Abstract
Current antipsychotic drugs (APDs) show efficacy with positive symptoms, but are limited in treating negative or cognitive features of schizophrenia. Whereas all currently FDA-approved medications target primarily the dopamine D2 receptor (D2R) to inhibit Gi/o-mediated adenylyl cyclase, a recent study has shown that many APDs affect not only Gi/o- but they can also influence β-arrestin- (βArr)-mediated signaling. The ability of ligands to differentially affect signaling through these pathways is termed functional selectivity. We have developed ligands that are devoid of D2R-mediated Gi/o protein signaling, but are simultaneously partial agonists for D2R/βArr interactions. The purpose of this study was to test the effectiveness of UNC9975 or UNC9994 on schizophrenia-like behaviors in phencyclidine-treated or NR1-knockdown hypoglutamatergic mice. We have found the UNC compounds reduce hyperlocomotion in the open field, restore PPI, improve novel object recognition memory, partially normalize social behavior, decrease conditioned avoidance responding, and elicit a much lower level of catalepsy than haloperidol. These preclinical results suggest that exploitation of functional selectivity may provide unique opportunities to develop drugs with fewer side effects, greater therapeutic selectivity, and enhanced efficacy for treating schizophrenia and related conditions than medications that are currently available.
Introduction
Schizophrenia is a severe neuropsychiatric disorder characterized by positive, negative, and cognitive symptoms (Andreasen, 1995). The current FDA-approved antipsychotic drugs (APDs) show efficacy in treating positive symptoms (Leucht et al, 2009, 2013); however, they have limited efficacy with negative and/or cognitive symptoms (Miyamoto et al, 2012; Citrome, 2014). APDs can induce a wide range of motor, metabolic, cardiovascular, and/or emotional side effects that lower the quality of life and lead to treatment noncompliance (Kroeze et al, 2003; Schimmelmann et al, 2005; Leucht et al, 2013; Moritz et al, 2013). Hence, the development of novel pharmacotherapies that have higher efficacies for treating schizophrenic symptoms and possess a low profile for side effects is urgently needed.
All currently FDA-approved APDs target primarily the dopamine (DA) D2 receptor (D2R) for their actions (Boyd and Mailman, 2012; Miyamoto et al, 2012). Parenthetically, the D2R is a G protein-coupled receptor (GPCR) whose activation inhibits cAMP production (Gingrich and Caron, 1993). APDs were identified originally by their abilities to bind the D2R and regulate cAMP synthesis (Creese et al, 1976; Boyd and Mailman, 2012). More recent studies indicate that D2R activities can influence several different signal transduction pathways (Beaulieu et al, 2004, 2007) and that most APDs have both G protein-dependent and -independent actions (Masri et al, 2008). The ability of ligands to differentially affect signaling through a GPCR is termed ‘functional selectivity' (Gay et al, 2004; Urban et al, 2007; Boyd and Mailman, 2012).
Although G protein-dependent signaling is a well-known property of GPCRs, G protein-independent signaling is mediated through β-arrestin (βArr) (Luttrell et al, 1999; Beaulieu et al, 2005; Lefkowitz and Shenoy, 2005). Stimulation of DA receptors by amphetamine or through deletion of the DA transporter (Dat1) gene in mice leads to inactivation of Akt kinase with concomitant activation of glycogen synthase kinase 3α/β (GSK3α/β) (Beaulieu et al, 2004). Similarly, phencyclidine (PCP)-induced hypoglutamatergia can also influence the phosphorylation status of Akt and GSK (Lei et al, 2008). These signaling events occur independently of the cAMP pathway and are reversed by D2R blockade and inhibition of DA synthesis. Interestingly, pharmacological inhibition of GSK3 depresses hyperlocomotion in DAT-knockout (KO) mice and the effects of amphetamine are blunted in GSK3β heterozygotes. Subsequent work has shown that in D2R-containing neurons βArr2 serves as a scaffolding protein for Akt and protein phosphatase 2A (PP2A; Beaulieu et al, 2005). In βArr2-KO mice behavioral responses to amphetamine are decreased, activation of Akt in striatum is reduced, and binding of Akt to PP2A is disrupted. In comparison, responses mediated through the cAMP system are preserved and are not differentiated from those in wild-type (WT) mice. Thus, G protein-independent signaling from the D2R requires βArr2 and signaling through Akt and GSK3 that influences motor activity in mice.
In humans, the Akt1/GSK3β signaling pathways are implicated in schizophrenia. For instance, the brain and lymphocytes from schizophrenic patients contain low levels of Akt1 protein and its substrate, GSK3β, has reduced phosphorylation (Emamian et al, 2004). In addition, mutation of AKT1 has been suggested as a susceptibility gene for schizophrenia (Emamian et al, 2004; Zheng et al, 2012) and APDs enhance phosphorylation of Akt1 and GSK3β in vitro and in vivo (Emamian et al, 2004; Park et al, 2011; Deslauriers et al, 2013). As Akt and GSK3 responses are mediated through ligands that are biased for βArr signaling, this may represent a novel approach to treat schizophrenia. To test this idea, we have recently developed D2R ligands that are devoid of D2R-mediated Gi/o activity and are simultaneously high-affinity partial agonists for βArr-mediated responses (Allen et al, 2011).
We tested two D2R βArr-biased ligands for APD-like activity in NR1-knockdown (KD) mice because they represent a hypoglutamatergic model for schizophrenia (Mohn et al, 1999). These mutants are hyperactive in the open field, deficient in prepulse inhibition (PPI), display abnormal social and sexual behaviors, and are impaired in cognitive performance (Mohn et al, 1999; Duncan et al, 2004, 2006a, 2006b; Halene et al, 2009; Milenkovic et al, 2014). Haloperidol, clozapine, and olanzapine reduce their hyperactivity and improve PPI, whereas clozapine partially restores their sexual behavior. Importantly, NMDA receptor levels are reduced by ~90%, DA neurons have faster spontaneous firing rates, and D2R autoreceptors are desensitized in NR1-KD relative to those in WT mice (Mohn et al, 1999; Ferris et al, 2014). Hence, these mutants are not only hypoglutamatergic but also hyperdopaminergic, and they have been proposed as a model for schizophrenia-like behaviors. The purpose of the present studies was to examine the efficacy of the βArr-biased UNC compounds across a series of behavioral domains related to schizophrenia-like responses in mouse models of hypoglutamatergia.
Materials and Methods
Animals
Adult male and female WT and NR1-KD, WT and βArr2-KO, and inbred C3H/HeJ mice were used in these experiments. The NR1 mice were maintained on separate C57BL/6J and 129X1/SvJ coisogenic backgrounds and F1 animals were used in experiments. The βArr2 mice had been backcrossed onto a C57BL/6J background for more than 10 generations and were generated by heterozygous (HET) matings. The C3H/HeJ mice were purchased from Jackson Labs (Bar Harbor, ME). All mice were housed 3–5 per cage in a temperature- and humidity-controlled room on a 14 : 10 h (lights on at 0600 h) light/dark cycle with food and water provided ad libitum. All experiments were conducted with an approved protocol from the Duke University Institutional Animal Care and Use Committee.
Drugs and Compounds
These details are located in the Supplementary Information.
Open Field Activity
Locomotor activities of NR1 mice were monitored in an automated Omnitech Digiscan apparatus (Omnitech Electronics, Columbus, OH). Baseline activities were assessed over the first 30 min, mice were injected with vehicle or different doses of haloperidol, UNC9975, or UNC9994, and immediately returned to the open field for 90 min. Locomotor activity was monitored using Fusion Integra software (Omnitech).
Prepulse Inhibition
PPI of the acoustic startle response was conducted as described previously (Ralph et al, 2001). WT and βArr2-KO mice were injected with vehicle or PCP (6 mg/kg) and returned to their home cages. After 10 min, WT and βArr2-KO mice or WT and NR1-KD animals were treated with vehicle or UNC9975, habituated to the PPI chamber for 10 min, and tested. Further details are located in the Supplementary Information.
