Abstract
Background/Aim:
Epidemiological studies of celiac disease (CD) among Saudi children have been performed only within some groups who are at a high risk of developing CD. The aim of this study was to determine the prevalence of CD among symptom-free children from the public schools of the military campus of National Guard in the Eastern Province of Saudi Arabia.
Patients and Methods:
Between 2012 and 2014, serum samples were collected from 1141 students (age 6–18 years) attending nine public schools of the military campus of National Guard in the Eastern Province of Saudi Arabia. Participants were screened for CD by testing for anti-tissue transglutaminase IgA (IgA-tTG) and IgG antibodies (IgG-tTG). Small intestinal biopsy was offered to all participants who tested positive for IgA-tTG [IgA-tTG >20 relative units (RU)/ml].
Results:
Of the 1141 participants, 32 were IgA-tTG positive. Thus, the estimated serology-positive prevalence was 3%. An intestinal biopsy was performed in 10 of the participants with antibody positivity. The biopsy findings of all 10 children were consistent with CD. Thus, the estimated biopsy-confirmed prevalence was about 1%.
Conclusions:
The prevalence of CD was estimated to be about 1% among symptom-free children from the public schools of the military campus of National Guard in the Eastern Province of Saudi Arabia.
Keywords: Anti-tissue transglutaminase IgA (IgA-tTG) and anti-tissue transglutaminase IgG (IgG-tTG), anti-tissue transglutaminase (IgG/IgA), celiac disease, endoscopy, Saudi Arabia
Celiac disease (CD) is a permanent intolerance to dietary gluten that is characterized by immune-mediated inflammatory lesions of the intestinal mucosa. Owing to its variable manifestations and age at onset, CD has emerged as a worldwide public health problem.[1] Although the “classical” malabsorption syndrome that is characterized by diarrhea, steatorrhea, weight loss, and fatigue may occur in severe cases, most patients present with a milder constellation of symptoms, such as abdominal discomfort and bloating that mimic irritable bowel syndrome or non-gastrointestinal symptoms that include anemia and osteoporosis, or no symptoms at all. In most patients with CD, a gluten-free diet assures full recovery.
Many patients with milder presentation of CD are diagnosed at a relatively advanced stage of the disease. The prompt diagnosis and treatment of CD are associated with symptomatic improvement, reduction of potential complications (including malignancies), and decreased mortality.[2,3] Therefore, it is necessary to increase the awareness of physicians about the variable manifestations of CD and to emphasize the importance of screening.
Till date, only one prevalence study for Saudi healthy adolescents has been reported. The study was performed by Aljabreen et al.[4] in three regions: Aseer, Madinah, and Al-Qaseem. They screened 1167 students aged between 16 and 18 years (out of 65,431 students), and showed the prevalence by region as follows: Aseer 2.1%, Madinah 1.8%, and Al-Qaseem 3.2%.
The aims of this study were to determine the prevalence of silent and asymptomatic CD in the young population (i.e., individuals aged 6–18 years), to compare the prevalence of CD among those with and without comorbidities, and to identify significant risk factors for CD in the studied age group. These aims were accomplished using active serologic screening for markers of CD and the diagnosis of CD was confirmed histologically using duodenal biopsy.
PATIENTS AND METHODS
After approval was obtained to conduct this study from King Abdullah International Medical Research Center (KAIMRC), the local research ethics board, and the Ministry of Education, the study was conducted in the schools that belong to the military campus of the Ministry of the National Guard in the Eastern Province of Saudi Arabia, between January 2012 and May 2014.
The campus has nine schools, which include three levels each for boys and girls: Elementary, intermediate, and secondary schools. The Saudi population includes many tribes, which are distributed throughout the Kingdom. Military housing campuses are among the places where most of the tribes are found in a single location. Thus, it was decided to conduct this study on children attending the campus schools.
A letter including the objectives of the study was sent to all parents in December 2011. Parents were asked to fill out a questionnaire to gather information on medical history and familial history of CD, and they were asked to sign an informd consent if they agreed to participate in the study.
Apparently healthy children from the nine schools, who were in grades 1–11 (age range, 6–17 years), were invited to participate in the study. Exclusion criteria included children with medical problems associated with CD, such as Down syndrome, Turner syndrome, autoimmune disease, or diabetes, and children with a positive family history of CD.
Serum samples were collected from the participating students and tested for anti-tissue transglutaminase IgA (IgA-tTG) and IgG antibodies (IgG-tTG) (QUANTA Flash® tTG-IgA/G; Inova Diagnostics, San Diego, USA (9900 Old Grove Road San Diego, CA, 92131-1638, USA. The cutoff value for positivity was set at 20 relative units (RU)/ml (maximum calibrator was 220 RU/ml).
