Abstract
Background:
Thyroid fine-needle aspiration cytology (FNAC) is a valuable test used for diagnosing diseases of the thyroid gland.
Aims:
Using all satisfactory categories of the Bethesda system, this study aimed to determine the accuracy with which FNAC diagnoses thyroid neoplasms. We also discuss the factors that affect diagnosis accuracy.
Settings and Design:
A comparison was drawn between FNAC results and final histological diagnosis using samples collected over a period of 3 years.
Materials and Methods:
For all patients, age, sex, cytological features, and histological types were determined. All cases of false negative (FN) and false positive (FP) diagnosis were reanalyzed.
Statistical Analysis Used:
The chi-square test and univariate analysis were performed to examine the relationship between different variables.
Results:
About 52% of the cases were found malignant, and they were of six different histological types. Papillary carcinoma was the commonest type of malignancy at 76.9%. The rate of malignancy was 63% in males and 49.4% in females. In two of the FN cases, the tumor had a diameter of ≥35 mm. Of the 12 FP cases, nine were in the follicular neoplasm or suspicious for follicular neoplasm Bethesda category. FNAC diagnosis had 95.2% sensitivity, 68.4% specificity, 83.3% positive predictive value, 89.6% negative predictive value, and 85.14% accuracy.
Conclusions:
FNAC was found to have a high level of sensitivity and an acceptable degree of specificity in diagnosing different types of thyroid neoplasms. The presence of microfollicular structures or crowded cellular clusters is a challenge to diagnosis, particularly in low-quality specimens.
Keywords: Bethesda, fine-needle aspiration cytology (FNAC), Iran, neoplasm, thyroid
Introduction
Thyroid nodule is a common clinical problem. It can be palpated in 5% of individuals during thyroid examination and can be detected in up to 60% of people who undergo thyroid ultrasound.[1,2] Most nodules are benign, but they are usually the first sign of thyroid cancer.[1,3] Thyroid cancer is the seventh commonest type of cancer among Iranian women.[4] Patient age and histology as well as stage of cancer are important prognostic factors.[3]
Fine-needle aspiration cytology (FNAC) is an easy, cost-effective test for cancer diagnosis, and its use has markedly decreased the number of unnecessary thyroid surgeries.[5]
The purpose of the present study is to compare the results of FNAC using the Bethesda system and all its categories except the unsatisfactory category with the final histological diagnosis in order to determine the accuracy with which FNAC diagnoses thyroid neoplastic nodules.[5] In addition, we want to discuss the possible causes of false negative (FN) or false positive (FP) diagnostic results in order to better identify the factors that may affect the accuracy of FNAC.
Materials and Methods
Currently, the Bethesda system of reporting thyroid cytology (TBSRTC) is used for reporting FNAC specimens of thyroid. According to Cibas (2009), this system was innovated in 2007 and consists of six categories:
Unsatisfactory (UNS) or nondiagnostic (ND),
Benign and nonneoplastic,
Atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS),
Follicular neoplasm or suspicious for follicular neoplasm (FN/SFN),
Suspicious for, but not diagnostic of, malignancy, and
Malignant. Bethesda improves interpretation of FNAC reports and allows a more precise study and diagnosis of thyroid nodules.[5]
Much investigation has been conducted on the accuracy with which FNAC diagnoses thyroid malignancy. However, only a small number of studies have used the Bethesda system, and even some of the studies that were based on this reporting system excluded some of its categories in their investigation of FNAC accuracy. More specifically, some studies aimed to determine FNAC accuracy in diagnosis of thyroid cancer; however, it should be noted that FNAC cannot differentiate between benign and malignant follicular neoplasms. Further, definite differentiation between follicular adenoma and follicular carcinoma is only possible after thyroid lobectomy.[6,7] In addition, a study of FNAC showed that 68% of the cases diagnosed by FNAC as follicular neoplasm turned out to be the follicular type of papillary carcinoma, indicting a considerable overlap between benign and malignant neoplasms.[7] Thus, instead of determining FNAC accuracy in diagnosis of malignancy, we decided to evaluate the accuracy of this test in the diagnosis of thyroid neoplasms and also included all diagnostic categories of the Bethesda system.