Novel Object Recognition Memory (NORM)
This test was modified from that of McDowell et al (2010). Briefly, WT and NR1-KD mice received 2 days of training and on each day they were given vehicle, haloperidol, or UNC9975 and returned to their home cages for 30 min before testing. Subsequently, mice were exposed for 5 min to 2 identical objects. Testing occurred 24 h later, when mice were injected with vehicle and given access to one of the familiar objects and a novel object for 5 min. A preference score for the novel object was calculated as: ((time spent with the novel object−time spent with the familiar object)/total time spent with both objects). Additional details are in the Supplementary Information.
Social Behavior
NR1 mice were tested for sociability as described previously (Wang et al, 2011). WT and NR1-KD mice were injected with vehicle, aripiprazole, clozapine, or UNC9975 and returned to their home cages. After 10 min, the mouse was given free access to the entire apparatus during each of three phases (10 min each): the nonsocial/nonsocial (NS–NS), social/nonsocial (S1–NS, social affiliation test), and familiar/novel social (S1–S2, social preference test) conditions. A preference score was calculated based on the total time spent exploring each cage in each test condition: [(S1−NS1)/(S1+NS1)] or [(S2−S1)/(S2+S1)]. Additional details are in the Supplementary Information.
Conditioned Avoidance Responding (CAR)
The βArr2 mice were trained and tested for CAR in two-way avoidance chambers. Briefly, mice were acclimated to both chambers for 5 min and trials began with the presentation of the conditioned stimulus (CS; 0.5 s of a 72 dB 2900 Hz tone with a 10 s house light). If the mouse did not cross to the adjoining chamber after 10 s, a scrambled foot-shock (US; 0.15 mAmp) was given. Animals received a total of 30 trials over 30 min each day. The numbers of avoidances, escapes, and failures were recorded. Additional details are in the Supplementary Information.
Fear Conditioning
At 1 day before conditioning, βArr2 mice were habituated to the chamber for 5 min. After 24 h, a 30 s tone (CS; 2900 Hz, 80 db) was presented and it coterminated with a 2 s, 0.4 mA foot-shock (US). Three CS–US pairings (60 s ITI) were given. The next day, mice were injected with vehicle or UNC9975 and tested 25 min later. Additional details are in the Supplementary Information.
Catalepsy
The βArr2 mice were examined in the horizontal rod test as described previously (Sanberg et al, 1988). Baseline catalepsy was set at time 0. Subsequently, mice were injected with haloperidol or UNC9975 and separate cohorts of mice were tested 30, 60, or 90 min later. Additional details are in the Supplementary Information.
Statistical Analysis
The data are presented as means±SEM and were analyzed with IBM SPSS Statistics (Armonk, NY). The data were analyzed by ANOVA or repeated measures ANOVA (RMANOVA), and post hoc analyses were by Bonferroni corrected pair-wise comparisons. A p<0.05 was considered significant.
Results
UNC9975 and UNC9994 Reduce NR1-KD Hyperlocomotion
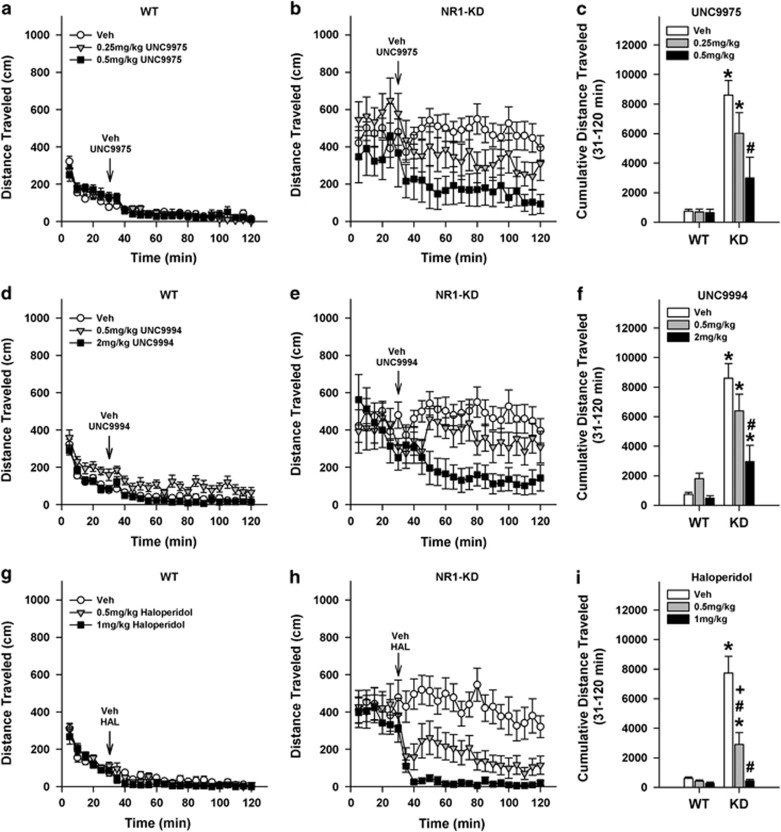
Reductions of hyperactivity in the open field are often used to assess the efficacy of APDs in rodents. The NR1-KD mice are hyperactive in the open field, and haloperidol, clozapine, and olanzepine are reported to decrease their hyperlocomotion (Mohn et al, 1999; Duncan et al, 2006a). As expected, baseline locomotion (0–30 min) was significantly higher in NR1-KD than WT mice at each time point (ps<0.018; Figure 1a and b). To visualize the UNC9975 effects more clearly over the 31–120 min treatment interval, the data were analyzed as cumulative distance traveled. Here, locomotion was higher in vehicle-treated NR1-KD than in corresponding WT controls (p<0.001; Figure 1c). The 0.5 mg/kg UNC9975 depressed hyperlocomotion in NR1-KD mice relative to their vehicle controls (p<0.001), and this activity was not significantly different from those of the WT groups that were also not different from each other. Nevertheless, 0.25 mg/kg UNC9975 failed to attenuate the hyperlocomotion in the NR1-KD animals compared with their vehicle control.
Figure 1.
