Abstract
Language-based learning disorders such as dyslexia affect millions of people, but there is little agreement as to their cause. New evidence from behavioral measures of the ability to hear tones in the presence of background noise indicates that the brains of affected individuals develop more slowly than those of their unaffected counterparts. In addition, it seems that brain changes occurring at ≈10 years of age, presumably associated with puberty, may prematurely halt this slower-than-normal development when improvements would normally continue into adolescence. The combination of these ideas can account for a wide range of previous results, suggesting that delayed brain development, and its interaction with puberty, may be key factors contributing to learning problems.
Language-based learning problems (LPs) affect ≈8% of the population (1), but their causes are still poorly understood. These problems comprise a variety of disorders, all of which hinder the ability of individuals with normal intelligence to produce or understand oral or written language. Prevalent among these diagnoses are dyslexia, specific language impairment, and central auditory processing disorder. The effects of these disorders are far-reaching. Beyond the psychosocial stress experienced by affected individuals and their families, LPs cost society billions of dollars annually in special education services, lost productivity, and un- and underemployment (2). Most current theories of these LPs focus on particular impairments in the neurological (3-6), perceptual (7-10), cognitive (11, 12), or linguistic (13-19) functioning of affected individuals. However, few attempts have been made to integrate the wide range of abnormalities seen in these populations (3, 15). Here, we propose that a broad array of these impairments arises from delayed neurological development and that, because of this delay, development that would normally continue into adolescence is halted at ≈10 years of age, presumably by sexual maturation. This theoretical framework allows us to predict a wide range of deficits in children and adults with LPs, suggesting that neuro-developmental immaturity may be a key contributor to LPs.
This proposal arises from our investigation of the performance of individuals with LPs on behavioral tasks that measure their ability to hear a brief tone in the presence of a noise masker. We previously observed deficiencies in this ability in a group of 8-year-old children with specific language impairment and suggested that these deficits may be a key contributor to LPs (10). In that experiment, we measured tone-detection thresholds in five conditions: a long tone presented during a noise and a brief tone presented before, at the beginning of, toward the middle of, or after that noise. Compared with age-matched controls, the mean detection thresholds for the affected children ranged from completely normal (for the long tone presented during the noise) to severely impaired (for the brief tone presented before the noise), seeming to indicate that the perceptual deficits of children with specific language impairment only occurred in particular sound contexts (10). The evidence for a developmental delay emerged from analyses of data on these same conditions obtained from individuals with different LPs. We initially collected these data with the intent of determining whether the same auditory deficits we observed in individuals with specific language impairment (10) also occurred in populations with dyslexia and central auditory processing disorder. Apparent from these analyses was that there was little difference between diagnostic groups but large differences with age. Here we report the data, combined across these and previous (20) experiments, from a total of 54 listeners with LPs and 61 unaffected controls. These listeners were assigned to one of five age groups that spanned the range from 6 years to adult (Table 1).
Table 1. Number of listeners tested in each condition.
| Age group, years | 6.5 | 8 | 10 | 13 | Adult |
| Age range, years | 6.2-6.9 | 7.1-8.9 | 9.0-11.7 | 12.1-15.5 | 16.8-43.6 |
| LP* | — | 8.1 (0.44) | 10.1 (0.75) | 13.1 (1.48) | 23.7 (4.79) |
| n† | — | 18 | 16 | 6 | 14 |
| n‡ | — | 18 | 16 | 6 | 14 |
| n§ | — | 10 | 8 | — | 12 |
| Controls* | 6.5 (0.23) | 8.0 (0.54) | 10.0 (0.65) | — | 26.7 (7.54) |
| n† | 15 | 18 | 11 | — | 17 |
| n‡ | — | 8 | — | — | 7 |
| n§ | — | 8 | — | — | 7 |
Mean age in years (SD).
Number of listeners tested in the long-tone, backward, and delay conditions.
Number of listeners tested in the forward condition.
