Abstract
The hearts of lower vertebrates such as fish and salamanders display scarless regeneration following injury, although this feature is lost in adult mammals. The remarkable capacity of the neonatal mammalian heart to regenerate suggests that the underlying machinery required for the regenerative process is evolutionarily retained. Recent studies highlight the epicardial covering of the heart as an important source of the signalling factors required for the repair process. The developing epicardium is also a major source of cardiac fibroblasts, smooth muscle, endothelial cells and stem cells. Here, we examine animal models that are capable of scarless regeneration, the role of the epicardium as a source of cells, signalling mechanisms implicated in the regenerative process and how these mechanisms influence cardiomyocyte proliferation. We also discuss recent advances in cardiac stem cell research and potential therapeutic targets arising from these studies.
Keywords: regeneration, epicardium, cardiac stem cells
1. Introduction
An emerging concept in cardiovascular biology is that the mammalian myocardium has the potential to regenerate; however, the repair processes are insufficient to address the extensive damage caused by a cardiac insult such as myocardial infarction (MI). MI culminates in severe ischaemic damage to the surrounding tissue with, in some cases, the loss of up to a billion cardiomyocytes (CMs) [1,2]. This results in invasion of immune cells and myofibroblasts promoting scar formation [3,4]. With many advances in medical treatment, the mortality rate post-MI has decreased [5,6]; however, this has resulted in a rise in the number of patients that now present with heart failure [7–9]. For these reasons, research is now focused on enhancing the existing repair processes to improve cardiac function and to prevent the advent of heart failure. One approach is to study animal models that undergo full cardiac regeneration and extrapolate these observations to the adult human heart.
2. Models of cardiac regeneration
The zebrafish, a member of the teleost family, is noted for its cardiac regenerative capacity, and as a result is exploited throughout the field. Structurally, the zebrafish heart differs from that of mammals insofar as it is a two chamber organ, consisting of one atrium and one ventricle [10,11]. As the zebrafish heart consists of numerous small vessels for oxygen uptake rather than the large coronary vasculature typical of mammalian hearts, methods for inducing cardiac injury are limited to apex resection, cryoprobe injury or genetic ablation [12–14]. Following resection, a fibrin clot forms, providing a platform for proliferating CMs [15,16]. Remarkably, as demonstrated using a transgenic model which is deficient in ventricular myoctes, atrial CMs undergo transdifferentiation and replace lost ventricle CMs, although it should be noted that this mechanism is lost by the age of four months [17]. Interestingly, the medaka, a close relative of the zebrafish, lacks the ability to regenerate following resection. Injured medaka hearts present with persistent collagen deposition, lack of vascularization and a limited number of proliferating CMs [18], demonstrating the variation in evolutionary conservation of regeneration even within closely related species.
Similar to zebrafish, axolotls are also capable of replacing tissue following apical amputation [19,20]. Here again the repair process begins with the formation of a blood clot followed by CM cell cycle re-entry. The newt also retains the ability to regenerate the heart post-resection; however, not all CMs re-enter the cell cycle [21,22]. Moreover, replenishment of CMs in the newt is initiated by tenascin-C, a component of the extracellular matrix (ECM) [23], rather than infiltration of resident progenitor cell populations such as is the case with other models of regeneration. For this reason, the newt heart is an attractive model for understanding the mechanism(s) involved in CM proliferation and, more importantly, inhibitors of this process.
Recent studies on the ability of the neonatal murine heart to regenerate following injury would suggest that the mammalian heart does in fact harbour regenerative capabilities, albeit limited to immature stages: murine hearts undergo full regeneration post-MI from post-natal day 1 (P1), with loss of regenerative capacity evident by P7 [24,25]. Irrespective of species differences, a common thread between the aforementioned in vivo models is their ability to maintain CM proliferation throughout adulthood. This mechanism is largely lost in adult mammalian hearts, and while there are reports of proliferating CMs [26–28], there are too few to make any impact on the repair process.
3. Epicardial signalling
A common theme with successful models of regeneration is their ability to facilitate CM proliferation and the perfusion of injured tissue via neovascularization, of which the epicardium plays a central role. The epicardium contributes to heart development through secretion of a number of factors and controlled expression of developmental genes that have been shown to be instrumental to normal heart development. Collectively, these epicardial makers identify a cell population that is capable of giving rise to cell lineages that are deemed to be epicardially derived and therefore of mesothelial descent, which goes to explain how once activated and under optimum conditions the epicardium can give rise to fibroblasts, smooth muscle cells and endothelial cells. In addition, the epicardium has been described as a source-pool for cardiac stem cells (CSCs) [29–32]. These unique features of the epicardium and their respective signalling mediators will be discussed in the following paragraphs.
(a). Wilms tumour gene
Wilms tumour gene 1 (Wt1) is a transcription factor that is expressed in many tissues, including the urogenital system, spleen, brain, spinal cord, mesothelial organs, diaphragm, limb, proliferating coelomic epithelium, epicardium and subepicardial mesenchyme, during development [33,34]. Disruption of Wt1 activity results in developmental abnormalities, and Wt1−/– mice are embryonic lethal at embryonic day 12.5 (E12.5), with heart failure being one of the contributing factors to their early demise [33]. In the adult mammalian heart, Wt1 is reactivated following MI [35,36], although the specificity of Wt1+ cells epicardial origin has been questioned. Wagner et al. identified Wt1+ endothelial and vascular smooth muscle cells in the infarct and border zone, and attributed observed de novo neogenesis to Wt1+cells activated by hypoxia [35]. In addition, Duim et al. have recently identified a population of Wt1+ endothelial cells that undergo proliferation in a hypoxic environment both in vitro and in vivo following MI [37]. In contrast, Zhou et al. attribute the beneficial impact of Wt1+ cells in infarcted hearts to paracrine signalling and secretion of proangiogenic factors, rather than to a rise in Wt1+ endothelial cells [36]. Despite these conflicting reports, there is substantial evidence to show that Wt1 is activated following injury and, once activated, contributes to angiogenesis. Fate mapping studies have revealed Wt1 is expressed in endothelial cells, which points to its unsuitability as an exclusive epicardial marker. Although initially hampered by the lack of a definitive lineage trace model [38], collectively these in vivo studies demonstrate the beneficial impact of reactivating Wt1 in the adult epicardium, and establish a link between Wt1 expression and vascular formation.
