Abstract
Accumulating evidence indicates that species interactions such as competition and predation can indirectly alter interactions with other community members, including parasites. For example, presence of predators can induce behavioural defences in the prey, resulting in a change in susceptibility to parasites. Such predator-induced phenotypic changes may be especially pervasive in prey with discrete larval and adult stages, for which exposure to predators during larval development can have strong carry-over effects on adult phenotypes. To the best of our knowledge, no study to date has examined possible carry-over effects of predator exposure on pathogen transmission. We addressed this question using a natural food web consisting of the human malaria parasite Plasmodium falciparum, the mosquito vector Anopheles coluzzii and a backswimmer, an aquatic predator of mosquito larvae. Although predator exposure did not significantly alter mosquito susceptibility to P. falciparum, it incurred strong fitness costs on other key mosquito life-history traits, including larval development, adult size, fecundity and longevity. Using an epidemiological model, we show that larval predator exposure should overall significantly decrease malaria transmission. These results highlight the importance of taking into account the effect of environmental stressors on disease ecology and epidemiology.
Keywords: Anopheles coluzzii, carry-over effects, host–parasite and predator–prey interactions, life history, non-consumptive effects, Plasmodium falciparum
1. Introduction
Disease ecologists increasingly realize that community structure and species interactions influence the intensity of epidemics in wildlife populations [1,2]. Among species interactions, special attention has recently been devoted to predation and its possible role in disease dynamics. In particular, both theoretical and empirical studies have demonstrated that predators can indirectly affect pathogen transmission through changes in host abundance (i.e. density-mediated effects) [1,3]. However, besides direct consumptive effects, the mere presence of predators in the environment can exert non-consumptive effects on prey with important consequences for the functioning of ecological communities [4,5].
The non-consumptive effects of predators are widespread [6] and include changes in prey development, morphology, physiology and behaviour [7,8]. These phenotypic responses to predation risk are costly and can alter the nature of the interactions with other species, such as pathogens [9]. Predator exposure can modify the key traits that govern host–pathogen interactions through trade-offs between anti-pathogen and anti-predator defences. First, exposure to predation can reduce immune response making prey more vulnerable to pathogens [10]. For example, sparrows exposed to a cat had a significant reduction in T-cell response compared with sparrows that were exposed to a rabbit [10]. Second, predation risk may also elicit behavioural changes in ways that increase the rate of contact with pathogens. For example, water fleas (Daphnia magna) exposed to predatory fish tend to spend more time near the sediment where predation is less frequent, but where the risk of infection by fungal parasites is higher than at the water surface [11]. Finally, predators can influence traits that are not involved in direct interactions with pathogens, but which can be important determinants of pathogen transmission (e.g. fecundity, longevity, size). For example, water fleas exposed to predation risk are bigger and release more infectious fungal spores upon death [12].
Predator-induced phenotypic changes can be especially pervasive in prey with discrete larval and adult stages for which exposure to biotic or abiotic stress during larval development can have strong carry-over effects on adult phenotypes [13,14]. In the wood frogs (Lithobates sylvaticus), for example, Groner et al. [15] showed that larval exposure to predation alters adult immune response to a fungal pathogen. However, to the best of our knowledge, no study to date has examined possible carry-over effects of predation exposure on pathogen transmission. Of particular interest is the possibility that such cascading effects shape the transmission of medically important vector-borne diseases. A growing body of literature, indeed, indicates that environmental stress (e.g. competition, predation and food shortage) experienced during vector larval development can have a profound impact on life-history traits (e.g. growth rate, fecundity and longevity) [16–19] and thus potentially impact pathogen transmission through changes in adult vectorial capacity. For example, recent studies have shown that intra- and interspecific competition during mosquito larval development can weigh heavily on the transmission of filarial worms and arboviruses through indirect carry-over effects on vector–pathogen interactions [20–22]. Despite the high predator diversity and abundance in mosquito larval environments, no study has yet explored whether predator exposure can have carry-over effects on the transmission potential of mosquito-borne diseases [23].
This study addresses this question using a natural food web consisting of the parasite Plasmodium falciparum, which causes the most severe form of human malaria, the mosquito Anopheles coluzzii (formerly the M molecular form of An. gambiae s.s.), which is one of the main vectors of P. falciparum in Africa, and the backswimmer Anisops jaczewskii, which is the most important predator of An. coluzzii larvae in Burkina Faso [24]. Previous studies on this system showed that Anopheles larvae display significant anti-predator behaviours in the presence of backswimmers, consisting of both lower feeding rates and activity [25,26]. We hypothesized that investment in larval anti-predatory defences has carry-over effects on a series of traits that play key roles in the spread of malaria (i.e. components of vectorial capacity). On the one hand, we predict that adult mosquitoes exposed to predators during their larval development may have reduced size, fecundity and longevity, making them less efficient vectors. On the other hand, they may show enhanced vector competence for P. falciparum, making them more efficient vectors. When combined, these conflicting effects can make it difficult to predict the overall consequences of predator exposure on malaria transmission. To explore this issue, we built an epidemiological model and examined how these effects interact to shape malaria transmission.
2. Material and methods
(a). Insect collections
Experiments were conducted on mosquito larvae obtained from wild An. coluzzii females collected in the village of Bama in southwestern Burkina Faso (West Africa; see electronic supplementary material for details).
The backswimmer A. jaczewskii (Hemiptera: Notonectidae) is the most abundant and widespread predatory bug in mosquito larval habitats in our study area [24]. Predators were collected in Bama rice fields and were fed daily ad libitum with An. coluzzii larvae.
(b). Exposure to predator
Initially, 17 400 first-instar mosquito larvae were randomly assigned to a predation group or to a control (i.e. not predated) group. Predator exposure was performed by placing a single A. jaczewskii individual in a plastic container with mosquito larvae (300 larvae per container). A total of 58 containers (29 experimental and 29 control) were used over two replicates (see electronic supplementary material for details). The predator was free to feed upon the mosquito larvae during the entire period of larval development. The presence of a predator and chemical cues associated with killing and consumption have been shown to induce anti-predator behaviours in An. coluzzii [25,27]. This design allowed the study of mosquito responses to total predation-derived stimuli (chemical, visual, mechanical cues) instead of fractions thereof [25,27]. We therefore favoured the most ecologically relevant situation in which both consumptive and non-consumptive effects occur, but only larvae that survived to predation (i.e. those that suffered from non-consumptive effects only) contribute to malaria transmission as adults.
Mosquito larvae were provided with Tetramin® Baby Fish Food ad libitum twice a day, and excess food was removed to avoid water pollution. Owing to predator consumption, mosquito larval densities varied over time in the experimental containers. To avoid the potential effects of changes in density on adult life-history traits [21,22], we standardized the larval density by randomly removing supernumerary larvae from the control containers on one occasion when larvae reached the late second instar (see electronic supplementary material). At the end of larval development, the nymphs were collected and placed in plastic cups at equal densities for emergence in 30 × 30 × 30 cm cages covered with a mesh and provided with water and a 5% glucose solution until experimental infection.
