Abstract
Background/Aims:
Airway difficulties leading to cardiac arrest are frequently encountered during propofol sedation in patients undergoing gastrointestinal (GI) endoscopy. With a noticeable increase in the use of propofol for endoscopic sedation, we decided to examine the incidence and outcome of cardiac arrests in patients undergoing gastrointestinal (GI) endoscopy with sedation.
Patients and Methods:
In this retrospective study, cardiac arrest data obtained from the clinical quality improvement and local registry over 5 years was analyzed. The information of patients who sustained cardiac arrest attributable to sedation was studied in detail. Analysis included comparison of cardiac arrests due to all causes until discharge (or death) versus the cardiac arrests and death occurring during the procedure and in the recovery area.
Results:
The incidence of cardiac arrest and death (all causes, until discharge) was 6.07 and 4.28 per 10,000 in patients sedated with propofol, compared with non–propofol-based sedation (0.67 and 0.44). The incidence of cardiac arrest during and immediately after the procedure (recovery area) for all endoscopies was 3.92 per 10,000; of which, 72% were airway management related. About 90.0% of all peri-procedural cardiac arrests occurred in patients who received propofol.
Conclusions:
The incidence of cardiac arrest and death is about 10 times higher in patients receiving propofol-based sedation compared with those receiving midazolam–fentanyl sedation. More than two thirds of these events occur during EGD and ERCP.
Keywords: Cardiac arrest, death, endoscopy, Endoscopc retrograde cholangiopancreatogrphy, Esophagoduodenoscopy, propofol
Gastrointestinal (GI) endoscopies are commonly performed across the globe and these procedures are on the increase. Over the years, they have evolved from simple diagnostic to complex therapeutic procedures, with increased duration and patient discomfort. To a large extent, the success of these procedures is due to improved sedation techniques including extensive use of propofol. However, they are associated with increased direct costs (drugs, provider's payment, and so on) along with reported sedation-related complications such as cardiac arrest.[1] The incidence of cardiac arrests during outpatient GI endoscopy is likely to be higher than that reported under general anesthesia. Closed claim studies suggest that propofol sedation (PS) is likely to be associated with a higher incidence of cardiorespiratory complications than non–propofol-based sedation (NPBS) during GI endoscopy.[2] However, studies comparing cardiac arrests and their outcome between PS and NPBS are unavailable. In this single center retrospective study, we compared the incidence, causation, and outcome of cardiac arrests in patients who underwent GI endoscopy over a period of 5 years.
PATIENTS AND METHODS
After obtaining the institutional review board's approval, patient and procedure data of GI endoscopic procedures performed between 9-08-2008 and 5-31-2013 (our new endoscopy center opened on 9-08-2008) were analyzed. Data from the hospital Clinical Quality Improvement (CQI) and the local registry for the same period were scrutinized for documented cardiac arrests, regardless of their duration and outcome. All adverse events, especially major adverse events like death or cardiopulmonary arrests are entered in a register kept at the nurses’ station. Additionally, the concerned physician documents in a hospital reporting system. Both are accessed by the physician presenting the data on a quarterly basis at the continuous quality improvement meetings. The data for the study was obtained from the records kept by both the physician presenting the register at the nurses’ station. Patients who displayed asystole, ventricular fibrillation, or pulseless electrical activity requiring cardiopulmonary resuscitation of any duration were included in the definition of cardiac arrest. In effect, cardiac arrest was defined as an event with cessation of pumping action of the heart, requiring CPR, however brief it was. However, the duration of loss of pumping action was not standardized. Many cardiac arrests proceeded with respiratory arrest.
The statistical analysis was performed using SPSS Ver 21 (IBM Inc., Chicago, IL, USA) for Macintosh.