Number of listeners tested in the onset condition.
The results are consistent with the idea that these auditory perceptual deficits reflect neurological immaturity and that deficits that persist into adulthood do so because development ceases if a critical level of performance has not been reached by puberty. Others have reported evidence that children with LPs are late to reach a broad range of developmental milestones (21) and suggested that developmental delay may play a key role in LPs (15, 17, 22-24). What we add is that, because of this delay, further development on attributes with normally long developmental courses seems to be halted by sexual maturation. The combination of these ideas seems to provide a unifying account of the wide array of disparate abnormalities observed in individuals with LPs.
Methods
Listeners. A total of 115 listeners, distributed among five age groups (Table 1), participated in the testing. Each listener was represented in only one age group. Thus, no longitudinal data were included. None of the listeners had any history of hearing loss or any previous experience with psychoacoustic tasks. Sixty-one listeners (31 males and 30 females) with no suspected LPs served as controls. The remaining 54 listeners (26 males and 28 females) had clinical diagnoses of specific language impairment (n = 12), dyslexia (n = 27), or central auditory processing disorder (n = 15) and formed the sample with LPs. We previously reported the data of 54 of the 61 controls (10, 20) and 8 of the 54 LP listeners (10).
Outside professionals had previously diagnosed all listeners with LPs and referred them to us for testing. We had confirmatory clinical records on 51 of the 54 listeners with LPs derived from recent clinical reports, our own clinical testing, or both. For all 12 of our listeners with specific language impairment, we had standardized measures of both nonverbal intelligence [mean = 106.5 (SD = 12.7)] and language [mean = 76.0 (SD = 6.1)] (10). For 24 of the 27 listeners with dyslexia, we had measures of both nonverbal intelligence and reading (19 listeners), only reading (4 listeners), or only nonverbal intelligence (1 listener). Overall, their average nonverbal intelligence standard score was 113.1 (SD = 13.7), and their average reading standard score was 93.4 (SD = 11.9). No scores were available for the remaining three listeners with dyslexia. Finally, we had clinical records for all 15 of the listeners with central auditory processing disorder. All were judged to have at least average intelligence, based primarily on school records available to the clinicians, and had performed at least two SDs below the mean on at least three of the auditory-skill tests commonly used to diagnose central auditory processing disorder (25). The standard scores for nonverbal intelligence ranged from 93 to 137 across all listeners for whom scores were available. These scores were higher for the adults than the children, on average, but were very similar between groups at each age (8-year-old group: LPs = 103.1, control 105.1; adult group: LPs = 116.0, control = 117.6). There seemed to be no relationship between nonverbal intelligence and performance among either listeners with LPs or controls in either the 8-year-old or adult age groups. Among these four listener-type and age-group combinations, Spearman correlations (rs) between intelligence and threshold in the backward and forward conditions ranged from -0.06 to 0.24.
We combined the listeners with the three different clinical diagnoses into one LP group because examination of mean performance for each age group indicated that the differences between these diagnoses were minimal. The number of listeners diagnosed with specific language impairment decreased and the number of listeners diagnosed with dyslexia increased with increasing age. We had sufficient numbers of listeners to statistically compare subgroups (n ≥ 6 per subgroup) for only two subgroups at each of two ages: 8-year-olds with dyslexia vs. 8-year-olds with specific language impairment and 10-year-olds with dyslexia vs. 10-year-olds with central auditory processing disorder. Of the 10 possible same-age comparisons (five conditions × two age groups), 8 were not statistically significant. The two significant differences both occurred in the backward condition; the thresholds of the listeners with dyslexia were significantly lower in each case. Note that the developmental trends reported for all listeners with LPs held for the subset of listeners with LPs that were diagnosed with dyslexia (the only subset for which there was a sufficient number of listeners per age group to evaluate development), with the exception that these listeners showed no improvement with age in the long-tone condition. We did not conduct formal analyses of gender differences because, for both listener types, there were insufficient numbers of listeners of one or the other gender within several age groups.