(c). Experimental infections
Infections of predator-exposed and control mosquitoes were carried out using natural P. falciparum isolates obtained from 5- to 10-year-old children from the villages of Soumousso and Dande in southwestern Burkina Faso [28]. Briefly, 4- to 6-day-old female mosquitoes were allowed to feed simultaneously on membrane feeders filled with infectious blood. As a negative control (non-infected mosquitoes), females were fed on the same blood in which the gametocytes (i.e. the infectious stages of parasites) were heat-inactivated [28]. Females that did not feed were discarded. Fully fed females were kept under insectary conditions and enclosed in cages measuring either 30 × 30 × 30 cm (50 mosquitoes per cage) or 20 × 20 × 20 cm (25 mosquitoes per cage) for infection and longevity assays, respectively. A total of three wild parasite isolates from three distinct gametocyte carriers were used (one for the first replicate with a gametocytemia of 168 parasites µl−1 of venous blood, and two for the second replicate with 216 and 560 parasites µl−1, respectively). After feeding on the blood, four groups of mosquitoes were obtained: (i) those exposed to both predator and infection; (ii) those exposed to predator only; (iii) those exposed to infection only and (iv) unexposed control mosquitoes.
(d). Mosquito traits
A series of mosquito traits that play key roles in determining the intensity of malaria transmission were measured, namely mosquito larval development time, adult size, fecundity, longevity and competence for P. falciparum. Development time—this is the number of days from egg to emergence as an adult. Competence—this is the mosquito's ability to develop and transmit malaria. More specifically, vector competence is characterized by infection prevalence (i.e. the proportion of mosquitoes that develop infection upon feeding on an infectious blood meal) and intensity (i.e. the average number of parasites in infected mosquitoes). Here, we gauged competence at two distinct points in time over the course of infection. On day 7 post-blood meal, the midguts of about 40 females from each group were dissected, and the presence and number of oocysts (immature, non-transmissible stage of malaria parasites) were recorded under the microscope. These female mosquitoes were also used to gauge adult size and fecundity. On day 14 post-blood meal, the heads and thoraces of about 50 other parasite-exposed females were dissected, and the presence and quantity of sporozoites (mature transmissible stage) were determined using qPCR (see electronic supplementary material for details). None of the control mosquitoes (heat-inactivated blood meal) became infected. After oocyst and sporozoite quantification, three groups of mosquitoes were obtained according to their infection status: uninfected control, exposed–uninfected and infected mosquitoes. Adult size—on day 7 post-blood meal, one wing per female was measured as a proxy for adult size (see electronic supplementary material for details). Fecundity—we measured three surrogates for fecundity: (i) egg prevalence defined as the proportion of females with developed eggs in their ovaries (i.e. gravid) on day 7 post-blood meal, (ii) egg load defined as the number of eggs in gravid females and (iii) egg size wherein five eggs per female were randomly chosen and measured from tip-to-tip and their mean length per female was used in our statistical analysis (see electronic supplementary material for details). Longevity—after the blood meal, females (n = 25 in two different cages for each group to avoid a possible cage effect) were provided with a 2.5% glucose solution every other day and water ad libitum every day. Dead females were counted and removed every 24 h. Whereas size, fecundity and competence were gauged over two replicates using three different gametocyte carriers (see electronic supplementary material for details), longevity was measured using only one carrier.
(e). Statistical analysis
All analyses were conducted in R (v. 2.12.1). Female wing size was analysed with a linear-mixed model (LMM) with larval treatment (exposed to predator or not; hereafter, ‘predator exposure’) as a fixed effect and replicate as a random effect. Larval development time was analysed with a Cox's mixed model with predator exposure, sex and their interactions coded as fixed effects and replicate as a random effect. Oocyst and sporozoite prevalence were analysed using binomial generalized linear-mixed models (GLMMs). Infection intensity was analysed separately at the oocyst and sporozoite stage with a negative binomial GLMM and a LMM, respectively. Wing size, predator exposure, gametocytemia and their interactions were considered fixed effects, and blood donor was considered a random effect. Egg prevalence was analysed using a binomial GLMM with wing size, predator exposure, female infection status and their interactions as fixed effects and blood donor as a random effect. Egg load and egg size were analysed separately using a LMM with wing size, predator exposure, female status, egg size (in egg load analysis) or egg load (in egg size analysis) and their interactions as fixed effects and blood donor as a random effect. A data subset made of gravid females only was used. The effect of oocyst intensity on mosquito fecundity was also assessed. Survivorship was analysed with a Cox's proportional hazard regression model with predator exposure, exposure to parasite and their interaction as explanatory variables. For model selection, we used the stepwise removal of terms, followed by likelihood ratio tests. Term removals that significantly reduced explanatory power (p < 0.05) were retained in the minimal adequate model [29]. Our design included pseudo-replications (mosquitoes belonging to same rearing container, cage, paper cups, etc.). However, for logistical reasons, it was not possible to keep track of the origin of each individual. Thus, mosquitoes were treated as independent and beside replicate and blood donor, larval containers and adult cages were not included as random effects.
(f). Theoretical exploration
To quantify the consequences of larval predator exposure on malaria transmission, we designed a mathematical model using the SIR approach [30]. We assumed that the mosquito population studied could be categorized into susceptible individuals (Sm, i.e. that could be infected), which then moved to the exposed category upon infection (Em, i.e. infected, but not yet infectious) and became infectious (Im, i.e. mosquitoes that can transmit the pathogen). All adult mosquitoes can produce larvae (Lm) that can emerge as susceptible adults after their development period. We also assumed similar categories for the human population (the model is fully described in the electronic supplementary material) leading to a classic model for vector-borne pathogens [31]. In the presence or absence of predators, we simulated the expected outbreak size in a human population (number of individuals that have been infected at the end of the season) when one infectious human was introduced into a population of 100 individuals. We explored the parameter space through a Latin hypercube sampling with 10 000 replicates for which all parameters were randomly chosen within their confidence interval based on the data measurements obtained experimentally here [32]. This approach takes into account the overall effect of predation on malaria transmission potential (i.e. both consumptive and non-consumptive effects). Using our model, we also estimated the relative importance of these two effects on pathogen transmission potential (see electronic supplementary material for details). Simulations were performed over both one and 10 seasons (short- and long-term effects).
3. Results
(a). Mosquito development and size
A total of 3231 out of 8700 (37.1%) larvae exposed to predation survived to the adult stage and 3297 control larvae were obtained. The male-to-female sex ratios at emergence were 0.94 and 0.95 for the control and predation group, respectively (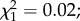 p = 0.88). The mean (±s.e.) larval developmental time was significantly affected by predator exposure, with exposed larvae having a longer period of development than controls (11.10 ± 0.01 versus 10.53 ± 0.01 days, respectively;
p = 0.88). The mean (±s.e.) larval developmental time was significantly affected by predator exposure, with exposed larvae having a longer period of development than controls (11.10 ± 0.01 versus 10.53 ± 0.01 days, respectively; 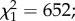 p < 0.0001; figure 1). Males emerged earlier than females (10.75 ± 0.01 versus 10.87 ± 0.01 days;
p < 0.0001; figure 1). Males emerged earlier than females (10.75 ± 0.01 versus 10.87 ± 0.01 days; 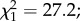 p < 0.0001; figure 1), regardless of predator exposure (i.e. no sex × predator exposure interaction;
p < 0.0001; figure 1), regardless of predator exposure (i.e. no sex × predator exposure interaction; 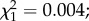 p = 0.9; figure 1). Finally, predator exposure also influenced adult body size, with females emerged from larvae exposed to predators having significantly shorter wings than control females (3195 ± 9 and 3338 ± 8 µm, respectively;
p = 0.9; figure 1). Finally, predator exposure also influenced adult body size, with females emerged from larvae exposed to predators having significantly shorter wings than control females (3195 ± 9 and 3338 ± 8 µm, respectively; 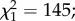 p < 0.0001; figure 2).
p < 0.0001; figure 2).
