholar]
- Kato T, Totsuka Y, Ishino K, Matsumoto Y, Tada Y, Nakae D, et al. Genotoxicity of multi-walled carbon nanotubes in both in vitro and in vivo assay systems. Nanotoxicology. 2013;7:452–461. doi: 10.3109/17435390.2012.674571. [DOI] [PubMed] [Google Scholar]
- Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, Van Nimwegen M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- Lacerda L, Ali-Boucetta H, Herrero MA, Pastorin G, Bianco A, Prato M, et al. Tissue histology and physiology following intravenous administration of different types of functionalized multiwalled carbon nanotubes. Nanomedicine (London) 2008;3:149–161. doi: 10.2217/17435889.3.2.149. [DOI] [PubMed] [Google Scholar]
- Lam CW, James JT, Mccluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflamma-somes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Lee JK, Sayers BC, Chun KS, Lao HC, Shipley-Phillips JK, Bonner JC, et al. Multi-walled carbon nanotubes induce COX-2 and iNOS expression via MAP kinase-dependent and -independent mechanisms in mouse RAW264.7 macrophages. Part Fibre Toxicol. 2012;9:14. doi: 10.1186/1743-8977-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhao Y, Sun B, Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46:702–713. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohcharoenkal W, Wang L, Stueckle TA, Park J, Tse W, Dinu CZ, et al. Role of H-Ras/ERK signaling in carbon nanotube-induced neoplastic-like transformation of human mesothelial cells. Front Physiol. 2014;5:222. doi: 10.3389/fphys.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luanpitpong S, Wang L, Stueckle TA, Tse W, Chen YC, Rojanasakul Y. Caveolin-1 regulates lung cancer stem-like cell induction and p53 inactivation in carbon nanotube-driven tumorigenesis. Oncotarget. 2014;5:3541–3554. doi: 10.18632/oncotarget.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol Ther. 2010;125:376–393. doi: 10.1016/j.pharmthera.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangum JB, Turpin EA, Antao-Menezes A, Cesta MF, Bermudez E, Bonner JC. Single-walled carbon nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part Fibre Toxicol. 2006;3:15. doi: 10.1186/1743-8977-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manke A, Luanpitpong S, Dong C, Wang L, He X, Battelli L, et al. Effect of fiber length on carbon nanotube-induced fibrogenesis. Int J Mol Sci. 2014;15:7444–7461. doi: 10.3390/ijms15057444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard AD, Kuempel ED. Airborne nanostructured particles and occupational health. J Nanopart Res. 2005;7:587–614. [Google Scholar]
- Maynard AD, Warheit DB, Philbert MA. The new toxicology of sophisticated materials: nanotoxicology and beyond. Toxicol Sci. 2011;120:S109–S129. doi: 10.1093/toxsci/kfq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Friend S, et al. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol. 2011;8:21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, et al. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, Mckinney W, Friend S, et al. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2013a;10:33. doi: 10.1186/1743-8977-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, Mckinney W, et al. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol. 2013b;10:38. doi: 10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier E, Coste A, Olagnier D, Authier H, Lefevre L, Dardenne C, et al. Double-walled carbon nanotubes trigger IL-1beta release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine. 2012;8:987–995. doi: 10.1016/j.nano.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, Mcdonald JD. Pulmonary and systemic immune response to inhaled multi-walled carbon nanotubes. Toxicol Sci. 2007;100:203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Lauer FT, Burchiel SW, Mcdonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WKC, Gee JBL. Asbestos-related diseases. In: Morgan WKC, Seaton A, editors. Occupational Lung Diseases. 3rd. Philadelphia: W.B. Saunders Company; 1995. [Google Scholar]
- Muller J, Decordier I, Hoet PH, Lombaert N, Thomassen L, Huaux F, et al. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis. 2008;29:427–433. doi: 10.1093/carcin/bgm243. [DOI] [PubMed] [Google Scholar]
- Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol Sci. 2009;110:442–448. doi: 10.1093/toxsci/kfp100. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Munshi A, Ramesh R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer. 2013;4:401–408. doi: 10.1177/1947601913485414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 2011;178:2587–2600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AR, Kisin E, Leonard SS, Young SH, Kommineni C, Kagan VE, et al. Oxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubes. Toxicology. 2009;257:161–171. doi: 10.1016/j.tox.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci USA. 2011;108:E1330–E1338. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, Ra C, et al. Blockade of transforming growth factor beta/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000;192:151–158. doi: 10.1084/jem.192.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerl HC, Cheng C, Goode AE, Bergin SD, Lich B, Gass M, et al. Imaging methods for determining uptake and toxicity of carbon nanotubes in vitro and in vivo. Nanomedicine (London) 2011;6:849–865. doi: 10.2217/nnm.11.87. [DOI] [PubMed] [Google Scholar]
- NIOSH. Current Intelligence Bulletin 65: occupational exposure to carbon naotubes and nanofibers. 2013a. [Google Scholar]
- NIOSH. Current Strategies for Engineering Controls in Nanomaterial Production and Downstream Handling Processes. Cincinnati: Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2013b. [Google Scholar]
- NSF. Nanotechnology research directions for societal needs in 2020: retrospective and outlook summary. National Science Foundation. In: Roco M, Mirkin C, Hersan M, editors. Science Policy Reports. New York: Springer; 2011. [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116:1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomaki J, Valimaki E, Sund J, Vippola M, Clausen PA, Jensen KA, et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Park EJ, Cho WS, Jeong J, Yi J, Choi K, Park K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology. 2009;259:113–121. doi: 10.1016/j.tox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Park EJ, Roh J, Kim SN, Kang MS, Han YA, Kim Y, et al. A single intratracheal instillation of single-walled carbon nanotubes induced early lung fibrosis and subchronic tissue damage in mice. Arch Toxicol. 2011;85:1121–1131. doi: 10.1007/s00204-011-0655-8. [DOI] [PubMed] [Google Scholar]
- Park EJ, Zahari NE, Lee EW, Song J, Lee JH, Cho MH, et al. SWCNTs induced autophagic cell death in human bronchial epithelial cells. Toxicol In Vitro. 2014;28:442–450. doi: 10.1016/j.tiv.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Patlolla AK, Hussain SM, Schlager JJ, Patlolla S, Tchounwou PB. Comparative study of the clastogenicity of functionalized and nonfunctionalized multiwalled carbon nanotubes in bone marrow cells of Swiss-Webster mice. Environ Toxicol. 2010;25:608–621. doi: 10.1002/tox.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters PM, Perkins TN, Wouters EF, Mossman BT, Reynaert NL. Silica induces NLRP3 inflammasome activation in human lung epithelial cells. Part Fibre Toxicol. 2013;10:3. doi: 10.1186/1743-8977-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Chen BT, Mckinney W, Mercer RR, Wolfarth MG, et al. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology. 2013;7:1179–1194. doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, et al. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168:58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ravichandran P, Baluchamy S, Sadanandan B, Gopikrishnan R, Biradar S, Ramesh V, et al. Multiwalled carbon nanotubes activate NF-kappaB and AP-1 signaling pathways to induce apoptosis in rat lung epithelial cells. Apoptosis. 2010;15:1507–1516. doi: 10.1007/s10495-010-0532-6. [DOI] [PubMed] [Google Scholar]
- Reddy AR, Reddy YN, Krishna DR, Himabindu V. Pulmonary toxicity assessment of multiwalled carbon nanotubes in rats following intratracheal instillation. Environ Toxicol. 2012;27:211–219. doi: 10.1002/tox.20632. [DOI] [PubMed] [Google Scholar]
- Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothen-Rutishauser B, Brown DM, Piallier-Boyles M, Kinloch IA, Windle AH, Gehr P, et al. Relating the physicochemical characteristics and dispersion of multiwalled carbon nanotubes in different suspension media to their oxidative reactivity in vitro and inflammation in vivo. Nanotoxicology. 2010;4:331–342. doi: 10.3109/17435390.2010.489161. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009a;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009b;40:349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager TM, Wolfarth MW, Andrew M, Hubbs A, Friend S, Chen TH, et al. Effect of multi-walled carbon nanotube surface modification on bioactivity in the C57BL/6 mouse model. Nanotoxicology. 2014;8:317–327. doi: 10.3109/17435390.2013.779757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Nakae D, Fukumori N, Tayama K, Maekawa A, Imai K, et al. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci. 2009;34:65–76. doi: 10.2131/jts.34.65. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Hubbs AF, Young SH, Kashon ML, Dinu CZ, Salisbury JL, et al. Single-walled carbon nanotube-induced mitotic disruption. Mutat Res. 2012;745:28–37. doi: 10.1016/j.mrgentox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli L, et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:3. doi: 10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Reynolds SH, Castranova V. Potential pulmonary effects of engineered carbon nanotubes: in vitro genotoxic effects. Nanotoxicology. 2010;4:396–408. doi: 10.3109/17435390.2010.500444. [DOI] [PubMed] [Google Scholar]
