Abstract
Objective:
This exploratory clinical trial evaluated the safety and clinical activity of a novel, sustained-exposure formulation of ciprofloxacin microparticulates in poloxamer (OTO-201) administered during tympanostomy tube placement in children.
Methods:
Double-blind, randomized, prospective, placebo- and sham-controlled, multicenter Phase 1b trial in children (6 months to 12 years) with bilateral middle ear effusion requiring tympanostomy tube placement. Patients were randomized to intraoperative OTO-201 (4 mg or 12 mg), placebo, or sham (2:1:1 ratio).
Results:
Eighty-three patients (52 male/31 female; mean age, 2.80 years) were followed for safety (otoscopic exams, cultures, audiometry, and tympanometry) and clinical activity, defined as treatment failure (physician-documented otorrhea and/or otic or systemic antibiotic use ≥3 days post surgery). At baseline, 14.3% to 36.8% of children showed positive cultures of middle ear effusion samples in at least 1 ear. Through day 15, treatment failures accounted for 14.3%, 15.8%, 45.5%, and 42.9% of patients (OTO-201 4 mg, OTO-201 12 mg, placebo, and sham, respectively); treatment failure reductions for OTO-201 doses were significant compared to pooled control (P values = .023 and .043, respectively). Observed OTO-201 safety profile was indistinguishable from placebo or sham.
Conclusions:
Results of this first clinical trial suggest that OTO-201 was well tolerated and shows preliminary clinical activity in treating tympanostomy tube otorrhea.
Keywords: middle ear effusion, otitis media, OTO-201, ciprofloxacin, sustained exposure, tympanostomy tube, culture, otorrhea
Introduction
Tympanostomy tube placement (TTP) is the most common pediatric ambulatory surgery in the US, with approximately 1 million annual cases.1 Children with recurrent acute otitis media or chronic otitis media with effusion (OME) commonly require TTP. Unfortunately, tympanostomy tube otorrhea (TTO) occurs in as many as half of patients within the first 2 weeks following surgery and more than 80% of patients in the late postoperative period (>1 month).2,3 Topical antibiotics are commonly administered during and for a short duration after TTP to reduce the rate of early TTO. Though a single intraoperative dose may reduce the rate of TTO, multiple days of therapy may be more effective in the presence of mucoid or purulent effusion.4,5 However, the safety and efficacy of perioperative topical antibiotics has not been demonstrated in prospective multicenter clinical trials and remains an unapproved use for these products. Thus, there is a need for robust clinical data to establish the role of perioperative topical antibiotic use that includes the microbiological assessment of baseline effusions by sensitive methods. Furthermore, there is an unmet medical need for an approved, single-dose antibiotic therapy that can be administered by the otolaryngologist during TTP, thereby eliminating the need for repeat otic drop administration by the caregiver, removing compliance issues, and ensuring sufficient drug exposure to effectively treat middle ear effusion (MEE) in pediatric patients requiring TTP.
OTO-201 (Otonomy, Inc, San Diego, California, USA) is a suspension of ciprofloxacin in a buffered solution containing a glycol polymer, poloxamer 407. At the concentration employed, the poloxamer vehicle exhibits thermo-reversible properties. OTO-201 exists as a liquid at or below room temperature and gels at body temperature. This provides prolonged (1-2 weeks) exposure of the middle ear to ciprofloxacin.6 Ciprofloxacin is approved for the topical treatment of acute otitis externa and acute OM in pediatric patients with tympanostomy tubes.7 The purpose of this exploratory trial was to describe the safety and clinical activity of OTO-201 in pediatric patients with bilateral MEE requiring TTP.
Methods
Trial Design
A double-blind, randomized, prospective, placebo- and sham-controlled, multicenter, Phase 1b clinical trial of OTO-201 administered as a single dose intraoperatively to children with bilateral MEE who required TTP was institutional review board approved. Given this was a pediatric trial, caregiver compliance with the protocol was required as well as written informed consent and Health Insurance Portability and Accountability Act (HIPAA of 1996) documents before initiation of trial-related procedures. The trial was conducted in accordance with the regulations and guidelines of the United States Food and Drug Administration (FDA), Declaration of Helsinki and current amendments, and the International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) guidelines
For patients 4 years or younger, hearing was tested, at a minimum, by distortion product otoacoustic emission (DPOAE) in both ears and visual reinforcement audiometry (VRA) in 1 ear at 2 frequencies, using both air and bone conduction to be eligible for enrollment.
Two dose levels of OTO-201 (4 and 12 mg of ciprofloxacin) were evaluated relative to placebo (vehicle only) and sham (air) in 2 dose cohorts, which were conducted sequentially, and patients were assigned to groups using a 2:1:1 ratio.
Randomization and Trial Intervention
Randomization to trial drug within each cohort was stratified by age (6 months to 2 years or >2 years). A permuted-block randomization algorithm was used to generate the patient’s randomized treatment assignment, which was deployed via an interactive web-response system. While assignment to dose cohort was not blinded, within each dose cohort, the random assignment to trial drug was double-blind.