Figure 1.
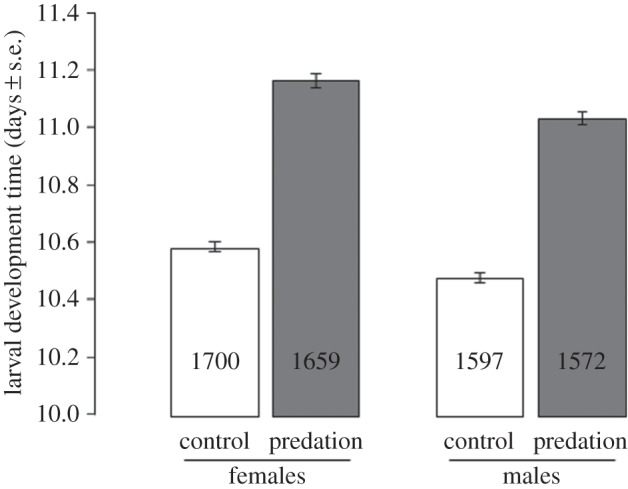
Effect of predator exposure on larval development time. Numbers in bars indicate sample size.
Figure 2.
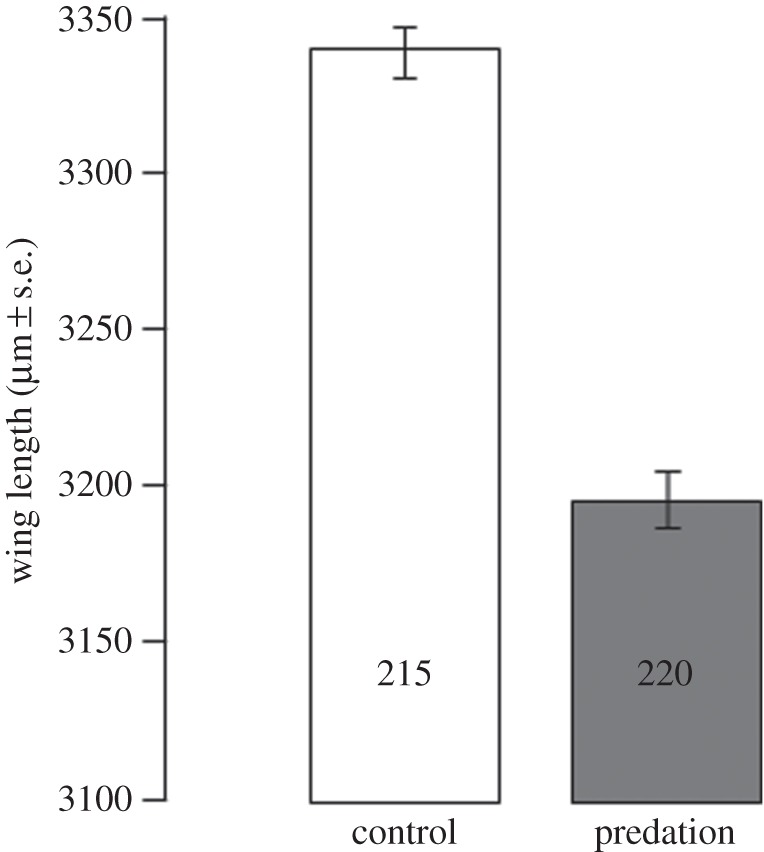
Effect of larval predator exposure on female mean wing size. Numbers in bars indicate sample size.
(b). Competence
Among the 272 females dissected 7 days post-infection, 156 (57.3%) harboured oocysts. Oocyst prevalence and intensity did not significantly vary between control (n = 134) and predator-exposed individuals (n = 138) (prevalence (±95% CI): 53.7 ± 8.4% versus 60.8 ± 8.1%; 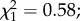 p = 0.44; intensity (mean ± s.e.): 21.3 ± 2.6 versus 23.6 ± 2.7 oocysts;
p = 0.44; intensity (mean ± s.e.): 21.3 ± 2.6 versus 23.6 ± 2.7 oocysts; 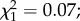 p = 0.78; electronic supplementary material, figures S1a and S1b). There was an effect of mosquito size on infection prevalence, with larger females being less likely to become infected than smaller females (
p = 0.78; electronic supplementary material, figures S1a and S1b). There was an effect of mosquito size on infection prevalence, with larger females being less likely to become infected than smaller females (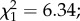 p = 0.012, electronic supplementary material, figure S2). On day 14 post-infection, among the 128 dissected females, 112 (87.5%) were infected with P. falciparum sporozoites. Sporozoite prevalence and intensity were not different between control (n = 77) and predator-exposed females (n = 21) (prevalence: 88.3 ± 7.2% versus 86.3 ± 9.4%;
p = 0.012, electronic supplementary material, figure S2). On day 14 post-infection, among the 128 dissected females, 112 (87.5%) were infected with P. falciparum sporozoites. Sporozoite prevalence and intensity were not different between control (n = 77) and predator-exposed females (n = 21) (prevalence: 88.3 ± 7.2% versus 86.3 ± 9.4%; 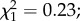 p = 0.62 and intensity (log of gene copy, mean ± s.e.): 3.38 ± 0.15 versus 3.23 ± 0.22;
p = 0.62 and intensity (log of gene copy, mean ± s.e.): 3.38 ± 0.15 versus 3.23 ± 0.22; 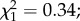 p = 0.5; electronic supplementary material, figures S1c and S1d). Oocyst prevalence on day 7 post-blood meal was significantly lower than sporozoite prevalence on day 14 (
p = 0.5; electronic supplementary material, figures S1c and S1d). Oocyst prevalence on day 7 post-blood meal was significantly lower than sporozoite prevalence on day 14 (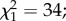 p < 0.001).
p < 0.001).
(c). Fecundity
Three proxies for fecundity were measured: egg prevalence, load and size. The overall egg prevalence was 58.5%. We found a significant effect of predator exposure on egg prevalence, with females exposed during their larval development being 1.6 times less likely to develop eggs after their first blood meal compared with the controls (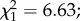 p = 0.009; figure 3a). Female infection status (i.e. uninfected control, exposed–uninfected or infected) and the interaction between infection status and exposure to predators were marginally non-significant (
p = 0.009; figure 3a). Female infection status (i.e. uninfected control, exposed–uninfected or infected) and the interaction between infection status and exposure to predators were marginally non-significant (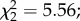 p = 0.06 and
p = 0.06 and 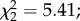 p = 0.06, respectively; figure 3a). However, among females exposed to an infectious blood meal, predator-exposed females that remained uninfected were less likely to be gravid (predation × infection interaction:
p = 0.06, respectively; figure 3a). However, among females exposed to an infectious blood meal, predator-exposed females that remained uninfected were less likely to be gravid (predation × infection interaction: 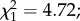 p = 0.029; figure 3a). Among infected females, oocyst intensity had no effect on egg prevalence (
p = 0.029; figure 3a). Among infected females, oocyst intensity had no effect on egg prevalence (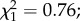 p = 0.38). Finally, wing size did not influence the proportion of gravid females (
p = 0.38). Finally, wing size did not influence the proportion of gravid females (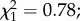 p = 0.37).
p = 0.37).
Figure 3.