Parametric data was compared using t-test and frequency data was compared using the Chi-square test, considering P value of 0.05 as significant in comparisons. The denominator included 251 different procedures among a total of 73,029 procedures. This large group was simplified into 14 broad categories based on the type and complexity of the procedures (Tables 1 and 2 in Appendix). The patients who were not given any type of sedation were excluded from the analysis. At the Hospital of the University of Pennsylvania, Philadelphia, propofol sedation is used at either patients’ request or referring physician's recommendation. Younger patients and patients who have failed non-propofol sedation are generally sedated with propofol. Some of the gastroenterologists use propofol sedation for all the procedures irrespective of patient- or procedure-related factors. Non–propofol-based sedation was provided using fentanyl, midazolam and occasionally diphenhydramine by the endoscopy nurse under the supervision of the endoscopist, whereas propofol was administered by a certified registered nurse anesthetist (CRNA) or a resident physician under the supervision of a physician anesthesiologist. Statistical comparisons were made between the cardiac arrest events recorded (all causes, irrespective of outcome) in either the propofol or nonpropofol sedation groups. Where available, data was analyzed to find relationships between the frequency of cardiac arrest and the American Society of Anesthesiology (ASA) physical status, Modified Mallampatti (MMP) airway classification, and Body Mass Index (BMI) of the patients.
Appendix Table 1.
Master chart displaying the procedures performed over about 5 years

Appendix Table 2.
Simplified procedure list

RESULTS
From a total of 73,029 GI (36,092 males and 36,937 females) endoscopic procedures performed, 20 cardiac arrests were reported [Table 1]. These were the patients who sustained cardiac arrest (irrespective of the outcome) during or after the procedure, irrespective of the length after the procedure. About 28,008 (14,083 males and 13,925 females) procedures received propofol-based sedation, whereas 45,021 (22,009 males and 23,012 females) procedures received non–propofol-based sedation (typically with midazolam, fentanyl, and rarely diphenhydramine). Propofol-based sedation was administered by either a nurse anesthetist or a resident (physician training in anesthesia) under the supervision of an experienced anesthesiologist, whereas non–propofol-based sedation was administered by a registered nurse under the guidance of the endoscopist performing the procedure. Irrespective of the cause of cardiac arrest and death (sedation related, procedure complication related, or unrelated to either of these), patients who received propofol-based sedation had a higher risk of cardiac arrest and death. As displayed in Table 1, the overall incidence of cardiac arrest in patients undergoing GI procedures with PS (6.069 per 10000) was 9.11 times greater when compared with those undergoing GI procedures with NPBS (0.666 per 10000, Chi-square test 12.46, P < 0.001). The odds ratio of patient developing cardiac arrest in PS group was 9.109 (95% CI, 2.67–31.079). The incidence of death was even higher, at 11.25 times greater in PS (4.28 per 10000) compared with that of NPBS (0.444 per 10000) with a P < 0.001 using Chi-square test.
Table 1.
Relationship between cardiac arrest (all causes irrespective of duration) and type of sedation (the incidence of cardiac arrest is per 10,000 procedures)
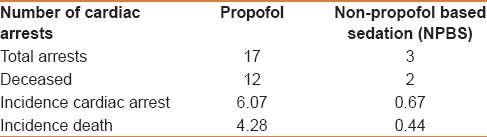
The incidence of peri-proceudural cardiac arrests in patients undergoing EGD and ERCP was 4.64 per 10,000 peri-procedural in patients receiving propofol sedation, whereas it was zero in patients who received nonpropofol sedation. Airway complications were responsible for 4.12 per 10,000 of these procedures.
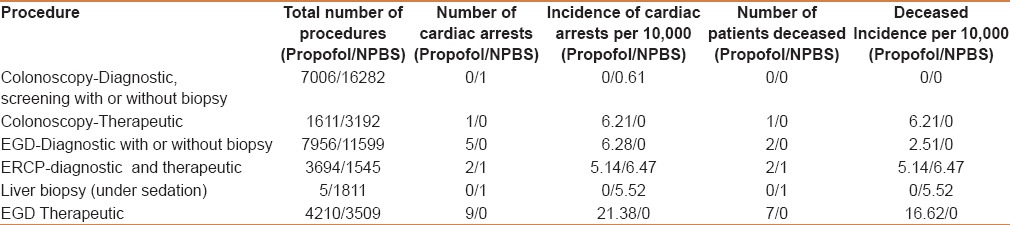
Table 2 shows the incidence of cardiac arrests in patients undergoing different endoscopic procedures. Screening and diagnostic colonoscopies had the lowest risk of cardiac arrest. Surprisingly, of the 45,021 procedures performed under nonpropofol sedation, only one patient experienced a brief asystole (possibly vasovagal) in the immediate postoperative period. He received brief cardiopulmonary resuscitation and admitted to emergency room; he was discharged to go home later. However, the therapeutic colonoscopy group had one intraprocedural aspiration that resulted in sepsis followed by death. The patient had received propofol sedation. ERCP had the highest incidence of mortality among all endoscopic procedures.