Stimuli and Procedure. The stimuli and procedure have been described (10, 20). Briefly, the tone to be detected had a frequency of 1,000 Hz and a total duration of 20 or 200 ms. The masking noise ranged from 600 to 1,400 Hz and had a total duration of 300 ms and a spectrum level of 40 dB sound pressure level. All stimuli were gated with either a 10-ms (new data) (10) or 6-ms (20) cosine-squared rise-fall time. The onset of the 20-ms tone came 20 ms before noise onset (backward condition), at noise onset (onset condition), 200 ms after noise onset (delay condition), or immediately after noise offset (forward condition). The onset of the 200-ms tone came 50 ms after noise onset (long-tone condition). We presented the stimuli to the right ear over Sennheiser (Old Lyme, CT) HD450 headphones.
We estimated the tone level necessary for 94% correct detections with a two-interval forced-choice procedure in which we adaptively adjusted the tone level in each 30-trial block by using the maximum-likelihood method (26). Tone thresholds for individual listeners are based on either one or the mean of two estimates for the long tone and on the mean of two or three estimates for the short tone. We omitted the most deviant of three estimates if the SD was >15 dB either across three estimates (new data) (10) or across the first two estimates (20). Such deviant thresholds have been obtained, and removed from analyses, in other investigations using the maximum-likelihood method (27-29). They have been reported to occur with approximately equal frequency (on ≈7% of estimates) for listeners with LPs and controls (29) and in approximately equal numbers of 8-year-old, 10-year-old, and adult controls (20). The average within-listener SD of the final threshold estimates ranged from ≈2 to 7 dB across conditions. Listeners completed the long-tone condition first, followed by the short-tone conditions. The order of the short-tone conditions was randomized across listeners.
Analysis. We analyzed each condition with a two-way, typically 2 (group) × 3 (age), ANOVA followed by Fisher's protected t tests. We compared between LP and control listeners within the selected age groups and within each listener type across age groups. We also calculated the effect size index (d), which is the between-groups difference expressed in SD units (30).
Results
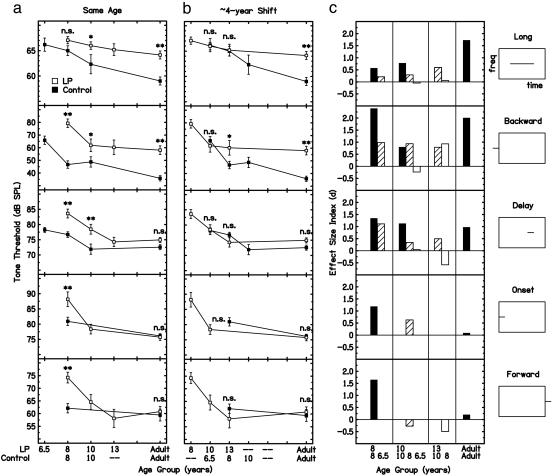
When we compared the thresholds of listeners with LPs with same-age controls, as is standard, the listeners with LPs often had significantly higher thresholds, but their impairment pattern across the five masking conditions varied with age. The listeners with LPs had significantly higher thresholds in 9 of the 13 possible comparisons between them and same-age controls (Fig. 1a). However, as indicated by the effect size indices of these comparisons (Fig. 1c, black bars), the pattern of impairments in the listeners with LPs varied with age, because across the five masking conditions, the deficits of these listeners were ultimately resolved (onset condition, row 4; forward condition; row 5), maintained (delay condition, row 3), and even acquired or magnified (long-tone condition, row 1; backward condition, row 2) as they progressed toward adulthood. Thus, what we had presumed to be a stable impairment pattern in fact varied with age. These developmental trends are consistent with data from other investigations in which multiple masking conditions, similar to the present ones, were tested. Compared with same-age controls, thresholds were significantly higher (effect sizes are not available) in the backward but not the onset condition for ≈9.3-year-old children with specific language impairment (31) and in the backward but not the forward or delay conditions for ≈13-year-old children with dyslexia (29). Additionally, thresholds for a briefer tone than was used here tended to decrease with increasing age in backward, forward, and delay conditions in controls between 5 and 11 years old (32).
