Abstract
Lymphangioleiomyomatosis (LAM) is a rare lung disease traditionally affecting women during their childbearing years. It can be sporadic or be associated with tuberous sclerosis syndrome. It is usually manifested in the lungs, kidneys, and/or lymphatic system. It consists of an overgrowth of abnormal smooth muscle-like cells, usually along the bronchovascular structures, resulting in the formation of cysts and the destruction of the lung parenchyma. We present the case of a 43-year-old woman with a history of pleural effusion and dyspnea. A computed tomographic scan revealed a mediastinal mass, chylothorax, and multiple pulmonary cysts. A diagnosis of LAM was rendered on a pleural fluid sample.
Keywords: Lymphangioleiomyomatosis, pleural effusion, spindle cells, tuberous sclerosis
Introduction
Lymphangioleiomyomatosis (LAM) is a rare disease usually seen in young females. The histopathologic features in the lung, which is a common location for this entity, are characterized by an overgrowth of abnormal smooth muscle-like cells, usually along the bronchial vasculature, resulting in the formation of cysts and the destruction of the parenchyma.[1,2,3] The clinical presentation of a patient with LAM is variable and the symptoms may include cough, shortness of breath, fatigue, and/or hemoptysis.[1] Without a high index of suspicion, an early and accurate diagnosis is difficult. The disease progression is slow and may be complicated by effusion, lung collapse, and even heart failure. Given the progressive decline in lung function, lung transplantation may be indicated.[1] In this manuscript, we would like to demonstrate that a cytologic diagnosis of LAM of the lung is possible, which may save the patient from an invasive procedure such as a biopsy. For this, a familiarity with the cytomorphologic features of LAM and correlation with imaging features in a correct clinical setting are essential.
Case Report
A 43-year-old female with past medical history significant for anxiety attacks presented to an outside institution emergency room with a short duration of chest pressure. A chest computed tomography (CT) revealed a 7.7-cm anterior mediastinal mass. This was biopsied at an outside institution. The findings were consistent with a low-grade spindle-cell neoplasm. Further in-house evaluation of the CT scan revealed numerous cysts in the lungs with associated pleural effusions. The biopsy slides were rereviewed at our institution and essentially verified the outside pathologic interpretation of the presence of spindled cells within smooth muscle fascicles [Figure 1a]. Given the patient's sex and age, LAM was considered in differential diagnosis; however, the immunostains performed at our institution on the outside material were not contributory due to the absence of sufficient tissue for a definitive diagnosis. The presence of a left pleural effusion triggered a pleural aspirate, which yielded a diagnostic cytopathology sample. The fluid was evaluated by a ThinPrep and cytospin samples supplemented by a formalin-fixed cellblock. These samples showed scattered clusters of bland spindled cells in a background of histiocytes and mesothelial cells [Figure 1b]. At a higher magnification, spindle cell balls were lined by lymphatic-like endothelial cells [Figure 1c and d]. Immunohistochemical stains were performed on the cell block and were positive for smooth muscle actin (SMA) (Ventana, Tucsan, Arizona, USA) [Figure 2a], Desmin (Ventana, Tucsan, Arizona, USA) [Figure 2b], HMB-45 (Dako, Carpinteria, California, USA) [Figure 2c], and ER and PR (Ventana, Tucsan, Arizona, USA). No immunoreactivity was noted with cytokeratin AE1/AE3 (Dako, Carpinteria, California, USA), Calretinin (Ventana, Tucsan, Arizona, USA), CD68 (Dako, Carpinteria, California, USA), and WT-1 (Ventana, Tucsan). The immunohistochemical staining pattern confirmed the suspected diagnosis of LAM. The patient was tested for tuberous sclerosis with a negative result. Treatment with sirolimus was suggested; however, the patient was reluctant to initiate therapy due to the side effects. Since the diagnosis, she remains asymptomatic.
Figure 1.
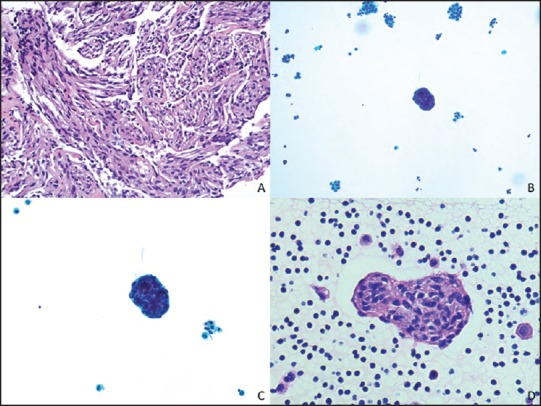
(a) Spindle cells within smooth muscle fascicles. (H and E, ×200) (b) Scattered clusters of bland spindled cells in a background of histiocytes and mesothelial cells. (ThinPrep, ×200) (c) Spindle cells cluster lined by lymphatic-like endothelial cells. (ThinPrep, ×400) (d) H and E, ×400
Figure 2.
