Abstract
Background:
Human papillomavirus (HPV) is known to be involved in the carcinogenesis of squamous cells in uterine cervix cancer, mostly by binding and inactivating the p53 and pRb tumor suppressor genes. Lately, evidence has emerged suggesting that HPV oncoproteins may interact with proteins involved in cellular apoptosis as well.
Aim:
This study aimed to investigate the expression of proapoptotic proteins Bax and Bak in women with low-risk and high-risk HPV types as opposed to HPV-negative women, and in women with normal pap smear compared to women with abnormal Papanicolau test (Pap) smear.
Materials and Methods:
A total of 120 liquid-based cervical samples were subtyped for HPV types with microarray hybridization and then stained and evaluated immunocytochemically for Bax and Bak expression. Statistical analysis was performed on the Bax and Bak scores (percentage of positive cells × staining intensity), the overall percentage of positive cells, and the most prevalent staining intensity group found in each sample.
Results:
A weak association between negative Bax staining and cytologically normal Pap smears was discovered, whereas cytologically abnormal samples tended to stain weakly or moderately positive. No other statistically significant difference was found in the other analyzed parameters.
Conclusion:
Cytologically normal pap smears seem to have a slight tendency to stain negative for Bax as opposed to cytologically abnormal pap smears. Although the association is weak, it is an indication that there might be a connection between the expression of Bax and the development of cervical intraepithelial dysplasia, which warrants further investigation in larger-scale studies.
Keywords: Bak, Bax, cytology, human papillomavirus (HPV), Papanicolaou test (Pap) smear
Introduction
Based on epidemiological, clinical, and laboratory data, human papillomavirus (HPV) has been identified as a necessary cause for the development of uterine cervical cancer,[1] the second most common female cancer worldwide.[2] More precisely, the high-risk subtypes of the alpha genus HPV virus play a critical role in the development of cervical cancer and are involved in the development of most other anogenital cancers and a substantial percentage of head and neck cancers, whereas beta-HPVs are considered to have less carcinogenic potential, although lately they have been linked to the development of skin cancers.[3] Although the mechanisms by which HPV triggers the immortalization of keratinocytes and thus promotes dysplasia and carcinogenesis have been exhaustingly analyzed, it is not yet entirely clear which factors modify the potential of the various HPV types for cancer development. It has been established that the high-risk HPV types are associated with carcinogenesis through their genes E5, E6, and E7 from the early region of the viral genome, which encode the oncoproteins of the same name.[4] Studies have shown that besides the two important suppressor genes p53 and pRb, E6 and E7 also bind other proteins such as the proapoptotic proteins Bax and Bak, and this might play an important role in the increase of their carcinogenic potential.[5,6,7] Therefore, Bax and Bak expression in women infected by low- and high-risk HPV types could be a potentially useful prognostic marker. The aim of this study was to compare the expression of the Bax and Bak proteins in women infected by low- and high-risk HPV types and to investigate the usefulness of examining this expression as part of the standard screening test. To that end, Bax and Bak expression was tested not on tissue but on cytocentrifuged ThinPrep (Cytyk, Crawley, UK) cervical cell samples, which were also used for HPV subtyping. Moreover, Bax and Bak expression was statistically analyzed with correlation to the cytological examination of the Papanicolaou test (Pap) smears of those women, to determine whether there was any statistically significant difference in the expression of those two proteins in women with abnormal pap smear [atypia of squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or high-grade squamous intraepithelial lesion (HSIL)] as opposed to women with normal Pap smear.
Materials and Methods
In this study, we examined samples of 120 women, 16-62 years of age (average age 33.4 years). After obtaining informed consent from them, these women had a conventional pap smear and a ThinPrep (PreservCyt Solution 20 mL) sample for HPV subtyping taken with a flexible brush. HPV subtyping was performed by microarrays hybridization. After HPV subtyping, 2 mL of the ThinPrep solution were taken from each case. Each sample was centrifuged in a cytocentrifuge (Shandon Cytospin, Thermo Shandon Ltd, Runcorn, UK) at 1200 rpm for 5 min. The resulting slides were air-dried at room temperature for 30 min and then stored in deep freeze (–8° to –15°C).
