Abstract
Like many animals, firebugs (Hemiptera, Pyrrhocoridae) rely on behavioural adaptations to successfully endow their offspring with microbial mutualists. To transmit the nutritionally beneficial Coriobacteriaceae symbionts, female firebugs smear egg surfaces with symbiont-containing faecal droplets that are subsequently ingested by newly hatched nymphs through active probing to initiate infection. Alternatively, the symbionts can be acquired horizontally through contact with faeces of infected conspecifics. Here, we report that these adaptations ensuring successful transmission of bacterial symbionts among firebugs are exploited by the specialized trypanosomatid parasite Leptomonas pyrrhocoris. Using comparative transcriptomics, fluorescence in situ hybridization (FISH) and controlled bioassays, we demonstrate that the transmission cycle of L. pyrrhocoris mirrors that of the bacterial mutualists, with high efficiency for both vertical and horizontal transmission. This indicates that the parasite capitalizes on pre-existing behavioural adaptations (egg smearing and probing) to facilitate its own transfer within host populations, adaptations that likely evolved to initiate and maintain an association with beneficial gut symbionts. Thus, the transmission of mutualistic microbes across host generations can entail a significant risk of co-transmitting pathogens or parasites, thereby exerting selective pressures on the host to evolve more specific mechanisms of transfer.
Keywords: host–parasite interaction, vertical and horizontal transmission, symbiont, mutualism
1. Introduction
Mutualisms with microorganisms have played an integral role in the evolution of animals [1]. As such, numerous adaptations have evolved, in both host and symbiont, to ensure the successful transfer of beneficial microbes to future host generations [2], thereby contributing to the maintenance and evolutionary stability of mutualisms. Similarly, among parasites that obligately depend on their hosts for survival, fitness is largely determined by their success in establishing infection and transmitting to other hosts [3]. Thus, adaptations for successful initiation and maintenance of infection are strongly selected for, and, as a result, represent a fundamental component of the parasites' ecology and evolution [4].
Firebugs associate with a highly stable gut bacterial community dominated by two actinobacterial symbionts belonging to the Coriobacteriaceae family, as well as members of the Firmicutes and Gammaproteobacteria [5,6]. The two Coriobacteriaceae symbionts Coriobacterium glomerans and Gordonibacter sp. are vertically transmitted across host generations through the faecal smearing of egg surfaces by females during oviposition, but can also be horizontally acquired through contact with conspecifics [7]. Experimental sterilization of egg surfaces disrupts the transmission cycle of the Coriobacteriaceae symbionts, resulting in aposymbiotic (symbiont-free) firebugs that suffer retarded growth, high mortality and low fecundity [8], which is owing to the deficiency in B vitamins that are provided by the symbionts [9].
Matching the highly conserved bacterial midgut community associated with firebugs is the specialized epidemiology of trypanosomatids across this insect family [10]. Most striking is the cosmopolitan distribution of a single, mildly virulent flagellate, Leptomonas pyrrhocoris, across at least 11 species and four genera of firebugs sampled from eight countries across four continents [10–12]. Among firebugs, infection by L. pyrrhocoris can induce paler coloration, lethargy and diarrhoea [11], as well as increased mortality, reduced starvation resistance and a reduced lifespan in insects that contract the parasite early in development [12].
Despite the global distribution of L. pyrrhocoris among firebugs, little is known about how infection is initiated and maintained. While it is presumed that L. pyrrhocoris is horizontally transferred between con- and heterospecifics as mediated by the large aggregations formed by the insects [10], no study to date has directly reported on the transmission route of L. pyrrhocoris within and between host populations.
In this study, we demonstrate that the transmission route of L. pyrrhocoris in firebugs mirrors that of the Coriobacteriaceae symbionts. Specifically, we report that while L. pyrrhocoris can be acquired horizontally through contact with infected firebugs, the parasite also exploits the symbionts' vertical transmission route via the egg surface for its own transfer among host individuals.