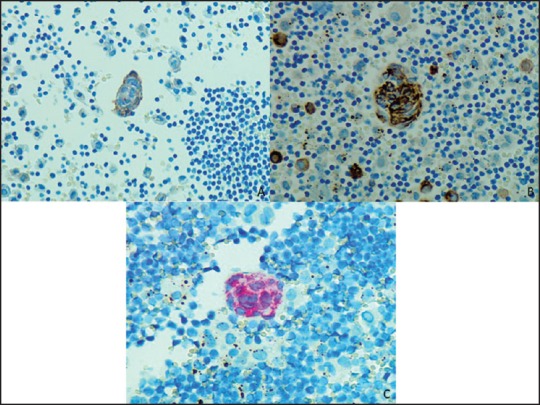
(a) Positive SMA (IHC, ×400) (b) Positive Desmin (IHC, ×400) (c) Positive HMB45 (IHC, ×400)
Discussion
LAM is an unusual disease, which can occur sporadically or in association with tuberous sclerosis complex (TSC). LAM and TSC are caused by a mutation of the tuberous sclerosis genes, TSC1 or TSC2. Sporadic LAM affects approximately 1 in 400,000 adult females. The incidence of LAM, associated with TSC is as high as 30-40% in adult females.[1] In both forms of the disease, extrathoracic angiomyolipomas are common.
On histologic examination, LAM is characterized by cystic lung destruction and proliferation of bundles of distinctive smooth muscle cells. LAM cells express metalloproteinases (MMPs) [such as membrane type 1 and matrix metalloproteinase 2 (MMP2)] and cathepsin K, which degrade the extracellular matrix protein and facilitate cell migration. The proliferation of LAM cells in lymphatics may cause airway obstruction and air-trapping, which induce cyst formation.[2]
Clinically, the patients present with shortness of breath on exertion, cough, hemoptysis, recurrent pneumothorax, chylous, pleural effusion, or chylous ascites. The most common clinical complaint is shortness of breath, as seen in our patient who additionally experienced chest pressure
The diagnosis by imaging is facilitated by high-resolution CT scan (HRCT) although recent studies suggest that conventional CT may also be helpful.[3] The diagnostic radiologic finding is the presence of diffuse thin-walled cysts (with diameters of 0.2-2 cm) throughout the bilateral lungs with a chylous effusion.[2] In our case, these features were present with an enlarged mediastinal mass. While in general, the confirmatory diagnosis relies upon a lung biopsy, several studies have demonstrated that cytologic diagnosis is also possible. The diagnostic features may be observed on a pleural effusion. These features in combination with clinical suspicion and confirmatory immunohistochemical support can be sufficient for the diagnosis of LAM as in our case.[3,4] The cytologic findings of LAM are characterized by globular clusters of oval to spindled cells, with moderate nuclear: Cytoplasmic ratio, enveloped by epithelioid-like flat cells. The spindle cells show reactivity with smooth muscle actin (SMA), HMB45, melan-A, estrogen receptor (ER), and progesterone receptor (PR). SMA, vimentin, and Desmin support the smooth muscle phenotype expression by LAM cells.[5]
Currently, no effective treatment is reported for LAM. A few medications have been tried to improve the symptoms and quality of life. Of these, sirolimus, is an immunosuppressant approved by the FDA to inhibit cell proliferation. Sirolimus therapy helps to reduce chylous effusions, and lymphangioleiomyomas and improves lung function.[2] Nevertheless, lung transplantation remains the best treatment option for advanced stages of the disease.
To summarize, LAM is a rare disease predominantly affecting females in their reproductive age, which can occur sporadically or in association with TSC. While there are no effective treatments yet, some medications may help improve the quality of life. An accurate diagnosis for treatment and TSC screening can be obtained by cytology thereby avoiding an invasive biopsy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, et al. Review Panel of the ERS LAM Task Force. European respiratory society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 2.Mavroudi M, Zarogoulidis P, Katsikogiannis N, Tsakiridis K, Huang H, Sakkas A, et al. Lymphangioleiomyomatosis: Current and future. J Thorac Dis. 2013;5:74–9. doi: 10.3978/j.issn.2072-1439.2013.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan D, Ding L, Liu H, Wang J, Ran W, Li Y, et al. Effusion cytology: An effective method for the diagnosis of pulmonary lymphangioleiomyomatosis. J Thorac Dis. 2014;6:E54–7. doi: 10.3978/j.issn.2072-1439.2014.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riojas RA, Bahr BA, Thomas DB, Perciballi J, Noyes L. A case report of Lymphangioleiomyomatosis presenting as spontaneous pneumothorax. Mil Med. 2012;177:477–80. doi: 10.7205/milmed-d-11-00333. [DOI] [PubMed] [Google Scholar]
- 5.Grzegorek I, Drozdz K, Podhorska-Okolow M, Szuba A, Dziegiel P. LAM cells biology and lymphangioleiomyomatosis. Folia Histochem Cytobiol. 2013;51:1–10. doi: 10.5603/FHC.2013.001. [DOI] [PubMed] [Google Scholar]