The main inclusion criteria required that patients were male or female, aged 6 months to 12 years, and had a clinical diagnosis of bilateral MEE requiring TTP. Exclusion criteria were: (1) history of prior ear or mastoid surgery, not including myringotomy or myringotomy with tympanostomy tube (TT); (2) designated for any other surgical procedure that would occur concurrently with TTP, such as but not limited to adenoidectomy or tonsillectomy; (3) history of sensorineural hearing loss; (4) history of chronic or recurrent bacterial infections other than otitis media that likely will require treatment with antibiotics during the course of the trial; (5) history of tympanic membrane perforation; (6) history of known immunodeficiency disease; (7) abnormality of the tympanic membrane or middle ear that would preclude precise placement of trial drug or intratympanic injection; (8) topical nonsteroidal otic agents within 1 day of randomization; (9) use of nasal, inhaled, and topical steroids during the trial; (10) any infection requiring systemic antimicrobial or antifungal agents; (11) topical or systemic antimicrobial or antifungal agents; amoxicillin with clavulanate potassium, cefdinir, ceftriaxone, and cephalexin within 3 days of randomization; doxycycline and fluoroquinolones within 7 days; and azithromycin within 14 days of randomization; (12) concurrent use of oral anti-inflammatory agents; (13) history of allergy to ciprofloxacin or any of the components of OTO-201; (14) clinically significant illness or medical condition that in the opinion of either the investigator or medical monitor would prohibit the patient from participating in the trial; (15) use of an investigational drug or device in the month prior to screening; (16) previous exposure to OTO-201; and (17) menarcheal or post-menarcheal female. In addition, for patients 4 years or younger, the patient must complete, at a minimum, DPOAE in both ears and VRA in 1 ear at 2 frequencies.
Eligible patients were randomized to treatment assignment on the surgery day (day 1), prior to TTP. Qualified medical personnel other than the treating physician prepared all syringes, including sham, to maintain the blind. Before trial drug injection, a MEE sample was aspirated from each ear for culture and to allow sufficient space for the trial drug. OTO-201, placebo, or sham was administered in both ears following myringotomy by an otolaryngologist who was unblinded based on the difference in the appearance of OTO-201 compared to placebo and sham.
Patients visited the trial center on days 4, 8, 15, and 29 for safety assessments and otoscopic examination. The assessment of TTO for the clinical activity endpoint (a visual external ear exam) occurred on days 4, 8, 15, and 29 by a blinded medical professional who was not present during surgery. A specimen for culture was obtained if TTO in the auditory canal was observed. Patients with otorrhea were eligible for otic antibiotic drop administration if it was determined by the investigator to be in the best interests of the health and welfare of the patient; the patient remained on trial to be monitored for safety.
Trial Outcomes
Safety assessments included treatment-emergent adverse events, otoscopy, audiometric testing, and tympanometry. For children able to complete audiometric testing (typically ≥4 years of age), hearing was assessed at baseline, day 15, and day 29. The degree of hearing loss was calculated using the pure tone average (ie, average of the air conduction thresholds measured at frequencies 500, 1000, and 2000 Hz by ear), with hearing loss categorized as normal (0-15 dB), Grade 1 (16-40 dB), Grade 2 (41-55 dB), Grade 3 (56-71 dB), or Grade 4 (≥72 dB). For children too young to complete conventional audiometric testing, VRA and DPOAE testing was performed at baseline, day 15, and day 29 to assess hearing function. Standard tympanometric assessments performed at screening and days 4, 8, 15, and 29 assessed the volume, mobility, peak pressure, and compliance of the ear canal and middle ear as an objective test of tube patency and middle ear status.
Standard microbiological cultures were performed on baseline MEE samples as well as on postsurgical TTO samples. If positive, microorganisms were identified by using Bruker matrix-assisted laser desorption ionization–time-of-flight (Maldi-TOF) Biotyper.
Clinical activity of OTO-201 was assessed by the proportion of treatment failures through the day 15 visit and was defined as the first event from among the following: (1) the presence of TTO noted by the nontreating physician during the visual external ear exam on or after 3 days postsurgery (day 4), (2) the requirement for otic antibiotic drop administration in the best interest of the patient as determined by the treating physician during the otoscopic exam, (3) the requirement for systemic antibiotic administration in the best interest of the patient as determined by the treating physician, or (4) loss to follow-up. Other clinical activity endpoints included the proportion of treatment failures at days 4, 8, and 15 and for patients with positive effusion cultures, microbiological eradication (ie, positive or negative) of pre-therapy bacterial pathogens.
Statistical Analysis
The planned sample size was 80 patients, with 40 patients per dose cohort, and 20, 10, and 10 patients receiving OTO-201, placebo, or sham, respectively. For this first in-human trial of OTO-201, the sample size was based on clinical experience relative to trial design and objectives. The trial was not designed to provide adequate power for hypothesis testing related to efficacy; therefore, the analytic focus for clinical activity endpoints was descriptive, and the interpretation was considered exploratory.