(b). Thymosin β4
The G-actin sequestering peptide thymosin β4 regulates actin-cytoskeletal organization necessary for cell motility, organogenesis and other cell functions. Following MI, thymosin β4 has been shown to induce epicardially derived cells (EPDCs) to form vascular precursors and prompt neovasculogenesis [39,40]. Rossdeutsch et al. identified thymosin β4 expression in embryonic endothelium, and demonstrated that it promotes mural cell maturation and differentiation, and embryos lacking thymosin β4 were subjected to severe haemorrhaging (which in some cases proved to be lethal) [39]. A follow up study by Smart et al. has demonstrated that the addition of exogenous thymosin β4 can enhance cardiac repair by directing Wt1+ cells to undergo cardiomyogenesis [41], confirming earlier findings [42]. The proangiogenic effects of thymosin β4 in the adult heart were confirmed in a study by Shrivastava et al. [43] where mice were given a systemic injection of thymosin β4 immediately following MI injury, resulting in an increase in vessel density at the border zone and remote zone, and demonstrating the global effects of thymosin β4. In vitro data from the same study attributed the proangiogenic effects of thymosin β4 to protein kinase C signalling and confirmed that thymosin β4 can reactivate epicardial embryonic genes, including Wt1 and Tbx18 [43].
In addition, Rui et al. [44] have extended the cardiac regenerative capacity of neonatal mice by pre-treatment with thymosin β4. Here, neonatal mice were subjected to daily intraperitoneal injections of thymosin β4 from P1 to P7; apical resection was performed at P7, and 19 days post-injury neonatal hearts displayed complete regeneration, which was attributed to an increase of WT1+ cells. Notably, post-injury mice continued to receive intraperitoneal injections of thymosin β4 on alternate days until the study was terminated, potentially affecting the validity of the claimed extension of the neonatal window.
Conversely, a report by Banerjee et al. failed to document any effect of thymosin β4 on mural cell migration, angiogenesis or embryo development. In this study, thymosin β4 knockout models were not embryonic lethal and a lack of thymosin β4 had no impact on vascular development [45]. These contrasting results have been attributed to variation of environment or genetic background, resulting in differing compensatory mechanisms in response to the loss of thymosin β4 between strains [46]. Contrasting data on mural cell activity has also been attributed to the different time points at which mural cell activity was assessed between these studies. Since thymosin β4 seems to have a global impact on the heart, particularly in response to injury, teasing out a specific role for this molecule is likely to generate a number of contrasting views. Future investigations into the role of cardiac thymosin β4 are eagerly anticipated.
(c). Follistatin-like-1
A recent study has demonstrated the potential clinical application of follistatin-like-1 (Fstl1) as a catalyst for CM replenishment [47]. Fstl1 is a secreted glycoprotein and has been described as a cardiokine (cardiac secreted protein that can be used as a biomarker of cardiac dysfunction). Fstl1 belongs to the follistatin protein family, which mediate their effects by binding to transforming growth factor β (TGFβ) [48]. It is expressed in the developing heart and in the epicardium of the adult heart. Following either MI or ischaemic reperfusion, Fstl1 is upregulated in murine models [49]. In addition, Fstl1 has been detected in circulation in patients with acute coronary syndrome and protein levels are increased in failing hearts, hence its suitability as a biomarker of cardiac dysfunction [50,51]. The cardioprotective effects of Fstl1 have been associated with suppression of apoptosis and inflammation [49,52]. Fstl1 activates AMPK signalling, which decreases expression of proinflammatory genes in both macrophages and CMs in vitro, and administration of Fstl1 to damaged myocardium reduces the expression of proinflammatory mediators in areas of ischaemic damage in vivo [52].
In an exciting extension of these observations, epicardial patches seeded with Fstl1 were sutured onto the mouse epicardium immediately following MI injury, which resulted in CM cell cycle re-entry and division of existing CMs [47]. Only epicardially derived FStl1 could initiate cell division of CMs, whereas myocardial Fstl1 did not evoke CM cell division. This effect was specific to naive CMs (i.e. adult ventricular CMs did not undergo cell division in response to Fstl1). Intriguingly, this study shows that the source of Fstl1 determines its regenerative (epicardial) versus cardioprotective (myocardial) potential.
Taken together, these studies shift the focus from Fstl1 as a biomarker to a viable therapeutic that is ripe for clinical exploration.
(d). T-box genes
Several T-box family transcription factor genes have been implicated in cardiac regeneration. Studies have demonstrated that epicardially derived Tbx18+ cells give rise to CMs [53,54], although it should be noted that these findings were later disputed as Tbx18 was subsequently detected in the myocardium [55]. Thus claims that new CMs solely originate from the epicardium cannot be substantiated. Nevertheless, Tbx18 has been shown to be upregulated following MI with a similar expression pattern to Wt1 [36]. Moreover, Tbx18 has been shown to be upregulated following priming with thymosin β4 [41].
Interestingly, Tbx20 has been identified in cardiac fibroblasts as a key regulator of myofibroblast differentiation [56]. Moreover, Tbx20 has been shown to regulate scar formation following MI; in vivo models devoid of fibroblasts expressing Tbx20 develop thicker scars [56]. This study highlights a unique role for cardiac fibroblasts in managing scar formation following MI.
(e). C/EBP
A study by Huang et al. has identified the C/EBP transcription factor family to be key for neutrophil-mediated activation of the epicardium following MI [57]. Disruption of C/EBP signalling in the epicardium blunted the inflammatory response following ischaemic reperfusion injury, resulting in inhibition of invading neutrophils, the net result of which was reduced scar formation. This study offers key insights into myocardial scar and epicardial communication, highlighting the fine balance between inflammation and repair, and defining a novel role for the epicardium as a modulator of the inflammatory response: an interesting hypothesis worthy of further investigation.
(f). Hypoxia-inducing factor
Hypoxia-inducing factor-1α (HIF-1α) is upregulated following MI and increases vascular endothelial growth factor (VEGF) expression in ischaemic tissues [58,59]. Furthermore, myocardial overexpression of HIF-1α increases vascular density, reduces scar formation and improves cardiac function [60]. Following MI, HIFs are expressed by numerous cell types, including CMs, interstitial cells and endothelial cells [61]. Notably, HIFs have been shown to occupy distinct sites within the epicardium that correspond with coronary vasculogenic patterning [62]; this may be in conjunction with HIF-mediated expression of Wt1 [63]. Furthermore, constituent expression of HIF-1α in avian epicardium via a viral vector showed that although EPDC epithelial mesenchymal transition (EMT) was enhanced, migration of EPDCs into the myocardium was impaired [64]. Thus, while HIF-1α may be important for driving vasculogenesis, it can also serve as a negative regulator of EPDC migration.
