Abstract
Active-sensing systems such as echolocation provide animals with distinct advantages in dark environments. For social animals, however, like many bat species, active sensing can present problems as well: when many individuals emit bio-sonar calls simultaneously, detecting and recognizing the faint echoes generated by one's own calls amid the general cacophony of the group becomes challenging. This problem is often termed ‘jamming’ and bats have been hypothesized to solve it by shifting the spectral content of their calls to decrease the overlap with the jamming signals. We tested bats’ response in situations of extreme interference, mimicking a high density of bats. We played-back bat echolocation calls from multiple speakers, to jam flying Pipistrellus kuhlii bats, simulating a naturally occurring situation of many bats flying in proximity. We examined behavioural and echolocation parameters during search phase and target approach. Under severe interference, bats emitted calls of higher intensity and longer duration, and called more often. Slight spectral shifts were observed but they did not decrease the spectral overlap with jamming signals. We also found that pre-existing inter-individual spectral differences could allow self-call recognition. Results suggest that the bats’ response aimed to increase the signal-to-noise ratio and not to avoid spectral overlap.
Keywords: biosonar, echolocation, acoustic interference, jamming-avoidance, Lombard effect
1. Introduction
Most animals do not emit energy to sense their environment and orient in it, but rather they rely on energy (e.g. light, sound and magnetic) present in the environment. In contrast, several groups of animals generate outgoing signals in order to sense their surroundings [1]. Some examples include weakly electric fish [2] and echolocating dolphins [3] and bats [4].
The main advantages of active sensory systems that rely on own-energy emission are the ability to extract information in environments that do not allow use of vision (e.g. a dark night or turbid waters) and the ability to control different aspects of the received information by adapting the outgoing emission: the rate of information acquisition and the signal's directionality and design [5–9]. These advantages, however, do not come without a price. Animals using their own energy are subject to disadvantages such as signal double-energy loss owing to energy propagation and attenuation [10], energetic costs [11,12] and detection by potential prey [13].
Another often mentioned disadvantage of sensory systems that rely on energy emission is jamming by conspecifics, which has been well studied in weakly electric fish [14,15]. In bats, when two or more individuals of the same species fly in close proximity while emitting spectrally similar echolocation calls, loud conspecific calls might mask the faint echoes returning from a small insect, thus impairing the bat's ability to detect the insect. Moreover, even without direct masking but with conspecific calls and echoes returning in temporal proximity, the task of matching one's outgoing call to its appropriate incoming echo becomes challenging. It has been suggested that similar to weakly electric fish, bats actively alter the spectral characteristics of their calls (a behaviour known as Jamming Avoidance Response—JAR) aiming to reduce the potential ambiguity and masking resulting from conspecific calls. In the field: Obrist [16] reported an increase in call start- and peak-frequencies and in inter-pulse intervals, and a decrease in call duration when two Lasiurus borealis bats were foraging nearby. Ulanovsky et al. reported bidirectional JAR for Tadarida teniotis flying in pairs, where individuals shifted the frequency of their calls either upwards or downwards relative to conspecifics [17], and a unidirectional JAR, where bats always shifted their frequency upwards, was reported for Tadarida brasiliensis [18]. In contrast to these reports, a recent study that recorded audio on-board free-flying Rhinopoma microphyllum bats in the wild did not find any evidence for a JAR [19]. In the laboratory: a JAR was reported for Eptesicus fuscus flying in pairs: including changes in start and terminal call frequencies, bandwidth (BW), duration and sweep rate [20], while Pipistrellus abramus presented with playback of recorded jamming sequences or artificial wide-band noise responded with a unidirectional frequency shift, and changes to emission timing [21]. Stationary T. brasiliensis were shown to reduce emission rate when jammed either by conspecific calls [22] or by wide-band noise [23]. Several other studies used playbacks of artificial signals to test jamming. Griffin et al. showed that Plecotus townsendii were remarkably resistant to jamming by artificial wide-band noise and attributed this mainly to the directionality of their ears [24]; and Bates et al. [25] showed that stationary E. fuscus performing a detection task employ bidirectional JAR by shifting the quasi-constant-frequency (QCF) component of their call either upwards or downwards relative to an artificial CF jamming tone.
Almost all of the above experiments restricted themselves to situations in which only two bats fly together. This is, however, the simplest jamming scenario, while it is well known that bats often forage in groups of several individuals [26] or even fly in swarms of dozens to many thousands [27,28]. The two exceptions that tested bats under more severe jamming did so with artificial (non-bat) signals [24,25]. To better understand how bats avoid extreme, yet naturally occurring jamming, we confronted bats with situations of severe jamming by multiple individuals, using natural bat-call playbacks. To this end, we flew bats in a confined space while jamming them with recordings of their own calls as well as recordings of one or more conspecifics, played back from an array of multiple speakers.
We used Pipistrellus kuhlii (Kuhl, 1817)—a small insectivorous bat (ca 5–9 g) common in the Mediterranean region, the Middle East and South Asia. This bat is commonly observed in tight groups of ca 5 individuals foraging around a street-light, while larger groups of up to dozens of individuals can often be seen foraging in high proximity over ponds. In such a dynamic situation, where individual bats pursue different targets using different trajectories and velocities, each bat is exposed to multiple constantly changing interfering conspecifics calls.
We trained four P. kuhlii to individually search for and land on a feeding platform in a small flight room. While the bat was flying, we played back intense bat calls from 12 speakers covering the room's walls and ceiling and recorded the bat's flight trajectory and echolocation signals. By using playbacks rather than actual flying conspecifics, we ensured that any modification in signal design was a response to acoustic jamming, and not an attempt to detect and localize other individuals. We consecutively played back a variety of echolocation sequences generating different types of jamming, including calls of the bat itself, calls of one or more conspecifics and calls that were reversed in time. These sequences were played at a duty-cycle of 40% or 100% imposing different degrees of jamming. In all cases, we ensured a maximum (peak to peak) sound level of at least 95 dB (SPL) at any location in the room, thus much more intense than any echo received by the bat.
