Abstract
Using a broad-scale automated telemetry array, we explored post-fledging movements of blackpoll warblers breeding in Atlantic Canada. We sought to determine the full spatial scale of post-fledging dispersal, to assess support for three hypotheses for regional-scale post-fledging movement, and to determine whether learning influenced movement during this period. We demonstrated that both young and adults moved over distances more than 200 km prior to initiating migration. Adults moved southwest, crossing the Gulf of Maine (GOM), consistent with the commencement of migration hypothesis. Hatch-year birds exhibited less directional movements constrained geographically by the GOM. Their movements were most consistent with exploration hypotheses—that young birds develop a regional-scale map to aid in habitat selection, natal dispersal and subsequent migrations.
Keywords: post-fledging, dispersal, blackpoll warbler, migration, radio telemetry
1. Introduction
The post-fledging period is the primary population bottleneck for both hatch-year and adult birds [1,2]. Post-fledging dispersal comprises three stages: a dependent period after leaving the nest, an independent period prior to dispersal and a dispersal period prior to migration. The latter period has been poorly studied because of logistical constraints of tracking small birds over broad areas, although there are incidental records of post-fledging movements of approximately 250 km [3,4].
Several hypotheses have been put forward regarding post-fledging movements. Most fine-scale studies support the habitat amelioration hypothesis, which suggests that movements are related to habitat needs. Both hatch-years and adults increase the size of their home ranges and occupy denser, earlier succession habitat immediately after breeding [5,6]. However, this hypothesis does not account for the scale of movement suggested by incidental recaptures sometimes hundreds of kilometres from breeding grounds [3,4]. It is plausible that there are multiple underlying reasons for post-fledging movements, with some acting at broader spatial or temporal scales. Two other hypotheses that account for broader-scale movements have been proposed, though few studies have assessed them [7]. The exploration hypothesis suggests that movements are exploratory, whereby individuals assess habitat and obtain social cues regarding productivity of future breeding sites [8], or familiarize themselves with the landscape to aid in navigation [9,10]. Alternately, the migration hypothesis suggests that individuals travel in an appropriate direction for migration without entering a physiological migratory state [3].
Prior to moving away from the breeding area, hatch-year birds have no innate knowledge of the geographical area that surrounds them, but do innately know the broad-scale orientation and distance required for their first migratory journey [11,12]. Adults, however, can remember locations and quality of geographical areas they have previously explored [13], allowing them to return to sites discovered during previous post-fledging or migratory periods [11,13]. This suggests that there could be a contrast between movements of adults and hatch-years during the dispersal period with adults potentially moving towards previously learned geographical areas, and hatch-years exploring more broadly.
The Atlantic population of blackpoll warblers (Setophaga striata) depart from the Atlantic coast during autumn migration, travelling approximately 3000 km overwater to South America, then returning in spring using a different, overland route up the Atlantic coast of North America [14,15]. Autumn migration thus starts with departure across the Atlantic Ocean, providing a simple way to distinguish between post-fledging and migratory periods. This loop migration pattern introduces adult birds to the geography of the Atlantic coast during spring, where hatch-years may not be innately compelled to travel during autumn. If only adult birds use the more southern part of the Atlantic coast (New England) during autumn migration, then it suggests that they are doing so based on information learned during spring migration.
We sought to determine for these Atlantic populations of blackpoll warbler (i) the full spatial scale of post-fledging dispersal, (ii) whether there was evidence to support the hypotheses for regional-scale post-fledging movement outlined above and (iii) how learning influences movement during this period.
To do this, we compared, at a regional scale, the post-breeding movements of hatch-year and adult blackpoll warblers from the same breeding area (where both ages would be expected to have similar innate preferences). If only the habitat hypothesis influences dispersal, then we expected to see similar movements of adults and hatch-years, with no regional-scale dispersal. Under the exploration hypothesis, we expected the movements of hatch-years to be less directional than adults, whereas under the commencement of migration hypothesis, we expected, for either age, to see movements that would minimize the overwater component of the migratory route. Finally, differential use of the New England coast by age would indicate the ability to alter innate migratory routes based on learning.