(g). Retinoic acid
Epicardially derived retinoic acid (RA) synthesizing enzyme 2 (Raldh 2) is responsible for CM proliferation and differentiation, as well as ventricular maturation and angiogenesis [65]. Furthermore, Raldh 2 plays an important role in cardiac development; indeed, mice lacking retinoid X receptors die mid-gestation due to detachment of the epicardium [66,67]. In adult zebrafish, endocardial Raldh 2 has been shown to be intrinsic to repair of the heart following resection [68]. Inhibition of RA receptors or RA-degrading enzyme blocked the regenerative response of the zebrafish heart [68]. Furthermore, a lack of Raldh 2 expression in the endocardium of the medaka is suggested as a key factor for their inability to regenerate [18]. In murine hearts, RA signalling is reactivated following MI, and may play an important role in the repair and remodelling processes post-ischaemic injury due to its anti-proliferative effects on cardiac fibroblasts [69]. The use of RA as an anti-proliferative treatment following MI is an exciting prospect for future therapeutic interventions; indeed, RA derivatives have been used to suppress proliferative diseases such as prostate, lung, breast, skin, ovarian, bladder and oral cancer [70]. Further investigations are warranted to elucidate the contribution of RA signalling to repair of the mammalian heart.
(h). Growth factors
Migration of EPDCs to the myocardium is key to regenerating damaged myocardial tissue; therefore manipulation of myocardial fibroblast growth factor (FGF) to epicardial FGF receptor 1 (FGFR1) presents an interesting model for myocardial mediated self-repair. Epicardial FGFR1 is upregulated following activation of myocardial FGF [71]. In addition, overexpression of myocardial FGF results in increased EPDC expression of FGFR [72]. Furthermore, the FGF family has also been implicated in regeneration of the zebrafish adult heart. Here, FGF 17b has been localized to the myocardium, while FGFR2 and FGFR4 were found in the epicardium [73]. These studies suggest that the expression of FGF in the myocardium can regulate FGFR expression in the epicardium—the result of which is epicardial cells undergoing EMT [72,73]. Interestingly, studies on explant hearts show that activation of FGFR is important for the migration of proepicardial cells to the subepicardium and myocardium [72].
Similarly, there is evidence to support involvement of growth factors such as platelet-derived growth factor (PDGF) and TGFβ and VEGF in myocardial to epicardial communication. As with FGFs, PDGF and VEGF factors promote EMT and favour the fate of vasculogenesis [74,75], while TGFβ favours smooth muscle cell differentiation [76].
The other major invading cell type following MI are macrophages, which are an abundant source of cytokines, chemokines and growth factors including insulin-like growth factor 1 (IGF-1). Several studies have demonstrated the beneficial impact IGF-1 has on the heart following MI [77–79]; forced cardiac-specific overexpression of IGF-1 improves cardiac function and reduces scar formation post-MI [77]. Macrophages also secrete proangiogenic factors including VEGF and TGFβ, and have been shown to contribute to angiogenesis post-injury [80–82]. Increasingly, macrophages are being recognized for more than their ability to invade and phagocytose debris, and indeed their ability to secrete cytokines and chemokines at injured sites makes them an attractive prospect for clinical interventions. Recently, macrophages have been implicated in the neonatal regenerative response [83]. Although there are difficulties in determining which populations are contributing to the repair process (resident versus circulatory), their role in the repair of the mammalian heart merits further investigation.
4. Epicardial signalling: clinical application
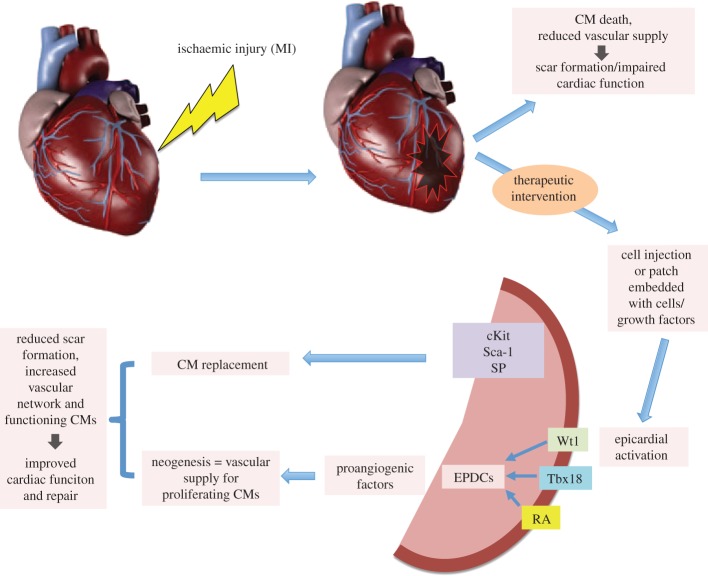
Providing an adequate vascular supply that can meet the metabolic demands of CMs and facilitate clearing of the debris from the immune response is a critical step towards restoring cardiac function. Therefore, the ability to initiate angiogenesis is key to the repair/regenerative processes. EPDCs have the ability to differentiate to coronary lineages and the majority of paracrine signalling from the epicardium is proangiogenic (figure 1). Given that endogenous repair mechanism(s) in the adult favour angiogenesis, methods to improve reperfusion should be a primary focus of regenerative medicine, as replacement of CMs will not be successful without the necessary infrastructure to meet the demands of these highly metabolic cells.
Figure 1.
Schematic of proposed repair following therapeutic intervention. Following ischaemic injury, the epicardium is activated following either cell injection or application of a patch loaded with cells or growth factors, switching on fetal genes Wt1, Tbx18, RA and progenitor pools cKit Sca-1 and SP, which signal back to damaged myocardium through the secretion of proangiogenic factors to evoke neogenesis. Activation of progenitor pools promotes CM proliferation. The net result is reduced scar formation, increased vascular network and a new CM population. CM, cardiomyocyte; Wt1, Wilms tumour gene; RA, retinoic acid; Tbx18, T box gene 18; EPDC, epicardially derived cell. (Online version in colour.)