We found that bats can deal with even the most extreme continuous jamming with almost no effect on their performance. We found that bats emitted longer and louder echolocation calls to deal with jamming. They also shifted call frequency, but they always increased the frequency whether the jamming signal was higher or lower than their own. Their response thus did not reduce the potential jamming. We therefore hypothesize that these modifications in call design aimed to increase signal-to-noise ratio (SNR) rather than increase the inter-individual spectral differences. We argue that the pre-existing inter-individual differences in call characteristics allow self-recognition even under such extreme conditions.
2. Material and methods
For extended details on animal training, jamming signal production, and echolocation parameters extraction and analysis, see electronic supplementary material, S1.
(a). Animals
Four adult female P. kuhlii were trained to land on an elevated wooden platform (120 cm above the floor) at a different, random location in an obstacle-free or cluttered (with vertical strings, see below) flight room.
(b). Flight room recordings
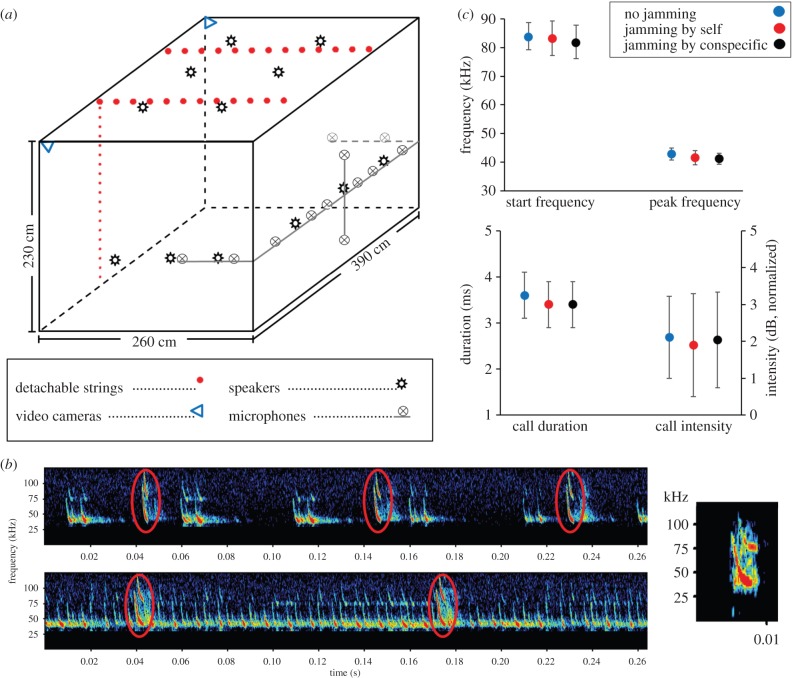
A detailed scheme of our flight room and its arrangement is given in figure 1. Room dimensions were 260 × 390 × 230 cm3. Walls and ceiling were covered with echo-attenuating acoustic foam. In two corners of the room, there were 3.6 mm-lens IR video cameras (Oasis, China) with an overlapping field of view covering about 60% of the room, enabling the reconstruction of three-dimensional flight paths. Video was recorded at 30 fps using GV-800 software (Geovision, Inc., Taiwan). Except for the IR illumination of the cameras, the flight room was completely dark. Twelve ultrasonic speakers (Vifa, Avisoft Bioacoustics) were spread around the room: six at a height of 100 cm along two walls and six in three rows on the ceiling (figure 1a). Jamming sequences were played using an AD converter (116H ultrasonic player, Avisoft Bioacoustics) at a sampling rate of 250 000 Hz. All speakers played the same sequence. Owing to the distances between the speakers, at any point in the room the bat therefore received 12 calls arriving shortly one after the other. Moreover, owing to the different position of the bat relative to each speaker, it experienced each call with a different spectrum, resulting in a complex acoustic scene (figure 1b,c). Note that these recordings were performed with a microphone behind the speakers (off-axis) while the bat experienced on-axis playbacks that contained much higher frequencies (figure 1b-right).
Figure 1.
Flight room set-up and jamming signals. (a) Flight room dimensions and arrangement. For simplification, only one vertical string is shown. (b) Spectrogram showing echolocation calls of a single bat (in red ovals) flying under low-duty-cycle (top left) and high-duty-cycle (bottom-left) jamming conditions. Note that these recordings were performed on the wall—behind the speakers, while the bat received the played-back signal directed towards it thus reaching much higher frequencies (see an example of an on-axis recording on the ‘right’). (c) Control of data analysis methods: the four echolocation parameters did not differ (mean ± s.d.) when a known echolocation sequence was played back without jamming playback, and with two jamming conditions. The sequence was recorded and analysed in the same manner as bats' echolocation was recorded and analysed in the experiments, confirming that our analysis did not introduce any bias.
We used playback sequences with different duty cycles and different types of signals simulating different situations of multiple bats echolocating in high density (see below). We used a calibrated microphone (GRAS, 40DP) connected to an AD converter (Avisoft, Hm116) to estimate the sound levels in the room during jamming. We calibrated the system such that at their most intense emission frequencies—35–50 kHz—bats experienced intensities of at least 95 dB (SPL) everywhere in the room. Bat vocalizations were recorded at a 250 kHz sampling rate using 12 omnidirectional synchronized ultrasonic microphones (Knowles with Avisoft Bioacoustics pre-amplifiers), which were spread along three walls at a height of approximately 96 cm and at distances of approximately 40 cm from each other. Recordings were always performed with 12 microphones, to allow high SNR recordings at any position of the bat in the room. The flight room was either empty or fitted with two arrays of 2.5 mm diameter strings hanging stretched from ceiling to floor, set 30 cm apart from wall to wall in two lines across the room's narrow dimension (figure 1a).
(c). Jamming signals
We used recordings of echolocation calls emitted by our bats to compose the jamming signals. For each individual, five jamming sequences were produced: (i) calls emitted by the jammed bat itself when flying in the empty silent room, termed ‘self’. (ii) Calls emitted by one conspecific (the ‘self’ recording of another bat). We chose conspecifics with both higher and lower peak frequencies (electronic supplementary material, figure S2), termed ‘conspecific’. (iii) A mixture of calls of several conspecifics (each recorded independently in the empty room), termed ‘multiple consp’. (iv) Calls emitted by the jammed bat itself when flying in the silent cluttered room (with hanging strings), termed ‘clutter-self’. (v) Reversed self-signal sequence (termed ‘reversed’): here each call was reversed along the time axis (played backwards), so the signal's spectral content was unaffected, but the temporal structure was completely different. These calls jammed the bat spectrally to the same extent, but could not be confused by the bats for bat calls. They therefore allowed testing of whether a response (if observed) was owing to spectral or spectro-temporal matching.
