Abstract
Cytoblocks prepared from residual tissue fluids and fine-needle aspirations can be useful adjuncts to smears for establishing a more definitive cytopathologic diagnosis. These paraffin embedded cytoblocks have been popular since these can be handled like any other histologic specimen. Rapid on-site evaluation (ROSE) can help in attaining adequate material in the cytoblock, which is a major concern to the cytopathologists. Ancillary studies can be done using cytoblocks including IHC and various molecular techniques. The opportunities for cytopathologists to influence therapy, and uncover strategies in the complex field of lung cancer are exciting and limitless especially in the presence of an adequate cytoblock
Keywords: Cytology, epidermal growth factor receptor (EGFR) mutations, fine-needle aspiration cytology (FNAC), immunochemistry, lung cancer
Lung Cancer
Lung cancer is one of the most common causes of cancer related mortality worldwide. It accounts for 1-5 million deaths in the world and 2.5 million deaths in developing countries. In India, approximately 63,000 new lung cancer cases are reported each year.[1] More than 70% lung cancers are unresectable and present clinically in an advanced stage. Therefore, many a time only a small biopsy or cytology samples such as fine-needle aspiration cytology (FNAC) and fluid specimens are available for diagnostic workup and molecular characterization. Lung cancer is broadly categorized into non-small cell lung carcinoma (NSCLC) and small-cell carcinoma. Approximately 85% lung cancers are of NSCLC type. NSCLCs are categorized into squamous-cell carcinoma (SCC), adenocarcinoma of the lung (LADC), and large-cell carcinoma that is a combination of poorly differentiated LADC and other uncommon cell types. Historically, all subtypes of NSCLCs were given similar chemotherapy, so further categorization of NSCLC into LADC and SCC was not important on fine-needle aspirates. But treatment in the present era for lung cancer is personalized and is based on the subtype of lung cancer (LADC vs SCC) and molecular status that is, epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements. Accurate subtyping of NSCLC into LADC and SCC and identification of the molecular status is mandatory for administrating the appropriate therapy.
Necessity to Subclassify NSCLC
Lung cancer therapy has become personalized based on specific subtypes of lung cancer. The number of recent clinical studies has shown that:
The response rate and survival with pemetrexed[2,3,4] are significantly better in patients with LADC and other “nonsquamous NSCLC.”
Severe life-threatening pulmonary hemorrhage with bevacizumab [a recombinant humanized monoclonal antibody inhibitor of vascular endothelial growth factor (VEGF)] can occur in patients with SCC.[5]
NSCLCs with EGFR mutations respond better to EGFR tyrosine kinase inhibitors (TKI) than wild-type tumors.[6,7,8,9,10] Histological subtyping of tumors is important, as a majority of EGFR mutant NSCLCs are adenocarcinomas
NSCLC harboring echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) translocations are responsive to ALK kinase inhibitors.[11,12,13] EML4-ALK is found almost exclusively in LADC.
So, it is very important to subclassify NSCLC into adenocarcinoma and squamous cell carcinoma for the benefit of patients to receive therapy with low adverse effect and high therapeutic response and for further diagnostic workup, that is, analysis of EGFR mutation and ALK rearrangement.
Fine-needle aspiration cytology
FNAC is a simple, relatively safe, and rapid reliable technique for the diagnosis of pulmonary lesions, particularly with the aid of computed tomography (CT) scan. This is a minimally painful, nonoperative procedure as compared to biopsy for the diagnostic purpose of lung lesions and outweighs the single major complication of the pneumothorax. Previous reports showed that the sensitivity of FNAC for lung carcinoma diagnosis ranged from 50% to more than 90%, and specificity was approximately 100%. The overall positive predictive value was close to 99% with false negative rate of around 10%.[14,15] The main reason for false negative rate is sampling error, that is, the failure to obtain adequate diagnostic material. Rapid on-site evaluation (ROSE) for cellularity is of value in reducing the number of false negative diagnoses due to nondiagnostic material.[16]
FNAC is also a well-established technique for providing excellent material for ancillary studies such as immunochemistry, fluorescence in situ hybridization (FISH), ultrastructural examination, cytogenetics, cell culture preparations, and molecular studies. The need of the hour is to obtain more information from less and less material obtained through minimally invasive procedures such as FNAC. FNAC also offers other advantages over core biopsies for molecular testing such as:
Multiple passes can be taken by FNAC for wider representation of tumor,
FNAC allows obtaining a higher number of tumor cells with less stroma,
Rapid on-site evaluation is possible in FNA so as to triage the sample and to assess tumor tissue, and
FNAC provides better archival DNA quality due to the use of air-dried and/or alcohol fixatives, as compared to formaldehyde-based fixatives used in histology.
