Abstract
Locating appropriate settlement habitat is a crucial step in the life cycle of most benthic marine animals. In marine fish, this step involves the use of multiple senses, including audition, olfaction and vision. To date, most investigations of larval fish audition focus on the hearing thresholds to various frequencies of sounds without testing an ecological response to such sounds. Identifying responses to biologically relevant sounds at the development stage in which orientation is most relevant is fundamental. We tested for the existence of ontogenetic windows of reception to sounds that could act as orientation cues with a focus on vulnerability to alteration by human impacts. Here we show that larvae of a catadromous fish species (barramundi, Lates calcarifer) were attracted towards sounds from settlement habitat during a surprisingly short ontogenetic window of approximately 3 days. Yet, this auditory preference was reversed in larvae reared under end-of-century levels of elevated CO2, such that larvae are repelled from cues of settlement habitat. These future conditions also reduced the swimming speeds and heightened the anxiety levels of barramundi. Unexpectedly, an acceleration of development and onset of metamorphosis caused by elevated CO2 were not accompanied by the earlier onset of attraction towards habitat sounds. This mismatch between ontogenetic development and the timing of orientation behaviour may reduce the ability of larvae to locate habitat or lead to settlement in unsuitable habitats. The misinterpretation of key orientation cues can have implications for population replenishment, which are only exacerbated when ontogenetic development decouples from the specific behaviours required for location of settlement habitats.
Keywords: soundscape, audition, behaviour, ontogeny, mangrove, orientation
1. Introduction
The transition from a pelagic to a benthic lifestyle is a crucial phase in the life cycle of many marine organisms. During early stages of development, dispersal of planktonic larvae may be mostly driven by currents; however, larvae progressively develop behavioural and physiological competencies that allow them to locate, orient towards and selectively settle in suitable benthic habitat [1–3]. Competencies acquired during development include increased body size, development of functional fins and strong musculature, as well as sensory capabilities necessary for navigation and habitat selection. This set of adaptations means that settlement is far from a stochastic event [1].
In the ocean, sound pressure waves carry qualitative (e.g. type of habitat) and quantitative (e.g. proximity) information over long distances (far-field) owing to low attenuation of acoustic energy in water. Sound, in the form of particle acceleration, instead does not travel such long distances and is the dominant component in the vicinity of the sound source (near-field). In recent years acoustic cues have been shown to aid settlement of marine fishes and some invertebrates [2,4–6]. Electrophysiological assessments of larval fishes show that auditory sensitivity increases during development [7,8], suggesting that acoustic cues may become more important through development. However, these studies demonstrate only that fish at a certain age can detect particular frequencies and do not explore how larval fish interpret and respond to biological sounds at different stages of development. Some species of fish appear to have a narrow window of competency for settlement and may be unable to delay settlement past this window [9,10]. Despite the apparent role of sound in guiding settlement and the likely influence on patterns of connectivity among populations, the timing of attraction towards auditory cues remains unknown for most fish species. Identifying when fish become responsive to sensory cues from settlement habitat is critical for predicting settlement and connectivity patterns of marine species and how they could be affected by human impacts [11].
Ocean acidification is predicted to have profound effects on marine life [12,13], but its potential effect on sound reception and swimming behaviour by free-swimming larvae remains largely untested. To date, only one study has directly investigated the effect of ocean acidification on auditory-mediated behaviour, finding that elevated CO2 (600–900 µatm) alters the auditory responses of juvenile clownfish to predator-rich daytime reef noise [14]. It is also known that elevated CO2 conditions can increase the size of otoliths [15], and that this could potentially affect hearing sensitivity [16].
However, nothing is known about the effects of elevated CO2 on the orientation of fishes to auditory cues during their pelagic larval stage. The effect of elevated CO2 on fish swimming behaviour is also unresolved. Some studies suggest that swimming performances of larval fishes could be unaffected by ocean acidification [17,18], whereas others find ocean acidification alters activity levels [19,20] and one study suggests that ocean acidification can have an indirect effect on maximum swimming speed of settlement-stage larvae through increasing body size [21]. However, most of these studies have used different swimming behaviour metrics, making comparisons difficult to interpret.
Fish possess well-developed physiological mechanisms to prevent acidosis of the blood and tissues, even at very high levels (greater than 5000 µatm) of CO2 [22]. However, the same physiological responses that fish use to prevent acidosis in a high CO2 environment appear to affect the processing of sensory information, resulting in profound and often striking behavioural alterations [23]. A wide spectrum of behaviours have been found to be affected by CO2 levels that are expected to be reached by the end of the century [24], including the senses of olfaction, audition and vision [14,25,26]. The common driver for all these effects is altered function of the GABAA neuroreceptor [23,27,28], which is the main inhibitory receptor in the vertebrate brain. However, few studies to date have investigated the effects of ocean acidification on behaviour in pre-metamorphic larvae [19,20,29] and only the olfactory ability of clownfish has been investigated throughout larval development [26]. Most other studies on ocean acidification and fish behaviour have tested behavioural competencies in post-metamorphic juveniles. This emphasis has left the potential effects of ocean acidification on behavioural traits in pre-metamorphic and metamorphic stages unexplored in most fish species. It is at this stage that the effects of ocean acidification on sound reception in larval fish may be most detrimental because it overlaps with the critical transition from a pelagic to benthic lifestyle [1,6].
To date, the majority of research on ocean acidification and fish behaviour has focused on coral reef species. Few behavioural studies have been conducted on species with euryhaline physiologies [30,31]. Many estuarine environments normally experience high and fluctuating CO2 levels owing to processes such as tidal exchange, eutrophication and freshwater input [32,33]. Therefore, the evolution of fish within these environments may have enabled adaptation to elevated levels of CO2 [34]. However, adaptation to extreme ranges of natural variability in seawater chemistry may not automatically translate into resistance to future conditions. As local processes can exacerbate the effect of ocean acidification, these environments may attain future CO2 levels that are much higher than currently experienced [35,36]. Yet, little is known about whether estuarine species are better adapted to ocean acidification than marine species owing to the dynamic and more extreme pH environment in which they live.
