Abstract
Ambush foragers use a hunting strategy that places them at risk of predation by both visual and olfaction-oriented predators. Resulting selective pressures have driven the evolution of impressive visual crypsis in many ambushing species, and may have led to the development of chemical crypsis. However, unlike for visual crypsis, few studies have attempted to demonstrate chemical crypsis. Field observations of puff adders (Bitis arietans) going undetected by several scent-orientated predator and prey species led us to investigate chemical crypsis in this ambushing species. We trained dogs (Canis familiaris) and meerkats (Suricata suricatta) to test whether a canid and a herpestid predator could detect B. arietans using olfaction. We also tested for chemical crypsis in five species of active foraging snakes, predicted to be easily detectable. Dogs and meerkats unambiguously indicated active foraging species, but failed to correctly indicate puff adder, confirming that B. arietans employs chemical crypsis. This is the first demonstration of chemical crypsis anti-predatory behaviour, though the phenomenon may be widespread among ambushers, especially those that experience high mortality rates owing to predation. Our study provides additional evidence for the existence of an ongoing chemically mediated arms race between predator and prey species.
Keywords: camouflage, predator–prey arms race, olfaction, scent-matching
1. Background
Successful heterotrophic organisms tread a fine line between finding sufficient food while limiting energy expenditure and avoiding predation. Several successful foraging strategies exist, including, at one ecological extreme, ambush foraging [1–3]. This foraging mode is characterized by a reliance on concealment and stealth to capture prey and avoid predation. Ambush foraging is taxonomically widespread, occurring in many animal taxa as phylogenetically diverse as spiders and felids. It is associated with infrequent movement [2], and usually requires extended periods of immobility while waiting for feeding opportunities [4,5]. Unsurprisingly, typical ambush foragers exhibit a diversity of ecological, morphological and physiological adaptations that maximize fitness in this context [6–8], and tend to be adapted to making a lunge, strike or short, rapid pursuit after passing prey [2]. Many also respond to danger by remaining concealed instead of fleeing from predators [9].
During periods of lying in wait, ambush foragers are themselves at risk of being discovered by predators and, as a consequence, many have evolved extremely effective visual camouflage as a means of avoiding detection [10–12]. However, visual crypsis offers little protection against macrosmatic, scent-oriented predators that use their keen sense of olfaction to locate prey. Among tetrapods, olfaction-oriented species are primarily represented by mammals [13,14], which evolved this characteristic early in their radiation [15], and some lineages of squamates, particularly actively foraging species [16], suggesting that the selective pressures exerted by these animals on their prey have likely been acting over millennia. An individual that remains in a specific location for an extended period of time in ambush should be easily detectable by such predators as it serves as a continuous odour source, irrespective of any visual crypsis.
In their capacity as prey species, ambush foragers would therefore likely be under strong selective pressure to reduce body odour in order to minimize the likelihood of being detected by an olfaction-oriented predator. Although chemical crypsis may provide selective advantage in both the context of avoiding detection by prey and predator animals, the latter is likely more pervasive [17]. Despite the abundance of both prey species that exhibit extended occupancy of a single location and macrosmatic, olfaction-oriented predators, little evidence for such selection exists. Even the frequently quoted example of odourless fawns (e.g. [18]), which remain motionless in response to threat, while supported by anecdotal accounts [19,20], does not appear to have been formally investigated. The scarcity of evidence for odourlessness may be a direct consequence of chemical crypsis being unlikely to evolve, as suggested by Conover [14], given that the by-products of essential metabolic pathways are often odourous.
Viperid snakes (Reptilia: Squamata: Viperidae) represent a diverse monophyletic radiation of over 330 species [21]. Ambush foraging is a strongly conserved ecological trait within the group [10] and is a foraging mode that, in snakes, is synonymous with long bouts of immobility, squat body form, infrequent prey intake, the ability to consume large meals and shut-down of digestive machinery between meals [6–8]. Vipers are abundant in many environments [22–24], and despite venomous defences, many are important prey species for numerous predators [25]. Moreover, natural selection through predation appears to be an important driver in the evolutionary history of the group [26].
The puff adder (Bitis arietans) is a large-bodied, ambush-foraging, viperid snake that is abundant and widespread in Africa [27,28]. Our extensive experience with radio-telemetered puff adders has highlighted that snakes move infrequently and distance travelled is strongly correlated with increased risk of mortality [29]. A wide variety of vertebrate species prey on puff adders (see the electronic supplementary material, table S1) and their visual crypsis appears elaborate. However, at least 15 of the 42 known predators of B. arietans rely on olfaction as their primary hunting modality. In spite of being preyed upon by a broad array of macrosmatic, olfaction-oriented predators, puff adders typically choose to remain motionless in response to approaching danger. Additionally, our observations and intensive videography of wild puff adders suggest that these snakes are not easily detected by canids, mongooses, genets (all of which are known puff adder predators), and some rodent species (e.g. Rattus spp. and Cape porcupine: Hystrix africaeaustralis).
Here, we follow Stevens & Merilaita's [11] definition of crypsis as an umbrella term applicable to any trait that serves to minimize an organism's detection in situations where possibility for detection exists. Based on our observations described above, we hypothesize that puff adders possess a form of chemical crypsis limiting their detection by macrosmatic predators. We investigated this hypothesized crypsis from the predator perspective in representatives of two important predator lineages using specially trained, scent-matching dogs (Canis familiaris) and meerkats (Suricata suricatta) as models. Canids and herpestids are renowned scent-orientated predators and several species include puff adders and other viperid snakes in their diet. Additionally, trained dogs have previously been used to find eastern diamondback rattlesnakes (Crotalus adamanteus [30]), brown tree snakes (Boiga irregularis [31]) and Burmese pythons (Python bivittatus [32]). We predicted that both dogs and meerkats would have difficulty in detecting puff adder scent, despite a demonstrable capacity to recognize the scent of snakes that forage actively and for which the potential selective pressures acting on chemical crypsis are presumed to be off-set by the ‘moving target’ effect.