Table 2.
Relationship between type of procedure and cardiac arrest (all causes)
Where possible, an attempt was made to evaluate the relationship between the cardiac arrest and the ASA status, MMP airway class, and BMI [Tables 3–5]. The majority of cardiac arrests and deaths occurred in patients assigned to ASA status 3. Chi-square test showed a significant association of frequency of arrest to both ASA status (P = 0.003, highest being ASA III) and MMP class (P = 0.03, highest being in MMP II). Unfortunately, ASA status and MMP status of all 73,029 patients who underwent the procedures was not available; therefore, it was not possible to calculate the risk of cardiac arrest associated with both ASA status and MMP class.
Table 3.
Relationship between ASA status and cardiac arrest
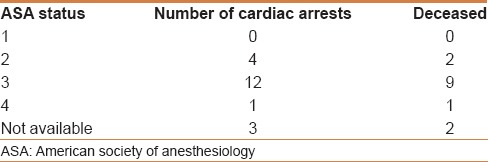
Table 5.
Relationship between body mass index and cardiac arrest
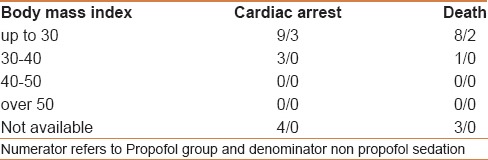
Table 4.
Relationship between Airway class and cardiac arrest
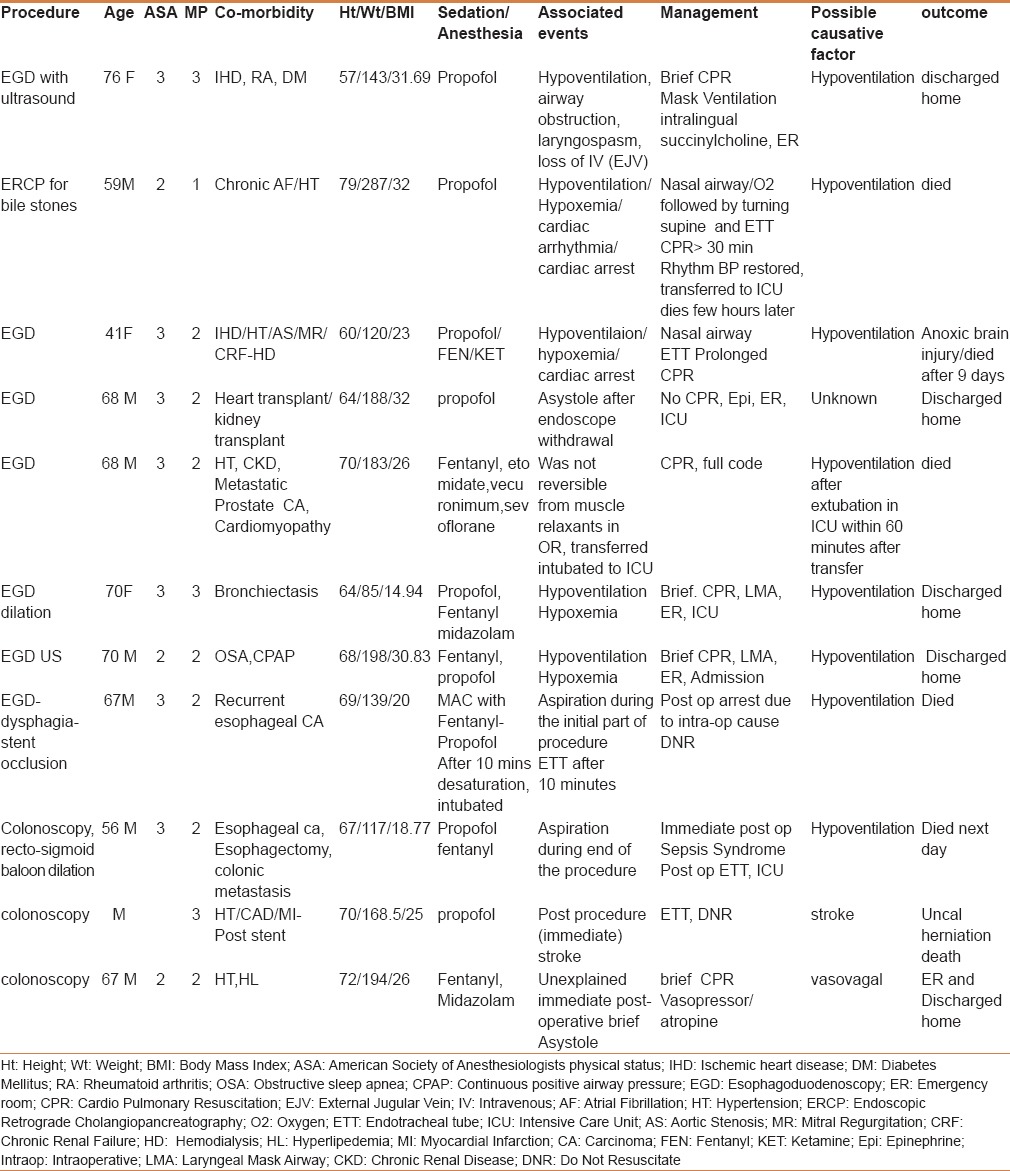
Table 6 documents the details of the patients who sustained cardiac arrests attributable to sedation-related causes (directly or indirectly). These are the patients sustaining cardiac arrest either during the procedure or in the postprocedure recovery area. One patient developed a cerebrovascular stroke during the colonoscopy and died later. Hypoxemia, as a result of hypoventilation, was found to be the leading cause of intraoperative cardiac arrest and all such arrests occurred only during propofol-based sedation. Although successfully resuscitated and discharged home in the majority, 2 patients died in the postoperative period. In 9 patients, cardiac arrests not attributable to sedation