The main challenge to a cytopathologist in the present era of personalized treatment is to be able to devise techniques that can provide more information with less tissue available.[17] We can differentiate between small-cell carcinoma and NSCLC in over 90% cases by FNAC. In 10-40% of NSCLC cases, it may not be possible to subtype NSCLC based on morphology alone that applies for both small biopsies as well as cytology samples. The strongest predictors of difficulty in the subtyping of NSCLC on morphology in FNAC include poor differentiation of the tumor, necrosis, low specimen cellularity, and squamous histology. Unclassifiable NSCLC-not otherwise specified (NOS) cases are usually not more than 10% after using immunohistochemistry (IHC) panel. Immunochemistry can be applied in small diagnostic samples such as effusion samples and transcutaneous FNAC or transbronchial needle aspiration (TBNA) as conventional smears, liquid-based cytology (LBC), or cell block preparations.
Effusions
Fluid samples are sent to the cytopathology laboratory as a routine test for diagnostic purposes. The main purpose of the cytological evaluation of effusion samples is to look for the presence of malignant cells. Many times, effusion samples that are reported as positive for malignant cells are highly cellular. The cell block preparation can easily be done in such cases and by applying a panel of immunostains, the primary site of malignancy can be evaluated. The diagnostic difficulty on the morphology to distinguish between mesothelioma and metastatic lung carcinoma can also be dealt with by IHC. A panel comprising two mesothelial markers [calretinin, WT-1 (Wilms tumor-1), cytokeratin 5/6 (CK5/6)] and two lung adenocarcinoma markers [cytokeratin 7 (CK7), thyroid transcription factor-1 (TTF-1), napsin A] should be used in such cases.
Cell block preparation
Cell block is a mini formalin-fixed paraffin-embedded (FFPE) biopsy obtained from fine-needle aspirate or fluid sediment. Preservation of cytologic material in the cell block for IHC and molecular studies adds to its diagnostic accuracy[18] and enables long-term archiving for future analyses. Cell block also helps in providing additional architectural information. LBC is another technique that can also be used to prepare cell blocks with better preservation of tumor cells.
Classification of lung cancer
The new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification was developed by international multidisciplinary panel comprising medical oncologists, physicians, thoracic surgeons, radiologists, pathologists, and molecular biologists.[19,20] It provides criteria and a standard terminology for the diagnosis of lung cancer in cytology and small biopsies. According to this classification, if clear squamous or adenocarcinoma differentiation is seen on morphology, a tumor can be diagnosed as SCC or adenocarcinoma.[21]
Cytologically, adenocarcinoma can have various architectural patterns including sheets, three-dimensional cell clusters, papillary clusters, acinar structures, “picket fence,” or “drunken honeycomb.”[22] Individual tumor cells have homogenous basophilic cytoplasm that may be granular and foamy and may show cytoplasmic vacuoles. The nuclei are usually eccentrically placed with uniform finely granular to coarse hyperchromatic chromatin. Majority of the tumor cells have macronucleoli. Squamous differentiation is manifested by three main morphological features: Keratinization, intercellular bridges, and pearls. Keratinization is seen as a characteristic inky blue color on the Romanowsky stains.[22] The cytoplasm is usually dense or opaque. Cells often have variable shapes ranging from round, oval, to elongated cells with well-demarcated cell borders. Nuclei are hyperchromatic and centrally located with dense homogenous pyknotic chromatin. Nucleoli are usually not seen. A diagnosis of large-cell carcinoma should not be offered on cytology and should be reserved only for resection specimens.