2. Results and discussion
Our first insights into the transmission route of trypanosomatids in pyrrhocorid bugs came from comparative transcriptomic analyses of midguts extracted from the firebug Dysdercus fasciatus that had either been subjected to egg surface sterilization to rid them of their beneficial Coriobacteriaceae symbionts or left untreated. While the untreated group featured transcripts that could be assigned to the Trypanosomatidae cluster TU61 (associated with the genus Blastocrithidia, see [10]), none could be retrieved from firebugs that had been subjected to egg surface sterilization (figure 1a), suggesting that the method for eliminating the bacterial symbionts also clears infection by the trypanosomatid. This hypothesis is corroborated by fluorescence in situ hybridization (FISH) images demonstrating the co-localization of L. pyrrhocoris and C. glomerans in faecal droplets collected from firebugs that had been artificially inoculated with L. pyrrhocoris in the laboratory (figure 1b).
Figure 1.
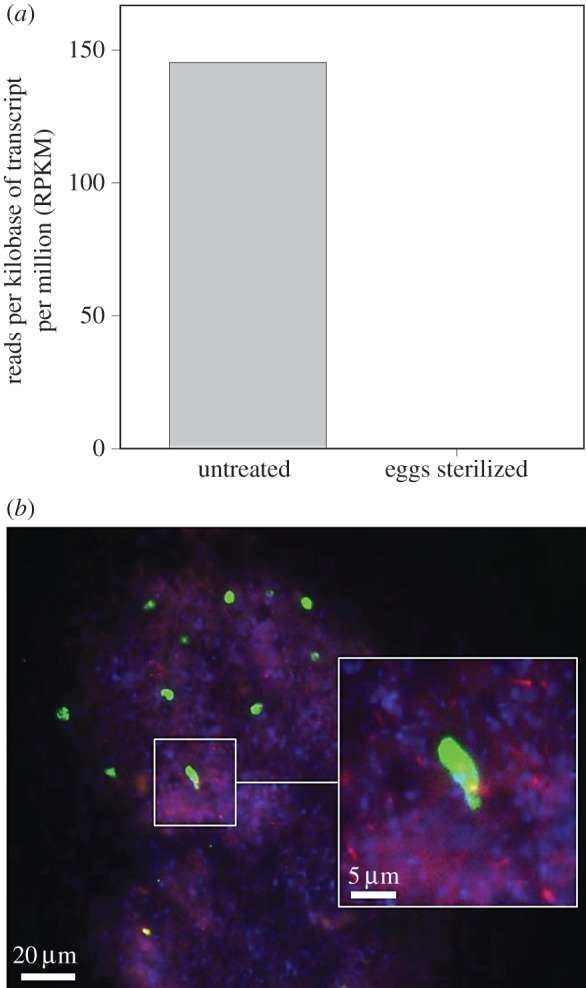
(a) Normalized expression values of reads per kilobase of transcript per million reads (RPKM) belonging to the Trypanosomatidae in guts of adult Dysdercus fasciatus that had hatched from untreated or surface-sterilized eggs. (b) Fluorescence micrograph of Leptomonas pyrrhocoris (green) and Coriobacterium glomerans (red) in a faecal droplet from D. fasciatus. Counterstaining of DNA was done with DAPI (blue).
To directly test for the vertical and horizontal transmission routes of L. pyrrhocoris in D. fasciatus, we harvested six egg clutches from L. pyrrhocoris-infected mating pairs and divided the eggs of each clutch into four groups: (i) untreated, (ii) egg surface-sterilized, (iii) egg surface-sterilized, followed by the inoculation of L. pyrrhocoris from pure culture over egg surfaces, and (iv) egg surface-sterilized, then reared upon hatching in the presence of two L. pyrrhocoris-infected adult D. fasciatus.