2. Material and methods
Our approach was to ask dogs and meerkats, using positive reinforcement, to recognize cotton cloths scented with the smell of various species of snakes (captive and radio-telemetered wild snakes for dogs, captive snakes for meerkats), from a line-up including both blank controls (cloths washed but not scented on any surface), and appropriate environmental (cloths scented on vegetation or washed terraria) controls. Including both environmental and blank controls in our design allowed us to test for effects of our scenting protocol, and to assess the relative importance of chemical background matching as a mechanism of crypsis. For our dog model, odour detectability was assessed for eight treatment groups: (i) free-ranging and (ii) captive puff adders, (iii) captive rhombic night adder (Causus rhombeatus: an active foraging viperid) and (iv–vii) four species of captive active foraging colubrid snakes (brown house snake: Boaedon capensis, aurora house snake: Lamprophis aurora, common file snake: Gonionotophis capensis and corn snake: Pantherophis guttatus). In addition to this, scent was also collected off (viii) freshly shed skin from a captive puff adder (table 1). Dogs clearly detected all active foraging snake species and demonstrated no differences in detectability between captive and free-ranging puff adders. We therefore only exposed our meerkat model to brown house snake and captive puff adder targets (and their controls). The geographical distributions of puff adder and brown house snake are broadly sympatric with that of meerkat.
Table 1.
Snake species assessed for chemical crypsis using scent-matching dogs.
| species | common name | no. of scent donors | family | hunting strategy | prediction |
|---|---|---|---|---|---|
| Bitis arietans | puff adder | 8 | Viperidae | ambusher | cryptic |
| Causus rhombeatus | rhombic night adder | 2 | Viperidae | active forager | non-cryptic |
| Boaedon capensis | brown house snake | 8 | Lamprophiidae | active forager | non-cryptic |
| Lamprophis aurora | aurora house snake | 1 | Lamprophiidae | active forager | non-cryptic |
| Gonionotophis capensis | common file snake | 2 | Lamprophiidae | active forager | non-cryptic |
| Pantherophis guttatus | corn snake | 1 | Colubridae | active forager | non-cryptic |
(a). Scent collection, cloth storage and handling
Cotton scent-cloths (200 × 200 mm) were collectively prepared in a single batch. All cloth-handling was performed using latex gloves and metal tongs cleaned in chemically pure 99% hexane [33] to avoid contamination with alternative non-target scents. After being machine-washed with mild detergent (Surf, Unilever, South Africa) and rinsed 10 times in boiling water to remove scent, cloths were tumbled-dried and then stored in sealed glass jars (also cleaned with hexane) before being used to capture target or environmental control scents.
Cloths were placed in direct contact with the donor (target, decoy or environmental control) for a period of 40 min, after which they were removed and stored in donor-specific glass jars (volume: 750 ml) at room temperature for use during trials or training sessions no later than 3 days post-collection. Prior to the collection of snake scents from captive donors, snakes were removed from their glass-fronted terraria (dimensions: 400 × 400 × 750 mm), which were then emptied of all fittings, thoroughly washed with soap and cleaned using hexane. Environmental control scents were collected from these empty terraria prior to the snakes being returned, and target scents were collected from the snakes through direct contact once they were returned to their newly cleaned terraria. For wild donors, control cloths were scented off nearby (≈ 5 m from a telemetered puff adder) vegetation. Cloths were considered blank controls if they were not used to capture any scent.
(b). Dog scent-matching protocol
Using positive reinforcement through the use of food- or toy-rewards, we trained four owner-handled pet dogs indoors for a maximum of 2 h once a week, over a three-month period, to accurately match dynamic target scents. Dogs were presented with the target scent, before being asked to find its matching equivalent in a six-option scent lineup. Lineups were presented to the dogs in a scent-wheel: a 0.6 m-high circular fence (ø: 0.75 m; figure 1 insert) to which six lidless glass jars containing one scent sample each, spaced equidistantly, were attached on its outside edge. A wire mesh screen to prevent direct contact from dogs surrounded each jar. Each scent lineup consisted of one target, three environmental controls and two blank controls. To indicate a match, dogs either sat or downed (dog-dependent) in front of the selected sample. In general, dogs investigated all options before making their indication, and thus their accuracy was calculated using their first indications only. Dogs qualified as ‘scent-matchers' if they were eventually able to maintain their individual accuracy at greater than or equal to 80% during training sessions, which was typically achieved after 10–12 training sessions. Testing procedures followed a double-blind methodology; neither the dog nor the handler were privy to scent order within lineups to ensure that dogs were not indicating the correct cloth based on visual memory or cues from their handlers.
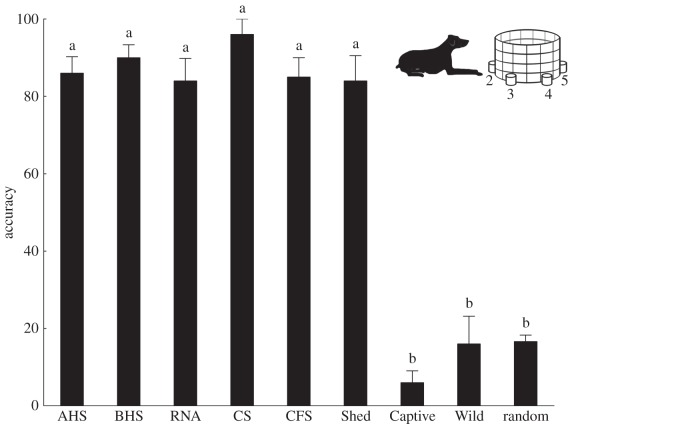
Figure 1.