2. Methods
Ten adults and 13 hatch-years were tagged between 13 August and 13 September 2014 at their breeding/natal grounds on Bon Portage Island (BP; 43°28′ N, 65°45′ W) in southwest Nova Scotia (figure 1; see electronic supplementary material). Each individual was aged according to Pyle et al. [16], and fitted with a digitally coded radio transmitter (Avian NanoTag NTQB—2, Lotek Wireless Inc., Newmarket, Canada) using a figure-eight leg loop harness [17]. Tags transmitted a coded burst at 166.380 MHz every 11.2 s. Tag life was approximately 40 days, and tags weighed 0.29 g, approximately 2.0% of body weight.
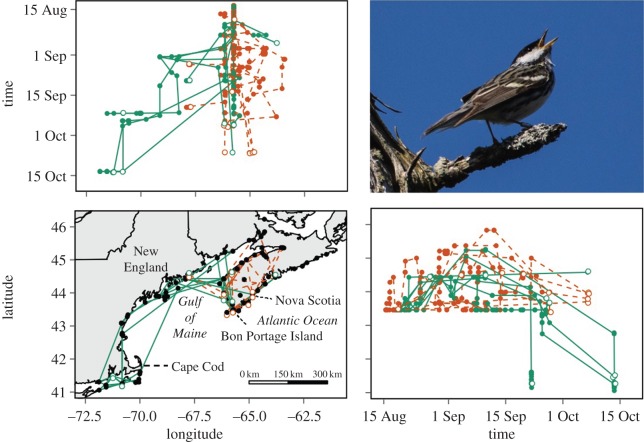
Figure 1.
Post-fledging dispersal paths and displacement through time of adult and hatch-year blackpoll warblers breeding in southwestern Nova Scotia, tracked using an automated telemetry array. Telemetry towers are represented by black dots on the map. Adults are represented by solid lines, and hatch-years by dashed lines. Final detections of each individual are represented with an open circle. (Online vesion in colour.)
Movements were tracked using an array of more than 60 automated digital telemetry towers in the Motus Wildlife Tracking System (www.motus-wts.org; figure 1). Each antenna on a tower was connected to a SensorGnome receiver (sensorgnome.org) that operated 24 h d−1. A tag detection comprised a GPS synchronized time and signal strength.
We used simple linear models to assess whether net displacement or cumulative net displacement differed with age. We used Gaussian, identity link generalized additive mixed models with temporally autocorrelated errors (gamm, R package mgcv [18], with individual as a random effect) to model latitude and longitude of hourly detections by smoothed functions of time and age. We used likelihood ratio tests to assess whether there was evidence that time, age or their interactions were related to the response (see electronic supplementary material).
3. Results
Individuals left BP between 20 August and 14 September (on average (±s.d.) 10 ± 5 days after tagging) and presumed migratory departures across the Atlantic occurred between 28 September and 13 October 2014 (38 ± 17 days after tagging). One adult and three hatch-years died before departing BP. Adults and hatch-years were detected as distant as 484 and 200 km from BP, respectively (figure 1). Sixty-six per cent of adults and 30% of hatch-years moved west across the Gulf of Maine (GOM). Of birds that crossed the GOM, 66% of adults and no hatch-years were detected as far south as Cape Cod. Mean (±s.d.) net displacement of adults (280 ± 202 km) was greater than hatch-years (91 ± 68 km; F1,17 = 7.91, p = 0.01), but cumulative distance travelled was not (adults: 567 ± 351 km versus hatch-years: 488 ± 348 km; F1,17 = 0.24, p = 0.64).
There was support for a time by age interaction in longitudinal (likelihood ratio test, LRT: χ2 = 15.3, p = 0.0001) but not latitudinal (LRT: χ2 = 2.9, p = 0.09) models. Longitudinal movements of adults were oriented west of BP (a linear relationship with time), whereas hatch-years tended to move slightly east of their starting position (figure 1). Adults that remained in Nova Scotia stayed close to the tagging site. Latitudinal movements of both age groups initially were directed north, with adults eventually moving further south than hatch-years (a nonlinear relationship with time; figure 1).