UNC9975 and UNC9994 reduce hyperlocomotion in the open field in NR1-KD mice. Baseline activities of WT and NR1-KD animals were monitored over 30 min, they were treated (arrow) with vehicle (Veh) or different doses of UNC9975, UNC9994, or haloperidol, and were returned immediately to the open field for 90 min. (a, b) Effects of UNC9975 on WT and NR1-KD locomotor activity. For baseline activity, RMANOVA found the within-subjects time-by-genotype interaction (F(5, 265)=4.67, p<0.001) and the between-subjects genotype effect (F(1, 53)=34.08, p<0.001) to be significant. Following UNC9975 injection, RMANOVA revealed the within-subjects time effect to be significant (F(17, 901)=3.40, p<0.001). (c) Cumulative locomotor activities of both genotypes following injection of Veh or UNC9975 (31–120 min). A two-way ANOVA observed the effects of genotype (F(1, 53)=46.47, p<0.001) and treatment (F(2, 53)=4.52, p=0.015), and the genotype-by-treatment interaction to be significant (F(2, 53)=4.26, p=0.019). (d, e) Effects of UNC9994 on WT and NR1-KD locomotion. For baseline activity RMANOVA reported a within-subjects effect of time (F(5, 265)=12.32, p<0.001) and the time-by-genotype interaction (F(5, 265)=3.34, p=0.006) to be significant. The between-subjects genotype effect (F(1, 53)=36.81, p<0.001) was also significant. Following UNC9994 injection, RMANOVA noted a significant within-subjects effect of time (F(17, 901)=3.47, p<0.001), and significant time-by-genotype (F(17, 901)=1.96, p=0.011), time-by-treatment (F(34, 901)=2.25, p<0.001), and time-by-genotype-by-treatment interactions (F(34, 901)=1.52, p=0.030). (f) Cumulative distance traveled by both genotypes following injection of Veh or UNC9994 (31–120 min). A two-way ANOVA observed significant effects of genotype (F(1, 53)=64.38 p<0.001) and treatment (F(2, 53)=8.52, p=0.001); the genotype-by-treatment interaction (F(2, 53)=6.66, p=0.003) was also significant. (g, h) Effects of haloperidol on WT and NR1-KD locomotion. The RMANOVA for baseline activity observed significant within-subject effects of time (F(5, 295)=13.927, p<0.001), and a significant time-by-genotype interaction (F(5, 295)=5.136, p<0.001). Following haloperidol treatment, RAMANOVA revealed a significant effect of time (F(17, 1003)=6.496, p<0.001), and significant time-by-genotype (F(17, 1003)=1.904, p<0.015) and time-by-treatment interactions (F(51, 1003)=1.526, p<0.011). Whereas the within-subjects effects for the time-by-genotype-by-treatment interaction was not significant, the cubic function detected a significant three-way interaction (F(3, 59)=2.946, p<0.040). (i) Cumulative locomotor activities of WT and NR1-KD mice following injection of Veh or haloperidol (31–120 min). A two-way ANOVA found significant effects of genotype (F(1, 45)=51.299, p=0.001), treatment (F(2, 45)=24.127, p=0.001), and a significant genotype-by-treatment interaction (F(2, 45)=19.096, p=0.001). N=8–11 mice for UNC9975, N=9–11 mice for UNC9994, and N=8–9 mice for haloperidol per genotype per treatment. The data are presented as mean±SEM; *p<0.05, WT vs NR1-KD for the same dose; #p<0.05, within-genotype comparison vs the Veh; +p<0.05 within-genotype 0.5 compared with 1 mg/kg haloperidol.
Consistent with the UNC9975 results, baseline activities of NR1-KD mice in the UNC9994 experiment were higher than those at WT mice at each time point (ps<0.047; Figure 1d and e). Analyses of cumulative locomotion following vehicle or UNC9994 administration revealed that vehicle-treated NR1-KD motor activity remained high relative to the WT vehicle controls (p<0.001; Figure 1f). UNC9994 at 2 mg/kg significantly suppressed hyperlocomotion in NR1-KD mice compared with the vehicle (p<0.001); however, locomotion was still higher than that in WT vehicle controls (p=0.026). In comparison, 0.5 mg/kg UNC9994 was without effect on NR1-KD hyperlocomotion.
The effects of UNC9975 and UNC9994 were compared with that for haloperidol. Both 0.5 and 1 mg/kg haloperidol reduced hyperlocomotion in NR1-KD mice at 40–120 min relative to the vehicle controls (ps<0.004), with the 1 mg/kg dose being more efficacious than 0.5 mg.kg at 45–85 min (ps<0.012; Figure 1g–i). Importantly, 1 mg/kg haloperidol suppressed locomotion in NR1-KD mice to levels of the WT controls. Together, these results show that haloperidol and both UNC9975 and UNC9994 reduce hyperlocomotion in the hypoglutaminatergic NR1-KD animals.
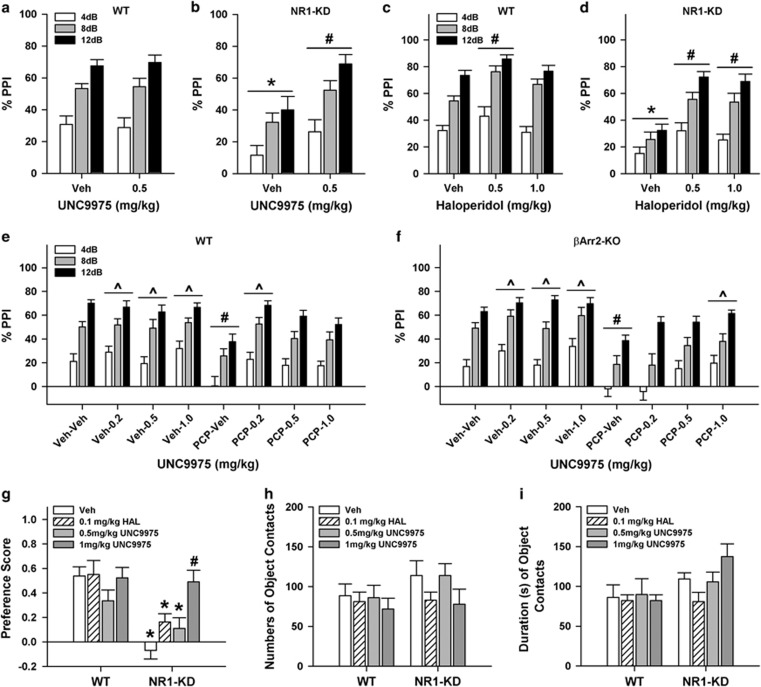
UNC9975 Restores PPI in Hypoglutamatergic Mice
PPI is disrupted in schizophrenia (Moghaddam et al, 1997; Krystal et al, 1999; Powell et al, 2012) and it is decreased in NR1-KD mice (Duncan et al, 2006a, 2006b). As expected, PPI was reduced in vehicle-treated NR1-KD compared with WT mice (p=0.001; Figure 2a and b). Although UNC9975 did not influence PPI in WT mice relative to vehicle controls, it restored PPI in mutants (p=0.005) to the levels of the WT animals. Similarly, haloperidol rescued the deficits in NR1-KD animals (ps<0.001) and it also augmented PPI in the WT controls at the 0.5 mg/kg dose (p<0.011; Figure 2c and d). For UNC9975, null activities were indistinguishable between genotypes (not shown); however, startle activities were increased in NR1-KD mice regardless of treatment condition (p<0.001; Supplementary Figure S1a). With haloperidol, null activities were enhanced in NR1-KD compared with WT mice (p<0.033; Supplementary Table S1); however, this difference was only 1.8–3.8 mAmp between WT and NR1-KD mice and it comprised <2% of the maximum startle responses in each group. In contrast, startle responses for the vehicle-treated NR1-KD mice were higher than those for WT animals (p=0.001) and 1 mg/kg haloperidol reduced this NR1-KD startle activity to the levels of the WT vehicle controls (Supplementary Figure S1b).
Figure 2.
UNC9975 enhances PPI and NORM in NR1-KD mice and higher doses of this compound are required to augment PPI in PCP-treated βArr2-KO than in WT mice. (a, b) PPI in WT and NR1-KD mice given the vehicle (Veh) or 0.5 of 1 mg/kg UNC9975. The RMANOVA found the within-subjects effects of prepulse intensity were significant (F(2, 86)=104.93, p<0.001). The between-subjects effects of genotype (F(1, 43)=6.36, p=0.015) and treatment (F(1, 43)=5.00, p=0.031) and the genotype-by-treatment interaction (F(1, 43)=4.59, p=0.038) were also significant. (c, d) PPI in WT and NR1-KD mice administered haloperidol. RMANOVA detected significant within-subject effects of prepulse intensity (F(2, 106)=177.099, p=0.001) and significant prepulse-intensity-by-genotype (F(2, 106)=3.525, p=0.033) and prepulse-intensity-by-treatment interactions (F(4, 106)=3.441, p=0.011). The between-subjects effects of genotype (F(1, 53)=32.152, p=0.001), treatment (F(2, 53)=17.444, p=0.001), and the genotype-by-treatment interaction (F(2, 53)=3.642, p=0.033) were also significant. (e, f) PPI in WT and βArr2-KO mice treated with the Veh or 6 mg/kg PCP followed by Veh or UNC9975. RMANOVA reported a significant within-subjects effect of prepulse intensity (F(2, 374)=295.31, p<0.001) and a significant between-subjects treatment effect (F(7, 187)=8.89, p<0.001) and treatment-by-genotype interaction (F(7, 187)=2.15, p=0.040). (g) Preference scores for NORM in WT and NR1-KD mice; mice were administered Veh, 0.1 mg/kg haloperidol (HAL), or 0.5 or 1 mg/kg UNC9975 during two training sessions but not at testing. ANOVA at testing revealed significant effects of genotype (F(1, 69)=25.639, p<0.001) and treatment (F(3, 69)=4.673, p=0.005); the genotype-by-treatment interaction was significant (F(3, 69)=3.864, p=0.013). (h) Total numbers of contacts with both objects at testing for WT and NR1-KD mice. ANOVA found no significant differences in the numbers of object contacts. (i) Total duration of contacts with both objects at testing for WT and NR1-KD mice. ANOVA for the duration of object contacts noted only a significant genotype effect (F(1, 69)=6.857, p<0.011). N=8–15 mice for the NR1 experiments and N=9–23 mice for the βArr2 study. The data are presented as mean±SEM; *p<0.05, WT vs NR1-KD for the same treatment condition; #p<0.05, within-genotype comparison vs the Veh or Veh–Veh group; ^p<0.05, within-βArr2 genotype comparison vs the PCP–Veh group.