The FNAC samples used in this study were taken from a pathology center in Iran. The samples were collected from a single laboratory because we wanted to ensure the uniformity of interpretation of cytological findings and reanalysis of the samples. We collected all cases of thyroidectomy performed in several local hospitals over a period of 3 years (2011-2014). Then, out of these patients, we identified those who had an associated thyroid FNAC record in our pathology center. We wanted to match thyroid surgery cases and thyroid FNAC cases. Histological reports were obtained from patients’ hospital records and considered as final diagnosis.
For all patients, age, sex, Bethesda cytological categories and subcategories, and histological types were determined from FNAC and pathology reports.
All Bethesda categories except the first one were included in the present study. The parameter used to determine the satisfactoriness of the specimens was the presence of at least six clusters of thyroidal follicular cells, with each cluster including at least 10 follicular cells with good preservation.[5] All specimens were obtained by an endocrinologist and without using ultrasound. The prepared slides were stained with the Papanicolaou stain.
The histological diagnoses were classified into three types:
Benign and nonneoplastic,
Benign and neoplastic, and
Malignant.
In our determination of the accuracy with which FNAC diagnosed malignancy, we considered four outcomes: True negative (TN), true positive (TP), FN, and FP. As Table 1 shows, these outcomes were based on matching between Bethesda categories and histological types.
Table 1.
Matching between Bethesda categories and histological types used to determine FNAC accuracy
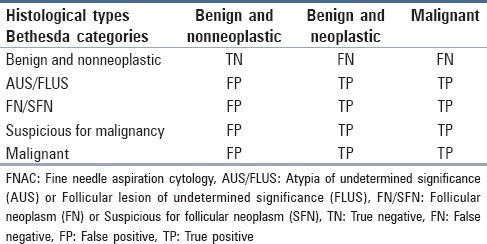
FN and FP cases were reanalyzed to determine possible causes of the false results. Descriptive statistics [frequency, mean, and standard deviation (SD)] was used for describing the data. The chi-square test and univariate analysis were performed to examine the relationship between different variables. The significance level was set at P < 0.05. Sensitivity, specificity, accuracy, and positive and negative predictive values were determined using SPSS 16 (IBM Corporation, Armonk, NY, USA, 2008) and related formulas.
Results
A total of 101 cases, comprised of 16 (15.8%) men and 85 (84.2%) women, were included in this study. The female/male (F/M) ratio was 5.31:1. The patients’ age ranged 19-84 years, with an average age of 41 (SD = 13.6).
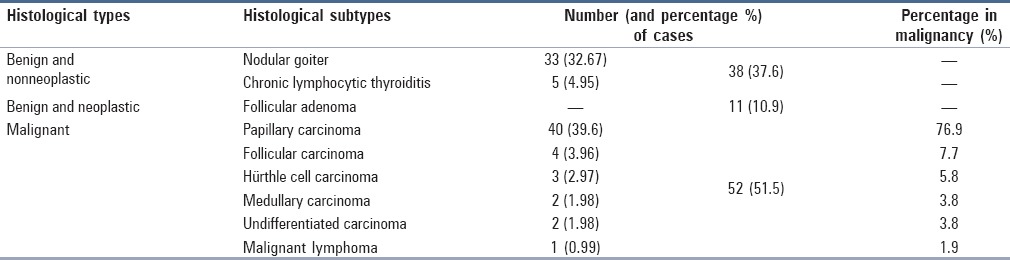
The distribution of the FNAC cases according to the Bethesda categories was as follows. There were 29 benign and nonneoplastic cases (28.7%), including 26 cases of nodular goiter and three cases of chronic lymphocytic thyroiditis. Moreover, there were four cases (4%) in the AUS/FLUS category. Furthermore, 27 of the cases (26.7%) were in the FN/SFN category, including 24 cases of the follicular cell type and three cases of the Hürthle cell type. Also, 16 cases (15.8%) were suspicious for malignancy, with 13 of them being suspicious for papillary carcinoma and the other three being suspicious for medullary carcinoma, undifferentiated carcinoma, and malignant lymphoma (one case per type). Lastly, there were 25 malignant cases (24.8%), including 22 cases of papillary carcinoma, one case of medullary carcinoma, and two cases of undifferentiated carcinoma. Table 2 gives the frequency of histological types and subtypes of the FNAC cases after thyroidectomy.