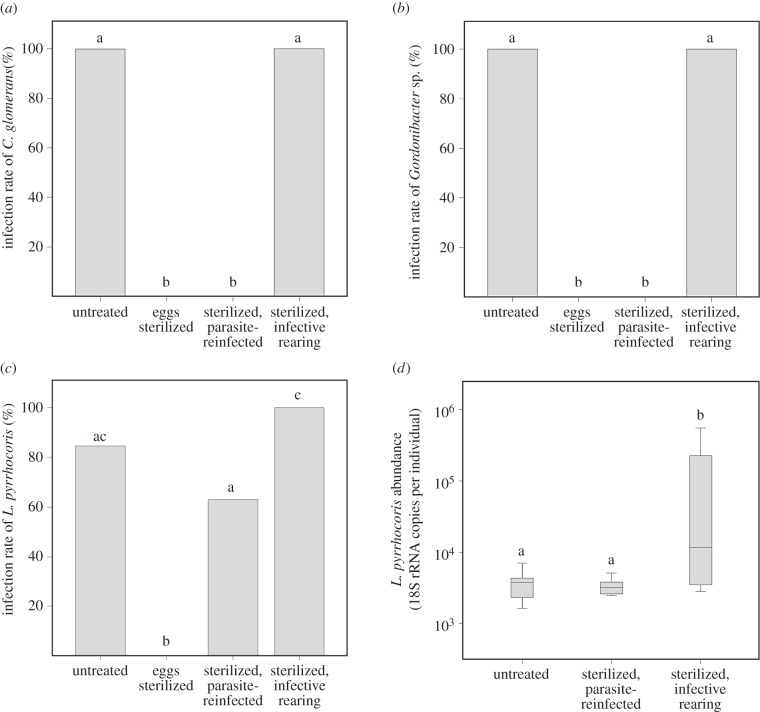
Matching the infection dynamics of the Coriobacteriaceae symbionts (figure 2a,b), L. pyrrhocoris (figure 2c) could be detected with high prevalence in the untreated group (82%). In contrast, the sterilization of egg surfaces resulted in adult firebugs that completely lacked the symbionts as well as L. pyrrhocoris (figure 2a–c; p < 0.001, χ2 test). Spreading cultured L. pyrrhocoris over previously sterilized eggs reinstituted the infection at a high frequency (63%; figure 2c), thereby confirming that the parasite can vertically transmit via the egg surface. Additionally, horizontal transfer of L. pyrrhocoris among conspecifics occurred with high efficiency (100%) in firebugs that were subjected to the egg surface sterilization procedure but were subsequently reared in the presence of infected conspecifics (figure 2c). Collectively, such findings demonstrate that L. pyrrhocoris is transmitted across host generations in a manner that is identical to the beneficial Coriobacteriaceae symbionts [7]: vertically via the egg surface and horizontally through contact with infected firebugs, specifically their faeces (figure 2a,b). This is consistent with recent findings in milkweed bugs and their trypanosomatid parasite, Leptomonas wallacei [13], where transovum propagation of the parasite was demonstrated to mediate vertical transmission. Despite the parallels to the transmission cycle of L. pyrrhocoris, it is unclear whether the egg probing behaviour in Oncopeltus fasciatus is relevant for symbiont transmission, considering the lack of evidence for vertically transmitted mutualists associated with milkweed bugs [14].
Figure 2.
Transmission rates of (a) Coriobacterium glomerans, (b) Gordonibacter sp. and (c) Leptomonas pyrrhocoris in Dysdercus fasciatus across four experimental treatments (untreated, eggs surface-sterilized, sterilized then reinfected with L. pyrrhocoris, and sterilized then reared in the presence of conspecifics infected with L. pyrrhocoris as well as both Coriobacteriaceae symbionts). (d) Titre of L. pyrrhocoris across infected D. fasciatus individuals in the three L. pyrrhocoris-harbouring experimental treatments. Parasite abundances represent estimated 18S rRNA gene copy numbers obtained from qPCR assays. Different letters above bars/boxes indicate significant differences ((a–c) pairwise χ2 tests, p < 0.05; (d) Kruskall–Wallis H-test, p < 0.05).