In all of the sequences above, calls were equally spread in time with intervals of 50 ms. This produced a jamming duty-cycle of ca 10% when played back from a single speaker, but because we used 12 speakers, owing to the delays in sound arrival from different speakers, the bats experienced an effective duty-cycle of 30–50% (figure 1b–top). We produced one more jamming sequence for each bat, using calls emitted by itself (the ‘self’ condition), this time equally spread in time with intervals of 10 ms. This produced a jamming duty-cycle of ca 40% experienced by the bat as an effective jamming duty-cycle of up to 100%, termed ‘100% duty-cycle’, (figure 1b–bottom; see also electronic supplementary material, S3).
In all cases, the sequence was played from all speakers simultaneously. Owing to the different locations of the speakers, the bats experienced each call from different directions (and with a different spectrum), thus being jammed from all around (figure 1b).
(d). Jamming experimental protocol
Reference (with no jamming playback) sessions were performed several times along the study period between manipulation (jamming) sessions. In each session, each bat was released in the flight room individually for at least 30 min. Each time the bat landed on the food platform, the platform was moved to a different, random location in the room. Manipulation sessions were performed on different days. At each manipulation session, a different jamming sequences was played (e.g. jamming by self-calls). Reference and manipulation sessions were performed both in an obstacle-free room (henceforth: ‘uncluttered’) and in a cluttered obstacle-rich room (two lines of vertical strings, henceforth: ‘cluttered’). For a detailed list of the various manipulations and sessions for each bat, see electronic supplementary material, figure S3.
(e). Data analysis: performance
Two parameters were used to quantify behavioural performance: (i) in ‘cluttered’, we used the collision rate with the strings to assess performance; Success rate = number of clean passes/number of passes per session. Collisions and clean passes were scored based on the video recordings. (ii) In ‘uncluttered’, we used the landing rate to assess performance: landing rate = number of landings/time of flight. This parameter could be thought of as an approximation of the bat's detection rate.
(f). Data analysis: echolocation parameters
We analysed six call parameters: pulse duration (−14 dB relative to the peak—Dur), peak frequency (frequency of highest intensity—FP), terminal frequency (frequency at the end of the call—FT), BW, peak intensity (In) and inter-pulse interval (time lapse from the start of one call to the start of the following call in a sequence—IPI). We used an in-house Matlab (Mathworks, Inc.) script for parameter extraction from the highest SNR microphone and calculated call parameters and signal design from the spectrograms of the detected bat calls (calculated with a window of 512 and an overlap of 99%). All detected calls were manually scrutinized and calls that were embedded within the playback signals were not analysed.
In addition, we ran control experiments to exclude the possibility that our analysis methods created any bias and thus affected the observed results. Here, playback signals were played as in the experiments while another speaker was used to mimic the bat. This speaker was moved in the room while playing a known sequence of signals. We could therefore run the same analysis as described above to test whether any bias was introduced. This control revealed no bias in our analysis (figure 1c).
(g). Data analysis: statistics
Statistical analyses were done using JMP software (SAS institute, Inc., USA). Discriminant function analyses (DFA) were performed in Matlab. We used a cross-validation procedure, training the classifier on 95% of the data and testing the remaining 5% (and repeating this process 20 times to estimate the variance).
3. Results
Our results, both behavioural and echolocation analysis, are based on extensive flight durations: bats performed a total of at least 300 minutes of actual flight and a total of 497 landings in all conditions, with a minimum of 10 landings per bat per condition (maximum—29 landings). In total, we analysed 35 250 search-phase echolocation calls and 2140 approach-phase calls.
We ensured that our results could not result from a bias created by our analysis (see §2, figure 1c) or from different flight directionality or spatial coverage of the room as these did not change between the different jamming and the control conditions (electronic supplementary material, figure S4).
(a). Behavioural performance
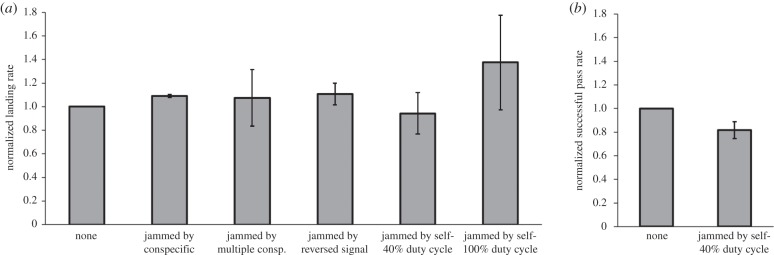
Under the different jamming conditions, the ability of all four bats to detect the platform and land on it (as expressed by their landing rate) was not reduced compared to their performance with no jamming (figure 2a), although they displayed much individual variation in response to different jamming conditions (electronic supplementary material, figure S5). Even with a 100% duty-cycle jamming, the landing rate did not deteriorate (electronic supplementary material, figure S5; Kruskal–Wallis ANOVA: H = 1.979, d.f. = 5, p = 0.852). Obstacle avoidance performance in clutter (as expressed by successful passes between strings) slightly decreased in the jammed condition (from 74 to 63%, figure 2b). The decrease was significant in three of the four bats (electronic supplementary material, figure S5), but the bats had no problem in manoeuvering the room, finding the landing platform and landing on it.
Figure 2.
Effect of jamming on behavioural performance. (a) Landing rate in uncluttered room was not reduced by various jamming conditions and severities. Data for each bat was pooled and normalized such that the landing rate (number of landings per seconds flown) without jamming was designated as ‘1’ (values are mean ± s.d. for all bats). (b) Obstacle avoidance performance in a cluttered room was slightly reduced when bats were jammed. Data were pooled and normalized as in (a).