To this end, the use of biomaterials such as hydrogels or patches could have a significant impact. Here, cells are incorporated within a gel or patch which can then be injected or applied to the epicardium [84–87]. In theory, cells can migrate to the injured myocardium-secreting paracrine factors to enhance the repair process. This approach may also have the additive effect of activating the epicardium to secrete key factors such as Wt1, RALDH, thymosin β4 or Fstl1. Indeed, a recent study using an IGF-1-loaded fibrin patch post-MI in a porcine model reported improved cardiac function [88]. The use of cardiac patches also has the added benefit of providing mechanical support to the weakened epicardium post-ischaemic injury [89]. Although this approach is still in its infancy, and considerations such as the immune response and potential rejection of these materials need to be fully assessed, it does seem an intriguing therapeutic avenue.
5. Cardiac stem cells
The heart contains a number of distinct CSC populations; EPDC, stem cell antigen-1 (Sca-1), c-Kit, cardiosphere-forming CSCs and side population (SP) progenitors. Although a variety of CSCs exist, they all share a common feature: their reported ability to give rise to all cardiac lineages. Here, the epicardium again plays a significant role. The epicardium itself is derived from mesothelial cells, thus it is reasonable to assume that it can give rise to progenitor cell types [29,42,54,90,91]. Furthermore, the reactivation of embryonic cell markers post-MI such as Wt1 demonstrates that the epicardium retains signature gene expression patterns that are intrinsic to fetal growth and development, and therefore potentially maintains the necessary machinery for repair. Moreover, studies have shown that EPDCs are capable of undergoing EMT in adult hearts giving rise to smooth muscle cells [90].
One CSC population that has divided opinion within the field is that of cKit+ cells, with recent investigations disputing earlier findings that demonstrated cKit+ cells give rise to CMs. Orlic et al. first reported that bone marrow-derived cKit+ cells regenerate the myocardium following MI and reported cKit+ cells as CSCs [92]. This was later disputed by Murry et al., who showed that cKit+ cells do not give rise to CMs [1]. Later studies confirmed these observations and went on to claim that CMs are derived from pre-existing CMs [27]. However, a study by Ellison et al. [32] argued that cKit+ cells are not only necessary but are sufficient to mediate repair to the damaged myocardium. Conclusively, a thorough investigation using genetic lineage-tracing models demonstrated that cKit+ cells do indeed give rise to CMs in both uninjured and injured tissue, but at a much lower rate than previously reported [93]. With this question outstanding, the field currently errs on the side of caution with regard to the potential of cKit+ cells as a pool of CM precursors.
Irrespective of these discrepancies, ongoing studies are investigating cardiospheres, essentially clusters of proliferating mesenchymal/stromal cells that express cKit. This unique cluster formation is thought to enhance ‘stemness' [94]. Interestingly, as cardiospheres diverge to a monolayer, they lose their cKit expression. Given that cluster formation recapitulates a stem cell-like microenvironment, it is not unreasonable to assume that cKit plays a role in this structure formation. Post-MI, cardiosphere injection has been shown to be more effective in improving cardiac function than injections of monolayer cells [95]. The therapeutic benefits of cardiospheres over cell monolayers is attributed to enhanced expression of ECM and adhesion molecules, as post-MI these clusters are retained at the site of injury with greater potency than injection of cells from a monolayer [94]. Thus, injection of multicellular spheres may prove to be the way forward for successful stem cell treatment.
Other CSCs have been identified based on their expression of Sca-1. Sca-1 is a ubiquitous stem cell marker associated with haematopoietic stem cell lineages. In the heart, Sca-1 expression is used to distinguish CSCs from differentiated cells. The Sca-1+ population was first observed in endothelial cells that also expressed cardiac transcription factors such as myocyte enhancer factor 2 (Mef 2) and GATA 4 [96]. Moreover, these cells were shown to give rise to CMs in vitro and in vivo [96,97]. Other studies have reported that Sca-1+ cells can give rise to CMs, endothelial cells and fibroblast cells [31,98]. Recent studies have identified a Sca-1 population derived from endothelial lineage that can migrate and give rise to CMs [99]. Notably, a Sca-1 gene analogue has yet to be found in the human genome and therefore caution should be taken when extrapolating these findings to the human heart. However, the murine Sca-1 antibody has been used to isolate Sca-1+ cells from the human heart, presumably cross-reacting with a related peptide, and these populations were also found to express GATA 4 and Mef 2 [100].
Further studies have led to the discovery of another progenitor population, referred to as SP. These cells are characterized by their unique ability to exclude Hoechst dye via the ATP-binding cassette transporter [101]. The cardiac SP population which is Sca-1+ and CD31− has been shown to be immunophenotypically distinct from bone marrow-derived SP population, and is capable of self-renewal and giving rise to functionally mature CMs [102].
A recent study has demonstrated that PDGRα is necessary for the clonogenicity of SP cells and proposes that SP cells may be isolated based on PDGRα expression [103]. SP express cardiac transcription factors such as GATA 4 [102], thereby demonstrating their tendency towards cardiac-specific lineages. Post-MI, SP cells are initially depleted but are restored again by day 7, evidenced by a rise in the proliferation marker Ki67 [104]. This would suggest that SP cells contribute to the repair process following MI; however, their marked depletion at the critical 24 h period could have a profound effect on the course of the repair process. Perhaps if the SP population is somehow maintained following the initial injury, then the loss of CMs may be more readily appeased as the resident progenitor pools can readily replace lost CMs (figure 1).
6. Clinical application
While the evidence supports the existence of CSCs in the adult heart, these endogenous populations are often insufficient to repair the heart following MI. It is likely that the primary function of these populations is to support homeostatic maintenance under normal physiological conditions as opposed to mediating repair following severe injury such as MI. Nonetheless, if it were possible to sustain these populations, particularly 24 h post-MI, then such a repair process could positively contribute in the face of such significant CM depletion post-MI. Certainly, the capacity of CSCs to give rise to various cardiac lineages is certainly a feature that needs to be exploited. Accordingly, the method by which CSCs are delivered to the site of injury appears to be a major influence on their regenerative effect.