To assess the specificity of UNC9975 for βArr in PPI, WT and βArr2-KO mice were tested. Prepulse-dependent responding was detected across all test conditions (ps<0.001; Figure 2e and f). As anticipated, PCP suppressed PPI in both genotypes relative to their vehicle controls (ps<0.040). Different doses of UNC9975 alone did not affect PPI in either genotype. Although 0.2 mg/kg UNC9975 restored PCP-disrupted PPI in WT mice to levels of the vehicle controls (p=0.003), no further effects were seen with the 0.5 or 1 mg/kg doses. In comparison, 1 mg/kg UNC9975 was required to rescue PPI in PCP-treated βArr2-KO mice to the levels of the WT and βArr2-KO vehicle controls. Although null activities in the PCP-treated animals increased relative to the vehicle controls (ps<0.05), these levels were <6% of their startle responses (Table 1). Animals given 1 mg/kg UNC9975 also had higher startle responses regardless of genotype compared with the vehicle controls (Supplementary Figure S1c).
Table 1. Null Activities of βArr2 Mice.
| Genotypea | Treatment conditionb | Mean null activity (mAmp) | SEM |
|---|---|---|---|
| WT | Veh–Veh | 2.60 | ±0.18 |
| Veh–0.2 mg/kg UNC9975 | 2.57 | ±0.39 | |
| Veh–0.5 mg/kg UNC9975 | 1.88 | ±0.39 | |
| Veh–1 mg/kg UNC9975 | 4.19 | ±0.39 | |
| PCP–Vehc | 5.45 | ±0.98 | |
| PCP–0.2 mg/kg UNC9975c | 5.97 | ±1.21 | |
| PCP–0.5 mg/kg UNC9975c | 4.09 | ±0.46 | |
| PCP–1 mg/kg UNC9975c | 6.88 | ±0.75 | |
| KO | Veh–Veh | 3.10 | ±0.51 |
| Veh–0.2 mg/kg UNC9975 | 2.68 | ±0.41 | |
| Veh–0.5 mg/kg UNC9975 | 2.34 | ±0.42 | |
| Veh–1 mg/kg UNC9975 | 5.29 | ±0.62 | |
| PCP–Vehc | 5.55 | ±0.74 | |
| PCP–0.2 mg/kg UNC9975c | 5.33 | ±1.06 | |
| PCP–0.5 mg/kg UNC9975c | 4.11 | ±0.46 | |
| PCP–1 mg/kg UNC9975c | 9.09 | ±1.46 |
N=9–23 mice/genotype/treatment condition.
A two-way ANOVA for null activity found only the main effect of treatment was significant (F(7, 187)=10.94, p<0.001).
PCP=phencyclidine, 6 mg/kg was given.
UNC9975 Rescues NORM in NR1-KD Mice
The NMDA receptor is known to play a role in cognition (Lee and Silva, 2009) and NR1-KD mice are impaired in tests in the Y-maze and puzzle box (Milenkovic et al, 2014). We examined the effects of haloperidol and UNC9975 in the NORM task with NR1 mice as this task examines processes similar to declarative memory in humans (Horiguchi and Meltzer, 2012). Mice received the vehicle, haloperidol, or UNC9975 over the 2 training days. As the numbers of object contacts and the time spent with objects for each of these 2 days were virtually identical, the data were collapsed across days. When the numbers of object contacts at training were examined for WT mice, no effects of treatment were observed (Supplementary Figure S2a). In comparison, although object contacts between vehicle- and haloperidol-treated NR1-KD mice were similar to each other and WT animals, mutants given UNC9975 made more objects contacts than these groups (ps<0.012). An examination of the duration of object contact times at training yielded similar results (Supplementary Figure S2b). Here, NR1-KD mice administered the vehicle or either dose of UNC9975 interacted with objects for longer periods of time than WT animals under the same treatment conditions (ps<0.003). When NORM was evaluated, previous treatment with UNC9975 exerted no effects on WT performance at testing (Figure 2g). In comparison, NR1-KD mice given vehicle, haloperidol, or 0.5 mg/kg UNC9975 at training had lower preference scores at testing than WT controls (ps<0.045). Importantly, 1 mg/kg augmented the preference scores of NR1-KD mice to those of WT controls and the scores at this dose were enhanced relative to mutants given vehicle, haloperidol, or the lower dose of UNC9975 (ps<0.042). Notably, the impairment in object preference for NR1-KD mice administered the vehicle, haloperidol, or 0.5 mg/kg UNC9975 was not due to reduced object exploration as both the numbers of object contacts and the duration of contacts at testing were not different from those of the WT mice (Figure 2h and i).
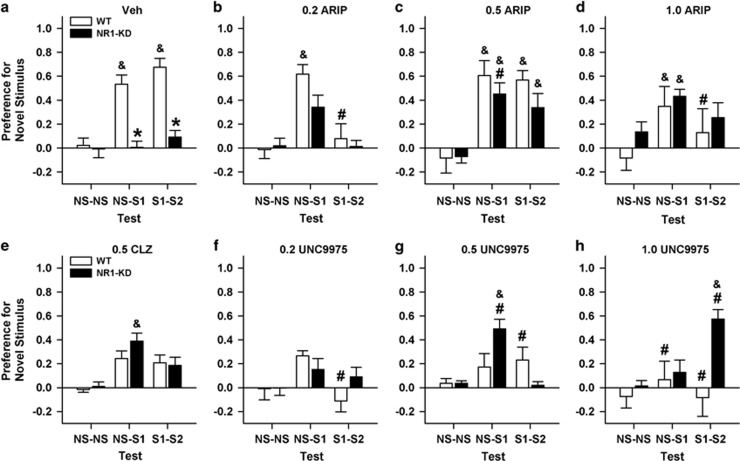
UNC9975 Attenuates the NR1-KD Deficiency in Social Behavior
Social behavior can be abnormal in schizophrenia (Couture et al, 2006) and NR1-KD mice rarely interact with cagemates in the home cage and they are impaired in the resident–intruder and sociability assays (Mohn et al, 1999; Duncan et al, 2004; Halene et al, 2009). Social behavior was evaluated in the sociability test to determine whether the NR1-KD mice would respond to UNC9975. Aripiprazole was included because UNC9975 was synthesized from an aripiprazole scaffold and because aripiprazole and UNC9975 have similar potencies in reducing amphetamine-stimulated hyperlocomotion (Allen et al, 2011). Preference scores during the non-social (NS) or NS–NS test were similar between genotypes regardless of treatment (Figure 3). However, vehicle-treated WT mice had much higher preference scores for the novel social (S1 or S2) stimuli during the NS–S1 and S1–S2 test sessions than the NR1-KD animals (ps<0.001). Notably, these mutants failed to show social affiliation (NS–S1) or social preference (S1–S2) during these tests (Figure 3a). Effects of aripiprazole, clozapine, and UNC9975 were next examined. Relative to the vehicle control, 0.5 mg/kg aripiprazole, clozapine, and UNC9975 did not significantly alter WT responses during the NS–S1 or S1–S2 tests (Figure 3a, c, e, and g). However, in the S1–S2 test, 0.2 and 1 mg/kg aripiprazole and UNC9975 depressed preference scores in WT mice compared with their vehicle control (ps<0.013; Figure 3a, b, d, f, and h). In the NR1-KD mice, 0.5 mg/kg aripiprazole increased preferences for the novel social stimulus in both the NS–S1 and S1–S2 relative to the NS–NS test (ps<0.005; Figure 3c). Similarly in the NS–S1 test, preferences for the novel social stimuli were augmented with 0.5 mg/kg clozapine and UNC9975 (ps<0.035); however, in the S1–S2 test, responses to 1 mg/kg aripiprazole were not enhanced (Figure 3d, e, and g). UNC9975 at 1 mg/kg was effective only during the S1–S2 test (p=0.001; Figure 3h). Both 0.2 mg/kg UNC9975 and 0.2 mg/kg aripiprazole were without effect in either the NS–S1 or S1–S2 test (Figure 3b and f).