The immunocytochemical staining of the slides was carried out according to the EnVision protocol 3-1 UNMASKING with MW for cellular smears (Dako, Glostrup, Denmark). For the detection of Bax and Bak proteins we used anti-human polyclonal rabbit antibodies (Rb Anti-Bax Pab, 7 mL, ready-to-use, diluted 1:1 and Rb Anti-Bak Pab, 7 mL, ready-to-use, Spring Bioscience, Pleasanton, California, USA), detection system EnVision/HRP Rabbit/Mouse, 100 mL, ready-to-use (Dako, Glostrup, Denmark) with peroxidase and secondary antibodies against rabbit and mouse immunoglobulin molecules, and chromogen Liquid DAB + Chromogen, 50× concentrate with Substrate Chromogen System, diluted 1:50 (Dako, Glostrup, Denmark). Mayer's hematoxylin was used for counterstain.
Statistical analysis of the results was performed with the SPSS Statistic Packs 17.0 program (SPSS Inc., Chicago, Illinois, USA). Statistical differences were calculated by t-test, chi-square test, and one-way analysis of variance (ANOVA). In all tests, P < 0.05 was taken as the limit of significance.
Results
Cytological examination
Cytological examination of the Pap tests showed that out of 120 cases, 88 were negative for intraepithelial lesion or malignancy (NILM), 12 had ASCUS, 18 had LSIL, and 2 had HSIL. More specifically, out of the 70 cases negative for HPV types, 60 were NILM, 6 ASCUS, and 4 LSIL, while out of the 50 cases positive for HPV types, 28 were NILM, 6 ASCUS, 14 LSIL, and 2 HSIL.
HPV subtyping
Of the 120 cases, 70 were negative for the examined HPV types (control group) and 50 were positive for 1 or more of the examined types (33 cases were positive for 1 type, 11 for 2 types, 2 for 3 types, 3 for 4 types, and 1 for 5 HPV types). Of the 50 cases positive for HPV types, low-risk HPV types were detected in 12, high-risk HPV types were detected in 27, and both low- and high-risk HPV types were detected in 11. Of the examined types, the one more frequently encountered was type 42 with 12 cases, followed by types 44/55, 51, 59, 16, 31, etc. The women infected with both low- and high-risk HPV types were grouped in the high-risk type group, thus making up a total of 38 cases in this group.
Bax and Bak score statistical analysis
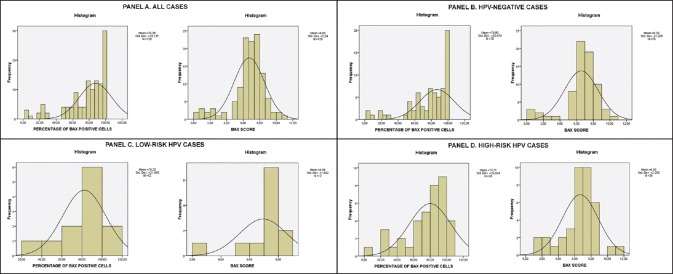
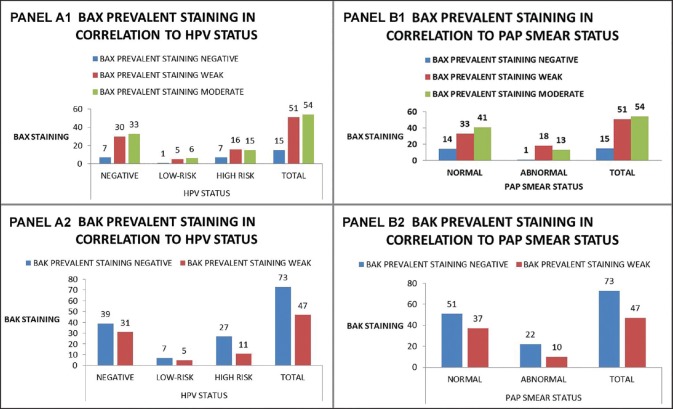
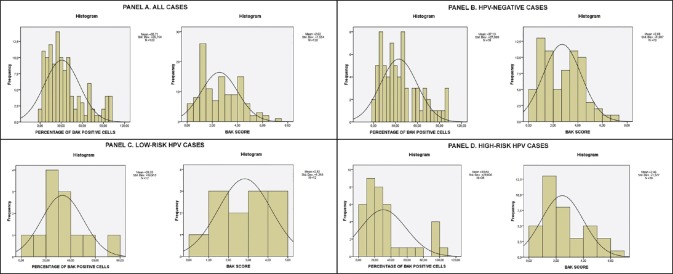
The evaluation of the expression of the Bax and Bak proteins was carried out by viewing at least 1000 cells per slide (fifty ×400 power fields) in order to be considered accurate. According to criteria cited in previous papers,[8,9] evaluation of expression depended on stain intensity and percentage of positive staining cells. Percentage of cells was graded as follows: 0: no reactive cells, 1: 1-25%, 2: 26-50%, 3: 51-75%, and 4: 76-100%. Stain intensity was graded as follows: 0: No staining, 1: Weak staining, 2: Moderate staining, and 3: Intense staining [See Figure 1 for Bax and Figure 2 for Bak]. The two values were multiplied and the result was the score for each field. If the field manifested heterogeneity, every different area of the field was independently graded and the results were added, e.g., if in a field 15% of the cells was intensely stained (1 × 3 = 3), 30% was moderately stained (2 × 2 = 4), and if 55% was weakly stained (3 × 1 = 3) the field score was 3 + 4 + 3 = 10. This method was preferred because it was considered by the authors as more precise and more liable to yield statistically significant results than the semiquantitative evaluation of stain intensity proposed by other authors.