We here tested the effect of ocean acidification expected for estuarine environments on multiple behavioural traits of a catadromous fish (barramundi, Lates calcarifer, which migrates from fresh water to the ocean to spawn) known to have a highly flexible physiology and to be associated with marine and estuarine habitats during its larval development [37]. The larvae of barramundi settle in estuaries, embayments and near-shore coastal areas that can experience high pCO2 owing to natural and anthropogenic processes such as tidal exchange, freshwater input, eutrophication and run-off of acid sulfate soils. As these processes could locally exacerbate the effect of ocean acidification, we expect that estuarine areas in the future will reach CO2 levels that are substantially higher than projections for the open ocean [32,33]. Future projections that take into account the combined effect of ocean acidification and heterotrophic degradation of organic matter in coastal and estuarine hypoxic regions estimate that pCO2 values of 1700–3200 µatm could be reached by the end of the century [35]. Given this observation, we exposed barramundi to pCO2 of approximately 1675 µatm to represent possible future elevated CO2 conditions in their habitat.
The aims of the study were to: (i) identify ontogenetic patterns of attraction to sound cues from potential settlement habitats; (ii) test the effects of ocean acidification on important behavioural traits characteristic of pre-settlement lifestyle, such as sound-driven orientation and swimming velocity, as well as post-settlement behavioural traits like sheltering; and (iii) test the effect of CO2 on development and timing of metamorphosis and how this might interact with the timing of attraction to settlement habitat sound and swimming velocity.
2. Material and methods
(a). Model species
Barramundi is a tropical fish whose range extends from the eastern Indian Ocean to the western Central Pacific and is highly valued both commercially and recreationally [38]. Barramundi is an obligatory catadromous species (migrates from fresh water to the ocean to spawn) whose eggs and larvae are typically found around river mouths and marine bays [37,38]. In this species, successful gonadal and larval development require saltwater (28–35 ppt) [38] and juveniles settle into mangroves and wetland habitats [37]. The egg stage duration is typically 12–17 h and metamorphosis occurs at approximately 19 days post hatching (dph), depending on diet and environmental factors [39].
Barramundi, like many other Perciformes, possess a rostral extension of the swim bladder and a gas-filled chamber in the otic region [40,41]. This trait appears to have evolved multiple times independently in this group of fish and allows the transit of sound pressure oscillations captured by the swim bladder to the otolithic region [41]. Although the hearing sensitivity of barramundi has not been tested, experimental studies have shown that the extension of the swim bladder to the otic region is linked to enhanced acoustic sensory performance typical of hearing specialists [41]. Dissection under a stereomicroscope confirmed the presence of this gas-filled chamber in the otic region of barramundi at settlement stage (n = 5, length 8–20 mm, T. Rossi 2015, personal observation).
Fertilized eggs were obtained from a commercial hatchery (Robarra, 7th generation broodstock) and reared at University of Adelaide. The larval rearing systems were duplicated for each treatment and comprised a 60 l rearing tank recirculating in a closed system with a 20 l sump that contained a biological filter, a protein skimmer WG-308 (Boyu, Guangdong, China) and a UV sterilizer UView (Blue Planet). Fish were fed ad libitum with rotifers for the first 12 dph, then with Artemia nauplii and a dry feed (Otohime) of increasing granule size as development progressed. CO2 treatments (table 1) were initiated on the second day post hatching. Temperature was maintained at approximately 27°C (table 1). See the electronic supplementary material for details on CO2 manipulation.
Table 1.
Summary of the water chemistry parameters measured in the laboratory experiment. Average (±s.e.) temperature (T), pH and total alkalinity (TA) measured in the laboratory. NBS, National Bureau of Standards.
| treatment | T (°C) | pH NBS | N | TA (µmol kg−1 SW) | pCO2 (µatm)a | N | salinity | N |
|---|---|---|---|---|---|---|---|---|
| control | 27.07 (±0.05) | 8.19 (±0.01) | 34 | 2595.6 (±25.4) | 400.9 (±43.6) | 3 | 38.8 (±0.2) | 34 |
| elevated | 27.11 (±0.05) | 7.70 (±0.01) | 34 | 2629.8 (±17.6) | 1675.1 (±135.0) | 3 | 38.8 (±0.2) | 34 |
aIndicates values of pCO2 calculated using CO2SYS.
(b). Effect of CO2 on larval fish audition and swimming velocity
The attraction to acoustic cues of habitat was investigated daily for a duration of 15 days (from pre-metamorphosis at 13 dph to post-metamorphosis at 28 dph) by testing the response of naive larval fish to recordings of estuarine soundscapes, which could act as a potential orientation cue leading to settlement habitat. The tests started at 13 dph because earlier stages showed low swimming competency during trials, which is typical of the early planktonic stages. For each individual fish we also visually assessed their developmental stage (electronic supplementary material).
The response to soundscapes was tested in fish raised under control and high CO2 conditions using an auditory choice chamber. A 5-min recording of mixed tropical and temperate estuarine habitats was used as the biologically relevant acoustic cue (electronic supplementary material, figures S1–S4). The recordings were dominated by typical broadband snapping shrimp sound [42], which rose sharply from zero to being the dominant acoustic feature in the soundscape around 500–800 Hz (electronic supplementary material, figure S2). The recordings contained very few abiotic sounds because the sea state at the time of the recordings was always calm. As sounds like those produced by snapping shrimp are highly dependent on habitat characteristics [42] and are known to be associated with mangroves [43], they probably provide a potentially valuable directional cue for barramundi larvae attempting to locate suitable settlement habitat. We also tested the auditory preferences of barramundi against white noise (constant amplitude at every frequency), which acted as a biologically irrelevant control sound. Additional information on the sound recordings is provided in the electronic supplementary material.
The auditory choice experiments were performed inside a plastic tank (100 × 50 × 20 cm) lined with polystyrene foam and containing a white acrylic auditory choice chamber (35 × 22 × 2 cm) divided in eight parallel lanes with a triangular section (35 × 3 × 2 cm; electronic supplementary material, figure S5). At each end of the chamber there was an underwater speaker (UW-30; maximal output 156 dB re 1 µPa at 1 m, frequency response 0.1–10 kHz, Lubell Labs Inc., Columbus, OH, USA). The sound pressure level was very similar between external and central lanes (electronic supplementary material, figure S6). The auditory chamber had mesh at the two ends facing the speakers, while the top was open to the surface. The fish larvae could not escape or see each other because the ridges between each lane were higher than the water level. Between each trial the chamber was flushed with fresh seawater in order to remove potential chemical cues left by the previous fish. During the experiments the chamber was placed at a fixed distance of 8 cm from the speakers. At the beginning of each trial, one fish larva was randomly placed in a removable enclosure in the centre of each of the eight lanes and given a two min habituation period during which recorded estuary sounds were played. The sound during the habituation time was played by the same speaker used for the trial and was intended to avoid the potential confounding effect of a startle response triggered by the abrupt increase of sound level in the tank during the trial. At the beginning of each trial, eight fish (four control and four elevated CO2, one per lane) were released simultaneously and their position was videotaped from above using a camcorder (HF R406 Legria, Canon, Japan).