Fig. 1.
(a and b) Average tone level required by listeners with LPs and controls to just detect a tone in the five masking conditions (rows; see schematics at far right). LP listeners (open squares) are compared with controls (filled squares) of the same age (a) and with controls ≈4 years younger (b). Between-group differences: **, P < 0.01; *, P < 0.05; n.s., P > 0.05. Error bars indicate ±SEM. (c) Effect size indices (d) on the same conditions calculated between LP listeners and (i) same-age controls (black bars), (ii) controls ≈2 years younger (hashed bars), and (iii) controls ≈4 years younger (white bars). SPL, sound pressure level.
This age-related variation in the impairment pattern can be accounted for by assuming that the perceptual development of children with LPs is delayed, and that some factor halts this (delayed) development in particular, predictable conditions. Supporting the assumption that the perceptual development of the children with LPs was delayed, all but one of the nine statistically significant differences between groups of the same age disappeared when we compared the thresholds of children with LPs to those of younger controls. We calculated effect sizes between the children with LPs (≤13-year-old age group) and two subsets of controls, those who were averages of 2 or 4 years younger than the children with LPs (Fig. 1c). These effect sizes were smaller with the 2-year-younger (hashed bars) than with the same-age (black bars) controls in five of six possible comparisons. The effect sizes were smaller yet, or even negative, with the 4-year-younger (white bars) than with the 2-year-younger (hashed bars) controls in another five of six possible comparisons. Correspondingly, the children with LPs had significantly higher thresholds in 7 of 8 possible comparisons with same-age controls (Fig. 1a) but only 3 of 11 comparisons with the controls who were 2 years younger (data not shown) and 1 of 7 comparisons with the controls who were 4 years younger (Fig. 1b). Also supporting the comparison with younger controls, the thresholds of the 8-year-old children with LPs make plausible predictions of the extrapolated thresholds of control children younger than those reported here (Fig. 1b, 8-year-old data). Overall, these comparisons suggest that the children with LPs were developmentally delayed by ≈2-4 years in their performance on these masking tasks.
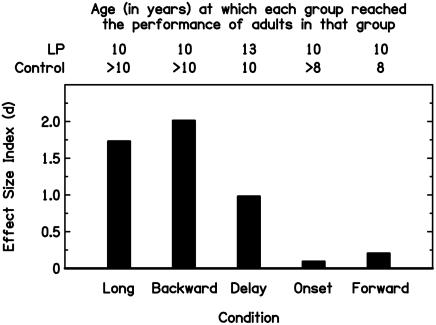
Finally, indicating that some factor halted the delayed development of listeners with LPs in some conditions, adults with LPs showed deficits only in conditions in which controls continued to improve after 10 years of age. In adulthood, the largest effect sizes between listeners with LPs and controls were associated with the longest developmental courses in controls (Fig. 2). The adults with LPs reached the performance of control adults in the three conditions in which normal development was either complete at or before 10 years of age (delay and forward conditions) or continued for an undetermined, although presumably brief, period after 8 years of age (onset condition; note that the 10-year-old listeners with LPs had already reached the level of adult controls). In contrast, the adults with LPs remained impaired in the two conditions in which development continued after 10 years of age in controls but stopped at 10 years of age in listeners with LPs (backward and long-tone conditions). Thus, it seems that something occurred at ≈10 years of age that transformed the LP listeners' delayed perceptual development in childhood into a difference in adulthood.
Fig. 2.
Effect size indices (d) calculated between adults with LPs and adult controls in each of the five conditions (black bars, replotted from Fig. 1) and the age at which listeners in each group reached the performance of adults in that group.