Table 2.
The frequency of histological types and subtypes of the FNAC cases (N = 101) after thyroidectomy
The benign nonneoplastic histological type affected 32 females and 6 males. All cases of benign neoplasm were women. Although the rate of malignancy was 63% in males and 49.4% in females, the relationship between sex and malignancy was insignificant (P = 0.28). The average patient ages associated with the three histological types were not significantly different from one another (P = 0.4). However, the average age of the malignant cases was 49.4 years for men (SD = 20.6) and 38.1 years for women (SD = 12.2), with the difference being significant (P = 0.008).
Papillary carcinoma cases were seen in 33 women and 7 men. The average age of these patients was 36.5 years (SD = 9.9). About one-third of the cases (35%) in this histological subtype were of the nonclassic type, and the follicular type was the commonest nonclassic variant (12 cases).
Table 3 gives the correlation between FNAC and histological diagnoses and also shows the accuracy with which FNAC diagnosed malignancy. As can be seen, there were three cases of FN diagnosis, two of which had ultimately been diagnosed as papillary carcinoma and the other one as follicular adenoma. It should be noted that in one of the FN papillary carcinoma cases, the tumor was 7 mm in diameter, and chronic lymphocytic thyroiditis was reported in histological diagnosis. Reanalysis of the FNAC diagnosis for this case only revealed the presence of the background disease. The other case of FN papillary carcinoma was a tumor with a diameter of 50 mm with cystic changes. FNAC reported this case as nodular goiter and did not show any evidence of malignancy. This diagnosis may have been due to the fact that the specimen was only minimally cellular. The last FN case was a follicular adenoma (the tumor having a diameter of 35 mm) and had been reported as nodular goiter during FNAC. Reanalysis of the FNAC diagnosis for this case revealed no evidence of follicular neoplasm.
Table 3.
Correlation between FNAC and histological diagnoses together with the risk of malignancy calculated for each Bethesda category
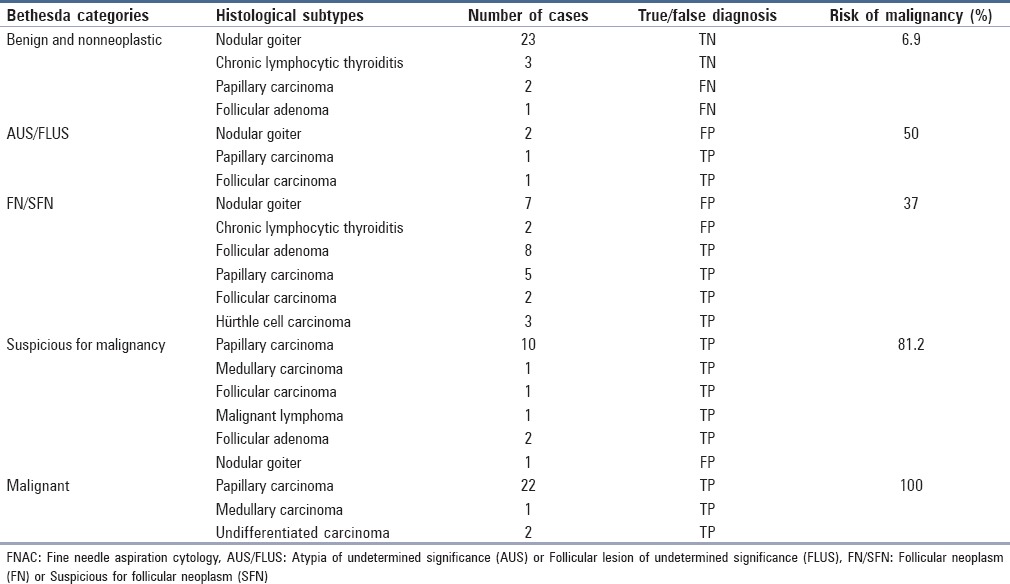
There were 12 cases of FP diagnosis, with most of them belonging to the FN/SFN Bethesda category. Most of these cases had previously been finally diagnosed as adenomatous goiter. Reanalysis showed that FNAC suggestion of follicular neoplasm was due to the presence of microfollicular structures or crowded cellular clusters. It was also revealed that this FNAC suggestion had only been made for the specimens that were thick, had relatively low cellularity, or did not have microfollicular structures as the predominant microscopic pattern. Reanalysis of three other FP cases (two in the AUS/FLUS Bethesda category and one in the suspicious category) showed the presence of only one or two cytological features of papillary carcinoma. Furthermore, in the case in the suspicious category, the FP suggestion can be attributed to the presence of an unusual form of squamous metaplasia. The risk of malignancy calculated for each Bethesda category, shown in Table 3, was obtained by dividing the number of malignant cases by the total number of cases in each category.