The position and velocity of the fish in the choice chamber were tracked continuously using EthoVision XT10 (Noldus Information Technology, Wageningen, The Netherlands) for 7 min. The combined use of video recordings and automated tracking eliminated the risk of observer bias and external influences on behaviour caused by the presence of the observer. Within Ethovision, each chamber was divided in two equal sections and the percentage of time that the fish spent in each section was obtained (electronic supplementary material, figure S5). Assuming that a fish with no acoustic preference spends an equal amount of time in each section (50%), any percentage of time spent in one of the sections that significantly differs from 50% indicates a response to the cue. The sound pressure levels of the playback in the chamber (below 4 kHz) were set so that they matched levels recorded in the field near to the speaker, and decreased towards ambient levels along the chamber [44] (electronic supplementary material, figures S1 and S3). A response to sound does not necessarily demonstrate that fish can resolve the directional origin of the sound as they may simply sense the gradient of sound pressure along the chamber by sampling multiple positions, and consequently choose to spend more time in the section of the chamber where they can hear a soundscape that they find ‘attractive’. In other words, we assume that if the fish were deterred by our playback, and this playback is audible only in a section of the chamber, the fish would move to the other section. From a drifting propagule perspective, the selection of habitat based on soundscape spatial heterogeneity is possible by simply sampling sound pressure at multiple time points as the larva moves closer to the source (see Lillis et al. [45], for conceptual model). Particle acceleration was measured in the experimental chamber based on sound pressure measurements obtained simultaneously with two hydrophones (same as above) held at 5.5 cm distance and the Euler equation, as in [46]. This established method is not prone to the limitations of using large accelerometers in tanks. The results show that particle acceleration was maximal in proximity to the speaker and decreases linearly along the chamber (electronic supplementary material, figure S3). To test for possible side preference effects in the chambers, we ran trials with control fish and no sound playing. The results showed the absence of a side preference (ANOVA, F1,30 = 0.22, p = 0.643, n = 15 at 23 dph).
We acknowledge the difficulty of replicating a far-field acoustic cue in a small tank [47]. However, in this study, we do not attempt to determine absolute values of sensitivity but rather relative auditory preferences in larval fish that had been exposed to elevated CO2 versus control conditions throughout their larval development.
A total of 20–24 (half from each treatment) randomly selected naive larval barramundi that had been reared under control and elevated CO2 conditions from the second day post hatching were used daily between 13 and 28 dph for trials. All testing took place between 14.00 and 18.00 h. Additional methods are present in the electronic supplementary material.
(c). Sheltering behaviour
Sheltering behaviour and thigmotaxis (edge-following) were tested as a proxy for boldness/anxiety [48]. The time to first emergence from the shelter and total time spent in the shelter were scored by a human observer (see the electronic supplementary material for details). A total of 56 (half from each treatment) naive post-settlement barramundi that had been reared under control and elevated CO2 conditions from 2 dph were used at 35 dph for trials.
(d). Statistical analysis
Percentage of time spent in the half of the chamber in proximity to the acoustic cue, fish standard length and swimming velocity data were pooled in 3-day blocks. Attraction or deterrence towards soundscape was then determined by testing each distribution of percentages of time spent in the half of the chamber close to the active speaker against the threshold for random response set at 50%. Percentage data were not normally distributed, as assessed by Shapiro–Wilk's test (p < 0.05); therefore, a non-parametric one-sample Wilcoxon signed-rank test was used. Fish standard length and swimming velocity were analysed using a two-way ANOVA with CO2 treatment and dph as factors. No significant tank effect was detected (three-way ANOVA with CO2 treatment, dph and tank nested in treatment as factors).
A log-linear model was used to test the relationships between the number of individuals in each developmental stage (S), day post hatching (dph) and CO2 treatment (CO2). Starting from the saturated model (containing all main effects and their interactions), higher-order terms were removed from the model until there was a significant increase in deviance from one model to the next.
The overall proportion of fish that emerged from the shelter during the trial was compared between treatments with a 2 × 2 χ2-test. Differences in sheltering behaviour traits (time to first emergence from the shelter and total time outside the shelter) were tested with ANOVA. No significant tank effect was detected (ANOVA with CO2 treatment and tank nested in treatment as factors). Logit transformation was applied to the percentage time in shelter data.
