Abstract
gp160 is a cell surface differentiation-related glycoprotein of 160 kDa expressed by epithelial cells of the glomerulus and proximal tubule cells of the human nephron but only by a subset of renal cell carcinomas (RCCs). We have reported that gp160 expression correlates with the resistance of cultured RCCs to the antiproliferative effects of alpha interferon, while lack of expression correlates with sensitivity to alpha interferon. In this study, we have purified gp160 protein, obtained partial sequences of random peptides, and isolated a full-length cDNA. The gp160 cDNA possesses 78% homology to the murine BP-1/6C3 antigen, a B-lymphocyte differentiation protein that exhibits aminopeptidase A (APA; EC 3.4.11.7) activity. Enzymatic assays on human RCC cell lines indicated a 100% concordance between APA activity and gp160 expression. APA activity of gp160-expressing RCC cells was increased or decreased by a panel of APA activators or inhibitors, respectively. Furthermore, anti-gp160 monoclonal antibodies immunoprecipitate APA activity from RCC cell lysates and selectively deplete APA activity from RCC cell extracts. These data indicate that the gp160 human kidney/RCC glycoprotein is human APA.
Full text
PDF

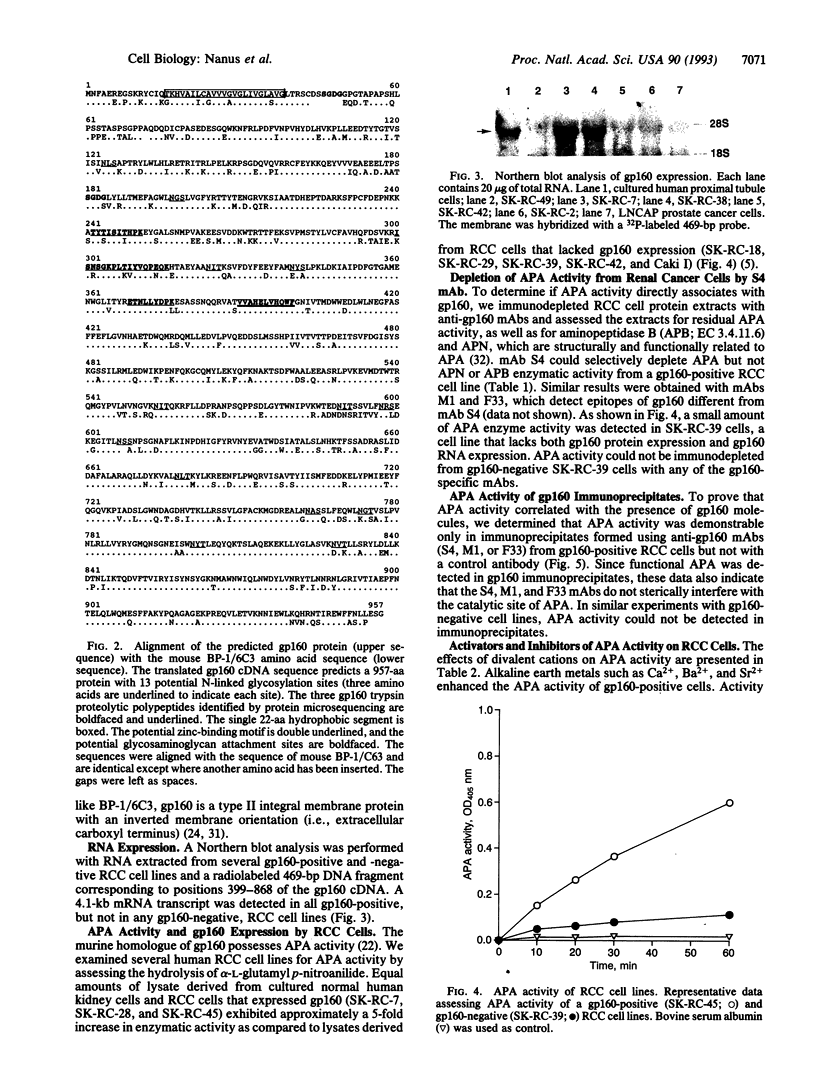
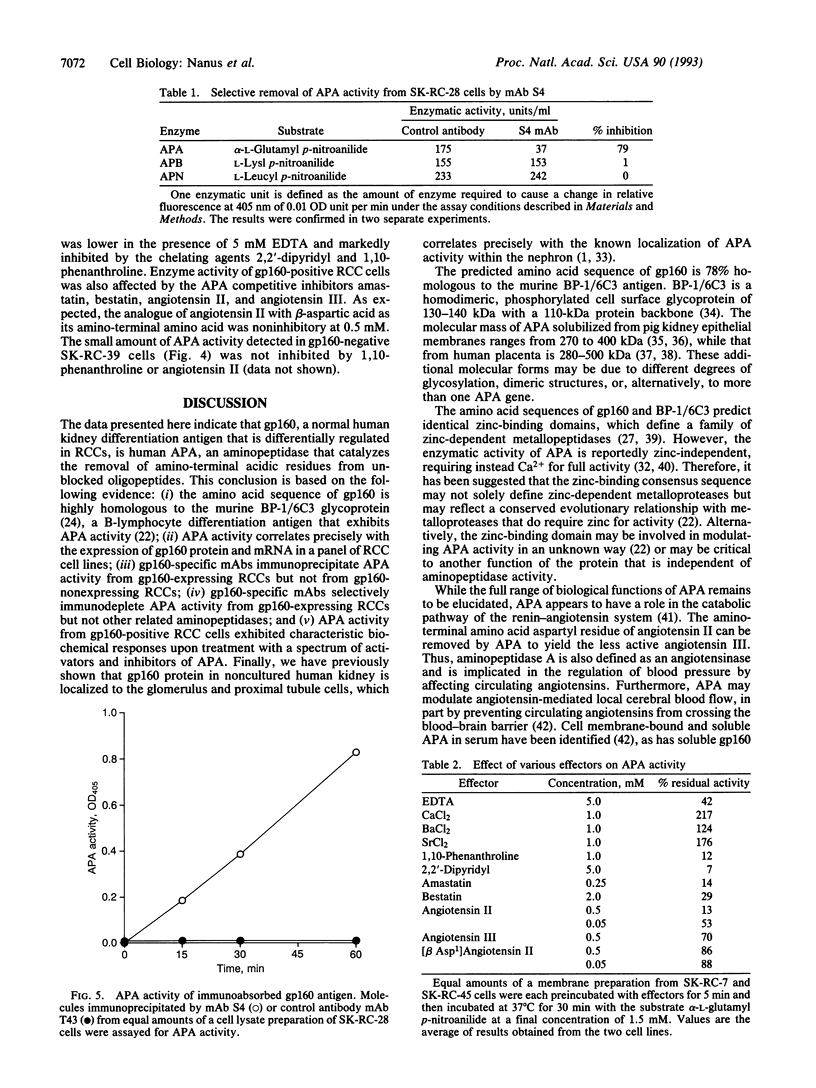

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Ward P. E. Role of aminopeptidase activity in the regulation of the pressor activity of circulating angiotensins. J Pharmacol Exp Ther. 1990 Feb;252(2):643–650. [PubMed] [Google Scholar]
- Alcalay J., Ullrich S. E., Kripke M. L. Local suppression of contact hypersensitivity in mice by a monofunctional psoralen plus UVA radiation. Photochem Photobiol. 1989 Aug;50(2):217–220. doi: 10.1111/j.1751-1097.1989.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Andy R. J., Finstad C. L., Old L. J., Lloyd K. O., Kornfeld R. The antigen identified by a mouse monoclonal antibody raised against human renal cancer cells is the adenosine deaminase binding protein. J Biol Chem. 1984 Oct 25;259(20):12844–12849. [PubMed] [Google Scholar]
- Bander N. H., Cordon-Cardo C., Finstad C. L., Whitmore W. F., Jr, Vaughan E. D., Jr, Oettgen H. F., Melamed M., Old L. J. Immunohistologic dissection of the human kidney using monoclonal antibodies. J Urol. 1985 Mar;133(3):502–505. doi: 10.1016/s0022-5347(17)49041-0. [DOI] [PubMed] [Google Scholar]