The Cochran-Mantel-Haenszel (CMH) test stratified by age group compared each OTO-201 dose group to the pooled placebo cohorts and pooled sham cohorts groups as well as a single control group consisting of the combined placebo and sham groups across all cohorts. Only the CMH results comparing each OTO-201 dose group to the single control group are reported here. Due to the small sample size, the CMH exact tests and corresponding P values also were calculated for the comparisons and are the P values quoted in this article. Due to the exploratory nature of the trial, no adjustments for multiple comparisons were made. Unless otherwise stated, the analysis of the treatment failure endpoint was conducted using all randomized patients who received at least 1 dose of trial drug.
Results
Patients and Baseline Characteristics
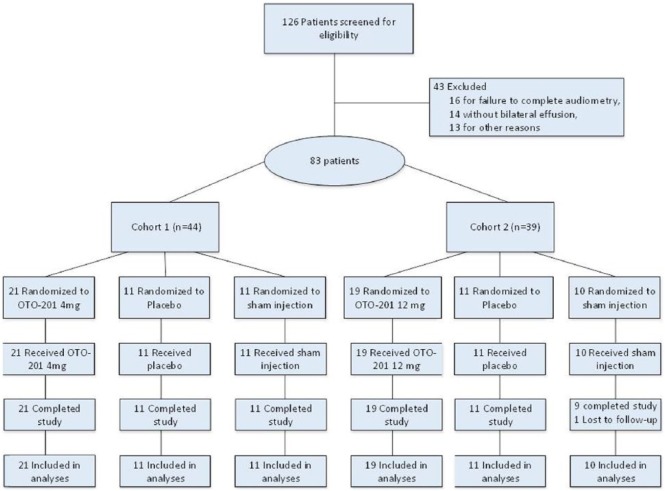
A total of 83 patients were randomized between January 8 and June 17, 2013, at 12 clinical sites in the US (Figure 1). Table 1 summarizes patient baseline demographics and disease characteristics. A total of 52 (62.7%) male and 31 (37.3%) female patients, with a mean age of 2.80 years (range, 0.6-10.0 years), participated in the trial, and all but 1 patient completed the trial through day 29 (1 patient was lost to follow-up). Baseline effusion types were generally balanced across all groups, and right and left ears for all patients were mostly characterized as serous (42.2% and 39.8%, respectively), mucoid (22.9% and 22.9%, respectively), or purulent (22.9% and 25.3%, respectively).
Figure 1.
Flow of patients through the trial.
Table 1.
Baseline Demographics and Disease Characteristics.
| Characteristic | OTO-2014 mg(N = 21)n (%) | OTO-20112 mg(N = 19)n (%) | PooledPlacebo(N = 22)n (%) | PooledSham(N = 21)n (%) | |
|---|---|---|---|---|---|
| Age, years | Mean (SD) | 2.98 (2.636) | 2.85 (2.229) | 2.52 (1.945) | 2.87 (2.326) |
| Male | No. (%) | 15 (71.4) | 10 (52.6) | 12 (54.5) | 15 (71.4) |
| Female | 6 (28.6) | 9 (47.4) | 10 (45.5) | 6 (28.6) | |
| Race | No. (%) | ||||
| White | 18 (85.7) | 15 (78.9) | 20 (90.9) | 17 (81.0) | |
| Black | 1 (4.8) | 3 (15.8) | 2 (9.1) | 2 (9.5) | |
| Other | 2 (9.5) | 1 (5.3) | 0 | 2 (9.5) | |
| Left ear | Effusion type | ||||
| Mucoid | 1 (4.8) | 8 (42.1) | 6 (27.3) | 4 (19.0) | |
| Purulent | 4 (19.0) | 8 (42.1) | 3 (13.6) | 6 (28.6) | |
| Sanguineous | 1 (4.8) | 0 | 0 | 0 | |
| Serous | 11 (52.4) | 3 (15.8) | 10 (45.5) | 9 (42.9) | |
| Other | 4 (19.0) | 0 | 3 (13.6) | 2 (9.5) | |
| Positive microbiology culture | 5 (23.8) | 5 (26.3) | 8 (36.4) | 4 (19.0) | |
| Right ear | Effusion type | ||||
| Mucoid | 1 (4.8) | 8 (42.1) | 6 (27.3) | 4 (19.0) | |
| Purulent | 5 (23.8) | 6 (31.6) | 3 (13.6) | 5 (23.8) | |
| Sanguineous | 1 (4.8) | 0 | 0 | 0 | |
| Serous | 10 (47.6) | 5 (26.3) | 10 (45.5) | 10 (47.6) | |
| Other | 4 (19.0) | 0 | 3 (13.6) | 2 (9.5) | |
| Positive Microbiology Culture | 2 (9.5) | 6 (31.6) | 5 (22.7) | 5 (23.8) | |
| One ear | Positive microbiology culture | 5 (23.8) | 7 (36.8) | 7 (31.8) | 3 (14.3) |
| Both ears | Positive microbiology culture | 1 (4.8) | 2 (10.5) | 3 (13.6) | 3 (14.3) |
Safety
There were no deaths, no serious adverse events related to trial drug, and no adverse events leading to discontinuation from the trial or from trial drug. Most adverse events were mild or moderate in severity. The proportions of patients who experienced treatment-emergent adverse events were 61.9% (OTO-201 4 mg), 47.4% (OTO-201 12 mg), 54.5% (placebo), and 85.7% (sham treatment); ear-related treatment-emergent adverse events were experienced by 23.8%, 21.1%, 40.9%, and 71.4%, respectively; Table 2). The most frequent treatment-emergent adverse events for the OTO-201 4 mg, OTO-201 12 mg, placebo, and sham groups were otorrhea (defined as discharge from the ear, 4.8%, 5.3%, 31.8%, and 38.1%, respectively), upper respiratory tract infection (14.3%, 0%, 9.1%, and 4.8%, respectively), pyrexia (0%, 21.2%, 4.5%, and 9.5%, respectively), and ear infection (most commonly otitis media, 4.8%, 5.3%, 13.6%, and 9.5%, respectively). The types of events in OTO-201–treated patients were indistinguishable from those observed in the placebo and sham groups.