3. Results
(a). Auditory behaviour, swimming velocity and development
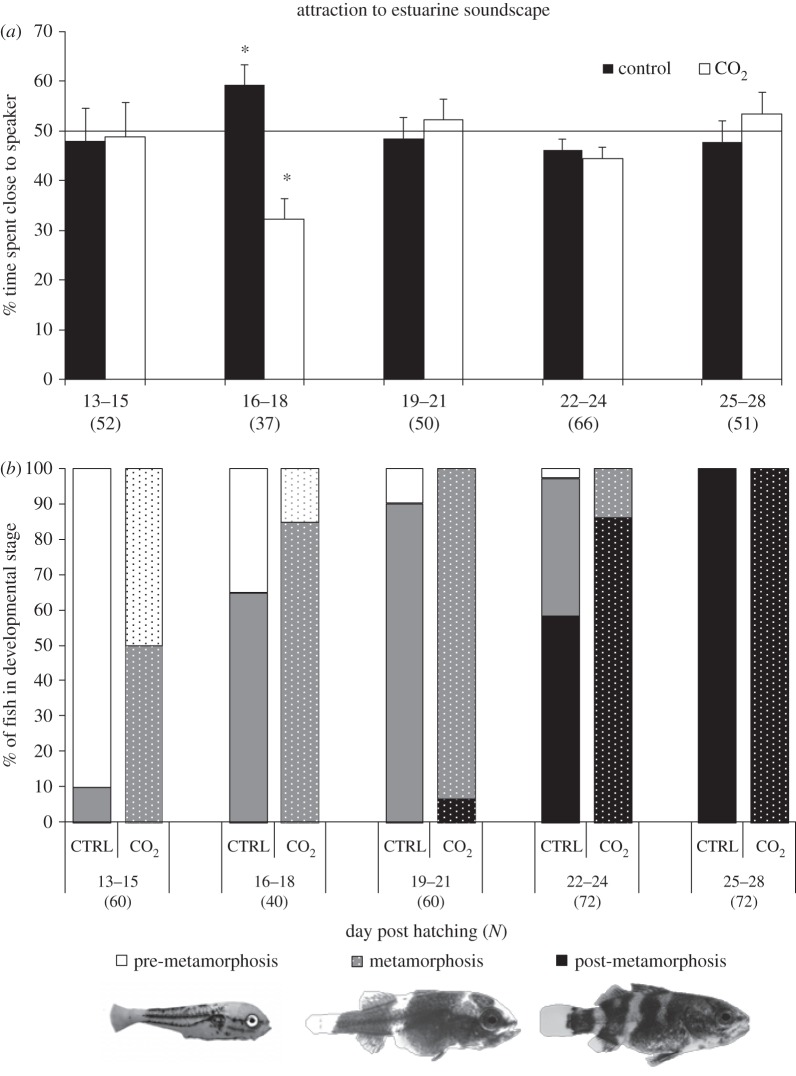
There was a significant attraction in control fish towards playback of settlement habitat soundscapes for the 3-day period from 16–18 dph (Wilcoxon signed-rank test, p = 0.022; figure 1a). This pattern was largely driven by fish spending more time in the loudest part of the chamber (electronic supplementary material, figure S7). The period of attraction towards settlement habitat in the control fish matched the period when most fish underwent metamorphosis (figure 1b). Swimming velocity of control fish was lowest during the early developmental stages (13–15 dph), before most fish had initiated metamorphosis, then significantly increased for a period of 9 days (16–24 dph) before decreasing again past day 25 when all fish had completed metamorphosis (figure 2a).
Figure 1.
Effect of ocean acidification on fish auditory preferences throughout larval development. (a) Mean (+s.e.) percentage of time spent in the half of the chamber closest to the speaker broadcasting an estuarine soundscape. Results are pooled in blocks of 3 days. Stars indicate statistically significant differences relative to a 50% threshold for random response represented by the horizontal bar. (b) Proportion of fish in each developmental stage as a function of number of dph. Results are pooled in blocks of 3 days. The elevated CO2 treatment is represented by dotted bars. Number of replicates for each 3-day block is reported in parentheses.
Figure 2.
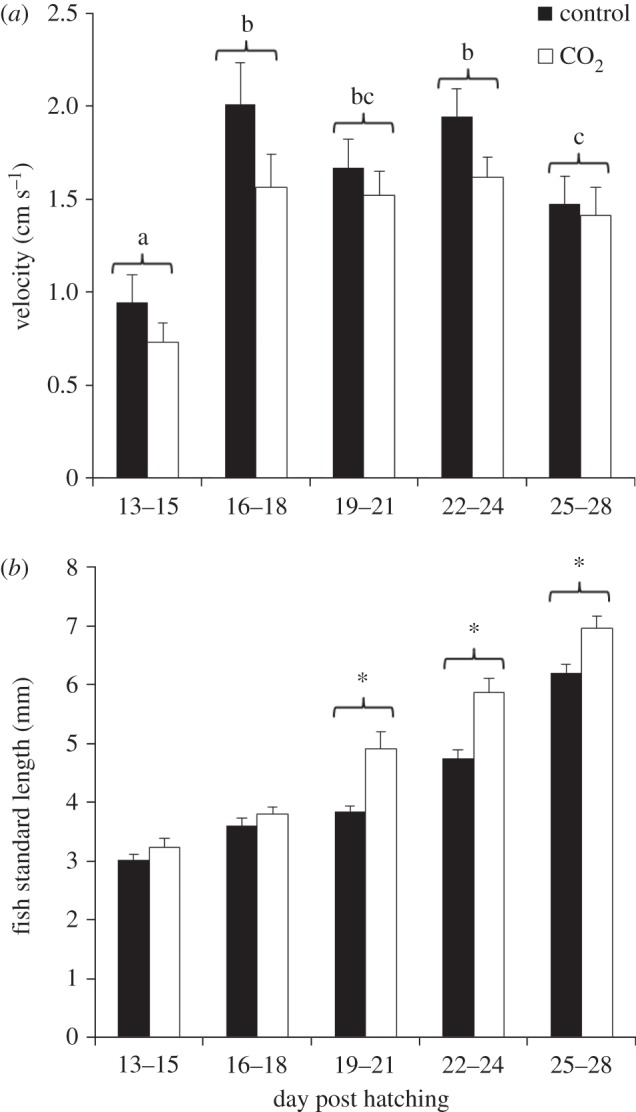
Effect of ocean acidification on swimming velocity and fish size throughout larval development. (a) Mean (+s.e.) swimming velocity during audition trials and (b) mean (+s.e.) fish standard length during development. Asterisks indicate significant (p < 0.05) differences between distributions. Letters indicate significant differences between groups of dph for combined CO2 treatments (pair-wise tests).
During the short temporal window when control fish showed attraction towards playback of settlement habitat sound, CO2-treated fish were instead significantly deterred by habitat sounds (Wilcoxon signed-rank test, p < 0.001; figure 1a). This pattern was largely driven by fish spending more time in the quietest part of the chamber (electronic supplementary material, figure S7). There was no significant attraction or avoidance exhibited towards the habitat acoustic cue, for either control or CO2-treated fish, at any other developmental stage (figure 1a). Furthermore, no attraction or avoidance, at any stage, towards playback of white noise was observed (electronic supplementary material, figure S8). Swimming velocity of CO2-treated fish during the audition trials followed the same ontogenetic pattern exhibited by the control fish but at significantly lower values (ANOVA, CO2 treatment: F1,246 = 6.7, p = 0.011; dph: F4,246 = 14.7, p < 0.001; figure 2a).