When examining parasite titres among infected individuals across the three L. pyrrhocoris-harbouring treatments (figure 2d), we found that parasite load was significantly different across groups (p = 0.012, Kruskal–Wallis H-test). Specifically, D. fasciatus reared in the presence of infected firebugs were found to harbour higher titres of L. pyrrhocoris compared with treatments where the trypanosomatids were provisioned over the egg surface, possibly as a consequence of repeated exposure to the parasite via contact with infected conspecifics.
The transmission dynamics reported in this study for L. pyrrhocoris are consistent with theoretical predictions implicating bimodal (horizontal and vertical) transfer, alongside low virulence, as hallmarks of globally distributed, specialized parasites [3]. Additionally, the high vertical transmission efficiency of the parasite may ultimately select for reduced virulence [15], owing to the alignment of interest in host and parasite, which may explain the predominance of asymptomatic infections caused by trypanosomatids in insects [16].
Many parasites exploit the ecology of their hosts to initiate infection. For example, Crithidia bombi, the trypanosomatid parasite of bumblebees, capitalizes on its host's social organization to spread among nest-mates following contact with infected individuals [17]. C. bombi can also propagate within populations of its hosts through the shared use of flowers [18], collectively highlighting the adaptive propensity of the parasite to the behavioural as well as feeding ecology of bumblebees. Coprophagy, which often not only contributes towards fulfilling the nutritional requirements of immature insects [19], also facilitates the horizontal transfer of trypanosomatids across a range of bug species [16]. In this study, however, we report on how a specialized parasite may have capitalized on pre-existing adaptations for mutualist transmission in an insect to facilitate its own transfer, thereby contributing to a cosmopolitan distribution mirroring that of the host [10] as well as of the beneficial bacterial associates of this insect family [6]. Given the widespread occurrence of extracellular symbiont transmission routes in insects [2], in particular through the smearing of egg surfaces with faeces or glandular secretions, co-transmission of intestinal parasites is likely a common phenomenon. Hence, the risk of parasite hitchhiking may result in trade-offs that favour the evolution of additional mechanisms ensuring specificity during transfer by the host or new defences against the parasite, particularly if the costs of parasite infection outweigh the benefits of acquiring mutualistic microbes.
3. Material and methods
(a). Insect sampling and rearing
Live D. fasciatus were originally collected in the Comoé National Park, Côte d'Ivoire, but have since been maintained in the laboratory at the University of Würzburg, Germany, and a subculture was later established at the Max Planck Institute for Chemical Ecology, Jena, Germany. The insects were reared in plastic containers (20 × 35 × 22 cm) at a constant temperature of 28°C and exposed to long light regimes (16 h L : 8 h D cycles).
(b). Illumina-based transcriptome sequencing
RNA was extracted from dissected whole midgut regions (M1–M4) from five symbiotic and aposymbiotic bugs, respectively, resulting in two pooled samples. Transcriptome sequencing of poly-A enriched mRNAs, assembly, annotation and analysis were described previously [9,20]. The sequence data were deposited in the European Nucleotide Archive, accession number PRJEB6171 (http://www.ebi.ac.uk/ena/data/view/PRJEB6171).
(c). Fluorescence in situ hybridization
Faecal droplets were collected from infected adults by pressing the insect's abdomen on a glass slide. Upon drying, the faeces were fixed with 70% and 99% ethanol in succession. Cor653 [7] and SSUR2 (5′-GAGTCAACACTGCTGGGTGT-3′) probes were used to localize C. glomerans and L. pyrrhocoris, respectively. The SSUR2 probe was designed using the 18S rRNA sequence of L. pyrrhocoris (GenBank accession no. JN036653). Hybridization was carried out as described previously [7].