Combined first-attempt indication accuracy of scent-matching dogs (n = 4) on the scents of five species of active foraging snakes and the puff adder (Bitis arietans) and its shed skin. All accuracy scores for active foraging snakes and puff adder shed (Shed) are significantly different from random chance, while accuracy scores for scent from both wild and captive puff adder (B. arietans) are not. AHS, aurora house snake (Lamprophis aurora); BHS, brown house snake (Boaedon capensis); RNA, rhombic night adder (Causus rhombeatus); CS, corn snake (Pantherophis guttatus); CFS, common file snake (Gonionotophis capensis); Captive and Wild, captive and wild, free-ranging puff adder (Bitis arietans). Error bars represent standard error and shared lettering indicates non-significant differences among samples.
All four scent-matching dogs were used to evaluate the chemical crypsis of each of the seven snake species and the puff adder shed during indoor, scent-matching trials. With the exception of common file snake scent, dogs were asked to perform 10 five-match sets per active foraging species and per puff adder scent type (i.e. captive, wild and shed skin). Fifty matches for each treatment (bar one) were therefore performed; dogs were only able to perform a total of 16 matches for the common file snake treatment owing to limited access to this secretive species [34].
(c). Meerkat scent-matching protocol
Five habituated, but not tame meerkats located at Monte Bird Gardens, South Africa, were trained to scent-match using positive reinforcement in the form of mealworm (Tenebrio molitor) and Madagascar hissing cockroach (Gromphadorhina portentosa) rewards. Training was conducted outdoors, for a maximum of 30 min per meerkat, twice a week over a two-month period. As with the dogs, testing procedures followed a double-blind methodology, and meerkats were asked to find a target scent among a six-option scent lineup. Six plastic test tubes (inside ø: 23 mm; length: 145 mm), each containing a scent sample, were attached, 300 mm apart from the next, to a melamine board (width: 380 mm, length: 1.85 m; figure 2, top insert). To prevent the meerkats from gaining access to the scent samples, test tubes were capped using plastic lids, each with a 6 mm hole drilled into it. Two additional 6 mm holes were drilled into the tubes themselves to allow sufficient airflow for scenting purposes (figure 2). Meerkats were trained to smell each option and indicate a ‘match’ by scratching on the matching tube. Each scent lineup consisted of one target, one environmental control and four blank control samples, and meerkats indicated on a sample-by-sample basis, resulting in a multi-indication design. Thus, during training and testing all indications were recorded. Given that repeated indications on targets in a multi-sample, multi-indication design can be an artefact of indiscriminate indications rather than accuracy [35,36], meerkats only qualified as ‘scent-matchers' if they met the Scientific Working Group for Dogs and Orthogonal detector Guidelines (SWGDOG) criteria [37], where correct identifications are maintained above 90% and incorrect below 10%. In general, meerkats required 10 training sessions each to achieve SWGDOG criteria, and all five scent-matching meerkats were used to evaluate the chemical crypsis of brown house snake (B. capensis) and captive puff adder during outdoor, scent-matching trials. For each snake species, meerkats were asked to perform five 10-match sets (ntotal = 50), during which all indications were recorded.
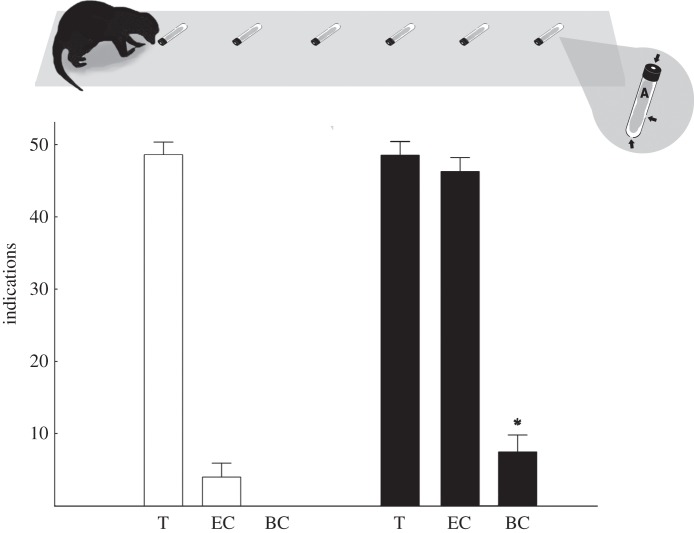
Figure 2.
Total number of indications by scent-matching meerkats (Suricata suricatta; n = 5) made on targets (T), environmental controls (EC) and blank controls (BC) for brown house snake (Boaedon capensis; clear bars) and puff adder (Bitis arietans; black bars) treatments. For brown house snake, indications on targets differed significantly from random choice and from indications on environmental controls. Indications on environmental controls were not significantly different from random choice. No blanks were indicated. For puff adder, indications on targets and environmental controls were not significantly different from each other, but were from indications made on blank controls (indicated by *). All indications differed significantly from random choice. Error bars represent standard error. Arrows in insert indicate the position of the holes made in the tubes and lids to allow for sufficient airflow for scenting purposes, and ‘A’ demonstrates the position of the scent cloth within each tube.