Fish in the high CO2 treatment initiated metamorphosis significantly earlier than control fish. The log-linear model showed that developmental stage was dependent on dph and CO2 treatment, but not an interaction between the two. The best-fitting model contained the interaction between developmental phase and dph and the two-way interaction between developmental phase and CO2 treatment (likelihood χ2 = 3.338, d.f. = 8, p = 0.911). Removal of the three-way interaction between developmental phase, dph and CO2 treatment did not lead to a significant increase in deviance. However, removal of either of the two way interactions involving developmental phase caused a significant increase in deviance. Removal of the interaction between developmental phase and dph had a much greater effect on the model deviance (χ2 = 398.52, d.f. = 8, p < 0.001) compared with removing the interaction between developmental phase and CO2 treatment (χ2 = 25.76, d.f. = 2, p < 0.001), indicating that dph has a larger effect on developmental phase than did CO2 treatment.
Fish length was significantly higher (ANOVA, CO2 treatment: F1,294 = 33.1, p < 0.001, dph: F4,294 = 119.8, p < 0.001, interaction: F4,294 = 98.1, p = 0.024) in the elevated CO2 treatment compared with the controls (figure 2b). A pair-wise test showed that the effect of CO2 on growth was significant only from dph 19–21 onward (figure 2b).
(b). Sheltering behaviour
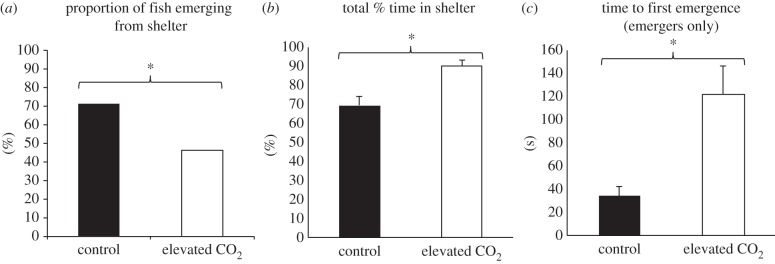
Elevated CO2 had a significant effect on fish sheltering behaviour. The proportion of fish that emerged from the shelter during the trial was significantly lower in the elevated CO2 treatment (13 out of 28 fish) compared to the controls (21 out of 28 fish) (Pearson's χ2 = 4.791, d.f. = 1, p = 0.029; figure 3a). The mean percentage of time spent in shelter was significantly higher for the fish in the elevated CO2 treatment (ANOVA, F1,54 = 7.38, p = 0.009; figure 3b). Furthermore, when only considering the fish that emerged from the shelter during the trial, the average time to first emergence was significantly longer in the elevated CO2 treatment (ANOVA, F1,32 = 17.8, p < 0.001; figure 3c). Thigmotaxis did not significantly differ between treatments (ANOVA, F1,31 = 2.71, p = 0.11; electronic supplementary material, figure S9).
Figure 3.
Fish sheltering behaviour during the post-settlement stage. (a) Percentage of fish that emerged at least once from shelter during the trials. (b) Mean percentage (±s.e.) time (seconds) spent in shelter during trials. (c) Mean (+s.e.) time to first emergence from shelter (emergers only). Asterisks indicate significant (p < 0.05) differences.
4. Discussion
The overarching goal of our study was to investigate whether a restricted ontogenetic window of opportunity exists during which larval fish respond to benthic habitat sound cues that could guide them from the ocean to their settlement habitat and whether ocean acidification might alter this critical process. Our approach addressed the effects of ocean acidification on sound-driven orientation, swimming behaviour and sheltering behaviour in the context of ontogenetic development. We expected barramundi to show some degree of tolerance to elevated CO2 owing to its residence in estuaries. However, our experiment revealed behavioural and life-history effects at CO2 levels that can be considered moderate for estuarine environments [35]. Auditory preferences, development, growth, swimming speed and boldness were all affected by elevated CO2 (approx. 1675 µatm) conditions. As settlement to adult habitat is a crucial step in the life cycle of coastal marine species, these results have implications for the replenishment and connectivity of populations of this euryhaline species.
The ontogenetic timing of responsiveness towards settlement cues is poorly known in most fish [49]. However, some species appear to have a narrow window of competency for settlement [9,10]. Our study found that under present-day CO2 conditions larval barramundi exhibited attraction to playback of settlement habitat soundscape only during a short temporal window of approximately three days, which also matched the timing of metamorphosis from larval to juvenile morphology. To our knowledge, this is the first study that investigates the behavioural response to soundscapes across multiple developmental phases in larval fish and identifies a specific window of attraction. We also provide evidence that barramundi is selectively attracted to biologically relevant habitat sounds but not to artificial white noise. During metamorphosis, larvae of many species like barramundi undergo dramatic morpho-physiological changes at the same time that they transition from a pelagic to a demersal lifestyle. Larval fish also become highly competent swimmers as they approach metamorphosis, able to move directionally and travel many kilometres despite ocean currents [1]. As expected, swimming speed during auditory trials increased along with the onset of metamorphosis and decreased post-metamorphosis. The lowering of swimming speed after metamorphosis may reflect the transition from pelagic to demersal lifestyle typical of post-settlement ambush predators like barramundi [50].
Elevated CO2 caused larval barramundi to grow faster and initiate metamorphosis earlier. However, a mismatch between the two occurred, because a significantly higher proportion of fish in the high CO2 treatment initiated metamorphosis between 13 and 15 dph, whereas the increase in length appeared 6 days later. Despite the earlier onset of metamorphosis, the window of response to soundscape playback in CO2-treated fish did not advance relative to the control fish. Instead, fish reared under elevated CO2 significantly avoided the playback of settlement habitat sound during the same days when control fish were attracted to these sounds. This reversal of auditory preference is different from the only other study on ocean acidification and sound orientation, which showed that post-settlement-stage juvenile clownfish that are normally deterred by daytime reef soundscape simply lose auditory preference when exposed to elevated CO2 levels [14]. Response to habitat sound during the pelagic larval stage (for orientation), however, is very different from that for settled stages (e.g. for communication, predator avoidance and finding food) [2,51,52]. Here we show that ocean acidification can disrupt the window of opportunity for sound-driven orientation by oceanic larvae towards settlement habitats. Such a behavioural disruption could lead to decreased chances of finding suitable adult habitat, leaving larvae exposed to predation and starvation for longer periods of time and potentially resulting in reduced population replenishment and connectivity.