Overall, the sensitivity of FNAC diagnosis was found to be 95.2%, specificity was 68.4%, positive predictive value was 83.3%, negative predictive value was 89.6%, and accuracy was 85.14%.
Discussion
The age and sex distributions of the patients in this research were close to those reported by similar studies.[2,8] In addition, the F/M ratio observed in this study for thyroid cancer (4.2:1) is in agreement with the fact that thyroid cancer is commoner among women.[3] It should also be noted that two other Iranian studies reported this ratio to be 1.8:1 and 2.5:1.[3,4] Furthermore, the average age we observed for the patients with malignancy was close to the age reported by these two studies. Moreover, like Muratli et al.,[2] we found that on average male patients were diagnosed with malignancy at an older age than females were.
In this study, 52% of the cases were malignant. This finding differs from Muratli et al.,[2] whose study reported a lower rate. However, this high rate of malignancy is not surprising if we know that FNAC is nowadays routinely performed for most cases of thyroid nodules. This has led to a reduction in the number of unnecessary surgeries and consequently to a rise in the percentage reported for malignancy.[5]
The percentages we observed for papillary carcinoma (75%) and follicular carcinoma (7.7%) were similar to the figures reported in the previous studies on thyroid cancer.[2,3,4] Follicular adenoma constituted 10.9% of our cases, close to the figure reported by another study performed on Iranian patients but lower than the percentage observed in some other studies (e.g., Muratli et al.).[1,2] In addition, Hürthle cell carcinoma made up 5.8% of the malignant cases in our study. However, previous research has reported a lower percentage for this type of follicular carcinoma or has failed to study it as a separate type of malignancy.[1,2,3,4,9]
Six histological types of malignancy were observed in our study, indicating that FNAC is a valuable tool for diagnosing different types of malignancy. In addition, it should be noted that FNAC is a tool for separating cases that are in need of surgery from other cases. In our study, there were 15 cases of follicular neoplasm, with 13 of them being identified as candidates for surgical resection with a cytological diagnosis of “suspicious for malignancy or follicular neoplasm.” One was wrongly reported as benign, and one fell in the AUS/FLUS category.
The risk of malignancy for each Bethesda category ranged from 6.9% (the “benign and nonneoplastic” category) to 100% (the “malignant” category). This wide range shows the power of the Bethesda system to differentiate and determine the probability of malignancy. The percentages obtained in our research were rather close to the figures reported in other studies: 6.9% versus 0-3% (the “benign and non-] neoplastic” category), 50% versus 5-15% (AUS/FLUS), 37% versus 15-30% (FN/SFN), 81.2% versus 60-75% (the “suspicious for malignancy” category), and 100% versus 97-99% (the “malignant” category).[5] The fact that the percentages obtained in this study are slightly higher is partly due to the pattern we used for selecting patients. In other words, we selected the cases from surgery wards, whereas other studies included in their experiments all the cases that were subjected to FNAC. Another observation in the present study was that two of the four AUS/FLUS cases were malignant. As noted above, the risk of malignancy in this group has been reported to be 5-15%; however, a higher rate has also been reported.[2,5,9]
As for FNAC strength, the FN rate was found to be as low as 3%. There were two cases of papillary carcinoma that were missed and diagnosed as nodular goiter. One of these cases was a nodule with cystic changes. It is worth noting that cystic changes may occur in neoplastic lesions, thus making it difficult to sample the solid portion of the tumor and in turn causing malignancy to be missed.[6,8,10] Many factors contribute to FN diagnosis, but in our study, the key factor was the sampling error associated with the large size of nodules. A study of factors that lead to FN diagnosis showed that there is a positive correlation between nodule size and the rate of FN diagnosis.[10] More specifically, if a tumor is less than 1 cm in diameter (one of the FN papillary carcinoma cases in our study was 7 mm in diameter), sampling error is possible.