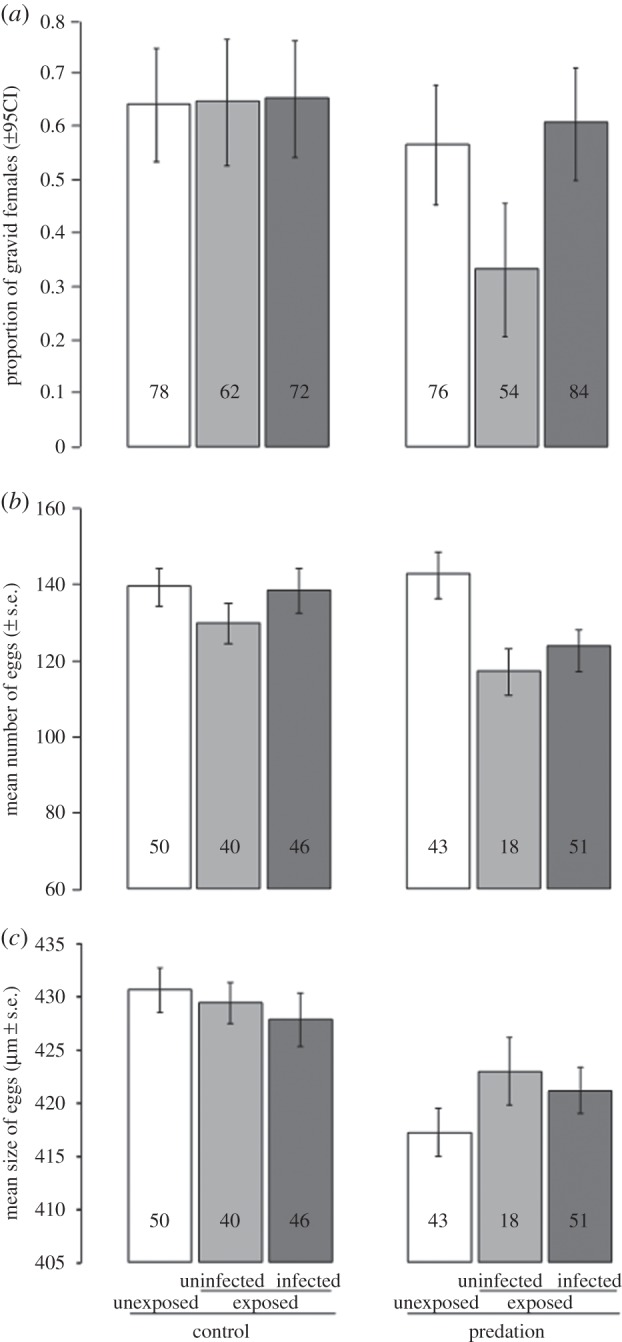
Effects of larval predator exposure and P. falciparum infection on female fecundity. (a) Proportion of gravid females; (b) mean number of eggs in gravid females and (c) mean size of eggs. ‘Control’ and ‘predation’: exposure to larval predator. ‘Unexposed’ and ‘exposed’: exposure to P. falciparum infectious blood meal. ‘Uninfected’ and ‘infected’: infection outcome in females exposed to an infectious blood meal. Numbers in bars indicate the sample size. CI, confidence interval.
In gravid females, the mean egg load (±s.e.) was not statistically different between control and predator-exposed individuals (136.6 ± 3.2 versus 130.1 ± 3.5; 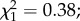 p = 0.53). Both the main effect of female infection status and the interaction between infection status and predator exposure were marginally non-significant (
p = 0.53). Both the main effect of female infection status and the interaction between infection status and predator exposure were marginally non-significant (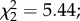 p = 0.06 and
p = 0.06 and 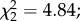 p = 0.08, respectively; figure 3b). However, females exposed to both parasites and predators developed fewer eggs (predator × parasite exposure interaction
p = 0.08, respectively; figure 3b). However, females exposed to both parasites and predators developed fewer eggs (predator × parasite exposure interaction 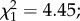 p = 0.035; figure 3b). Finally, among infected females, egg load was positively affected by oocyst intensity (
p = 0.035; figure 3b). Finally, among infected females, egg load was positively affected by oocyst intensity (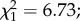 p = 0.009, electronic supplementary material, figure S3). This effect was dependent on egg size, with highly infected females developing numerous small eggs (oocyst intensity × egg size interaction,
p = 0.009, electronic supplementary material, figure S3). This effect was dependent on egg size, with highly infected females developing numerous small eggs (oocyst intensity × egg size interaction, 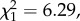 p = 0.012). There also was a significant interaction between predator exposure and oocyst intensity (
p = 0.012). There also was a significant interaction between predator exposure and oocyst intensity (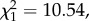 p = 0.001; electronic supplementary material, figure S4). Finally, egg load was positively associated with wing size (
p = 0.001; electronic supplementary material, figure S4). Finally, egg load was positively associated with wing size (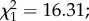 p < 0.0001; electronic supplementary material, figure S5).
p < 0.0001; electronic supplementary material, figure S5).
The mean egg size in predator-exposed females was smaller than in controls (420 ± 1.4 versus 429.4 ± 1.2 µm; 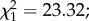 p < 0.0001; figure 3c). However, there was no effect of female infection status on egg size (
p < 0.0001; figure 3c). However, there was no effect of female infection status on egg size (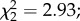 p = 0.23) and no interaction with predator exposure
p = 0.23) and no interaction with predator exposure 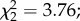 p = 0.15). Among infected females, oocyst intensity had no effect on egg size (
p = 0.15). Among infected females, oocyst intensity had no effect on egg size (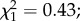 p = 0.5). No trade-off was found between egg size and egg load (
p = 0.5). No trade-off was found between egg size and egg load (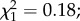 p = 0.97).
p = 0.97).
(d). Longevity
Following experimental infection, 50 females (25 per cage) from each group were specifically kept for survival assays. The blood donor used for this longevity assay had a high gametocytemia (560 parasites µl−1) and yielded a sporozoite prevalence of 95%. Because almost all exposed mosquitoes became infected (i.e. there were very few exposed–uninfected mosquitoes), we considered only the distinction between infected and uninfected control mosquitoes. Predator-exposed females had less longevity than controls (7.7 ± 0.3 versus 8.8 ± 0.3 days; 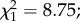 p = 0.003; figure 4). Overall, infection with P. falciparum had no effect on mosquito survival (
p = 0.003; figure 4). Overall, infection with P. falciparum had no effect on mosquito survival (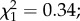 p = 0.55). However, when distinguishing the two main phases of P. falciparum development (i.e. oocyst development from day 1 to 9, and sporozoites in salivary glands from day 10 to 20), infected females survived better during the period of oocyst development than uninfected individuals (
p = 0.55). However, when distinguishing the two main phases of P. falciparum development (i.e. oocyst development from day 1 to 9, and sporozoites in salivary glands from day 10 to 20), infected females survived better during the period of oocyst development than uninfected individuals (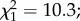 p = 0.001; figure 4 light grey zone), thus confirming the difference in the infection prevalence observed between the two developmental phases. In contrast, at the sporozoite stage, infected females had less longevity compared with uninfected individuals (
p = 0.001; figure 4 light grey zone), thus confirming the difference in the infection prevalence observed between the two developmental phases. In contrast, at the sporozoite stage, infected females had less longevity compared with uninfected individuals (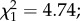 p = 0.029; figure 4 dark grey zone). Finally, over the entire period of parasite development (oocyst + sporozoite), there was no interaction between infection and predation (
p = 0.029; figure 4 dark grey zone). Finally, over the entire period of parasite development (oocyst + sporozoite), there was no interaction between infection and predation (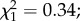 p = 0.55). However, during the sporozoite phase, the shortest lifespan was observed in predator-exposed and infected females, whereas the longest lifespan was observed among control females (Tukey's post hoc for multiple comparison: z = 2.827; p = 0.024).
p = 0.55). However, during the sporozoite phase, the shortest lifespan was observed in predator-exposed and infected females, whereas the longest lifespan was observed among control females (Tukey's post hoc for multiple comparison: z = 2.827; p = 0.024).