(d). Statistical analyses
(i). Dog scent-matching protocol
Each dog's accuracy was calculated based on their overall percentage of correct first indications in each five-run trial for each treatment group. These data were then arcsine-transformed, and tested against a random-choice model (i.e. a dataset of 50 random samples with replacement from a selection of six options: one target, three controls, two blanks) using a 2-factor ANOVA where dog and treatment were coded as factors. Significant differences among dogs and treatment groups were identified using Tukey HSD post-hoc test. Errors on environmental and blank controls for captive and wild puff adder treatments were analysed using χ2, and owing to differences here we investigated the error of detection by comparing the ratio of environmental and blank control errors to a null model in which errors occurred at a ratio of 3 : 2 to test the hypothesis that dogs were making errors randomly. Since errors were significantly biased in favour of environmental controls, we simulated a dataset of 50 random samples with replacement from a selection of four options (one target, three controls) and tested whether detection differed from random choice.
(ii). Meerkat scent-matching protocol
The total number of indications on target, environmental control and blank control cloths made by each meerkat within treatments were analysed using χ2. These indications were tested against a random-choice model that considered the average number of indications made by each meerkat within a treatment and the frequency at which the scent options (i.e. target versus environmental versus blank controls) occurred.
All statistical analyses were performed using either Statistica v. 8 (STATISTICA Data Analysis Software System 2001, http://www.statsoft.com) or SPSS v. 23 (IBM SPSS Statistics 2015, http://www.spss.co.in). Means ± s.e. accuracy are reported throughout.
3. Results
(a). Dog scent-matching protocol
Individual dogs had no effect on the outcome of results (2-factor ANOVA: F3,83 = 1.21, p = 0.31), while treatment did (2-factor ANOVA: F7,83 = 20.99, p < 0.001). Dogs correctly indicated scents from all active foraging snakes with greater than 80% accuracy, which is significantly better than chance (Tukey HSD, p < 0.001 for all comparisons). However, they failed to indicate either wild (mean accuracy ± s.e.: 16.0 ± 5.2%) or captive (mean accuracy ± s.e.: 6.0 ± 3.4%) puff adder scent correctly at an accuracy level different from chance (figure 1; mean accuracy ± s.e.: 16.6 ± 5.2%; Tukey HSD: Pwild ≈ 1, Pcaptive = 0.99). For the active foraging snake scents, accuracy ranged from 96.0 ± 4.0% (mean ± s.e.) for corn snake scent to 81.3 ± 10.1% for common file snake scent. We detected no significant differences among the five active foraging species tested (Tukey HSD: p > 0.05 for all comparisons), but all were significantly different from puff adders (Tukey HSD: p < 0.001). Despite failing to correctly indicate wild or captive puff adder scents at accuracy levels significantly different from chance, dogs located the scent of puff adder shed skin with high accuracy (mean ± s.e: 84.0 ± 5.2%, Tukey HSD: p < 0.001).
Errors made by the dogs when attempting to detect puff adder scent were not evenly distributed between environmental and blank controls. Dogs incorrectly indicated environmental control cloths disproportionately (ncaptive = 45; nwild = 37) more frequently than blank controls (ncaptive = 2; nwild = 5) in both captive (χ2 = 15.1, d.f. = 2, p < 0.001) and wild puff adders (χ2 = 8.87, d.f. = 2, p = 0.003). In the light of this finding, we tested the dogs' accuracy of identifying captive and wild puff adder scent against a one-in-four random model (n = 50, mean ± s.e.: 20.0 ± 5.96%). Accuracy remained non-significantly different from random (Tukey HSD: Pcaptive = 0.59; Pwild ≈ 1.00), while all other snake species and puff adder shed were different (Tukey HSD: p < 0.001).
(b). Meerkat scent-matching protocol
For brown house snake, meerkats indicated 53 tubes as matches, of which 49 contained targets (92.5%) and four contained environmental controls (7.6%). No blank controls were indicated as matches (figure 2). Individual meerkats had no effect on the outcome within this treatment (χ2 = 0.175, d.f. = 4, p = 0.996), with all meerkats indicating targets at frequencies significantly greater than would be expected by chance (χ2 = 40.39, d.f. = 5, p < 0.001). Environmental controls were however indicated at frequencies non-significantly different from those expected by chance (χ2 = 7.999, d.f. = 5, p = 0.156). These results clearly demonstrate their capacity to detect brown house snake using olfaction.
For puff adder, individual meerkats had no effect on the outcome (χ2 = 5.301, d.f. = 5, p = 0.380). One hundred and four tubes were indicated as matches, 49 of which contained target cloths, 47 environmental controls and only eight blank controls. Both targets and environmental controls were indicated significantly more frequently than would be expected by chance (target: χ2 = 40.393, d.f. = 5, p < 0.001; environmental controls: χ2 = 32.550, d.f. = 5, p < 0.001). Indications on target and environmental controls were however not significantly different from each other (χ2 = 0.089, d.f. = 1, p = 0.372). Blank controls were indicated significantly less frequently than target and environmental controls (targets: χ2 = 74.707, d.f. = 1, p < 0.001; environmental controls: χ2 = 65.047, d.f. = 1, p < 0.001) at rates lower than would be expected by chance (χ2 = 33.369, d.f. = 5, p < 0.001). These results demonstrate that the meerkats were actively selecting both target and environmental control options at equal rates, while actively avoiding blank controls. Meerkats were therefore able to clearly distinguish between scented options and blanks, but were unable to discern between targets and environmental controls, and clearly show that puff adders are chemically cryptic to this herpestid predator.