resulted from the following: Procedure-related bleeding (3/9), guts perforation (1/9), pancreatitis (1/9), pulmonary embolism (1/9), and undiagnosed cause (3/9). Excluding surgical causes, the sedation-related cardiac arrest incidence was 0.357 per 10,000 in PS and 0.022 per 10,000 in NPBS.
Table 6.
Details of the patients who sustained possible sedation related cardiac arrest during the procedure and in post procedure recovery area
The effectiveness of the following components (for their ability to predict cardiac arrest) was estimated using automatic linear modeling in SPSS: Age at the time of procedure, BMI, ASA status, gender, and type of sedation (propofol vs nonpropofol). The adjusted r2 value using above modeling was 0.406, with 2 variables displaying possible predictive association with cardiac arrests. Automated predictor importance in SPSS reported a value of 0.96 for “Age at time of procedure” and 0.04 for “increasing ASA” status. However, none of the above factors showed any possible predictive efficacy using the data of patients who sustained cardiac arrest.
DISCUSSION
This is the first study to systematically analyze the frequency, mechanisms, management, and outcome of cardiac arrests during GI endoscopy. The reported incidence of cardiac arrest during general anesthesia and regional anesthesia, is 5.5 and 1.5 per 10,000, respectively.[3,4] Of 5.5 per 10,000 reported by Sprung et al., approximately 50% were related to airway complications. In our analysis, although the total incidence of peri-procedure-related cardiac arrests in patients undergoing upper endoscopic procedures (EGD and ERCP) was 4.64 per 10,000, about 90% (4.12 per 10,000) of these cardiac arrests were related to airway management.