In the present research, there were five cases in the FN/SFN category that were finally diagnosed as papillary carcinoma. This begs the question of why they were not placed in the “suspicious for malignancy” category. An explanation is that these cases were indeed variants of papillary carcinoma: Three were follicular variants, one had a background of Hashimoto's thyroiditis, and one was the Warthin-like variant. As a matter of fact, there are multiple variants of papillary carcinoma, including the follicular variant, which may be wrongly diagnosed as follicular neoplasm. If the characteristic features of papillary carcinoma, such as true papillary structures or psomma bodies are absent, the differentiation of papillary carcinoma from follicular neoplasm may be difficult given the fact that the nuclear changes of papillary carcinoma may be slight or focal, and also because features such as nuclear groove may be seen in other lesions, particularly in low-cellular smears.[5]
Another observation in this research is that there were two cases in the FN/SFN category that turned out to be chronic lymphocytic thyroiditis in histological examination. Both cases exhibited marked Hürthle cell metaplasia in addition to lymphocytic infiltration. Thyroid follicular cells in this type of thyroiditis may show a mild to moderate degree of cellular atypia. In addition, Hürthle cell metaplasia may produce cytological atypia. Overemphasis on the cellularity that may be seen in this disease may lead us to categorize it as follicular neoplasm.[6]
The rate of FP cases in our study was 11.8%. Similar or higher rates have also been reported, particularly for follicular neoplasm.[6,11] Like Schreiner et al.,[11] we found adenomatoid nodules to be the main cause of poor histological correlation in the case of follicular neoplasm diagnosed by FNAC. Clumping and crowding of follicular cells may be seen if aspiration is performed on a hyperplastic nodule.[8] Among helpful criteria for the differentiation of nodular goiter from follicular adenoma are higher cellularity, uniform cellularity, uniform nuclear enlargement, synsytial clusters, predominant microfollicles, and scanty colloid.[6] Another point is that, like Pandey et al.[6] and Yang et al.,[7] we found out that overemphasizing the presence of microfollicular structures or crowded cellular clusters in low-quality specimens may result in FP diagnosis.
Concerning FP results for papillary carcinoma, we can argue that although there are multiple cytological features that can be used for diagnosing this type of carcinoma, we cannot be sure that they are invariably available or sufficiently specific.[12] Furthermore, like Pandey et al.,[6] we found the focal presence of some of the features mentioned above to be the cause of FP diagnosis for papillary carcinoma.
Moreover, some studies have reported higher rates of sensitivity and specificity than we observed in this research. This contradiction is because these studies dealt with a smaller number of cases and a lower percentage of malignancy.[13,14] In addition, in some studies, some or all of the undetermined or suspicious cases (i.e., Bethesda categories of AUS/FLUS, FN/SFN, and suspicious for malignancy) were excluded from statistical computation.[1,8,15] However, the problem is that this will lead to a reduction in the number of reported FN and FP cases and thus result in an exaggerated rate of accuracy.[7] Finally, specificity of the FNAC test in a study with a method completely similar to our study was 64.6%.[2]
Furthermore, our study had a lower FN rate and a higher FP rate in comparison with some other studies.[6,8,13] It may be noted at this point that although follow-up observation of patients for at least a few years is necessary for determination of the true number of FN cases, this was not done in our study. Another limitation of this study is that we could not analyze some other features of thyroid cancer, such as risk factors and laboratory and imaging findings.
The outcomes of the present study are important when considering the following:
Although there was relatively a small number of cases (N = 101), we observed six types of malignancy (i.e., papillary carcinoma, follicular cell type, Hürthle cell type, medullary carcinoma, undifferentiated carcinoma, and malignant lymphoma);
Although the number of cases was small, we observed a significantly large percentage of malignancy (52% of the cases); and
Unlike most of the studies previously performed, we considered all diagnostic categories of the Bethesda system to determine the FP and FN rates.