(d). Experimental set-up
Six egg clutches (approx. 30 eggs each) from different females of L. pyrrhocoris-infected D. fasciatus were harvested three days after oviposition and kept separately. We then split each of the collected clutches into four experimental treatments: (i) untreated, (ii) surface-sterilized, (iii) surface-sterilized then re-infected with a pure inoculum of L. pyrrhocoris (30 µl of approx. 105 flagellates µl−1) and (iv) surface-sterilized and subsequently reared in contact with two L. pyrrhocoris-infected adult bugs. The four treatments were provided ad libitum with autoclaved water and a nutrient-rich artificial diet [9].
The sterilization of egg surfaces followed the procedure used in [8]. Briefly, the eggs were submerged in ethanol for 5 min, followed by bleach (12% NaOCl) for 45 s. Residual bleach was removed by washing in autoclaved water.
(e). DNA extraction and PCR screening for Leptomonas pyrrhocoris and the Coriobacteriaceae symbionts
All individuals from every experimental treatment were subjected to DNA extraction three days after adult emergence as previously described [8]. Primers targeting L. pyrrhocoris' 18S rRNA gene, SSU Fwd_2 (5′-CTGGTTGATCCTGCCAGTAG-3′) and SSU Rev_2 (5′-GAGTCAACACTGCTGGGTGT-3′) were then used to screen for the parasite, using the following cycle parameters: 3 min at 94°C, followed by 32 cycles of 94°C for 40 s, 56°C for 1 min and 72°C for 1 min, and a final extension time of 4 min at 72°C. Screening for C. glomerans and Gordonibacter sp. was performed as previously described [8], using the primers Cor_2F/Cor_1R and fD1/Egg_1R, respectively.
(f). Quantitative PCR
To assess parasite infection titres, quantitative PCR for L. pyrrhocoris was conducted for samples that were positive for the parasite per diagnostic PCR, using a RotorGene-Q cycler (Qiagen, Hilden, Germany). The final reaction volume of 25 ml included the following components: 1 ml of DNA template, 2.5 ml of SSU Fwd_2 and SSU Rev_2 primers (10 mM), 6.5 ml of autoclaved distilled H2O and 12.5 ml of SYBR Green Mix (Qiagen, Hilden, Germany).
(g). Statistical analysis
Infection rates of L. pyrrhocoris, C. glomerans and Gordonibacter sp. across the four experimental treatments were compared using pairwise χ2 tests (SPSS, Chicago, IL). To compare L. pyrrhocoris 18S copy numbers estimated in the qPCRs, Kruskal–Wallis H-test with Dunn's post hoc comparisons was used as implemented in BiAS v. 7.40 (Epsilon Verlag, Hochheim-Darmstadt, Germany).
Acknowledgements
We thank Benjamin Weiss for his assistance in caring for the bugs, and Jan Votýpka for providing a pure culture of L. pyrrhocoris.
Ethics
The work conducted complies with the ethical regulations in Germany.
Data accessibility
Original data on PCR and qPCR results of symbiont and parasite infection are available in the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.187mt.
Authors' contributions
H.S., T.O.O. and M.K. conceived of the study. T.O.O. and H.S. performed the experiments. H.S. analysed the data. E.B. performed the transcriptomic analysis. H.S. drafted the manuscript, and all authors reviewed, approved and are accountable for the final version for publication.
Competing interests
The authors declare that they have no competing interests.
Funding
Financial support from the Max Planck Society (H.S., T.O.O., M.K.) and the Jena School for Microbial Communication (to T.O.O.) is gratefully acknowledged.