Current knowledge suggests that ocean acidification affects larval growth in a species-dependent fashion. Some studies have found reduced growth [53], while others find increased growth [17,54] and others no effect [18]. Similarly, swimming performances have been found to be unaffected by ocean acidification in some studies [17,18] or decreased in others [20]. Here, contrary to expectations [1], increased length in high CO2-reared fish was accompanied by a decrease in swimming velocity. This could be owing to the fact that in our study (as in Pimentel et al. [20]), we quantified spontaneous swimming activity rather than forced maximum sustained swimming velocity (Ucrit). Additionally, in this study the fish may have compensated the cost of hypercapnia by decreasing swimming velocity. Increased anxiety, as found in later developmental stages in this study (see below), might also decrease the willingness of fish to explore the chamber, resulting in a lower velocity. As swimming competency during late larval stages is an important trait that allows fish larvae to swim directionally, overcome currents and ultimately reach desired settlement habitats [1], a decrease could compromise successful settlement, with detrimental impacts on likelihood of survival.
Ocean acidification also had a negative effect on the behaviour of post-metamorphic fish. Juvenile barramundi live in mangroves and wetlands [37] and typically hide in submerged vegetation where they adopt an ambush predatory strategy [50]. Whereas some studies have found increased boldness in fish exposed to elevated CO2 [55], other studies found decreased boldness [28,30], indicating that ocean acidification might affect the same behavioural trait differently in different species. Our observations of sheltering behaviour showed that post-settlement barramundi exposed to elevated CO2 are less bold (or more anxious) compared to control fish. From an ecological perspective, increased anxiety might result in decreased foraging success owing to extended time spent hiding. As this anxiety test was performed with post-metamorphic fish that had experienced high CO2 during most of their pre-metamorphic stage, it provides evidence for a lack of acclimation to elevated CO2 as fish progress to their juvenile stage. Juvenile reef fish at natural CO2 seeps exhibited some of the same suite of behavioural problems (e.g. reversal of olfactory and sheltering preferences, and reduced anti-predator behaviour) as those observed in laboratory experiments [56,57], indicating that the fish did not acclimate despite continuous exposure to elevated CO2 since settlement. Furthermore, another study [58] found that parental exposure to elevated CO2 did not ameliorate behavioural impacts of elevated CO2 on juveniles, as has been observed in some life-history traits [31,59], while a meta-analysis of studies based on short- versus long-term CO2 exposure revealed little scope for acclimation for various species traits [13]. Consequently, rapid acclimation of behaviour to high CO2 does not appear likely and fish may need to rely on slower mechanisms like selection and evolutionary adaptation to overcome future effects of ocean acidification.
Fish can use a suite of senses to locate suitable settlement habitat. This includes sound, olfaction and vision [2,5]. Sound is one of the cues that propagates furthest, and it attenuates most predictably with distance from the source [2]. While other senses like olfaction and vision could potentially compensate for the ineffective processing of auditory sensory information caused by increased CO2, they too are likely to be impaired [60–62]. It is well known that larval fish settlement in many species is synchronized with lunar cycles [63]. However, ocean acidification has been shown to also affect timing of settlement by forcing fish to settle at unfavourable times [64]. The inability to successfully or quickly locate settlement habitat is likely to reduce survival and undermine successful establishment owing to increased predation risk, delayed occupancy of suitable benthic habitats and settlement to unsuitable habitats. As orientation towards suitable settlement habitat is a key process in the life of most coastal marine organisms, our findings could have far-reaching implications for the replenishment of marine populations and ecosystems and for population connectivity.
Supplementary Material
Acknowledgements
We thank Jordan Jones and William Nichols for help in the laboratory, and M. Igulu for collecting the habitat sounds in Tanzania.
Ethics
Research was carried out under approval of the University of Adelaide animal ethics committee (permit: S-2012-171) and according to the University's animal ethics guidelines.
Data accessibility
Data are available at Data Dryad: http://dx.doi.org/10.5061/dryad.2cf6s.
Authors' contributions
All authors contributed to the study design and writing of the article. T.R. collected and analysed the data. P.L.M. and L.M. contributed to data analysis.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by an Australian Research Council Future Fellowship to I.N. (grant no. FT120100183). S.D.C. was supported by Future Fellowship grant no. FT0991953.
References
- 1.Leis JM. 2006. Are larvae of demersal fishes plankton or nekton? Adv. Mar. Biol. 51, 57–141. ( 10.1016/S0065-2881(06)51002-8) [DOI] [PubMed] [Google Scholar]
- 2.Montgomery JC, Jeffs A, Simpson SD, Meekan M, Tindle C. 2006. Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv. Mar. Biol. 51, 143–196. ( 10.1016/S0065-2881(06)51003-X) [DOI] [PubMed] [Google Scholar]
- 3.Dixson DL, Abrego D, Hay ME. 2014. Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345, 892–897. ( 10.1126/science.1255057) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Vermeij MJA, Marhaver KL, Huijbers CM, Nagelkerken I, Simpson SD. 2010. Coral larvae move toward reef sounds. PLoS ONE 5, e10660 ( 10.1371/journal.pone.0010660.g001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huijbers CM, Nagelkerken I, Lössbroek PA, Schulten IE, Siegenthaler A, Holderied MW, Simpson SD. 2012. A test of the senses: fish select novel habitats by responding to multiple cues. Ecology 93, 46–55. ( 10.1890/10-2236.1) [DOI] [PubMed] [Google Scholar]
- 6.Simpson SD, Meekan M, Montgomery J, McCauley R, Jeffs A. 2005. Homeward sound. Science 308, 221 ( 10.1126/science.1107406) [DOI] [PubMed] [Google Scholar]