4. Discussion
Dogs and meerkats were able to repeatedly and accurately detect the scent of all respective experimental snakes except puff adders, irrespective of whether scent samples were drawn from wild or captive individuals. The remarkable and stark difference in detectability between puff adders and other snake species provides strong evidence for our hypothesis of chemical crypsis in puff adders, and given that its detectability remained unchanged under different scenarios (i.e. wild versus captive), the underlying mechanism is unlikely to be one of mimicry. This is, to our knowledge, the first evidence of the employment of chemical crypsis by a vertebrate organism as a defence against detection by macrosmatic predators, and the first example of such crypsis in a terrestrial vertebrate.
Our findings are at odds with Conover's [14] prediction that chemical crypsis is unlikely to have evolved. However, Vermeij's hypothesis states that the greatest selective agents acting on an organism are those imposed by its own predators [17], making run-away selection for an adaptation that provides even incremental improvement on survival commonplace in systems with high predation rates. Our own radio-telemetry studies of wild puff adders have shown that animals from our study population exhibit very low estimated annual survival rates (males: 43–58%; females: 50–63%; G. J. Alexander 2011, unpublished data), with a large number of telemetered animals falling prey to one of several species of predator during the study [29]. In this context, adaptations that provide even minor reductions to the production of metabolic volatiles (either from the organism itself or its microbiota) or their persistence in the air plume are likely to be strongly selected.
Molecules become volatile when their molecular weights are less than 300 and their vapour pressure is greater than 1.33 Pa at ambient temperatures [38]. However, more heavily weighted odour molecules will drop out of the air plume sooner [14], thereby decreasing the potential of their detection. Such odour manipulation is not without precedent: the composition of preening wax in some species of birds shifts towards a less volatile, heavier molecular weight during periods of breeding and incubation [39]. Similarly, reducing the production and subsequent release of metabolic odourants to levels below a detectable threshold, even if only temporarily, may also make detection difficult [14], and may well be the driving force behind temporary bradycardia, bradypnea and even aponea (e.g. [40–42]) seen in many taxa in response to perceived threat.
Some ambush-foraging viperids are known to have lower field metabolic rates than active foraging snake species [8]. As such, it is likely that puff adders also exhibit relatively low metabolic rates, although this remains to be tested. Low metabolic rate could result in reduced production of metabolically derived odourants, providing the basis for selection should those reductions result in even small increases in crypsis. In this context, chemical crypsis could be relatively widespread among ambush-foraging species that experience high predation rates from macrosmatic predators. This metabolic suppression hypothesis also raises the possibility that body temperature may have a direct impact on a puff adder's detectability, since warmer snakes would have higher metabolic rates [43,44], resulting in increased volatile metabolite production. If this were the case, high body temperatures represent an odour-detectability cost in the cost/benefit ratio of thermoregulation (sensu [45,46]) that has not previously been considered.
Despite our dogs not being able to detect puff adder scent, they were easily able to detect the scent of recently shed puff adder skin. This finding suggests that scent collected from shed skin may not always serve as a suitable proxy for the body odour of snakes, and we caution against making this assumption in experiments (e.g. [47]). However, the high detectability of shed skins is particularly relevant in the context of two important field observations of telemetered free-ranging puff adders: puff adders always moved to new lie-up positions following shedding; puff adders typically defecated at the site of shedding before moving. These observations suggest that puff adders consolidate the production of shed skin and faeces, both of which would serve as chemical-beacons to macrosmatic predators and prey species. Our ‘odour products consolidation hypothesis' provides an alternative explanation to that of Lillywhite et al.'s [48] ‘adaptive ballast’ hypothesis as to why ambush-foraging species often retain faeces for long periods of time. For this hypothesis, Lillywhite et al. [48] argue that ambushing snakes retain their faeces to provide additional mass as ballast against which to strike. The much shorter retention times reported by Lillywhite et al. [48] for arboreal species is also easily explained by our hypothesis since the faeces of arboreal snakes would generally fall to the ground and thus not act as scent beacon indicating the location of the snake.
Avoiding detection by prey animals may synergistically provide selection for chemical crypsis in ambush predators through increased hunting success and as an escape from prey defensive attacks. Resetarits & Binckley [49] showed that pirate perch (Aphredoderus sayanus) remain undetected by several species of aquatic beetles on which they feed, and several species of reptiles use chemical mimicry to gain unchallenged access to ant colonies on which they feed [50]. The detectability of puff adder scent by prey species remains to be tested; however, our video footage of rodents remaining seemingly unaware of ambushing puff adders despite being in direct contact with the snakes is highly suggestive that they are chemically cryptic towards at least some prey species.
It remains unclear how ubiquitous chemical crypsis may be in viperid snakes. Warner [51] used a scent dog trained to detect snakes to search for closely related gaboon adders (Bitis gabonica) with no success, suggesting that they too may be cryptic and that chemical crypsis may be more widespread in this African genus. However, we have shown that the active foraging, and ecologically derived [52] C. rhombeatus does not appear to exhibit chemical crypsis and the fact that dogs are used to locate C. adamanteus [30] suggests that chemical crypsis is not necessarily widespread among viperids. The apparent absence of chemical crypsis in Crotalus may stem from the comparatively low number of predatory species [30] that prey on Crotalus, and because these snakes take an active approach to warning off potential predators. Further investigations should focus on other African vipers (specifically other Bitis and Echis), as well as Asian viperids (e.g. Daboia) that are likely to experience high predation rates because of the diverse macrosmatic, scent-orientated predatory communities in those regions.