Biopsies
A small intestinal biopsy was offered to participating students who tested positive for IgA-tTG or IgG-tTG. Endoscopic biopsies were obtained using an Olympus GIF P230 videogastroscope (Olympus Optical, Tokyo, Japan). Five biopsy specimens were collected from the bulbus and the second part of the duodenum of each patient and carefully oriented on filter paper prior to submersion in 10% neutral buffered formalin. Biopsy samples were stained with hematoxylin–eosin and immunostained with anti-CD3 monoclonal antibodies to obtain an intraepithelial lymphocyte count.
All biopsies were evaluated by the same expert histopathologist and classified using the Marsh classification. Normal mucosa with more than 30 intraepithelial lymphocytes per 100 epithelial cells was defined as Marsh type 1, infiltrative or hyperplastic lesions were defined as Marsh type 2, and villous atrophy was defined as Marsh type 3.[5]
A diagnosis of CD was made in children who tested positive for IgA-tTG and/or IgG-tTG, and showed features of mucosal changes (ranging from grade I to III) upon small intestinal biopsy.
Statistical analysis
The sample size was based on the 4430 students in the military campus (95% Confidence Level, and 2.5 confidence interval have been used).
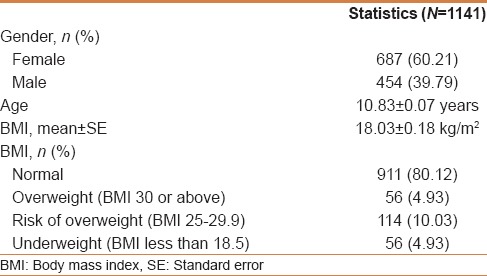
All variables were summarized and reported across the study cohorts using descriptive statistics. Interval variables, such as age and body mass index (BMI), were summarized and reported in terms of mean and standard error. Categorical variables, such as gender, BMI, and comorbidity status, were summarized and reported in terms of frequency distribution [Table 1].
Table 1.
Cohort characterization
Univariate analysis was used to estimate the prevalence of CD using both diagnostic methods (i.e., serology and endoscopy). The results were reported in terms of proportion and percentage. A one-sample binomial test was used to determine whether the prevalence of CD was different from a hypothetical value that was set a priori. The results were reported in terms of the estimate, standard error, 95% confidence interval, and P value. Significance was declared at α <0.05.
The Chi-square test of independence was used to measure the relationship between CD and comorbidity status. The results were reported in terms of proportion, percentage, and P value. Significance was declared at α <0.05.
A binary logistic regression model was used to identify significant predictors of serology-confirmed CD. Potential covariates (e.g., age, gender, and BMI) were included in the statistical model. The results were reported in terms of the odds ratio, standard error, 95% confidence interval, and P value. Significance was declared at α <0.05. Logistic regression could not be performed for patients in whom CD was confirmed by endoscopy, owing to the small sample size.
All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
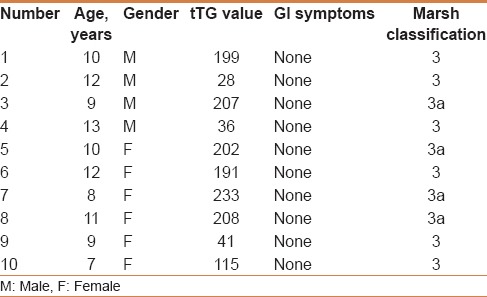
Totally 1141 students agreed to participate in the study, with a mean age of 11 years ± SD 0.07 years (687 girls and 454 boys) [Table 1]. Of the 1141 participants, 32 were IgA-tTG positive. Thus, the estimated serology-positive prevalence was 3%. Twenty-two of the 32 students refused the Endoscopy and biopsies [Table 2]. Ten of the 32 students accepted to undergo endoscopy and biopsies; all the 10 students had duodenal biopsies consistent with CD [Table 3]. Thus, the estimated biopsy-confirmed prevalence was about 1%. All the 10 patients had normal growth parameters and did not suffer any gastrointestinal symptoms at the time of diagnosis.
Table 2.
Clinical and serologic characteristics of the 22 students with positive IgA-tTG, but refused upper endoscopy
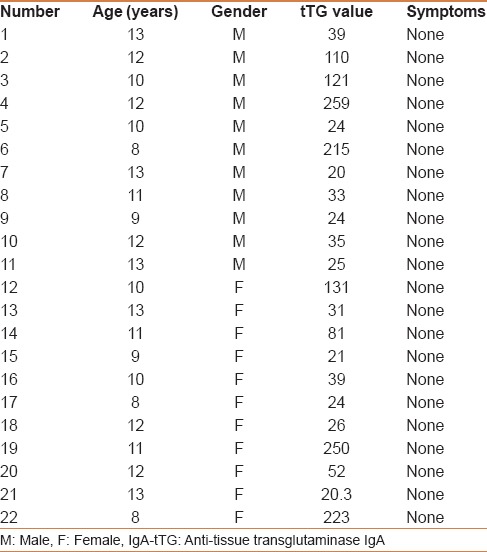
Table 3.