Figure 4.
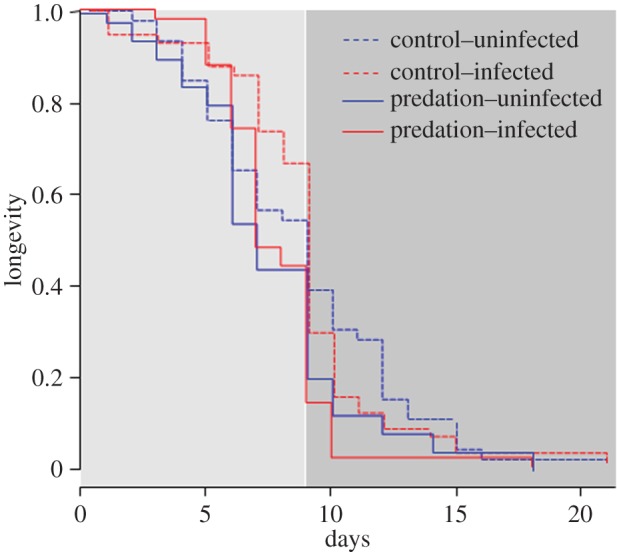
Effect of predator exposure and P. falciparum infection on mosquito longevity during parasite development (oocyst phase: light grey and sporozoite phase: dark grey). (Online version in colour.)
(e). Theoretical modelling
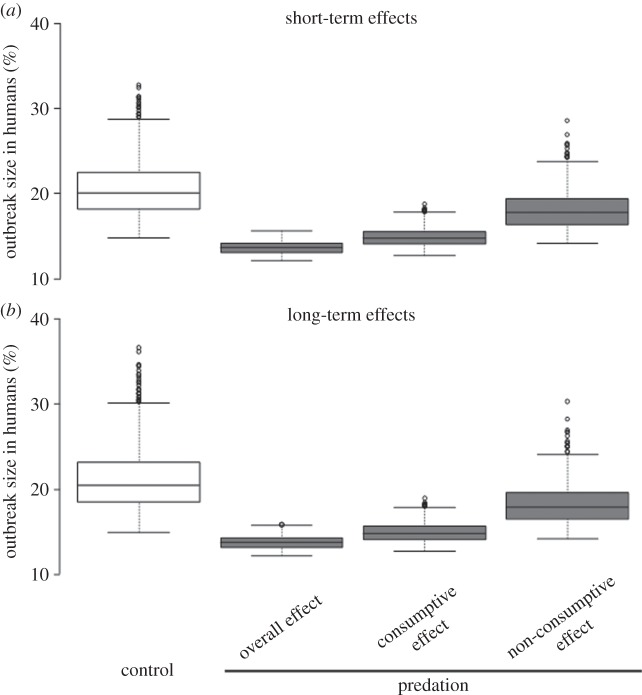
The epidemiological consequences of the presence of predators were highlighted by focusing on outbreak size. When larvae are exposed to predation during their larval development (i.e. overall effect of predation: consumptive + non-consumptive effects), outbreak size in human populations significantly decreases by 34% at both short- and long-terms (control versus overall predation effect: Wilcoxon test, W = 999 738; p < 0.001 and W = 999 741; p < 0.001, respectively; figure 5). When considering consumptive effects alone (i.e. decrease in vector density owing to predation-derived larval mortality), outbreak size significantly decreased by 29% at both short- and long-terms (control versus consumptive effect: W = 984 574; p < 0.001 and W = 984 501; p < 0.001, respectively; figure 5). Finally, when considering non-consumptive effects alone (i.e. carry-over effects of predation exposure), outbreak size decreased by 14% at both short- and long-terms (control versus predation non-consumptive effect: W = 756 701; p < 0.001 and W = 758 474; p < 0.001, respectively; figure 5). The three outbreak sizes, corresponding to the (i) overall effect of predation, (ii) consumptive effect only and (iii) non-consumptive effects, were significantly different from each other (all pairwise comparisons p < 0.001). No significant difference in outbreak size between short- and long-terms were found for all scenarios (overall effect: W = 496 203; p = 0.76, consumptive effect: W = 493 534; p = 0.61 and non-consumptive effect: W = 474 851; p = 0.051; figure 5).
Figure 5.
(a,b) Theoretical difference in the distribution of outbreak sizes for predation and control groups at both short- and long-terms. Outbreak size is the proportion of humans that has been infected at the end of one (short-term) or 10 (long-term) transmission seasons, after one infectious human was introduced into the population. A first model fed with our experimental results (values listed in electronic supplementary material, table S1) was run. This model, including both consumptive and non-consumptive effects, predicts the overall effect of predation exposure on outbreak size. To account for consumptive effects only, the value of all parameters of the predation group was replaced in the model with those of control individuals, except the developmental success (i.e. the Y parameter in electronic supplementary material, table S1). To account for non-consumptive effects only, the value of the Y parameter of the predation group was replaced by that of the control group. The box plots indicate the median (large horizontal bars), the 25th and 75th percentiles (boxes), the minimum and maximum values (whiskers) and outliers (circles).
4. Discussion
Our results indicate that the presence of the predator A. jaczewskii during mosquito larval development can reduce the transmission of malaria through both consumptive and non-consumptive effects. Although predator exposure did not significantly influence mosquito susceptibility to P. falciparum, it incurred strong fitness costs in terms of larval development, adult size, fecundity and longevity. Collectively, these effects on key epidemiological factors reduce the rate of malaria spread. Our findings highlight the importance of taking into account the mosquito larval environment in malaria ecology and epidemiological studies.
In our experimental design, the predator was allowed to feed upon the mosquito larvae during the entire period of larval development. As a result, several potential mechanisms could explain the observed negative effects of predator exposure on mosquito life-history traits. First, the backswimmers may have selectively fed upon big larvae such that adult mosquitoes emerging from the predation group were on average smaller than mosquitoes from the control group. However, our data indicate that the distributions of mosquito life-history traits in the predation group are shifted compared with that in the control group (see electronic supplementary material, figure S6), hence suggesting that selection alone probably could not explain the observed results. Second, although we removed supernumerary larvae from the control containers to compensate the loss owing to predation in the treated group, we cannot completely rule out the possibility that larval density was slightly reduced in the predation group (i.e. relaxed intraspecific competition). However, under this scenario, we would expect fitness benefits (i.e. bigger adult size, fecundity, etc.) of predation exposure rather expect than fitness costs. Finally, exposure to predation risk may have induced a high level of stress and the expression of costly larval anti-predatory behaviours. As a result, larvae possibly spent less time performing other activities, such as foraging, and this had crucial consequences for mosquito life-history traits.