- Bander N. H., Finstad C. L., Cordon-Cardo C., Ramsawak R. D., Vaughan E. D., Jr, Whitmore W. F., Jr, Oettgen H. F., Melamed M. R., Old L. J. Analysis of a mouse monoclonal antibody that reacts with a specific region of the human proximal tubule and subsets renal cell carcinomas. Cancer Res. 1989 Dec 1;49(23):6774–6780. [PubMed] [Google Scholar]
- Bander N. H. Monoclonal antibodies: state of the art. J Urol. 1987 Apr;137(4):603–612. doi: 10.1016/s0022-5347(17)44154-1. [DOI] [PubMed] [Google Scholar]
- Bausback H. H., Churchill L., Ward P. E. Angiotensin metabolism by cerebral microvascular aminopeptidase A. Biochem Pharmacol. 1988 Jan 15;37(2):155–160. doi: 10.1016/0006-2952(88)90712-5. [DOI] [PubMed] [Google Scholar]
- Bourdon M. A., Krusius T., Campbell S., Schwartz N. B., Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Norén O., Sjöström H., Ingram J., Kenny A. J. Proteins of the kidney microvillar membrane. Aspartate aminopeptidase: purification by immunoadsorbent chromatography and properties of the detergent- and proteinase-solubilized forms. Biochem J. 1980 Sep 1;189(3):591–603. doi: 10.1042/bj1890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker A. P., Volkenandt M., Albino A. P. Mutational analysis of human NRAS genes in malignant melanoma: rapid methods for oligonucleotide hybridization and manual and automated direct sequencing of products generated by the polymerase chain reaction. Genes Chromosomes Cancer. 1990 Mar;1(4):257–269. doi: 10.1002/gcc.2870010402. [DOI] [PubMed] [Google Scholar]
- Ebert T., Bander N. H., Finstad C. L., Ramsawak R. D., Old L. J. Establishment and characterization of human renal cancer and normal kidney cell lines. Cancer Res. 1990 Sep 1;50(17):5531–5536. [PubMed] [Google Scholar]
- Feracci H., Benajiba A., Gorvel J. P., Doumeng C., Maroux S. Enzymatic and immunological properties of the protease form of aminopeptidase N and A from pig and rabbit intestinal brush border. Biochim Biophys Acta. 1981 Mar 13;658(1):148–157. doi: 10.1016/0005-2744(81)90258-8. [DOI] [PubMed] [Google Scholar]
- Finstad C. L., Cordon-Cardo C., Bander N. H., Whitmore W. F., Melamed M. R., Old L. J. Specificity analysis of mouse monoclonal antibodies defining cell surface antigens of human renal cancer. Proc Natl Acad Sci U S A. 1985 May;82(9):2955–2959. doi: 10.1073/pnas.82.9.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C. Purification of glycoproteins. Methods Enzymol. 1990;182:529–539. doi: 10.1016/0076-6879(90)82042-z. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- Kugler P., Wolf G., Scherberich J. Histochemical demonstration of peptidases in the human kidney. Histochemistry. 1985;83(4):337–341. doi: 10.1007/BF00684380. [DOI] [PubMed] [Google Scholar]