Figure 3.
UNC9975 partially restores social behavior in NR1-KD mice. A NR1 mouse had access to the entire test arena with two empty wire mesh cages in the NS–NS test. In the NS–S1 test, the mouse had access to an empty wire mesh cage and a cage containing a novel C3H mouse. In the S1–S2 test, the NR1 mouse could interact with the cage containing the now familiar C3H mouse and a cage with a novel C3H mouse. Social responses of the WT and NR1-KD mice were compared under vehicle (Veh); 0.2, 0.5, or 1 mg/kg aripiprazole (ARIP); 0.5 mg/kg clozapine (CLZ); or 0.2, 0.5, or 1 mg/kg UNC9975. (a) The RMANOVA for NR1-KD Veh controls showed significant within-subjects effects of test (ie, NS–NS, S1–NS, and S1–S2) (F(2, 42)=20.25, p<0.001) and a significant test-by-genotype interaction (F(2, 42)=12.63, p<0.001). The between-subjects test revealed a significant genotype effect (F(1, 21)=35.18, p<0.001). (b–f) RMANOVA compared effects of the vehicle to aripiprazole, clozapine, or UNC9975 and found the within-subjects test effect (F(2, 304)=42.22, p<0.001) and the test-by-treatment (F(14, 304)=24.76, p=0.001) and test-by-genotype-by-treatment interactions (F(14, 304)=24.13, p=0.011) were significant. N=8–16 mice/genotype/treatment. The data are presented as mean±SEM; *p<0.05, WT vs NR1-KD in the Veh group; #p<0.05, within genotype vs Veh; &p<0.05, within genotype compared with the NS–NS group within the treatment condition.
To determine whether aripiprazole, clozapine, or UNC9975 could restore the social deficit in NR1-KD mice, comparisons were made with the WT vehicle control (ie, Figure 3a). Here, all three doses of aripiprazole, 0.5 mg/kg clozapine, and 0.5 mg/kg UNC9975 restored social affiliation (NS–S1) in NR1-KD mice (Figure 3a–e and g). Similarly, 1 mg/kg UNC9975 normalized social preferences (S1–S2) in these mutants (Figure 3a and h). Importantly, the duration of total object contacts was similar between genotypes treated with vehicle (Supplementary Figure S3a). However, compared with this vehicle control, the duration of contacts was significantly reduced in WT mice under all treatment conditions (ps<0.01) except clozapine (Supplementary Figure S3). In contrast, the duration of contacts in NR1-KD mice was unaffected by any treatment condition.
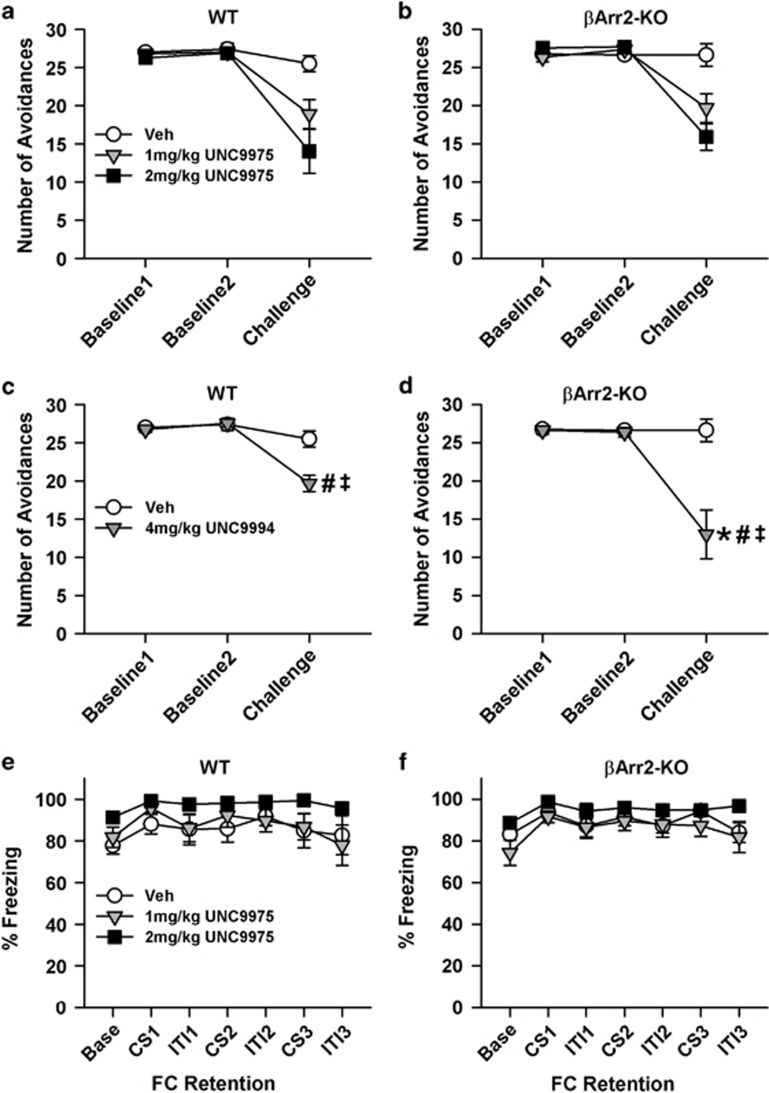
UNC Compounds Reduce CAR in WT and βArr2-KO Mice
CAR has high predictive validity for detecting antipsychotic properties as demonstrated with haloperidol and other antipsychotic drugs (Fibiger et al, 1975; Wadenberg, 2010). Baseline responses over the 2 training days before treatment were similar between the βArr2 genotypes and among the assigned treatment groups (Figure 4a and b). Both 1 and 2 mg/kg UNC9975 decreased CAR compared with baseline and the vehicle control regardless of genotype (ps<0.001), and this reduction in avoidances produced a corresponding increase in escapes (ps<0.01; Supplementary Figure S4a and b) without affecting failures (data not shown). With UNC9994, baseline training was also similar for both genotypes (Figure 4c and d). UNC9994 at 4 mg/kg significantly reduced CAR compared with baseline and the vehicle controls in WT and βArr2-KO mice (ps<0.024). However, avoidance responses in the UNC9994-treated βArr2-KO mice were lower than those in WT animals (p=0.012), and this effect was reflected by increased escapes (Supplementary Figure S4c and d).
Figure 4.