In organisms with larval aquatic stages and an aerial adult stage, development can be shortened to allow individuals to reach the aerial adult stage sooner and, in that way, avoid threats such as aquatic predators by leaving the aquatic environment [8]. In contrast to this prediction, our results rather indicate a longer period of development under a predation risk. This can occur when anti-predatory defences are traded off against foraging rate [33]. In our system, the larvae, indeed, tended to adopt low-risk behaviours to be less conspicuous when under the threat of predation and thus limit their movements and activities such as foraging ([25] and references therein). Reduced foraging activity in response to predation risk can not only increase development time, but can also result in a number of other secondary consequences (cascade effects). First, mosquito larvae exposed to predators were significantly smaller than control individuals. Similar effects of natural stressors in larval habitats often arise in other mosquito systems [18,20,21]. Second, mosquito body size is often positively correlated with fecundity [34], and accordingly, we found that predator-exposed individuals had lower fecundity levels possibly owing to the few resources acquired during their larval development. A decrease in mosquito reproduction would dampen the vector population dynamics and hence the spread of disease [35], all else being equal. Third, smaller-sized adult females may have less survivorship because of fewer metabolic resources and/or enhanced oxidative stress [7,18]. In our study, larval predator exposure, indeed, impeded An. coluzzii survival, a frequently reported carried-over effect of larval predation in other mosquito species [16,18]. Longevity is a core component of disease spread: the longer the vector lives, the more the parasite can be transmitted. Predator exposure at larval stages can, therefore, render adult mosquitoes less efficient in transmitting malaria parasites because they may complete fewer gonotrophic cycles.
Vector competence, a fourth component of malaria spread, did not respond to predator exposure. Reduced host resistance and immune function under predation risk have been demonstrated in a number of invertebrate host–pathogen systems ([36] and references therein). In mosquitoes, previous studies have shown that larval environmental factors, including competition and nutritional deprivation, can have latent effects on susceptibility to pathogens in subsequent adult stages [20,37]. Here, we found that predator exposure during immature mosquito developmental stages does not modify adult susceptibility to malaria parasites, suggesting that in this system there is no trade-off between anti-predatory and anti-malaria defences. This further suggests that an investment in an immune response to P. falciparum may be relatively important and take priority over other life-history traits, including mosquito lifespan and fecundity.
Although predator exposure had no direct effect on mosquito competence for P. falciparum, cumulative effects between predator exposure and infection on mosquito fecundity and longevity were observed. First, exposure to both predators and parasites induced a lower number of eggs in a synergistic way (i.e. separately, these two factors did not affect egg load). Negative Plasmodium effects on mosquito fecundity are generally thought to arise from mounting energetically costly immune responses [38]. In our study, differences in quality between predator-exposed and control mosquitoes may have led to differences in the ability to pay the costs associated with an immune response. Generally, individuals in better condition should be better able to pay these costs and still have a good performance overall [39]. Accordingly, control mosquitoes may have been able to bear these costs because they had enough energy to fuel both the immune response and egg production. In contrast, predator-exposed individuals had a reduced energy budget and had to pay an investment in an immune response in the form of lower fecundity. Second, predator-exposed female mosquitoes that remained uninfected after ingesting an infectious blood meal (i.e. resistant females) were less likely to carry eggs. This result suggests that females exposed to infectious gametocytes mounted an immune response efficient in preventing the establishment of the parasite, and that this resistance was costly only when they were confronted with predation during their larval development. This confirms findings by previous studies on mosquitoes showing that anti-infection resistance can incur fitness costs in terms of lower fecundity [38,40]. Third, infected and predator-exposed mosquitoes had less longevity compared with control mosquitoes during the sporozoite infection phase only. This further suggests that predator-exposed females were in poor condition and were unable to maintain their fitness once infected. In this study, the overall longevity of mosquitoes was rather short (10 days on average). However, this assay was conducted under nutritionally stressful conditions, because the mosquitoes received a 2.5% glucose solution every other day. We cannot exclude the possibility that providing the mosquitoes with the traditional 5% glucose solution ad libitum would have reduced the difference observed in survival between mosquitoes exposed to both infection and predator and control mosquitoes.
Regardless of predator exposure, infected females displayed greater survivorship rates during the oocyst infection phase. Consistent with this finding, the proportion of infected females (parasite prevalence) in the competence assay was higher on day 14 post-infection compared with day 7 post-infection, suggesting that infected females, indeed, survived better than uninfected individuals between day 7 and day 14 post-infection. A recent study showed that An. gambiae infected with the rodent malaria parasite P. berghei enhance their sucrose uptake and accumulate more glycogens during oocyst development, thereby increasing their survival under starvation conditions [41]. These findings support the hypothesis according to which the parasite must manipulate its insect vector in a way that reduces mortality before the parasite reaches maturity and is ready to be transmitted [42]. In contrast, at the sporozoite stage, malaria infection is expected to increase mosquito activity and mortality [42,43] and, accordingly, we found that infected females had lower survivorship rates compared with uninfected individuals. In addition to a possibly greater activity-associated mortality rate, previous studies showed that malaria sporozoites can damage salivary glands and, hence, make the vector more prone to infections by harmful bacteria and viruses ([44,45] and references therein).
Altogether, our findings strongly suggest that the costs of P. falciparum infection on mosquito fecundity and longevity become apparent in stressful environmental conditions only. Whether malaria parasites cause fitness costs to the mosquito vectors remains disputed [45,46]. Most studies on the costs of malaria infection have been conducted under optimal laboratory conditions. However, in their natural habitats, mosquitoes are constantly challenged by various biotic and abiotic pressures, including resource limitation, competition, predation, temperature variations and pesticides. It therefore becomes imperative to examine infection costs on mosquito fitness in conditions reflecting those occurring in nature. Some recent studies have begun to fill this gap and demonstrated that malaria parasites reduce the overall fitness of their natural mosquito vectors when in stressful conditions [40,44,47]. Our findings complement these studies to show that malaria infection is generally costly for their natural vectors.
In apparent contradiction to this statement was the observation of an increased number of eggs in highly infected mosquitoes (positive relationship between fecundity and infection intensity). This finding may illustrate the terminal investment hypothesis (also known as fecundity compensation), which postulates that when the future reproductive opportunities of an individual decline because of high mortality risk such as high infection levels, organisms increase their current reproductive investment [48]. We examined the first reproductive event only and further studies are needed to investigate whether this increased egg production indeed corresponds to a case of terminal investment.
Overall, the theoretical model, fed with our experimental results, showed that the presence of predators in larval habitat could decrease malaria transmission in human populations. Both consumptive and non-consumptive effects contributed significantly to this decrease. The direct consumption of mosquito larvae by predators reduced the number of emerging adults and hence the number of females able to transmit malaria. The non-consumptive effects resulted in changes in vector life-history traits playing key roles in malaria transmission. Our findings suggest that direct consumptive effects are relatively more important than non-consumptive effects in reducing malaria transmission potential.