Clinical, serologic, and histopathologic characteristics of the 10 patients with biopsy-proven celiac disease
DISCUSSION
The prevalence of CD varies widely across different countries. This variability reflects population-based differences in the risk of CD, as well as differences in study design (e.g., serological screening vs. symptom-based diagnosis, or screening of general vs. high-risk populations). With these limitations in mind, the prevalence of CD in Western populations (based on serological screening) appears to be approximately 1%, with a reasonable range of 0.71–1.25%. However, the prevalence of CD is lower in other parts of the world, such as South America and Asia,[6] and the disease rarely affects people of purely Chinese or Japanese origin.
No national epidemiological studies of mass screening for CD in Saudi Arabia have been reported. However, Al Attas[7] reported a seroprevalence of 7.6% for CD in a reference laboratory setting among 145 patients with clinically suspected disease and 2.5% among 18 patients with various autoimmune diseases. None of the patients with inflammatory bowel disease and none of the healthy blood donors were seropositive for CD.
Data on prevalence of CD in children with Type 1 diabetes mellitus (T1D) in Saudi Arabia are limited to a few studies. Al-Ashwal et al., showed a 4.9% prevalence rate of biopsy-proven CD in T1D children (anti-gliadin antibodies-based screening study).[8] In a prospective study, Al-Hussaini et al. used both IgA-tTG and anti-endomyseal antibodies to screen for CD among T1D children; they reported a prevalence rate of 11.3% for biopsy-proven CD.[9] Saadah[10] reported a 10% prevalence rate of biopsy-proven CD in T1D children.
Outside of Saudi Arabia and other Gulf countries, Dalgic et al. estimated that the prevalence of CD is at least 0.47% among apparently healthy Turkish school children.[11] Ben Hariz et al.[12] estimated the prevalence of CD among Tunisian school children to be approximately 1/157. Vijgen et al. reported that the seroprevalence of IgA-tTG in non-IgA-deficient children and adolescents from Belgium is 1:114.[13] In Italy, Catassi et al. reported that the prevalence of CD among undiagnosed school-aged children is 1:210.[14] Farahmand et al. determined the prevalence of occult CD among healthy Iranian school-aged children to be 0.5%.[15] Mäki et al. estimated that the prevalence of CD among Finnish school children is at least 1 case per 99 children.[16] Alarida et al. reported that the prevalence of CD among Libyan school children is 1%.[17] Karagiozoglou-Lampoudi et al. reported the prevalence of CD in the Greek pediatric population to be 1:154.[18] Nusier estimated the serological prevalence of CD among Jordanian school children to be 1:124.[19] Al Jabreen et al. reported the seroprevalence of CD among healthy adolescents in Saudi Arabia by region as follows: Aseer 2.1%, Madinah 1.8%, and Al-Qaseem 3.2%.[4] The sample size was 1167 out of the school population of 65,431.
To our knowledge, this study represents the first to carry out screening for CD in healthy school children in Saudi Arabia. In our study of 1141 school children, 32 participants were antibody positive (3%). Comorbidity status and serology-confirmed CD were not significantly associated (P value: 0.401). All participants with endoscopy-confirmed CD (n = 10) were free of comorbid conditions. None of the potential covariates (i.e., age, gender, and BMI) were significant predictors of serology-confirmed CD (P value: 0.847, 0.316, and 0.119, respectively).
Intestinal biopsy was performed for 10 participants. The biopsy findings of all 10 children were consistent with CD. Thus, the estimated biopsy-proven prevalence was 1%. This value is similar to the reported European prevalence.
Our study is important to raise awareness of this disease at the level of general pediatrician and community. This awareness will help in decreasing the incidence of many known complications of the disease like malnutrition, osteoporosis, lactose intolerance, depression, intestinal lymphoma, and others. His study would be a start to more nation-based studies.
This study had some limitations. The screening was limited only to Ministry of National Guard military campus in the Eastern Province of Saudi Arabia, and therefore, the data obtained from this study cannot be generalized to the entire Saudi population of the Eastern Province.
This study will increase awareness of both the care-giver and the management about the disease prevalence, especially the type of food to be given for those affected with the disease. For patients suffering from the high cost of treatment, since it is not covered by most of governmental hospitals, this study will increase the community awareness about their sufferings.