- Sayers BC, Taylor AJ, Glista-Baker EE, Shipley-Phillips JK, Dackor RT, Edin ML, et al. Role of cyclooxygenase-2 in exacerbation of allergen-induced airway remodeling by multiwalled carbon nanotubes. Am J Respir Cell Mol Biol. 2013;49:525–535. doi: 10.1165/rcmb.2013-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schins RP, Knaapen AM. Genotoxicity of poorly soluble particles. Inhal Toxicol. 2007;19:189–198. doi: 10.1080/08958370701496202. [DOI] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Seaton A, Tran L, Aitken R, Donaldson K. Nanoparticles, human health hazard and regulation. J R Soc Interface. 2010;7:S119–S129. doi: 10.1098/rsif.2009.0252.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kapralov AA, Feng WH, Kisin ER, Murray AR, Mercer RR, et al. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperox-idase-deficient mice. PLoS One. 2012a;7:e30923. doi: 10.1371/journal.pone.0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008a;295:L552–L565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Murray AR, Gorelik O, Arepalli S, Castranova V, et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol. 2007;221:339–348. doi: 10.1016/j.taap.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Murray AR, Kommineni C, Castranova V, Fadeel B, et al. Increased accumulation of neutrophils and decreased fibrosis in the lung of NADPH oxidase-deficient C57BL/6 mice exposed to carbon nanotubes. Toxicol Appl Pharmacol. 2008b;231:235–240. doi: 10.1016/j.taap.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol Appl Pharmacol. 2012b;261:121–133. doi: 10.1016/j.taap.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist KJ, Reynolds SH, Kashon ML, Lowry DT, Dong C, Hubbs AF, et al. Genotoxicity of multi-walled carbon nanotubes at occupationally relevant doses. Part Fibre Toxicol. 2014;11:6. doi: 10.1186/1743-8977-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Talkington BN, Schwegler-Berry D, Castranova V, Qian Y, Guo NL. Multi-walled carbon nanotubes induce human microvascular endothelial cellular effects in an alveolar-capillary co-culture with small airway epithelial cells. Part Fibre Toxicol. 2013;10:35. doi: 10.1186/1743-8977-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton MF. Some etiological considerations of fibre carcinogenesis. In: Bogovski P, Gilson JC, Timbrell V, Wagner JC, editors. Biological Effects of Asbestos. Lyon, France: WHO IARC; 1973. [Google Scholar]
- Stone V, Donaldson K. Nanotoxicology: signs of stress. Nat Nanotechnol. 2006;1:23–24. doi: 10.1038/nnano.2006.69. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Youtie J, Porter AL, Shapira P. Is there a shift to “active nanostructures"? J Nanopart Res. 2010;12:1–10. doi: 10.1007/s11051-009-9729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Futakuchi M, Tsuda H, Kanno J. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer Sci. 2012;103:1440–1444. doi: 10.1111/j.1349-7006.2012.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, et al. Induction of mesothelioma in p53+/— mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Mcclure CD, Shipkowski KA, Thompson EA, Hussain S, Garantziotis S, et al. Atomic layer deposition coating of carbon nanotubes with aluminum oxide alters pro-fibrogenic cytokine expression by human mononuclear phagocytes in vitro and reduces lung fibrosis in mice in vivo. PLoS One. 2014;9:e106870. doi: 10.1371/journal.pone.0106870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnherr T, Brandenberger C, Fischer K, Diener L, Manser P, Maeder-Althaus X, et al. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol Lett. 2011;200:176–186. doi: 10.1016/j.toxlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Ursini CL, Cavallo D, Fresegna AM, Ciervo A, Maiello R, Buresti G, et al. Differences in cytotoxic, genotoxic, and inflammatory response of bronchial and alveolar human lung epithelial cells to pristine and COOH-functionalized multiwalled carbon nanotubes. Biomed Res Int. 2014;2014:359506. doi: 10.1155/2014/359506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berlo D, Clift MJ, Albrecht C, Schins RP. Carbon nanotubes: an insight into the mechanisms of their potential genotoxicity. Swiss Med Wkly. 2012;142:w13698. doi: 10.4414/smw.2012.13698. [DOI] [PubMed] [Google Scholar]