- Look A. T., Ashmun R. A., Shapiro L. H., Peiper S. C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest. 1989 Apr;83(4):1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanus D. M., Ebrahim S. A., Bander N. H., Real F. X., Pfeffer L. M., Shapiro J. R., Albino A. P. Transformation of human kidney proximal tubule cells by ras-containing retroviruses. Implications for tumor progression. J Exp Med. 1989 Mar 1;169(3):953–972. doi: 10.1084/jem.169.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanus D. M., Mentle I. R., Motzer R. J., Bander N. H., Albino A. P. Infrequent ras oncogene point mutations in renal cell carcinoma. J Urol. 1990 Jan;143(1):175–178. doi: 10.1016/s0022-5347(17)39905-6. [DOI] [PubMed] [Google Scholar]
- Nanus D. M., Pfeffer L. M., Bander N. H., Bahri S., Albino A. P. Antiproliferative and antitumor effects of alpha-interferon in renal cell carcinomas: correlation with the expression of a kidney-associated differentiation glycoprotein. Cancer Res. 1990 Jul 15;50(14):4190–4194. [PubMed] [Google Scholar]
- Nishikawa B. K., Fowlkes D. M., Kay B. K. Convenient uses of polymerase chain reaction in analyzing recombinant cDNA clones. Biotechniques. 1989 Jul-Aug;7(7):730–735. [PubMed] [Google Scholar]
- Okuyama T., Ishiura S., Nojima M., Tsukahara T., Yanagida M., Sugita H. Aminopeptidase A in human placenta and pregnant serum. Clin Chim Acta. 1991 Feb 15;196(2-3):207–215. doi: 10.1016/0009-8981(91)90074-m. [DOI] [PubMed] [Google Scholar]
- Olsen J., Cowell G. M., Kønigshøfer E., Danielsen E. M., Møller J., Laustsen L., Hansen O. C., Welinder K. G., Engberg J., Hunziker W. Complete amino acid sequence of human intestinal aminopeptidase N as deduced from cloned cDNA. FEBS Lett. 1988 Oct 10;238(2):307–314. doi: 10.1016/0014-5793(88)80502-7. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L., Wu Q., Yue A., Cooper M. D., Rosenberg N. BP-1/6C3 expression defines a differentiation stage of transformed pre-B cells and is not related to malignant potential. J Immunol. 1990 Sep 1;145(5):1603–1608. [PubMed] [Google Scholar]
- Rosenberg H. F., Corrette S. E., Tenen D. G., Ackerman S. J. Rapid cDNA library screening using the polymerase chain reaction. Biotechniques. 1991 Jan;10(1):53–54. [PubMed] [Google Scholar]