Immunochemistry
Immunochemistry can be done on alcohol-fixed cytology smears or cell blocks. The IHC done on cell blocks offers better results and therefore, cell block preparation should be done in cases where IHC or other ancillary techniques are needed.
Use of immunohistochemical stains to distinguish LADC and SCC
IHC should be performed in NSCLC lacking proper adenocarcinoma or squamous morphology. A panel of antibodies should be used rather than using a single antibody to increase sensitivity and specificity. There are several markers for LADC and SCC with different sensitivity and specificity. A few of them are highlighted in the Table 1.
Table 1.
Immunohistochemical markers used to subtype NSCLC, NOS
TTF-1
TTF-1 is a 38-kDa homeodomain protein. Gene expression in the thyroid, lungs, and diencephalon during embryogenesis is regulated by TTF-1. It is expressed in ciliated respiratory epithelial cells, alveolar pneumocytes, basal cells of the lung, and clara cells.[23,24] TTF-1 is used to differentiate between primary and metastatic LADCs of the lung.[25] TTF-1 is a nuclear stain that is reported in 87% of LADCs and 2% of SCCs.
p63
p63 is located on chromosome 3q27-29 and is a member of the p53 family. p63 positivity is seen normally in basal cells of the urothelium, in basal cells of the squamous epithelium, and in basal cells of the prostate epithelium.[26,27] p63 can be detected in SCCs of different primary sites including SCCs of the lung. Notably, 80-97% of lung SCCs show nuclear positivity for p63. Up to 18% of lung LADCs can show positivity for p63.[26,27,28] Only strong nuclear positivity should be considered as positive to interpret p63.
Cytokeratins
Cytokeratins are intermediate filament cytoskeletal proteins needed for the development and differentiation of epithelial cells. CK7 is a type II cytokeratin, seen in most glandular epithelia and in transitional epithelia. Anti-CK7 is of value to subtype various carcinomas and tumors of epithelial origin.
CK5/6 can be seen in normal cells including prostate basal cells, breast myoepithelial cells, basal cells of the epidermis, and salivary glands. CK5/6 shows a a membranous staining pattern. Marson et al.[29] found 100% of primary lung SCCs showing CK5/6 positivity.
There are number of studies done with panel of IHC markers to differentiate adenocarcinoma and squamous cell carcinoma. The six-antibody panel consisting of napsin A, desmoglein 3, TTF-1, CK5/6, p63, and tripartite motif-containing protein 29 in one study[30] classified 96% and 87% of moderately differentiated and poorly differentiated lung cancer, respectively. In a study by Terry J et al.[31] to distinguish lung adenosine deaminase (ADA) from SCC in small specimens, the reported sensitivity and specificity of these markers are shown in Table 2.
Table 2.
Sensitivity and specificity of the immunomarkers[31]
Napsin A is relatively specific for LADC and stains approximately 80% of the cases. It is a cytoplasmic stain. A dual staining for TTF-1 and napsin A has been shown in LADC cell blocks in previous reports.[32]
IHC panel — Thrombomodulin (CD141), p63, 34βE12, and CK5/6 have been shown to be sensitive markers for SCC. The coexpression of p63 and CK5 has high sensitivity with 100% specificity for SCC [Figure 1]. TTF-1 and napsin A are considered to be specific markers for LADC [Figure 2]. Therefore, a preferred panel of immunostains comprising TTF-1, p63, CK5/6, CK7, and napsin A should provide high specificity and sensitivity in differentiating SCC versus LADC.
Figure 1.