(b). Echolocation: approach and landing
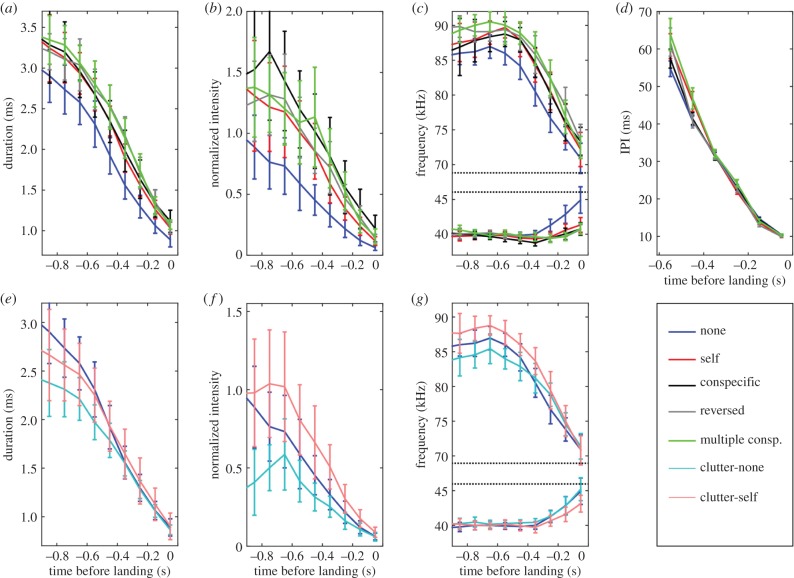
Bats adjusted their echolocation when jammed (figure 3; electronic supplementary material, figure S6). All four bats shifted to using significantly longer and more intense calls during their approach to landing, as well as increased call BW. The average increase in duration was 19.8% ± 1 (=0.4 ms) (mean ± s.e.), in intensity 4.5 ± 0.9 dB, and the increase in BW averaged 3.9 ± 0.4 kHz. IPI, however, was unaffected during the approach. These responses were evident in both the uncluttered and cluttered environments (mixed model ANCOVA with jamming condition and time-before-landing as factors and bat as random effect. For uncluttered/cluttered, respectively: Dur—d.f. = 4/1, F = 46.4/14.8, p = 0.0001/ = 0.0001. In—d.f. = 4/1, F = 39.3/73.4, p = 0.0001. BW—d.f. = 4/1, F = 47.3/62.2, p = 0.0001. IPI—d.f. = 4, F = 0.675, p = 0.609. In all cases except IPI, the difference between no jamming and all jamming conditions was significant, whereas the differences between jamming conditions were small and mostly non-significant (Tukey HSD post hoc). Owing to the frequent overlap of the playback signals and the bat calls, we were unable to analyse the echolocation parameters from the approach phase in the high-duty-cycle sessions and to measure IPI in the cluttered environment because of the multiple echoes.
Figure 3.
Effect of jamming on echolocation during approach. Data shown in (a–g) are for all bats, mean ± s.e. When jammed, the bats delayed and restrained their transition to approach phase in both uncluttered (a–d) and cluttered (e–g) environments. (a) Call durations decreased during approach to landing (marked as time ‘0’), but stayed longer when bats were jammed when compared with no jamming (blue line). (b) Pulse intensity was higher when jammed. (c) BW during approach increased under jamming. Start frequency (higher plots) and terminal frequency (lower plots). (d) IPI did not change when jammed. Similar patterns were observed in the cluttered environment. Interestingly, in a cluttered environment, the same response in pulse duration, intensity and BW (e–g, respectively) produced values closer to the bats' no-clutter no-jamming values (blue line in all panels).
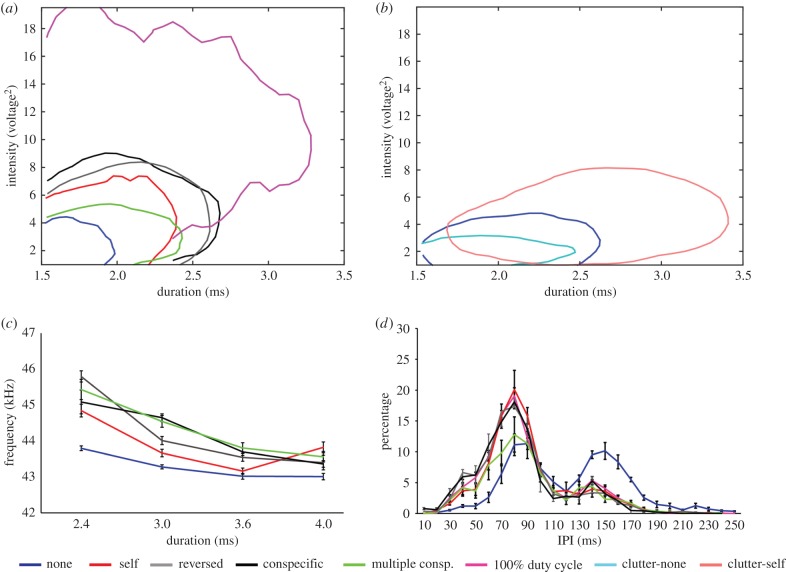
(c). Echolocation: search phase
Bats also used significantly longer (by an average of 26% ± 5.5 = 0.6 ms) and more intense (by an average of 5.4 ± 0.8 dB) calls during search phase (figure 4a,b; electronic supplementary material, figure S7) when jammed in both cluttered and un-cluttered environments. This was the response under all different jamming conditions, with the highest response elicited by the high-duty-cycle jamming signal (for uncluttered: mixed model one-way ANOVA with bat as random effect, Dur—d.f. = 5, F = 1875.3, p < 0.001. In—d.f. = 5, F = 5724.8, p < 0.001. In both duration and intensity, all jamming conditions were different from no jamming—Dunnett's post hoc. For cluttered [difference between none and jammed by self]: t-test, Dur—d.f. = 6317.4, t = 1.69, p < 0.0001. In—d.f. = 7833.9, t = 1.96, p < 0.0001). Unlike during the approach, IPI did change when the bats were jammed (figure 4d): the bats decreased IPI when jammed from 118.8 ± 1.1 ms in the control to an average of 81.9 ± 84 ms (mean ± s.e.). The difference between no jamming and all jamming conditions was significant (mixed model one-way ANOVA with bat as random effect, d.f. = 5, F = 288.6, p < 0.001, followed by Tukey HSD post hoc).
Figure 4.