Table 2.
Summary of Treatment-Emergent Adverse Events (TEAE).
| Preferred Terma | OTO-2014 mg(N = 21)n (%) | OTO-20112 mg(N = 19)n (%) | PooledPlacebo(N = 22)n (%) | PooledSham(N = 21)n (%) |
|---|---|---|---|---|
| Patients with any TEAE | 13 (61.9) | 9 (47.4) | 12 (54.5) | 18 (85.7) |
| Patients with any ear-related TEAE | 5 (23.8) | 4 (21.1) | 9 (40.9) | 15 (71.4) |
| Most frequent TEAEs | ||||
| Otorrhoea | 1 (4.8) | 1 (5.3) | 7 (31.8) | 8 (38.1) |
| Pyrexia | 0 | 4 (21.1) | 1 (4.5) | 2 (9.5) |
| Upper respiratory tract infection | 3 (14.3) | 0 | 2 (9.1) | 1 (4.8) |
| Ear infection | 1 (4.8) | 1 (5.3) | 3 (13.6) | 2 (9.5) |
| Diarrhea | 1 (4.8) | 2 (10.5) | 0 | 0 |
Adverse events were classified using MedDRA version 15.0.
Most patients had normal otoscopic examinations, including TT patency and no displacement at the end of trial, and there was no apparent difference between exams in OTO-201-treated patients and those exams in the placebo and sham groups.
For audiometric assessments at baseline, most patients had an air-bone gap >10 dB observed across frequencies 500 to 2000 Hz (generally >50% of patients had an air-bone gap >10 dB for each treatment group and frequency) (Table 3). By the end of the trial, the proportion of patients with an air-bone gap >10 dB was generally reduced to approximately 30% or less for each treatment group and frequency. At baseline, most patients in each treatment group had Grade 1 hearing loss (16-40 dB), which improved to levels within normal limits (<40 dB) for most patients by day 29.
Table 3.
Summary of Audiometric Assessments (Conventional or Visual Reinforcement Audiometry), Air-Bone Gap at Baseline.a
| Ear | Frequency (Hz) | Category | OTO-2014 mg(N = 21)n (%) | OTO-20112 mg(N = 19)n (%) | Pooled Placebo (N = 22)n (%) | Pooled Sham (N = 21)n (%) |
|---|---|---|---|---|---|---|
| Left | 500 | ≤10 dB | 0 | 1 (16.7) | 1 (16.7) | 1 (16.7) |
| >10 dB | 5 (100) | 5 (83.3) | 5 (83.3) | 5 (83.3) | ||
| Missing | 16 | 13 | 16 | 15 | ||
| 1000 | ≤10 dB | 0 | 1 (16.7) | 2 (33.3) | 1 (16.7) | |
| >10 dB | 6 (100) | 5 (83.3) | 4 (66.7) | 5 (83.3) | ||
| Missing | 15 | 13 | 16 | 15 | ||
| 2000 | ≤10 dB | 3 (50.0) | 2 (33.3) | 6 (100) | 2 (33.3) | |
| >10 dB | 3 (50.0) | 4 (66.7) | 0 | 4 (66.7) | ||
| Missing | 15 | 13 | 16 | 15 | ||
| 4000 | ≤10 dB | 0 | 2 (33.3) | 5 (83.3) | 1 (16.7) | |
| >10 dB | 5 (100) | 4 (66.7) | 1 (16.7) | 5 (83.3) | ||
| Missing | 16 | 13 | 16 | 15 | ||
| Right | 500 | ≤10 dB | 0 | 2 (33.3) | 0 | 2 (33.3) |
| >10 dB | 5 (100) | 4 (66.7) | 5 (100) | 4 (66.7) | ||
| Missing | 16 | 13 | 17 | 15 | ||
| 1000 | ≤10 dB | 1 (16.7) | 1 (16.7) | 2 (40.0) | 2 (33.3) | |
| >10 dB | 5 (83.3) | 5 (83.3) | 3 (60.0) | 4 (66.7) | ||
| Missing | 15 | 13 | 17 | 15 | ||
| 2000 | ≤10 dB | 3 (60.0) | 2 (33.3) | 3 (60.0) | 3 (50.0) | |
| >10 dB | 2 (40.0) | 4 (66.7) | 2 (40.0) | 3 (50.0) | ||
| Missing | 16 | 13 | 17 | 15 | ||
| 4000 | ≤10 dB | 1 (16.7) | 2 (33.3) | 3 (60.0) | 2 (33.3) | |
| >10 dB | 5 (83.3) | 4 (66.7) | 2 (40.0) | 4 (66.7) | ||
| Missing | 15 | 13 | 17 | 15 | ||
| Nonspecific | 500 | ≤10 dB | 3 (30.0) | 1 (11.1) | 0 | 2 (22.2) |
| >10 dB | 7 (70.0) | 8 (88.9) | 12 (100) | 7 (77.8) | ||
| Missing | 11 | 10 | 10 | 12 | ||
| 1000 | ≤10 dB | 1 (10.0) | 2 (25.0) | 3 (25.0) | 4 (44.4) | |
| >10 dB | 9 (90.0) | 6 (75.0) | 9 (75.0) | 5 (55.6) | ||
| Missing | 11 | 11 | 10 | 12 | ||
| 2000 | ≤10 dB | 2 (22.2) | 2 (33.3) | 1 (14.3) | 4 (50.0) | |
| >10 dB | 7 (77.8) | 4 (66.7) | 6 (85.7) | 4 (50.0) | ||
| Missing | 12 | 13 | 15 | 13 | ||
| 4000 | ≤10 | 3 (42.9) | 1 (20.0) | 1 (14.3) | 2 (33.3) | |
| >10 dB | 4 (57.1) | 4 (80.0) | 6 (85.7) | 4 (66.7) | ||
| Missing | 14 | 14 | 15 | 15 |