CONCLUSIONS
It was found that the prevalence of CD is about 1% among symptom-free children from the public schools of military campus of National Guard in the Eastern Province of Saudi Arabia. Mass screening is recommended in the other provinces of Saudi Arabia, because early diagnosis and diet restriction can prevent the complications of CD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Baker SS. Rethinking strategies to screen for celiac disease. Pediatrics. 2014;133:331–2. doi: 10.1542/peds.2013-3631. [DOI] [PubMed] [Google Scholar]
- 3.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–8. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 4.Aljebreen AM, Almadi MA, Alhammad A, Al Faleh FZ. Seroprevalence of celiac disease among healthy adolescents in Saudi Arabia. World J Gastroenterol. 2013;19:2374–8. doi: 10.3748/wjg.v19.i15.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 6.Makharia GK, Mulder CJ, Goh KL, Ahuja V, Bai JC, Catassi C, et al. World Gastroenterology Organization-Asia Pacific Association of Gastroenterology Working Party on Celiac Disease. Issues associated with the emergence of coeliac disease in the Asia-Pacific region: A working party report of the World Gastroenterology Organization and the Asian Pacific Association of Gastroenterology. J Gastroenterol Hepatol. 2014;29:666–77. doi: 10.1111/jgh.12514. [DOI] [PubMed] [Google Scholar]
- 7.Al Attas RA. How common is celiac disease in Eastern Saudi Arabia? Ann Saudi Med. 2002;22:315–9. doi: 10.5144/0256-4947.2002.315. [DOI] [PubMed] [Google Scholar]
- 8.Al-Ashwal AA, Shabib SM, Sakati NA, Attia NA. Prevalence and characteristics of celiac disease in type I diabetes mellitus in Saudi Arabia. Saudi Med J. 2003;24:1113–5. [PubMed] [Google Scholar]
- 9.Al-Hussaini A, Sulaiman N, Al-Zahrani M, Al-Enazi A, Khan M. Prevalence of Celiac disease among type 1 diabetic children. BMC Gastroenterology. 2012;12:180. doi: 10.1186/1471-230X-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saadah OI. Celiac disease in children and adolescents at a singe center in Saudi Arabia. Ann Saudi Med. 2011;31:51–7. doi: 10.4103/0256-4947.75779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalgic B, Sari S, Basturk B, Ensari A, Egritas O, Bukulmez A, et al. Turkish Celiac Study Group. Prevalence of celiac disease in healthy Turkish school children. Am J Gastroenterol. 2011;106:1512–7. doi: 10.1038/ajg.2011.183. [DOI] [PubMed] [Google Scholar]
- 12.Ben Hariz M, Kallel-Sellami M, Kallel L, Lahmer A, Halioui S, Bouraoui S, et al. Prevalence of celiac disease in Tunisia: Mass-screening study in schoolchildren. Eur J Gastroenterol Hepatol. 2007;19:687–94. doi: 10.1097/MEG.0b013e328133f0c1. [DOI] [PubMed] [Google Scholar]
- 13.Vijgen S, Alliet P, Gillis P, Declercq P, Mewis A. Seroprevalence of celiac disease in Belgian children and adolescents. Acta Gastroenterol Belg. 2012;75:325–30. [PubMed] [Google Scholar]
- 14.Catassi C, Fabiani E, Rätsch IM, Coppa GV, Giorgi PL, Pierdomenico R, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 15.Farahmand F, Mir-Nasseri MM, Shahraki T, Yourdkhani F, Ghotb S, Modaresi V, et al. Prevalence of occult celiac disease in healthy Iranian school age children. Arch Iran Med. 2012;15:342–5. [PubMed] [Google Scholar]
- 16.Mäki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, et al. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003;348:2517–24. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 17.Alarida K, Harown J, Ahmaida A, Marinelli L, Venturini C, Kodermaz G, et al. Coeliac disease in Libyan children: A screening study based on the rapid determination of anti-transglutaminase antibodies. Dig Liver Dis. 2011;43:688–91. doi: 10.1016/j.dld.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Karagiozoglou-Lampoudi T, Zellos A, Vlahavas G, Kafritsa Y, Roma E, Papadopoulou A, et al. Screening for coeliac disease in preschool Greek children: The feasibility study of a community-based project. Acta Paediatr. 2013;102:749–54. doi: 10.1111/apa.12241. [DOI] [PubMed] [Google Scholar]
- 19.Nusier MK, Brodtkorb HK, Rein SE, Odeh A, Radaideh AM, Klungland H. Serological screening for celiac disease in schoolchildren in Jordan. Is height and weight affected when seropositive? Ital J Pediatr. 2010;36:16. doi: 10.1186/1824-7288-36-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