Effect of jamming on echolocation during search. (a,b) Ninety per cent contour of the two-dimensional histogram of call intensity versus call duration for bat 1 during its search phase in uncluttered (a) and cluttered (b) environments. Bats used longer, more intense calls when jammed. (c) Peak frequency increased under jamming (all bats, mean ± s.e.). (d) Bats decreased IPI when jammed (all bats, mean ± s.e.). The bi-modal distribution implies that bats emit either one or two echolocation pulses per wing-beat cycle. When jammed bats increase the proportion of two-pulse cycles.
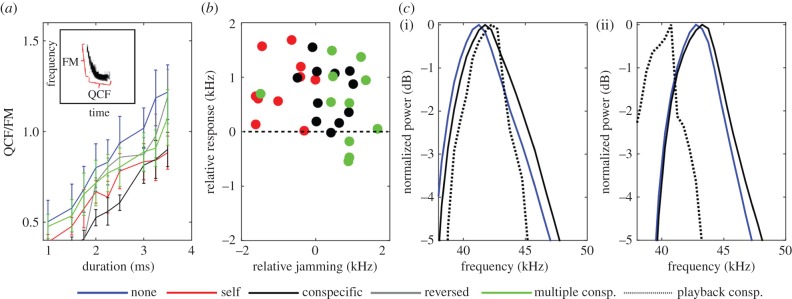
Calls were lengthened mostly by increasing the duration of the QCF component (defined as the part of the call beginning 5 kHz above the call's terminal frequency—FT, figure 5a-top left) with only a slight increase in FM duration (figure 5a-top left). The QCF component is characterized by much energy concentrated in a narrow low-frequency band and is hypothesized to improve detection [29,30]. In accordance, bat search calls that aim to detect prey (or objects) are typically characterized by longer QCF components [7,30]. This suggests that our bats increased call duration to improve detection (via better SNR) when jammed.
Figure 5.
Call design and spectral response to jamming. (a) QCF/FM duration ratio (mean ± s.e.). Increasing pulse duration was mostly achieved by prolonging the QCF tail. (b) Spectral response for all bats in three jamming conditions. The shift in peak frequency (Y-axis) is presented as a function of the difference in peak frequency between the jamming signal and the own peak frequency of the bat (prior to jamming—X-axis). In almost all cases, the bats shifted upwards (31 out of 38), while in four cases they did not change their frequency and in only three they slightly decreased it. In contrast with the JAR prediction, there was no (negative) correlation between the jamming stimulus (X-axis) and the response (Y-axis). The panel shows 38 points in total—five call durations (between 2 and 3.5 ms) for four bats in three experimental conditions. Not all durations were present in all jamming sequences. (c) Bats shifted their call frequency content upwards when jammed by a signal with a higher frequency content ((i)—bat 1 jammed by bat 4) thus increasing spectral overlap, and when jammed by a signal with lower frequency content ((ii)—bat 4 by bat 3), thus decreasing spectral overlap. The mean call spectrum is presented before jamming (blue) and when jammed by one conspecific (black). Grey dashed line shows the jamming signal which was once higher (i) and once lower (ii).
(d). Spectral changes
During target approach, bats changed the spectral content of their calls. For a given time point before landing, the bats in the different jamming conditions emitted calls with higher starting frequencies and lower peak frequencies (figure 3c,g). This was in accordance with emitting longer calls as such spectral features are characteristic of longer calls during an approach [31].
We also found a spectral shift in calls of the same duration under different jamming conditions. Bats slightly shifted their calls upwards in frequency (by 550 ± 680 Hz on average) regardless of the jamming signal (figures 4c and 5b,c). The classical spectral JAR predicts that when jammed, bats will shift their call frequencies away from the jamming signal to maximize the spectral difference between their own echoes and between echoes (and calls) of conspecifics. Our bats, however, almost always shifted their start-, peak- and terminal frequencies upwards independent of the jamming signals, which were sometimes higher and sometimes lower in frequency than their own calls (out of the 60 conditions, in 51 there was a shift upwards and only in three was there a downward shift, while in six there was no change; figure 5b). In many cases, the bat's response actually increased the spectral overlap of their calls with the jamming signal (figure 5c(i,ii)). To further validate these results, we added another control experiment, this time flying two additional bats, jammed by their own calls artificially shifted 2.5 kHz upwards. Here too, the bats shifted their call frequencies upwards (in the direction of the jamming signal) and used longer, more intense calls (electronic supplementary material, figure S8).
How then can bats handle severe jamming without decreasing spectral overlap between their own and conspecifics’ calls? Examining bat spectral call parameters revealed that individual differences between bats are large enough to allow own call recognition without any active spectral adjustments. A discriminant function analysis (DFA) showed that when using only two spectral parameters (the terminal and peak frequencies), the identity of the emitting bat could be correctly recognized for 61% of the calls, across the different jamming conditions (figure 6; electronic supplementary material, figure S9). This was much higher than chance level (25%) indicating that inter-individual differences were greater than the differences between treatments within individuals. Using more acoustic parameters to better describe the echolocation calls would likely improve the classification performance as we have shown in the past [32]. Testing a DFA on a set of seven spectral parameters already yielded better classification (67%, electronic supplementary material, figure S9).
Figure 6.
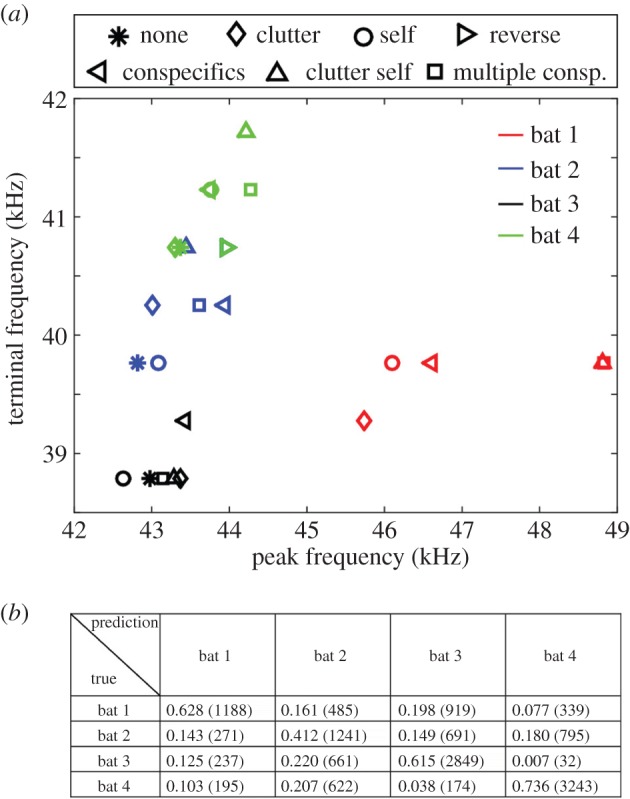
Inter-individual spectral differences allow individual recognition. (a) Peak- and terminal frequencies for the different bats and treatments (medians are presented). A DFA based on these parameters enabled the correct classification of 61% of the calls to their emitter across treatments (e.g. jammed by conspecifics, etc.). (b) Confusion matrix of correct and incorrect identification of each bat. Number of calls used in parentheses. Values in the table are for all jamming conditions combined. Individual conditions are provided in electronic supplementary material, figure S9.