Percentages are calculated based on the number of patients in safety analysis set who have nonmissing air-bone gap data for that relevant visit, ear, and frequency. The air-bone gap is equal to the air conduction threshold minus the bone conduction threshold. Air-bone gap will not be calculated for patients who have ear-specific data for air (bone) conduction but nonspecific data for bone (air) conduction for the same visit.
At baseline, for both the left and right ears, absent (<1 dB) otoacoustic emissions (OAE) activity was observed across all frequencies (1000 to 6000 Hz) for >60% of patients in each treatment group. By the end of the trial, the proportion of patients with absent OAE activity was generally reduced to approximately 30% or less for each treatment group and frequency.
Evaluation of tympanogram types on day 15 demonstrated that the majority (~80%) of patients progressed to a Type B-Large, as expected in an ear cleared of fluid with a patent TT.
Table 4 presents the microbiologic results at baseline and following treatment, as assessed by culture. For all children, 28.9% showed at least 1 ear with a positive baseline cultures of MEE samples, with the following pathogens identified: Haemophilus influenza (16.9%), Moraxella catarrhalis (7.2%), Streptococcus pneumonia (3.6%), and Streptococcus aureus (2.4%).
Table 4.
Microbiologic Results by Culture at Baseline and Following Treatment.
| Ear | Visit | Culture Result | OTO-2014 mg(N = 21)n (%) | OTO-20112 mg(N = 19)n (%) | PooledPlacebo(N = 22)n (%) | PooledSham(N = 21)n (%) |
|---|---|---|---|---|---|---|
| Left | Baseline | Negative | 16 (76.2) | 14 (73.7) | 14 (63.6) | 17 (81.0) |
| Positive | 5 (23.8) | 5 (26.3) | 8 (36.4) | 4 (19.0) | ||
| Post baseline | Negative | 1 (50.0) | 1 (100) | 1 (25.0) | 0 | |
| Positive | 1 (50.0) | 0 | 3 (75.0) | 3 (100) | ||
| Right | Baseline | Negative | 19 (90.5) | 13 (68.4) | 17 (77.3) | 16 (76.2) |
| Positive | 2 (9.5) | 6 (31.6) | 5 (22.7) | 5 (23.8) | ||
| Post baseline | Negative | 2 (50.0) | 2 (100) | 1 (14.3) | 0 | |
| Positive | 2 (50.0) | 0 | 6 (85.7) | 4 (100) |
Clinical Activity
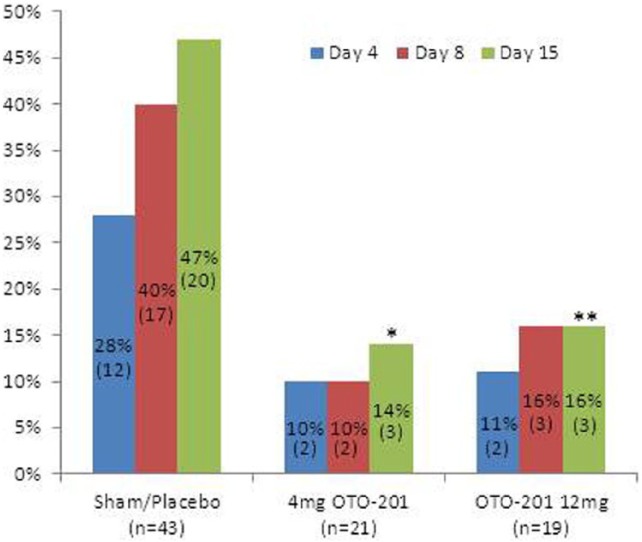
The control groups (sham and placebo) were each pooled across cohorts as no clinically meaningful or statistically significant differences between the proportion of patients with treatment failure in the low dose cohort or high dose cohort were observed at any visit for the placebo groups (Fisher’s exact test P values > .646) or for the sham injection groups (Fisher’s exact test P values > .183). At each visit, the proportion of treatment failures was lower in both OTO-201 dose groups compared with the patients in the pooled placebo or pooled sham injection groups (Table 5, Figure 2). Through day 15, 14.3%, 15.8%, 45.5%, and 42.9% of patients who received OTO-201 4 mg, OTO-201 12 mg, placebo, and sham, respectively, were treatment failures. Reduced proportions of treatment failures for the 2 OTO-201 dose groups compared to the placebo and sham groups were apparent as early as day 4 (see Figure 2). When compared to the single control group (the pooled placebo and sham groups combined), both OTO-201 dose groups had statistically significant reductions in the proportion of treatment failures by day 15 (CMH exact test P values = .023 and .043, respectively). As treatment failure was comprised of patients with otorrhea in any ear or the use of otic or systemic antibiotics (rescue medication), the proportions of patients with otorrhea through day 15 in the OTO-201 4 mg, OTO-201 12 mg, placebo, and sham groups were 9.5%, 10.5%, 36.4%, and 23.8%, respectively, and those who failed due to required rescue medication were 4.8%, 5.3%, 9.1%, and 19.0%, respectively (Table 5). No patient was a treatment failure due to loss to follow-up.