- Sherwood P. J., Weissman I. L. The growth factor IL-7 induces expression of a transformation-associated antigen in normal pre-B cells. Int Immunol. 1990;2(5):399–406. doi: 10.1093/intimm/2.5.399. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Maher P. A., Yaffe M. P. On the transfer of integral proteins into membranes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1960–1964. doi: 10.1073/pnas.84.7.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic V., Vlahovic P., Ardaillou N., Ronco P., Ardaillou R. Cell surface aminopeptidase A and N activities in human glomerular epithelial cells. Kidney Int. 1992 Jun;41(6):1571–1580. doi: 10.1038/ki.1992.227. [DOI] [PubMed] [Google Scholar]
- Tempst P., Link A. J., Riviere L. R., Fleming M., Elicone C. Internal sequence analysis of proteins separated on polyacrylamide gels at the submicrogram level: improved methods, applications and gene cloning strategies. Electrophoresis. 1990 Jul;11(7):537–553. doi: 10.1002/elps.1150110704. [DOI] [PubMed] [Google Scholar]
- Tempst P., Riviere L. Examination of automated polypeptide sequencing using standard phenyl isothiocyanate reagent and subpicomole high-performance liquid chromatographic analysis. Anal Biochem. 1989 Dec;183(2):290–300. doi: 10.1016/0003-2697(89)90482-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe H., Kojima F., Aoyagi T., Umezawa H. Purification by affinity chromatography using amastatin and properties of aminopeptidase A from pig kidney. Biochim Biophys Acta. 1980 Jun 13;613(2):459–468. doi: 10.1016/0005-2744(80)90100-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda R., Ogata S., Morrissey D. M., Finstad C. L., Szkudlarek J., Whitmore W. F., Oettgen H. F., Lloyd K. O., Old L. J. Cell surface antigens of human renal cancer defined by mouse monoclonal antibodies: identification of tissue-specific kidney glycoproteins. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5122–5126. doi: 10.1073/pnas.78.8.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G. B., Scherberich J. E., Schoeppe W. Peptidase-associated antigens as markers of differentiation in human kidney and renal adenocarcinoma. Cell Mol Biol. 1988;34(3):311–321. [PubMed] [Google Scholar]
- Wu Q., Lahti J. M., Air G. M., Burrows P. D., Cooper M. D. Molecular cloning of the murine BP-1/6C3 antigen: a member of the zinc-dependent metallopeptidase family. Proc Natl Acad Sci U S A. 1990 Feb;87(3):993–997. doi: 10.1073/pnas.87.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Li L., Cooper M. D., Pierres M., Gorvel J. P. Aminopeptidase A activity of the murine B-lymphocyte differentiation antigen BP-1/6C3. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):676–680. doi: 10.1073/pnas.88.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]