4. Discussion
Flying with conspecifics in close proximity, whether during commuting, foraging or social interactions, can present echolocating bats with two main problems: first, the bat has to discern the faint echoes from the more intense calls of the conspecifics. Second, it has to be able to recognize its own echoes from those of other bats in order to properly assign them to its outgoing calls. Several solutions have been suggested to these problems; the most common one includes changing the frequency content of the calls to decrease their spectral overlap with the interfering signal [16–18,25].
Our results indicate that in situations of severe acoustic interference, bats use a different solution: bats did not alter their emitted calls to decrease spectral overlap with the jamming signals, but shifted to using longer and more intense echolocation signals and to calling more often. This response seems consistent with the aim of increasing the SNR of the returning echoes, as the bat invests more energy in its outgoing calls and increases the proportion of low frequencies (which suffer from less atmospheric attenuation) in the signals. The increased duration of the calls also contributes to detection, as the longer echo is more likely to be detected by the bat [7]. An increase of 0.2–0.6 ms, as we observed, should account for an increase of 1.5–4 dB in detectability [33].
The response we observed will not be enough to ensure that a faint echo returning from a small target is above the noise level (electronic supplementary material, figure S10). However, if bats rely on some template-matching mechanism to recognize their own echoes, as our current and previous [32] results imply, these measures will assist this mechanism. Bats can also apply additional acquisition measures to improve SNR such as modulating the emitted beam [9] or the ear's received-beam directionality [24].
Increasing the intensity of a vocalization is known as a measure to deal with masking noise and has been termed the Lombard effect. It has been described in many animals, including humans and horseshoe bats, which use constant frequency calls [34–36] and has been recently reported in FM bats under jamming conditions as well [21]. Interestingly, the Lombard effect is also sometimes accompanied by a rise in the vocalization pitch, which could explain the increase in frequency we found in this study. The typical explanation for this rise in pitch is physiological, i.e. increasing sub-glottal pressure to generate higher intensity results in a widening of glottal width and a shortening of the vocal folds, and an increase in frequency [37]. Our results cannot be viewed as a classical example of the Lombard effect as it normally refers to masking by broadband white noise that is not correlated to the desired signal, while here the bats were jammed with a signal very similar to their own. Nevertheless, we argue that the benefits of the Lombard effect hold in our case as well.
Bats did not respond temporally when approaching an object (figure 3d), and during the 100% duty-cycle jamming bats could still perform well, demonstrating that they can even deal with signals that overlap with their own emissions (and returning echoes). During the search phase, bats responded temporally; when jammed, all bats decreased IPI, thereby increasing emission rate. This result is in contrast to previously reported findings [22] and, like the increase in duration and intensity, increasing emission rate contributes to improving the SNR simply by multiplying the available signals. Interestingly, in other contexts (e.g. changing from search to approach phase or moving from open to cluttered environment), decreased IPI is accompanied by a decrease in call duration. Since during search phase, emission is often coupled with wing-beat [13], the bats probably reduced their IPI by emitting two pulses per wing cycle more often than in the control condition (figure 4d; note the shift in the bi-modal distribution towards left peak depicting shorter intervals).
Bats responded strongly to jamming by calls that were reversed in time, indicating that spectral, rather than spectro-temporal content is key for jamming. This suggests that the problem the bats face is one of spectral masking, rather than of recognizing their own echoes from the calls of conspecifics, an observation reported earlier by Masters & Raver [38], suggesting that bats view other bat calls as noise rather than potentially confusing ‘own’ calls.
Importantly, the longer, more intense calls emitted by the jammed bats were not beyond their normal echolocation repertoire. Such calls are typically emitted by these bats when flying in more open, less cluttered environments (as we confirmed experimentally by flying bats in a larger room; electronic supplementary material, figure S11). We therefore suggest that bats operate along an acoustic axis, shortening and elongating the duration of their calls based on several environmental parameters (figure 7). It is already well known that bats will shorten their calls when closing in on a target [7] or in a cluttered environment. Here, we show that acoustic masking will induce the opposite effect of increasing call duration. The bats that we jammed in the cluttered environment increased call duration, making it more similar to that of calls they emitted in the uncluttered environment; and when approaching the landing platform, bats used longer calls as if they were delaying the initiation of their approach phase. Increasing call duration is one component of the typical acoustic shift observed in echolocating bats that are moving away from clutter, which also includes higher intensity and spectral changes—as we have observed in our bats as well.
Figure 7.
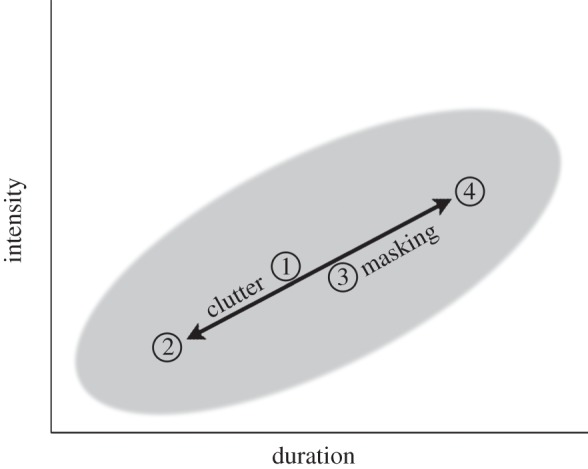
The clutter–masking trade-off. Scheme showing the effect of masking on bat echolocation calls along the duration–intensity axis. The grey oval represents the natural repertoire of the bats. In our flight room, with no clutter and no masking, bats used calls from the middle of this repertoire (1). Clutter drives the calls to be shorter and less intense (2), but when masking is added to clutter, the bats are ‘pushed’ back towards the centre (3). Masking without clutter causes bats to use longer and more intense calls (4), which are also typical for more open environments.