UNC9975 and UNC9994 reduce CAR in βArr2 mice but do not affect associative fear conditioning. Once CAR responses were stable, mice were treated the next day with vehicle (Veh) or a UNC compound, and assessed 30 min later. (a, b) Effects of UNC9975 on CAR responses by WT and βArr2-KO mice. RMANOVA for CAR with UNC9975 revealed a significant within-subjects day effect (F(2, 90)=71.42, p<0.001) and a significant day-by-treatment interaction (F(4, 90)=15.70, p<0.001). In addition, the between-subjects effect of treatment was significant (F(2, 45)=12.56, p<0.001). (c, d) Effects of UNC9994 on CAR responses by WT and βArr2-KO mice. RMANOVA for CAR with UNC9994 demonstrated a significant within-subjects day effect (F(2, 62)=36.34, p<0.001) and significant day-by-treatment (F(2, 62)=26.00, p<0.001) and day-by-treatment-by-genotype interactions (F(2, 62)=4.10, p=0.021). The between-subjects treatment effect was significant (F(1, 31)=22.22, p<0.001); the effects of genotype (F(1, 31)=3.38, p=0.076) and the treatment-by-genotype interaction (F(1, 31)=3.60, p=0.067) were marginally significant. (e, f) Effects of UNC9975 on fear conditioning in WT and βArr2-KO mice. RMANOVA at retention testing revealed significant within-subjects effects of time (F(6, 324)=9.89, p<0.001). Base, baseline; CS, conditioned stimulus; ITI, intertrial interval; FC, fear conditioning. N=8–11 mice/genotype/treatment condition. The data are presented as mean±SEM; *p<0.05, WT vs βArr2-KO; #p<0.05, within genotype vs Veh; ‡p<0.05, within genotype vs baseline responses.
One reason why CAR may be reduced by the UNC compounds is that they may interfere with aversive memories. To test this idea, βArr2 mice were fear conditioned. All animals showed increased freezing across conditioning and this was not differentiated by genotype or treatment condition (Supplementary Figure S4e and f). Freezing was enhanced with the first CS presentation compared with baseline and it was reduced during the last intertribal interval (ps<0.030; Figure 4e and f). Together, these data show that both UNC9975 and UNC9994 are effective in reducing CAR and this decrease cannot be attributed to interference with aversive memories.
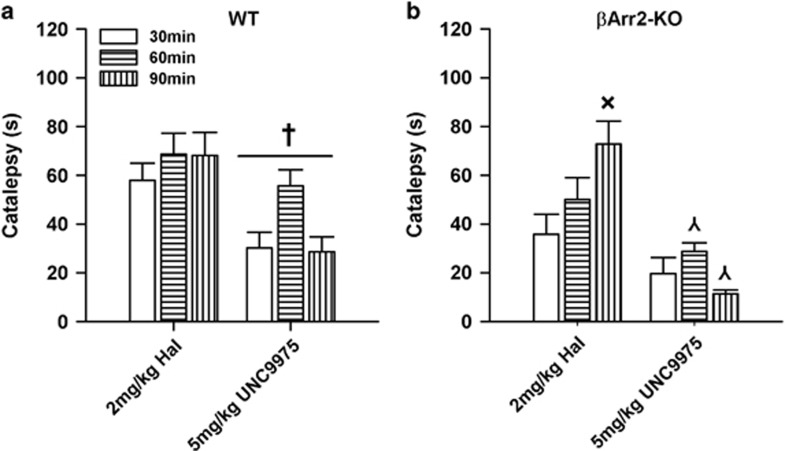
UNC9975 Elicits Low Cataleptic Responses
Catalepsy is often used to determine the potential of a drug to possess extrapyramidal side effects (Sanberg et al, 1988). When responses of βArr2 mice were examined, their latencies to initiate voluntary movement at time 0 were ~2.8 s longer for haloperidol than with UNC9975. Hence, cataleptic times were subtracted from all groups at 30, 60, and 90 min and analyzed. Regardless of genotype, catalepsy was significantly lower in UNC9975- than in haloperidol-treated mice at each time point (ps<0.011; Figure 5). Within treatment, the UNC9975 animals displayed higher catalepsy at 60 min than at 30 or 90 min (ps<0.023), whereas haloperidol-treated mice had higher catalepsy at 90 min than at 30 min (p=0.006). βArr2-KO had less catalepsy than WT mice regardless of treatment condition or time (p<0.001). Although the ANOVA did not discern any significant genotype-by-treatment differences, WT and βArr2-KO mice clearly responded differently in catalepsy (Figure 5). Thus, we compared responses within genotype. UNC9975-treated WT mice had shorter latencies to initiate movement than haloperidol-treated animals (p<0.001; Figure 5a). In addition, with UNC9975, catalepsy in WT mice was more pronounced at 60 min than at 30 min (p<0.044). In comparison, catalepsy at 60 and 90 min was lower in UNC9975 than in haloperidol-treated βArr2-KO animals (ps<0.016; Figure 5b). In addition, catalepsy was higher at 90 min than at 30 min in haloperidol-treated mutants (p=0.001), whereas no differences were observed at the various time points among the UNC9975-treated animals.
Figure 5.
Catalepsy to UNC9975 is reduced compared with haloperidol in βArr2 mice. (a, b) Catalepsy to haloperidol and UNC9975 in WT and βArr2-KO mice monitored 30, 60, and 90 min after injection. ANOVA detected significant effects of treatment (F(1, 133)=57.30, p<0.001), genotype (F(1, 133)=14.72, p<0.001), and time (F(2, 133)=4.85, p=0.009), and the treatment-by-time interaction was significant (F(2, 133)=7.26, p=0.001). For WT mice, ANOVA found significant effects of treatment (F(1, 66)=20.02, p<0.001) and time (F(2, 66)=3.47, p=0.037). For βArr2-KO mice, ANOVA discerned a significant treatment effect (F(1, 67)=40.72, p<0.001) and a significant treatment-by-time interaction (F(2, 67)=7.59, p=0.001). N=9–15 mice/genotype/treatment condition. The data are presented as mean±SEM; †p<0.05, within WT mice for haloperidol vs UNC9975; ⋏p<0.05, within βArr2-KO mice for haloperidol vs UNC9975 at 60 and 90 min; ×p<0.05, within βArr2-KO mice for haloperidol treatment at 30 vs 90 min.
Discussion
The glutamatergic hypothesis for schizophrenia is derived from observations that subanesthetic doses of PCP or ketamine can induce schizophrenia-like symptoms in healthy individuals and they exacerbate symptoms in schizophrenic patients (Tsai and Coyle, 2002; Krystal et al, 2003). Moreover, NMDA antagonists or genetic knockdown of the common NR1 subunit of the NMDA receptor induce responses in rodents that bear similarities to positive and negative symptoms of schizophrenia (Moghaddam et al, 1997; Krystal et al, 1999; Mohn et al, 1999). In this study, we tested the effects of a D2R β-arrestinergic compound in pharmacological or genetic models of hypoglutamatergia and found that it reduced hyperlocomotion in the open field, rescued PPI, normalized NORM, partially restored social behavior, decreased CAR at a dose that did not affect aversive memories, and elicited a lower level of catalepsy than haloperidol.
In a previous study (Allen et al, 2011), UNC9975 and UNC9994 reduced acute PCP-stimulated hyperlocomotion. In the present experiment we used NR1-KD mice that are persistently hypoglutamatergic and found that acute administration of both UNC compounds decreased their hyperactivity. UNC9994 was less potent than UNC9975 in these mutants; an effect consistent with that reported for PCP-stimulated hyperlocomotion in βArr2 mice (Allen et al, 2011). As with the UNC compounds, haloperidol dose-dependently reduced hyperactivity in the NR1-KD mice while leaving WT motor responses intact. Similar effects on WT and NR1-KD activities have been reported with olanzapine and clozapine (Mohn et al, 1999; Duncan et al, 2006a). Because of the effects of NMDA receptor antagonists on schizophrenia-like symptoms in humans and the induction of hyperactivity in rodents, our findings suggest that UNC9975 and UNC9994 may have therapeutic efficacy for treating certain schizophrenic symptoms.