Ruxton [53] concluded that even though the investigation of crypsis has been applied almost exclusively to visual systems in biology, the concept can be applied to many other modalities (e.g. sound, olfaction, electrical fields, pressure change and vibration). The bias for vision comes primarily from the fact that this modality is the predominant human sense [53]. Because olfaction is often a largely unconscious process in humans [54], perceptual dimensions of odours are not well understood and olfaction lacks an intrinsic spatial topology in comparison to other modalities [55]. This has resulted in the importance of olfaction being ignored in many systems. The power of using dogs as tools to augment research in the field of ecology, and specifically chemical ecology, should not be underestimated. Their use in forensics as scent-matchers is well established [56,57], but few studies have applied this ability within an ecological framework (e.g. [58,59]). Through their use, we have demonstrated that olfaction and chemical crypsis can be investigated in a scientific setting. Although headspace analyses can provide important insight into chemical crypsis by revealing the volatiles associated with species, and is considered the next step within this ongoing investigation, in isolation these data do not necessarily consider ecological influences given that odour perception is receptor-driven [60], and varied across species [61]. Furthermore, we contend that chemical crypsis is likely to be a far more important autecological trait of many species, especially for predator and prey species that spend extended periods immobile. Future investigations should consider the implications for such crypsis on the natural history, ecology and evolution of such organisms.
Supplementary Material
Supplementary Material
Acknowledgements
Maria Branco, Marietjie Coetzee and Louise Kaye-Eddie and their dogs gave of their time so selflessly. Peter Place Scouts Group allowed us to train and work our dogs at their indoor facilities. Dinokeng Game Reserve and Kwalata Game Ranch allowed us access to their property. Chris Cooke and Monte Bird Gardens allowed us access to their meerkat colony.
Ethics
Experimental protocols were cleared by the University of the Witwatersrand's Animal Ethics Committee under permit number 2011/26/2A. Animals were collected under research permit CPF6 No. 0024.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.5nf45.
Authors' contributions
A.K.M.: Experimental design, animal training, data collection, statistical analyses and manuscript preparation; B.M.: puff adder predation review, statistical analyses and manuscript preparation; S. M.: dog training and dog protocol data collection; X.G.: scent sample collection from and videography of free-ranging puff adders; G.J.A.: concept development, experimental design and manuscript preparation.
Competing interests
We declare we have no competing interests.
Funding
Funds were provided by the University of the Witwatersrand.
References
- 1.Pianka ER. 1966. Convexity, desert lizards, and spatial heterogeneity. Ecology 47, 1055–1059. ( 10.2307/1935656) [DOI] [Google Scholar]
- 2.Huey RB, Pianka ER. 1981. Ecological consequences of foraging mode. Ecology 62, 991–999. ( 10.2307/1936998) [DOI] [Google Scholar]
- 3.Perry G, Pianka ER. 1997. Animal foraging: past, present and future. Trends Ecol. Evol. 12, 360–364. ( 10.1016/S0169-5347(97)01097-5) [DOI] [PubMed] [Google Scholar]
- 4.Grobecker DB. 1983. The ‘lie-in-wait’ feeding mode of a cryptic teleost, Synanceia verrucosa. In Predators and prey in fishes (eds Noakes DLG, Lindquist DG, Helfman GS, Ward JA), pp. 29–40. Dordercht, The Netherlands: Springer. [Google Scholar]
- 5.Clark RW. 2006. Fixed videography to study predation behavior of an ambush foraging snake, Crotalus horridus. Copeia 2006, 181–187. ( 10.1643/0045-8511%282006%296%5B181%3AFVTSPB%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 6.Secor SM, Nagy KA. 1994. Bioenergetic correlates of foraging mode for the snakes Crotalus cerastes and Masticophis flagellum. Ecology 75, 1600–1614. ( 10.2307/1939621) [DOI] [Google Scholar]
- 7.Secor SM. 1995. Ecological aspects of foraging mode for the snakes Crotalus cerastes and Masticophis flagellum. Herpetol. Monogr. 9, 169–186. ( 10.2307/1467004) [DOI] [Google Scholar]
- 8.Secor SM, Diamond J. 1998. A vertebrate model of extreme physiological regulation. Nature 395, 659–662. ( 10.1038/27131) [DOI] [PubMed] [Google Scholar]
- 9.Maritz B. 2012. To run or hide?: Escape behaviour in a cryptic African snake. Afr. Zool. 47, 270–274. ( 10.3377/004.047.0215) [DOI] [Google Scholar]
- 10.Greene HW. 1997. Snakes: the evolution of mystery in nature. Berkley and Los Angeles, CA: University of California Press. [Google Scholar]
- 11.Stevens M, Merilaita S. 2009. Animal camouflage: mechanisms and function. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Spinner M, Kovalev A, Gorb SN, Westhoff G. 2013. Snake velvet black: hierarchical micro- and nanostructure enhances dark colouration in Bitis rhinoceros. Sci. Rep. 3, 1846 ( 10.1038/srep01846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenberg JF. 1981. The mammalian radiations: an analysis of trends in evolution, adaptation, and behavior. Chicago, IL: University of Chicago Press. [Google Scholar]
- 14.Conover MR. 2007. Predator–prey dynamics: the use of olfaction. Boca Raton, FL: Taylor and Francis. [Google Scholar]
- 15.Apfelbach R, Blanchard C, Blanchard R, Hayes R, McGregor I. 2005. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144. ( 10.1016/j.neubiorev.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 16.Zug GR, Vitt LJ, Caldwell JP. 2001. Herpetology: an introductory biology of the amphibians and reptiles. San Diego, CA: Academic Press. [Google Scholar]
- 17.Dietl GP, Kelley PH. 2002. The fossil record of predator–prey arms races: coevolution and escalation hypotheses. Paleontol. Soc. Pap. 8, 353–374. [Google Scholar]
- 18.Panzacchi M, Linnell J, Odden M, Odden J, Andersen R. 2009. Habitat and roe deer fawn vulnerability to red fox predation. J. Anim. Ecol. 78, 1124–1133. ( 10.1111/j.1365-2656.2009.01584.x) [DOI] [PubMed] [Google Scholar]
- 19.Johnson DE. 1951. Biology of the Elk Calf, Cervus canadensis nelsoni. J. Wildlife Manag. 15, 396–410. ( 10.2307/3796583) [DOI] [Google Scholar]
- 20.Lent PC. 1974. Mother–infant relationships in ungulates. In The behavior of ungulates and its relation to management (eds Geist V, Walther F), pp. 14–55. Morges, Switzerland: International Union for the Conservation of Nature and Natural Resources. [Google Scholar]
- 21.Uetz P. 2015. The reptile database. See http://www.reptile-database.org (accessed October, 2015).