Discussion
In summary, these results suggest that individuals with LPs often perform poorly on auditory masking tasks, because their perceptual development is delayed in childhood and then halted, for some tasks, at ≈10 years of age. The present data thus add a set of nonlinguistic perceptual abilities to a growing list of wide-ranging skills reported to be delayed in children with LPs (15, 17, 21-24). They also are consistent with a recent proposal that reduced processing efficiency can explain the excessive amounts of auditory masking in individuals with LPs (33), because processing efficiency improves with increasing age (34). In addition, the current results indicate that some factor prevents these individuals from overcoming their delayed perceptual development and reaching the performance levels of their unaffected counterparts on skills that normally continue to develop through adolescence.
The observed developmental delay may arise from either genetic or environmental factors. If genetic factors are responsible, it may be that at least some of the specific genes already associated with LPs (35) play a role in determining developmental rate. Similarly, a number of environmental factors linked to LPs (36) might also contribute to the developmental delay. In any event, the generality of these auditory deficits across various diagnostic categories suggests that an understanding of the genetic and environmental contributions to LPs might be facilitated by examining a specific deficit across seemingly disparate subgroups that share it rather than within a single subgroup.
Neurobiological changes associated with puberty (37) are a likely cause for the halt in development on slowly acquired skills. Two pieces of circumstantial evidence support this idea: Human puberty begins at approximately the same time as the developmental arrest (38), and puberty in humans and other animals is associated with neurological changes that are thought to reduce brain plasticity (39, 40). To account for the current results, we assume that listeners with LPs and controls both reach the stage of puberty that affects perceptual development at ≈10 years of age. We are unaware of any data on the timing of puberty in individuals with LPs. However, illustrating that disordered neurological development need not delay sexual maturation, individuals with Down's syndrome reach puberty at, or perhaps before, the normal age (41, 42). We also assume that puberty only has the potential to influence the development of perceptual skills that normally continue to improve after the onset of puberty, a situation that could occur if, for example, different mechanisms were to govern slowly versus rapidly developing skills. In this scenario, children whose perceptual skills are developmentally delayed would acquire, more or less completely, auditory abilities that fully develop before puberty in controls (onset, delay, and forward conditions). They would just do so later than normal. However, these children would not fully acquire auditory abilities that normally continue to develop after puberty (backward and long-tone conditions). Two possible explanations for this failure would be that (i) puberty has a stronger effect on perceptual development in listeners with LPs than controls or (ii) listeners need to reach some critical level of performance by the time of puberty to continue improving after puberty and a delay in perceptual development prevents children with LPs from reaching this critical level. Intrinsic to the latter explanation is the possibility that puberty may actually enable further improvement on slowly acquired skills in normal development. Thus, the present data provide indirect evidence that puberty affects brain development in humans on skills other than language acquisition (39), as is expected from animal studies (39, 40), and indicate that investigations of individuals with perceptual delays but otherwise intact intellectual skills might help determine the role of puberty in human brain development.
One implication of these ideas is that the combination of delayed (15, 17, 21-24) and prematurely arrested development may account for an array of abnormalities observed in LP individuals compared with same-age controls. Consistent with this view, abnormalities in LP adults often occur on measures on which normal development extends into adolescence and thus might be halted by puberty. Paralleling the primary areas of focus of theories of language-based LPs, there are examples of this pattern in neurology [brain asymmetry (5, 43), white matter distribution (4, 44), and cerebellar activation (6, 45)] as well as in perceptual [auditory backward and long-tone masking (here and refs. 10 and 20) amplitude modulation detection (46, 47), and intensity discrimination (48, 49)], cognitive [working memory (12, 50) and rapid naming (18, 19, 51)], and linguistic [phonological awareness (13, 14, 16, 51)] functioning. Indeed, the mixture of normal and abnormal characteristics often observed in LP individuals, as well as the partial overlap between LP individuals and controls at any given age, may arise, in part, from an interaction among differences in the normal developmental time courses of those characteristics, the delayed development of LP individuals on those characteristics, and puberty. Of course, this interaction could be quite complicated if delay on one measure early in life were to alter the developmental course on other measures. Nevertheless, this developmental perspective might help unite the array of disparate individual abnormalities observed in individuals with LPs.