[10,11] Furthermore, for statistical verification purposes, in each sample the most prevalent staining intensity group and the overall percentage of cells positive for Bax and Bak regardless of staining intensity was calculated and those two parameters were also statistically analyzed. It should be noted that all previously mentioned studies were on paraffin-embedded tissue samples, whereas in our study we used cytocentrifuged ThinPrep samples of cervical smears, for the reasons mentioned in the Introduction section. To our knowledge, this is the first time this analysis was performed on cell smears instead of tissue samples. The Bax and Bak scores; percentage of positive cells; and prevalent staining groups in all cases including HPV negative, low- and high-risk cases; and normal and abnormal Pap smear cases are listed in Figures 3–6. Regarding the correlation of Bax and Bak expression in women with low-risk and high-risk HPV types, the question statistically analyzed was whether there was any statistically significant difference between the Bax and Bak scores of women with low-risk HPV types and those of women with high-risk HPV types. Therefore, cases with high-risk HPV types and both low- and high-risk HPV types were compared by means of the t-test with cases with low-risk HPV types. In addition, each of those groups was compared with the control group, again by means of the t-test. In all cases, statistical analysis showed that there is no statistically significant difference in the Bax and Bak scores between any of the aforementioned compared groups (in the t-test between low- and high-risk HPV types the P values were P = 0.586 for Bax and P = 0.479 for Bak, between high-risk HPV types and control group they were P = 1 for Bax and P = 0.494 for Bak and between low-risk HPV types and control group they were P = 0.541 for Bax and P = 0.771 for Bak). Likewise, no statistically significant difference was found in the one-way ANOVA tests for the overall percentage of Bax and Bak positive cells (P = 0.844 and P = 0.791 between high-risk HPV women, low-risk HPV women, and the control group for Bax and Bak, respectively) or in the chi-square tests comparing the most prevalent staining intensity groups (negative/weak/moderate staining for Bax and negative/weak staining for Bak), in which P = 0.739 and P = 0.291 between high-risk HPV women, low-risk HPV women, and the control group for Bax and Bak, respectively.
Figure 1.
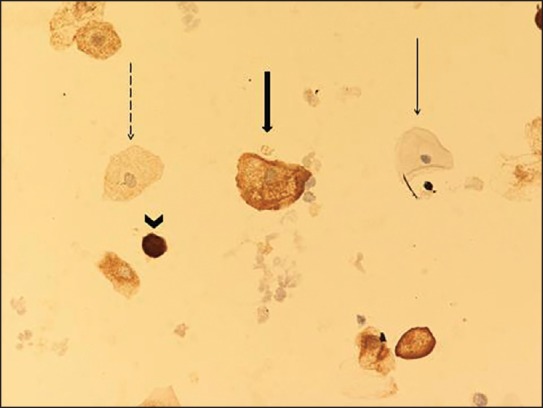
Negative, weak, moderate, and strong Bax staining intensity (Bax stain, ×200). Negative, score 0 (thin arrow). Weak, score 1 (dashed arrow): Fine hypochromatic staining. Moderate, score 2 (wide arrow): Denser, more hyperchromatic staining. Strong, score 3 (arrowhead): Intensely hyperchromatic staining
Figure 2.