In a closed claim study, it was found that about 50% of all claims in the GI suite were related to propofol-based sedation.[2] Moreover, claims related to death or brain damage resulted predominantly from oversedation, which led to hypoxemia. Our findings are in agreement, with many of the cardiac arrests heralded by sedation-related hypoxemia. However, closed claim studies have two major drawbacks. Firstly, they do not have a denominator. As a result, calculating incidence is not possible. For the first time, we have provided such an incidence from a large sample of patients, sedated with both PS and NPBS. Secondly, closed claim studies often underestimate the actual incidences due to under-reporting of complications.
Other large studies mainly involving endoscopist-directed propofol sedation are available. In a retrospective study (by Rex et al.) involving 646,080 patients, the number of patients with sedation-related hypoxemia requiring endotracheal intubation was 11.[5,6] Rex et al. did not report any neurological injuries and only 4 deaths. Moreover, among deceased were 2 patients with pancreatic cancer, a severely handicapped patient with mental retardation, and a patient with severe cardiomyopathy. In another prospective study involving 10,000 patients undergoing GI endoscopy with endoscopist-guided propofol, only 3 needed assisted ventilation.[7] No patient experienced laryngospasm or needed endotracheal intubation. There were no deaths or neurological injuries. Another study involving 446 patients undergoing advanced endoscopic procedures, including ASA III/IV patients, found a very low incidence of complications.[8] Apart from minor complications including a 7.9% incidence of <90% oxygen saturation, the safety record was outstanding. No patients required endotracheal intubation or sustained cardiac arrest. The readers are referred to other studies with similar outcomes.[9,10,11,12]
The only endoscopist-directed PS involving ERCP patients reported one case of hypoxemia requiring bag mask ventilation and another patient requiring endotracheal intubation.[13] However, a sample size of 156 is too small to make meaningful conclusions.
Data examining the incidence of cardiac arrest in patients undergoing GI endoscopy under propofol sedation administered by anesthesia providers is absent. In a prospective study involving 799 patients undergoing advanced endoscopic procedures,[14] the incidence of hypoxemia was about 13% and no patient required either bag mask ventilation or endotracheal intubation. There were no major complications such as cardiac arrest.
It might come as a surprise that the incidence of cardiac arrest in our study is higher than the only large study referenced above (Rex et al.). An explanation is that the endoscopists are likely to be more conservative than anesthesia providers, especially with regard to depth of sedation. With extensive experience in intravenous conscious sedation, they are more likely to provide mild-to-moderate sedation, even with propofol. This is in contrast to anesthesia providers, where the sedation is likely to be deeper. However, when anesthesia providers are utilized, gastroenterologists expect their patients to be sedated to the point of being unresponsive. Lastly, endoscopists might have used anesthesia providers to treat any severe hypoxemia episodes, thereby preventing further deterioration. Additionally, anesthesiologists seem to accept lower oxygen saturations (longer apnea times leading to desaturation) than endoscopists.
Our study has certain inadequacies. It is a retrospective study with its inevitable limitations. The documentation was done in patients chart with no plans to analyze at a later date. However, retrospective studies are more likely to underestimate the incidence of morbidity and mortality. The patient's data such as ASA status and BMI were not available for all the patients. Although hypoxemia was a factor in many cardiac arrests, the factor that triggered hypoxemia was not clear from the documentation. The patients’ comorbidity might have played a significant role in the outcome of the resuscitation efforts. The mortality in ERCP group is higher in patients sedated without propofol and is unexpected. However, considering the total deaths (2 and 1) and smaller denominator, they are understandable and of questionable significance.