Conclusions
This study revealed a high sensitivity and an acceptable specificity for the FNAC test in diagnosis of different types of neoplasia. Most cases of FP diagnosis were in the FN/SFN Bethesda category. Overemphasis on the presence of microfollicular structures was the main cause of FP diagnosis. Taking samples from different regions of the nodule and fulfilling the criteria for adequacy and appropriateness can decrease the FN rate.[6] In addition, ultrasound and ancillary testing in the form of molecular genetics and immunocytochemistry can improve diagnostic accuracy.[13]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Esmaili HA, Taghipour H. Fine-needle aspiration in the diagnosis of thyroid diseases: An appraisal in our institution. ISRN Pathology 2012. 2012 912728. [Google Scholar]
- 2.Muratli A, Erdogan N, Sevim S, Unal I, Akyuz S. Diagnostic efficacy and importance of fine-needle aspiration cytology of thyroid nodules. J Cytol. 2014;31:73–8. doi: 10.4103/0970-9371.138666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larijani B, Aghakhani S, Haghpanah V, Mosavi-Jarrahi A, Bastanhagh M. Review of thyroid cancer in Iran. Aus-Asian J Cancer. 2005;4:199–203. [Google Scholar]
- 4.Khayamzadeh M, Khayamzadeh M, Tadayon N, Salmanian R, Zham H, Razzaghi Z, et al. Survival of thyroid cancer and social determinants in Iran, 2001-2005. Asian Pac J Cancer Prev. 2011;12:95–8. [PubMed] [Google Scholar]
- 5.Cibas ES, Ali SZ. NCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–65. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 6.Pandey P, Dixit A, Mahajan NC. Fine-needle aspiration of the thyroid: A cytohistologic correlation with critical evaluation of discordant cases. Thyroid Res Pract. 2012;9:32–9. [Google Scholar]
- 7.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: A study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–15. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 8.Sinna EA, Ezzat N. Diagnostic accuracy of fine needle aspiration cytology in thyroid lesions. J Egypt Natl Canc Inst. 2012;24:63–70. doi: 10.1016/j.jnci.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Mondal SK, Sinha S, Basak B, Roy DN, Sinha SK. The Bethesda system for reporting thyroid fine needle aspirates: A cytologic study with histologic follow-up. J Cytol. 2013;30:94–9. doi: 10.4103/0970-9371.112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agcaoglu O, Aksakal N, Ozcinar B, Sarici IS, Ercan G, Kucukyilmaz M, et al. Factors that affect the false-negative outcomes of fine-needle aspiration biopsy in thyroid nodules. Int J Endocrinol 2013. 2013 doi: 10.1155/2013/126084. 126084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schreiner AM, Yang GC. Adenomatoid nodules are the main cause for discrepant histology in 234 thyroid fine-needle aspirates reported as follicular neoplasm. Diagn Cytopathol. 2012;40:375–9. doi: 10.1002/dc.21499. [DOI] [PubMed] [Google Scholar]
- 12.Chandanwale SS, Kumar H, Buch AC, Vimal SS, Soraisham P. Papillary thyroid carcinoma, a diagnostic approach in fine needle aspiration: Review of literature. Clin Cancer Investig J. 2013;2:339–43. [Google Scholar]
- 13.Bagga PK, Mahajan NC. Fine needle aspiration cytology of thyroid swellings: How useful and accurate is it? Indian J Cancer. 2010;47:437–42. doi: 10.4103/0019-509X.73564. [DOI] [PubMed] [Google Scholar]
- 14.Handa U, Garg S, Mohan H, Nagarkar N. Role of fine needle aspiration cytology in diagnosis and management of thyroid lesions: A study on 434 patients. J Cytol. 2008;25:13–7. [Google Scholar]
- 15.Wong LQ, Baloch ZW. Analysis of the Bethesda system for reporting thyroid cytopathology and similar precursor thyroid cytopathology reporting schemes. Adv Anat Pathol. 2012;19:313–9. doi: 10.1097/PAP.0b013e3182666398. [DOI] [PubMed] [Google Scholar]