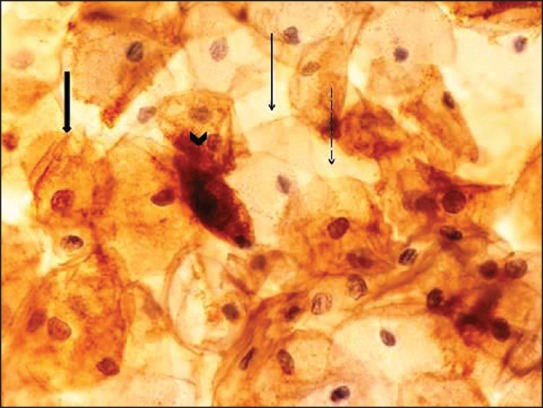
Negative, weak, moderate, and strong Bak staining intensity (Bak stain, ×400). Negative, score 0 (thin arrow). Weak, score 1 (dashed arrow). Moderate, score 2 (wide arrow). Strong, score 3 (arrowhead). Staining features as described in Figure 1
Figure 3.
Bax staining score and overall positive cells percentage histograms for Panel A. All cases Panel B. HPV-negative cases (control group) Panel C. Low-risk HPV cases Panel D. High-risk HPV cases. Mean score and standard deviation are displayed
Figure 6.
Bax and Bak prevalent staining groups in correlation with HPV status (negative/low-risk/high-risk) (Panel A1 and A2) and Pap smear status (normal/abnormal) (Panel B1 and B2)
Figure 4.
Bak staining score and overall positive cells percentage histograms for Panel A. All cases Panel B. HPV-negative cases (control group) Panel C. Low-risk HPV cases Panel D. High-risk HPV cases. Mean score and standard deviation are displayed
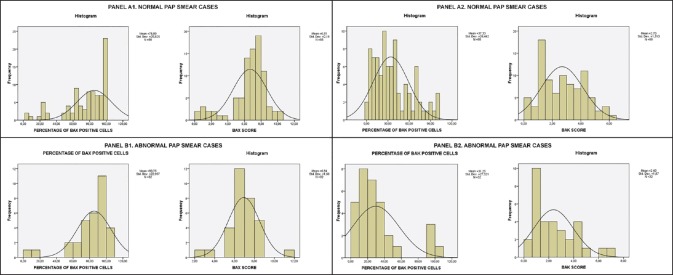
Figure 5.
Bax and Bak staining score and overall positive cells percentage histograms for normal Pap smear cases (Panel A1 and A2) and abnormal Pap smear cases (Panel B1 and B2). Mean score and standard deviation are displayed
Regarding the correlation of Bax and Bak expression with the cytological diagnosis of the Pap smear, the question statistically analyzed was whether there was a statistically significant difference between the Bax and Bak scores of women with a cytologically normal Pap test and those of women with a cytologically abnormal Pap test (ASCUS, LSIL, or HSIL). Statistical analysis with t-test showed that there is no statistically significant difference in the Bax and Bak scores between the aforementioned compared groups (P = 0.442 for Bax and P = 0.403 for Bak) and no statistically significant difference in the one-way ANOVA tests for the overall percentage of Bax and Bak positive cells (P = 0.253 and P = 0.273 between women with normal Pap test and women with abnormal Pap test for Bax and Bak, respectively). In the chi-square tests comparing the most prevalent staining intensity groups (negative/weak/moderate staining for Bax and negative/weak staining for Bak), there was no statistically significant difference for Bak (P = 0.390). However, it was noted that 14 of the 15 women staining negative for Bax had normal Pap smears (93.3%), whereas only 1 had abnormal Pap smear (6.7%). The resulting P value was P = 0.074, which is higher than the designated statistically significant limit of P < 0.05 but still indicates a low presumption against the null hypothesis, especially considering the low number of cases. The potential significance of this finding is discussed below.