Table 5.
Proportion of Treatment Failures and Treatment Failure Causes.
| Time PeriodTreatment Failure Cause | OTO-2014 mg(N = 21)n (%) | OTO-20112 mg(N = 19)n (%) | PooledPlacebo(N = 22)n (%) | PooledSham(N = 21)n (%) |
|---|---|---|---|---|
| Through day 4 | 2 (9.5) | 2 (10.5) | 6 (27.3) | 6 (28.6) |
| Otorrhea (any ear) | 1 (4.8) | 1 (5.3) | 4 (18.2) | 3 (14.3) |
| Rescue medicationa | 1 (4.8) | 1 (5.3) | 2 (9.1) | 3 (14.3) |
| Through day 8 | 2 (9.5) | 3 (15.8) | 8 (36.4) | 8 (38.1) |
| Otorrhea (any ear) | 1 (4.8) | 2 (10.5) | 6 (27.3) | 5 (23.8) |
| Rescue medication | 1 (4.8) | 1 (5.3) | 2 (9.1) | 3 (14.3) |
| Through day 15 | 3 (14.3) | 3 (15.8) | 10 (45.5) | 9 (42.9) |
| Otorrhea (any ear) | 2 (9.5) | 2 (10.5) | 8 (36.4) | 5 (23.8) |
| Rescue medication | 1 (4.8) | 1 (5.3) | 2 (9.1) | 4 (19.0) |
Rescue medication refers to any otic or systemic antibiotic prescribed for reasons other than otorrhea observed by the blinded assessor.
Figure 2.
Proportion of treatment failures through days 4 to 15. Treatment failure is defined as any otorrhea, otic, or systemic antibiotics or loss-to-follow-up. Cochran-Mantel-Haenszel exact test P values = 0.023(*) and 0.043(**) versus pooled placebo/sham.
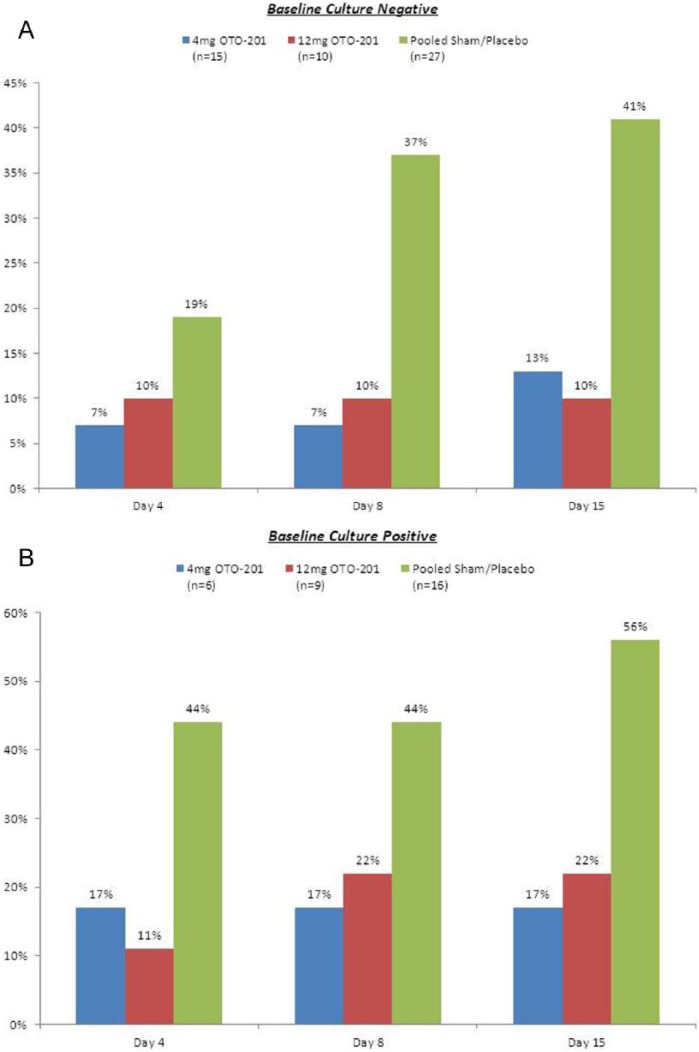
OTO-201 exhibited clinical activity regardless of bacterial culture status (Figure 3). Despite the small sample size, there was a clear indication that OTO-201 was effective regardless of baseline effusion type (data not shown).