References
- 1.McFall-Ngai M, et al. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236. ( 10.1073/pnas.1218525110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem H, Flórez L, Gerardo N, Kaltenpoth M. 2015. An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. Proc. R. Soc. B 282, 20142957 ( 10.1098/rspb.2014.2957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altizer SM, Augustine DJ. 1997. Interactions between frequency-dependent and vertical transmission in host–parasite systems. Proc. R. Soc. Lond. B 264, 807–814. ( 10.1098/rspb.1997.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poulin R. 2011. Evolutionary ecology of parasites. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Sudakaran S, Salem H, Kost C, Kaltenpoth M. 2012. Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae). Mol. Ecol. 21, 6134–6151. ( 10.1111/mec.12027) [DOI] [PubMed] [Google Scholar]
- 6.Sudakaran S, Retz F, Kikuchi Y, Kost C, Kaltenpoth M. 2015. Evolutionary transition in symbiotic syndromes enabled diversification of phytophagous insects on an imbalanced diet. ISME J. 9, 2587–2604. ( 10.1038/ismej.2015.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaltenpoth M, Winter SA, Kleinhammer A. 2009. Localization and transmission route of Coriobacterium glomerans, the endosymbiont of pyrrhocorid bugs. FEMS Microbiol. Ecol. 69, 373–383. ( 10.1111/j.1574-6941.2009.00722.x) [DOI] [PubMed] [Google Scholar]
- 8.Salem H, Kreutzer E, Sudakaran S, Kaltenpoth M. 2013. Actinobacteria as essential symbionts in firebugs and cotton stainers (Hemiptera, Pyrrhocoridae). Environ. Microbiol. 15, 1956–1968. ( 10.1111/1462-2920.12001) [DOI] [PubMed] [Google Scholar]
- 9.Salem H, Bauer E, Strauss AS, Vogel H, Marz M, Kaltenpoth M. 2014. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc. R. Soc. B 281, 20141838 ( 10.1098/rspb.2014.1838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Votýpka J, Klepetková H, Yurchenko VY, Horák A, Lukeš J, Maslov DA. 2012. Cosmopolitan distribution of a trypanosomatid Leptomonas pyrrhocoris. Protist 163, 616–631. ( 10.1016/j.protis.2011.12.004) [DOI] [PubMed] [Google Scholar]
- 11.Lipa JJ. 1963. Infections caused by protozoa other than sporozoa. Insect pathology: an advanced treatise. New York, NY: Academic Press. [Google Scholar]
- 12.Schaub GA. 1994. Pathogenicity of trypanosomatids on insects. Parasitol. Today 10, 463–468. ( 10.1016/0169-4758(94)90155-4) [DOI] [PubMed] [Google Scholar]
- 13.De Almeida Dias F, da Costa Vasconcellos LR, Romeiro A, Attias M, Souto-Padrón TC, Lopes AH. 2014. Transovum transmission of trypanosomatid cysts in the milkweed bug, Oncopeltus fasciatus. PLoS ONE 8, e108746 ( 10.1371/journal.pone.0108746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feir D. 1963. Midgut bacteria of Oncopeltus fasciatus (Hemiptera: Lygaeidae). Ann. Entomol. Soc. Am. 56, 829–830. ( 10.1093/aesa/56.6.829) [DOI] [Google Scholar]
- 15.Sachs JL, Wilcox TP. 2006. A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. Proc. R. Soc. B 273, 425–429. ( 10.1098/rspb.2005.3346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maslov DA, Votýpka J, Yurchenko V, Lukeš J. 2013. Diversity and phylogeny of insect trypanosomatids: all that is hidden shall be revealed. Trends Parasitol. 29, 43–52. ( 10.1016/j.pt.2012.11.001) [DOI] [PubMed] [Google Scholar]
- 17.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 18.Durrer S, Schmid-Hempel P. 1994. Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B 258, 299–302. ( 10.1098/rspb.1994.0176) [DOI] [Google Scholar]
- 19.Scriber JM, Slansky F. 1981. The nutritional ecology of immature insects. Annu. Rev. Entomol. 26, 183–211. ( 10.1146/annurev.en.26.010181.001151) [DOI] [Google Scholar]
- 20.Bauer E, Salem H, Marz M, Vogel H, Kaltenpoth M. 2014. Transcriptomic immune response of the cotton stainer Dysdercus fasciatus to experimental elimination of vitamin-supplementing intestinal symbionts. PLoS ONE 9, e114865 ( 10.1371/journal.pone.0114865) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data on PCR and qPCR results of symbiont and parasite infection are available in the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.187mt.