Discussion
Cancer of the uterine cervix accounts for 6% of all malignancies in women,[12] and is the second leading cause of cancer-related deaths among women.[13,14,15]
Interestingly enough, not all high-risk HPV types are equally responsible for cervical cancer.[16] The eight most common HPV types detected in cervical cancers were 16, 18, 45, 31, 33, 52, 58, and 35, which are responsible for 90% of cervical cancers worldwide.[17,18]
The HPV oncoproteins E6 and E7 have been proved to sequester and inactivate p53 and pRb respectively, two of the most important tumor suppressor genes,[19] thus causing the infected cell to replicate at an abnormally high rate and ignore the external signals that would normally stop it from dividing. However, at this point the cell is still not cancerous because it does not possess metastatic potential, but if, during a chronic infection, the viral genome becomes integrated into the human cell genome and the altered cell survives while retaining the E6 and E7 genes, then the cell continues to divide without control and eventually acquires additional mutations, which make it cancerous.[4,20] A recent study by Vega-Peña et al. corroborated that E6 overexpression is associated with this integration and with increased risk of carcinogenic progression.[21] The factors deciding how such altered cells will evade apoptosis and eventually become carcinogenic have not yet been determined, but they should probably be sought among the molecules regulating cellular apoptosis.
Bax and Bak are members of the Bcl-2 family of proteins, which are key regulators of mitochondria-related apoptosis.[22] The Bcl-2 family proteins regulate the permeability of the outer mitochondrial membrane, constituting a checkpoint for the release of apoptosis-inducing mitochondrial proteins such as cytochrome c into the cytoplasm.[23] Bax and Bak belong to the proapoptotic subgroup of the family; heterodimerization of Bax with the antiapoptotic Bcl-2 modulates apoptosis, and the Bcl-2/Bax ratio determines survival or death of the cell after receiving an apoptotic signal.[24] Bak also interacts with Bcl-xL and Mcl-1 to mediate apoptosis.[25] Upon receiving an apoptotic signal, Bax-Bax homodimers are formed;[10] evidence shows that another proapoptotic Bcl-2 family member, BID, is responsible for inducing this oligomerization.[26] Those Bax oligomers have been shown to be a structural component of the mitochondrial apoptosis-induced channel (MAC), which facilitates the leakage of cytochrome c from the mitochondria to the cytoplasm, thus activating the caspase cascade, leading to cell death.[27] There is evidence suggesting that Bak can substitute Bax as a MAC component, and that deactivation of both proteins is required for a cell to develop resistance to mitochondria-induced apoptosis.[27,28]
Therefore, as key proteins of the intrinsic mitochondrial apoptotic pathway, the Bax and Bak proteins would be very likely candidates for inactivation prior to carcinogenesis, especially as there is already evidence of many viruses and viral proteins that are implicated in both the induction and suppression of apoptosis.[29] Regarding HPV in particular, Thomas and Banks have presented evidence supporting that the oncogenic HPV E6 proteins are more efficient in binding Bak in vivo, stimulating its degradation and thus abrogating Bak-induced apoptosis compared to nononcogenic HPV E6 proteins, although the latter also have this ability to a lesser degree. A probable cause offered by the authors for this was the fact that the oncogenic HPV types replicate in the higher levels of the stratified squamous epithelium, where the cells no longer divide, whereas the low-risk types replicate in the lower levels where there is still cell division.[30,31] Moreover, high-risk HPV E6 oncoprotein has been shown to form a complex with p53, leading to its inactivation and consequently affecting the functionality of a number of transcriptional target proteins of p53 modulating growth arrest and apoptosis, including Bax. This activity is limited to the high-risk HPV types, whereas the low-risk types do not inactivate p53 by this mechanism.[32,33] The difference between the effect of high-risk and nononcogenic HPV E6 on p53 and Bak was also demonstrated by Leverrier et al., where it was shown that HPV18 E6 targeted both p53 and Bak for proteolysis, whereas the β-HPV5 E6 targeted only Bak.[34] This was also observed in other beta-papillomaviruses.[35] Some more indirect supporting evidence was offered by Struijk et al., who demonstrated that beta-papillomaviruses HPV8 and HPV20 are associated with reduced steady-state expression of Bax compared to other beta-papillomaviruses, which begs the question whether such differences might exist among the alpha-papillomaviruses as well.[36]