These ideas could be tested more thoroughly by retrospective or, ideally, longitudinal examinations of a variety of neurological, perceptual, cognitive, and linguistic measures in groups of individuals who are matched for gender and socioeconomic status and are well characterized both diagnostically and in terms of their sexual maturation. If correct, the data of children with LPs should resemble those of controls 2-4 years younger on at least a subset of these measures. Additionally, for the affected measures, the data of adults with LPs should match those of adult controls on measures that ordinarily reach asymptote before puberty but should be more similar to those of children at the age of puberty on measures that normally continue to develop during adolescence.
What remains to be determined is the precise relationship between delayed development and LPs. Two possibilities deserve consideration. Delayed development on any given measure may (i) accompany but not cause other developmental or nondevelopmental impairments that lead to LPs or (ii) cause LPs either by itself or in concert with other impairments. If there is a causal relationship, differences in clinical presentation across diagnostic subgroups could result from differences in which key neurological, perceptual, cognitive, and linguistic characteristics are developing later than normal or in the magnitude of the delay. Given the focus of the present experiment on auditory perceptual development, it is interesting to note that the earlier a deaf child receives a cochlear implant, the more likely that child is to acquire age-appropriate language skills (52). This pattern suggests that delays in perceptual development may play a key role in LPs.
Acknowledgments
We thank Miriam Reid, Kathryn Murrell, Robert Madory, Judy Paton, and Dr. Linda Lombardino for assistance with data collection and listener recruitment; Christopher Stewart for preparing the final figures; and Karen Banai, Matthew Fitzgerald, Julia Huyck, Julia Mossbridge, Jeanette Ortiz, Dr. Mario Ruggero, Dr. Catherine Woolley, Yuxuan Zhang, and two anonymous reviewers for helpful comments on previous drafts of this paper. This work was supported by the National Institutes of Health.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: LP, learning problem.
References
- 1.Department of Education (1985) Seventh Annual Report to Congress on the Implementation of Public Law 94-142: The Education for All Handicapped Children Act (Dept. Educ., Washington, DC).
- 2.Adelman, P. & Vogel, S. (1998) in Learning About Learning Disabilities, ed. Wong, B. (Academic, San Diego), 2nd Ed., pp. 657-701.
- 3.Galaburda, A. M. (1999) Dyslexia 5, 183-191. [Google Scholar]
- 4.Klingberg, T., Hedehus, M., Temple, E., Salz, T., Gabrieli, J. D. E., Moseley, M. E. & Poldrack, R. A. (2000) Neuron 25, 493-500. [DOI] [PubMed] [Google Scholar]