Figure 3.
Proportion of treatment failures by baseline culture status. (A) Baseline culture negative. (B) Baseline culture positive.
As an exploratory trial, the sample size is small, but despite the limited sample size, the eradication pattern of organisms measured in postsurgical effusions by treatment group (Table 4) demonstrated that 50% to 100% of otorrhea specimens from OTO-201–treated patients showed bacterial eradication, while 75% to 85.7% and 100% of the effusion specimens derived from the placebo and sham groups, respectively, showed continued presence of microorganisms.
Discussion
This trial provided the first clinical evidence that OTO-201 was well tolerated in pediatric patients with MEE requiring TTP and that both doses of OTO-201 had similar clinical activity, as measured by treatment failure rate through the day 15 visits compared with the placebo and sham (pooled) groups. OTO-201 in this relative small group of patients was not associated with loss of TT patency or early TT displacement. For each endpoint considered, there was no apparent dose-dependent relationship, which was expected since the projected middle ear ciprofloxacin concentrations following OTO-201 treatment were anticipated to far exceed the minimum inhibitory concentration (MIC) of pathogens commonly found in MEE.8,9 The pharmacokinetic values that correlate with optimal clinical and microbiologic results as well as prevent emergence of bacterial resistance are enhanced ratios of maximum drug concentration (Cmax) to MIC and area under the concentration curve (AUC) to MIC. Pharmacokinetic values influence rational therapeutic decisions in the selection, dosage, and administration of topical fluoroquinolone drugs. The OTO-201 Cmax/MIC and AUC data for common middle ear pathogens has been shown in animal models to exhibit superior drug exposure for days to approximately 2 weeks (depending on dose) compared to the pulsatile, short-lasting exposure from ciprofloxacin/dexamethasone drops now commonly used.6
The magnitude of the difference in baseline effusions observed in this multicenter trial using conventional culture methods is almost exactly in agreement with a single-center trial.10 The high middle ear Cmax of ciprofloxacin from OTO-201 (compared to ciprofloxacin/dexamethasone drops) may also enable treatment against biofilm formation in the middle ear.6,10 In an animal model, administration of OTO-201 yielded significantly higher Cmax values, ranging from 45.4 to 99.9 kg/mL, than either of 2 forms of ciprofloxacin/dexamethasone drops (22.6 and 24.1 kg/mL, respectively), translating into a higher degree of exposure (as measured by AUC).6
The AAO-HNS strongly recommends otic antibiotics drops be administered to treat TTO because of greater efficacy, diminished systemic side effects, and lack of antibiotic resistance with up to 1000 times greater antibiotic concentration at the infection site.11 In a recent open-label trial, topical otic antibiotics for the treatment of TTO were shown to be significantly superior to both oral antibiotics and observation. At 2 weeks, TTO in children diminished to 5% after 7 days of topical antibiotics yet remained at 44% after oral antibiotics and 55% after observation only.12 Fluoroquinolones (eg, ciprofloxacin) target the main bacterial pathogens associated with chronic OME, are not ototoxic (unlike aminoglycosides), and do not have significant systemic absorption when given topically. Antibiotic ear drops are commonly prescribed for up to a week perioperatively to minimize pediatric TTO5,12; however, the drops are not FDA approved for this use.7 Ear drops commonly fail to penetrate the TT, depending on technique of placement, drop viscosity, the presence of MEE, and multiple patient-related factors.13,14 OTO-201, ciprofloxacin suspended in a glycol polymer, given at time of TTP permits prolonged exposure of ciprofloxacin in the middle ear without fluctuating peak and trough levels associated with multiple topical drop applications.6 A single intraoperative application of OTO-201 may eliminate the issue of compliance and minimizes patient (and caregiver) discomfort.
Conclusion
This first human trial suggests that intratympanically administered OTO-201 is well tolerated and shows clinical activity by reducing treatment failure as well as treatment failure due solely to TTO in pediatric patients with bilateral MEE receiving TTP. Additional studies are ongoing to confirm the safety and efficacy of OTO-201 in tympanostomy tube otorrhea and other settings.