- 7.Wright K, Higgs D, Leis J. 2011. Ontogenetic and interspecific variation in hearing ability in marine fish larvae. Mar. Ecol. Prog. Ser. 424, 1–13. ( 10.3354/meps09004) [DOI] [Google Scholar]
- 8.Kenyon T. 1996. Ontogenetic changes in the auditory sensitivity of damselfishes (Pomacentridae). J. Comp. Physiol. A 179, 553–561. ( 10.1007/BF00192321) [DOI] [Google Scholar]
- 9.Thresher RE, Colin PL, Bell LJ. 1989. Planktonic duration, distribution and population structure of western and central Pacific damselfishes (Pomacentridae). Copeia 1989, 420–434. ( 10.2307/1445439) [DOI] [Google Scholar]
- 10.Wellington G, Victor B. 1989. Planktonic larval duration of one hundred species of Pacific and Atlantic damselfishes (Pomacentridae). Mar. Biol. 101, 557–567. ( 10.1007/BF00541659) [DOI] [Google Scholar]
- 11.Cowen R, Paris C, Srinivasan A. 2006. Scaling of connectivity in marine populations. Science 311, 522–527. ( 10.1126/science.1122039) [DOI] [PubMed] [Google Scholar]
- 12.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Mar. Sci. 1, 169–192. ( 10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 13.Nagelkerken I, Connell SD. 2015. Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions. Proc. Natl Acad. Sci. USA 112, 13 272–13 277. ( 10.1073/pnas.1510856112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY. 2011. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–920. ( 10.1098/rsbl.2011.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Checkley DM, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R. 2009. Elevated CO2 enhances otolith growth in young fish. Science 324, 1683 ( 10.1126/science.1169806) [DOI] [PubMed] [Google Scholar]
- 16.Bignami S, Enochs IC, Manzello DP, Sponaugle S, Cowen RK. 2013. Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc. Natl Acad. Sci. USA 110, 7366–7370. ( 10.1073/pnas.1301365110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bignami S, Sponaugle S, Cowen RK. 2014. Effects of ocean acidification on the larvae of a high-value pelagic fisheries species, mahi-mahi Coryphaena hippurus. Aqua. Biol. 21, 249–260. ( 10.3354/ab00598) [DOI] [Google Scholar]
- 18.Bignami S, Sponaugle S, Cowen RK. 2012. Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Glob. Change Biol. 19, 996–1006. ( 10.1111/gcb.12133) [DOI] [PubMed] [Google Scholar]
- 19.Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MC, Chivers DP. 2010. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12 930–12 934. ( 10.1073/pnas.1004519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pimentel M, Pegado M, Repolho T, Rosa R. 2014. Impact of ocean acidification in the metabolism and swimming behavior of the dolphinfish (Coryphaena hippurus) early larvae. Mar. Biol. 161, 725–729. ( 10.1007/s00227-013-2365-7) [DOI] [Google Scholar]
- 21.Munday PL, Donelson JM, Dixson DL, Endo GG. 2009. Effects of ocean acidification on the early life history of a tropical marine fish. Proc. R. Soc. B 276, 3275–3283. ( 10.1098/rspb.2009.0784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heuer RM, Grosell M. 2014. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. 307, R1061–R1084. ( 10.1152/ajpregu.00064.2014) [DOI] [PubMed] [Google Scholar]
- 23.Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson S-A, Munday PL. 2012. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Change 2, 201–204. ( 10.1038/nclimate1352) [DOI] [Google Scholar]
- 24.Briffa M, de la Haye K, Munday PL. 2012. High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Mar. Pollut. Bull. 64, 1519–1528. ( 10.1016/j.marpolbul.2012.05.032) [DOI] [PubMed] [Google Scholar]
- 25.Chung W-S, Marshall NJ, Watson S-A, Munday PL, Nilsson GE. 2014. Ocean acidification slows retinal function in a damselfish through interference with GABAA receptors. J. Exp. Biol. 217, 323–326. ( 10.1242/jeb.092478) [DOI] [PubMed] [Google Scholar]
- 26.Dixson DL, Munday PL, Jones GP. 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75. ( 10.1111/j.1461-0248.2009.01400.x) [DOI] [PubMed] [Google Scholar]
- 27.Chivers DP, McCormick MI, Nilsson GE, Munday PL, Watson SA, Meekan MG, Mitchell MD, Corkill KC, Ferrari MC. 2014. Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob. Change Biol. 20, 515–522. ( 10.1111/gcb.12291) [DOI] [PubMed] [Google Scholar]
- 28.Hamilton TJ, Holcombe A, Tresguerres M. 2014. CO2-induced ocean acidification increases anxiety in Rockfish via alteration of GABAA receptor functioning. Proc. R. Soc. B 281, 20132509 ( 10.1098/rspb.2013.2509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsgren E, Dupont S, Jutfelt F, Amundsen T. 2013. Elevated CO2 affects embryonic development and larval phototaxis in a temperate marine fish. Ecol. Evol. 3, 3637–3646. ( 10.1002/ece3.709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jutfelt F, de Souza KB, Vuylsteke A, Sturve J. 2013. Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS ONE 8, e65825 ( 10.1371/journal.pone.0065825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray CS, Malvezzi A, Gobler CJ, Baumann H. 2014. Offspring sensitivity to ocean acidification changes seasonally in a coastal marine fish. Mar. Ecol. Prog. Ser. 504, 1–11. ( 10.3354/meps10791) [DOI] [Google Scholar]
- 32.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 ( 10.1371/journal.pone.0028983.t002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feely RA, Alin SR, Newton J, Sabine CL, Warner M, Devol A, Krembs C, Maloy C. 2010. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast. Shelf Sci. 88, 442–449. ( 10.1016/j.ecss.2010.05.004) [DOI] [Google Scholar]
- 34.Melzner F, Gutowska M, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner H-O. 2009. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331. ( 10.5194/bg-6-2313-2009) [DOI] [Google Scholar]
- 35.Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska M, Bange H, Hansen H, Körtzinger A. 2012. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888. ( 10.1007/s00227-012-1954-1) [DOI] [Google Scholar]
- 36.Shaw EC, Munday PL, McNeil BI. 2013. The role of CO2 variability and exposure time for biological impacts of ocean acidification. Geophys. Res. Lett. 40, 4685–4688. ( 10.1002/grl.50883) [DOI] [Google Scholar]
- 37.Moore R. 1982. Spawning and early life history of burramundi, Lates calcarifer (Bloch), in Papua New Guinea. Mar. Freshw. Res. 33, 647–661. ( 10.1071/MF9820647) [DOI] [Google Scholar]
- 38.Russell DJ, Rimmer MA. 2004. Stock enhancement of barramundi in Australia. In FAO Fishery Technical Paper 429: Marine Ranching (eds DM Bartley and KM Leber), pp. 73–108. Rome, Italy: FAO.