- Vietti G, Ibouraadaten S, Palmai-Pallag M, Yakoub Y, Bailly C, Fenoglio I, et al. Towards predicting the lung fibrogenic activity of nanomaterials: experimental validation of an in vitro fibroblast proliferation assay. Part Fibre Toxicol. 2013;10:52. doi: 10.1186/1743-8977-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun P, Bao Y, Liu J, An L. Cytotoxicity of single-walled carbon nanotubes on PC12 cells. Toxicol In Vitro. 2011a;25:242–250. doi: 10.1016/j.tiv.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Wang L, Luanpitpong S, Castranova V, Tse W, Lu Y, Pongrakhananon V, et al. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011b;11:2796–2803. doi: 10.1021/nl2011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mercer RR, Rojanasakul Y, Qiu A, Lu Y, Scabilloni JF, et al. Direct fibrogenic effects of dispersed single-walled carbon nanotubes on human lung fibroblasts. J Toxicol Environ Health A. 2010a;73:410–422. doi: 10.1080/15287390903486550. [DOI] [PubMed] [Google Scholar]
- Wang P, Nie X, Wang Y, Li Y, Ge C, Zhang L, et al. Multiwall carbon nanotubes mediate macrophage activation and promote pulmonary fibrosis through TGF-beta/Smad signaling pathway. Small. 2013;9:3799–3811. doi: 10.1002/smll.201300607. [DOI] [PubMed] [Google Scholar]
- Wang X, Xia T, Ntim SA, Ji Z, George S, Meng H, et al. Quantitative techniques for assessing and controlling the dispersion and biological effects of multiwalled carbon nanotubes in mammalian tissue culture cells. ACS Nano. 2010b;4:7241–7252. doi: 10.1021/nn102112b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168:121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Winter M, Beer HD, Hornung V, Kramer U, Schins RP, Forster I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011;5:326–340. doi: 10.3109/17435390.2010.506957. [DOI] [PubMed] [Google Scholar]
- Wu L, Zhang Y, Zhang C, Cui X, Zhai S, Liu Y, et al. Tuning cell autophagy by diversifying carbon nanotube surface chemistry. ACS Nano. 2014;8:2087–2099. doi: 10.1021/nn500376w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Li R, Bian X, Zhu Z, Ding D, Li X, et al. Covalently combining carbon nanotubes with anticancer agent: preparation and antitumor activity. ACS Nano. 2009;3:2740–2750. doi: 10.1021/nn9005686. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Futakuchi M, Shimizu H, Alexander DB, Yanagihara K, Fukamachi K, et al. Multi-walled carbon nanotubes translocate into the pleural cavity and induce visceral mesothelial proliferation in rats. Cancer Sci. 2012;103:2045–2050. doi: 10.1111/cas.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Fujitani T, Ohyama K, Nakae D, Hirose A, Nishimura T, et al. Effects of sustained stimulation with multi-wall carbon nanotubes on immune and inflammatory responses in mice. J Toxicol Sci. 2012;37:177–189. doi: 10.2131/jts.37.177. [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Guarda G, Riteau N, Drexler SK, Tardivel A, Couillin I, et al. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc Natl Acad Sci USA. 2010;107:19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Jiang Y, Zhang H, Wang Y, Wu Y, Hou Z, et al. Multi-walled carbon nanotubes induce apoptosis in RAW 264.7 cell-derived osteoclasts through mitochondria-mediated death pathway. J Nanosci Nanotechnol. 2012;12:2101–2112. doi: 10.1166/jnn.2012.5677. [DOI] [PubMed] [Google Scholar]
- Ye SF, Wu YH, Hou ZQ, Zhang QQ. ROS and NF-kappaB are involved in upregulation of IL-8 in A549 cells exposed to multi-walled carbon nanotubes. Biochem Biophys Res Commun. 2009;379:643–648. doi: 10.1016/j.bbrc.2008.12.137. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Huang JQ, Qian WZ, Zhang YY, Wei F. The road for nanomaterials industry: a review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small. 2013;9:1237–1265. doi: 10.1002/smll.201203252. [DOI] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu R. Recent progress and perspectives on the toxicity of carbon nanotubes at organism, organ, cell, and biomacromolecule levels. Environ Int. 2012;40:244–255. doi: 10.1016/j.envint.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Zhiqing L, Zhuge X, Fuhuan C, Danfeng Y, Huashan Z, Bencheng L, et al. ICAM-1 and VCAM-1 expression in rat aortic endothelial cells after single-walled carbon nanotube exposure. J Nanosci Nanotechnol. 2010;10:8562–8574. doi: 10.1166/jnn.2010.2680. [DOI] [PubMed] [Google Scholar]