4. Discussion
Our results provide evidence that post-breeding movements are related to commencement of migration for adults and regional exploration for hatch-years. Net displacement from the tagging location differed strikingly with age even though both groups travelled similar distances. Both age groups moved considerably more than usually observed during the post-fledging period (but see [3,4]).
The New England route used by adults supports the commencement of migration hypothesis, because it shortens the length of their non-stop open ocean crossing [15]. The scale of movement observed suggests habitat amelioration is not the only cause of movement during this time, although it likely acts on a finer spatial scale within the observed regional-scale movement [19,20].
Consistent with the exploratory hypothesis, hatch-years exhibited more indirect, circular movements, moving north after leaving natal areas and returning south prior to undertaking transoceanic migratory flight. We cannot determine the purpose of the exploratory movements with our data, although we speculate they relate to a combination of prospecting and navigation. Adults dispersed at the same time as hatch-years, meaning that social information from conspecifics is unavailable to prospecting hatch-years [8,9], although heterospecific social cues and other habitat features may be learned [21]. To enable them to return quickly to breeding areas the following spring, hatch-years must also learn navigational landmarks [9]. Exploration along coastlines provides information about visual landmarks [9] as well as fine-scale habitat information of potential breeding areas; and interestingly, movement along a north–south axis is also perpendicular to the magnetic gradient in Nova Scotia, which would maximize information regarding the regional magnetic field.
Differential use of the New England coastline between adult and hatch-years provides indirect evidence that individuals alter innate migratory routes based on learning. At our tagging site, hatch-years are unlikely to innately know details of the geography of the Atlantic coastline further south. Thus, observed movements of adults (west then south) are most logical if we consider that these individuals learned the shape of the coastline during their initial spring migration. In other studies, adults have been shown to improve orientation and alter migratory strategy based on learned information [22,23]. Furthermore, even though they travelled similar distances, movements of most hatch-year birds appeared constrained by Nova Scotia's coastline. The overwater distance across to New England has not yet been learned by hatch-years, so hatch-years may also perceive the open water as risky, compared with adults.
Few other studies have tracked regional-scale post-fledging movements. Typical fine-scale studies may be biased towards supporting hypotheses regarding site selection, food availability and habitat structure as key variables determining post-fledging movements [19]. As in other ecological phenomena, a hierarchy of factors likely interact at multiple scales [20], so choosing appropriate scales to observe ecological processes is crucial.
Supplementary Material
Acknowledgements
We thank D. Bell, T. Brown and M. Furgoch for assistance in the field, Bird Studies Canada for management of the Motus project and towers, and researchers at UMass, UMaine and US Fisheries and Oceans for sharing telemetry data from their towers.
Ethics
This study was approved by Acadia University's Animal Care Committee (15-11R#3A#2) and Environment Canada (10169BT).
Data accessibility
Data supporting this article are available at www.motus-wts.org.
Authors' contributions
J.M.B. and P.D.T. conceived and designed the study. J.M.B. collected and analysed data. J.M.B. and P.D.T. wrote and revised the paper. All authors approve the final version of the manuscript and agree to be held accountable for the work performed.
Competing interests
We have no competing interests.
Funding
Funding was provided by Canadian Foundation for Innovation and NSERC Discovery grant to P.D.T., and an NSERC PGS scholarship to J.M.B.