Acknowledgments
The authors express their sincere gratitude to the following principal investigators who participated in the trial: Thomas Andrews, MD, Pediatric Ear, Nose and Throat Research Foundation, Tampa, FL; Ann Edmunds, MD, Omaha Ear, Nose & Throat Clinic, Omaha, NE; Samuel Engel, MD, Coastal Ear, Nose & Throat, Neptune, NJ; David Evans, MD, Sacramento Ear, Nose & Throat Surgical and Medical Group, Sacramento, CA; Brent Lanier, MD, Central California Ear, Nose & Throat Group, Fresno, CA; Kenneth Maxwell, MD, Piedmont Ear, Nose & Throat Associates, Winston-Salem, NC; J. Lewis Romett, MD, Colorado Ear, Nose & Throat & Allergy/Audubon Surgery Center, Colorado Springs, CO; Zorik Spektor, MD, Center for Pediatric Ear, Nose & Throat, Boynton Beach, FL; Donald Welsh, MD, and Steven Schotts, MD, Advanced Ear, Nose, Throat & Allergy Associates, Louisville, KY; David White, MD, Medical University of South Carolina, Charleston, SC. The authors thank the patients for their participation in this trial; the clinical trial site staff members, trial coordinators, statisticians, and nursing and supporting staff; the INC Research project managers, trial monitors, and remaining members of the trial team. The authors also thank Joan O’Byrne for her assistance in the article preparation.
Footnotes
Authors’ Note: Eric A. Mair, American Society of Pediatric Otolaryngology, May 16-18, 2014, Las Vegas, Nevada. Abstract ID ASPO-2014-0240. Trial registration: clinicaltrials.gov Identifier: NCT01755286 (http://clinicaltrials.gov/ct2/show/NCT01755286?term=otonomy%2C+pediatric&rank=3).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.A.M. was the lead investigator on the trial, has received financial compensation for regulatory consultation from Otonomy, Inc, but not related to his role on this trial, and drafted multiple versions of the manuscript. J.R.M. was an investigator on the trial, provided technical guidance on the trial, and was involved in manuscript review. J.E.D. and P.J.A. were members of the data safety monitoring board and were financially compensated for time spent reviewing, discussing, interpreting patient safety data, and in manuscript review. M.B. is a paid consultant to Otonomy, Inc and was involved in the trial design, statistical analysis, and manuscript review. C.L. is currently an employee of Otonomy, Inc and was involved in the trial design, operation, and manuscript drafting and review.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The trial was financially supported by Otonomy, Inc.
References
- 1. Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat. 1998;13(139):1-127. [PubMed] [Google Scholar]
- 2. Hellström S, Groth A, Jörgensen F, et al. Ventilation tube treatment: a systematic review of the literature. Otolaryngol Head Neck Surg. 2011;145(3):383-395. [DOI] [PubMed] [Google Scholar]
- 3. Ah-Tye C, Paradise JL, Colborn DK. Otorrhea in young children after tympanostomy-tube placement for persistent middle-ear effusion: prevalence, incidence, and duration. Pediatrics. 2001;107(6):1251-1258. [DOI] [PubMed] [Google Scholar]
- 4. Zipfel TE, Wood WE, Street DF, et al. The effect of topical ciprofloxacin on postoperative otorrhea after tympanostomy tube insertion. Am J Otol. 1999;20(4):416-420. [PubMed] [Google Scholar]
- 5. Hester TO, Jones RO, Archer SM, Haydon RC. Prophylactic antibiotic drops after tympanostomy tube placement. Arch Otolaryngol Head Neck Surg. 1995;121(4):445-448. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Fernandez R, Tsivkovskaia N, et al. OTO-201: nonclinical assessment of a sustained-release ciprofloxacin hydrogel for the treatment of otitis media. Otol Neurotol. 2014;35(3):459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alcon, Inc. CIPRODEX (ciprofloxacin 0.3% and dexamethasone 0.1%) sterile otic suspension. Package insert. 2009. [Google Scholar]
- 8. Jacobs MR, Bajaksouzian S, Zilles A, Lin G, Pankuch GA, Appelbaum PC. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. Surveillance study. Antimicrob Agents Chemother. 1999;43(8):1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thornsberry C, Jones ME, Hickey ML, Mauriz Y, Kahn J, Sahm DF. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 1997-1998. J Antimicrob Chemother. 1999;44(6):749-759. [DOI] [PubMed] [Google Scholar]
- 10. Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296(2):202-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenfeld RM, Schwartz SR, Pynnonen MA, et al. Clinical practice guidelines: tympanostomy tubes in children. Otolaryngol Head Neck Surg. 2013;149(1 suppl):S1-S35. [DOI] [PubMed] [Google Scholar]
- 12. Van Dongen TM, van derHeijden GJ, Venekamp RR, Rovers MM, Schilder AG. A trial of treatment for acute otorrhea in children with tympanostomy tubes. N Eng J Med. 2014;370(8):722-733. [DOI] [PubMed] [Google Scholar]
- 13. Mills RP, Albizzati C, Todd AS. Ear drops and grommets. Clin Otolaryngol Allied Sci. 1990;15(4):315-319. [DOI] [PubMed] [Google Scholar]
- 14. Boyd NH, Gottachall JA. Assessing the efficacy of tragal pumping: a randomized controlled trial. Otolaryngol Head Neck Surg. 2011;144(6):891-893. [DOI] [PubMed] [Google Scholar]