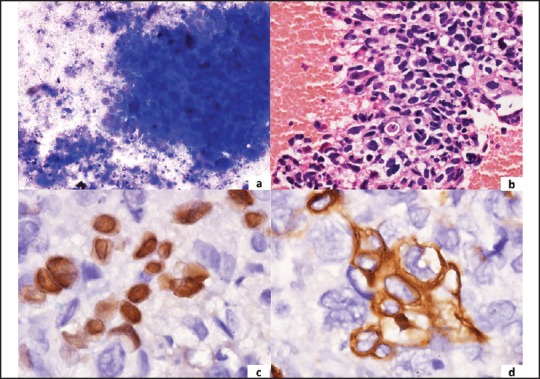
A panel of microphotographs of pulmonary squamous-cell carcinoma (a) Tumor cell cluster with necrosis in the background (MGG, ×400); (b) Cell block showing squamoid differentiation of tumor cells (H and E, ×400); (c) Nuclear positivity for p63 (IHC, ×1000) (d) Cytoplasmic positivity for CK5/6 (IHC, ×1000)
Figure 2.
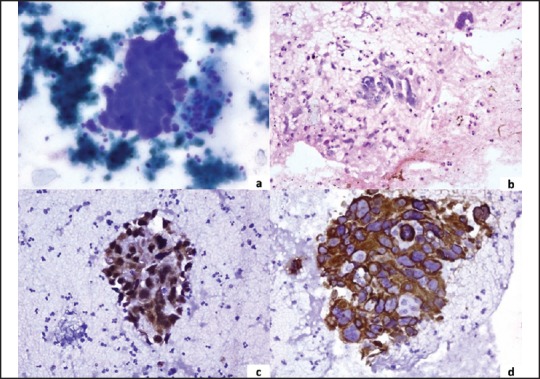
A group of microphotographs of pulmonary adenocarcinoma (a) Tumor cell cluster (MGG, ×400) (b) Cell block showing a cluster of tumor cells (H and E, ×400) (c) Nuclear positivity for TTF-1 (IHC, ×400) (d) Cytoplasmic positivity for CK7 (IHC, ×400)
EGFR mutation analysis
The presence of mutations in exons 18-21, deletions in exon 19 (52%), and a missense mutation L858R in exon 21 (26%) are the most common mutations. Mutations in the TK domain of EGFR are common in NSCLC in the Asian population.[33] The most common mutation found in approximately 46% cases with EGFR mutations is a short in-frame deletion of 9, 12, 15, 18, or 24 nucleotides in exon 19. The other common mutation, seen in about 43% of cases with EGFR mutations, is a point mutation (CTG to CGG) in exon 21 at nucleotide 2573 that results in the substitution of leucine by arginine at codon 858 (L858R). EGFR molecular testing on cytology samples can well be done with the availability of cell blocks. Depending on the methods used, investigators in previous reports were able to detect these mutations in cytologic material containing as little as 0.1-10% of tumor cells or in specimens containing at least 100 tumor cells. Failure of EGFR molecular testing on cytology samples ranges from 2-8% to approximately a quarter of cases in some studies and failure rates are more likely technique dependent. The main reasons for test failure in cytology samples are scant cellularity and necrotic tissue.[34]
EGFR mutation by immunohistochemistry
Immunohistochemistry using monoclonal antibodies specific to exon 19 E746-A750 (15bp) deletion and exon 21 (L858R) mutation is available. These antibodies were used on cytology samples (FNA and effusion fluid), small biopsies, LBC samples, cell blocks, and resection specimens.[34] IHC with these antibodies can be used as a screening method for EGFR mutations and all negative cases can be sent for molecular analysis to confirm mutation status.
EML4-ALK rearrangement testing
The EML4-ALK fusion gene is seen in approximately 5% of LADC cases. Patients with the EML4-ALK fusion gene do not harbor EGFR or KRAS mutations and hence demonstrating mutual exclusion of these mutations. FISH using break-apart probes is the gold standard for the detection of EML4-ALK. It has been seen that due to the relatively high cost, limited availability, and technical complexity of FISH, IHC can be carried out as a screening method and FISH may be reserved for cases with equivocal results. Sakairi et al.[35] examined 109 samples obtained by endobronchial ultrasound (EBUS)-TBNA and found that the material obtained was adequate for ALK fusion gene assessment by IHC, FISH, and reverse transcription polymerase chain reaction (RT-PCR). Cell block can provide persevered cytology material for such studies including IHC, FISH, and RT-PCR.