The observed shift in echolocation that we describe might be responsible for some of the results that have been associated with JAR in previous studies, such as an upward shift in frequency. To our knowledge, this is the first study to examine the effect of jamming on the approach phase, as previous studies did not examine the response when aligned to landing. Moreover, in most other studies that used natural bat calls as a jamming signal, there were other bats flying in vicinity of the examined bat, a situation that does not allow distinguishing between a response to the presence of the other bat (which is a nearby moving object) and to the jamming that potentially arises from the signals of the other bat. Our results document the response to the conspecific signals per se, which is probably different from the response to actual flying bats.
In a recent field study [19], we documented an opposite response to jamming in free-ranging R. microphyllum: when flying in close proximity to conspecifics, these bats used calls of shorter duration and lower intensity. We hypothesize that the different response in the two cases results from the presence of real flying bats in the field: the close proximity of conspecifics required the use of typical approach response. The lack of actual bats in the laboratory allowed us to isolate the response to jamming from the response to the physical presence of conspecifics. Bats may experience masking without a masker in the immediate vicinity in nature: since conspecifics' calls are much more intense than the faint echoes of a bat's own call, remote conspecifics are often loud enough to mask the faint echoes of an insect while still too far to be detected by the jammed bat's bio-SONAR.
While jamming presented bats with the problem of detecting the faint echoes of their calls, the problem of recognizing the bat's own call from those of conspecifics seems to be less difficult. In fact, our analysis shows that the pre-existing individual differences in frequency content provide vast information for self-call recognition with no need for an active response (figure 6).
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the following individuals for their assistance in this research: Mor Taub of the Department of Zoology, Tel-Aviv University, helped with graphics and helped with training bats. Lana Ngyev helped training the bats.
Ethics
Bats were captured under permit from the Israel Nature and Parks Authority, permit no. 2011/38137. Procedures were carried out under permit of the Institutional Animal Care and Use Committee operating according to the Israel Health Ministry, permit no. L-11–043.
Authors' contributions
E.A., G.B. and Y.Y. designed the experiments; E.A. performed the experiments and extracted behavioural results and echolocation parameters; G.B. produced the jamming signals, contributed new analytic tools and extracted echolocation parameters; E.A., G.B. and Y.Y. analysed data; E.A. and Y.Y. wrote the manuscript.
Competing interests
We have no competing interests.
References
- 1.Nelson ME, MacIver MA. 2006. Sensory acquisition in active sensing systems. J. Comp. Physiol. A 192, 573–586. ( 10.1007/s00359-006-0099-4) [DOI] [PubMed] [Google Scholar]
- 2.Heiligenberg W. 1973. Electrolocation of objects in the electric fish Eigenmannia (Rhamphichthyidae, Gymnotoidei). J. Comp. Physiol. 87, 137–164. ( 10.1007/BF01352158) [DOI] [Google Scholar]
- 3.Evans WE. 1973. Echolocation by marine delphinids and one species of fresh-water dolphin. J. Acoust. Soc. Am. 54, 191–199. ( 10.1121/1.1913562) [DOI] [Google Scholar]
- 4.Griffin D. 1944. Echolocation by blind men, bats and radar. Science 100, 589–590. ( 10.1126/science.100.2609.589) [DOI] [PubMed] [Google Scholar]
- 5.Petrites AE, Eng OS, Mowlds DS, Simmons JA, DeLong CM. 2009. Interpulse interval modulation by echolocating big brown bats (Eptesicus fuscus) in different densities of obstacle clutter. J. Comp. Physiol. A 195, 603–617. ( 10.1007/s00359-009-0435-6) [DOI] [PubMed] [Google Scholar]
- 6.Habersetzer J. 1981. Adaptive echolocation sounds in the bat Rhinopoma hardwickei: a field-study. J. Comp. Physiol. 144, 559–566. ( 10.1007/BF01326841) [DOI] [Google Scholar]
- 7.Kalko EV, Schnitzler HU. 1993. Plasticity in echolocation signals of European pipistrelle bats in search flight: implications for habitat use and prey detection. Behav. Ecol. Sociobiol. 33, 415–428. ( 10.1007/BF00170257) [DOI] [Google Scholar]
- 8.Jakobsen L, Brinkløv S, Surlykke A. 2013. Intensity and directionality of bat echolocation signals. Front. Physiol. 4, 89 ( 10.3389/fphys.2013.00089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kounitsky P, et al. 2015. Bats adjust their mouth gape to zoom their biosonar field of view. Proc. Natl Acad. Sci. USA 112, 6724–6729. ( 10.1073/pnas.1422843112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence BD, Simmons JA. 1982. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J. Acoust. Soc. Am. 71, 585–590. ( 10.1121/1.387529) [DOI] [PubMed] [Google Scholar]
- 11.Speakman JR, Anderson ME, Racey PA. 1989. The energy cost of echolocation in pipistrelle bats (Pipistrettus pipistrellus). J. Comp. Physiol. A 165, 679–685. ( 10.1007/BF00610999) [DOI] [Google Scholar]
- 12.Voigt C, Lewanzik D. 2012. ‘No cost of echolocation for flying bats’ revisited. J. Comp. Physiol. B 182, 831–840. ( 10.1007/s00360-012-0663-x) [DOI] [PubMed] [Google Scholar]
- 13.Miller LA, Surlykke A. 2001. How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. BioScience 51, 570–581. ( 10.1641/0006-3568(2001)051%5B0570:HSIDAA%5D2.0.CO;2) [DOI] [Google Scholar]