- 5.Morgan, A. E. & Hynd, G. W. (1998) Neuropsychol. Rev. 8, 79-93. [DOI] [PubMed] [Google Scholar]
- 6.Nicolson, R. I., Fawcett, A. J., Berry, E. L., Jenkins, I. H., Dean, P. & Brooks, D. J. (1999) Lancet 353, 1662-1667. [DOI] [PubMed] [Google Scholar]
- 7.Benasich, A. A. & Tallal, P. (2002) Behav. Brain Res. 136, 31-49. [DOI] [PubMed] [Google Scholar]
- 8.Stein, J. & Walsh, V. (1997) Trends Neurosci. 20, 147-152. [DOI] [PubMed] [Google Scholar]
- 9.Wright, B. A., Bowen, R. W. & Zecker, S. G. (2000) Curr. Opin. Neurobiol. 10, 482-486. [DOI] [PubMed] [Google Scholar]
- 10.Wright, B. A., Lombardino, L. J., King, W. M., Puranik, C. S., Leonard, C. M. & Merzenich, M. M. (1997) Nature 387, 176-178. [DOI] [PubMed] [Google Scholar]
- 11.Catts, H. W., Gillispie, M., Leonard, L. B., Kail, R. V. & Miller, C. A. (2002) J. Learn. Disabil. 35, 510-525. [DOI] [PubMed] [Google Scholar]
- 12.Swanson, H. L. (2003) J. Exp. Child Psychol. 85, 1-31. [DOI] [PubMed] [Google Scholar]
- 13.Bradley, L. & Bryant, P. (1981) Psychol. Res. 43, 193-199. [DOI] [PubMed] [Google Scholar]
- 14.Goswami, U. (2002) Ann. Dyslexia 52, 141-163. [Google Scholar]
- 15.Locke, J. L. (1994) J. Speech Hear. Res. 37, 608-616. [DOI] [PubMed] [Google Scholar]
- 16.Pennington, B. F., Van Orden, G. C., Smith, S. D., Green, P. A. & Haith, M. M. (1990) Child Dev. 61, 1753-1778. [PubMed] [Google Scholar]
- 17.Rice, M. L. (2004) in Developmental Language Disorders: From Phenotypes to Etiologies, eds. Rice, M. L. & Warren, S. F. (Erlbaum, Mahwah, NJ), pp. 207-240.
- 18.Wolf, M. (1999) Ann. Dyslexia 49, 3-28. [Google Scholar]
- 19.Wolff, P. H., Michel, G. F. & Ovrut, M. (1990) Brain Lang. 39, 556-575. [DOI] [PubMed] [Google Scholar]
- 20.Hartley, D. E. H., Wright, B. A., Hogan, S. C. & Moore, D. R. (2000) J. Speech Lang. Hear. Res. 43, 1402-1415. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro, B. K., Palmer, F. B., Antell, S., Bilker, S., Ross, A. & Capute, A. J. (1990) Pediatrics 85, 416-420. [PubMed] [Google Scholar]
- 22.Bishop, D. V. M. & Edmundson, A. (1987) Dev. Med. Child Neurol. 29, 442-459. [DOI] [PubMed] [Google Scholar]
- 23.Bishop, D. V. M. & McArthur, G. M. (2004) Dev. Sci. 7, F11-F18. [DOI] [PubMed] [Google Scholar]
- 24.Saugstad, L. F. (1999) Schizophr. Res. 39, 183-196. [DOI] [PubMed] [Google Scholar]
- 25.Bellis, T. J. (1996) Assessment and Management of Central Auditory Processing Disorders in the Educational Setting: From Science to Practice (Singular, San Diego).
- 26.Green, D. M. (1990) J. Acoust. Soc. Am. 87, 2662-2674. [DOI] [PubMed] [Google Scholar]
- 27.Wright, B. A. (1996) J. Acoust. Soc. Am. 100, 1717-1721. [DOI] [PubMed] [Google Scholar]
- 28.Wright, B. A. (1996) J. Acoust. Soc. Am. 100, 3295-3303. [DOI] [PubMed] [Google Scholar]
- 29.Rosen, S. & Manganari, E. (2001) J. Speech Lang. Hear. Res. 44, 720-736. [DOI] [PubMed] [Google Scholar]
- 30.Cohen, J. (1988) Statistical Power Analysis for the Behavioral Sciences (Erlbaum, Hillsdale, NJ), 2nd Ed.