FISH in respiratory cytology
FISH is the gold standard to identify ALK rearrangements for treatment with the ALK inhibitor crizotinib. FISH can be applied to almost all types of cytologic specimens such as conventional smears, cytospins, or LBC preparations and cell blocks.
Conclusions
In conclusion, cytoblocks prepared from residual tissue fluids and fine-needle aspirations (transcutaneous FNAC, TBNA, or EBUS-TBNA) can be useful adjuncts to smears for establishing a more definitive cytopathologic diagnosis. These paraffin-embedded cytoblocks have been popular since these can be handled like any other histologic specimen. Rapid on-site evaluation (ROSE) can help in attaining adequate material in the cytoblock that is a major concern to the cytopathologists. Ancillary studies can be done using cytoblocks including IHC and various molecular techniques. The opportunities for cytopathologists to influence therapy and uncover strategies in the complex field of lung cancer are exciting and limitless, especially in the presence of an adequate cytoblock.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Noronha V, Dikshit R, Raut N, Joshi A, Pramesh CS, George K, et al. Epidemiology of lung cancer in India: Focus on the differences between non-smokers and smokers: A single-centre experience. Indian J Cancer. 2012;49:74–81. doi: 10.4103/0019-509X.98925. [DOI] [PubMed] [Google Scholar]
- 2.Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomized, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 3.Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, et al. The differential efficacy of pemetrexed according to NSCLC histology: A review of two phase III studies. Oncologist. 2009;14:253–63. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small- cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 7.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harboring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomized Phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open label, randomized phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomized, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki T, Jänne PA. New strategies for treatment of ALK-rearranged non-small-cell lung cancers. Clin Cancer Res. 2011;17:7213–8. doi: 10.1158/1078-0432.CCR-11-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17:2081–6. doi: 10.1158/1078-0432.CCR-10-1591. [DOI] [PubMed] [Google Scholar]
- 13.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase Inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nizzoli R, Tiseo M, Gelsomino F, Bartolotti M, Majori M, Ferrari L, et al. Accuracy of fine needle aspiration cytology in the pathological typing of non-small-cell lung cancer. J Thorac Oncol. 2011;6:489–93. doi: 10.1097/JTO.0b013e31820b86cb. [DOI] [PubMed] [Google Scholar]
- 15.Zakowski MF. Fine-needle aspiration cytology of tumors: Diagnostic accuracy and potential pitfalls. Cancer Invest. 1994;12:505–15. doi: 10.3109/07357909409021411. [DOI] [PubMed] [Google Scholar]
- 16.Stewart CJ, Stewart IS. Immediate assessment of fine needle aspiration cytology of lung. J Clin Pathol. 1996;49:839–43. doi: 10.1136/jcp.49.10.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakowski MF, Bibbo M. Lung carcinoma in the era of personalized medicine: The role of cytology. Acta Cytol. 2012;56:587–9. doi: 10.1159/000345183. [DOI] [PubMed] [Google Scholar]
- 18.Sanz-Santos J, Serra P, Andreo P, Llatjós M, Castellà E, Monsó E. Contribution of cell blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration to the diagnosis of lung cancer. BMC Cancer. 2012;12:34. doi: 10.1186/1471-2407-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: Strategic management of tissue for molecular testing. Semin Respir Crit Care Med. 2011;32:22–31. doi: 10.1055/s-0031-1272866. [DOI] [PubMed] [Google Scholar]