There have been many studies that demonstrate the regenerative effects from intramyocardial injection of stem cells (generally bone marrow-derived) following MI [105–107]. However, translating this approach to the clinic is hampered first by the difficulty in stem cell isolation and expansion. As previously discussed, many parameters are used to define CSCs, and isolating these cells is often a laborious task, with great care needed to ensure a pure population is obtained, although it has been demonstrated that SP cells can be effectively isolated based on PDGFRα expression as opposed to the current dye exclusion methods [103]. Second, the number of cells that are successfully engrafted is often much lower than those administered, so the mechanism by which stem cells exert their effects remains unclear, with varying reports of a paracrine effect [108,109], a result of fusion with endogenous cells [110] or transdifferentiation [111]. Third, recent data from clinical trials point to the ineffectiveness of stem cells, particularly bone marrow-derived stem cells, in treatment of cardiovascular disease, with many of these trials describing a positive outcome that was later found to be flawed by inaccurate reporting [112]. These setbacks in the clinical application of CSCs serve to reinforce the necessity for caution when interpreting results. However, recent clinical revelations should not impede preclinical advancements being made with CSCs. Initial studies with allogeneic cardiospheres using rodent models noted improved cardiac function, reduced scar formation and overall less damage to the myocardium [95]. The CADUCEUS (cardiosphere-derived autologous stem cells to reverse ventricular dysfunction) clinical trial reported similar findings and further clinical trials are being considered [113].
Given the diverse populations that make up the heart, perhaps a combination approach, such as that as demonstrated by Ye et al. [88] may prove to be a more worthwhile intervention to assist the repair process. Here, a trilineage engraftment consisting of CMs, ECs and SMCs derived from human-induced pluripotent stem cells (hiPSCs) was supported by pre-application of an IGF-1-loaded fibrin patch. The overall result was improved cardiac function with a greater engraftment rate than previously reported. This study was terminated four weeks post-cell injection; therefore, it is difficult to determine the long-term benefits. However, it does set a precedent for future in vivo approaches.
7. Conclusion
While cardiac regeneration appears to be easily achieved by lower vertebrates and amphibians, the adult mammalian heart struggles to function and mediate repair simultaneously. Until recently, the epicardium has been overlooked as being a key player in the repair/regenerative process. Today it is the focus of intense research, with many studies concentrating on the stem cell potential of this region of the heart. Although resident CSCs have been identified, the insufficiencies of endogenous stem cell populations to alleviate acute and chronic damage to mammalian cardiac tissue remain to be overcome. Therapeutically speaking, identifying methods to sustain stem cell populations during ischaemic damage may also prove fruitful in terms of replenishing lost CMs. Moreover, combination approaches exploiting the use of biomaterials and cell-based therapies are making remarkable advances. In addition, there have been various studies examining epicardial development, and these have aided our understanding of the adult mammalian heart injury response. Evidently, the epicardium is key to mediating repair; as a signalling powerhouse and cell reservoir, it is certainly equipped to do so. However, cardiac repair requires a speedy and robust approach, and although advances are being made we are still far from contriving an ‘off the shelf’ cure for the masses affected by heart failure.
Authors' contributions
T.K.-L. conceived and wrote the manuscript. N.R. made substantial revisions to the manuscript and both authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Leducq Foundation: Transatlantic Networks of Excellence in Cardiovascular Research.
References
- 1.Murry CE, Reinecke H, Pabon LM. 2006. Regeneration gaps: observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 47, 1777–1785. ( 10.1016/j.jacc.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 2.Reinecke H, Minami E, Zhu W-Z, Laflamme MA. 2008. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ. Res. 103, 1058–1071. ( 10.1161/CIRCRESAHA.108.180588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. 2008. The immune system and cardiac repair. Pharmacol. Res. 58, 88–111. ( 10.1016/j.phrs.2008.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swirski FK. 2014. Inflammation and repair in the ischaemic myocardium. Hamostaseologie 35, 34–36. ( 10.5482/HAMO-14-09-0045) [DOI] [PubMed] [Google Scholar]
- 5.Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, Winkelmayer WC. 2008. Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis. J. Am. Coll. Cardiol. 51, 1247–1254. ( 10.1016/j.jacc.2007.10.063) [DOI] [PubMed] [Google Scholar]
- 6.Briffa T, Hickling S, Knuiman M, Hobbs M, Hung J, Sanfilippo FM, Jamrozik K, Thompson PL. 2009. Long term survival after evidence based treatment of acute myocardial infarction and revascularisation: follow-up of population based Perth MONICA cohort, 1984–2005. BMJ 338, b36 ( 10.1136/bmj.b36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellermann JP, et al. 2003. Incidence of heart failure after myocardial infarction: is it changing over time? Am. J. Epidemiol. 157, 1101–1107. ( 10.1093/aje/kwg078) [DOI] [PubMed] [Google Scholar]
- 8.Jhund PS, McMurray JJV. 2008. Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation 118, 2019–2021. ( 10.1161/CIRCULATIONAHA.108.813493) [DOI] [PubMed] [Google Scholar]
- 9.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. 2009. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J. Am. Coll. Cardiol. 53, 13–20. ( 10.1016/j.jacc.2008.08.067) [DOI] [PubMed] [Google Scholar]
- 10.Hu N, Sedmera D, Yost HJ, Clark EB. 2000. Structure and function of the developing zebrafish heart. Anat. Rec. 260, 148–157. () [DOI] [PubMed] [Google Scholar]
- 11.Asnani A, Peterson RT. 2014. The zebrafish as a tool to identify novel therapies for human cardiovascular disease. Dis. Model. Mech. 7, 763–767. ( 10.1242/dmm.016170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Rosa JM, Martín V, Peralta M, Torres M, Mercader N. 2011. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138, 1663–1674. ( 10.1242/dev.060897) [DOI] [PubMed] [Google Scholar]