- 31.Marler, J. A., Champlin, C. A. & Gillam, R. B. (2002) Psychophysiology 39, 767-780. [DOI] [PubMed] [Google Scholar]
- 32.Buss, E., Hall, J. W., III, Grose, J. H. & Dev, M. B. (1999) J. Speech Lang. Hear. Res. 42, 844-849. [DOI] [PubMed] [Google Scholar]
- 33.Hartley, D. E. H. & Moore, D. R. (2002) J. Acoust. Soc. Am. 112, 2962-2966. [DOI] [PubMed] [Google Scholar]
- 34.Schneider, B. A., Trehub, S. E., Morrongiello, B. A. & Thorpe, L. A. (1989) J. Acoust. Soc. Am. 86, 1733-1742. [DOI] [PubMed] [Google Scholar]
- 35.Olson, R. K. (2002) Dyslexia 8, 143-159. [DOI] [PubMed] [Google Scholar]
- 36.Adelman, H. S. & Taylor, L. (1983) Learning Disabilities in Perspective (Foresman and Company, Glenview, IL).
- 37.Bourgeois, J.-P., Goldman-Rakic, P. S. & Rakic, P. (2000) in The New Cognitive Neurosciences, ed. Gazzaniga, M.S. (MIT Press, Cambridge, MA), 2nd Ed., pp. 45-53.
- 38.Jones, R. E. (1984) Human Reproduction and Sexual Behavior (Prentice-Hall, Englewood Cliffs, NJ).
- 39.Doupe, A. J. & Kuhl, P. K. (1999) Annu. Rev. Neurosci. 22, 567-631. [DOI] [PubMed] [Google Scholar]
- 40.Linkenhoker, B. A. & Knudsen, E. I. (2002) Nature 419, 293-296. [DOI] [PubMed] [Google Scholar]
- 41.Hsiang, Y. H., Berkovitz, G. D., Bland, G. L., Migeon, C. J. & Warren, A. C. (1987) Am. J. Med. Genet. 27, 449-458. [DOI] [PubMed] [Google Scholar]
- 42.Arnell, H., Gustafsson, J., Ivarsson, S. A. & Anneren, G. (1996) Acta Pediatr. 85, 1102-1106. [DOI] [PubMed] [Google Scholar]
- 43.Sowell, E. R., Peterson, B. S., Thompson, P. M., Welcome, S. E., Henkenius, A. L. & Toga, A. W. (2003) Nat. Neurosci. 6, 309-315. [DOI] [PubMed] [Google Scholar]
- 44.Paus, T., Collins, D. L., Evans, A. C., Leonard, G., Pike, B. & Zijdenbos, A. (2001) Brain Res. Bull. 54, 255-266. [DOI] [PubMed] [Google Scholar]
- 45.Castellanos, F. X., Lee, P. P., Sharp, W., Jeffries, N. O., Greenstein, D. K., Clasen, L. S., Blumenthal, J. D., James, R. S., Ebens, C. L., Walter, J. M., et al. (2002) J. Am. Med. Assoc. 288, 1740-1748. [DOI] [PubMed] [Google Scholar]
- 46.Hall, J. W., III, & Grose, J. H. (1994) J. Acoust. Soc. Am. 96, 150-154. [DOI] [PubMed] [Google Scholar]
- 47.Menell, P., McAnally, K. I. & Stein, J. F. (1999) J. Speech Lang. Hear. Res. 42, 797-803. [DOI] [PubMed] [Google Scholar]
- 48.Maxon, A. B. & Hochberg, I. (1982) Ear Hear. 3, 301-308. [DOI] [PubMed] [Google Scholar]
- 49.McArthur, G. M. & Hogben, J. H. (2001) J. Acoust. Soc. Am. 109, 1092-1100. [DOI] [PubMed] [Google Scholar]
- 50.Swanson, H. L. (1999) Dev. Psychol. 35, 986-1000. [DOI] [PubMed] [Google Scholar]
- 51.Wagner, R. K., Torgeson, J. K. & Rashotte, C. A. (1999) The Comprehensive Test of Phonological Processing (Pro-Ed, Austin, TX).
- 52.Hammes, D. V., Novak, M. A., Rotz, L. A., Willis, M., Edmondson, D. M. & Thomas, J. F. (2002) Ann. Otol. Rhinol. Laryngol. Suppl. 189, 74-78. [DOI] [PubMed] [Google Scholar]