References
- 1.Cox WA, Thompson FR, Cox AS, Faaborg J. 2014. Post-fledging survival in passerine birds and the value of post-fledging studies to conservation. J. Wildl. Manag. 78, 183–193. ( 10.1002/jwmg.670) [DOI] [Google Scholar]
- 2.Grüebler MU, Korner-Nievergelt F, Naef-Daenzer B. 2014. Equal nonbreeding period survival in adults and juveniles of a long-distant migrant bird. Ecol. Evol. 4, 756–765. ( 10.1002/ece3.984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherry JD. 1985. Early autumn movements and prebasic molt of Swainson's thrushes. Wilson Bull. 97, 368–370. [Google Scholar]
- 4.Morton ML. 1991. Postfledging dispersal of green-tailed towhees to a subalpine meadow. Condor 93, 466–468. ( 10.2307/1368971) [DOI] [Google Scholar]
- 5.Anders AD, Faaborg J, Thompson FR. 1998. Postfledging dispersal, habitat use, and home-range size of juvenile wood thrushes. Auk 115, 349–358. ( 10.2307/4089193) [DOI] [Google Scholar]
- 6.Vega Rivera J, McShea W, Rappole J, Haas C. 1999. Postbreeding movements and habitat use of adult wood thrushes in northern Virginia. Auk 116, 458–466. ( 10.2307/4089379) [DOI] [Google Scholar]
- 7.Mitchell GW, Taylor PD, Warkentin IG. 2010. Assessing the function of broad-scale movements made by juvenile songbirds prior to migration. Condor 112, 644–654. ( 10.1525/cond.2010.090136) [DOI] [Google Scholar]
- 8.Betts MG, Hadley AS, Rodenhouse N, Nocera JJ. 2008. Social information trumps vegetation structure in breeding-site selection by a migrant songbird. Proc. R. Soc. B 275, 2257–2263. ( 10.1098/rspb.2008.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker RR. 1993. The function of post-fledging exploration: a pilot study of three species of passerines ringed in Britain. Ornis Scand. 24, 71–79. ( 10.2307/3676413) [DOI] [Google Scholar]
- 10.Wiltschko W, Wiltschko R. 1978. A theoretical model for migratory orientation and homing in birds. Oikos 30, 177–187. ( 10.2307/3543477) [DOI] [Google Scholar]
- 11.Perdeck A. 1958. Two types of orientation in migrating starlings, Sturnus vulgaris L., and chaffinches, Fringilla coelebs L., as revealed by displacement experiments. Ardea 46, 1–37. [Google Scholar]
- 12.Helbig A. 1996. Genetic basis, mode of inheritance and evolutionary changes of migratory directions in palaearctic warblers (Aves: Sylviidae). J. Exp. Biol. 199, 49–55. [DOI] [PubMed] [Google Scholar]
- 13.Mettke-Hofmann C, Gwinner E. 2003. Long-term memory for a life on the move. Proc. Natl Acad. Sci. USA 100, 5863–5866. ( 10.1073/pnas.1037505100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nisbet IC, McNair DB, Post W, Williams TC. 1995. Transoceanic migration of the blackpoll warbler: summary of scientific evidence and response to criticisms by Murray. J. Field Ornithol. 66, 612–622. [Google Scholar]
- 15.DeLuca WV, Woodworth BK, Rimmer CC, Marra PP, Taylor PD, McFarland KP, Mackenzie SA, Norris DR. 2015. Transoceanic migration by a 12 g songbird. Biol. Lett. 11, 20141045 ( 10.1098/rsbl.2014.1045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pyle P, Howell SN, DeSante D. 1997. Identification guide to North American birds. Bolinas, CA: Slate Creek Press. [Google Scholar]
- 17.Rappole JH, Tipton AR. 1991. New harness design for attachment of radio transmitters to small passerines. J. Field Ornithol. 62, 335–337. [Google Scholar]
- 18.Wood SN. 2006. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 19.Buler JJ, Moore FR, Woltmann S. 2007. A multi-scale examination of stopover habitat use by birds. Ecology 88, 1789–1802. ( 10.1890/06-1871.1) [DOI] [PubMed] [Google Scholar]
- 20.Wiens JA. 1989. Spatial scaling in ecology. Funct. Ecol. 3, 385–397. ( 10.2307/2389612) [DOI] [Google Scholar]
- 21.Kivelä S, Seppänen J-T, Ovaskainen O, Doligez B, Gustafsson L, Mönkkönen M, Forsman J. 2014. The past and the present in decision-making: the use of conspecific and heterospecific cues in nest site selection. Ecology 95, 3428–3439. ( 10.1890/13-2103.1) [DOI] [Google Scholar]
- 22.Ralph CJ. 1981. Age ratios and their possible use in determining autumn routes of passerine migrants. Wilson Bull. 93, 164–188. [Google Scholar]
- 23.Crysler Z. 2015. Breeding ground dispersal and fall migratory movements of Ipswich Sparrows (Passerculus sandwichensis princeps). MSc thesis, Acadia University.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this article are available at www.motus-wts.org.