CONCLUSIONS
In this single-center large retrospective study, cardiac arrest and death are seemingly higher in patients provided with propofol sedation, when compared with non–propofol-based intravenous conscious sedation. Although sedation-related hypoxemia was one of the associated factors during intraprocedural cardiac arrests, a cause and effect relationship could not be consistently established. Case selection might have contributed, although it could not be demonstrated in our analysis. A multicentric prospective study might address the contribution of sedation, especially propofol-mediated deep sedation to cardiac arrests.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Goudra BG, Singh PM. Cardiac arrests during endoscopy with anesthesia assistance. JAMA Intern Med. 2013;173:1659–60. doi: 10.1001/jamainternmed.2013.8756. [DOI] [PubMed] [Google Scholar]
- 2.Metzner J, Posner KL, Domino KB. The risk and safety of anesthesia at remote locations: The US closed claims analysis. Curr Opin Anaesthesiol. 2009;22:502–8. doi: 10.1097/ACO.0b013e32832dba50. [DOI] [PubMed] [Google Scholar]
- 3.Sprung J, Flick RP, Gleich SJ, Weingarten TN. Perioperative Cardiac Arrests. SIGNA VITAE. 2008;3:8–12. [Google Scholar]
- 4.Kopp SL, Horlocker TT, Warner ME, Hebl JR, Vachon CA, Schroeder DR, et al. Cardiac arrest during neuraxial anesthesia: Frequency and predisposing factors associated with survival. Anesth Analg. 2005;100:855–65. doi: 10.1213/01.ANE.0000144066.72932.B1. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, et al. Endoscopist-directed administration of propofol: A worldwide safety experience. Gastroenterology. 2009;137:1229. doi: 10.1053/j.gastro.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK. Endoscopist-directed propofol. Tech Gastrointest Endosc. 2009;11:177–80. doi: 10.1016/j.giec.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich K, Stremmel W, Sieg A. Endoscopist-administered propofol sedation is safe-a prospective evaluation of 10,000 patients in an outpatient practice. J Gastrointest Liver Dis. 2012;21:259–63. [PubMed] [Google Scholar]
- 8.Redondo-Cerezo E, Sánchez-Robaina A, Martínez Cara JG, Ojeda-Hinojosa M, Matas-Cobos A, Sánchez Capilla AD, et al. Gastroenterologist-guided sedation with propofol for endoscopic ultrasonography in average-risk and high-risk patients: A prospective series. Eur J Gastroenterol Hepatol. 2012;24:506–12. doi: 10.1097/MEG.0b013e328350fcbd. [DOI] [PubMed] [Google Scholar]
- 9.Fanti L, Agostoni M, Arcidiacono PG, Albertin A, Strini G, Carrara S, et al. Target-controlled infusion during monitored anesthesia care in patients undergoing EUS: Propofol alone versus midazolam plus propofol. A prospective double-blind randomised controlled trial. Dig Liver Dis. 2007;39:81–6. doi: 10.1016/j.dld.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Dewitt J, McGreevy K, Sherman S, Imperiale TF. Nurse-administered propofol sedation compared with midazolam and meperidine for EUS: A prospective, randomized trial. Gastrointest Endosc. 2008;68:499–509. doi: 10.1016/j.gie.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Yusoff IF, Raymond G, Sahai AV. Endoscopist administered propofol for upper-GI EUS is safe and effective: A prospective study in 500 patients. Gastrointest Endosc. 2004;60:356–60. doi: 10.1016/s0016-5107(04)01711-0. [DOI] [PubMed] [Google Scholar]
- 12.Fatima H, DeWitt J, LeBlanc J, Sherman S, McGreevy K, Imperiale TF. Nurse-administered propofol sedation for upper endoscopic ultrasonography. Am J Gastroenterol. 2008;103:1649–56. doi: 10.1111/j.1572-0241.2008.01906.x. [DOI] [PubMed] [Google Scholar]
- 13.Khan HA, Umar M, Tul-Bushra H, Nisar G, Bilal M, Umar S. Safety of non-anaesthesiologist-administered propofol sedation in ERCP. Arab J Gastroenterol. 2014;15:32–5. doi: 10.1016/j.ajg.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Coté GA, Hovis RM, Ansstas MA, Waldbaum L, Azar RR, Early DS, et al. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010;8:137–42. doi: 10.1016/j.cgh.2009.07.008. [DOI] [PubMed] [Google Scholar]