- 22.Trape JF, Pison G, Guyavarch E, Mane Y. 2001. High mortality from snakebite in south-eastern Senegal. Trans. R. Soc. Trop. Med. Hyg. 95, 420–423. ( 10.1016/S0035-9203(01)90202-0) [DOI] [PubMed] [Google Scholar]
- 23.Jones TR, Babb RD, Hensley FR, LiWanPo C, Sullivan BK. 2011. Sonoran Desert snake communities at two sites: concordance and effects of increased road traffic. Herpetol. Conserv. Biol. 6, 61–71. [Google Scholar]
- 24.Maritz B, Alexander GJ. 2012. Population density and survival estimates of the African viperid, Bitis schneideri. Herpetologica 68, 195–202. ( 10.1655/HERPETOLOGICA-D-11-00043.1) [DOI] [Google Scholar]
- 25.Branch WR. 1998. Field guide to snakes and other reptiles of southern Africa. Cape Town, South Africa: Struik Publishers. [Google Scholar]
- 26.Greene HW. 1992. The ecological and behavioral context for pitviper evolution. In Biology of the pitvipers (eds Campbell JA, Brodie ED Jr), pp. 107–118. Tyler, TX: Selva. [Google Scholar]
- 27.Spawls S, Branch WR. 1995. The dangerous snakes of Africa: natural history, species directory, venoms and snakebite. London, UK: Blandford Press. [Google Scholar]
- 28.Phelps T. 2010. Old World Vipers, a natural history of the azemiopinae and viperinae. Frankfurt, Germany: Edition Chimaira. [Google Scholar]
- 29.Alexander GJ, Maritz B.2011. Predation risk in relation to behaviour in Bitis arietans: does it pay to keep your head down? In 10th Herpetological Association of Africa Conf., p. 8, 11–14 January 2011, Cape Town, South Africa. Cape Town, South Africa: Herpetological Association of Africa.
- 30.Klauber LM. 1956. Rattlesnakes: their habits, life histories, and influence on mankind (vol. 1). Berkeley, CA: University of California Press. [Google Scholar]
- 31.Engeman RM, Vice DS, Rodriguez DV, Gruver KS, Santos WS, Pitzler ME. 1998. Effectiveness of the detector dogs used for deterring the dispersal of brown tree snakes. Pac. Conserv. Biol. 4, 256–260. [Google Scholar]
- 32.Dorcas ME, Willson JD. 2011. Invasive pythons in the United States: ecology of an introduced predator. Athens, GA: The University of Georgia Press. [Google Scholar]
- 33.Mason RT, Fales HM, Jones TH, Pannell LK, Chinn JW, Crews D. 1989. Sex pheromones in snakes. Science 245, 290–293. ( 10.1126/science.2749261) [DOI] [PubMed] [Google Scholar]
- 34.Alexander GA, Maritz B. 2015. Sampling interval affects the estimation of movement parameters in four species of African snakes. J. Zool. ( 10.1111/jzo.12280) [DOI] [Google Scholar]
- 35.Helton WS. 2009. Canine ergonomics: the science of working dogs. London, UK: CRC Press. [Google Scholar]
- 36.Miller AK, Hensman M, Hensman S, Schultz K, Reid P, Shore M, Brown J, Furton K, Lee S. 2015. African elephant (Loxodonta africana) can detect TNT using olfaction: implications for biosensor application. J. Appl. Anim. Behav. Sci. 171, 177–183. ( 10.1016/j.applanim.2015.08.003) [DOI] [Google Scholar]
- 37.Furton KG, Holness H. 2010. The Scientific Working Group on Dog and Orthogonal Detector Guidelines (SWGDOG). National Criminal Justive Reference Service, 155 Rockville, MD: US Dept of Justice. [Google Scholar]
- 38.Rawson NE. 2000. Human olfaction. In The neurobiology of taste and smell, 2nd edn (eds Finger TE, Silver WL, Restrepo D), pp. 257–284. New York, NY: Wiley-Liss. [Google Scholar]
- 39.Reneerkens J, Piersma T, Damsté JS. 2005. Switch to diester preen waxes may reduce avian nest predation by mammalian predators using olfactory cues. J. Exp. Biol. 208, 4199–4202. ( 10.1242/jeb.01872) [DOI] [PubMed] [Google Scholar]
- 40.Smith EN, Sweet DJ. 1980. Effect of atropine on the onset of fear bradycardia in eastern cottontail rabbits (Sylvilagus floridanus). Comp. Biochem. Physiol. C Comp. Pharmacol. 66, 239–241. ( 10.1016/0306-4492(80)90133-1) [DOI] [PubMed] [Google Scholar]
- 41.Causby LA, Smith EN. 1981. Control of fear bradycardia in the swamp rabbit, Sylvillagus aquaticus. Comp. Biochem. Physiol. C Comp. Pharmacol. 69, 367–370. ( 10.1016/0306-4492(81)90151-9) [DOI] [PubMed] [Google Scholar]