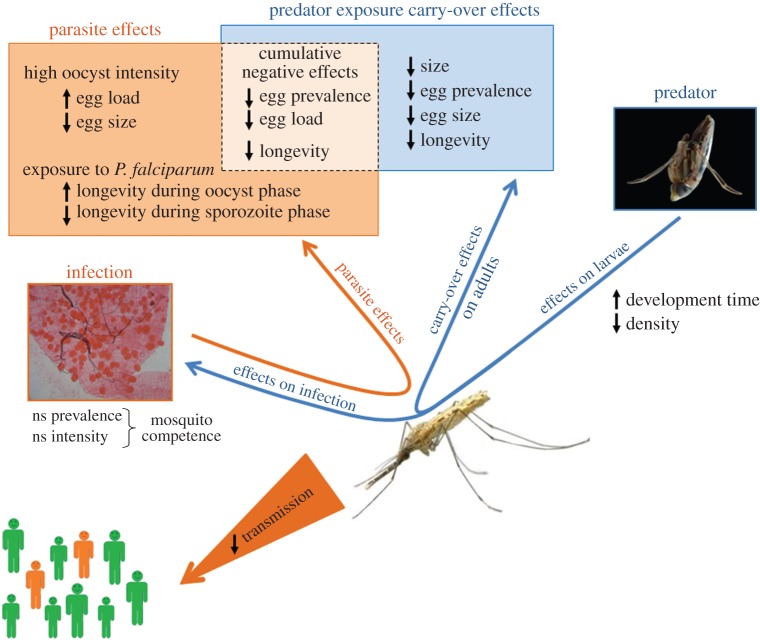
In summary, our findings suggest that the backswimmer, a common predator of mosquito larvae, can influence malaria epidemiology not only via decrease in vector density (consumptive effects), but also via non-consumptive effects on mosquito development, size, fecundity and longevity (figure 6). This may provide us a possible explanation to the paddies paradox, which describes situations where land irrigation increases mosquito populations without any increase in malaria transmission [49]. In permanent water collections, the density of predators is generally higher than in temporary water [24] and it may, therefore, contribute to reducing malaria transmission. In a time of renewed scientific and political commitment to malaria control and against biodiversity erosion, the biological conservation of predators in natural mosquito larval habitats is an obvious need. Finally, our work highlights the need to consider larval, and, more widely, environmental stressors to better evaluate vectorial capacity and produce more reliable estimates of transmission. This will be useful, not only to predict in a more natural way the impact on vector-borne disease transmission, which until now has assumed that all individuals are equally likely to acquire and to transmit parasites, but also to better understand the complex, physiological outcomes of multiple stressors.
Figure 6.
Summary of direct and carry-over effects of larval predator exposure on mosquito life-history traits, on the infection outcome in the mosquito and on transmission potential in human population. ns denotes statistically non-significant effects. Oocysts of Plasmodium falciparum in dissected mosquito midguts: picture by Da Dari, IRSS. Anopheles coluzzii female: picture by Nil Rahola, IRD. Backswimmer, Anisops jaczewskii eating a mosquito larva: picture by Olivier Roux, IRD. (Online version in colour.)
Supplementary Material
Acknowledgements
We thank the Associate Editor Dr Pejman Rohani and two anonymous reviewers for their valuable comments that greatly improved the manuscript. We thank Boubacar Nikiema for the RFLP-PCR, Jean Bazié and Raymond Hien for technical help with dissections and Andrea Yockey-Dejean for proofreading the paper.
Ethics
Ethical approval was obtained from the Centre Muraz Institutional Ethics Committee under agreement no. A003-2012/CE-CM.
Data accessibility
Data files: DRYAD provisional doi: http://dx.doi.org/10.5061/dryad.1c817.
Authors' contributions
O.R., A.V. and T.L. designed and conducted the experiments, B.R. carried out the mathematical modelling, K.B.Y. carried out the qPCR, O.R. and T.L. wrote the manuscript and all of the authors contributed to the revisions.
Competing interests
We have no competing interests.
Funding
O.R. received financial support through a post-doctoral fellowship from the IRD. Experimental infections were supported by the ANR grant no. 11-PDOC-006-01.
References
- 1.Keesing F, Holt RD, Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. ( 10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 2.Johnson PTJ, Hartson RB, Larson DJ, Sutherland DR. 2008. Diversity and disease: community structure drives parasite transmission and host fitness. Ecol. Lett. 11, 1017–1026. ( 10.1111/j.1461-0248.2008.01212.x) [DOI] [PubMed] [Google Scholar]
- 3.Packer C, Holt RD, Hudson PJ, Lafferty KD, Dobson AP. 2003. Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecol. Lett. 6, 797–802. ( 10.1046/j.1461-0248.2003.00500.x) [DOI] [Google Scholar]
- 4.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86, 501–509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 5.Schmitz OJ. 2008. Effects of predator hunting mode on grassland ecosystem function. Science 319, 952–954. ( 10.1126/science.1152355) [DOI] [PubMed] [Google Scholar]
- 6.Werner EE, Peacor SD. 2003. A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100. ( 10.1890/0012-9658(2003)084%5B1083:arotii%5D2.0.co;2) [DOI] [Google Scholar]
- 7.Slos S, Stoks R. 2008. Predation risk induces stress proteins and reduces antioxidant defense. Funct. Ecol. 22, 637–642. ( 10.1111/j.1365-2435.2008.01424.x) [DOI] [Google Scholar]
- 8.Benard MF. 2004. Predator-induced phenotypic plasticity in organisms with complex life histories. Annu. Rev. Ecol. Evol. Syst. 35, 651–673. ( 10.1146/annurev.ecolsys.35.021004.112426) [DOI] [Google Scholar]
- 9.Raffel TR, Hoverman JT, Halstead NT, Michel PJ, Rohr JR. 2010. Parasitism in a community context: trait-mediated interactions with competition and predation. Ecology 91, 1900–1907. ( 10.1890/09-1697.1) [DOI] [PubMed] [Google Scholar]
- 10.Navarro C, de Lope F, Marzal A, Moller AP. 2004. Predation risk, host immune response, and parasitism. Behav. Ecol. 15, 629–635. ( 10.1093/beheco/arh054) [DOI] [Google Scholar]
- 11.Decaestecker E, De Meester L, Ebert D. 2002. In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc. Natl Acad. Sci. USA 99, 5481–5485. ( 10.1073/pnas.082543099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy MA, Housley JM, Penczykowski RM, Cáceres CE, Hall SR. 2011. Unhealthy herds: indirect effects of predators enhance two drivers of disease spread. Funct. Ecol. 25, 945–953. ( 10.1111/j.1365-2435.2011.01872.x) [DOI] [Google Scholar]
- 13.De Block M, Stoks R. 2005. Fitness effects from egg to reproduction: bridging the life history transition. Ecology 86, 185–197. ( 10.1890/04-0116) [DOI] [Google Scholar]
- 14.Crean JA, Monro K, Marshall DJ. 2011. Fitness consequences of larval traits persist across the metamorphic boundary. Evolution 65, 3079–3089. ( 10.1111/j.1558-5646.2011.01372.x) [DOI] [PubMed] [Google Scholar]
- 15.Groner ML, Buck JC, Gervasi S, Blaustein AR, Reinert LK, Rollins-Smith LA, Bier ME, Hempel J, Relyea RA. 2013. Larval exposure to predator cues alters immune function and response to a fungal pathogen in post-metamorphic wood frogs. Ecol. Appl. 23, 1443–1454. ( 10.1890/12-1572.1) [DOI] [PubMed] [Google Scholar]
- 16.Costanzo KS, Muturi EJ, Alto BW. 2011. Trait-mediated effects of predation across life-history stages in container mosquitoes. Ecol. Entomol. 36, 605–615. ( 10.1111/j.1365-2311.2011.01302.x) [DOI] [Google Scholar]
- 17.Muriu SM, Coulson T, Mbogo CM, Godfray HCJ. 2013. Larval density dependence in Anopheles gambiae s.s., the major African vector of malaria. J. Anim. Ecol. 82, 166–174. ( 10.1111/1365-2656.12002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Uitregt VO, Hurst TP, Wilson RS. 2012. Reduced size and starvation resistance in adult mosquitoes, Aedes notoscriptus, exposed to predation cues as larvae. J. Anim. Ecol. 81, 108–115. ( 10.1111/j.1365-2656.2011.01880.x) [DOI] [PubMed] [Google Scholar]