- 39.Dhert P, Lavens P, Duray M, Sorgeloos P. 1990. Improved larval survival at metamorphosis of Asian seabass (Lates calcarifer) using ω3-HUFA-enriched live food. Aquaculture 90, 63–74. ( 10.1016/0044-8486(90)90283-S) [DOI] [Google Scholar]
- 40.Petersen C, Jurevicius D. 2009. Environmental noise impact of a major transport corridor on a barramundi fish farm. In Proc. of ACOUSTICS 2009, 23–25 November 2009, Adelaide, Australia. Toowong, Australia: Australian Acoustical Society.
- 41.Braun CB, Grande T. 2008. Evolution of peripheral mechanisms for the enhancement of sound reception. In Fish bioacoustics (eds JF Webb, RR Fay, AN Popper), pp. 99–144. Berlin, Germany: Springer. [Google Scholar]
- 42.Knowlton RE, Moulton JM. 1963. Sound production in the snapping shrimps Alpheus (Crangon) and Synalpheus. Biol. Bull. 125, 311–331. ( 10.2307/1539406) [DOI] [Google Scholar]
- 43.Anker A. 2003. Alpheid shrimps from the mangroves and mudflats of Singapore. Part I. Genera Salmoneus, Athanas and Potamalpheops, with the description of two new species (Crustacea: Decapoda: Caridea). Raffles Bull. Zool. 51, 283–314. [Google Scholar]
- 44.Akamatsu T, Okumura T, Novarini N, Yan HY. 2002. Empirical refinements applicable to the recording of fish sounds in small tanks. J. Acoust. Soc. Am. 112, 3073–3082. ( 10.1121/1.1515799) [DOI] [PubMed] [Google Scholar]
- 45.Lillis A, Eggleston DB, Bohnenstiehl D. 2014. Soundscape variation from a larval perspective: the case for habitat-associated sound as a settlement cue for weakly swimming estuarine larvae. Mar. Ecol. Prog. Ser. 509, 57–70. ( 10.3354/meps10917) [DOI] [Google Scholar]
- 46.Radford CA, Montgomery JC, Caiger P, Higgs DM. 2012. Pressure and particle motion detection thresholds in fish: a re-examination of salient auditory cues in teleosts. J. Exp. Biol. 215, 3429–3435. ( 10.1242/jeb.073320) [DOI] [PubMed] [Google Scholar]
- 47.Rogers P, Hawkins A, Popper A, Fay R, Gray M. 2015. Parvulescu revisited: small tank acoustics for bioacousticians. In Effects of noise on aquatic life II (eds AN Popper, A Hawkins), pp. 933–941 New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 48.Maximino C, de Brito TM, de Mattos Dias CAG, Gouveia A, Morato S. 2010. Scototaxis as anxiety-like behavior in fish. Nat. Protocols 5, 209–216. ( 10.1038/nprot.2009.225) [DOI] [PubMed] [Google Scholar]
- 49.Leis JM, Siebeck U, Dixson DL. 2011. How Nemo finds home: the neuroecology of dispersal and of population connectivity in larvae of marine fishes. Integr. Comp. Biol. 51, 826–843. ( 10.1093/icb/icr004) [DOI] [PubMed] [Google Scholar]
- 50.Dowling N, Hall S, Mitchell J. 2000. Foraging kinematics of barramundi during early stages of development. J. Fish Biol. 57, 337–353. ( 10.1111/j.1095-8649.2000.tb02176.x) [DOI] [Google Scholar]
- 51.Simpson SD, Radford AN, Tickle EJ, Meekan MG, Jeffs AG. 2011. Adaptive avoidance of reef noise. PLoS ONE 6, e16625 ( 10.1371/journal.pone.0016625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tricas TC, Boyle KS. 2014. Acoustic behaviors in Hawaiian coral reef fish communities. Mar. Ecol. Prog. Ser. 511, 1–16. ( 10.3354/meps10930) [DOI] [Google Scholar]
- 53.Baumann H, Talmage SC, Gobler CJ. 2011. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Change 2, 38–41. ( 10.1038/nclimate1291) [DOI] [Google Scholar]
- 54.Chambers R, Candelmo A, Habeck E, Poach M, Wieczorek D, Cooper K, Greenfield C, Phelan B. 2014. Effects of elevated CO2 in the early life stages of summer flounder, Paralichthys dentatus, and potential consequences of ocean acidification. Biogeosciences 11, 1613–1626. ( 10.5194/bg-11-1613-2014) [DOI] [Google Scholar]
- 55.Munday PL, McCormick MI, Nilsson GE. 2012. Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? J. Exp. Biol. 215, 3865–3873. ( 10.1242/jeb.074765) [DOI] [PubMed] [Google Scholar]
- 56.Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE. 2014. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat. Clim. Change 4, 487–492. ( 10.1038/nclimate2195) [DOI] [Google Scholar]
- 57.Nagelkerken I, Russell BD, Gillanders BM, Connell SD. 2015. Ocean acidification alters fish populations indirectly through habitat modification. Nat. Clim. Change. ( 10.1038/nclimate2757) [DOI] [Google Scholar]
- 58.Welch MJ, Watson S-A, Welsh JQ, McCormick MI, Munday PL. 2014. Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat. Clim. Change 4, 1086–1089. ( 10.1038/nclimate2400) [DOI] [Google Scholar]
- 59.Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL. 2012. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2, 858–861. ( 10.1038/nclimate1599) [DOI] [Google Scholar]
- 60.Ferrari MC, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lönnstedt O, Chivers DP. 2012. Effects of ocean acidification on visual risk assessment in coral reef fishes. Funct. Ecol. 26, 553–558. ( 10.1111/j.1365-2435.2011.01951.x) [DOI] [Google Scholar]
- 61.Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB. 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852. ( 10.1073/pnas.0809996106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagelkerken I, Munday PL. In press. Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob. Change Biol. [DOI] [PubMed] [Google Scholar]
- 63.Robertson D. 1992. Patterns of lunar settlement and early recruitment in Caribbean reef fishes at Panama. Mar. Biol. 114, 527–537. ( 10.1007/BF00357250) [DOI] [Google Scholar]
- 64.Devine B, Munday P, Jones G. 2012. Rising CO2 concentrations affect settlement behaviour of larval damselfishes. Coral Reefs 31, 229–238. ( 10.1007/s00338-011-0837-0) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at Data Dryad: http://dx.doi.org/10.5061/dryad.2cf6s.