- 42.Gabrielsen GW, Smith EN. 1985. Physiological responses associated with feigned death in the American opossum. Acta Physiol. Scand. 123, 393–398. ( 10.1111/j.1748-1716.1985.tb07605.x) [DOI] [PubMed] [Google Scholar]
- 43.Bennett AF, Dawson WR. 1976. Metabolism. In Biology of the reptilia, physiology A, vol. 5 (eds Gans C, Dawson WR), pp. 127–223. London, UK: Academic Press. [Google Scholar]
- 44.Randall D, Burggren W, French K. 1997. Eckert animal physiology: mechanisms and adaptations. New York, NY: W.H. Freeman and Company. [Google Scholar]
- 45.Huey RB, Slatkin M. 1976. Costs and benefits of lizard thermoregulation. Q. Rev. Biol. 51, 363–384. ( 10.1086/409470) [DOI] [PubMed] [Google Scholar]
- 46.Huey RB. 1974. Behavioral thermoregulation in lizards: importance of associated costs. Science 184, 1001–1003. ( 10.1126/science.184.1001) [DOI] [PubMed] [Google Scholar]
- 47.Phillips MA, Waterman JM, Ebensperger L. 2013. Olfactory snake-predator discrimination in the Cape Ground Squirrel. Ethology 119, 278–285. ( 10.1111/eth.12059) [DOI] [Google Scholar]
- 48.Lillywhite HB, de Delva P, Noonan BP. 2002. Patterns of gut passage time and the chronic retention of faecal mass in viperid snakes. In Biology of the vipers (eds Schuett GW, Höggren M, Douglas ME, Greene HW), pp. 497–506. Eagle Mountain, UT: Eagle Mountain Publishing. [Google Scholar]
- 49.Resetarits W, Binckley C. 2013. Is the pirate really a ghost? Evidence for generalized chemical camouflage in an aquatic predator, Pirate Perch Aphredoderus sayanus. Amer. Nat. 181, 690–699. ( 10.1086/670016) [DOI] [PubMed] [Google Scholar]
- 50.Mason MC, Alexander GJ. 1996. Oviposition site selection in Tetradactylus africanus africanus: a relationship with the ant Anochetus faurei. Afr. J. Herpetol. 45, 31–37. ( 10.1080/21564574.1996.9649960) [DOI] [Google Scholar]
- 51.Warner JK. 2009. Conservation biology of the gaboon adder (Bitis gabonica) in South Africa. Unpublished MSc thesis, University of the Witwatersrand, Joahnnesburg, South Africa.
- 52.Ineich I, Bonnet X, Shine R, Shine T, Brischoux F, Lebreton M, Chirio L. 2006. What, if anything, is a ‘typical’ viper? Biological attributes of basal viperid snakes (genus Causus Wagler, 1830). Biol. J. Linnean Soc. 89, 575–588. ( 10.1111/j.1095-8312.2006.00690.x) [DOI] [Google Scholar]
- 53.Ruxton GD. 2009. Non-visual crypsis: a review of the empirical evidence for camouflage to senses other than vision. Phil. Trans. R. Soc. B 364, 549–557. ( 10.1098/rstb.2008.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sela L, Sobel N. 2010. Human olfaction: a constant state of change-blindness. Exp. Brain Res. 205, 13–29. ( 10.1007/s00221-010-2348-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auffarth B. 2013. Understanding smell—the olfactory stimulus problem. Neurosci. Biobehav. Rev. 37, 1667–1679. ( 10.1016/j.neubiorev.2013.06.009) [DOI] [PubMed] [Google Scholar]
- 56.Settle RH, Sommerville BA, McCormick J, Broom DM. 1994. Human scent matching using specially trained dogs. Anim. Behav. 48, 1443–1448. ( 10.1006/anbe.1994.1380) [DOI] [Google Scholar]
- 57.Schoon GAA. 1997. Scent identifications by dogs (Canis familiaris): a new experimental design. Behaviour 134, 531–550. ( 10.1163/156853997X00511) [DOI] [Google Scholar]
- 58.Wasser SK, Davenport B, Ramage ER, Hunt KE, Parker M, Clarke C, Stenhouse G. 2004. Scat detection dogs in wildlife research and management: application to grizzly and black bears in the Yellowhead Ecosystem, Alberta, Canada. Can. J. Zool. 82, 475–492. ( 10.1139/z04-020) [DOI] [Google Scholar]
- 59.Kerney LL, Salkina GP. 2007. Using scent-matching dogs to identify individual Amur Tigers from scats. J. Wildlife Manag. 71, 1349–1356. ( 10.2193/2006-361) [DOI] [Google Scholar]
- 60.Rouquier S, Blancher A, Giorgi D. 2000. The olfactory receptor gene repertoire in primates and mouse: evidence for reduction of the functional fraction in primates. Proc. Natl Acad. Sci. USA 97, 2870–2874. ( 10.1073/pnas.040580197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niimura Y, Matsui A, Touhara K. 2014. Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res. 24, 1485–1496. ( 10.1101/gr.169532.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.5nf45.