- 19.Telang A, Qayum AA, Parker A, Sacchetta BR, Byrnes GR. 2012. Larval nutritional stress affects vector immune traits in adult yellow fever mosquito Aedes aegypti (Stegomyia aegypti). Med. Vet. Entomol. 26, 271–281. ( 10.1111/j.1365-2915.2011.00993.x) [DOI] [PubMed] [Google Scholar]
- 20.Alto BW, Lounibos LP, Mores CN, Reiskind MH. 2008. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc. R. Soc. B 275, 463–471. ( 10.1098/rspb.2007.1497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bevins SN. 2008. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae). Biol. Invasions 10, 1109–1117. ( 10.1007/s10530-007-9188-8) [DOI] [Google Scholar]
- 22.Breaux JA, Schumacher MK, Juliano SA. 2014. What does not kill them makes them stronger: larval environment and infectious dose alter mosquito potential to transmit filarial worms. Proc. R. Soc. B 281, 20140459 ( 10.1098/rspb.2014.0459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefèvre T, Vantaux A, Dabiré KR, Mouline K, Cohuet A. 2013. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 9, e1003365 ( 10.1371/journal.ppat.1003365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diabaté A, Dabiré RK, Heidenberger K, Crawford J, Lamp WO, Culler LE, Lehmann T. 2008. Evidence for divergent selection between the molecular forms of Anopheles gambiae: role of predation. BMC Evol. Biol. 8, 5 ( 10.1186/1471-2148-8-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roux O, Diabaté A, Simard F. 2013. Larvae of cryptic species of Anopheles gambiae respond differently to cues of predation risk. Freshwater Biol. 58, 1178–1189. ( 10.1111/fwb.12117) [DOI] [Google Scholar]
- 26.Gimonneau G, Pombi M, Dabire R, Diabate A, Morand S, Simard F. 2012. Behavioural responses of Anopheles gambiae sensu stricto M and S molecular form larvae to an aquatic predator in Burkina Faso. Parasites Vectors 5, 65 ( 10.1186/1756-3305-5-65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux O, Diabaté A, Simard F. 2014. Divergence in threat-sensitivity among aquatic larvae of cryptic mosquito species. J. Anim. Ecol. 83, 702–711. ( 10.1111/1365-2656.12163) [DOI] [PubMed] [Google Scholar]
- 28.Vantaux A, Dabire K, Cohuet A, Lefevre T. 2014. A heavy legacy: offspring of malaria-infected mosquitoes show reduced disease resistance. Malar. J. 13, 442 ( 10.1186/1475-2875-13-442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawley MJ. 2007. The R book, 950 p. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 30.Keeling MJ, Rohani P. 2008. Modeling infectious diseases. Princeton, NJ: Princeton University Press. [Google Scholar]
- 31.Reiner RCR, et al. 2013. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970−2010. J. R. Soc. Interface 10, 20120921 ( 10.1098/rsif.2012.0921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iman RL, Helton JC, Campbell JE. 1981. An approach to sensitivity analysis of computer models, Part 1. Introduction, input variable selection and preliminary variable assessment. J. Qual. Technol. 13, 174–183. [Google Scholar]
- 33.Werner EE, Anholt BR. 1993. Ecological consequences of the trade-off between growth and mortality-rates mediated by foraging activity. Am. Nat. 142, 242–272. ( 10.1086/285537) [DOI] [PubMed] [Google Scholar]
- 34.Briegel H. 1990. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. J. Med. Entomol. 27, 839–850. ( 10.1093/jmedent/27.5.839) [DOI] [PubMed] [Google Scholar]
- 35.MacDonald G. 1957. The epidemiology and control of malaria. London, UK: Oxford University Press. [Google Scholar]
- 36.Stoks R, De Block M, Slos S, Van Doorslaer W, Rolff J. 2006. Time constraints mediate predator-induced plasticity in immune function, condition, and life history. Ecology 87, 809–815. ( 10.1890/0012-9658(2006)87%5B809:tcmppi%5D2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 37.Takken W, Smallegange R, Vigneau A, Johnston V, Brown M, Mordue-Luntz A, Billingsley P. 2013. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasites Vectors 6, 345 ( 10.1186/1756-3305-6-345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed AM, Hurd H. 2006. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect. 8, 308–315. ( 10.1016/j.micinf.2005.06.026) [DOI] [PubMed] [Google Scholar]
- 39.Demas G, Nelson R. 2012. Ecoimmunology. London, UK: Oxford University Press. [Google Scholar]
- 40.Sangare I, Dabire R, Yameogo B, Da DF, Michalakis Y, Cohuet A. 2014. Stress dependent infection cost of the human malaria agent Plasmodium falciparum on its natural vector Anopheles coluzzii. Infect. Gen. Evol. 25, 57–65. ( 10.1016/j.meegid.2014.04.006) [DOI] [PubMed] [Google Scholar]
- 41.Zhao YO, Kurscheid S, Zhang Y, Liu L, Zhang L, Loeliger K, Fikrig E. 2012. Enhanced survival of Plasmodium-infected mosquitoes during starvation. PLoS ONE 7, e40556 ( 10.1371/journal.pone.0040556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koella JC. 1999. An evolutionary view of the interactions between anopheline mosquitoes and malaria parasites. Microbes Infect. 1, 303–308. ( 10.1016/S1286-4579(99)80026-4) [DOI] [PubMed] [Google Scholar]
- 43.Anderson RA, Knols BGJ, Koella JC. 2000. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae s.l. Parasitology 120, 329–333. ( 10.1017/S0031182099005570) [DOI] [PubMed] [Google Scholar]
- 44.Lalubin F, Delédevant A, Glaizot O, Christe P. 2014. Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. J. Anim. Ecol. 83, 850–857. ( 10.1111/1365-2656.12190) [DOI] [PubMed] [Google Scholar]
- 45.Ferguson HM, Read AF. 2002. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 18, 256–261. ( 10.1016/S1471-4922(02)02281-X) [DOI] [PubMed] [Google Scholar]
- 46.Vézilier J, Nicot A, Gandon S, Rivero A. 2012. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc. R. Soc. B 279, 4033–4041. ( 10.1098/rspb.2012.1394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aboagye-Antwi F, Tripet F. 2010. Effects of larval growth condition and water availability on desiccation resistance and its physiological basis in adult Anopheles gambiae sensu stricto. Malar. J. 9, 225 ( 10.1186/1475-2875-9-225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agnew PC, Koella J, Michalakis Y. 2000. Host life history responses to parasitism. Microbes Infect. 2, 891–896. ( 10.1016/S1286-4579(00)00389-0) [DOI] [PubMed] [Google Scholar]
- 49.Ijumba JN, Lindsay SW. 2001. Impact of irrigation on malaria in Africa: paddies paradox. Med. Vet. Entomol. 15, 1–11. ( 10.1046/j.1365-2915.2001.00279.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data files: DRYAD provisional doi: http://dx.doi.org/10.5061/dryad.1c817.