- 13.Rodius S, et al. 2014. Transcriptional response to cardiac injury in the zebrafish: systematic identification of genes with highly concordant activity across in vivo models. BMC Genomics 15, 852 ( 10.1186/1471-2164-15-852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poss KD, Wilson LG, Keating MT. 2002. Heart regeneration in zebrafish. Science 298, 2188–2190. ( 10.1126/science.1077857) [DOI] [PubMed] [Google Scholar]
- 15.Poss KD. 2007. Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 18, 36–45. ( 10.1016/j.semcdb.2006.11.009) [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi K, et al. 2010. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464, 601–605. ( 10.1038/nature08804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, et al. 2013. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 498, 497–501. ( 10.1038/nature12322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito K, Morioka M, Kimura S, Tasaki M, Inohaya K, Kudo A. 2014. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev. Dyn. 243, 1106–1115. ( 10.1002/dvdy.24154) [DOI] [PubMed] [Google Scholar]
- 19.Flink IL. 2002. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat. Embryol. 205, 235–244. ( 10.1007/s00429-002-0249-6) [DOI] [PubMed] [Google Scholar]
- 20.Cano-Martínez A, Vargas-González A, Guarner-Lans V, Prado-Zayago E, León-Oleda M, Nieto-Lima B. 2010. Functional and structural regeneration in the axolotl heart (Ambystoma mexicanum) after partial ventricular amputation. Arch. Cardiol. Mex. 80, 79–86. [PubMed] [Google Scholar]
- 21.Bettencourt-Dias M, Mittnacht S, Brockes JP. 2003. Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. J. Cell Sci. 116, 4001–4009. ( 10.1242/jcs.00698) [DOI] [PubMed] [Google Scholar]
- 22.Oberpriller JO, Oberpriller JC. 1974. Response of the adult newt ventricle to injury. J. Exp. Zool. 187, 249–253. ( 10.1002/jez.1401870208) [DOI] [PubMed] [Google Scholar]
- 23.Mercer SE, Odelberg SJ, Simon H-G. 2013. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev. Biol. 382, 457–469. ( 10.1016/j.ydbio.2013.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM. 2012. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 4, 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porrello ER, et al. 2013. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl Acad. Sci. USA 110, 187–192. ( 10.1073/pnas.1208863110/-/DCSupplemental) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, et al. 2010. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS ONE 5, e12559 ( 10.1371/journal.pone.0012559.s011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senyo SE, et al. 2013. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436. ( 10.1038/nature11682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollova M, et al. 2013. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl Acad. Sci. USA 110, 1446–1451. ( 10.1073/pnas.1214608110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wessels A, Pérez-Pomares JM. 2004. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat. Rec. 276A, 43–57. ( 10.1002/ar.a.10129) [DOI] [PubMed] [Google Scholar]
- 30.Winter EM, Gittenberger-de Groot AC. 2007. Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell. Mol. Life Sci. 64, 692–703. ( 10.1007/s00018-007-6522-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong JJH, et al. 2011. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 9, 527–540. ( 10.1016/j.stem.2011.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellison GM, et al. 2013. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842. ( 10.1016/j.cell.2013.07.039) [DOI] [PubMed] [Google Scholar]
- 33.Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB. 1993. The expression of the Wilms’ tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 40, 85–97. ( 10.1016/0925-4773(93)90090-K) [DOI] [PubMed] [Google Scholar]
- 34.Wagner K-D, Wagner N, Schedl A. 2003. The complex life of WT1. J. Cell. Sci. 116, 1653–1658. ( 10.1242/jcs.00405) [DOI] [PubMed] [Google Scholar]
- 35.Wagner K-D, Wagner N, Bondke A, Nafz B, Flemming B, Theres H, Scholz H. 2002. The Wilms’ tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J. 16, 1117–1119. ( 10.1096/fj.01-0986fje) [DOI] [PubMed] [Google Scholar]
- 36.Zhou B, et al. 2011. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Invest. 121, 1894–1904. ( 10.1172/JCI45529DS1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duim SN, Kurakula K, Goumans MJ, Kruithof BPT. 2015. Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J. Mol. Cell. Cardiol. 81, 127–135. ( 10.1016/j.yjmcc.2015.02.007) [DOI] [PubMed] [Google Scholar]
- 38.Rudat C, Kispert A. 2012. Wt1 and epicardial fate mapping. Circul. Res. 111, 165–169. ( 10.1161/CIRCRESAHA.112.273946) [DOI] [PubMed] [Google Scholar]
- 39.Rossdeutsch A, Smart N, Dubé KN, Turner M, Riley PR. 2012. Essential role for thymosin β4 in regulating vascular smooth muscle cell development and vessel wall stability. Circul. Res. 111, e89–102. ( 10.1161/CIRCRESAHA.111.259846) [DOI] [PubMed] [Google Scholar]
- 40.Smart N, Risebro CA, Melville AAD, Moses K, Schwartz RJ, Chien KR, Riley PR. 2007. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445, 177–182. ( 10.1038/nature05383) [DOI] [PubMed] [Google Scholar]
- 41.Smart N, et al. 2011. De novo cardiomyocytes from within the activated adult heart after injury. Nature 474, 640–644. ( 10.1038/nature10188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou B, et al. 2008. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113. ( 10.1038/nature07060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrivastava S, Srivastava D, Olson EN, DiMaio JM, Bock-Marquette I. 2010. Thymosin β4 and cardiac repair. Ann. NY Acad. Sci. 1194, 87–96. ( 10.1111/j.1749-6632.2010.05468.x) [DOI] [PubMed] [Google Scholar]
- 44.Rui L, Yu N, Hong L, Feng H, Chunyong H, Jian M, Zhe Z, Shengshou H. 2014. Extending the time window of mammalian heart regeneration by thymosin β4. J. Cell. Mol. Med. 18, 2417–2424. ( 10.1111/jcmm.12421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banerjee I, Moore-Morris T, Evans SM, Chen J. 2013. Thymosin β4 is not required for embryonic viability or vascular development. Circul. Res. 112, e25–e28. ( 10.1161/CIRCRESAHA.111.300197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smart N, Riley PR. 2013. Thymosin β4 in vascular development response to research commentary. Circul. Res. 112, e29–e30. ( 10.1161/CIRCRESAHA.112.300555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei K, et al. 2015. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525, 479–485. ( 10.1038/nature15372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibanuma M, Mashimo J, Mita A. 1993. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-β1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur. J. Biochem. 217, 13–19. ( 10.1111/j.1432-1033.1993.tb18212.x) [DOI] [PubMed] [Google Scholar]