However, it has not yet been determined whether there are similar differences in Bax and Bak functionality among women infected with low-risk alpha-papillomaviruses compared to high-risk ones. The purpose of our study was to determine whether such differences exist, and if so, whether they can be detected with a simple immunocytochemistry examination performed on the very same sample used for HPV subtyping. Of all our results, the only statistically interesting finding was a weak association between negative staining for Bax and cytologically normal Pap smears, whereas cytologically abnormal Pap smears were more likely to stain weakly or moderately positive. This statistical difference between the prevalent staining groups was only marginally significant (P = 0.074), but it should be borne in mind that it was detected on a small number of abnormal Pap smear cases (N = 32). Although the expression of Bax or Bak was not proven to be affected by the presence of low- or high-risk HPV types, this slight tendency indicates a possible connection between Bax expression and the development of dysplasia. Because our hypothesis was that Bax and Bak expression and consequently the apoptosis regulation is affected by the presence of high- or low-risk HPV DNA, this association we discovered does not constitute conclusive evidence: It is linked only with dysplasia as manifested by abnormal cells in the Pap smear and not the presence of HPV DNA. Nevertheless, as squamous cell dysplasia is inherently associated with the presence of HPV DNA and there is already considerable evidence of Bax and Bak involvement in the process of cell immortalization initiated by HPV, this finding cannot be dismissed. It is the authors’ opinion that further larger-scale studies focusing on the molecular level of apoptosis mechanisms in correlation with the clinical manifestations of squamous intraepithelial lesions may shed more light on the role of Bax and Bak in the development of dysplasia and carcinoma in the anogenital tract and especially the uterine cervix, and on their potential usefulness as prognostic markers for this particular carcinogenic process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Galani E, Christodoulou C. Human papilloma viruses and cancer in the post-vaccine era. Clin Microbiol Infect. 2009;15:977–81. doi: 10.1111/j.1469-0691.2009.03032.x. [DOI] [PubMed] [Google Scholar]
- 2.Stamataki P, Papazafiropoulou A, Elefsiniotis I, Giannakopoulou M, Brokalaki H, Apostolopoulou E, et al. Prevalence of HPV infection among Greek women attending a gynecological outpatient clinic. BMC Infect Dis. 2010;10:27. doi: 10.1186/1471-2334-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol. 2008;82:10408–17. doi: 10.1128/JVI.00902-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenblatt RJ. Human papillomaviruses: Diseases, diagnosis, and a possible vaccine. Clin Microbiol Newsl. 2005;27:139–45. [Google Scholar]
- 5.Oh JM, Kim SH, Cho EA, Song YS, Kim WH, Juhnn YS. Human papillomavirus type 16 E5 protein inhibits hydrogen-peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis. 2010;31:402–10. doi: 10.1093/carcin/bgp318. [DOI] [PubMed] [Google Scholar]
- 6.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: Its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–62. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 7.Pim D, Tomaic V, Banks L. The human papillomavirus (HPV) E6* proteins from high-risk, mucosal HPVs can direct degradation of cellular proteins in the absence of full-length E6 protein. J Virol. 2009;83:9863–74. doi: 10.1128/JVI.00539-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, et al. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio J, Ramos D, López-Guerrero JA, Iborra I, Collado A, Solsona E, et al. Immunohistochemical expression of Ki-67 antigen, cox-2 and Bax/Bcl-2 in prostate cancer; prognostic value in biopsies and radical prostatectomy specimens. Eur Urol. 2005;48:745–51. doi: 10.1016/j.eururo.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Wortham NC, Alam NA, Barclay E, Pollard PJ, Wagner BE, Manek S, et al. Aberrant expression of apoptosis proteins and ultrastructural aberrations in uterine leiomyomas from patients with hereditary leiomyomatosis and renal cell carcinoma. Fertil Steril. 2006;86:961–71. doi: 10.1016/j.fertnstert.2006.02.106. [DOI] [PubMed] [Google Scholar]