Although PPI is perturbed in several different neuropsychiatric disorders (Braff et al, 2001), it has high translational appeal and is disrupted in schizophrenia (Moghaddam et al, 1997; Krystal et al, 1999; Powell et al, 2012). We found that 0.5 mg/kg UNC9975 restored PPI in NR1-KD mice. In addition, we confirmed that haloperidol normalized PPI in the NR1-KD mice to the levels of the WT vehicle controls. This finding is consistent with others showing that nonfunctionally selective APDs such as haloperidol, olanzapine, and risperidone increased PPI in these mutants (Duncan et al, 2006a, 2006b).
To examine UNC9975 specificity, PPI was evaluated in βArr2 mice. Whereas 0.2 mg/kg restored PCP-disrupted PPI in WT animals, a 1 mg/kg dose was required in βArr2-KO mice. This finding provides support for the functional selectivity of UNC9975 and its antipsychotic efficacy as suggested by Allen et al (2011). However, the PPI results with 1 mg/kg UNC9975 suggest there may be some off-target actions at this dose. In the paper of Allen et al (2011), we show that UNC9975 binds not only the D2R, but also the 5-HT2A and D3R and it has Gq activity at the former receptor. Nevertheless, it is unlikely that the action of UNC9975 in PPI is through 5-HT2A because in the Supplementary Information to the paper of Allen et al (2011), we report that SR46349B (a 5-HT2A/C agonist) suppresses PCP-stimulated hyperlocomotion to similar extents in WT and βArr2-KO mice. Another consideration is that of the D3R. As UNC9975 binds the D2R and D3R with similar affinities (Allen et al, 2011) and because selectivity of ligands is poor between these receptors, behavioral responses cannot be distinguished between them. Hence, some of the actions of UNC9975 attributed to the D2R may include the D3R. Another issue pertains to actions at βArr2 compared with βArr1. We feel that the actions of UNC9975 are mediated primarily through βArr2 rather than βArr1 because βArr2-KO mice are relatively unresponsive to an acute injection of amphetamine in the open field and amphetamine-stimulated hyperlocomotion in DAT-βArr2 double KO mice is reduced relative to that of DAT-KO animals (Beaulieu et al, 2005). Regardless, the ability of UNC9975 to restore PPI in both NR1-KD and PCP-treated C57BL/6 mice further indicates that UNC9975 has efficacy in treating conditions that involve chronic and acute hypoglutamatergia.
Recognition memory was also examined in NR1-KD mice. It has been proposed that there are similarities between declarative memory in humans and NORM in rodents (Horiguchi and Meltzer, 2012). We found that NR1-KD mice were deficient on this task, and although 0.1 mg/kg haloperidol was ineffective, 1 mg/kg UNC9975 restored their NORM to WT levels. This performance was achieved, however, after 2 training days with the compound and testing conducted in its absence. Hence, UNC9975 may enhance the acquisition or consolidation of NORM in NR1-KD mice. Despite these procognitive effects, the 1 mg/kg dose may possess D2R β-arrestinergic as well as some off-target actions.
We evaluated social behavior as this can be abnormal in individuals with schizophrenia (Couture et al, 2006) and in NR1-KD mice (Mohn et al, 1999; Halene et al, 2009). In addition, rodents given NMDA antagonists display social withdrawal that can be partially rescued with certain APDs (Geyer and Ellenbroek, 2003; Porsolt et al, 2010; Gobira et al, 2013). In the sociability test, vehicle-treated NR1 WT mice demonstrated high social affiliation and social preference for the novel mouse, whereas NR1-KD animals showed no preferences in either test. These genotype differences were not confounded by competing behaviors in mutants as the duration and numbers of social contacts were similar between genotypes. Mohn et al (1999) reported that 0.5 mg/kg clozapine augmented social investigation and reduced escapes in NR1-KD mice in the resident–intruder test. We observed the same clozapine dose partially restored social affiliation; however, social preference was unaffected. In contrast, 0.5 mg/kg aripiprazole enhanced both social affiliation and social preference in NR1-KD mice. Interestingly, 0.5 mg/kg UNC9975 also normalized social affiliation, whereas the 1 mg/kg dose restored social preference. The effect of this higher dose may be because of β-arrestinergic as well as off-target actions as 1 mg/kg UNC9975 normalized PCP-disrupted PPI in βArr2-KO mice.
A behavioral test that has high predictive validity for detecting APD properties is CAR (Wadenberg, 2010). Both 1 and 2 mg/kg UNC9975 and 4 mg/kg UNC9994 depressed CAR. It is significant that the UNC-treated mice engaged in some escape behaviors, indicating these doses of UNC9975 and UNC9994 did not produce immobility. Although 1 and 2 mg/kg UNC9975 were equipotent in both genotypes, 4 mg/kg UNC9994 was more suppressive in βArr2-KO mice. However, the effects at these doses may not be due solely to β-arrestinergic actions (Allen et al, 2011). Nevertheless, the reductions in CAR with the UNC compounds are consistent with APD actions in this test. Regardless of action, it is possible that UNC compounds reduce CAR by perturbing retention or recall of associative memory. To address this point we examined the effects of UNC9975 on fear memory in βArr2 mice. At 24 h after fear conditioning, the 1 and 2 mg/kg UNC9975 freezing responses were distinguished neither by genotype nor from the vehicle control. Hence, similar to that of APDs (Li et al, 2004), UNC9975 does not influence fear memories.
Catalepsy evaluates possible motoric side effects of APDs (Sanberg et al, 1988). We observed cataleptic responses with 5 mg/kg UNC9975; however, they were significantly lower than with 2 mg/kg haloperidol. This is significant as both UNC9975 and haloperidol bind to the D2R with similar affinities (Caron et al, 1978; Allen et al, 2011). Haloperidol-induced catalepsy in WT mice was high at 30 min and it remained at this level throughout testing, whereas catalepsy increased over time in βArr2-KO animals. In comparison, UNC9975-induced catalepsy was highest at 60 min in both genotypes but it was much lower in mutants than WT mice, suggesting that the cataleptic effects of UNC9975 may be mediated through the βArr signaling pathways. This finding is different from a previous report (Allen et al, 2011) where catalepsy was higher with UNC9975 in βArr2-KO than WT mice. The discrepancy between these studies may be because of the protocol where they used the inclined screen test with the same cohort of mice at each time point, whereas we used the horizontal rod test with different cohorts of naive mice at each time. Regardless of paradigm, UNC9975 possesses low cataleptic activity.
Schizophrenia is a complex disorder where many different alleles are postulated to underlie the condition. A recent genome-wide study with >36 000 schizophrenia cases has found the highest associations are with the D2R gene, as well as other genes concerned with glutamatergic neurotransmission (Ripke et al, 2014). The D2R association is significant because all currently approved APDs bind to this receptor and alter cAMP production. Interestingly, many APDs also antagonize βArr recruitment to the D2R (Masri et al, 2008). Despite multiple actions at the D2R and other receptors, most APDs are efficacious in treating positive symptoms and, at best, a few exert mild effects on negative and cognitive symptoms of schizophrenia (Leucht et al, 2009; Miyamoto et al, 2012). The recognition that GPCR ligands can be functionally selective provides us with an unprecedented opportunity to develop new drugs that selectively target the G protein or βArr pathways. In the present studies, we demonstrate that the β-arrestinergic compounds—UNC9975 and UNC9994—are efficacious in ameliorating a broad range of schizophrenia-like behaviors in mice. Importantly, these compounds show efficacy in mice in this study with persistent hypoglutamatergia and in the hyperdopaminergic amphetamine model (Allen et al, 2011). Although we do not know the efficacy of biased compounds in treating patients, the ability to manipulate the functional selectivity of ligands may provide a unique opportunity to develop drugs with fewer side effects, greater therapeutic selectivity, and enhanced efficacy for treating schizophrenia and related disorders than curently available medications.
Funding and Disclosure
The authors declare no conflict of interest. MGC has compensation from Lundbeck as a member of their Psychopharmacology Advisory Board and is a consultant for Omeros. MGC also owns stock in Acadia Pharmaceutical. A Sponsored Research Agreement from Hoffmann LaRoche to Duke University has supported investigations in the Caron laboratory unrelated to this work.