- 49.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. 2008. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 117, 3099–3108. ( 10.1161/CIRCULATIONAHA.108.767673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lara-Pezzi E, et al. 2008. Expression of follistatin-related genes is altered in heart failure. Endocrinology 149, 5822–5827. ( 10.1210/en.2008-0151) [DOI] [PubMed] [Google Scholar]
- 51.Widera C, et al. 2009. Circulating concentrations of follistatin-like 1 in healthy individuals and patients with acute coronary syndrome as assessed by an immunoluminometric sandwich assay. Clin. Chem. 55, 1794–1800. ( 10.1373/clinchem.2009.129411) [DOI] [PubMed] [Google Scholar]
- 52.Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y. 2012. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation 126, 1728–1738. ( 10.1161/CIRCULATIONAHA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng B, Ren X-F, Cao F, Zhou X-Y, Zhang J. 2011. Developmental patterns and characteristics of epicardial cell markers Tbx18 and Wt1 in murine embryonic heart. J. Biomed. Sci. 18, 67 ( 10.1186/1423-0127-18-67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai C-L, et al. 2008. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454, 104–108. ( 10.1038/nature06969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christoffels VM, Grieskamp T, Norden J, Mommersteeg MTM, Rudat C, Kispert A. 2009. Tbx18 and the fate of epicardial progenitors. Nature 458, E8–E9. ( 10.1038/nature07916) [DOI] [PubMed] [Google Scholar]
- 56.Furtado MB, et al. 2014. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circul. Res. 114, 1422–1434. ( 10.1161/CIRCRESAHA.114.302530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang GN, et al. 2012. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science 338, 1599–1603. ( 10.1126/science.1228186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim SH, Moon H-H, Kim HA, Hwang K-C, Lee M, Choi D. 2011. Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Mol. Ther. 19, 741–750. ( 10.1038/mt.2010.301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. 2000. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 342, 626–633. ( 10.1056/NEJM200003023420904) [DOI] [PubMed] [Google Scholar]
- 60.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. 2005. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J. Am. Coll. Cardiol. 46, 2116–2124. ( 10.1016/j.jacc.2005.08.045) [DOI] [PubMed] [Google Scholar]
- 61.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. 2003. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108, 79–85. ( 10.1161/01.CIR.0000078635.89229.8A) [DOI] [PubMed] [Google Scholar]
- 62.Wikenheiser J, Karunamuni G, Sloter E, Walker MK, Roy D, Wilson DL, Watanabe M. 2013. Altering HIF-1α through 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure affects coronary vessel development. Cardiovasc. Toxicol. 13, 161–167. ( 10.1007/s12012-012-9194-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner KD, Wagner N, Wellmann S, Schley G, Bondke A, Theres H, Scholz H. 2003. Oxygen-regulated expression of the Wilms’ tumor suppressor Wt1 involves hypoxia-inducible factor-1 (HIF-1). FASEB J. 17, 1364 ( 10.1096/fj.02-1065fje) [DOI] [PubMed] [Google Scholar]
- 64.Tao J, Doughman Y, Yang K, Ramirez-Bergeron D, Watanabe M. 2013. Epicardial HIF signaling regulates vascular precursor cell invasion into the myocardium. Dev. Biol. 376, 136–149. ( 10.1016/j.ydbio.2013.01.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merki E, et al. 2005. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc. Natl Acad. Sci. USA 102, 18 455–18 460. ( 10.1073/pnas.0504343102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. 1994. RXR-alpha mutant mice establish a genetic-basis for vitamin-a signaling in heart morphogenesis. Genes Dev. 8, 1007–1018. ( 10.1101/gad.8.9.1007) [DOI] [PubMed] [Google Scholar]
- 67.Gruber PJ, Kubalak SW, Pexieder T, Sucov HM, Evans RM, Chien KR. 1996. RXR alpha deficiency confers genetic susceptibility for aortic sac, conotruncal, atrioventricular cushion, and ventricular muscle defects in mice. J. Clin. Invest. 98, 1332–1343. ( 10.1172/JCI118920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD. 2011. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 20, 397–404. ( 10.1016/j.devcel.2011.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bilbija D, Haugen F, Sagave J, Baysa A, Bastani N. 2012. Retinoic acid signalling is activated in the postischemic heart and may influence remodelling. PLoS ONE 7, e44740 ( 10.1371/journal.pone.0044740.s006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen MC, Hsu SL, Lin H, Yang TY. 2014. Retinoic acid and cancer treatment. Biomedicine 4, 22 ( 10.7603/s40681-014-0022-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smart N, Riley PR. 2012. The epicardium as a candidate for heart regeneration. Future Cardiol. 8, 53–69. ( 10.2217/fca.11.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pennisi DJ, Mikawa T. 2009. FGFR-1 is required by epicardium-derived cells for myocardial invasion and correct coronary vascular lineage differentiation. Dev. Biol. 328, 148–159. ( 10.1016/j.ydbio.2009.01.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. 2006. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127, 607–619. ( 10.1016/j.cell.2006.08.052) [DOI] [PubMed] [Google Scholar]
- 74.Tomanek RJ, Christensen LP, Simons M, Murakami M, Zheng W, Schatteman GC. 2010. Embryonic coronary vasculogenesis and angiogenesis are regulated by interactions between multiple FGFs and VEGF and are influenced by mesenchymal stem cells. Dev. Dyn. 239, 3182–3191. ( 10.1002/dvdy.22460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith CL, Baek ST, Sung CY, Tallquist MD. 2011. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circul. Res. 108, e15–e26. ( 10.1161/CIRCRESAHA.110.235531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bax NAM, van Oorschot AAM, Maas S, Braun J, van Tuyn J, de Vries AAF, Groot ACG-D, Goumans MJ. 2011. In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFβ-signaling and WT1. Basic Res. Cardiol. 106, 829–847. ( 10.1007/s00395-011-0181-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santini MP, et al. 2007. Enhancing repair of the mammalian heart. Circul. Res. 100, 1732–1740. ( 10.1161/CIRCRESAHA.107.148791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koudstaal S, et al. 2014. Sustained delivery of insulin-like growth factor-1/hepatocyte growth factor stimulates endogenous cardiac repair in the chronic infarcted pig heart. J. Cardiovasc. Trans. Res. 7, 232–241. ( 10.1007/s12265-013-9518-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scharin Täng M, Redfors B, Lindbom M, Svensson J, Ramunddal T, Ohlsson C, Shao Y, Omerovic E. 2012. Importance of circulating IGF-1 for normal cardiac morphology, function and post infarction remodeling. Growth Horm. IGF Res. 22, 206–211. ( 10.1016/j.ghir.2012.09.002) [DOI] [PubMed] [Google Scholar]