- 11.Batinac T, Zamolo G, Hadzisejdić I, Zauhar G, Brumini G, Ruziæ A, et al. Expression of Bcl-2 family proteins in psoriasis. Croat Med J. 2007;48:319–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Fehrmann F, Laimins LA. Human papillomaviruses: Targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22:5201–7. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- 13.Shai A, Pitot HC, Lambert PF. E6-associated protein is required for human papillomavirus type 16 E6 to cause cervical cancer in mice. Cancer Res. 2010;70:5064–73. doi: 10.1158/0008-5472.CAN-09-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavlidou E, Zafrakas M, Papadakis N, Benos A, Agorastos T. Cervical, uterine corpus, and ovarian cancer mortality in Greece during 1980 to 2005: A trend analysis. Int J Gynecol Cancer. 2010;20:482–7. doi: 10.1111/IGC.0b013e3181d80a8f. [DOI] [PubMed] [Google Scholar]
- 15.Kroupis C, Thomopoulou G, Papathomas TG, Vourlidis N, Lazaris AC. Population-based study of human papillomavirus infection and cervical neoplasia in Athens, Greece. Epidemiol Infect. 2007;135:943–50. doi: 10.1017/S095026880700876X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtig H, Algrisi M, Botzer LE, Abadi T, Verbitzky J, Jackman A, et al. HPV16 E6 natural variants exhibit different activities in functional assays relevant to the carcinogenic potential of E6. Virology. 2006;350:216–27. doi: 10.1016/j.virol.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl 3):S3/1–10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 19.Zheng ZM, Baker CC. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci. 2006;11:2286–302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: Concepts and clinical implications. J Pathol. 2006;208:152–64. doi: 10.1002/path.1866. [DOI] [PubMed] [Google Scholar]
- 21.Vega-Peña A, Illades-Aguiar B, Flores-Alfaro E, López-Bayghen E, Leyva-Vázquez MA, Castañeda-Saucedo E, et al. Risk of progression of early cervical lesions is associated with integration and persistence of HPV-16 and expression of E6, Ki-67, and telomerase. J Cytol. 2013;30:226–32. doi: 10.4103/0970-9371.126644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–92. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai D, Jin C, Huang Z, Satterthwait AC, Reed JC. Differential regulation of Bax and Bak by anti-apoptotic Bcl-2 family proteins, Bcl-B and Mcl-1. J Biol Chem. 2008;283:9580–6. doi: 10.1074/jbc.M708426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanke J. Apoptosis and occurrence of Bcl-2, Bak, Bax, Fas and FasL in the developing and adult rat endocrine pancreas. Anat Embryol (Berl) 2000;202:303–12. doi: 10.1007/s004290000112. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher JI, Huang DC. Controlling the cell death mediators Bax and Bak: Puzzles and conundrums. Cell Cycle. 2008;7:39–44. doi: 10.4161/cc.7.1.5178. [DOI] [PubMed] [Google Scholar]
- 26.Gross A. BCL-2 proteins: Regulators of the mitochondrial apoptotic program. IUBMB Life. 2001;52:231–6. doi: 10.1080/15216540152846046. [DOI] [PubMed] [Google Scholar]
- 27.Dejean LM, Martinez-Caballero S, Kinnally KW. Is MAC the knife that cuts cytochrome c from mitochondria during apoptosis? Cell Death Differ. 2006;13:1387–95. doi: 10.1038/sj.cdd.4401949. [DOI] [PubMed] [Google Scholar]
- 28.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX or BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teodoro JG, Branton PE. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–46. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene. 1998;17:2943–54. doi: 10.1038/sj.onc.1202223. [DOI] [PubMed] [Google Scholar]
- 31.Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J Gen Virol. 1999;80:1513–7. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- 32.Münger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–28. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 33.Kehmeier E, Rühl H, Voland B, Stöppler MC, Androphy E, Stöppler H. Cellular steady-state levels of “high risk” but not “low risk” human papillomavirus (HPV) E6 proteins are increased by inhibition of proteasome-dependent degradation independent of their p53- and E6AP-binding capabilities. Virology. 2002;299:72–87. doi: 10.1006/viro.2002.1502. [DOI] [PubMed] [Google Scholar]
- 34.Leverrier S, Bergamaschi D, Ghali L, Ola A, Warnes G, Akgül B, et al. Role of HPV E6 proteins in preventing UVB-induced release of pro-apoptotic factors from the mitochondria. Apoptosis. 2007;12:549–60. doi: 10.1007/s10495-006-0004-1. [DOI] [PubMed] [Google Scholar]
- 35.Simmonds M, Storey A. Identification of the regions of the HPV 5 E6 protein involved in Bak degradation and inhibition of apoptosis. Int J Cancer. 2008;123:2260–6. doi: 10.1002/ijc.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Struijk L, van der Meijden E, Kazem S, ter Schegget J, de Gruijl FR, Steenbergen RD, et al. Specific betapapillomaviruses associated with squamous cell carcinoma of the skin inhibit UVB-induced apoptosis of primary human keratinocytes. J Gen Virol. 2008;89:2303–14. doi: 10.1099/vir.0.83317-0. [DOI] [PubMed] [Google Scholar]