- 14.Heiligenberg W. 1974. Electrolocation and jamming avoidance in a Hypopygus (Rhamphichthyidae, Gymnotoidei), an electric fish with pulse-type discharges. J. Comp. Physiol. 91, 223–240. ( 10.1007/BF00698054) [DOI] [Google Scholar]
- 15.Bullock T, Hamstra R Jr, Scheich H. 1972. The jamming avoidance response of high frequency electric fish. J. Comp. Physiol. B 77, 1–22. ( 10.1007/BF00696517) [DOI] [Google Scholar]
- 16.Obrist MK. 1995. Flexible bat echolocation: the influence of individual, habitat and conspecifics on sonar signal design. Behav. Ecol. Sociobiol. 36, 207–219. ( 10.1007/BF00177798) [DOI] [Google Scholar]
- 17.Ulanovsky N, Fenton MB, Tsoar A, Korine C. 2004. Dynamics of jamming avoidance in echolocating bats. Proc. R. Soc. Lond. B 271, 1467–1475. ( 10.1098/rspb.2004.2750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillam EH, Ulanovsky N, McCracken GF. 2007. Rapid jamming avoidance in biosonar. Proc. R. Soc. B 274, 651–660. ( 10.1098/rspb.2006.0047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cvikel N, et al. 2015. On-board recordings reveal no jamming avoidance in wild bats. Proc. R. Soc. B 282, 20142274 ( 10.1098/rspb.2014.2274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu C, Xian W, Moss CF. 2009. Adaptive echolocation behavior in bats for the analysis of auditory scenes. J. Exp. Biol. 212, 1392–1404. ( 10.1242/jeb.027045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi E, Hyomoto K, Riquimaroux H, Watanabe Y, Ohta T, Hiryu S. 2014. Adaptive changes in echolocation sounds by Pipistrellus abramus in response to artificial jamming sounds. J. Exp. Biol. 217, 2885–2891. ( 10.1242/jeb.101139) [DOI] [PubMed] [Google Scholar]
- 22.Jarvis J, Jackson W, Smotherman M. 2013. Groups of bats improve sonar efficiency through mutual suppression of pulse emissions. Front. Physiol. 4, 140 ( 10.3389/fphys.2013.00140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis J, Bohn KM, Tressler J, Smotherman M. 2010. A mechanism for antiphonal echolocation by free-tailed bats. Anim. Behav. 79, 787–796. ( 10.1016/j.anbehav.2010.01.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin DR, McCue JJG, Grinnell AD. 1963. The resistance of bats to jamming. J. Exp. Zool. 152, 229–250. ( 10.1002/jez.1401520303) [DOI] [Google Scholar]
- 25.Bates ME, Stamper SA, Simmons JA. 2008. Jamming avoidance response of big brown bats in target detection. J. Exp. Biol. 211, 106–113. ( 10.1242/jeb.009688) [DOI] [PubMed] [Google Scholar]
- 26.Amichai E, Levin E, Kronfeld-Schor N, Roll U, Yom-Tov Y. 2013. Natural history, physiology and energetic strategies of Asellia tridens (Chiroptera). Mammal. Biol. 78, 94–103. ( 10.1016/j.mambio.2012.06.006) [DOI] [Google Scholar]
- 27.Levin E, Roll U, Dolev A, Yom-Tov Y, Kronfeld-Shcor N. 2013. Bats of a gender flock together: sexual segregation in a subtropical bat. PLoS ONE 8, e54987 ( 10.1371/journal.pone.0054987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons K, Jones G, Greenaway F. 2003. Swarming activity of temperate zone microchiropteran bats: effects of season, time of night and weather conditions. J. Zool. 261, 257–264. ( 10.1017/S0952836903004199) [DOI] [Google Scholar]
- 29.Schnitzler H-U, Kalko EKV. 2001. Echolocation by insect-eating bats: we define four distinct functional groups of bats and find differences in signal structure that correlate with the typical echolocation tasks faced by each group. BioScience 51, 557–569. ( 10.1641/0006-3568(2001)051%5B0557:EBIEB%5D2.0.CO;2) [DOI] [Google Scholar]
- 30.Jung K, Kalko EKV, Von Helversen O. 2007. Echolocation calls in Central American emballonurid bats: signal design and call frequency alternation. J. Zool. 272, 125–137. ( 10.1111/j.1469-7998.2006.00250.x) [DOI] [Google Scholar]
- 31.Melcón ML, Denzinger A, Schnitzler H-U. 2007. Aerial hawking and landing: approach behaviour in Natterer's bats, Myotis nattereri (Kuhl 1818). J. Exp. Biol. 210, 4457–4464. ( 10.1242/jeb.007435) [DOI] [PubMed] [Google Scholar]
- 32.Yovel Y, Melcon ML, Franz MO, Denzinger A, Schnitzler H-U. 2009. The voice of bats: how greater mouse-eared bats recognize individuals based on their echolocation calls. PLoS Comput. Biol. 5, e1000400 ( 10.1371/journal.pcbi.1000400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heil P, Neubauer H. 2003. A unifying basis of auditory thresholds based on temporal summation. Proc. Natl Acad. Sci. USA 100, 6151–6156. ( 10.1073/pnas.1030017100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junqua J-C. 1996. The influence of acoustics on speech production: a noise-induced stress phenomenon known as the Lombard reflex. Speech Commun. 20, 13–22. ( 10.1016/S0167-6393(96)00041-6) [DOI] [Google Scholar]
- 35.Hage SR, Jiang T, Berquist SW, Feng J, Metzner W. 2013. Ambient noise induces independent shifts in call frequency and amplitude within the Lombard effect in echolocating bats. Proc. Natl Acad. Sci. USA 110, 4063–4068. ( 10.1073/pnas.1211533110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summers WV, Pisoni DB, Bernacki RH, Pedlow RI, Stokes MA. 1988. Effects of noise on speech production: acoustic and perceptual analyses. J. Acoust. Soc. Am. 84, 917–928. ( 10.1121/1.396660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Titze IR. 1989. On the relation between subglottal pressure and fundamental frequency in phonation. J. Acoust. Soc. Am. 85, 901–906. ( 10.1121/1.397562) [DOI] [PubMed] [Google Scholar]
- 38.Masters WM, Raver KAS. 1996. The degradation of distance discrimination in big brown bats (Eptesicuc fuscus) caused by different interference signals. J. Comp. Physiol. A 179, 703–713. ( 10.1007/BF00216134) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.