Acknowledgments
We thank Theodore Rhodes, Zayd Ahmed, Christopher Means, Paul Skiba, and Brittany Thompson for assisting with some of the behavioral testing and Ms Jiechun Zhou for genotyping and maintaining the mice. Some of the behavioral experiments were conducted with equipment and software purchased with a North Carolina Biotechnology Center grant. We obtained the βArr2 mice from Dr Laura Bohn (Scripps Research Institute, Juniper, FL) and they were further backcrossed with C57BL/6J mice. We also received the NR1 mice from Dr Amy J Ramsey (University of Toronto, Ontario, Canada) and they were further backcrossed to C57BL/6 and 129 mice as separate lines. This work was supported by the NIMH grant U19-MH082441. Drs Caron, Jin, and Wetsel received NIH funding.
Author contributions
Most of the experiments were conducted by SMP, MC, and CMS; the sociability test was run by RSD, SMP, CMS, and RMR, NORM was run by RMR, and all of these investigators participated in statistically analyzing the data. JJ supplied the UNC9975 and UNC9994 and helped write the manuscript. MGC supplied the NR1 mice that had been backcrossed onto the C57BL/6 and 129 backgrounds and he helped write the manuscript. WCW designed the behavioral experiments, helped to make the graphs, and with SMP and others wrote the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M et al (2011). Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA 108: 18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC (1995). Symptoms, signs, and diagnosis of schizophrenia. Lancet 346: 477–481. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG (2005). An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122: 261–273. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR et al (2004). Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA 101: 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J, Gainetdinov RR, Caron MG (2007). The Akt–GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci 28: 166–172. [DOI] [PubMed] [Google Scholar]
- Boyd KN, Mailman RB (2012). Dopamine receptor signaling and current and future antipsychotic drugs. Handb Exp Pharmacol 212: 53–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR (2001). Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 156: 234–258. [DOI] [PubMed] [Google Scholar]
- Caron MG, Beaulieu M, Raymond V, Gagne B, Drouin J, Lefkowitz RJ et al (1978). Dopaminergic receptors in the anterior pituitary gland. Correlation of [ 3H]dihydroergocryptine binding with the dopaminergic control of prolactin release. J Biol Chem 253: 2244–2253. [PubMed] [Google Scholar]
- Citrome L (2014). Unmet needs in the treatment of schizophrenia: new targets to help different symptom domains. J Clin Psychiatry 75(Suppl 1): 21–26. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL (2006). The functional significance of social cognition in schizophrenia: a review. Schizophr Bull 32: S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH (1976). Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192: 481–483. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Desmarais C, Sarret P, Grignon S (2013). α-Lipoic acid interaction with dopamine D2 receptor-dependent activation of the Akt/GSK-3β signaling pathway induced by antipsychotics: potential relevance for the treatment of schizophrenia. J Mol Neurosci 50: 134–145. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA et al (2004). Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res 153: 507–519. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH (2006. a). Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharm Biochem Behav 85: 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH (2006. b). Effects of haloperidol, clozapine, and quetinapine on sensorimotor gating in a genetic model of reduce NMDA receptor function. Psychopharmacology (Berl) 184: 190–200. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA (2004). Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 36: 131–137. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Milenkovic M, Liu S, Mielnik CA, Beerepoot P, John CE et al (2014). Sustained N-methyl-D-aspartate receptor hypofunction remodels the dopamine system and impairs phasic signaling. Eur J Neurosci 40: 2255–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger HC, Zis AP, Phillips AG (1975). Haloperidol-induced disruption of conditioned avoidance responding: attenuation by prior training or by anticholinergic drugs. Eur J Pharmacol 30: 309–314. [DOI] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB (2004). Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol 66: 97–105. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B (2003). Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry 27: 1071–1079. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Caron MG (1993). Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci 16: 299–321. [DOI] [PubMed] [Google Scholar]
- Gobira PH, Ropke J, Aguiar DC, Crippa JA, Moreira FA (2013). Animal models for predicting the efficacy and side effects of antipsychotic drugs. Rev Bras Psiquiatr 35: S132–S139. [DOI] [PubMed] [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL et al (2009). Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav 8: 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M, Meltzer HY (2012). The role of 5-HT1A receptors in phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. Psychopharmacology (Berl) 221: 205–215. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P et al (2003). H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28: 519–526. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003). NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 169: 215–233. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS et al (1999). NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry 7: 125–143. [PubMed] [Google Scholar]
- Lee YS, Silva AJ (2009). The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci 10: 1260140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK (2005). Transduction of receptor signals by β-arrestins. Science 308: 512–517. [DOI] [PubMed] [Google Scholar]
- Lei G, Xia Y, Johnson KM (2008). The role of Akt-GSK3β signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacology 33: 1343–1353. [DOI] [PubMed] [Google Scholar]
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM (2009). How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 14: 429–447. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F et al (2013). Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382: 951–962. [DOI] [PubMed] [Google Scholar]
- Li M, Parkes J, Fletcher PJ, Kapur S (2004). Evaluation of the motor initiation hypothesis of APD-induced conditioned avoidance decreases. Pharm Biochem Behav 78: 811–819. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ et al (1999). β-arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283: 655–661. [DOI] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR et al (2008). Antagonism of dopamine D2 receptor/β-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA 105: 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KA, Hutchinson AN, Wong-Goodrich SJE, Presby MM, Su D, Rodriguiz RM et al (2010). Reduced cortical BDNF expression and aberrant memory in Carf knock-out mice. J Neurosci 30: 7453–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic M, Mielnik CA, Ramsey AJ (2014). NMDA receptor deficient mice display sexual dimophism in the onset and severity of behavioural abnormalities. Genes Brain Behav 13: 850–862. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA (2012). Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry 17: 1206–1227. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH (1999). Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98: 427–436. [DOI] [PubMed] [Google Scholar]
- Moritz S, Andreou C, Klingberg S, Thoering T, Peters MJ (2013). Assessment of subjective cognitive and emotional effects of antipsychotic drugs. Effect by defect? Neuropharmacology 72: 179–186. [DOI] [PubMed] [Google Scholar]
- Park SW, Seo MK, Cho HY, Lee JG, Lee BJ, Seol W et al (2011). Differential effects of amisulpride and haloperidol on dopamine D2 receptor-mediated signaling in SH-SY5Y cells. Neuropharmacology 61: 761–769. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Moser PC, Castagne V (2010). Behavioral indices in antipsychotic drug discovery. J Pharmacol Exp Ther 333: 632–638. [DOI] [PubMed] [Google Scholar]
- Powell SB, Weber M, Geyer MA (2012). Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders. Curr Top Behav Neurosci 12: 251–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA (2001). Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci 21: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA et al (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg PR, Bunsey MD, Giordano M, Norman AB (1988). The catalepsy test: its ups and downs. Behav Neurosci 102: 748–759. [DOI] [PubMed] [Google Scholar]
- Schimmelmann BG, Paulus S, Schacht M, Tilgner C, Schulte-Markwort M, Lambert M (2005). Subjective distress related to side effects and subjective well-being in first admitted adolescents with early-onset psychosis treated with atypical antipsychotics. J Child Adolesc Psychopharmacol 15: 249–258. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT (2002). Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42: 165–179. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H et al (2007). Functional selectivity and classical concepts of quantitative pharmacology. J Pharm Exp Ther 320: 1–13. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML (2010). Conditioned avoidance response in the development of new antipsychotics. Curr Pharm Des 16: 358–370. [DOI] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC et al (2011). Synaptic dysfunction and abnormal behaviors in mice lacking the major isoforms of Shank3. Hum Mol Gene 20: 3093–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Wang H, Zeng Z, Lin J, Little PJ, Srivastava LK et al (2012). The possible role of the Akt signaling pathway in schizophrenia. Brain Res 1470: 145–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.