- 20.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. American Thoracic Society. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International multidisciplinary classification of lung adenocarcinoma: Executive summary. Proc Am Thorac Soc. 2011;8:381–5. doi: 10.1513/pats.201107-042ST. [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Müller-Hermelink HK, Harris CC, editors . Lyon, France: IARC; 2004. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart; pp. 26–30. [Google Scholar]
- 22.Johnston WW, Frable WJ. The cytopathology of the respiratory tract. A review. Am J Pathol. 1976;84:372–424. [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura N, Miyagi E, Murata S, Kawaoi A, Katoh R. Expression of thyroid transcription factor-1 in normal and neoplastic lung tissues. Mod Pathol. 2002;15:1058–67. doi: 10.1097/01.MP.0000028572.44247.CF. [DOI] [PubMed] [Google Scholar]
- 24.Lau SK, Luthringer DJ, Eisen RN. Thyroid transcription factor-1: A review. Appl Immunohistochem Mol Morphol. 2002;10:97–102. doi: 10.1097/00129039-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Ng WK, Chow JC, Ng PK. Thyroid transcription factor-1 is highly sensitive and specific in differentiating metastatic pulmonary from extrapulmonary adenocarcinoma in effusion fluid cytology specimens. Cancer. 2002;96:43–8. doi: 10.1002/cncr.10310. [DOI] [PubMed] [Google Scholar]
- 26.Massion PP, Taflan PM, Jamshedur Rahman SM, Yildiz P, Shyr Y, Edgerton ME, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63:7113–21. [PubMed] [Google Scholar]
- 27.Pelosi G, Pasini F, Olsen Stenholm C, Pastorino U, Maisonneuve P, Sonzogni A, et al. p63 immunoreactivity in lung cancer: Yet another player in the development of squamous cell carcinomas? J Pathol. 2002;198:100–9. doi: 10.1002/path.1166. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh HA, Fuhrer K, Cieply K, Yousem S. p63 expression in assessment of bronchioloalveolar proliferations of the lung. Mod Pathol. 2004;17:1134–40. doi: 10.1038/modpathol.3800163. [DOI] [PubMed] [Google Scholar]
- 29.Jerome Marson V, Mazieres J, Groussard O, Garcia O, Berjaud J, Dahan M, et al. Expression of TTF-1 and cytokeratins in primary and secondary epithelial lung tumours: Correlation with histological type and grade. Histopathology. 2004;45:125–34. doi: 10.1111/j.1365-2559.2004.01893.x. [DOI] [PubMed] [Google Scholar]
- 30.Tacha D, Yu C, Bremer R, Qi W, Haas T. A 6-antibody panel for the classification of lung adenocarcinoma versus squamous cell carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:201–7. doi: 10.1097/PAI.0b013e31823d7f0e. [DOI] [PubMed] [Google Scholar]
- 31.Terry J, Leung S, Laskin J, Leslie KO, Gown AM, Ionescu DN. Optimal immunohistochemical markers for distinguishing lung adenocarcinomas from squamous cell carcinomas in small tumor samples. Am J Surg Pathol. 2010;34:1805–11. doi: 10.1097/PAS.0b013e3181f7dae3. [DOI] [PubMed] [Google Scholar]
- 32.Fatima N, Cohen C, Lawson D, Siddiqui MT. TTF-1 and Napsin A double stain: A useful marker for diagnosing lung adenocarcinoma on fine-needle aspiration cell blocks. Cancer Cytopathol. 2011;119:127–33. doi: 10.1002/cncy.20135. [DOI] [PubMed] [Google Scholar]
- 33.Sahoo R, Harini VV, Babua VC, Patil Okaly GV, Rao S, Nargund A, et al. Kumar BSA. Screening for EGFR mutations in lung cancer, a report from India. Lung Cancer. 2011;73:316–9. doi: 10.1016/j.lungcan.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Moreira AL, Hasanovic A. Molecular charactrization by immunocytochemistry of lung adenocarcinoma on cytology specimens. Acta Cytol. 2012;56:603–10. doi: 10.1159/000339794. [DOI] [PubMed] [Google Scholar]
- 35.Sakairi Y, Nakajima T, Yasufuku K, Ikebe D, Kageyama H, Soda M, et al. EML4-ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res. 2010;16:4938–45. doi: 10.1158/1078-0432.CCR-10-0099. [DOI] [PubMed] [Google Scholar]