- 80.Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD. 1990. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J. Cell Biol. 111, 743–755. ( 10.1083/jcb.111.2.743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ribatti D, Nico B, Crivellato E, Vacca A. 2007. Macrophages and tumor angiogenesis. Leukemia 21, 2085–2089. ( 10.1038/sj.leu.2404900) [DOI] [PubMed] [Google Scholar]
- 82.Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y. 2011. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 117, 5264–5272. ( 10.1182/blood-2011-01-330720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aurora AB, Porrello ER, Tan W. 2014. Macrophages are required for neonatal heart regeneration. Clin. Invest. 124, 1382–1392. ( 10.1172/JCI72181DS1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camci-Unal G, Aubin H, Ahari AF, Bae H, Nichol JW, Khademhosseini A. 2010. Surface-modified hyaluronic acid hydrogels to capture endothelial progenitor cells. Soft Matter 6, 5120–5126. ( 10.1039/c0sm00508h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boopathy AV, Martinez MD, Smith AW, Brown ME, García AJ, Davis ME. 2015. Intramyocardial delivery of notch ligand-containing hydrogels improves cardiac function and angiogenesis following infarction. Tissue Eng. Part A 21, 2315–2322. ( 10.1089/ten.TEA.2014.0622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez EC, et al. 2013. Grafts enriched with subamnion-cord-lining mesenchymal stem cell angiogenic spheroids induce post-ischemic myocardial revascularization and preserve cardiac function in failing rat hearts. Stem Cells Dev. 22, 3087–3099. ( 10.1089/scd.2013.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiong Q, et al. 2011. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells 29, 367–375. ( 10.1002/stem.580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye L, et al. 2014. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15, 750–761. ( 10.1016/j.stem.2014.11.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serpooshan V, Wu SM. 2014. Patching up broken hearts: cardiac cell therapy gets a bioengineered boost. Cell Stem Cell 15, 671–673. ( 10.1016/j.stem.2014.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wada AM, Smith TK, Osler ME, Reese DE, Bader DM. 2003. Epicardial/mesothelial cell line retains vasculogenic potential of embryonic epicardium. Circul. Res. 92, 525–531. ( 10.1161/01.RES.0000060484.11032.0B) [DOI] [PubMed] [Google Scholar]
- 91.Schlueter J, Brand T. 2012. Epicardial progenitor cells in cardiac development and regeneration. J. Cardiovasc. Trans. Res. 5, 641–653. ( 10.1007/s12265-012-9377-4) [DOI] [PubMed] [Google Scholar]
- 92.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. 2003. Bone marrow stem cells regenerate infarcted myocardium. Pediatr. Transplant. 7(Suppl 3), 86–88. ( 10.1034/j.1399-3046.7.s3.13.x) [DOI] [PubMed] [Google Scholar]
- 93.van Berlo JH, Molkentin JD. 2014. An emerging consensus on cardiac regeneration. Nat. Med. 20, 1386–1393. ( 10.1038/nm.3764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li T-S, et al. 2010. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells 28, 2088–2098. ( 10.1002/stem.532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marbán L, Luthringer D, Marbán E. 2013. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J. Am. Coll. Cardiol. 61, 1108–1119. ( 10.1016/j.jacc.2012.10.052) [DOI] [PubMed] [Google Scholar]
- 96.Oh H, et al. 2003. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. USA 100, 12 313–12 318. ( 10.1073/pnas.2132126100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H, et al. 2014. Isolation and characterization of a Sca-1+/CD31− progenitor cell lineage derived from mouse heart tissue. BMC Biotechnol. 14, 75 ( 10.1186/1472-6750-14-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takamiya M, Haider KH, Ashraf M. 2011. Identification and characterization of a novel multipotent sub-population of Sca-1+ cardiac progenitor cells for myocardial regeneration. PLoS ONE 6, e25265 ( 10.1371/journal.pone.0025265.s003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fioret BA, Heimfeld JD, Paik DT, Hatzopoulos AK. 2014. Endothelial cells contribute to generation of adult ventricular myocytes during cardiac homeostasis. Cell Rep. 8, 229–241. ( 10.1016/j.celrep.2014.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ. 2009. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat. Protoc. 4, 232–243. ( 10.1038/nprot.2008.229) [DOI] [PubMed] [Google Scholar]
- 101.Zhou S, et al. 2001. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7, 1028–1034. ( 10.1038/nm0901-1028) [DOI] [PubMed] [Google Scholar]
- 102.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. 2005. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circul. Res. 97, 52–61. ( 10.1161/01.RES.0000173297.53793.fa) [DOI] [PubMed] [Google Scholar]
- 103.Noseda M, et al. 2015. PDGFRα demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat. Commun. 6, 6930 ( 10.1038/ncomms7930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. 2005. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circul. Res. 97, 1090–1092. ( 10.1161/01.RES.0000194330.66545.f5) [DOI] [PubMed] [Google Scholar]
- 105.Schaechinger V, et al. 2006. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 355, 1210–1221. ( 10.1056/NEJMoa060186) [DOI] [PubMed] [Google Scholar]
- 106.Wollert KC, et al. 2004. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364, 141–148. ( 10.1016/S0140-6736(04)16626-9) [DOI] [PubMed] [Google Scholar]
- 107.Chen S-L, et al. 2004. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 94, 92–95. ( 10.1016/j.amjcard.2004.03.034) [DOI] [PubMed] [Google Scholar]
- 108.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. 2009. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63. ( 10.1016/j.stem.2009.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. 2011. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell. Cardiol. 50, 280–289. ( 10.1016/j.yjmcc.2010.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nygren JM, et al. 2004. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 10, 494–501. ( 10.1038/nm1040) [DOI] [PubMed] [Google Scholar]
- 111.Duran JM, et al. 2013. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circul. Res. 113, 539–552. ( 10.1161/CIRCRESAHA.113.301202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nowbar AN, et al. 2014. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ 348, g2688 ( 10.1136/bmj.g2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Malliaras K, et al. 2014. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 63, 110–122. ( 10.1016/j.jacc.2013.08.724) [DOI] [PMC free article] [PubMed] [Google Scholar]